Abstract
Background
Deceptive methods of falsifying urine samples are of concern for anyone who relies on accurate urine toxicology results. A novel method to combat these efforts utilizes polyethylene glycol (PEG) markers administered orally prior to providing a urine sample. By using various PEG combinations to create a tracer capsule of unique composition, each urine sample can be matched to that individual. The goal of this study was to determine the effectiveness of using the PEG marker system among active heroin users screening for research studies.
Methods
Upon each screening visit, participants (N=55) were randomized to provide an unobserved urine sample, or the PEG tracer procedure was used. LCMS analysis was used to distinguish the PEG combinations, and allowed us to provide a unique qualitative analysis of patterns of drug use (N=168, total urine specimens).
Results
The unique composition of the tracer capsules was accurately detected in 83.5% of the urine specimens. Analyses of inconsistencies implicated a number of possible attempts at fraudulence (11.4%) and investigator/lab error (5.1%). Among this sample, the concurrent use of multiple classes of psychoactive drugs was more common than not, though concomitant drug use was often underreported.
Conclusion
Urine drug testing should be the minimum standard for obtaining information about drug use as self-report was unreliable even in a situation where there were no perceived adverse consequences for full disclosure. In cases where there are significant pressures for individuals to falsify these data, more protective collection methods such as the PEG marker system should be considered.
Keywords: urine drug testing, urine toxicology, sample adulteration, polydrug use, polyethylene glycols
1. INTRODUCTION
Urine drug testing is a quick and easy method for clinicians and researchers to gain information about risk-related behaviors concerning substance abuse. Among opioid users in treatment, regular urine drug testing can identify aberrant drug-related behaviors and help to ensure treatment adherence and effectiveness (U.S. Food and Drug Administration (FDA), 2011). Among active opioid users, urine toxicology (Utox) results can inform us about drug use patterns, such as polysubstance abuse, that are thought to significantly increase risk of disease transmission and overdose (Roux et al., 2013; Gjersing et al., 2013). Accordingly, regular urine drug testing has been advocated by many state, policy, and organizational guidelines (Chou et al., 2009; Gudin et al., 2013; Manchikanti et al., 2012; Utah Dept. of Health 2009). However, urine sample adulteration may be a problem in any clinical population that has an interest in false results (e.g., pre-employment and workplace screening; Owen et al., 2012).
There are several documented methods of tampering with urine samples: dilution by drinking excessive amounts of water or external dilution, and adulteration by mixing the urine with oxidants, soaps etc. (Honour et al., 1996; Mikkelsen and Ash, 1988). These methods can be detected with modern laboratory techniques (Federal Register, 2001). However, substitution of one’s urine with a “clean” sample from another individual or synthetic urine remains a serious concern (Jaffe et al., 2007). The most common approach to prevent these methods of Utox falsification is supervision of the urination process. However, supervised urine collection can be burdensome to personnel and embarrassing to clients. Also, observed collection does not entirely ensure against fraudulence aided by clever devices, such as those containing life-like penises, synthetic urine, and heat packs (to keep the fake urine at body temperature; http://www.thewhizzinator.com/).
The current study tested a different method to detect sample manipulation, a labelling procedure that allows samples to be matched with a particular person (Gauchel et al., 2003; Huppertz et al., 2004). With this novel method, a tracer/marker substance is taken orally prior to the participant providing a urine sample. Urine samples can be matched to the particular patient by assessing for the specific marker substance previously ingested.
The marker substances are low molecular weight polyethylene glycols (PEGs) used for years as a galenic basis for drugs and considered “inactive ingredients” by the FDA. PEGs appear in urine 30 min after ingestion and are undetectable after 6–8 hours (Christensen et al., 2014). By combining polyethylene glycols of different molecular weights, a large number of different polyethylene glycol chain mixtures can be obtained. Therefore, for a group of participants, unique tracer capsules can be offered that can be discriminated from each other, matching a participant to his or her sample.
