Abstract
This review aims to provide a broad overview of the targets, challenges and potential for gene therapy in the CNS, citing specific examples. There are a broad range of therapeutic targets, with very different requirements for a suitable viral vector. By utilizing different vector tropisms, novel routes of administration and engineered promoter control, transgenes can be targeted to specific therapeutic applications. Viral vectors have proven efficacious in preclinical models for several disease applications, spurring several clinical trials. While the field has pushed the limits of existing adeno-associated virus-based vectors, a next generation of vectors based on rational engineering of viral capsids should expand the application of gene therapy to be more effective in specific therapeutic applications.
General introduction to CNS gene therapy
The use of viral vectors to deliver genes to patients affected with neurological disorders is an attractive concept to researchers and clinicians. Since the sequencing of the human genome, opportunities to correct genetic imbalances seem to be at our fingertips; however, many hurdles remain before DNA can be used as a drug. Simply introducing naked DNA into the body is inefficient [1]. Due to the natural ability of viruses to infect cells with nucleic acid, they have gained much attention as a vector for delivery of genetic material since the 1980s. As of December 2009, a total of 1579 gene therapy clinical trials have been initiated; a majority of these trials are Phase I, but only 3.6% of trials are Phase III or beyond [201]. If gene therapy has been around for nearly three decades, why then are so few cases in Phase III trials?
Progress was handicapped in 1999 when a nonfatal disorder, treated with an adenoviral vector, led to an unfortunate fatal outcome [2]. Safety has become the key criterion for acceptable gene therapy. Other criteria can be exploited by different viruses’ advantages and limitations. The ideal vector should have:
High-transduction efficiency
Specificity for the target area
An acceptable safety profile
An appropriate level and length of transgene expression
Before viral vectors can be commonly used to treat neurological disorders, the complications of delivery to the brain and safety concerns must be addressed. One such delivery complication is the impedance of the blood–brain barrier (BBB) to the CNS [3]. These complications may explain why less than 2% of gene therapy clinical trials are aimed at neurological disorders [201]. This article briefly reviews some viral vectors currently in use, and then focuses on how adeno-associated virus (AAV) is being used as the prominent vector for gene delivery to the brain.
Introduction & overview of viral vectors
Herpes simplex virus type 1 (HSV-1) vectors are enveloped 100 nm particles with a foreign DNA packaging capacity of more than 100 kb. The greatest advantages are the high packaging capacity and natural neurotropism via retrograde axonal transport.
Lentiviral vectors are enveloped 100 nm particles with a foreign DNA packaging capacity of 9 kb. When pseudotyped, they have high neuronal tropism.
Adenoviral vectors are nonenveloped 100 nm particles with a foreign DNA packaging capacity of 25 kb. One of the first viral-based gene therapy vectors, but are generally not suited to CNS application because of high cytotoxicity.
AAV vectors are nonenveloped 25 nm particles with a foreign DNA packaging capacity of 4.6 kb. They have been clinically demonstrated to be safe in the CNS, and certain serotypes display strong neural tropism.
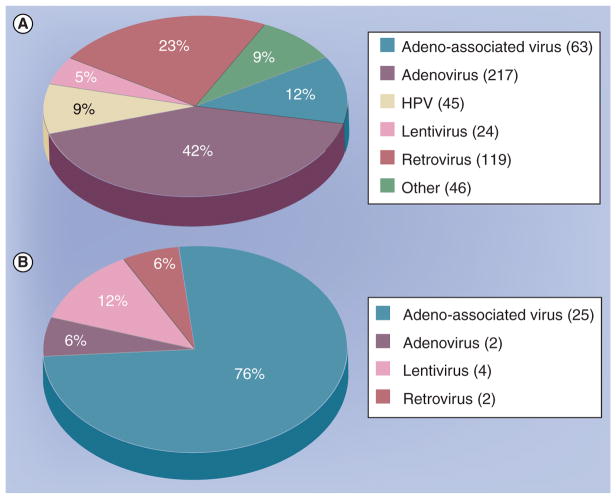
Figure 1 shows the vectors used in clinical trials for all or CNS-only applications.
Figure 1. Gene therapy trials sinze 2000.
(A) Total gene therapy clinical trials from 2000–2009 by vector, excluding nonviral delivery. (B) Gene therapy trials involving the central or peripheral nervous system (excluding cancer), from 2000–2009 by vector.
This figure was compiled by searching the database at [201].
Herpes simplex virus type 1 vector
The HSV-1 vector’s transduction pathway and neuronal tropism have been previously described [4]. Only 3.5% of current clinical trials use HSV-1, aimed mostly at targeting cancer cells. Two trials are aimed at treating chronic pain [5,201], taking advantage of HSV-1’s natural neurotropism via retrograde transport to dorsal root or trigeminal ganglions. Wider application to the CNS has been limited mainly due to the vector’s inflammatory profile. Concerns over HSV-1 immune responses have led to newer vectors and production designs to produce relatively safe recombinant HSV-1 (rHSV-1) vectors.
The rHSV-1 vectors have been made considerably safer through the removal of multiple immediate-early (IE) genes (ICP4, ICP22, ICP27, and ICP47) in the viral genome [6]. The absence of these IE genes inhibits the activation of other downstream viral genes. rHSV-1 vectors are ideal because they express few wild-type viral genes, show minimal cytotoxicity and have high trans-gene expression [7]. Vectors have been developed that show high sensitivity to the herpes antiviral drug acyclovir [8], thus prophylactic acyclovir can be administered in the event that recombination leads to the generation of a pathogenic virus. In addition to this safety feature, expression cell lines are able to produce replication-deficient HSV-1 [9]. Production can also be done in high titer and with low levels of contaminating, non-pathogenic helper virus [10]; however, even low levels could still prove problematic.
While rHSV-1 is becoming easier and safer to use for gene therapy, transgene expression length needs to be increased. Liu et al. showed that deletion of UL46 and UL47, which modulate the IE promoter, enhanced expression duration to 2 months [11]. Other research groups were able to obtain expression lasting 24 weeks in HSV-1 vectors deleted for both latency-associated transcripts and ICP4. Therefore, optimization of HSV-1 for increased transgene expression duration, production and purification methods need to be improved before HSV-1 vector is suitable for a wide number of applications in the clinic.
Lentiviral vector
The advantages of the lentiviral vector are numerous [3,12]. Despite these advantages, lentivirus is used in 1.5% of current clinical trials, with only one trial aimed at neurological disorders [201]. Similar to HSV-1, lentiviral potential seems largely unutilized. What are the technological hindrances of lentivirus, and how have advancements opened up avenues for vector use?
Cells of the monocyte/macrophage lineage are the natural target of lentivirus. The range of transducible tissue can be broadened by pseudo-typing the vector [13], and pseudotyped lentivirus can stably transduce cells without a decrement in transgene expression [14]. Researchers not only can broaden tropism, but also target specific cell types. Colin et al. showed the ability to target astrocytes in the hippocampus, striatum and cerebellum [15], and neuronal specificity can be achieved by using a neuronal-specific promoter [16]. In addition, the use of tetracycline-regulatory systems allow for an even greater control of transgene expression [17].
As most lentiviral vectors originated from HIV, safety is a major concern. In addition, traditional retroviruses raise safety concerns of insertional mutations that may lead to activation of cellular oncogenes. The concern of oncogenesis was realized with the occurrence of T-cell leukemia in 20% of children treated for severe combined immunodeficiency syndrome (SCID) using a retroviral vector [18]. To address this issue, nonintegrating lentiviruses (NIL) have a nonfunctional integrase, yet transduce neurons equally well as their integrating complement [19]. No decrement was observed in transduction efficiency or transgene expression, and expression in the brain and eye has been documented out to 1 year in vivo [20–22]. Several platforms have been developed to virtually eliminate the possibility of generating replication-competent HIV during vector manufacture without sacrificing transduction efficiency. Examples of this are a three-plasmid system, nonprimate-based lentivirus [23] or self-inactivating (SIN) lentiviral vectors [24,25].
Adenovirus
While a majority of the current clinical trials (24%) use adenovirus (Ad), no trials target neurological disorders [201]. Most likely, the main cause of this is the highly toxic nature of Ad, so this virus is more apt to cancer targeting rather than restoring damaged areas in the brain. While Ad offers numerous advantages including high neural tropism [26], the first advancement of the Ad vector is the need to reduce cytotoxicity, both in response to the vector and the viral genes.
Cytotoxicity is a result of the activation of the immune response, so reduction in antigenicity should lead to safer vectors. Although immunosuppression of patients concomitant with gene therapy would fit such criterion, it could exacerbate current conditions or promote the pathogenesis of foreign invaders that are not involved in the disease. An additional method is to electrostatically coat the vector with a multivalent copolymer that reduces both antigenicity and detection from the immune system [27,28]. This coating also allows for changes in vector tropism [28,29]. ‘Gutless’ (helper-free) vectors devoid of all viral genes have a 28-kb transgene carrying capacity [30] and long-term expression [31]. Coated ‘gutless’ vectors may be the golden combination to advance Ad into more clinical trials.
