Abstract
Objective
Positive remodeling (PR), a coronary artery characteristic associated with risk for myocardial infarction (MI), may be more prevalent in HIV-infected (HIV+) people. We evaluated the prevalence of PR using coronary CT angiography (CCTA) in HIV+ and HIV-uninfected (HIV−) men.
Methods/Results
Men enrolled in the Multicenter AIDS Cohort Study underwent CCTA if they were 40–70 years, had normal kidney function and no history of coronary revascularization. Multivariable logistic regression models were used to estimate the odds ratio (OR) of PR by HIV serostatus, adjusting for demographics and coronary artery disease (CAD) risk factors. Analysis of PR among atherosclerotic segments further adjusted for plaque type and stenosis. The prevalence of PR was 8.4% versus 12.1% (p=0.10) for HIV− and HIV+ men, respectively. After demographic adjustment, HIV+ men had twice the odds of PR [OR 2.01(95% CI 1.20–3.38)], which persisted after CAD risk factor adjustment [1.76(1.00–3.10)]. Higher systolic blood pressure, total cholesterol, diabetes medication use, older age, segment number with plaque present, mixed and non-calcified plaque, and stenosis>50%, were associated with increased odds of PR, while higher HDL cholesterol, higher nadir CD4 count, and black race were associated with lower PR odds. Among atherosclerotic segments, the association between HIV infection and PR persisted, but was not statistically significantly.
Conclusion
HIV+ men have more positively remodeled arterial segments, which may be due to more coronary segments with atherosclerosis or HIV-related immunosuppression. Further studies are needed to evaluate whether PR contributes to higher rates of MI in HIV+ individuals.
Keywords: Coronary disease, imaging, epidemiology, AIDS
1. Introduction
HIV infection is associated with an increased risk for cardiovascular disease, including myocardial infarction and sudden cardiac death [1–3]. Proposed mechanisms include a higher prevalence of traditional coronary artery disease (CAD) risk factors, the use of antiretroviral therapy, especially protease inhibitors (PI), with associated dyslipidemia and insulin resistance [4–6], and HIV infection itself, which results in chronically elevated inflammation and immune activation [7,8]. A higher prevalence of non-calcified coronary plaque on coronary CT angiography (CCTA) has been associated with HIV infection [9–11]. CCTA allows assessment of plaque morphology [12], and can identify plaque characteristics known to be potentially “vulnerable” to rupture [13], thus possibly leading to an increased risk for acute coronary syndromes. High-risk plaque characteristics that are detectable using CCTA include positive arterial remodeling (Figure 1), low attenuation plaque (LAP), and spotty calcifications. Plaques with these features have been associated with acute myocardial infarction in autopsy studies and with invasive imaging such as intravascular ultrasound [14–16]. A previous smaller study found a higher prevalence of positive remodeling in HIV-infected individuals compared with uninfected controls [17]. Our study builds on this work by examining the associations between positive remodeling and HIV serostatus, demographic characteristics, CAD risk factors, HIV-associated factors, coronary plaque composition, and inflammatory biomarkers in the Multicenter AIDS Cohort Study (MACS).
Figure 1.
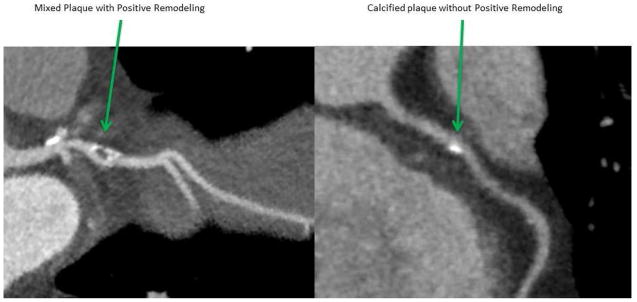
Coronary CT angiography showing an example of a vessel with and without positive remodeling.
