Abstract
Neuroinflammation plays an important role in the pathophysiology of surgical brain injury (SBI). Phosphoinositide 3-kinase gamma (PI3Kγ), predominately expressed in immune and endothelial cells, activates multiple inflammatory responses. In the present study, we investigated the role of PI3Kγ and PI3Kγ-activated phosphodiesterase 3B (PDE3B) in neuroinflammation in a rat model of SBI. One hundred and fifty-two male Sprague Dawley rats (weight 280–350 g) were subjected to a partial right frontal lobe corticotomy model of SBI. A PI3Kγ pharmacological inhibitor (AS252424 or AS605240) was administered intraperitoneally. PI3Kγ siRNA, human recombinant active-PI3Kγ protein, or human recombinant active-PDE3B protein were administered intracerebroventricularly. Post-SBI assessments included neurobehavioral tests, brain water content, Western blot, and immunohistochemistry. Endogenous PI3Kγ levels were increased within peri-resection brain tissues after SBI, accompanied by increased brain water content and neurological functional deficits. There was a trend toward increased endogenous PDE3B phosphorylation after SBI. The selective PI3Kγ inhibitors AS252424 and AS605240 reduced brain water content surrounding corticotomy and improved neurological function after SBI. SBI increased and PI3Kγ inhibitor decreased levels of myeloperoxidase, cluster of differentiation 3, mast cell degranulation, E-selectin, and IL-1 in peri-resection brain tissues. Direct administration of human recombinant active-PI3Kγ protein and active-PDE3B protein countered the protective effect of AS252424. PI3Kγ siRNA reduced PI3Kγ levels, decreased brain water content within peri-resection brain tissues, and improved neurological function after SBI. Collectively, our findings suggest that PI3Kγ contributed to neuroinflammation after SBI. The use of selective PI3Kγ inhibitors may be a novel approach to ameliorating SBI via their anti-inflammation effects.
SIGNIFICANCE STATEMENT Life-saving or elective neurosurgeries often involve unavoidable damages to neighboring, nondiseased brain tissues. Such surgical brain injury (SBI) is attributable exclusively to the neurosurgical procedure itself and may cause postoperative complications that exacerbate neurological function. Although the importance of this medical problem is fully acknowledged, intraoperative administration of adjunctive treatment such as steroids and mannitol to patients undergoing neurosurgery appear not to be efficient remedies for SBI. To date, the issue of perioperative neuroprotection specifically against SBI has not been well studied. Using a clinically relevant rat model of SBI, we are exploring a new neuroprotective strategy targeting phosphoinositide 3-kinase gamma (PI3Kγ). PI3Kγ activates multiple inflammatory responses. By attenuating neuroinflammation, selective PI3Kγ inhibition would limit postoperative complications and benefit neurological outcomes.
Keywords: blood–brain barrier, brain edema, inflammation, neurosurgery, PDE3B, PI3Kγ
Introduction
Surgical brain injury (SBI) that is attributable exclusively to the neurosurgical procedure itself may cause postoperative complications such as brain edema, ischemia, and intracranial hematoma, worsening neurological and behavioral outcomes (Manninen et al., 1999; Solaroglu et al., 2004). We demonstrated previously that neuroinflammation played important roles in the pathophysiology of SBI, characterized by activation of resident immune cells, leukocyte infiltration, and release of inflammatory mediators within brain tissues adjacent to the resection (Lo et al., 2007; Hyong et al., 2008; Ayer et al., 2012). Therapeutic strategies targeting neuroinflammation may limit postoperative complications and thus enable a more aggressive surgical approach.
