Abstract
Alternariol (AOH) is an important mycotoxin from the Alternaria fungi. AOH was detected for the first time in the wheat pathogen Parastagonospora nodorum in a recent study. Here, we exploited reverse genetics to demonstrate that SNOG_15829 (SnPKS19), a close homolog of Penicillium aethiopicum norlichexanthone (NLX) synthase gene gsfA, is required for AOH production. We further validate that SnPKS19 is solely responsible for AOH production by heterologous expression in Aspergillus nidulans. The expression profile of SnPKS19 based on previous P. nodorum microarray data correlated with the presence of AOH in vitro and its absence in planta. Subsequent characterization of the ΔSnPKS19 mutants showed that SnPKS19 and AOH are not involved in virulence and oxidative stress tolerance. Identification and characterization of the P. nodorum SnPKS19 cast light on a possible alternative AOH synthase gene in Alternaria alternata and allowed us to survey the distribution of AOH synthase genes in other fungal genomes. We further demonstrate that phylogenetic analysis could be used to differentiate between AOH synthases and the closely related NLX synthases. This study provides the basis for studying the genetic regulation of AOH production and for development of molecular diagnostic methods for detecting AOH-producing fungi in the future.
INTRODUCTION
Mycotoxins pose serious health risks to humans and animals (1, 2). Consumption of food and feed contaminated with mycotoxins can lead to various mycotoxicoses. Mycotoxins also cause significant economic losses in agriculture due to reduced crop value resulting from mycotoxin contamination and losses in animal productivity from mycotoxin-induced health problems (2). Furthermore, there are increasing concerns about mycotoxins in indoor environments (3), the contamination of water with mycotoxins in agricultural land affected by mycotoxin-producing plant pathogens (4), and the effect of climate change on mycotoxin contamination of pre- and postharvest food (5, 6).
Deciphering the molecular genetic basis of mycotoxin production in fungi will improve our understanding of its genetic regulation and facilitate the identification of potential mycotoxin-producing fungi in the environment (7, 8). There has been considerable interest in developing PCR-based diagnosis methods for detection of mycotoxin-producing fungi in agricultural commodities and in food and feed (9). Such diagnosis will also help determine if a fungal strain is suitable for use in the food industry or as a biocontrol agent. With the wide availability of genomic technologies, the genome of a fungal strain can be rapidly scanned for potential genes encoding mycotoxin biosynthesis. The genetic and molecular bases for biosynthesis of several important mycotoxins are well known (10), such as those for aflatoxins (11, 12), fumonisins (13, 14), zearalenone (15, 16), ergot alkaloids (17, 18), and trichothecenes (19, 20). Nonetheless, the molecular genetic bases for the production of many other mycotoxins, such as ochratoxins, altertoxins, and alternariol, remain elusive.
Alternariol (AOH) and alternariol-9-methyl ether (AME) are two important mycotoxins that are well known to be produced by fungi in the Alternaria genus, which are capable of infecting a variety of crop plants. The two mycotoxins are common contaminants of food such as grain and grain-based products, fruits/fruit juice, and vegetable products (in particular, tomato products) (21–23). They have been reported to exhibit cytotoxic, fetotoxic, teratogenic, and possible mutagenic and estrogenic effects (24–27) and may contribute to development of human esophageal cancer (28). AOH has been demonstrated to inhibit cholinesterase (29) and topoisomerases I and II (30). Furthermore, it was shown that AOH induces DNA damage and cell cycle arrest in vitro in the murine macrophage RAW 264.7 cell line, and the treated cells exhibited an abnormal nuclear morphology (31, 32).
Recently, AOH was reported to be produced by Parastagonopora nodorum (synonymous to Stagonospora nodorum and Phaeosphaeria nodorum), an important wheat pathogen in Australia and worldwide. AOH was first detected in P. nodorum in a metabolomics study of a mutant lacking a short-chain dehydrogenase gene, Sch1, which accumulated AOH at a 200-fold-greater concentration than the wild type (WT) (33). Sch1 was demonstrated to be regulated by G-protein signaling and is required for asexual sporulation (34). Subsequently, a transcription factor gene, StuA, has also been shown to be a positive regulator of AOH production (35). The discovery of AOH in P. nodorum raised new concerns about the health implication of this wheat pathogen.
Two polyketide synthase (PKS) genes have been recently inferred in an RNA-silencing knockdown study to be involved in AOH biosynthesis in Alternaria alternata (36). In this study, we have unequivocally identified the PKS gene responsible for the production of AOH in P. nodorum by both targeted genetic disruption and heterologous expression. The P. nodorum AOH PKS gene is different from that identified previously but is highly conserved with another PKS gene in A. alternata. Furthermore, identification of the P. nodorum PKS AOH gene has enabled the survey of homologs in other fungal genomes.
MATERIALS AND METHODS
P. nodorum strains and culturing conditions.
The wild-type P. nodorum strain SN15 was obtained from Department of Agriculture and Food Western Australia (DAFWA) and had been previously deposited in American Type Culture Collection (ATCC MYA-4574) and the Fungal Genetics Stock Center (FGSC 10173). Both the wild-type and mutant strains generated in this study were maintained on V8-supplemented potato dextrose agar (V8-PDA) plates at 22°C under a 12-h dark/light regime, which induces sporulation (37). For screening of AOH and AME production in P. nodorum wild-type strain SN15 and ΔSnPKS19 mutants, the strains were grown in defined minimal medium agar (MMA) (37) in the dark at 22°C for 7 days.
Aspergillus nidulans strain TNO2A3 (from the Fungal Genetics Stocks Centre, FGSC A1149) was maintained on Aspergillus glucose minimal medium (GMM) agar (38) supplemented with 0.5 mM pyridoxine, 0.01 μg/ml riboflavin, 5 mM uridine, and 5 mM uracil. Aspergillus nidulans TNO2A3 and the transformed mutants were cultured in liquid GMM at 28°C with shaking 150 rpm.
Metabolite profile analysis.
For the detection of AOH and AME in the P. nodorum SN15 extracts from various growth conditions, liquid chromatography-mass spectrometry (LC-MS) was performed on an Agilent 1200 LC system (Agilent, Santa Clara, CA, USA) coupled to an Agilent 6520 quadrupole time of flight (QToF) system with a Jetstream electrospray ionization (ESI) source to achieve higher sensitivity (see the methods in the supplemental material).
For comparison of the metabolite profiles of P. nodorum ΔSnPKS19 strains against that of the wild type, agar blocks cut out from a 10-day-old MMA culture were extracted with an approximately equal volume of ethyl acetate (EtOAc). For A. nidulans expressing SnPKS19, 10 ml of liquid GMM cultures was sampled at 48 and 72 h postinoculation and extracted with an equal volume of EtOAc. The extracts were dried in vacuo and redissolved in methanol (MeOH). The metabolite profile analyses were performed on an Agilent 1200 LC system coupled to a diode array detector (DAD) and an Agilent 6120 quadrupole MS with an ESI source. Chromatographic separation and conditions were exactly as described previously (39). The authenticity of AOH was confirmed by comparing the m/z ratio, UV spectrum, and retention time with those of the corresponding commercial standards (LGC standards).
Transformation and screening of P. nodorum ΔSnPKS19 mutants.