2. Methods
Established in 1984, the MACS was created with 3 fundamental goals; 1) to identify the natural history of AIDS, 2) to identify risk factors and expression of the infection and, 3) to collect biologic specimens for future study [18]. This study enrolled HIV-infected (HIV+) and –uninfected (HIV−) men who have sex with men during three enrollment periods from 1984–5, 1987–91, and 2001–2003 in Baltimore, Chicago, Pittsburgh, and Los Angeles. A cross-sectional cardiovascular study within MACS enrolled participants aged 40–70 years, weight < 300 lbs, and without prior history of heart surgery (coronary artery bypass surgery, valve surgery, or coronary angioplasty). Participants in the cardiovascular study completed a non-contrast computed tomography (CT) scan to assess coronary artery calcium (CAC) and CCTA between 2010–2013 unless contraindicated by chronic kidney disease (estimated glomerular filtration rate <60 ml/min/m2 using the MDRD equation within 30 days or at prior MACS examinations), atrial fibrillation or IV contrast allergy. The analytic sample includes all participants who underwent CCTA.
Participants were seen during routine MACS research visits every 6 months for standardized interviews, physical examination, and blood and urine collection for concurrent laboratory analyses and storage. Data were collected regarding demographic, HIV clinical parameters, and CAD risk factors, including age, race, measured blood pressure, fasting glucose, fasting lipid panel, and body mass index (BMI = weight/height2), self-reported current smoking and use of medications (antiretrovirals and non-HIV medications) from the study visit prior to the CT measurements. Measures of HIV disease activity in HIV+ men included plasma HIV RNA levels (50 copies/mL limit of detection using Roche ultrasensitive assay), CD4+ T-cell counts, and history of a medical record confirmed AIDS-defining malignancy or opportunistic infection.
Details about CT scanning procedures and analysis have been previously described [19]. Briefly, non-contrast CT scans were analyzed for CAC using the Agatston method [20]. CCTA images were analyzed using the modified 15-segment model of the American Heart Association for plaque presence and extent, coronary artery stenosis, plaque composition and remodeling index [21]. Manual inspection, in both cross-section and longitudinal reconstruction, was used for determining the remodeling index (lesion diameter/reference diameter). For each segment with plaque present, coronary arterial remodeling was defined as an increase in vessel diameter at least 10% larger at the plaque site in comparison with the reference segment set proximal to the lesion in a normalappearing vessel segment (reference segment) [13]. Calcified atherosclerotic plaque was defined as any structure with attenuation >130 HU visualized separately from the intravascular lumen, identified in at least two independent planes. Non-calcified atherosclerotic plaque was defined as any discernible structure that could be clearly assignable to the vessel wall, with a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue, and identified in at least two independent planes. Finally, mixed plaque included lesions with less than 50% of plaque area occupied by calcium. Coronary stenosis was defined as greater than 50% in any coronary segment. The segment involvement score (SIS) was defined as the number of coronary segments with plaque present. The CT readers were blinded to participant characteristics, including HIV serostatus.
A panel of biomarkers was measured from blood samples drawn at the time CT images, including inflammatory biomarkers that have been associated with risk for CAD and/or HIV infection or therapy including high sensitivity C-reactive protein (CRP), fibrinogen, monocyte chemotactic protein 1 (MCP-1), interleukin 6 (IL-6), D-dimer, intercellular adhesion molecule 1 (ICAM-1), soluble tumor necrosis factor alpha receptor type I (sTNFαRI) and type 2 (sTNFαRII), sCD14, and sCD163. The assays were performed in Dr. Russell Tracy’s laboratory in Vermont.