Adeno-associated virus
Wild-type AAV has a 4.7-kb single-stranded (ss) DNA genome and belongs to the parvovirus family [32,33]. Although AAV infects humans, it is nonpathogenic and it is classified as a dependo-virus because it is unable to execute a lytic infection without co-infection with a helper virus such as Ad or herpesvirus [32,33]. Importantly for CNS gene therapy applications, AAV can transduce nondividing cells and has the ability to confer long-term stable gene expression without associated inflammation or toxicity [32,34,35]. The AAV-delivered genome persists mostly as an extrachromosomal episome [36,37], but the approximately 1% of genomes that integrate in the target genome raises the hypothetical risk of insertional mutagenesis and oncogenesis. A possible relationship between AAV vectors and cancer has been somewhat controversial [38], but the most comprehensive study in over 600 mice showed no increased incidence of cancer after AAV delivery [39]. Recombinant AAV packages any DNA cassette within its size constraints as long as the DNA is flanked by approximately 145 bp inverted terminal repeats (ITR). Due to the highly conserved nature of the ITR packaging elements, identical ITR-flanked DNA cassettes are easily packaged in almost any AAV capsid. Over 100 different AAV variants have been identified, each with potentially different cell tropism, providing a broad toolkit of vectors for optimized delivery to the target cells [40]. Of the current gene therapy clinical trials, 4.5% use an AAV vector delivery system; 20% of which focus on gene transfer specific to neurological disorders, notably including Parkinson’s disease (PD) [201].
Clinical applications of viral gene therapy to the CNS
The following is a brief overview of selected gene therapy applications to the CNS that have been applied in clinical trials, including PD, Batten disease and adrenoleukodystrophy (ALD):
Parkinson’s disease treatment with AAV and lentivirus vectors has been the largest and most expansive push into CNS gene therapy
The Batten disease trial with an AAV vector demonstrates an approach to correct a global brain disorder
The ALD trial exemplifies an approach to use a lentiviral vector to enhance the application of the standard of care for ALD, namely a bone marrow transplant
Parkinson’s disease
Parkinson’s disease poses a conceptually simple scenario in terms of viral vector delivery. PD is basically a loss of dopaminergic (DA) neurons in the substantial nigra; therefore, using a vector targeted to DA cells in this specific region should be achievable (clinical symptoms described in [41]). Nevertheless, the complexity of the disorder and the numerous genes involved make this anything but a simple problem to solve. Despite this complexity, gene therapy for PD has made its way into clinical trials, and the replacement of a few key enzymes and tropic factors appear to have great promise. Of the seven clinical trials active, all except one use AAV2 as the preferred delivery vector carrying either glutamic acid decarboxylase (GAD) or a neurotropic factor. The outlying trial utilizes a lentiviral vector. GAD works to modify neurotransmitter levels and neurotropic factors work to protect neurons from atrophy. Below we will discuss the rationale for the use of these transgenes and the current status of these trials (also reviewed in [42]).
Treatment with the pharmacological agent L-dopa is initially successful, but as substantia nigra neuronal atrophy progresses, the loss of aromatic L-amino acid decarboxylase (AADC) impedes the conversion of L-dopa to dopamine, rendering the L-dopa treatment ineffective over time. AAV2-hAADC enzyme replacement in conjunction with L-dopa treatment leads to sustained transgene expression lasting at least 6 years with long-term preclinical improvements in nonhuman primates [43]. In nonhuman primates that were hemiparkinsonian after unilateral intracarotid injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), the use of AAV2-hAADC showed a marked reduction in both the amount of L-dopa needed to be benefical and the side effects. However, these improvements are only seen once AADC activity reaches a level where it is no longer the rate-limiting step in the dopamine synthesis pathway [44]. Recent clinical studies have shown AAV-mediated delivery of human AADC was well tolerated and lead to motor improvements [45–47]. Further directions will be to test higher doses of AAV-hAADC and move into placebo-controlled Phase II clinical trials.
Due to advanced patients that present with complications due to prolonged L-dopa treatment, nondopaminergic strategies have been explored. DA neuronal loss causes reduction in the GABAergic inputs to the subthalamic nucleus; therefore, augmentation of GABA may provide benefits without L-dopa side effects [48]. Within GABA synthesis, GAD is the rate-limiting step. Although functionally similar, clinical trials use both GAD isoforms: GAD65 and GAD67. Both isoforms were used in the Phase I clinical trial where twelve advanced PD patients underwent AAV2-GAD treatment [48]. All patients showed significant improvements with no adverse side effects up to final testing at 12 months postsurgery, thus showing AAV2-GAD gene therapy is a viable, safe treatment option. Recently, a Phase II clinical trial was completed that showed significant motor improvements in advanced PD patients that received the gene therapy NLX-P101 versus sham surgery. Patients remained nonmedicated during this 6 month, double-blind study and significant improvements were observed at 1- and 6-month time points with no serious adverse effects related to the therapy [202].
Whereas the above gene therapies work to correct the dopamine imbalance, neurotropic factors such as glia-derived neurotropic factor (GDNF) or its homolog neurturin (NTN) promote the survival of neurons that can slow, or even reverse, disease progression. Kordower et al. showed 80–90% recovery of MPTP-induced motor defects using AAV2-NTN (also known as CERE-120) and a marked preservation of substantia nigral neurons and striatal DA innervations [49]. These effects are long lasting for at least 6 months [50]. AAV2-NTN does not induce side effects for at least 12 months post-treatment, and is tolerated at concentrations 125-times the lowest dose that produces efficient results [50].
A more recent strategy uses tyrosine hydroxylase and its co-factor enzyme GTP-cyclohydrolase 1 packaged in AAV5 vectors. This delivery system results in enhanced DOPA production and recovery of motor functions [51]. These results were maintained for at least 6 months in rats. Although AAV2 has become the standard vector for PD treatment, other serotypes, such as AAV5, offer the ability to transduce neuronal populations at much greater efficiency.
Finally, the additional packaging capacity of lentivirus has allowed a combinatorial approach, leading to the only lentivirus-based CNS human trial. This Phase I/II trial targets delivery of ProSavin to PD patients. ProSavin is able to deliver three enzymatic genes (tyrosine hydroxylase, GTP-cyclohydrolase I and dopa decarboxylase) that are key in the dopamine synthesis pathway, thereby converting transduced cells to DA cells to replace the waning DA neuron population in the substantia nigra [52].
Batten disease
Late infantile neuronal ceriod lipofuscinosis (LINCL) is one of the three major forms of neuronal ceriod lipofuscinosis (NCL) that are collectively known as Batten disease [53,54]. LINCL has received recent attention due to a clinical trial in ten children that showed a modest but significant slowing of disease progression [55]. Complementary DNA (cDNA) of CLN2 produces the enzyme tripeptidyl peptidase 1 (TPP-1), whose absence leads to the formation of toxic intracellular inclusion bodies. LINCL patients lack functional TPP-1. In vitro cultures demonstrated that expressed TPP-1 spread to cells not originally transduced with AAV2CUhCLN2 [56]. In a LINCL murine model, delivery of CLN2 using AAV2 or other serotype vectors increased survival compared with LINCL mice receiving no treatment [57–59]. When AAV2CUhCLN2 was administered to rat striatum, TPP-1 detection was observed in other brain regions that had axonal projections to the striatum with expression lasting at least 18 months [56]. Within nonhuman primates, TPP-1 expression was exclusively neuronal and lasted at least 13 weeks [56]. Other than minor, ephemeral edema in nonhuman primates, there were no serious adverse side effects with the use of AAV2CUhCLN2 in either rats or nonhuman primates [60]. All of these preclinical studies demonstrated the safety and efficacy of the delivery system of CLN2 and paved the way for the Worgall et al. study of AAV2CUhCLN2 in humans [55]. The approach was highly invasive, requiring 12 stereotaxic vector injection through six burr holes, and adverse events were reported. One child developed status epilepticus on day 14 and died on day 49 days postsurgery, but it is not clear whether this was directly attributed to the AAV vector. 40% of subjects developed minor, ephemeral humoral responses to the vector. However, administration of vector led to significantly reduced rates of neurological decline compared with historical controls, and entices further assessment of safety and efficacy of AAV2CUhCLN2 in LINCL patients [55]. Additional studies show that early treatment is essential for maximal gene correction [57,61].
Adrenoleukodystrophy
In a recent encouraging clinical trial, two ALD patients showed cerebral demyelination stagnation after they received gene therapy using a SIN lentiviral vector [62]. This study used the strategy of ex vivo transduction of the deficient ABCD1 gene into autologous CD34+ cells, myeloablative treatment of the patients, followed by re-infusion of genetically corrected cells. Within 14–16 months of infusion, both patients showed neurological benefits, and these results were conferred with only 15% of monocytes having long-lasting ALD protein expression. While the pre-existing therapeutic strategy of hematopoietic cell transplantation is effective, it imposes constraints in terms of donor relation and a developmental window where treatment is most effective [63].