The goal of this study was to determine the feasibility and effectiveness of using the PEG marker system among active heroin users screening for clinical studies within the Opioid Research Laboratory, part of the Division on Substance Abuse at the College of Physicians and Surgeons of Columbia University/New York State Psychiatric Institute (NYSPI). Throughout our normal screening procedure urine toxicology tests are performed numerous times in order to: verify experience with the drug under investigation, assess and diagnose abuse, and identify potential adverse drug interactions.
Falsification is a serious concern in cases where opioid users may lose money, privileges, or their freedom (Owen et al., 2012). However, in the present setting, specific inclusion/exclusion criteria related to drug use and toxicology results were not disclosed to potential study participants. As such, this study allowed us to examine the need for objective and protective methods of assessing drug use among a population with little perceived incentive to be deceptive. Finally, the analyses performed on the urine samples provided an objective way to assess drug use trends and concomitant drug use among a unique population of heroin users not currently in treatment or seeking treatment.
2. METHODS
2.1 Overview
Data were collected between 2013 and 2014 at the NYSPI Substance Use Research Center located in upper Manhattan. Urine samples used in the current analysis were obtained from volunteers screening for six experimental studies with the Opioid Research Laboratory (IRB#s 6255, 6107, 5879, 6021, 6883R, 6400). Our clinical studies investigate the subjective and reinforcing effects of various opioids, and novel treatment medications among various populations of opioid users who are not seeking treatment for drug abuse (at time of their study participation). See Jones et al. (2011; 2014) for examples of this research.
Potential participants were recruited locally with newspaper advertisements and word-of-mouth referrals. Although the exact wording of the advertisements differed from study to study, the verbiage typically sought “intravenous and intranasal heroin users,” or “healthy heroin users.” After completing an initial telephone screening interview, eligible participants were scheduled for in-person screening at NYSPI that included: detailed medical history and drug use questionnaires, medical evaluation, psychiatric evaluation, a naloxone challenge to assess opioid dependence, and an interview with a research psychologist to discuss patterns of drug use in detail. Screening typically required 4–5 visits, and was conducted over the course of 3–4 weeks to determine eligibility (with urine collected at each visit). As an addendum to the screening process for the 6 inpatient studies mentioned above, participants were offered the opportunity to participant in the current study (IRB# 6817). Those who agreed signed separate study consent and completed the procedures described below. Participants were paid between $20 and $45 for each visit ($20 for the inpatient study screening procedures, plus a possible $25 for days they received a PEG tracer). All study procedures were approved by the NYSPI IRB.
2.2 Procedures
Prior to providing each individual urine sample, participants were randomized to one of two conditions: Testing as Usual (TAU) and Marker group (i.e., participants could have provided urine samples using both procedures). Participants were randomized to a specimen collection procedure upon each visit using a Latin Square randomization scheme (Bailey, 2008). Urine specimens from participants assigned to the TAU sample were collected using our current practice, without direct observation. Participants were provided with a urine cup and given access to a private bathroom. When participants were assigned to provide a urine sample with Marker, they were given a gel capsule containing 100 mg of PEG marker material, which they consumed with 100 ml of a flavored beverage (e.g., soda, fruit juice, Gatorade), under the supervision of a study nurse (Figure 1). Participants waited 30 min – 1 hr and then provided a urine sample in a standard urine cup (without supervision). All participants, when assigned to the Marker condition, received an active PEG tablet (i.e., there was no placebo tablet).
Figure 1.
Blister pack containing PEG marker capsule and barcode identifying its unique constituents.
Participants met with a research nurse to assess for any immediate adverse effects following consumption of the Marker capsule. At their next visit (or via phone), they were asked if they experienced any adverse drug effects after leaving the research center.
All urine samples were initially tested using an 11-Panel DrugCheck® Dip Drug Tests with the following positive result cut-offs: Amphetamine: 1000 ng/mL, Barbiturate: 300 ng/mL, Benzodiazepine: 300 ng/mL, Buprenorphine: 10 ng/mL, Cocaine: 150 ng/mL, Methamphetamine: 500 ng/mL, Methadone: 200 ng/mL, Opiates (morphine, codeine, heroin): 300 ng/mL, Oxycodone: 100 ng/mL, PCP: 25 ng/mL, THC: 50 ng/mL. The results of this test were entered into the participants’ study chart and on the sample reporting form that accompanied the urine sample for confirmatory Liquid Chromatography-Mass Spectrometry (LCMS) assessment for drugs of abuse and detection of the PEG tracers (when applicable).