Statistical methods
The distributions of demographic, behavioral, and clinical characteristics among HIV+ and HIV- men were compared using the chi-square or Wilcoxon rank sum tests for categorical and continuous variables, respectively. The distribution of the number of coronary arteries with remodeling was graphed overall and by HIV serostatus. We used multiple imputation to estimate values for nine CAD risk factor covariates with missing values (each <4% missing). The predictors for the multivariate imputation model included lipid-lowering, antihypertensive, and hypoglycemic medication use after or within 5 years prior to the CT scan, in addition to all covariates and outcome variables from the fully adjusted models. Missing values were imputed five times based on the distribution of covariates using the data augmentation Markov chain Monte Carlo (MCMC) method assuming a multivariate normal distribution [22]. We used logistic regression models, pooled across the multiple imputation datasets, to assess the association between HIV and the presence of any coronary artery remodeling. We fit minimally adjusted models that accounted for age, race, study site, and cohort enrollment period (pre- vs. post-2001). Fully adjusted models further accounted for established CAD risk factors: BMI; current smoking; use of hypertension, diabetes or lipid-lowering medications; and systolic blood pressure (SBP), fasting glucose, total and HDL cholesterol (HDL-C), among participants not receiving hypertension, diabetes or lipid-lowering medications, respectively. To quantify the extent to which the number of segments with plaque (SIS) affects the risk of PR (i.e., a segment could not have PR if there was no plaque present in that segment), we included SIS as a continuous variable in a multivariate model. The biomarkers previously described were individually added into the multivariable model and retained if they were statistically significant at the 0.05 level. The biomarker analyses were also stratified by HIV status to explore any differential effects of the biomarkers in HIV-infected compared to HIV-uninfected men. A sub-analysis was limited to men with CAC scores of 0. Among HIV+ men, we further expanded the model to assess the association of detectable HIV RNA, current and nadir CD4+ T-cell counts, history of an AIDS-defining illness, and duration of highly active antiretroviral therapy (HAART) and protease inhibitor use with positive remodeling. These factors were missing for less than 4% of HIV-infected men and were not imputed. Each HIV disease characteristic or treatment factor was included sequentially to the fully adjusted multivariate prediction model, and all variables with a p-value of 0.05 or less were included together in a final model for HIV+ men.
To account for previously described differences in the number of atherosclerotic segments and plaque characteristics by HIV serostatus [9], we used a multilevel model to assess the effect of plaque characteristics (calcified plaque, non-calcified plaque without stenosis > 50%, mixed plaque without stenosis, non-calcified plaque with stenosis, and non-calcified plaque without stenosis) and HIV infection on PR among coronary artery segments with plaque. The analysis used the generalized linear latent and mixed model (gllamm program) with a binomial distribution, logit link, and adaptive quadrature, with the artery segment as the primary unit of analysis and the individual as the second level [23]. In addition to HIV infection, plaque type, and stenosis, the models included the demographic and cardiovascular risk factors used in the individual-level analyses. Statistical significance was defined by a p-value <0.05. All analyses used Stata 13.1 (StataCorp, College Station, TX).
3. Results
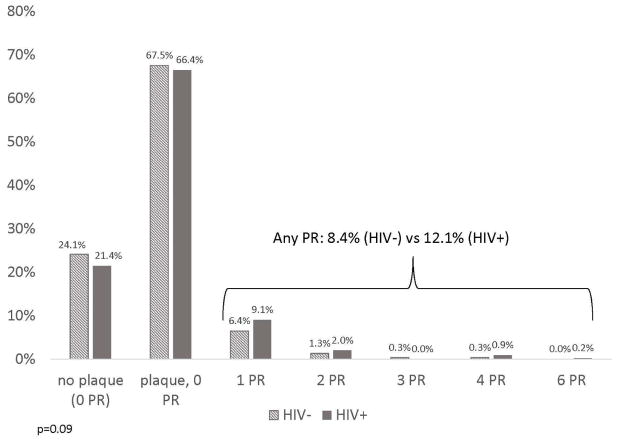
A total of 764 CCTA results were available for analysis. Characteristics of the study population by HIV serostatus are described in Table 1. As previously published, HIV+ men had a higher prevalence of non-calcified plaque [8]. At least one positively remodeled arterial segment on CCTA was seen in 8.4% (26/311) of HIV− men and 12.1% (55/453) of HIV+ men (Chi-square test p=0.10) (Figure 2). Two or more arterial segments with positive remodeling were found in 1.9% (6/311) of HIV− men and 3.1% (14/453) of HIV+ men, but the distribution of the number of segments with positive remodeling did not significantly differ by HIV serostatus in unadjusted analysis (p=0.09). HIV+ men had twice the odds of any positive remodeling compared with HIV− men (OR: 2.01 [95% CI: 1.20–3.38]; p=0.008) after adjustment for age, race, study site, and cohort status (minimally adjusted model) (Table 2). After additional adjustment for CAD risk factors (fully adjusted model), this relationship slightly attenuated but remained statistically significant (OR 1.76 [1.00–3.10]; p=0.049).