A double-homologous SnPKS19 knockout (KO) cassette carrying a hygromycin resistance marker was assembled using the Gibson assembly method (40) on a pGEM-T easy vector backbone (Promega). The hygromycin resistance marker was amplified from pAN7-1 (41) using GPE1-F and TtrpC-R primers (see Table S1 in the supplemental material). The 5′ and 3′ homologous regions of SnPKS19 (1.6 kbp each) were amplified with the SnPKS19-KO-P2/P3 and SnPKS19-KO-P4/P5 primer pairs, respectively. The three overlapping DNA fragments and the linearized vector backbone were assembled using the Gibson assembly master mix (NEB) following the manufacturer's protocol. The resulting plasmid, pGEM-SnPKS19-KO, was sequenced to confirm the construct. Finally, the 5.8-kbp SnPKS19 KO cassette (see Fig. S3 in the supplemental material) was liberated by digesting the plasmid with NotI enzyme (NEB) and gel purified for P. nodorum transformation. Transformation of P. nodorum with the SnPKS19 KO cassette was achieved by the polyethylene glycol (PEG)-mediated protoplast transformation protocol as described previously (42). The integration of the KO cassette in the correct locus was confirmed by diagnostic PCR using two pairs of primers, SnPKS19-KO-P1/Hyg-N-R and Hyg-C-F/SnPKS19-KO-P6, as described previously (43). The deletion of SnPKS19 was further confirmed by Southern blotting using a digoxigenin (DIG) DNA labeling and detection kit (Roche) according to the manufacturer's instructions.
Characterization of the virulence and oxidative stress tolerance of P. nodorum ΔSnPKS19 mutants.
A modified detached-leaf assay (DLA) was used to assess the virulence of P. nodorum ΔSnPKS19 mutants against wheat. The protocol was as described previously (44), with the exception of using 0.25% gelatin instead of 0.2% Tween 20 for spore suspension. The oxidative tolerance of P. nodorum ΔSnPKS19 mutants compared to wild type was determined by placing a 5-mm-diameter agar block on MMA containing 0, 2, 5, and 10 mM hydrogen peroxide in triplicates, as described previously (45).
Cloning and heterologous expression of SnPKS19 in Aspergillus nidulans.
The original annotations of the SnPKS19 translated protein in the GenBank and JGI databases lack the N-terminal starter unit:acyl carrier protein (ACP) transacylase (SAT) domain. The missing SAT domain conserved among nonreducing PKSs (NR-PKSs) was located by TBLASTX analysis with an additional 1,000 bp upstream of the SnPKS19 coding region added as a query sequence. The SAT domain was restored by manual annotation with the corrected annotation, placing the new ATG start codon 656 bp upstream of the original start codon.
SnPKS19 was cloned into the pBARGPE1-LIC expression plasmid. pBARGPE1-LIC was modified from pBARGPE1 (from FGSC) for ligation-independent cloning (LIC) as described previously (46). The plasmid contains an gpdA promoter and trpC terminator for gene expression and a bar gene for glufosinate resistance. The revised full-length SnPKS19 coding region, including introns (see Fig. S5 in the supplemental material), was PCR amplified from P. nodorum SN15 genomic DNA using primers LIC-SnPKS19-F_new and LIC-SnPKS19-R (see Table S1 in the supplemental material). DNA assembly using LIC was performed as described previously (46). Transformation of A. nidulans TNO2A3 with the resulting plasmid, pBGP-SnPKS19, was performed using the PEG-mediated transformation protocol as described previously (47). For selection of glufosinate-resistant transformants, the glufosinate was prepared from the commercial herbicide Basta (Bayer CropScience) as described previously and added at 25 μl/ml of Aspergillus glucose minimal medium with 10 mM ammonium tartrate as the sole nitrogen source (48).
The transcription of SnPKS19, pkgA (ANIA_07071), and pkgB (ANIA_07070) in the A. nidulans strain transformed with pBG-SnPKS19 was checked using reverse transcriptase PCR (RT-PCR), as described previously (46), with primer pairs SnPKS19-RT-F/R, AN7071-RT-F/R, AN7070-RT-F/R, which flank the last (3′-end) introns in the three respective genes (see Table S1 in the supplemental material).
Phylogenetic analysis.
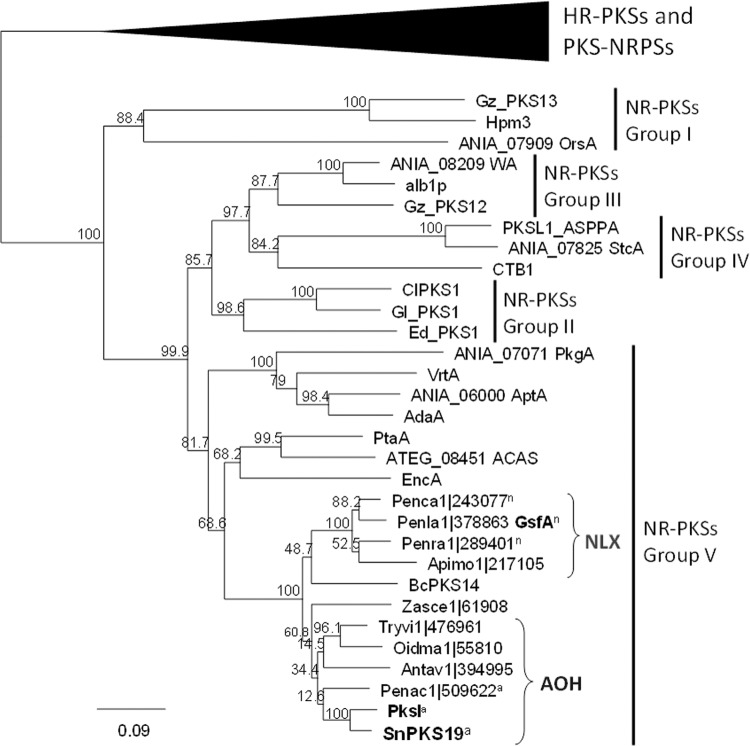
The conserved β-keto-acyl synthase (KS) domain sequences were used to infer the phylogenetic relationship between SnPKS19 and other fungal NR-PKSs. A total of 19 NR-PKSs characterized in previous studies were included as reference sequences, while seven additional characterized highly reducing PKSs (HR-PKSs)/PKS-NRPSs were included as outgroup (see Table S2 in the supplemental material). To investigate the distribution of AOH and norlichexanthone (NLX) synthases, the phylogenetic analysis further included all NR-PKSs with KS domains that shared >80% identity with SnPKS19 from the NCBI GenBank and JGI Ascomycota (http://genome.jgi-psf.org/ascomycota/ascomycota.info.html) databases. Multiple-protein alignment was performed using CLUSTALX, and the resulting alignment was trimmed (corresponds to amino acids 418 to 821 of the corrected SnPKS19 protein sequence). The phylogenetic analysis was performed using the neighbor-joining method (49) embedded in the Geneious software 7.17 software (Biomatters Ltd.). The tree was constructed with 1,000 bootstrap replicates, and branches corresponding to partitions that were reproduced in fewer than 50% of bootstrap replicates are collapsed.
Nucleotide sequence accession number.
The corrected annotation for SnPKS19 has been deposited in GenBank under accession no. KP941080.
RESULTS
Detection of alternariol under various growth conditions.
We first investigated for growth conditions that favor AOH production in the P. nodorum SN15 wild-type strain. The strain was cultured on different media and under different growth conditions and checked for AOH production by LC-DAD-MS. P. nodorum produced AOH (m/z 259) when grown in defined minimal agar medium (MMA) at 22°C after 7 days (Fig. 1). AOH could also be detected in shaking P. nodorum Fries medium cultures (see the methods and Fig. S1 in the supplemental material). To detect the possible production of AOH in planta, two experiments were performed, i.e., detached-leaf assay (DLA) and in vivo glume infection assay (see the methods in the supplemental material). However, we failed to detect any AOH in either the infected wheat leaves from DLAs or the infected wheat grains from glume infection assays (see Fig. S1 in the supplemental material).