Phosphoinositide 3-kinase gamma (PI3Kγ), a PI3K class IB isoform, controls a wide range of immune cell activities and vascular functions (Hirsch et al., 2000; Hirsch et al., 2006). Specifically with regard to inflammation, the predominant expression of PI3Kγ in leukocytes and endothelial cells participated in leukocyte chemotaxis and neutrophil oxidative burst (Hirsch et al., 2000; Puri et al., 2005; Hirsch et al., 2006; Rückle et al., 2006; Ghigo et al., 2010), T-cell proliferation and cytokine production (Hirsch et al., 2000; Sasaki et al., 2000; Smith et al., 2007; Thomas et al., 2008), and mast cell activation and degranulation (Hirsch et al., 2000; Laffargue et al., 2002). Emerging evidence has shown that gene ablation of PI3Kγ or pharmacological inhibition of PI3Kγ exerted protective effects in preclinical models of systemic inflammation including systemic lupus, rheumatoid arthritis, lung disease, sepsis, and colitis (Barber et al., 2005; Camps et al., 2005; Martin et al., 2010; van Dop et al., 2010; Kim et al., 2012). Recently, several studies identified the detrimental effects of PI3Kγ in neuroinflammation in brain ischemic injury and Alzheimer's disease (Jin et al., 2010b; Passos et al., 2010; Jin et al., 2011). Moreover, PI3Kγ has been shown to activate phosphodiesterase 3 (PDE3B), which suppresses cyclic adenosine monophosphate (cAMP) signaling (Patrucco et al., 2004; Perino et al., 2011; Schmidt et al., 2013).
In this study, we investigated the roles of PI3Kγ in neuroinflammation in a rat model of SBI by PI3Kγ pharmacological inhibition, by human recombinant active-PI3Kγ protein and active-PDE3B protein to counter the effect of the PI3Kγ inhibitor, and by PI3Kγ siRNA-mediated PI3Kγ knock-down.
Materials and Methods
Animals.
All procedures in this study were approved by the Institutional Animal Care and Use Committee at Loma Linda University and complied with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Adult male Sprague Dawley rats (weight 280–350 g) were housed in a vivarium for a minimum of 3 d before surgery with a 12 h light/dark cycle and ad libitum access to food and water.
Experimental design.
Experiments were performed in a rat model of SBI, as shown in Figure 1. A total of 152 rats were used.
Figure 1.
Experimental design and animal groups. WB, Western blot; IHC, immunohistochemistry.
Experiment 1 was to characterize the time course of endogenous changes in four PI3K isoforms, including PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ, as well as total/phosphorylated PDE3B levels within residual frontal lobe brain tissues at 1, 3, and 7 d after SBI. Twenty rats were randomized into four groups: sham, SBI-1d, SBI-3d, and SBI-7d (n = 5/group). The additional two rats in the SBI-1d group were used for immunohistochemistry.
Experiment 2 was to evaluate the detrimental role of PI3Kγ in the pathophysiology of SBI as follows. First, we tested the effect of the selective PI3Kγ inhibitor AS252424. Seventy-six rats were used in the following groups: sham (n = 13), SBI + vehicle (n = 21), SBI + AS252424 3 mg/kg (n = 8), SBI + AS252424 10 mg/kg (n = 21), and SBI + AS252424 10 mg/kg + human recombinant active-PI3Kγ (n = 13). AS252424 was dissolved in 24% dimethyl sulfoxide/0.5% carboxymethylcellulose (CMC)/0.25% Tween 20 saline with final concentration of 4.2 mg/ml. The inhibitor or same volume of vehicle was injected intraperitoneally 1 h before and 30 min after brain resection. Human recombinant active-PI3Kγ protein (200 ng, 2 μl; SignalChem) was administered by intracerebroventricular injection 30 min before SBI. Second, we investigated whether the effects of the PI3Kγ inhibitor are mediated through repression of PI3Kγ-PDE3B signaling. An additional group of SBI + AS252424 + human recombinant active PDE3B (n = 6) was added. Human recombinant active-PDE3B (100 ng, 2 μl; SignalChem) was administered intracerebroventricularly 30 min before SBI. Third, we tested the protective effects of PI3Kγ inhibition using another selective PI3Kγ inhibitor, AS605240, or siRNA-mediated PI3Kγ knock-out. Forty-eight rats in four additional groups were added: SBI + vehicle (n = 11), SBI + AS605240 10 mg/kg (n = 7), SBI + scramble siRNA (n = 15), and SBI + PI3K p110γ siRNA (n = 15). AS605240 was dissolved in 0.5% CMC/0.25% Tween 20 saline with a final concentration of 5 mg/ml. The inhibitor or same volume of vehicle was injected intraperitoneally 1 h before and 30 min after brain resection. The 500 pmol of p110γ siRNA (Accell SMART pool; Thermo Fisher Scientific) was dissolved in 2 μl of sterilized water and injected intracerebroventricularly 24 h before SBI. The same volume of scramble siRNA (siGENOME nontargeting siRNA Thermo Fisher Scientific) was administered as a control. The dosages of AS252424 and AS605240 were determined according to previous studies (Pomel et al., 2006; Kobayashi et al., 2011). Treatment and sample size in all experiment groups are summarized in Table 1.