Table 1.
Participant characteristics, by HIV serostatus
| HIV-Infected N=453 | HIV-Uninfected N=311 | P-value | |
|---|---|---|---|
| Race | <0.001 | ||
| Caucasian | 50.1% | 68.5% | |
| Black | 34.7% | 23.8% | |
| Hispanic/Other | 15.2% | 7.7% | |
| Age (years) | 52.0 ± 6.5 | 55.2 ± 7.3 | <0.001 |
| On Hypertensive Medications | 32.1% | 29.8% | 0.51 |
| On Diabetes Medications | 7.8% | 6.5% | 0.50 |
| On Lipid Lowering Medications | 33.7% | 31.7% | 0.56 |
| Smoking Status | 0.01 | ||
| Current | 30.4% | 21.5% | |
| Former | 43.5% | 54.7% | |
| Never | 26.2% | 23.8% | |
| Systolic Blood Pressure (mmHg) | 126 ± 14 | 128 ± 15 | 0.08 |
| Fasting Glucose (mg/dL) | 102 ± 24 | 99 ± 26 | 0.04 |
| Total Cholesterol (mg/dL) | 186 ± 41 | 194 ± 38 | <0.01 |
| HDL-Cholesterol (mg/dL) | 48 ± 15 | 54 ± 15 | <0.001 |
| LDL-Cholesterol (mg/dL) | 108 ± 36 | 116 ± 33 | <0.01 |
| Triglycerides (mg/dL) median (IQR) | 127 (93, 193) | 106 (74, 147) | <0.001 |
| Body mass index (kg/m2) | 26.1 ± 4.3 | 27.3 ± 4.9 | <0.01 |
| HIV Clinical Factors | |||
| Undetectable Viral Load (< 50 copies) | 80.4% | ||
| HIV RNA (copies/mL)& median (IQR) | 794 (139, 26350) | ||
| CD4+ T-cell count (cells/mm3) median (IQR) | 599 (426, 774) | ||
| CD4+ T-cell nadir (cells/mm3) median (IQR) | 293 (178, 416) | ||
| On HAART | 88.6% | ||
| Time on HAART (years) median (IQR) | 9.5 (6.4, 12.4) | ||
| Time on Protease Inhibitor (years) median (IQR) | 4.8 (0.3, 8.9) | ||
| History of clinical AIDS | 11.5% | ||
| CT Scan Parameters | |||
| Coronary Artery Calcium (Agatston score > 0) | 50.1% | 52.3% | 0.56 |
| Any coronary artery plaque on CCTA | 77.7% | 74.9% | 0.37 |
| Non-calcified plaque | 63.1% | 52.7% | <0.01 |
| Mixed plaque | 34.7% | 31.8% | 0.42 |
| Calcified plaque | 34.9% | 40.2% | 0.14 |
| Coronary artery stenosis > 50% | 16.8% | 14.5% | 0.39 |
| Segment involvement score (SIS) median (IQR) | 2 (1, 4) | 2 (0, 4) | 0.44 |
HDL= high-density lipoprotein, LDL = low-density lipoprotein, IQR = interquartile range, HAART = highly active antiretroviral therapy. Prevalence (%) or mean ± standard deviation unless otherwise noted. P-values determined using the Wilcoxon rank sum or chi-square test as appropriate.
Among the 88 HIV+ men with detectable current HIV RNA (>50 copies/mL) levels.
Figure 2.
Distribution of the number of positively remodeled coronary arterial segments per participant, by HIV status. Abbreviations:HIV=human immunodeficiency virus, PR=positive remodeling. Difference in the number of positive remodeled coronary arterial segments by HIV status was assessed by Wilcoxon rank sum test.
Table 2.