FIG 1.
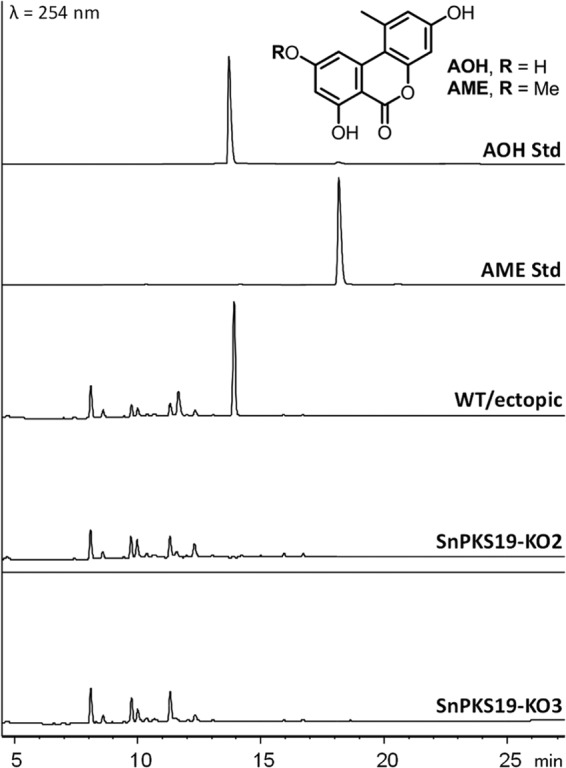
Comparison of metabolite profiles of the P. nodorum wild-type (WT) strain and ΔSnPKS19 mutants grown on minimal medium agar. UV chromatograms of P. nodorum WT/ectopic mutant and ΔSnPKS19 mutants are shown in comparison to AOH and AME standards.
Bioinformatic analysis of putative PKS genes in P. nodorum.
We previously performed a detailed survey of the P. nodorum genome for secondary metabolite genes and have compiled the polyketide synthase (PKS) gene inventory for this wheat pathogen (50). To identify the PKS gene encoding AOH in P. nodorum, we first searched for P. nodorum PKSs that are homologous to PksH and PksJ, which were previously implied to be involved in AOH production in A. alternata by RNA interference (RNAi) silencing experiments (36). However, a BLASTP search did not identify any close homolog of A. alternata PksH and PksJ in P. nodorum.
To further expand the search for the AOH PKS, a genome-wide comparison of all the PKSs in P. nodorum and A. alternata (36) identified another PKS from A. alternata (PksI) sharing significant homology (86% identity) to SNOG_15829 (abbreviated as SnPKS19) in P. nodorum. SnPKS19 is a typical nonreducing PKS (NR-PKS) (51, 52), consisting of a SAT domain, a β-keto-acyl synthase (KS) domain, a malonyl coenzyme A (malonyl-CoA):ACP transferase (MAT) domain, a product template (PT) domain, and an acyl carrier protein (ACP) domain. The original annotation of SnPKS19 in the GenBank and JGI databases lacked the SAT domain, but this N-terminal domain was restored by manual annotation (Fig. 2; see Fig. S4 in the supplemental material). PksI from A. alternata, however, is truncated at the 3′ end based on the annotation by Saha et al., and the predicted PKS protein lacks the C-terminal ACP domain (Fig. 2) (36). The closest characterized homolog for the two PKSs in the GenBank protein database is the norlichexanthone (NLX) synthase (GsfA) in the griseofulvin pathway in Penicillium aethiopicum (synonym, Penicillium lanosocoeruleum) (53, 54), which share high percentages of protein identity (overall protein, 65% identity; KS domain, 83% identity). Both AOH and NLX are derived from a heptaketide chain and involve a C-8–C-13 aldol cyclization in the biosynthesis, but the regiospecificity of the second cyclization diverges (Fig. 3). As AOH and NLX are structural isomers, we reasoned that it is highly likely that the PKS gene SnPKS19 may encode the biosynthesis of AOH.
FIG 2.
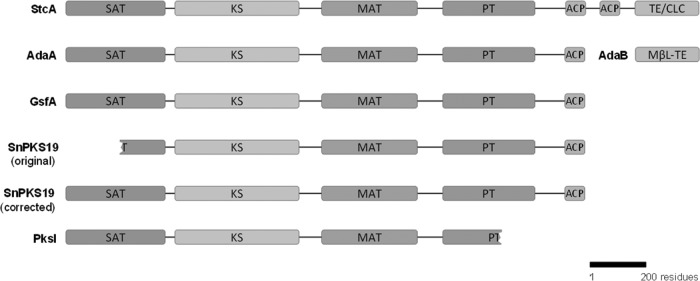
Comparison of the SnPKS19 domain architecture with those of other nonreducing polyketide synthases (NR-PKSs). SAT, starter unit:ACP transacylase domain; KS, β-keto-acyl synthase domain; MAT, malonyl-CoA:ACP transferase domain; PT, product template domain; ACP, acyl carrier protein domain; TE/CLC, thioesterase/Claisen cyclase domain; MβL-TE, metallo-β-lactamase-like thioesterase. MβL-TE is a discrete protein.
FIG 3.
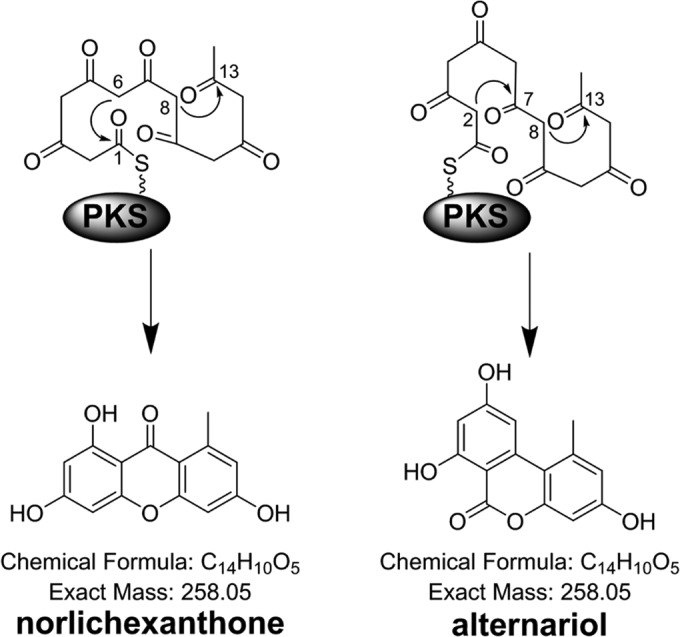
Biosynthesis of alternariol (AOH) in comparison to norlichexanthone (NLX) by polyketide synthases.
The genes in the vicinity of SnPKS19 encode two putative O-methyltransferases, a putative β-lactamase, a putative short-chain dehydrogenase, a putative regulatory protein, and a putative transporter (see Fig. S2 in the supplemental material). We extracted the expression profiles of SnPKS19 and its neighboring genes from previous microarray data (55, 56). The production of AOH in P. nodorum MMA culture and its absence in DLA samples correlated with the expression of SnPKS19 during the growth in vitro and its silence in planta (see Fig. S2 in the supplemental material). One of the putative O-methyltransferase genes (SNOG_15830), along with the colocated regulatory protein (SNOG_15831) and the putative short-chain dehydrogenase (SNOG_15832) genes, appears to be coregulated with SnPKS19, all with higher expression in vitro at 16 days postinoculation (dpi) than at 4 dpi, and all were silent in planta. Despite the presence of the putative O-methyltransferase gene in the gene cluster, we did not detect any O-methylated derivative of AOH in P. nodorum MMA culture (Fig. 1) and under the various other growth conditions tested.