Target diseases: categories of challenge
A gene therapy approach can differ tremendously based on the paradigm of the target disease, which can be divided into four general categories:
Localized therapy, cell nonautonomous
Localized therapy, cell autonomous
Diffuse therapy, cell nonautonomous
Diffuse therapy, cell autonomous
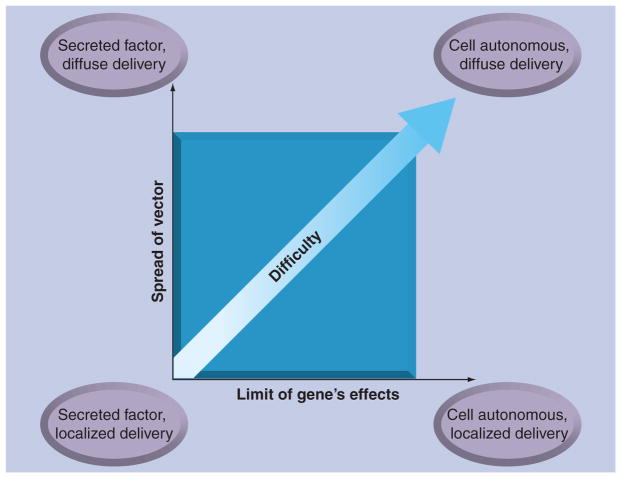
With the wide spectrum of neurological disorders comes a wide range of challenges from the perspective of gene delivery and expression. Without going into the genetic, pathological and phenotypic classes of neurological diseases, these diseases can be broken down into several categories based only on the challenges of therapeutic gene delivery. The therapeutic gene’s effect will either be cell autonomous or cell non-autonomous, which in most cases would refer to whether the gene product is secreted and taken up by neighboring cells. In addition, the therapeutic gene delivery would target a specific area of the CNS or require broad delivery throughout the CNS. This creates four general categories of diseases, which are listed as follows in increasing order of challenge and also illustrated in Figure 2.
Figure 2. General categories of CNS gene delivery.
In a broad sense, the difficulty of a gene therapy for any given disease depends on three factors: the range of vector delivery that must be achieved, the range of therapeutic effect that the transgene will impart, and how much knowledge about the disease is available to guide the choice of transgene and the extent that regulation is necessary. In this regard, a localized therapy with a secreted factor represents the easiest therapeutic approach, while a cell-autonomous transgene requiring widespread efficient vector delivery represents the most difficult. The third dimension (not shown) to predict the success of a gene therapy approach for any given disease is the available data about the disease to guide the design of an appropriate therapeutic gene cassette.
Localized therapy, cell nonautonomous
The most prominent examples of this are the therapeutic approaches for PD utilizing secreted neurotropic factors. In this case, delivery is localized to the striatum and transduced cells can become factories for the production of neurotropic factors to improve the health of neighboring nontransduced cells and areas of striatal projections, including the substantia nigra. Gene delivery does not need to reach all the affected cells and the vector can be dosed to provide a therapeutic level of secreted factor with the minimum amount of vector. Another example is epilepsy, which affects approximately 1% of the US population and in which one-third of patients do not respond well to medication [64]. The simplest means of delivery is achieved by stereotaxic injection directly to the affected area. It has been demonstrated that stereotaxic injection directly into foci for epileptogenesis, with AAV vectors expressing secreted galanin or neuropeptide Y, can greatly attenuate seizures in a rodent epilepsy model [65–67]. A new approach targeted areas of seizure damage by engineering an AAV vector to enter breaks in the BBB caused by seizures, localizing the treatment to damage foci after intravascular delivery [68]. As with PD, epilepsy gene therapy offers promise in an area of medicine not easily treated by traditional pharmacological approaches.
This approach is readily achieved with current vector technology. Gene regulation is an area of development to increase safety. Since AAV confers long-term gene expression that is permanent for all practical purposes, the long-term effects of therapeutic gene expression need to be carefully considered. As a safety mechanism, it would be valuable to have the ability to regulate the activation or inactivation of the therapeutic gene, but such a system has to exist within the packaging constraints of the delivery vector. The tetracycline-inducible system has become popular for regulating transgene expression using lentiviral [69] and AAV vectors [35]. The tetracycline response cis element and repressor together total 2.1 kb. The system is particularly useful since tetracycline can cross the BBB, tetracycline response is dose dependent [70] and the tetracycline system does not evoke an immune response, at least when used with an AAV vector [71]. This inducible system has been modified to regulate the expression of two genes [72], which is useful in disorders that are not monogenetic. Fitting such a system into the 4.6 kb packaging limit of AAV requires careful molecular engineering, but this is much less of a concern in lentiviral vectors which package up to 8–9 kb.
Localized therapy, cell autonomous
This approach is similar in all regards to localized therapy, noncell autonomous, except the efficacy of the therapeutic gene product is restricted to only the transduced cells. This adds a layer of challenge to a proposed therapy, because a vector must be chosen that specifically targets the intended cells in the region of interest. This is especially true if expression of the therapeutic gene in nontarget cells is undesirable. Moreover, a higher dose is necessary to ensure a high transduction efficiency of the target cell population. AADC gene delivery for PD blurs the boundaries of a cell-autonomous and cell nonautonous approach. Although AADC would only be expressed and act directly on the cell it enters, these cells will more effectively metabolize L-dopa to dopamine, thus raising overall dopamine levels and conferring a cell nonautonomous benefit.
Broad therapy, cell nonautonomous
Strong success of AAV vectors has come from preclinical studies with lysosomal storage diseases (LSDs), where the therapeutic metabolic enzyme is secreted and works either in the bloodstream, in the cerebrospinal fluid (CSF) or through uptake by neighboring cells. Since the gene product can work in a cell nonautonomous fashion, a limited number of transduced cells can have a therapeutic impact on a larger area. For example, in the mucopolysaccharidosis VII (MPS VII) murine model, complete correction of the pathological storage lesions throughout the entire brain, and an improvement in neurological function was achieved with an intracranial injection of the therapeutic AAV9/GUSB vector [73]. In MPSIIIb, improved behavior and neuropathology were attained in the diseased murine model after intrastriatal delivery of a therapeutic AAV5 vector [74]. In a canine model for MPS I, reduced lysosomal storage pathology was observed throughout much of the brain following intracranial injection of a therapeutic AAV vector [75].
Most gene therapy approaches for amyotrophic lateral sclerosis (ALS) would also fall into this general category. Secreted neuroprotective factors such as IGF-1, VEGF and GDNF have been delivered to the spinal cord of mice modeling ALS, in order to prolong survival of motor neurons and in turn slow the disease progression. Using lentivirus or AAV vectors, these attempts have modestly increased mouse survival, but efficacy was likely limited by the extent of delivery achieved [76–79]. Approximately 1–2% of ALS patients have a familial form of the disease linked to mutations in the SOD1 gene causing toxic accumulation of the mutant super-oxide dismutase 1 (SOD1) protein. In this case, an approach to use siRNA to knockdown SOD1 has been investigated [80,81], but this approach would be cell autonomous (see after discussion), and efficacy has been limited by the extent of delivery possible.
As exemplified by the preclinical studies with LSDs and the clinical trials with LINCL and ALD, current vector technology seems capable of impacting this class of disorders. The extent at which efficacy is achieved depends on:
How broadly the vector is delivered
The efficiency of transduction
The distance and mechanism that the secreted therapeutic factor is able to exert a therapeutic effect
How much gene product is necessary to reach therapeutic levels
Broad therapy, cell autonomous
This class of diseases represents the largest challenge from a gene delivery standpoint. Included in this are gene replacement strategies for Fragile X syndrome, spinal muscular atrophy (SMA), Rett syndrome and Huntington’s disease (HD), to name a few. These are diseases that, from a genetic standpoint, could be hypothetically treated by replacing the defective gene or, in the case of HD, to knockdown or otherwise remove toxic aggregates formed by the mutant protein. The biggest problem is with delivery, since the affected cells are pervasive through large areas of the CNS, sometime severely affecting the entire brain and/or spinal cord. Only areas where cells are directly transduced by the therapeutic vector can be expected to show efficacy, which makes any therapeutic approach limited by the extent that the vector can be targeted to the most critical areas and/or spread most effectively to as many areas of the CNS as possible. Efficacy would be expected to correlate with the efficiency at which cells in the most critical regions are transduced.
Diseases such as Fragile X syndrome and Rett syndrome offer an additional challenge. The genetic cause in 80% of Rett syndrome cases is various mutations that occur in the MECP2 gene on the X chromosome [82]. Although this gene is expressed ubiquitously, phenotypic expression of the disorder is limited to the nervous system. MECP2 mutations in males are lethal and only heterozygous females are symptomatic and viable. Owing to random X inactivation in females, some cell populations have normal MECP2 protein expression, while others are aberrant. This expression variation leads to the complexity of treating Rett syndrome patients with gene therapy. Postnatal gene replacement of MECP2 has been successfully demonstrated using carefully engineered genetic models, but not with vectors [83,84]. This result is still encouraging since it shows that gene therapy after birth will be sufficient to reverse the phenotype and eliminates the need for prenatal correction. However, overexpression, by even twice the endogenous levels, can lead to separate progressive neurological effects distinct from classical Rett syndrome and early mortality in mice [85]. It is unclear how to target specific cells based on their X chromosome inactivation or whether neuronal correction will be enough to alleviate symptoms. A hypothetical MECP2 replacement therapy would need to successfully transduce a large population of neurons (and perhaps glia) across a large area of the brain, while also maintaining tight control of gene expression.