The marker substances are low molecular weight polyethylene glycols. The chemical structure of polyethylene glycols is HO-(CH2-CH2-O)n –H with “n” varying between 8 and 1000 or more. Polyethylene molecules of chain lengths between 8 and 17 repeating units resulting in molecular weights ranging from 370 to 766. For the purposes of the current study, PEGs of 4 different molecular weights were used: PEG370 (PEG-8), PEG414 (PEG-9), PEG458 (PEG-10), and PEG503.3 (PEG-11). An individual marker capsule could contain a single PEG or any combination of the 4. The barcode on each gel capsule identified the PEG or PEG combination used (Figure 1). The unique PEG identifier was only known to Avee laboratory staff.
2.3 Aims
This study was designed to determine the safety and efficacy of the PEG marker system by assessing adverse events related to PEG capsule consumption, reliable identification of the PEG combination administered in the urine sample, and a comparison of attempts at fraudulence/substitution between TAU and Marker conditions. In addition, patterns of drug use among heroin users not currently seeking treatment were assessed using self-report, urine dip tests, and LCMS.
2.4 Statistical Analyses
Continuous and categorical participant variables were summarized descriptively (Table 1). Independent-samples T-test was planned to compare fake urine falsification attempts between the Marker and TAU groups, though this analysis was found to be unnecessary (see Results). The significance level of α was set at 0.05. All data analyses were performed using SPSS® version 18.
Table 1.
Demographic characteristics of study participants (N =55)
| Demographics
| |
|---|---|
| Mean (SD) or Participants (%)
|
|
| Age | 46.78 (7.45) |
| Sex | |
| Male | 51 (93) |
| Female | 4 (7) |
| Ethnic/Racial Category | |
| African American | 28 (51) |
| Caucasian | 7 (13) |
| Hispanic | 15 (27) |
| Multiracial | 5 (9) |
| Opioid Use
| |
|---|---|
| Mean (SD) or Participants (%) | |
| Heroin Bags per Day | 5.17 (2.48) |
| Years of Use | 18.12 (11.62) |
| Route of Administration Preference | |
| Intranasal | 35 (63.6) |
| Intravenous | 20 (36.4) |
3. RESULTS
3.1 Participants
In total, 55 heroin users (without chronic pain) were consented and provided Utox data for the current analysis. Participants were predominately male (93%), and in their mid 40s (mean: 46.7 yrs). On average, participants had been using heroin for 18 years and all but one reported daily heroin use with an average of 5.1 bags per day. This sample provided a total of 168 urine specimens, with each participant providing an average of 3.3 samples, collected on average 3.6 days apart. Table 1 presents a more extensive list of demographic data.
3.2 Detection of PEG and Urine Falsification Attempts
Eighty-nine total urine samples were collected under the TAU collection procedure, while 79 total urine samples were obtained using the Marker system. No fraudulent urine samples (e.g., synthetic or watered-down urine) were found among any of the samples collected.
Of the 79 samples collected under the Marker conditions, there were 65 cases (83.5%) where the PEG marker consumed was accurately identified in the urine sample. This leaves 13 (16.5%) where PEG results were inconsistent. In 9 samples, it was noted that a PEG marker had been administered, but not found in the urine specimen. In 4 samples, no PEG marker was indicated as being given yet PEGs were detected in the urine sample. Possible explanations for these inconsistencies are provided in the discussion. There was no association between the consistency/inconsistency of the samples and the total number of samples provided by a participant, or the samples’ order in the participants’ randomization sequence.
3.3 Side Effects and Adverse Events
No adverse side effects of PEG capsule consumption were reported in the period of observation immediately after consumption or after the participant left the research center (assessed at their next visit).
3.4 Consistency of Self-reported Drug Use with Utox Results
Urine toxicology results were matched by date to an in-person interview with a research psychologist. Only 23.5% of participants’ self-reported “current and/or 30-day” drug use was found to be consistent with their Utox results.