Associations between HIV serostatus, cardiovascular disease risk factors, and positive remodeling (n=764)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Odds Ratio | (95% CI) | Odds Ratio | (95% CI) | |
| HIV Infection | 2.01** | (1.20,3.38) | 1.76* | (1.00,3.10) |
| Age (per 10 years) | 1.73* | (1.14,2.63) | 1.81** | (1.16,2.84) |
| Black | 0.34** | (0.16,0.70) | 0.35** | (0.16,0.77) |
| Hispanic/Other | 0.69 | (0.28,1.69) | 0.68 | (0.27,1.72) |
| SBP1 (per 10 mmHg) | 1.23* | (1.00,1.52) | ||
| On Hypertensive Medication | 0.59 | (0.33,1.07) | ||
| On Diabetes Medication | 2.49* | (1.08,5.76) | ||
| Fasting Glucose2 (per 10 mg/dL) | 1.04 | (0.85,1.27) | ||
| Total Cholesterol3 (per 5 mg/dL) | 1.05* | (1.01,1.10) | ||
| HDL Cholesterol3 (per 5 mg/dL) | 0.74*** | (0.64,0.86) | ||
| On Lipid Lowering Medication | 1.55 | (0.85,2.80) | ||
| Body mass index | 1.02 | (0.96,1.09) | ||
| Current Smoker | 1.39 | (0.76,2.53) | ||
OR=Odds Ratio. CI=Confidence Interval.
p < 0.05,
p < 0.01,
p < 0.001. All models controlled for study site and cohort (output not shown).
Among participants not on hypertensive medications,
Among participants not on diabetes medication,
Among participants not on lipid-lowering medication
Results of a model that includes cardiovascular risk factors as predictors of PR is shown in Table 2. PR was positively associated with increasing age, total cholesterol, systolic blood pressure, and receiving diabetes medications and was inversely associated with HDL cholesterol and black race in the multivariable model (p< 0.05 for all). PR was not associated with fasting glucose, BMI, use of lipid lowering medication, or being a current smoker. In a model that further included SIS, each additional artery segment with atherosclerosis incrementally increased the participants’ odds of PR by 33 percent (p<0.001). The selected inflammatory biomarkers (CRP, ICAM-1, sTNFαRI, sTNFαRII, IL-6, sCD14, sCD163, MCP-1, D-dimer and fibrinogen) were not associated with positive remodeling, and were thus not retained in the multivariate model (all p>0.05, data not shown). The biomarkers were not associated with PR in the HIV-stratified models.
Restricting the analysis to 586 participants with coronary plaque present, a multilevel analysis that incorporated within-person correlation for multiple segments contributed by the same person was conducted to identify specific plaque characteristics associated with PR in each segment. A total of 1959 coronary segments with plaque present were included in the multivariable analyses after excluding 75 segments from 23 individuals missing some CAD covariates (data was not imputed for the segment-level dataset). The proportion of atherosclerotic segments with positive remodeling was higher among HIV+ compared to HIV− men (0.06 vs 0.04; Chi-square test p=0.03). After adjustment for age, race, and the previously defined CAD risk factors, and plaque type, the effect of HIV infection on PR in atherosclerotic segments became more attenuated and was no longer statistically significant (OR 1.52 [0.79, 2.95]). The odds of PR was 12.5 times greater in segments with mixed plaque and stenosis >50% (95% CI 4.45, 35.14), and 6.3 times greater in segments with mixed plaque but without stenosis (95% CI 2.62, 15.24), when compared to coronary segments with calcified plaque (both p<0.001); however the difference in the effect of mixed plaque with compared to without stenosis was not statistically significant (p=0.12). The odds of PR was 11.9 times greater in segments with non-calcified plaque and stenosis >50% (95% CI 4.09, 34.48), and 4.2 times greater in segments with non-calcified plaque but without stenosis (1.81, 9.55), when compared to coronary segments with calcified plaque (both p<0.001); and the difference in the effect of non-calcified plaque with compared to without stenosis was statistically significant (p=0.02).
Since non-contrast CT scans are sometimes utilized to screen for subclinical atherosclerosis, we evaluated the prevalence of positive remodeling in men with a CAC score of zero and its association with HIV infection in this setting. Among 374 men with a CAC score of zero, 7.1% (16/226) of the HIV+ and 2.7% (4/148) of the HIV− men had at least one positively remodeled arterial segment (p=0.07). Although of borderline statistical significance, HIV+ serostatus was associated with a three- fold increase in the odds of PR after adjustment for only demographics, OR 3.06 (0.96–9.74, p=0.06), and further for CAD risk factors (OR 3.33 [0.90–12.31]; p= 0.07).