While the challenges of this general disease paradigm are large, some successes have already been realized in preclinical models. Passini et al. used an AAV8 vector to replace the SMN1 gene in newborn mice by an intraventricular injection, which extended their median lifespan from 16 to 157 days [86]. Foust et al. used an intravascular approach with a high dose of AAV9 to replace the SMN1 gene in mice with SMA. By this approach, they were able to successfully replace SMN1 in newborn mice at high enough levels to enable the mice to survive over 250 days when the average lifespan of control untreated mice was 15.5 days [87]. This approach translated well to one newborn nonhuman primate injected with an AAV9/green fluorescent protein vector as part of the study, suggesting the strategy could in principal be applied to humans. Caution should be taken with an intravascular approach, however, which will be discussed below.
Routes of viral vector delivery to the CNS
Multiple routes of vector administration have been explored to target specific disease application:
Direct injection offers a simple but invasive means to efficiently transduce a small area
Injection into the CSF within the brain allows broad distribution of the vector across the CNS, with limited penetration into the brain parenchyma
Intrathecal injection distributes the vector through the CSF, but mostly targets the spinal cord and dorsal root ganglia (DRGs)
Intramuscular injection targets motor and sensory neurons via retrograde transport
Intravascular administration can achieve widespread transduction throughout the CNS, but requires high vector doses and peripheral exposure to the vector
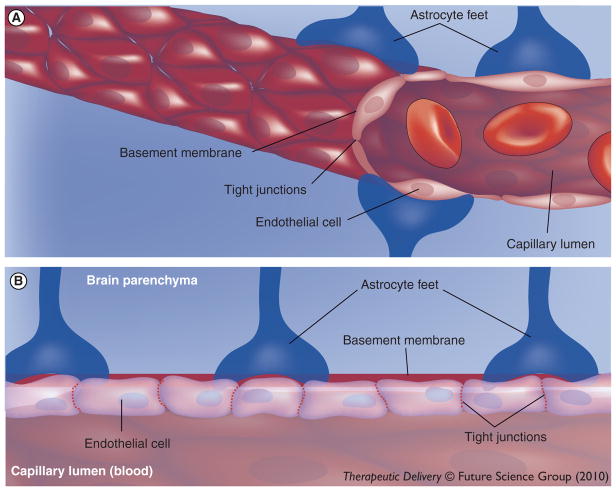
In order to deliver viral vectors to the CNS, several routes have been explored as means to either mechanically or biologically bypass the BBB. The BBB consists of three layers that separate the brain/spinal cord and CSF from the blood and is primarily localized to the unique tight junctions of endothelial cells (Figure 3) [88]. These layers, known as the cerebral capillary endothelium, choroids plexus epithelium and arachnoid membranes, are each composed of a layer of cells connected by tight junctions. Large or hydrophobic molecules, such as viruses, large drugs, and many proteins, are normally unable to passively cross from the blood across the BBB and into the brain. Mechanical means to bypass the BBB include stereotaxic injection directly into the brain parenchyma, injection into the CSF, or altering the BBB to allow intravascular delivery. Biological means include utilizing vectors that undergo retrograde transport to transduce motor neurons after intramuscular injection and, recently, employing vectors that can cross an intact BBB.
Figure 3. The blood–brain barrier represents a significant obstacle to CNS gene delivery.
(A) Blood vessel through the brain parenchyma. (B) Cross-section of the blood vessel and the barriers a molecule would encounter crossing from the bloodstream into the brain parenchyma. A molecule would have to pass through the endothelial cell tight junctions or undergo transport across the endothelial cells themselves. Next the molecule would encounter the basal lamina and astrocytic endfeet.
Direct injection
When injected into the brain parenchyma, AAV serotypes display distinct properties that make each serotype more or less amenable to CNS gene delivery. AAV2 is the serotype used in most human clinical trials, mostly because it has been studied for the longest time and most detail. AAV2 has a strong neuronal tropism but limited spread when injected intraparenchymally. AAV4 injected into the subventricular zone preferentially transduces astrocytes and ependymal cells [89]. AAV5 has been reported to transduce both astrocytes and neurons. The extent of AAV5’s neuronal transduction is greater than that of AAV2, and AAV5 will spread to a significantly larger volume [90,91]. AAV1, AAV8, and AAV9 have primarily neuronal tropism, and when injected intraparenchymally they transduce significantly more cells than AAV2, with the following general efficiency for CNS delivery: AAV9>AAV8>AAV1 [92,93]. AAV9 in particular has unique characteristics that lend it to its utility as a CNS vector. In addition to properties of high neuronal tropism and good volumetric spread after intraparenchymal injection, AAV9 appears able to undergo axonal transport throughout the brain at higher efficiency than previously described serotypes [94,95]. The axonal transport and transduction of cells at axon terminals was remarkably efficient, and a single injection of AAV9 into the ventral tegmental area led to widespread distribution of AAV9 vector genomes to numerous distal projection sites throughout the brain [73]. While very specific, in humans a stereotaxic injection requires highly invasive neurosurgery with significant risk to patients. Somewhat widespread delivery was achieved in an AAV-mediated trial for Canavan’s disease by entering the brain via six separate burr holes and depositing vector at three separate locations along each needle track for a total of 18 individual injections throughout the brain [96].
It is worth noting that the current generation of lentiviral vectors (SIN, NIL) show similar transduction profiles to AAV in the CNS. Integrating lentiviral vectors show superior gene expression, but at the risk of insertional mutagenesis and oncogenesis. For reviews of CNS applications of lentivirus, see elsewehere [97,98].
Intracisternal & intraventricular injection
Intraventricular injection of AAV4 and AAV5 vectors into the CSF results in efficient transduction of the ependymal cell layer with little to no penetration into the brain parenchyma [91]. Systemic administration of mannitol at the time of intraventricular vector injection can allow penetration of the vector into the brain parenchyma [99]. Instracisternal injection of AAV2 vectors in the absence of mannitol was reported to result in minimal transduction neurons and astrocytes throughout the CNS, but mostly transduction was concentrated around those cells surrounding the ventricular system in the cerebellum and brainstem [100]. The spread and transduction efficiency increased dramatically when mice were given an intravenous (i.v.) administration of 25% mannitol 15–20 min prior to the intracisternal injection [100,101]. Intracisternal injection of AAV1 vectors also showed diffuse global transduction of ependymal cells and Purkinje neurons mostly localized along areas of the brain in proximity to the CSF, but also efficient transduction of cervical dorsal root ganglia [102].
Intrathecal delivery
Storek et al. compared self-complementary AAV1, AAV2, AAV3 and AAV5 for gene delivery to the spinal cord after lumbar puncture in rats [103]. No expression was detected for AAV2 and AAV3, minimal expression was detected with AAV5 and highest expression was seen with AAV1. Gene expression was mostly seen in the caudate equina and weakly in the lumbar spinal cord, but some expression could also be detected in the thoracic and cervical spinal cord. While the specific cell type(s) transduced were not identified, reporter expression was visualized in the nerve roots of the lumbar spinal cord, an important target for gene therapy of spinal cord diseases. In a separate study, intra-thecal (IT) administration of AAV2 within the thoracic region of the spinal cord led to transduction of neurons distributed throughout the entire brain, albeit at very low efficiency [104]. This study demonstrated the possibility of vector transport through the CSF into the brain from an intrathecal injection. A second report by Storek et al. compared AAV1 and AAV8 for intrathecal delivery to the DRG, and found AAV8 to be approximately fivefold superior to AAV1 [105]. Following lumbar puncture, reporter gene expression was mostly seen in the sacral/lumbar region of the spinal cord, but also to an approximate tenfold lesser extent in the cervical spinal cord. Comparing several routes of delivery (intramuscular [IM], intranerve [IN] and IT), Towne et al. found that IT injection of AAV6 vectors led to the most efficient and widespread transduction of cervical and lumbar DRG [106].
IM injection and retrograde transport
Intramuscular and IN vector injections have been investigated as a way to target gene delivery to motor and/or sensory neurons. This approach has been proposed as a means to treat ALS, SMA and chronic pain. Initial investigations utilized AAV2 [76], which was later found to be inefficient for retrograde transduction of motor neurons [107]. A comparison of AAV1-AAV6 by IM and IN injection in rats found AAV1 to be most efficient by either injection strategy (with AAV6 second), and IN being more efficient than IM [107]. Zheng et al. reported efficient retrograde delivery of AAV8 to spinal motor neurons and dorsal root ganglia in mice [108]. Important for the clinical translation of this approach, Towne et al. injected 1.3 × 1012 vector genome (vg) in the gas-trocnemius muscle of African green monkeys and achieved reproducible transduction of motor neurons over 0.5–1 cm of the spinal cord, with a peak efficiency of approximately 30–40% motor neuron transduction [109].