SnPKS19 is required for alternariol production in P. nodorum.
To confirm that the SnPKS19 gene is involved in AOH biosynthesis, P. nodorum ΔSnPKS19 mutants were generated by polyethylene glycol (PEG)-mediated transformation with an SnPKS19 knockout cassette. Diagnostic PCR identified three positive transformants where SnPKS19 had been disrupted by double-homologous crossover recombination and replaced with a hygromycin resistance marker (see Fig. S3 in the supplemental material). The deletion of SnPKS19 in two transformants (SnPKS19-KO2 and -KO3) was further confirmed by Southern blotting (see Fig. S4 in the supplemental material). No observable growth defect or differences in the growth rate between the mutants and the wild-type (WT) P. nodorum were observed. The colony morphology of the mutants on V8-PDA and minimal medium plates were also indistinguishable from that of the WT.
Five transformants, including three ΔSnPKS19 mutants and two ectopic mutants, were selected and grown on MMA under the above-mentioned condition. Comparative LC-MS metabolite profile analysis of the EtOAc extracts from the cultures showed that production of AOH was abolished in all the ΔSnPKS19 mutants but remained present in both ectopic mutants and the WT (Fig. 1). Thus, this confirmed that SnPKS19 is required for AOH biosynthesis in P. nodorum.
SnPKS19 alone is sufficient for biosynthesis of alternariol.
The predicted PKS protein encoded by SnPKS19 lacks the polyketide product-releasing C-terminal thioesterase (TE)/Claisen cyclase (CLC) domain commonly found in fungal NR-PKSs (57, 58). Previous biosynthetic studies have shown that some fungal NR-PKSs lacking a releasing domain require an additional discrete metallo-β-lactamase-like TE (MβL-TE) protein to facilitate polyketide chain release or cyclization (Fig. 2) (54, 59–61). Thus, the putative β-lactamase gene (SNOG_15826) in the vicinity of SnPKS19 may be required for releasing of AOH via lactone formation. However, upon close inspection, SNOG_15826 is seen to share little similarity to the characterized MβL-TEs, as it belongs to a different family of β-lactamases. Furthermore, the previous microarray data showed that SNOG_15826 was not coregulated with the PKS gene SnPKS19 (see Fig. S3 in the supplemental material), which supported its noninvolvement in AOH biosynthesis.
To determine if SnPKS19 alone was sufficient for AOH synthesis, SnPKS19 with the restored 5′ region was cloned into plasmid pBARGPE1-LIC for heterologous expression. The resulting plasmid, pBG-SnPKS19, was used to transform A. nidulans strain TNO2A3 (from FGSC). Two glufosinate-resistant transformants were verified to contain the SnPKS19 gene by PCR and were cultured in Aspergillus minimal liquid medium for 72 h at 28°C. LC-DAD-MS analysis of the EtOAc extracts from the cultures showed that both transformants produced a unique peak with m/z (M + H)+ of 259, corresponding to AOH, which was absent in the A. nidulans control strain transformed with the empty pBARGPE1-LIC plasmid (Fig. 4). The result showed that SnPKS19 alone is sufficient for biosynthesis of AOH in A. nidulans.
FIG 4.
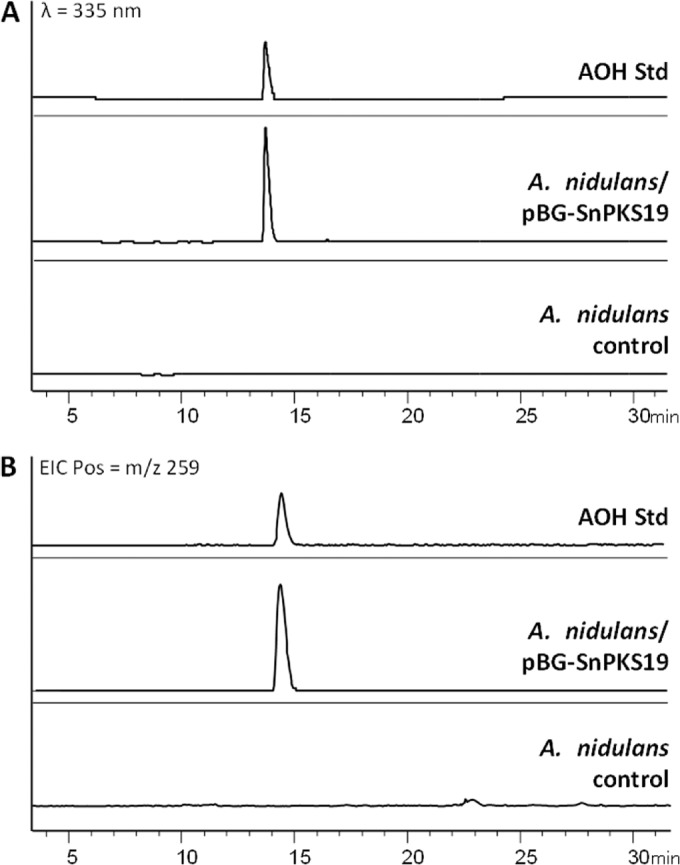
Heterologous production of AOH in Aspergillus nidulans. A UV chromatogram (A) and an extracted ion chromatogram (EIC) (m/z 259) (B) of the culture extracts from A. nidulans transformed with pBG-SnPKS19 in comparison to a control and an AOH standard are shown.
In a previous study, promoter replacement of the NR-PKS gene pkgA (ANIA_07071) and the MβL-TE gene pkgB (ANIA_07070) in A. nidulans resulted in production of AOH along with three other isocoumarins. To rule out that these two genes are involved in AOH production in the A. nidulans strains harboring SnPKS19, we performed an RT-PCR to determine the expression of SnPKS19 and the two genes at 72 h postinoculation. RT-PCR showed that SnPKS19, but not pkgA and pkgB, was transcribed and spliced in the A. nidulans/pBG-SnPKS19 strains (see Fig. S6 in the supplemental material).
SnPKS19 is not involved in the virulence and oxidative stress protection of P. nodorum.
The ability of the ΔSnPKS19 mutant strains (SnPKS19-KO2 and -KO3) to cause disease was assessed using detached-leaf assays. No significant difference in disease symptoms was observed on detached wheat leaves sprayed with spores of ΔSnPKS19 mutants compared to the controls (wild-type and ectopic integration strains) (see Fig. S7A in the supplemental material).
Some secondary metabolites are known to possess antioxidant activities and are thought to serve as protectants against oxidative stress (62). However, we did not observe a significant change in the ability to tolerate increasing concentration of hydrogen peroxide between ΔSnPKS19 mutant strains and wild-type strain SN15 (data not shown).
Alternatiol does not exhibit phytotoxic or antimicrobial activities.