3.5 Assessment of Drug Use
A total of 168 urine specimens were collected as a part of this investigation. Although all participants reported being current heroin users, seven participants provided samples that were never positive for opioids. As such, 87.3% of our participants provided at least one urine sample that was positive for an opioid and 91.6% of the total individual samples tested positive for an opioid. Following opioids, nicotine was the most commonly used drug with 81.8% of participants providing at least one urine sample that tested positive and 88.0% of all samples testing positive.
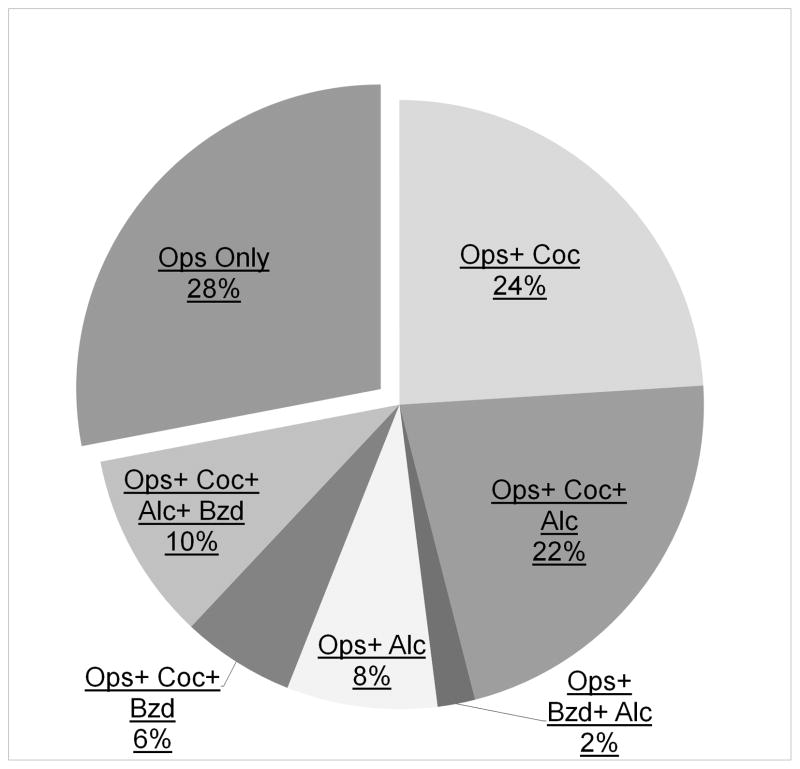
Cocaine was the next most commonly found drug with 54.5% of participants providing at least one urine sample that tested positive and 53.8% of all samples testing positive. Cocaine was followed by: delta-9-tetrahydrocannabinol (THC: 34.4% of participants, 24.5% of total samples), alcohol (30.9% of participants, 29.3% of samples), benzodiazepines (BZD: 20.0% of participants, 13.1% of samples), and gabapentin (10.9% participants, 8.3% samples). Two participants (3.6%) provided a sample positive (1.2% of total samples) for methamphetamine and one participant (1.8%) provided a single sample positive for phencyclidine (PCP; 0.59% of total samples). Results of quantitative drug levels are shown in Table 2. Figure 2 displays the percentage of participants who provided urine samples that tested positive only for opioid drugs vs. opioids in addition to another type of drug.
Table 2.