Intravascular delivery
Several studies have demonstrated some level of AAV delivery to the CNS following i.v. injection of AAV vectors. Foust et al. delivered very high doses (2 × 1014 vg/kg) of AAV9 i.v. and observed mostly astrocytic transduction in the CNS with isolated neuronal transduction [110]. Injection into neonatal (P2) mice or a newborn (P1) cynomolgus macaque at 2 × 1014 vg/kg resulted in extensive and efficient transduction of spinal motor neurons, enough to achieve significant efficacy in a mouse model for SMA [87]. Duque et al. observed transduction of 5–19% lower motor neurons in adult mice using 1 × 1014 vg/kg of AAV9 and 3–15% in adult cats at a dose of 2 × 1013 vg/kg [111]. Towne et al. i.v. injected 1 × 1013 vg/kg of AAV6 in adult mice and observed transduction of 3–5% of lower motor neurons as well as some astrocytic transduction in the spinal cord [81]. It should be noted that the high doses of AAV9 used in 20 g mice from the Foust and Duque studies would translate to approximately 2–4 × 1015 vg in a 20 kg (44 lb) child. Scaling these doses up to increase delivery efficiency would present a significant manufacturing challenge, if the therapies were meant to be translated to humans. Moreover, in each case, high loads of virus are delivered to systemic organs such as the heart, musculature, and liver in addition to the CNS, raising the probability of undesired side effects (e.g., off-target gene expression or immune response against the virus capsid).
Under reversible hyperosmotic stress such as i.v. infusion of D-mannitol, the endothelial cells that make up the BBB literally shrink, creating gaps in the tight junctions between the cells [88]. When the normal osmotic balance is restored, the endothelial cells swell to their original volumes and the tight junctions are restored. Two studies by Fu et al. have utilized mannitol to allow i.v.-injected AAV2 (2 × 1013 vg/kg) to penetrate the CNS and transduce cells [100,101]. By optimizing the timing of mannitol infusion relative to vector injection, significant and widespread CNS transduction was observed. AAV2 has a much lower transduction efficiency for peripheral organs, giving this approach an advantage over direct infusion of AAV9, at least in terms of vector peripheral biodistribution.
Engineering AAV vectors for therapeutic applications
Adeno-associated virus vectors have a broad range of natural tropisms, but these may become limiting for specific therapeutic applications
Self-complementary AAV is much more efficient than traditional AAV vectors, but at the cost of half the packaging capacity
Modification of the capsid provides a way to create AAV vectors with novel properties
Directed evolution allows for the development of new AAV capsids distinct from those found in nature, targeted specifically towards therapeutic applications
Although over 100 AAV variants from many species have been isolated [112], these capsids have evolved in nature for the purpose of virus maintenance and propagation. While we attempt to use these as therapeutic gene vectors, this is akin to using a high-performance sports car as a postal delivery vehicle. Certain features of the virus are useful for our purpose, but it is very far from being optimized with some specialized viral components being wasted and others falling short of fulfilling the therapeutic need. The field is approaching a point where the natural tropism of the virus may become a limiting factor in the types of applications that could benefit from a gene therapy approach.
Capsid modification
With a stronger understanding of the features of AAV (and other viral vectors) that confer tropism, evasion of immune surveillance, efficient intracellular trafficking and uncoating and genome stabilization/persistence, vectors can be engineered toward therapeutic applications and away from mechanisms of natural selective pressures that the virus allowed the virus to survive through evolution. In one example, phosphorylation of tyrosines on the capsid surface of AAV by EGF receptor protein tyrosine kinase was found to negatively impact the ability of AAV to traffic to the nucleus and undergo successful transduction [113]. By mutating targeted tyrosine residues, transduction efficiency of AAV was enhanced up to 11-fold in vitro and 29-fold in vivo [114]. In another example, a six-amino acid stretch of the heparin sulfate-binding motif of AAV2 was replaced with the corresponding six amino acids of AAV8 [115]. The resulting mutant, dubbed 2i8, had superior muscle and cardiac tropism with low liver tropism, a combination not seen in any known AAV serotypes. Similar swaps with other serotypes resulted in mostly poorly performing vectors, highlighting the complexities of the capsid structural interactions.
The virus coat is an obvious molecular target to engineer the virus toward a specific therapeutic application. One rational approach is to incorporate a known ligand for the target cell(s) on the surface of the capsid. To make an AAV2-based vector for enhanced retrograde transport and neuron targeting, peptides derived from an N-methyl-D-aspartic acid receptor agonist and a dynein binding motif were incorporated into the capsid [116]. These peptides synergistically enhanced retrograde transport of AAV2 10–100-fold, and allowed retrograde delivery to the CNS from peripheral injection in vivo whereas unmodified AAV2 delivery to the CNS was undetectable. This strategy can be taken one step further to bypass any knowledge of the target cell and utilize a phage display library to generate a peptide with tropism for the given tissue. Chen et al. cycled a phage display library of random peptides in normal or MPS VII mice, injecting the library into the tail vein and recovering it from the brain [117]. The dominant recovered peptide was incorporated into an AAV2 capsid, then tested in vivo. The peptide specifically targeted AAV2 to the cerebral vascular endothelial cells after i.v. injection in mice. By packaging β-glucuronidase in AAV, the authors were able to utilize cerebral vasculature transduction with the modified vector to express therapeutic levels of β-glucuronidase into the CNS of MPS VII mice. To expand the proof-of-concept of the study, the selection was repeated in a mouse model for LINCL and similar results were obtained (i.e., a novel peptide was recovered to target AAV2 to the cerebral vasculature in LINCL mice [117]). Both the strength and weakness of this approach was the specificity of the selection process. The engineered vector had a striking preference for the CNS vasculature of the disease mouse model where the phage-display selection was performed, so much so that it did not work in a wild-type mouse and vice versa. This raises a question of how effective the vector would be in another species (i.e., human) or whether it is so specific that it only works in mice.
Directed evolution
The generation of random AAV capsid libraries, termed ‘directed evolution,’ was pioneered by Schaffer and Maheshri [118,119], borrowing from DNA-shuffling techniques described by Stemmer [120,121]. Multiple variations of the AAV-directed evolution process have since been utilized, but the overall strategy is similar. First, the capsid genes are randomly mutagenized or mixed to form a library of pooled capsid variants in the context of a replication-competent backbone. Next, this library is subjected to multiple rounds of selective pressure. At the end, the recovered library clone(s) should be enhanced for whatever characteristic was selected for, above that of the parent serotype(s). Described methods for producing the library include random mutagenesis of the capsid gene of a single serotype by error-prone PCR, randomly mixing capsids from multiple serotypes by DNA shuffling or a combination of the two methods [120,122]. Gray et al. applied the directed evolution process directly to a CNS application, namely to specifically target a therapeutic vector to sites of seizure damage [68]. The authors took advantage of the disruption of the BBB that occurs at sites of seizures and selected for an AAV capsid that could enter the brain only at these sites of seizure damage. This was done by inducing seizures in a rat with kainic acid, then injected the library of shuffled capsids i.v. Viral genomes were recovered from sites of seizure damage, then used to make an enhanced library for another round of injection/recovery. After three rounds, two clones were recovered that met the selection criteria: they could be injected i.v. and transduced neurons at sites of seizure damage but not elsewhere in the brain. These clones had the additional benefit of a near-complete loss of liver, heart and muscle tropism, giving them a favorable safety and biodistribution profile.
Self-complementary AAV
Due to AAV having a single-strand genome, the second strand must be synthesized following uncoating in the cell before transcription occurs [32,33]. Second-strand synthesis of AAV represents a rate-limiting step in transduction and gene expression [123]. A double-stranded, or self-complementary (sc), version of AAV was developed overcome this limitation, but at the cost of half the packaging capacity; sc AAV vectors have a packaging limit of only 2.3 kb. These vectors achieve greater transduction efficiency as well as faster and more robust transgene expression in brain, muscle, liver and eye [123]. Increased transduction of over 100-fold has been observed for sc AAV vectors compared with conventional ss AAV [124]. This increase in transduction efficiency translates to a significantly lower dose of vector administered to achieve the same level of gene product or number of transduced cells, as compared with traditional ss AAV vectors [124].
Limitations of gene therapy
The most challenging applications of AAV vectors for gene therapy involve diseases where global cell-autonomous CNS delivery is necessary and uncontrolled gene expression is deleterious. Examples of this are Fragile X syndrome and Rett syndrome, where underexpression of the implicated gene leads to the disease phenotype while overexpression leads to a different but also adverse phenotype [85]. Such diseases require the development of new vectors and/or routes of administration to achieve efficient global CNS transduction while simultaneously engineering a tight regulation of the transgene. This is further complicated in that the only studies coming close to global CNS delivery required sc AAV vectors [87,100,110,111], which have a reduced packaging capacity of only around 2.3 kb. Lentiviral vectors have an increased packaging capacity over AAV, which may make them amenable to a more complex gene regulatory design if these can be developed into global delivery vectors.