We evaluated the associations between HIV-related factors and the presence of PR among HIV+ men (Table 4). A history of AIDS increased the odds of PR by 2.40 (CI 1.04–5.57, p=0.04) after adjusting for CAD risk factors. The odds of PR decreased by about 20% (p=0.03) for each 100 CD4+ T cells/mm3 increase in nadir CD4+ T cell count. When both a history of AIDS and nadir CD4+ T cell count were included in the same model, the effect size persisted, but was no longer statistically significant for history of AIDS. PR was not associated with current CD4+ T cell count or detectable HIV viral load, nor duration on HAART or PI use.
Table 4.
Association of HIV clinical and treatment factors with positive remodeling
| n1 | Individual Model Odds Ratio |
95% CI | Combined Model Odds Ratio (n=436) |
95% CI | |
|---|---|---|---|---|---|
| Detectable HIV RNA (> 50 copies/mL) | 450 | 0.69 | 0.27, 1.78 | ||
| History of AIDS | 453 | 2.40* | 1.04, 5.57 | 2.04 | 0.84, 4.93 |
| Duration on HAART (years) | 448 | 1.03 | 0.96, 1.12 | ||
| Protease Inhibitor Use (years) | 448 | 1.04 | 0.98, 1.11 | ||
| Current CD4+ T cell count (per 100 cells) | 449 | 1.06 | 0.94, 1.19 | ||
| Nadir CD4+ T cell count (per 100 cells) | 436 | 0.81* | 0.67, 0.98 | 0.82* | 0.67, 0.99 |
OR=Odds Ratio. CI=Confidence Internal.
p < 0.05,
p < 0.01,
p < 0.001.
Samples vary slightly from the 453 HIV-infected men due to missing values. Results are from separate models for each HIV-specific factor. Each regression models is adjusted for age, race, enrollment cohort, site, SBP, fasting glucose, total and HDL cholesterol, smoking, medication use, and body mass index (BMI).
4. Discussion
Positive remodeling has been shown to be associated with high-risk coronary artery plaque, which may predispose affected individuals to an increased risk of acute coronary syndromes [13]. In the general population, the presence of positive remodeling has been associated with incident coronary events even after accounting for the Framingham risk score [24,25]. We found that HIV+ men were 76 percent more likely to have positive remodeling after adjustment for demographic and CAD risk factors compared with HIVmen. Segment involvement score (SIS), which represents the number of coronary segments with plaque present, was strongly associated with positive remodeling, and a previous study in this population demonstrated that the SIS is on average 0.14 points higher on the natural log scale for HIV+ compared to HIV− men (p=0.02) [9]. After accounting for the number of atherosclerotic segments and plaque characteristics in a multilevel model, the odds of PR remained elevated in HIV+ men, but attenuated and was no longer statistically significant. These results demonstrate that HIV+ men have a higher prevalence of coronary arterial segments with PR, and that this increased risk is partially attributable to their having more atherosclerotic coronary segments and more non-calcified plaques (which are associated with PR).
Increasing age, systolic blood pressure, elevated total cholesterol, and the use of diabetes medications were associated with positive remodeling. In contrast, black men and men with higher levels of HDL-C were less likely to have positive remodeling. We previously demonstrated a lower prevalence of CAC, calcified plaque, and stenosis greater than 50% among black participants compared to white participants [26]. Among HIV-specific factors, PR was positively associated with a history of AIDS and inversely associated with nadir CD4+ T cell count, although there were no associations with years on HAART or protease inhibitors. These results suggest that more advanced HIV infection, through mechanisms yet to be determined, might increase the likelihood of the development of positively remodeled coronary arteries.
In our multilevel analysis after adjustment for HIV infection and CAD risk factors, we found that artery segments with noncalcified or mixed plaque and stenosis greater than 50% were far more likely to have positive remodeling compared to segments with calcified plaque. This may partially explain the higher rates of acute coronary syndrome associated with arteries demonstrating positive remodeling [13].