The phytotoxic activity of AOH on wheat leaves was examined by leaf infiltration on whole plant wheat seedlings (see the methods in the supplemental material). No necrosis was observed on wheat leaves at concentrations up to 200 μg/ml of AOH (see Fig. S7B in the supplemental material). The capacity of AOH to affect seed germination was also assessed. Unlike what we had observed for (R)-mellein in a previous study (39), AOH did not inhibit the germination of wheat and barrel medic seeds (data not shown). We have also tested the antimicrobial activity of AOH against Escherichia coli, Saccharomyces cerevisiae, Zymoseptoria tritici, and three environmental bacterial strains isolated from field wheat samples (Bacillus cereus, a Flavobacterium sp., and Sphingobacterium multivorum) (see the methods in the supplemental material). AOH did not inhibit the growth of any of the bacterial and fungal strains tested at concentrations up to 200 μg/ml.
Phylogenetic analysis can differentiate alternariol synthases from other fungal PKSs.
Previous studies have shown that fungal NR-PKSs (without a C-methyltransferase domain) can be classified by phylogeny into five major groups plus two additional groups that harbor a C-methyltransferase domain (59, 63). The two studies showed that the phylogenetic grouping correlated with the polyketide product chain length and cyclization regioselectivity of NR-PKSs. To determine the phylogenetic relationship of SnPKS19 with NLX synthase GsfA and in relation to other NR-PKSs, three PKSs from each group of group I to IV NR-PKSs, along with six PKS sequences from group V, were selected as reference sequences for phylogenetic analysis (see Table S2 in the supplemental material). The phylogenetic tree showed that the NR-PKSs were separated into the five respective groups (groups I to V), as expected (Fig. 5).
FIG 5.
Phylogeny and distribution of AOH synthase homologs in fungi in relation to other fungal NR-PKSs. KS sequences used to construct the phylogenetic tree are listed in Table S2 in the supplemental material.
GsfA and SnPKS19 along with their close homologs grouped together with other group V NR-PKSs but formed a separate subclade. Within this subclade, SnPKS19 and GsfA homologs were further separated into two smaller subclades, referred to here as the AOH and NLX subclades, respectively. Within the NLX subclades, there are two other NR-PKSs that originated from known NLX/griseofulvin-producing fungi, i.e., Penicillium canescens (Penca1|243077) and Penicillium raistrickii (Penra1|289401) (Fig. 4) (64). Likewise, the AOH subclade is supported by NR-PKSs from AOH producers, including A. alternata (PksI) and Talaromyces aculeatus (Penac1|509622) (65). These analyses suggest that the NR-PKS homologs that fall in the AOH and NLX subclades may produce the respective compounds.
DISCUSSION
Using both reverse genetics and heterologous expression, we recently identified the P. nodorum PKS gene responsible for production of R-(−)-mellein, which exhibited antigerminative activity (39). Here, using a similar approach, we unequivocally demonstrated that SnPKS19, which is highly similar to the A. alternata pksI product (86% protein identity), is solely responsible for the biosynthesis of AOH in P. nodorum. Saha et al. previously reported the involvement of pksH and pksJ in AOH biosynthesis in A. alternata based on RNAi silencing experiments (36). In contrast to PksH and PksJ, which are HR-PKSs commonly involved in biosynthesis of aliphatic compounds, both the SnPKS19 and PksI genes encode an NR-PKS that is typically involved in biosynthesis of aromatic polyketides (51). Curiously, the pksI in A. alternata is annotated as a truncated PKS gene (Fig. 2) (36). Quantitative real-time reverse transcriptase PCR (qRT-PCR) from this previous study by Saha et al. showed that pksI was transcribed and that its expression correlated to the timing of AOH production, suggesting that pksI is unlikely to be a pseudogene. Nevertheless, pksI was not investigated further (36). It is not uncommon that RNA silencing could result in off-target effects (66–68). Given that the highly conserved KS domain was targeted in the study by Saha et al., it is possible that the transcription of other PKS genes was knocked down as well due to the potential complementary nature of the nucleotide sequences. For example, transcription of both pksI and -J was silenced in the pksH knockdown strain (36), which may also explain the observed reduced AOH production if pksI is responsible for AOH production in A. alternata. It is possible that the presumed truncation of the pksI coding sequence is due to automated annotation error (like in the original SnPKS19 annotation) or frameshift errors in sequencing. Based on our results from this study, it is very likely that pksI is the gene responsible for AOH production in A. alternata. Further investigation is required to verify the identity of AOH synthase in A. alternata.
An NR-PKS gene (pkgA) from A. nidulans has been recently reported to produce AOH along with a mixture of several hexa- and heptaketide isocoumarin products, but only in the presence of the partner MβL-TE gene (pkgB) (59). The abundances of the heptaketides citreoisocoumarin and dehydrocitreoisocoumarin are comparable to that of AOH in the pkgA and -B overexpression strain. Overexpression of the PKS gene pkgA alone by promoter exchange did not result in any observable product (59). Here, we used the A. nidulans system to characterize SnPKS19 by heterologous expression and showed that the SnPKS19-expressing strains accumulated significant quantities of AOH at 72 h. No related isocoumarins identified in the previous study by Ahuja et al. (59) were detected in the SnPKS19-expressing A. nidulans strains, and the involvement of pkgA and -B is ruled out based on the RT-PCR results (see Fig. S6 in the supplemental material) and the absence of AOH in the control strain. Thus, we reasoned that AOH is the major and final product of SnPKS19, while AOH along with the hexaketide isocoumarins may be aberrant or derailed products of PkgA and -B, possibly due to overexpression of both genes at the same level (a nonoptimal gene/enzyme ratio) (69).
The PT domain of NR-PKSs mediates the cyclization of nonreduced polyketide chains into various aromatic systems (52, 70). All the isocoumarin products produced by PkgA and -B shared the C-2–C-7 cyclized ring in AOH. In contrast, NLX shared the C-8–C-13 cyclized ring in AOH (Fig. 3). It is possible that the polyketide biosynthesis in PkgA involved PT domain-controlled C-2–C-7 cyclization followed by-product release via hydrolysis or lactonization catalyzed by PkgB. AOH was likely formed in the pkgA- and B-overexpressing strain via spontaneous aldol cyclization after polyketide release, whereas the PT domain of NLX and AOH synthases is likely to mediate the C-8–C-13 cyclization instead; the two pathways diverged during the second ring cyclization (C-6–C-1 Claisen cyclization or C-2–C-7 aldol cyclization) to yield NLX or AOH, respectively.
Previous phylogenetics analysis showed that the NLX synthase GsfA fell within the group V NR-PKSs (59). The present study showed that GsfA grouped together with group V NR-PKSs as well but formed a separate subclade with SnPKS19 and other putative NLX/AOH synthases from the group V NR-PKSs that require a discrete MβL-TE for product release, which include ACAS (60), AptA (71), VrtA (54), AdaA (61), PkgA (59), and EncA (72). Thus, the branching between these two subgroups of group V NR-PKSs could represent the point where the partnership with MβL-TE was lost in the NLX/AOH synthases. It appears that the NLX/AOH synthases have evolved the ability to off-load PKS products by Claisen cyclization or hydrolysis/lactonization without C-terminal appended TEs or discrete MβL-TEs. The molecular mechanism and the functional domain responsible for the polyketide release in these TE-less NLX/AOH synthases remain to be elucidated.