Future perspective
This section of the review is devoted purely towards the authors’ speculation on where the field of CNS gene therapy will head in the next 5 years. This perspective is based on the most recent manuscripts published on vector design and delivery, the areas of need for viral vectors, and recent successes in preclinical models that are likely to spur clinical trials:
Clinical success in PD appears on the horizon
Better global delivery of AAV vectors
After numerous encouraging preclinical results, LSD gene therapy clinical trials are likely
Better capsid engineering should improve the safety and efficacy of vectors
Increased use and acceptance of lentivirus for CNS applications
Clinical success in PD
Parkinson’s disease has garnered large pharmaceutical and academic investment, leading to seven current trials. This heavy investment will propel the science forward to identify the most effective transgene cargo, and the pristine safety record in these trials with AAV will accelerate the regulatory approval of these ‘next-generation’ optimized approaches using serotypes more effective than AAV2, such as AAV5 or AAVrh10. The hundreds of patients tested will provide a wealth of human data, providing a much clearer picture of the best path forward than was gained from animal models.
Better global delivery of AAV vectors
Global delivery is one of the areas with the greatest unmet need, and it is also an area with the most encouraging delivery breakthroughs. The next 5 years will bring considerable advances in this area, as new vectors are being tested and developed for intravascular and intra-CSF delivery. Advances in this area will lead to breakthroughs in ALS, SMA and HD, where relatively large patient populations will encourage corporate interest and investment. In the next 5 years considerable advances are likely in the development of new vector and delivery technologies and these advances will likely translate to preclinical and eventually clinical studies within the next 10 years.
LSD clinical trials
Lysosomal storage diseases in general lend themselves well to a gene therapy approach. Preclinical studies in large animal models for glycogen storage disease type 1a, MPS type I, MPS type VI and MPS type VII have been strikingly successful, permanently (and sometimes completely) alleviating the disease phenotype long after untreated controls have died from the disease [125–128]. It should be noted that some of these approaches utilized retroviral vectors and others utilized AAV vectors.
Enzyme replacement therapies (ERT) for these disorders are economically promising for pharmaceutical companies. Each patient would require regular infusions of the ERT, so even a small patient population could generate considerable revenue over life-long dosing of the ERT. A gene therapy approach with a single vector administration in a small patient population has little financial incentive for profit, and this lack of incentive is reflected in the fast development of ERTs and the slow development of gene therapy approaches. With clear and striking efficacy seen for these and other disorders with gene therapy in large animal models, governmental and private funding initiatives for orphan diseases will slowly bring these approaches into the clinic.
Better capsid engineering
Capsid modifications to AAV and pseudotyping lentiviral vectors have significantly expanded the therapeutic utility of these vectors. This approach is still in its infancy, and this is an area primed for rapid advancement. As more is learned about the structure and function of the AAV capsid, rational mutagenesis of receptor binding domains may allow more efficient targeting of the vectors. Moreover, technologies such as directed evolution and phage display peptide libraries allow the creation of novel targeted vectors without a priori knowledge of the required capsid modifications or receptor interactions.
Increased use of lentiviral vectors for CNS applications
Lentiviral vectors initially had to overcome their stigmatic association with HIV, from which the vectors are derived. As retroviruses, lentiviral vectors initially utilized the property of chromosomal integration to confer superior long-term transgene expression. This advantage turned to a liability when 20% of children treated for SCID developed leukemia, demonstrating a significant risk for the broad application of retroviral vectors. Today’s lentiviral vectors are SIN to remove the risk of HIV revertants and nonintegrating to address the risk of oncogenesis. The new generation of lentiviral vectors will overcome the taint attached to them from their predecessors and emerge as a strong and effective gene delivery vehicle. Their success in the ALD human trial is the first major milestone along this path.
Conclusion
Recent near-miraculous clinical trial results for Leber’s congenital amaurosis [129] and ALD [62] are finally demonstrating the promises of gene therapy made three decades ago. These successes, along with initiatives from agencies such as the NIH to push translational research, will embolden academic investigators, companies, and patient-advocacy groups to pursue gene therapy clinical trials with renewed vigor. With the exceptional safety record of vectors such as AAV and the newest generation of NIL and SIN, regulatory approval of these trials will accelerate. With more human data, the safety and effectiveness of vectors will grow. However, with accelerated regulatory approval and the clamor of investigators to push their therapies into the clinic, the chance of a serious adverse event will increase as well. The field of gene therapy has learned very hard lessons as it matured, and we hope that the coming years will meet our expectations of success.
Executive summary.
Adeno-associated virus (AAV) is the most utilized vector for CNS gene delivery due to its favorable safety profile, long-term expression, and ability to target neurons and glia.
Lentiviral vectors are emerging as viable tools for therapeutic CNS gene delivery, with a larger packaging capacity compared with AAV vectors.
AAV and lentivirus vectors are being used in a number of CNS clinical trials, including Parkinsons disease, Batten disease and adrenoleukodystrophy.
The route of administration and vector choice greatly influence the efficiency and scope of gene delivery.
The required gene delivery paradigm will vary greatly from one disease to another; from a delivery standpoint, localized diseases like PD are relatively simple while diffuse cell-autonomous diseases such as Fragile X syndrome or Rett syndrome represent a high degree of challenge.
Modification of the viral capsid, by rational mutagenesis or use of random libraries, allows the engineering of next-generation vectors for specific therapeutic applications.
Acknowledgments
The authors would like to thank Melissa Ellermann for help in the preparation of Figure 3.
Key terms
- Viral vector
Genetically modified virus engineered to carry and deliver foreign genetic information
- Gene therapy
Introduction of nucleic acid into cells of an animal for therapeutic benefit. This definition generally excludes small oligonucleotides
- Blood–brain barrier
Limits the exchange of molecules between the vasculature and the CNS
- Adeno-associated virus
Commonly used as a vector for CNS gene transfer
- Transduce
Describes the successful transfer and expression of genetic material to a target cell
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Wang Y, Fraefel C, Protasi F, et al. HSV-1 amplicon vectors are a highly efficient gene delivery system for skeletal muscle myoblasts and myotubes. Am J Physiol Cell Physiol. 2000;278(3):C619–626. doi: 10.1152/ajpcell.2000.278.3.C619. [DOI] [PubMed] [Google Scholar]
- 2.Raper SE, Yudkoff M, Chirmule N, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13(1):163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- 3.Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006;17(1):1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 4.Berges BK, Wolfe JH, Fraser NW. Transduction of brain by herpes simplex virus vectors. Mol Ther. 2007;15(1):20–29. doi: 10.1038/sj.mt.6300018. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe D, Wechuck J, Krisky D, Mata M, Fink DJ. A clinical trial of gene therapy for chronic pain. Pain Med. 2009;10(7):1325–1330. doi: 10.1111/j.1526-4637.2009.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu H, Roizman B. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J Virol. 2009;83(1):181–187. doi: 10.1128/JVI.01940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe D, Brockman MA, Ndung’u T, et al. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357(2):186–198. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Broberg E, Watanabe D, Dudek T, Deluca N, Knipe DM. Genetic engineering of a modified herpes simplex virus 1 vaccine vector. Vaccine. 2009;27(21):2760–2767. doi: 10.1016/j.vaccine.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant KG, Krisky DM, Ataai MM, Glorioso JC., 3rd Engineering cell lines for production of replication defective HSV-1 gene therapy vectors. Biotechnol Bioeng. 2009;102(4):1087–1097. doi: 10.1002/bit.22123. [DOI] [PubMed] [Google Scholar]
- 10.Zaupa C, Revol-Guyot V, Epstein AL. Improved packaging system for generation of high-level noncytotoxic HSV-1 amplicon vectors using Cre-loxP site-specific recombination to delete the packaging signals of defective helper genomes. Hum Gene Ther. 2003;14(11):1049–1063. doi: 10.1089/104303403322124774. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Tang J, Wang X, Yang T, Geller AI. Enhanced long-term expression from helper virus-free HSV-1 vectors packaged in the presence of deletions in genes that modulate the function of VP16, UL46 and UL47. J Neurosci Methods. 2005;145(1–2):1–9. doi: 10.1016/j.jneumeth.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsson J, Lundberg C. Lentiviral vectors for use in the central nervous system. Mol Ther. 2006;13(3):484–493. doi: 10.1016/j.ymthe.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5(4):387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71(9):6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colin A, Faideau M, Dufour N, et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia. 2009;57(6):667–679. doi: 10.1002/glia.20795. [DOI] [PubMed] [Google Scholar]
- 16.Hioki H, Kameda H, Nakamura H, et al. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 2007;14(11):872–882. doi: 10.1038/sj.gt.3302924. [DOI] [PubMed] [Google Scholar]