Several biomarkers have been postulated to be associated with vulnerable plaques, given the presence of inflammatory cell infiltration, especially macrophages, and overexpression of matrix metalloproteinases in coronary plaque [27,28]. However, none of the inflammatory biomarker levels we evaluated were associated with positive remodeling. This analysis was limited by having only one measurement of each biomarker that was measured from samples collected at the time of the scan and not prior to coronary CT evaluation.
Coronary artery calcium is a surrogate marker for atherosclerosis and a potent predictor of future cardiac events in the general population [29,30]. However, it is not a perfect tool and does not allow for assessment of other plaque types, specifically non-calcified and some mixed plaques, which may have a higher risk for rupture [31]. We found HIV+ men had an increased but not statistically significantly different odds of having positively remodeling despite a CAC score of zero. The clinical predictive utility of CAC measurement in HIV+ men requires further study, compared to the strong negative predictive value for future coronary events that is seen in the general population.
Our findings are consistent with a previous study of 102 HIV+ and 41 HIV− men from the Massachusetts General Hospital [17]. However, the prevalence of positive remodeling was significantly lower in our study, likely due to using a positive remodeling index definition of 1.1 compared to the 1.05 definition used by the previous study. We used the remodeling index of 1.1 because Motoyama and colleagues found that this definition of positive remodeling was predictive of acute coronary syndrome [13]. To our knowledge, this is the largest study using CCTA to evaluate the presence of positive remodeling in HIV+ individuals. Furthermore, the MACS is a well-characterized cohort that includes HIV+ and HIV− men drawn from the same reference population. Limitations of our study include the cross-sectional study design, inclusion of men only, and lack of assessment for other types of vulnerable plaque including low attenuation plaque and spotty calcification.
5. Conclusion
HIV+ men were more likely to have coronary artery segments that were positively remodeled, a potential mechanism for the reported increased incidence of cardiovascular events in this population. Furthermore, positive remodeling was associated with a history of AIDS and inversely with nadir CD4+ T-cell count, suggesting that the duration of HIV infection or the severity of immunodeficiency prior to initiation of antiretroviral treatment are linked etiologically to high-risk plaque. The greater burden of atherosclerosis among HIV-infected men, in terms of the number of atherosclerotic segments and extent of non-calcified plaque, accounted for some but not all of the association between HIV infection and positive remodeling. Future studies should investigate whether the presence of high-risk plaques identified on CCTA lead to more cardiovascular events in HIV+ individuals, and whether earlier initiation of effective antiretroviral therapy helps prevent the development of high-risk coronary plaque.
Table 3.
Proportion and adjusted odds ratio of positive remodeling by HIV and plaque characteristics among atherosclerotic artery segments
| n with PR (n=114) | n with plaque (n=2034) | % with PRa | Adjusted Odds Ratiob | (95% CI) | |
|---|---|---|---|---|---|
| HIV Infection | |||||
| No | 34 | 799 | 4.3% | 1 [reference] | |
| Yes | 80 | 1235 | 6.5% | 1.52 | (0.79,2.95) |
| Plaque type and stenosis interactions | |||||
| Calcified plaque (with or without stenosis) | 11 | 655 | 1.7% | 1 [reference] | |
| Non-calcified plaque without stenosis | 48 | 861 | 5.6% | 4.16*** | (1.81, 9.55) |
| Mixed plaque without stenosis | 27 | 344 | 7.8% | 6.32*** | (2.62, 15.24) |
| Non-calcified plaque with stenosis | 12 | 80 | 15% | 11.87***,† | (4.09, 34.48) |
| Mixed plaque with stenosis | 16 | 94 | 17% | 12.51*** | (4.45, 35.14) |
CI=Confidence Interval. PR=Positive Remodeling.
p < 0.05,
p < 0.01,
p < 0.001.
The association between non-calcified plaque and PR is statistically significantly greater when the segment also has a stenosis >50%, p=0.02.
Observed proportion at the segment-level, not adjusted for clustering within individuals.
Adjusted odds ratios for each variable are reported from the same model that includes age, race, and coronary artery disease risk factors, and that accounts for clustering within individuals. N=1959 segments with non-missing covariates included in the adjusted analysis.
Highlights.