Based on the phylogenetic analysis, SnPKS19 and GsfA can be further divided into two smaller subclades consisting of putative AOH and NLX synthases, respectively. Both subclades are supported by two additional NR-PKSs from known AOH or NLX producers. Besides the high similarity shared between SnPKS19 and Penac1|509622 from the AOH/AME producer T. aculeatus, the two PKS gene clusters also encode a homologous O-methyltransferase (SNOG_15830/Penac1|509623; 71% protein identity), suggesting that these two enzymes are likely involved in methylation of AOH. Although we did not detect any AME in P. nodorum under the growth conditions tested in this study, the ability of P. nodorum to produce AME cannot be excluded. Based on the gene cluster map reported by Saha et al., an O-methyltransferase is encoded next to A. alternata pksI as well (36), but the corresponding gene was not deposited in a public database and therefore cannot be compared. Similarly, the gene clusters containing the putative NLX synthase gene in the NLX/griseofulvin producers P. canescens and P. raistrickii encode all the homologous enzymes in the P. aethiopicum gsf gene cluster required for griseofulvin biosynthesis (53, 54). These data together strongly support that phylogenetic analysis can be used to predict whether an NR-PKS gene is responsible for production of AOH or NLX using SnPKS19 and GsfA as reference sequences.
It is difficult to determine the exact number of fungal genome sequences deposited in the NCBI GenBank database (312 are ascomycetes listed in NCBI Genome), but the search for AOH synthase homologs in the JGI database (http://genome.jgi-psf.org/ascomycota/ascomycota.info.html) included 259 ascomycetes. Although the AOH synthase gene does not appear to be ubiquitous, the orthologs are not restricted to a single ascomycete class but distributed across Dothideomycetes, Leotiomycetes, Eurotiomycetes, and Sordariomycetes (Fig. 5; see Table S2 in the supplemental material). It is less certain whether the two PKSs (Botrytis cinerea BcPKS14 and Zasmidium cellare Zasce1|61908) basal to the NLX and AOH clades are responsible for production of NLX or AOH. Interestingly, a dibenzopyrone botrallin, structurally related to AOH, has been reported from Botrytis allii (73, 74). There is also at least one instance where a lichenized fungus was reported to produce both AOH and NLX (75). Thus, the possible existence of such NR-PKSs that have the promiscuity to produce both structural isomers cannot be discounted. AOH had also been reported in an endophytic Colletotrichum sp. (76), but no close homolog of AOH synthase has been found among the sequenced Colletotrichum genomes.
Due to their occurrence in plant-pathogenic Alternaria species, AOH and AME had also been investigated for potential phytotoxic activities, but most reports are negative (77, 78). Interestingly, AME has been reported to suppress cyanobacterial growth by inhibiting the photosynthetic electron transport chain (79). In this study, we showed that the virulence of ΔSnPKS19 against wheat leaves in vitro is comparable to that of the wild type; thus, AOH is unlikely to play a role in pathogenicity. This is not surprising given that the transcription of SnPKS19 is silent in planta based on previous microarray data and that we did not detect any AOH in infected wheat leaves or grains. Likewise, we did not detect any phytotoxic activity against wheat for AOH using leaf infiltration and seed germination. We have tested AOH against common laboratory microorganisms and environmental isolates of bacteria and fungi as well, but we did not detect any growth-inhibitory activity. Thus, the biological and ecological roles of AOH remain a mystery.
AOHs are often detected in wheat, sorghum, and barley grains contaminated with Alternaria fungi and pose a health risk to humans and animals (23). However, an analysis of P. nodorum-infected grains in this study failed to detect any AOH. Furthermore, there has been no prior report of AOH in P. nodorum-contaminated grains, suggesting that AOH mycotoxin production is not part of the disease cycle. Given that P. nodorum has the genetic potential to produce AOH and has been shown to produce AOH under laboratory culture conditions, it cannot be discounted that the fungus may produce AOH on wheat grain when the conditions become favorable, especially in the face of changing climatic environments. Recently, it has shown that AOH production in A. alternata is affected by light conditions and partially regulated by the blue-light receptor LreA (80). The identification of the PKS gene encoding AOH production in this study opens up the opportunity for investigating the molecular genetic regulation of AOH in P. nodorum and other AOH-producing fungi. This discovery will also facilitate the assessment of the genetic potential for production of AOH in other fungi and the development of molecular diagnostic methods for detection and quantification of AOH-producing fungi in agricultural commodities and in food and feed.
Supplementary Material
ACKNOWLEDGMENTS
Y.-H.C. is supported by an Australian Research Council Discovery Early Career Researcher Award (DECRA) fellowship. M.J.M.-G. is a recipient of an Australian Government Endeavor Award and a Mexican CONACyT scholarship. P.S.S. is an Australian Research Council Future Fellow.
Y.-H.C. thanks Jack Elix for helpful discussion.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00278-15.
REFERENCES
- 1.Marin S, Ramos AJ, Cano-Sancho G, Sanchis V. 2013. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Wu F, Groopman JD, Pestka JJ. 2014. Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol 5:351–372. doi: 10.1146/annurev-food-030713-092431. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis BB, Miller JD. 2005. Mycotoxins as harmful indoor air contaminants. Appl Microbiol Biotechnol 66:367–372. doi: 10.1007/s00253-004-1753-9. [DOI] [PubMed] [Google Scholar]
- 4.Schenzel J, Forrer HR, Vogelgsang S, Hungerbuhler K, Bucheli TD. 2012. Mycotoxins in the environment. I. Production and emission from an agricultural test field. Environ Sci Technol 46:13067–13075. [DOI] [PubMed] [Google Scholar]
- 5.Paterson RRM, Lima N. 2011. Further mycotoxin effects from climate change. Food Res Int 44:2555–2566. doi: 10.1016/j.foodres.2011.05.038. [DOI] [Google Scholar]
- 6.Magan N, Medina A, Aldred D. 2011. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol 60:150–163. doi: 10.1111/j.1365-3059.2010.02412.x. [DOI] [Google Scholar]
- 7.Moretti A, Susca A, Mule G, Logrieco AF, Proctor RH. 2013. Molecular biodiversity of mycotoxigenic fungi that threaten food safety. Int J Food Microbiol 167:57–66. doi: 10.1016/j.ijfoodmicro.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Woloshuk CP, Shim WB. 2013. Aflatoxins, fumonisins, and trichothecenes: a convergence of knowledge. FEMS Microbiol Rev 37:94–109. doi: 10.1111/1574-6976.12009. [DOI] [PubMed] [Google Scholar]
- 9.Niessen L. 2008. PCR-based diagnosis and quantification of mycotoxin-producing fungi. Adv Food Nutr Res 54:81–138. doi: 10.1016/S1043-4526(07)00003-4. [DOI] [PubMed] [Google Scholar]
- 10.Huffman J, Gerber R, Du L. 2010. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 93:764–776. doi: 10.1002/bip.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW. 2004. Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J. 2012. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins (Basel) 4:1024–1057. doi: 10.3390/toxins4111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proctor RH, Brown DW, Plattner RD, Desjardins AE. 2003. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet Biol 38:237–249. doi: 10.1016/S1087-1845(02)00525-X. [DOI] [PubMed] [Google Scholar]