- 17.Falkowska-Hansen B, Kollar J, Gruner BM, et al. An inducible Tet-Off-H2B-GFP lentiviral reporter vector for detection and in vivo isolation of label-retaining cells. Exp Cell Res. 2010;316(11):1885–1895. doi: 10.1016/j.yexcr.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 19.Rahim AA, Wong AM, Howe SJ, et al. Efficient gene delivery to the adult and fetal CNS using pseudotyped nonintegrating lentiviral vectors. Gene Ther. 2009;16(4):509–520. doi: 10.1038/gt.2008.186. [DOI] [PubMed] [Google Scholar]
- 20.Yanez-Munoz RJ, Balaggan KS, MacNeil A, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12(3):348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 21.Sarkis C, Philippe S, Mallet J, Serguera C. Nonintegrating lentiviral vectors. Curr Gene Ther. 2008;8(6):430–437. doi: 10.2174/156652308786848012. [DOI] [PubMed] [Google Scholar]
- 22.Banasik MB, McCray PB., Jr Integrase-defective lentiviral vectors: progress and applications. Gene Ther. 2010;17(2):150–157. doi: 10.1038/gt.2009.135. [DOI] [PubMed] [Google Scholar]
- 23.Wong LF, Azzouz M, Walmsley LE, et al. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther. 2004;9(1):101–111. doi: 10.1016/j.ymthe.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72(12):9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montini E, Cesana D, Schmidt M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119(4):964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jager L, Ehrhardt A. Emerging adenoviral vectors for stable correction of genetic disorders. Curr Gene Ther. 2007;7(4):272–283. doi: 10.2174/156652307781369074. [DOI] [PubMed] [Google Scholar]
- 27.Kim PH, Kim TI, Yockman JW, Kim SW, Yun CO. The effect of surface modification of adenovirus with an arginine-grafted bioreducible polymer on transduction efficiency and immunogenicity in cancer gene therapy. Biomaterials. 2010;31(7):1865–1874. doi: 10.1016/j.biomaterials.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8(5):341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 29.Sweigard JH, Cashman SM, Kumar-Singh R. Adenovirus vectors targeting distinct cell types in the retina. Invest Ophthalmol Vis Sci. 2010;51(4):2219–2228. doi: 10.1167/iovs.09-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and β-galactosidase. Proc Natl Acad Sci USA. 1996;93(12):5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morral N, O’Neal W, Rice K, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96(22):12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 34.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11 (Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 35.Haberman RP, McCown TJ, Samulski RJ. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998;5(12):1604–1611. doi: 10.1038/sj.gt.3300782. [DOI] [PubMed] [Google Scholar]
- 36.Duan D, Sharma P, Yang J, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72(11):8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi VW, McCarty DM, Samulski RJ. Host cell DNA repair pathways in adeno-associated viral genome processing. J Virol. 2006;80(21):10346–10356. doi: 10.1128/JVI.00841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay MA. AAV vectors and tumorigenicity. Nat Biotechnol. 2007;25(10):1111–1113. doi: 10.1038/nbt1007-1111. [DOI] [PubMed] [Google Scholar]
- 39.Bell P, Wang L, Lebherz C, et al. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther. 2005;12(2):299–306. doi: 10.1016/j.ymthe.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 42.Feng LR, Maguire-Zeiss KA. Gene therapy in Parkinson’s disease: rationale and current status. CNS Drugs. 2010;24(3):177–192. doi: 10.2165/11533740-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43■■.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14(4):564–570. doi: 10.1016/j.ymthe.2006.05.005. Adeno-associated virus (AAV) gene expression can be detected for at least 6 years in primates, and intrastriatal injection of AAV–aromatic l-amino acid decarboxylase, in conjunction with l-dopa, can improve clinical rating scores, reduction in side effects, and confer reduced l-dopa concentrations needed to show improvements. [DOI] [PubMed] [Google Scholar]
- 44.Forsayeth JR, Eberling JL, Sanftner LM, et al. A dose-ranging study of AAV-hAADC therapy in Parkinsonian monkeys. Mol Ther. 2006;14(4):571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christine CW, Starr PA, Larson PS, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson’s disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eberling JL, Jagust WJ, Christine CW, et al. Results from a Phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 47.Muramatsu SI, Fujimoto KI, Kato S, et al. A Phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther. 2010;18(9):1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48■.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, Phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. AAV-glutamic acid decarboxylase injected into 12 patients led to improved motor scores with no adverse side effects beginning at 3 months and lasting to at least 12 months. [DOI] [PubMed] [Google Scholar]
- 49.Kordower JH, Herzog CD, Dass B, et al. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60(6):706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 50■.Gasmi M, Brandon EP, Herzog CD, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27(1):67–76. doi: 10.1016/j.nbd.2007.04.003. AAV2 delivery of neurturin led to protection of substantia nigra cells, behavioral recovery, and no adverse effects for up to 12 months and with high dosage concentrations in rats. [DOI] [PubMed] [Google Scholar]
- 51.Bjorklund T, Carlsson T, Cederfjall EA, Carta M, Kirik D. Optimized adeno-associated viral vector-mediated striatal DOPA delivery restores sensorimotor function and prevents dyskinesias in a model of advanced Parkinson’s disease. Brain. 2010;133(Pt 2):496–511. doi: 10.1093/brain/awp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52■.Jarraya B, Boulet S, Ralph GS, et al. Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009;1(2):2ra4. doi: 10.1126/scitranslmed.3000130. Demonstrates a major utility of using a lentiviral vector with increased packaging capacity for treating Parkinsons disease, namely that it used a tricistronic genome containing tyrosine hydroxylase, GTP-cyclohydrolase I, and AADC in the same vector. [DOI] [PubMed] [Google Scholar]
- 53.Kohlschutter A, Schulz A. Towards understanding the neuronal ceroid lipofuscinoses. Brain Dev. 2009;31(7):499–502. doi: 10.1016/j.braindev.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793(4):697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 55■.Worgall S, Sondhi D, Hackett NR, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19(5):463–474. doi: 10.1089/hum.2008.022. Details an encouraging clinical trial for the treatment of a global CNS disease (Batten’s disease), where the delivery approach was 12 intracranial injections of an AAV vector. [DOI] [PubMed] [Google Scholar]
- 56.Sondhi D, Peterson DA, Giannaris EL, et al. AAV2-mediated CLN2 gene transfer to rodent and nonhuman primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 2005;12(22):1618–1632. doi: 10.1038/sj.gt.3302549. [DOI] [PubMed] [Google Scholar]
- 57.Cabrera-Salazar MA, Roskelley EM, Bu J, et al. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile batten disease. Mol Ther. 2007;15(10):1782–1788. doi: 10.1038/sj.mt.6300249. [DOI] [PubMed] [Google Scholar]
- 58.Passini MA, Dodge JC, Bu J, et al. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J Neurosci. 2006;26(5):1334–1342. doi: 10.1523/JNEUROSCI.2676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sondhi D, Hackett NR, Peterson DA, et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther. 2007;15(3):481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 60.Hackett NR, Redmond DE, Sondhi D, et al. Safety of direct administration of AAV2(CU)hCLN2, a candidate treatment for the central nervous system manifestations of late infantile neuronal ceroid lipofuscinosis, to the brain of rats and nonhuman primates. Hum Gene Ther. 2005;16(12):1484–1503. doi: 10.1089/hum.2005.16.1484. [DOI] [PubMed] [Google Scholar]
- 61.Sondhi D, Peterson DA, Edelstein AM, del Fierro K, Hackett NR, Crystal RG. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp Neurol. 2008;213(1):18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62■■.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. One of the first successful gene therapy trials, the novel approach of this study was to enhance an existing therapeutic approach (bone marrow transplant) by modifying the CD34+ bone marrow stem cells ex vivo with lentivirus vectors before re-injecting them into the patient to treat a CNS disease. [DOI] [PubMed] [Google Scholar]
- 63.Semmler A, Kohler W, Jung HH, Weller M, Linnebank M. Therapy of X-linked adrenoleukodystrophy. Expert Rev Neurother. 2008;8(9):1367–1379. doi: 10.1586/14737175.8.9.1367. [DOI] [PubMed] [Google Scholar]
- 64.McCown TJ. The clinical potential of antiepileptic gene therapy. Expert Opin Biol Ther. 2004;4(11):1771–1776. doi: 10.1517/14712598.4.11.1771. [DOI] [PubMed] [Google Scholar]
- 65.Foti S, Haberman RP, Samulski RJ, McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of NPY or NPY13–36 suppresses seizure activity in vivo. Gene Ther. 2007;14(21):1534–1536. doi: 10.1038/sj.gt.3303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haberman RP, Samulski RJ, McCown TJ. Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat Med. 2003;9(8):1076–1080. doi: 10.1038/nm901. [DOI] [PubMed] [Google Scholar]
- 67.McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14(1):63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 68■■.Gray SJ, Blake BL, Criswell HE, Nicolson SC, Samulski RJ, McCown TJ. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood–brain barrier (BBB) Mol Ther. 2010;18(3):570–578. doi: 10.1038/mt.2009.292. Innovative study detailing the use of DNA shuffling and directed evolution to create new AAV-based vectors specifically tailored to a CNS disease application. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou BY, Ye Z, Chen G, Gao ZP, Zhang YA, Cheng L. Inducible and reversible transgene expression in human stem cells after efficient and stable gene transfer. Stem Cells. 2007;25(3):779–789. doi: 10.1634/stemcells.2006-0128. [DOI] [PubMed] [Google Scholar]