Quantitative levels of drugs of abuse found using LCMS analysis
| Opioidsa
| ||
|---|---|---|
| Mean | (SEM) | |
| 6-MAM | 1523.00 | (241.00) |
| Hydrocodone | 450.00 | (198.00) |
| Oxycodone | 3530.00 | (434.00) |
| Buprenorphine | 54.78 | (15.00) |
| Fentanyl | 46.00 | (18.00) |
| Methadone | 2412.51 | (544.00) |
| Tramadol | 562.75 | (395.00) |
| Stimulants
| ||
|---|---|---|
| Mean | (SEM) | |
| Methamphetamine | 168.00 | (17.00) |
| Cocaine | 1937.00 | (145.00) |
| Alcohol Metabolites
| ||
|---|---|---|
| Mean | (SEM) | |
| Ethyl Glucoronide | 6557.00 | (350.00) |
| Ethyl Sulfate | 1507.00 | (92.00) |
| Benzodiazepines
| ||
|---|---|---|
| Mean | (SEM) | |
| Alprazolam | 123.00 | (57.30) |
| Clonazepam | 126.00 | (24.00) |
| Lorazepam | 2171.33 | (824.00) |
| Diazepam | 82.00 | (30.00) |
| Oxazepam | 984.00 | (288.00) |
| Other Drugs
| ||
|---|---|---|
| Mean | (SEM) | |
| THC | 196.00 | (92.00) |
| PCP | 124.00 | (40.70) |
| Zolpidem | 42.00 | (30.00) |
| Nicotine (cotinine) | 3321.89 | (144.94) |
| Gabapentin | 8500.71 | (815.51) |
As participants are primarily heroin users, we excluded opioids whose presence could have resulted from metabolism of heroin (i.e., codeine, morphine, hydromorphone) (http://www.mayomedicallaboratories.com/articles/drugbook/opiates.html).
Figure 2.
Percentage of participants who provided urine samples that tested positive only for opioid drugs and opioids in addition to: cocaine, alcohol and benzodiazepines. Nicotine was excluded due to its ubiquitous use among this sample. THC was excluded because the duration of time that it remains detectable in the urine makes it difficult to quantify frequency of use. Each participant was only counted once; that is, the categories do not overlap.
4. DISCUSSION
The results of this study demonstrate that PEG markers are a safe and effective means of ensuring against urine toxicology fraudulence. In over 83% of cases, the unique marker administered to each participant was accurately detected in the urine sample later collected. Two types of inconsistencies were found. In 9 samples, it was noted that a PEG marker had been administered, but was not found in the urine specimen. Cross referencing PEG marker results with our Opioid Lab records could find no evidence of human error (e.g., labeling errors, sample mix up). Reporting forms for all 9 samples indicated the day and time the PEG marker was given, matching the details in the laboratory report. Among these samples, urine specimens were obtained between 31 and 42 minutes following PEG administration. According to the manufacturer, PEGs should appear in urine 30 min after ingestion, thus, sufficient time was allowed for the marker to be detectible. As such, the remaining likely cause of these inconsistencies could be that participants did not swallow the capsules (“cheeking”) or provided a urine sample that was not theirs. In either case, the Marker system shows its utility as these samples were correctly flagged as “inconsistent.”
In 4 samples, no PEG marker was indicated as being given, but PEGs were detected in the urine sample. Exploration into possible causes of these errors revealed that in two cases PEG markers had been given on the previous visit, and the type of PEG markers found in the inconsistent samples matched that given previously. In both cases PEG marker had been given over 30 hrs previously. PEGs should completely clear from the blood and urine after 6–8 hours, making it unlikely that PEGs remained detectable in the urine from the previous sample (Christensen et al., 2014). This leads the investigators to conclude that these errors were most likely due to investigator labeling errors or lab mix-ups. Human error may have also been the cause of the remaining two erroneous samples. In both cases, the urine samples provided were the participants’ first as a part of this study and there is no indication of previous PEG exposure. As multiple participants often provided samples concurrently, samples from participants who received PEGs that day may have been substituted. Because of the unique nature of the PEG composition of the marker capsules, it is unlikely that false positives would result from other participant medications (e.g., antihypertensives) that use PEGs to prepare galenic forms of tablets and capsules. An equally unlikely, yet possible, source of the false positives could have been individuals in the non-marker group substituting their sample with that of another study participant who had recently received a PEG marker. Unpublished data from other clinical studies involving PEGs have not found a single verified false positive in over 70,000 cases (Personal Communication, Dr. Ruprecht Keller; Christensen, 2014). Further assessment of the LCMS chromatograph would allow for a more definitive determination of the source of these erroneous positive results.