- 14.Seo JA, Proctor RH, Plattner RD. 2001. Characterization of four clustered and coregulated genes associated with fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet Biol 34:155–165. doi: 10.1006/fgbi.2001.1299. [DOI] [PubMed] [Google Scholar]
- 15.Gaffoor I, Trail F. 2006. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl Environ Microbiol 72:1793–1799. doi: 10.1128/AEM.72.3.1793-1799.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YT, Lee YR, Jin J, Han KH, Kim H, Kim JC, Lee T, Yun SH, Lee YW. 2005. Two different polyketide synthase genes are required for synthesis of zearalenone in Gibberella zeae. Mol Microbiol 58:1102–1113. doi: 10.1111/j.1365-2958.2005.04884.x. [DOI] [PubMed] [Google Scholar]
- 17.Wallwey C, Li SM. 2011. Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat Prod Rep 28:496–510. doi: 10.1039/C0NP00060D. [DOI] [PubMed] [Google Scholar]
- 18.Correia T, Grammel N, Ortel I, Keller U, Tudzynski P. 2003. Molecular cloning and analysis of the ergopeptine assembly system in the ergot fungus Claviceps purpurea. Chem Biol 10:1281–1292. doi: 10.1016/j.chembiol.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Hohn TM, McCormick SP, Desjardins AE. 1993. Evidence for a gene cluster involving trichothecene-pathway biosynthetic genes in Fusarium sporotrichioides. Curr Genet 24:291–295. doi: 10.1007/BF00336778. [DOI] [PubMed] [Google Scholar]
- 20.Desjardins AE. 2009. From yellow rain to green wheat: 25 years of trichothecene biosynthesis research. J Agric Food Chem 57:4478–4484. doi: 10.1021/jf9003847. [DOI] [PubMed] [Google Scholar]
- 21.Ackermann Y, Curtui V, Dietrich R, Gross M, Latif H, Martlbauer E, Usleber E. 2011. Widespread occurrence of low levels of alternariol in apple and tomato products, as determined by comparative immunochemical assessment using monoclonal and polyclonal antibodies. J Agric Food Chem 59:6360–6368. doi: 10.1021/jf201516f. [DOI] [PubMed] [Google Scholar]
- 22.Scott PM. 2001. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J AOAC Int 84:1809–1817. [PubMed] [Google Scholar]
- 23.Alexander J, Benford D, Boobis A, Ceccatelli S, Cottrill B, Cravedi J, Di Domenico A, Doerge D, Dogliotti E, Edler L. 2011. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J 9:2407–2504. [Google Scholar]
- 24.Lehmann L, Wagner J, Metzler M. 2006. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem Toxicol 44:398–408. doi: 10.1016/j.fct.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Brugger EM, Wagner J, Schumacher DM, Koch K, Podlech J, Metzler M, Lehmann L. 2006. Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol Lett 164:221–230. doi: 10.1016/j.toxlet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Griffin GF, Chu FS. 1983. Toxicity of the Alternaria metabolites alternariol, alternariol methyl ether, altenuene, and tenuazonic acid in the chicken embryo assay. Appl Environ Microbiol 46:1420–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pero RW, Posner H, Blois M, Harvan D, Spalding JW. 1973. Toxicity of metabolites produced by the “Alternaria”. Environ Health Perspect 4:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu GT, Qian YZ, Zhang P, Dong WH, Qi YM, Guo HT. 1992. Etiological role of Alternaria alternata in human esophageal cancer. Chin Med J (Engl) 105:394–400. [PubMed] [Google Scholar]
- 29.Mohammed YS, Osman M, Gabr Y. 1974. Alternariol, a new fungal anticholinesterase drug. I Arzneimittelforschung 24:121–122. [PubMed] [Google Scholar]
- 30.Fehr M, Pahlke G, Fritz J, Christensen MO, Boege F, Altemoller M, Podlech J, Marko D. 2009. Alternariol acts as a topoisomerase poison, preferentially affecting the IIalpha isoform. Mol Nutr Food Res 53:441–451. doi: 10.1002/mnfr.200700379. [DOI] [PubMed] [Google Scholar]
- 31.Solhaug A, Holme JA, Haglund K, Dendele B, Sergent O, Pestka J, Lagadic-Gossmann D, Eriksen GS. 2013. Alternariol induces abnormal nuclear morphology and cell cycle arrest in murine RAW 264.7 macrophages. Toxicol Lett 219:8–17. doi: 10.1016/j.toxlet.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Solhaug A, Vines LL, Ivanova L, Spilsberg B, Holme JA, Pestka J, Collins A, Eriksen GS. 2012. Mechanisms involved in alternariol-induced cell cycle arrest. Mutat Res 738-739:1–11. doi: 10.1016/j.mrfmmm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Tan KC, Trengove RD, Maker GL, Oliver RP, Solomon PS. 2009. Metabolite profiling identifies the mycotoxin alternariol in the pathogen Stagonospora nodorum. Metabolomics 5:330–335. doi: 10.1007/s11306-009-0158-2. [DOI] [Google Scholar]
- 34.Tan KC, Heazlewood JL, Millar AH, Thomson G, Oliver RP, Solomon PS. 2008. A signaling-regulated, short-chain dehydrogenase of Stagonospora nodorum regulates asexual development. Eukaryot Cell 7:1916–1929. doi: 10.1128/EC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IpCho SV, Tan KC, Koh G, Gummer J, Oliver RP, Trengove RD, Solomon PS. 2010. The transcription factor StuA regulates central carbon metabolism, mycotoxin production, and effector gene expression in the wheat pathogen Stagonospora nodorum. Eukaryot Cell 9:1100–1108. doi: 10.1128/EC.00064-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha D, Fetzner R, Burkhardt B, Podlech J, Metzler M, Dang H, Lawrence C, Fischer R. 2012. Identification of a polyketide synthase required for alternariol (AOH) and alternariol-9-methyl ether (AME) formation in Alternaria alternata. PLoS One 7:e40564. doi: 10.1371/journal.pone.0040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon PS, Thomas SW, Spanu P, Oliver RP. 2003. The utilisation of di/tripeptides by Stagonospora nodorum is dispensable for wheat infection. Physiol Mol Plant Pathol 63:191–199. doi: 10.1016/j.pmpp.2003.12.003. [DOI] [Google Scholar]
- 38.Shimizu K, Keller NP. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chooi YH, Krill C, Barrow RA, Chen S, Trengove R, Oliver RP, Solomon PS. 17 October 2014. An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl Environ Microbiol doi: 10.1128/AEM.02745-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson DG, Young L, Chuang RY, Venter JC, CAHutchison 3rd, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 41.Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA. 1987. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- 42.Solomon PS, Tan KC, Sanchez P, Cooper RM, Oliver RP. 2004. The disruption of a Galpha subunit sheds new light on the pathogenicity of Stagonospora nodorum on wheat. Mol Plant Microbe Interact 17:456–466. doi: 10.1094/MPMI.2004.17.5.456. [DOI] [PubMed] [Google Scholar]