- 70.Chtarto A, Yang X, Bockstael O, et al. Controlled delivery of glial cell line-derived neurotrophic factor by a single tetracycline-inducible AAV vector. Exp Neurol. 2007;204(1):387–399. doi: 10.1016/j.expneurol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Han Y, Chang QA, Virag T, et al. Lack of humoral immune response to the tetracycline (Tet) activator in rats injected intracranially with Tet-off rAAV vectors. Gene Ther. 2010;17(5):616–625. doi: 10.1038/gt.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang JJ, Niu DB, Zhang T, Wang K, Xue B, Wang XM. A tetracycline-regulatable adeno-associated virus vector for double-gene transfer. Neurosci Lett. 2005;378(2):106–110. doi: 10.1016/j.neulet.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 73.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27(37):9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cressant A, Desmaris N, Verot L, et al. Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum. J Neurosci. 2004;24(45):10229–10239. doi: 10.1523/JNEUROSCI.3558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciron C, Desmaris N, Colle MA, et al. Gene therapy of the brain in the dog model of Hurler’s syndrome. Ann Neurol. 2006;60(2):204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 76.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 77.Lu YY, Wang LJ, Muramatsu S, et al. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci Res. 2003;45(1):33–40. doi: 10.1016/s0168-0102(02)00195-5. [DOI] [PubMed] [Google Scholar]
- 78.Franz CK, Federici T, Yang J, et al. Intraspinal cord delivery of IGF-I mediated by adeno-associated virus 2 is neuroprotective in a rat model of familial ALS. Neurobiol Dis. 2009;33(3):473–481. doi: 10.1016/j.nbd.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Azzouz M, Ralph GS, Storkebaum E, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 80.Raoul C, Abbas-Terki T, Bensadoun JC, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11(4):423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 81.Towne C, Raoul C, Schneider BL, Aebischer P. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther. 2008;16(6):1018–1025. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- 82.Quaderi NA, Meehan RR, Tate PH, et al. Genetic and physical mapping of a gene encoding a methyl CpG binding protein, MECP2, to the mouse X chromosome. Genomics. 1994;22(3):648–651. doi: 10.1006/geno.1994.1442. [DOI] [PubMed] [Google Scholar]
- 83.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315(5815):1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci USA. 2007;104(6):1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Collins AL, Levenson JM, Vilaythong AP, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13(21):2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 86.Passini MA, Bu J, Roskelley EM, et al. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest. 2010;120(4):1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87■■.Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28(3):271–274. doi: 10.1038/nbt.1610. First demonstration that a motor neuron disorder (spinal muscular atrophy) could be effectively rescued by an intravenous injection of an AAV vector. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Rapoport SI. Osmotic opening of the blood–brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20(2):217–230. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12(20):1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 90.Burger C, Gorbatyuk OS, Velardo MJ, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2 and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 91.Davidson BL, Stein CS, Heth JA, et al. Recombinant adeno-associated virus type 2, 4 and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97(7):3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92■.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9 and rh10 in the mouse brain. Mol Ther. 2006;13(3):528–537. doi: 10.1016/j.ymthe.2005.11.015. Good primary reference to show the mouse brain transduction capabilities of some of the best AAV brain vectors, namely AAV7, AAV8, AAV9 and AAVrh10. [DOI] [PubMed] [Google Scholar]
- 93.Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138(2):501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 94.Passini MA, Macauley SL, Huff MR, et al. AAV vector-mediated correction of brain pathology in a mouse model of Niemann-Pick A disease. Mol Ther. 2005;11(5):754–762. doi: 10.1016/j.ymthe.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Dodge JC, Clarke J, Song A, et al. Gene transfer of human acid sphingomyelinase corrects neuropathology and motor deficits in a mouse model of Niemann-Pick type A disease. Proc Natl Acad Sci USA. 2005;102(49):17822–17827. doi: 10.1073/pnas.0509062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96■.Janson C, McPhee S, Bilaniuk L, et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002;13(11):1391–1412. doi: 10.1089/104303402760128612. Details the first clinical trial to use to use a viral vector (AAV) to try to treat a neurodegenerative disorder (Canavan disease). The approach for ‘global’ delivery was to use 18 intracranial injections. [DOI] [PubMed] [Google Scholar]
- 97.Dissen GA, Lomniczi A, Neff TL, et al. In vivo manipulation of gene expression in nonhuman primates using lentiviral vectors as delivery vehicles. Methods. 2009;49(1):70–77. doi: 10.1016/j.ymeth.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nanou A, Azzouz M. Gene therapy for neurodegenerative diseases based on lentiviral vectors. Prog Brain Res. 2009;175:187–200. doi: 10.1016/S0079-6123(09)17513-1. [DOI] [PubMed] [Google Scholar]
- 99.Ghodsi A, Stein C, Derksen T, Martins I, Anderson RD, Davidson BL. Systemic hyperosmolality improves β-glucuronidase distribution and pathology in murine MPS VII brain following intraventricular gene transfer. Exp Neurol. 1999;160(1):109–116. doi: 10.1006/exnr.1999.7205. [DOI] [PubMed] [Google Scholar]
- 100.Fu H, Muenzer J, Samulski RJ, et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8(6):911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 101.Fu H, Kang L, Jennings JS, et al. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14(14):1065–1077. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- 102.Iwamoto N, Watanabe A, Yamamoto M, et al. Global diffuse distribution in the brain and efficient gene delivery to the dorsal root ganglia by intrathecal injection of adeno-associated viral vector serotype 1. J Gene Med. 2009;11(6):498–505. doi: 10.1002/jgm.1325. [DOI] [PubMed] [Google Scholar]
- 103.Storek B, Harder NM, Banck MS, et al. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain. 2006;2:4. doi: 10.1186/1744-8069-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watson G, Bastacky J, Belichenko P, et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13(11):917–925. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- 105.Storek B, Reinhardt M, Wang C, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci USA. 2008;105(3):1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5(1):52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16(2):296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- 108.Zheng H, Qiao C, Wang CH, et al. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum Gene Ther. 2010;21(1):87–97. doi: 10.1089/hum.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Towne C, Schneider BL, Kieran D, Redmond DE, Jr, Aebischer P. Efficient transduction of nonhuman primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17(1):141–146. doi: 10.1038/gt.2009.119. [DOI] [PubMed] [Google Scholar]
- 110.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111■■.Duque S, Joussemet B, Riviere C, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17(7):1187–1196. doi: 10.1038/mt.2009.71. Study showed intravenous delivery of self-complementary AAV9 vectors transduced motor neurons in the spine of both mice and cats without pharmacological disruption of the blood–brain barrier and with expression lasting at least 5 months. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5(3):285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 113.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(2):194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Asokan A, Conway JC, Phillips JL, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol. 2010;28(1):79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu J, Ma C, Bass C, Terwilliger EF. A combination of mutations enhances the neurotropism of AAV-2. Virology. 2005;341(2):203–214. doi: 10.1016/j.virol.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 117■■.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15(10):1215–1218. doi: 10.1038/nm.2025. Innovative study used a phage display peptide library screen coupled to AAV2 capsid engineering, to design an AAV-based vector tailored to a specific and novel CNS disease treatment approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24(2):198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 119.Schaffer DV, Maheshri N. Directed evolution of AAV mutants for enhanced gene delivery. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3520–3523. doi: 10.1109/IEMBS.2004.1403990. [DOI] [PubMed] [Google Scholar]
- 120.Stemmer WP. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370(6488):389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 121.Stemmer WP. DNA shuffling by random fragmentation and reassembly: in vitro recombination for molecular evolution. Proc Natl Acad Sci USA. 1994;91(22):10747–10751. doi: 10.1073/pnas.91.22.10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koerber JT, Maheshri N, Kaspar BK, Schaffer DV. Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nat Protoc. 2006;1(2):701–706. doi: 10.1038/nprot.2006.93. [DOI] [PubMed] [Google Scholar]
- 123.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 124.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 125.Traas AM, Wang P, Ma X, et al. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15(8):1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 126.Sleeper MM, Fornasari B, Ellinwood NM, et al. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110(7):815–820. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 127.Koeberl DD, Pinto C, Sun B, et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol Ther. 2008;16(4):665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- 128.Tessitore A, Faella A, O’Malley T, et al. Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle. Mol Ther. 2008;16(1):30–37. doi: 10.1038/sj.mt.6300325. [DOI] [PubMed] [Google Scholar]
- 129■.Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.Gene Therapy Clinical Trials Worldwide. www.wiley.com/legacy/wileychi/genmed/clinical.
- 202.Neurologix press release. www.neurologix.net/