These data confirm the necessity of validating self-reported drug use. Omission of many drugs of abuse was common even when there were no perceived adverse consequences for full disclosure. The results of the current investigation found that the abuse of multiple drug classes is far more common than not among active heroin users. Urine toxicology tests revealed that the majority of those sampled were using multiple opioids in addition to another type of drug. Among the heroin users sampled, there was evidence of regular buprenorphine and methadone use. These data suggest that use of these two medications is common among heroin users, even when out of treatment. Although this suggests a street market for these drugs, the authors would argue that this is beneficial to the health of heroin users. First, both drugs carry a lower risk of overdose in comparison to heroin (Brugal et al., 2005; Mattick et al., 2008; Megarbane et al., 2010; Vormfelde et al., 2001; Wolff et al., 2002). Second, studies suggest that the diversion of these drugs is for the purpose of self-detoxification or self-medication of opioid withdrawal, rather than for recreational purposes (Johanson et al., 2012; Monte et al., 2009).
As expected, high rates of nicotine use were found among this sample with the vast majority (88%) of urine samples indicating recent use. There was also robust evidence of regular alcohol use, with 29% of the sample indicating alcohol use within the past 3 days among 31% of study participants (Helander et al., 2009). Cocaine was the most commonly found illicit drug, followed by THC. The co-occurring use of cocaine and BZDs was the most likely to be unreported by the users.
Of most concern to the investigators was the indication of frequent BZD use among this opioid-dependent sample. The use of BZDs among this population may be unintentional, as anecdotal reports from heroin users and sellers indicate that street heroin is often “cut” with BZDs (http://www.bluelight.org/vb/threads/675556-Recent-Heroin-Cut-with-Benzo; http://www.talkingdrugs.org/benzodiazepines-sold-as-heroin). Alternatively, BZDs may be intentionally used for medicinal (i.e., the self-medication of underlying mental conditions) or recreational (i.e., potentiate the reinforcing/rewarding effect of the opioid) purposes (Jones et al., 2012). Either case is cause for concern, as there is a growing body of literature to suggest that the addition of BZD drugs significantly increases risk of opioid overdose and overdose lethality (Chan et al., 2006; Lintzeris et al., 2007; Pirnay et al., 2004; Reynaud et al., 1998; Ross et al., 2000; New York City Dept. of Health and Mental Hygiene (NYCDHMH), 2013). With some studies finding that BZDs were detected in a quarter of overdose deaths (Zador et al., 1996; Darke et al., 1997).
Among samples testing positive for both opioids and BZD, the mean heroin (6-MAM) levels were 738.96 ng/ml, while the mean BZD levels were 629.89 ng/ml. Among samples that did not also test positive for BZDs the 6-MAM levels were much higher (1677.72 ng/ml). These data may imply that the addition of a BZD may preclude the need for more heroin. BZDs may also be used in times when access to heroin is difficult, or its purity low. This analysis did not include levels of other opioids, which were common, but varied from individual to individual. The clinical significance of these data is difficult to interpret because of differences in strength and half-life across the various BZD drugs. However, given the increased risk of overdose and possibility of BZD withdrawal, accurate knowledge of regular BZD use is important in a number of clinical scenarios (e.g., opioid detoxification, when prescribing opioid agonist medications or opioid analgesics).
With respect to drug-using populations, having accurate, comprehensive information on drug use may improve safety and treatment adherence rates, facilitate patient–provider communication, and reduce opioid misuse (Substance Abuse and Mental Health Services Administration (SAMHSA), 2012). This study, along with several others, revealed that urine drug testing should be the minimum standard for obtaining information about drug use (Gudin et al., 2013). However, in cases where there are significant pressures for individuals to falsify these data, more protective collection methods should be considered. As methods of thwarting drug tests become savvier and more nuanced, the PEG marker system may prove to be a valuable tool to combat these efforts.
Highlights.
There are several documented methods of tampering with urine samples
Unique oral PEG markers allow urine samples to be matched to a particular person
The unique composition of the PEG was accurately detected in 83.5% of samples
The PEG marker system may prove to be a valuable tool to combat urine falsification
Acknowledgments
Role of the Funding Source: This study was supported by Marker-Test Diagnostics Inc. (via a grant to SDC). Marker-Test also provided the PEG markers used in the study. The funding source played no role in the collection, analysis and interpretation of data, in the writing of the article, or in the decision to submit it for publication. Additional financial support for the preparation of the manuscript was provided by NIDA grant DA030446 to JDJ.