- 43.Chooi YH, Hong YJ, Cacho RA, Tantillo DJ, Tang Y. 2013. A cytochrome P450 serves as an unexpected terpene cyclase during fungal meroterpenoid biosynthesis. J Am Chem Soc 135:16805–16808. doi: 10.1021/ja408966t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe RG, Lord M, Rybak K, Trengove RD, Oliver RP, Solomon PS. 2009. Trehalose biosynthesis is involved in sporulation of Stagonospora nodorum. Fungal Genet Biol 46:381–389. doi: 10.1016/j.fgb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Mead O, Thynne E, Winterberg B, Solomon PS. 2013. Characterising the role of GABA and its metabolism in the wheat pathogen Stagonospora nodorum. PLoS One 8:e78368. doi: 10.1371/journal.pone.0078368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chooi YH, Fang J, Liu H, Filler SG, Wang P, Tang Y. 2013. Genome mining of a prenylated and immunosuppressive polyketide from pathogenic fungi. Org Lett 15:780–783. doi: 10.1021/ol303435y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin WB, Chooi YH, Smith AR, Cacho RA, Hu Y, White TC, Tang Y. 2013. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth Biol 2:629–634. doi: 10.1021/sb400048b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 50.Chooi YH, Muria-Gonzalez MJ, Solomon PS. 2014. A genome-wide survey of the secondary metabolite biosynthesis genes in the wheat pathogen Parastagonospora nodorum. Mycology 5:192–206. doi: 10.1080/21501203.2014.928386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chooi YH, Tang Y. 2012. Navigating the fungal polyketide chemical space: from genes to molecules. J Org Chem 77:9933–9953. doi: 10.1021/jo301592k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford JM, Townsend CA. 2010. New insights into the formation of fungal aromatic polyketides. Nat Rev Microbiol 8:879–889. doi: 10.1038/nrmicro2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cacho RA, Chooi YH, Zhou H, Tang Y. 2013. Complexity generation in fungal polyketide biosynthesis: a spirocycle-forming P450 in the concise pathway to the antifungal drug griseofulvin. ACS Chem Biol 8:2322–2330. doi: 10.1021/cb400541z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chooi YH, Cacho R, Tang Y. 2010. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem Biol 17:483–494. doi: 10.1016/j.chembiol.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ipcho SV, Hane JK, Antoni EA, Ahren D, Henrissat B, Friesen TL, Solomon PS, Oliver RP. 2012. Transcriptome analysis of Stagonospora nodorum: gene models, effectors, metabolism and pantothenate dispensability. Mol Plant Pathol 13:531–545. doi: 10.1111/j.1364-3703.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chooi YH, Solomon PS. 2014. A chemical ecogenomics approach to understand the roles of secondary metabolites in fungal cereal pathogens. Front Microbiol 5:640. doi: 10.3389/fmicb.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujii I, Watanabe A, Sankawa U, Ebizuka Y. 2001. Identification of Claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chem Biol 8:189–197. doi: 10.1016/S1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- 58.Korman TP, Crawford JM, Labonte JW, Newman AG, Wong J, Townsend CA, Tsai SC. 2010. Structure and function of an iterative polyketide synthase thioesterase domain catalyzing Claisen cyclization in aflatoxin biosynthesis. Proc Natl Acad Sci U S A 107:6246–6251. doi: 10.1073/pnas.0913531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahuja M, Chiang YM, Chang SL, Praseuth MB, Entwistle R, Sanchez JF, Lo HC, Yeh HH, Oakley BR, Wang CC. 2012. Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J Am Chem Soc 134:8212–8221. doi: 10.1021/ja3016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awakawa T, Yokota K, Funa N, Doi F, Mori N, Watanabe H, Horinouchi S. 2009. Physically discrete beta-lactamase-type thioesterase catalyzes product release in atrochrysone synthesis by iterative type I polyketide synthase. Chem Biol 16:613–623. doi: 10.1016/j.chembiol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, Chooi YH, Sheng Y, Valentine JS, Tang Y. 2011. Comparative characterization of fungal anthracenone and naphthacenedione biosynthetic pathways reveals an alpha-hydroxylation-dependent Claisen-like cyclization catalyzed by a dimanganese thioesterase. J Am Chem Soc 133:15773–15785. doi: 10.1021/ja206906d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aguirre J, Hansberg W, Navarro R. 2006. Fungal responses to reactive oxygen species. Med Mycol 44:S101–S107. [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Image II, Xu W, Image I, Tang Y. 2010. Classification, prediction, and verification of the regioselectivity of fungal polyketide synthase product template domains. J Biol Chem 285:22764–22773. doi: 10.1074/jbc.M110.128504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frisvad JC, Filtenborg O. 1990. Revision of Penicillium subgenus Furcatum based on secondary metabolites and conventional characters, p 159–172. In Samson RA, Pitt JI (ed), Modern concepts in Penicillium and Aspergillus classification. Springer, Berlin, Germany. [Google Scholar]
- 65.Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. 2014. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol 78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. 2003. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 67.Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. 2006. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Senthil-Kumar M, Mysore KS. 2011. Caveat of RNAi in plants: the off-target effect. Methods Mol Biol 744:13–25. doi: 10.1007/978-1-61779-123-9_2. [DOI] [PubMed] [Google Scholar]
- 69.Lazarus CM, Williams K, Bailey AM. 2014. Reconstructing fungal natural product biosynthetic pathways. Nat Prod Rep 31:1339–1347. doi: 10.1039/C4NP00084F. [DOI] [PubMed] [Google Scholar]
- 70.Crawford JM, Korman TP, Labonte JW, Vagstad AL, Hill EA, Kamari-Bidkorpeh O, Tsai SC, Townsend CA. 2009. Structural basis for biosynthetic programming of fungal aromatic polyketide cyclization. Nature 461:1139–1143. doi: 10.1038/nature08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szewczyk E, Chiang YM, Oakley CE, Davidson AD, Wang CC, Oakley BR. 2008. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl Environ Microbiol 74:7607–7612. doi: 10.1128/AEM.01743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim FY, Hou Y, Chen Y, Oh JH, Lee I, Bugni TS, Keller NP. 2012. Genome-based cluster deletion reveals an endocrocin biosynthetic pathway in Aspergillus fumigatus. Appl Environ Microbiol 78:4117–4125. doi: 10.1128/AEM.07710-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kameda K, Aoki H, Namiki M, Overeem JC. 1974. Alternative structure for botrallin a metabolite of Botrytis Allii. Tetrahedron Lett 15:103–106. doi: 10.1016/S0040-4039(01)82147-X. [DOI] [Google Scholar]
- 74.Overeem JC, Vandijkm A. 1968. Botrallin, a novel quinone produced by Botrytis Allii. Rec Travaux Chim Pays-Bas 87:940–944. [Google Scholar]
- 75.Archer AW, Elix JA. 1998. The lichen genus Pertusaria (lichenised Ascomycotina) in Papua New Guinea: three new species and two new reports. Mycotaxon 69:311–318. [Google Scholar]
- 76.Yang Z-J, Yin Y, Wang Z-Q, Yang T, Chen D-J. 2012. Cytotoxic metabolites of endophytic fungus Colletotrichum sp. from Aristolochia sp. Nat Prod Res Dev 24:329–332. [Google Scholar]
- 77.Abbas HK, Vesonder RF, Boyette CD, Peterson SW. 1993. Phytotoxicity of AAL-toxin and other compounds produced by Alternaria alternata to jimsonweed (Datura stramonium). Can J Bot 71:155–160. doi: 10.1139/b93-017. [DOI] [Google Scholar]
- 78.Visconti A, Bottalico A, Solfrizzo M. 1989. Activity of Alternaria alternata metabolites on tomato leaves and Geotrichum candidum, p 457–459. In Graniti A, Durbin RD, Ballio A (ed), Phytotoxins and plant pathogenesis. Springer, Berlin, Germany. [Google Scholar]
- 79.Demuner AJ, Barbosa LC, Miranda AC, Geraldo GC, da Silva CM, Giberti S, Bertazzini M, Forlani G. 2013. The fungal phytotoxin alternariol 9-methyl ether and some of its synthetic analogues inhibit the photosynthetic electron transport chain. J Nat Prod 76:2234–2245. doi: 10.1021/np4005882. [DOI] [PubMed] [Google Scholar]
- 80.Pruss S, Fetzner R, Seither K, Herr A, Pfeiffer E, Metzler M, Lawrence CB, Fischer R. 2014. Role of the Alternaria alternata blue-light receptor LreA (white-collar 1) in spore formation and secondary metabolism. Appl Environ Microbiol 80:2582–2591. doi: 10.1128/AEM.00327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.