HIV+ men had more coronary artery segments with positively remodeling (PR)
PR is associated with a history of AIDS and inversely with nadir CD4+ T-cell count
PR is associated with age, systolic BP, elevated cholesterol, and diabetes meds
PR is more likely in coronary segments with non-calcified plaque
Acknowledgments
We wish to thank Dr. Rine Nakanishi of the Cedars-Sinai Medical Center for Figure 1 from MACS.
Funding Sources: The MACS CVD study is funded by NHLBI: RO1 HL095129 (Post). Data in this manuscript were collected by the MACS with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). MACS data collection is also supported by UL1 TR 001079 (JHU CTSA). Website located at http://www.statepi.jhsph.edu/macs/macs.html. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health
Footnotes
Disclosures: Dr. Jacobson is a consultant for Bristol-Myers Squibb. Dr. Palella is on the speaker’s bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck & Co., Inc., and Bristol- Myers Squibb. Dr. Todd Brown is a consultant for Gilead Sciences, Bristol-Myers Squibb, Merck & Co., Inc., AbbVie, Theratechnologies, and ViiV Healthcare. The other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabib A, Leroux C, Mornex JF, et al. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–46. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mary-Krause M, Cotte L, Simon A, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 6.Rossi R, Nuzzo A, Guaraldi G, Squillace N, Orlanda G, Esposito R, Lattanzi A, Modena MG. Metabolic disorders induced by highly active antiretroviral thereapy and their relationship with vascular remodeling of the brachial artery in a population of HIV-infected patients. Metabolism. 2009;58:927–933. doi: 10.1016/j.metabol.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Baker JV, Neuhaus J, Duprez D, et al. INSIGHT SMART Study Group. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ascenzo F, Cerrato E, Calcagno A, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: A meta-analysis. Atherosclerosis. 2015;240:197–204. doi: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging. 2011;4:537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Motoyama S, Sarai M, Harigaya H, et al. Computed tomography angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable vs unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 16.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 17.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–1272. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaslow RA, Ostrow DG, Detels R, et al. The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 19.Hacioglu Y, Gupta M, Choi T-Y, et al. Use of cardiac CT angiography imaging in an epidemiology study – the methodology of the multicenter aids cohort study cardiovascular disease substudy. Anadolu Kardiyol Derg. 2013 May;13(3):207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 22.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman & Hill; New York: 1997. [Google Scholar]
- 23.Rabe-Hesketh S, Skrondal A, Pickles A. Maximum likeklihood estimation of limited and discrete dependent variable models with nested random effects. J Econ. 2005;128:301–323. [Google Scholar]
- 24.Yamamoto H, Kitagawa T, Ohashi N, Utsunomiya H, Kunita E, Oka T, Urabe Y, Tsushima H, Awai K, Kihara Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J Cardiovasc Comput Tomogr. 2013;7:192–199. doi: 10.1016/j.jcct.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto S, Kondo T, Kodama T, Orihara T, Sugiyama J, Kondo M, Endo A, Fukazawa H, Nagaoka H, Oida A, Ikeda T, Yamazaki J, Takase S, Narula J. Coronary computed tomography angiography-based coronary risk stratification in subjects presenting with no or atypical symptoms. Circ J. 2012;76:2419–2425. doi: 10.1253/circj.cj-12-0157. [DOI] [PubMed] [Google Scholar]
- 26.Miller PE, Budoff M, Zikusoka M, Li X, Pallea F, Kingsley LA, Witt MA, Sharrett AR, Jacobson LP, Post WS. Comparison of racial differences in plaque composition and stenosis among HIV positive and negative men (From the Multicenter AIDS Cohort Study [MACS]) Am J Cardiol. 2014 doi: 10.1016/j.amjcard.2014.04.049. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsheikh-Ali AA, Kitsios GD, Balk Em, Lau J, Ip S. The vulnerable atherosclerotic plaque: scope of the literature. Ann Intern Med. 2010;153:387–395. doi: 10.7326/0003-4819-153-6-201009210-00272. [DOI] [PubMed] [Google Scholar]
- 28.Hellings We, Peeters W, Moll FL, Pasterkamp G. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med. 2007;17:162–171. doi: 10.1016/j.tcm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffmann U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107(1):10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]