Footnotes
Contributors: JDJ and SDC designed and planned the study. JDJ, GM, and VEM, were responsible for screening and assessing study participants. JDJ and JA performed analyses of study results. JDJ developed the first draft of the manuscript that all authors edited, and also approved the submitted draft.
Conflicts of Interest: Over the past three years, SDC has received compensation (in the form of partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova and served as a consultant to the following companies: AstraZeneca, Camarus, Grunenthal USA, Guidepoint Global, Janssen, Mallinckrodt, Neuromed, Orexo, Pfizer, and Salix. The other authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey RA. Design of Comparative Experiments. Cambridge University Press; 2008. 6 Row-Column Designs And 9 More About Latin Squares. [Google Scholar]
- Brugal MT, Domingo-Salvany A, Puig R, Barrio G, De Garcia-Olalla P, de la Fuente L. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction. 2005;100:981–989. doi: 10.1111/j.1360-0443.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- Chan GM, Stajic M, Marker EK, Hoffman RS, Nelson LS. Testing positive for methadone and either a tricyclic antidepressant or a benzodiazepine is associated with an accidental overdose death: analysis of medical examiner data. Acad Emerg Med. 2006;13:543–547. doi: 10.1197/j.aem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG. American Pain Society–American Academy of Pain Medicine Opioids Guideline Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, Tardiff K. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990–98. Addiction. 2003;98:739–747. doi: 10.1046/j.1360-0443.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, Mogali S, Saccone PA, Askalsky P, Martinez D, Walker EA, Jones JD, Vosburg SK, Cooper ZD, Roux P, Sullivan MA, Manubay JM, Rubin E, Pines A, Berkower EL, Haney M, Foltin RW. Effects of acute oral naltrexone on the subjective and physiological effects of oral D- amphetamine and smoked cocaine in cocaine abusers. Neuropsychopharmacolgy. 2013;38:2427–2438. doi: 10.1038/npp.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K. [Last Accessed on April 23rd, 2015];Shifting The Paradigm: A Blueprint For Value Recognition And Process Improvement In The U.S. Drug Testing Market. 2014 http://www.markertest.com/file_616-169783-2685/shifting-the-paradigm.pdf.
- Darke S, Zador D, Sunjic S. Heroin related deaths in south western Sydney. Med J Australia. 1997;167:107. doi: 10.5694/j.1326-5377.1997.tb138794.x. [DOI] [PubMed] [Google Scholar]
- Federal Register. US Federal Register Notice to Revise the Mandatory Guidances for Specimen Validity Testing. 2001 Aug 21;66(162):43876–82. [Google Scholar]
- Gauchel G, Huppertz B, Feiertag H, Keller R. Clinical use of polyethylene glycols as marker substances and determination in urine by liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:271–279. doi: 10.1016/s1570-0232(02)00925-x. [DOI] [PubMed] [Google Scholar]
- Gjersing L, Jonassen KV, Biong S, Ravndal E, Waal H, Bramness JG, Clausen T. Diversity in causes and characteristics of drug-induced deaths in an urban setting. Scand J Public Health. 2013;41:119–125. doi: 10.1177/1403494812472007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125:115–130. doi: 10.3810/pgm.2013.07.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A, Bottcher M, Fehr C, Dahmen N, Beck O. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44:55–61. doi: 10.1093/alcalc/agn084. [DOI] [PubMed] [Google Scholar]
- Honour JW. Testing for drug abuse. Lancet. 1996;348:41–43. doi: 10.1016/s0140-6736(96)05336-6. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Gauchel G, Feiertag H, Schweizer H, Krieger H, Richter F, Heinz H, Blanke J, Gastpar M, Keller R. Urine labeling with orally applied marker substances in drug substitution therapy. Clin Chem Lab Med. 2004;42:621–626. doi: 10.1515/CCLM.2004.107. [DOI] [PubMed] [Google Scholar]
- Jaffee WB, Trucco E, Levy S, Weiss RD. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33:33–42. doi: 10.1016/j.jsat.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Arfken CL, di Menza S, Schuster CR. Diversion and abuse of buprenorphine: findings from national surveys of treatment patients and physicians. Drug Alcohol Depend. 2012;120:190–195. doi: 10.1016/j.drugalcdep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125:8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay JM, Vosburg SK, Comer SD. The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacology. 2011;36:411–422. doi: 10.1038/npp.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Vosburg SK, Manubay JM, Mogali S, Metz V, Comer SD. Abuse potential of intranasal buprenorphine versus buprenorphine/naloxone in buprenorphine-maintained heroin users. Addict Biol. 2014 doi: 10.1111/adb.12163. (E-Pub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Abdi S, Atluri S. American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2—guidance. Pain Physician. 2012;15(Suppl 3):S67–S116. [PubMed] [Google Scholar]
- Me’garbane B, Buisine A, Jacobs F, Re’sie’re D, Chevillard L, Vicaut E, Baud FJ. Prospective comparative assessment of buprenorphine overdose with heroin and methadone: clinical characteristics and response to antidotal treatment. J Subst Abuse Treat. 2010;38:403–407. doi: 10.1016/j.jsat.2010.01.006. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav. 2008;33:1297–1305. doi: 10.1016/j.addbeh.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte A, Mandell T, Wilford B, Tennyson J, Boyer E. Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J Addict Dis. 2009;28:226–231. doi: 10.1080/10550880903014767. [DOI] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene. [Last accessed Nov 4th, 2014];Unintentional Drug Poisoning (Overdose) Deaths in New York City, 2000–2012. 2013 http://www.nyc.gov/html/doh/downloads/pdf/epi/databrief33.pdf.
- Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15:ES119–ES133. [PubMed] [Google Scholar]
- Pirnay S, Borron S, Giudicelli C, Tourneau J, Baud F, Ricordel I. A critical review of the causes of death among post-mortem toxicological investigations: analysis of 34 buprenorphine-associated and 35 methadone associated deaths. Addiction. 2004;99:978–988. doi: 10.1111/j.1360-0443.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- Reynaud M, Petit G, Potard D, Courty P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addiction. 1998;93:1385–1392. doi: 10.1046/j.1360-0443.1998.93913859.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Technical Assistance Publication (TAP) 32. HHS publication (SMA) 12–4668. Rockville, MD: 2012. Clinical Drug Testing in Primary Care. [Google Scholar]
- Stancliff S, Joseph H, Fong C, Furst T, Comer SD, Roux P. Opioid maintenance treatment as a harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J Addictive Dis. 2012;31:278–287. doi: 10.1080/10550887.2012.694603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux R, Fugon L, Jones JD, Comer SD. Hepatitis C infection in non- treatment seeking heroin users: the burden of cocaine injection. Am J Addict. 2013;22:613–618. doi: 10.1111/j.1521-0391.2013.12058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA) FDA Acts To Reduce Harm From Opioid Drugs. Washington, DC: 2011. [Last accessed Jan 2nd, 2015]. Updated April 2013. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm251830.htm. [Google Scholar]
- Utah Department of Health. Utah Clinical Guidelines on Prescribing Opioids for Treatment of Pain. Salt Lake City, UT: 2009. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Poser W. Death attributed to methadone. Pharmacopsychiatry. 2001;34:217–222. doi: 10.1055/s-2001-18032. [DOI] [PubMed] [Google Scholar]
- Wolff K. Characterization of methadone overdose: clinical considerations and the scientific evidence. Thera Drug Monit. 2002;24:457–470. doi: 10.1097/00007691-200208000-00001. [DOI] [PubMed] [Google Scholar]
- Zador D, Sunjic S, Darke S. Heroin-related deaths in New South Wales, 1992: toxicological findings and circumstances. Med J Australia. 1996;164:204–207. doi: 10.5694/j.1326-5377.1996.tb94136.x. [DOI] [PubMed] [Google Scholar]