Table 1.
Summary of experimental groups, interventions, and sample sizes
| Experimental group | Treatment | Animal no. |
|---|---|---|
| Sham | None | 13 |
| SBI + vehicle | Vehicle, i.p. | 32 |
| SBI + AS252424 3 mg/kg | AS252424 3 mg/kg, i.p. | 8 |
| SBI + AS252424 10 mg/kg | AS252424 10 mg/kg, i.p. | 21 |
| SBI + AS252424 10 mg/kg + human recombinant active-PI3Kγ | AS252424 10 mg/kg, i.p.; human recombinant active-PI3Kγ 200 ng, i.c.v. | 13 |
| SBI + AS252424 10 mg/kg + human recombinant active-PDE3B | AS252424 10 mg/kg, i.p.; human recombinant active-PDE3B 100 ng, i.c.v. | 6 |
| SBI + AS605240 10 mg/kg | AS605240 10 mg/kg, i.p. | 7 |
| SBI + scramble siRNA | Scramble siRNA, i.c.v. | 15 |
| SBI + PI3K p110γ siRNA | PI3K p110γ siRNA 500 pmol, i.c.v. | 15 |
i.p., Intraperitoneally; i.c.v., intracerebroventricularly.
SBI model.
Anesthesia was induced via induction chamber with 4% isoflurane and maintained with 2.5% via nasal mask. Temperature was controlled throughout surgery with thermal heat lamps. Rats were placed prone in a stereotactic frame. SBI was induced as described previously (Yamaguchi et al., 2007). Briefly, a midline incision was made and the periosteum was reflected to expose the bregma and the right frontal skull. A 5 × 5 mm square craniotomy with the lower left corner at the bregma was made by a microdrill on the right frontal skull. Care was taken to keep the dura intact. For SBI animals, the dura was incised and a dissection of brain tissue was made to create a partial right frontal lobectomy. The margins of the brain tissue cut were 2 mm lateral to the sagittal suture and 1 mm proximal to the coronal suture, with the depth extending to the base of the skull. Intraoperative packing and normal saline irrigation was used until hemostasis was obtained. Afterward, the incision was closed with sutures. Sham surgeries underwent the same surgical procedure without craniotomy and brain resection. Vital signs were monitored throughout surgery and recovery. Twenty-four or 72 h after surgery, neurological functions were assessed, after which time the animals were killed for brain water content measurement, Western blot, or immunohistochemistry assay as described below.
Intracerebroventricular injection.
As described previously (Chen et al., 2013, Liu et al., 2007), rats were anesthetized with isoflurane (4% induction, 2.5% maintenance) and mounted on a stereotaxic frame. The needle of a 10 μl Hamilton syringe was inserted through a burr hole perforated through the skull into the right lateral ventricle using the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of the bregma. siRNA was injected 24 h before SBI. According to the manufacturer's instructions, a total volume of 2 μl (500 pmol) of accell SMAT pool siRNA pik3cg (Thermo Fisher Scientific) in sterile saline was injected into the ipsilateral ventricle at a rate of 0.5 μl/min. Four different sequences targeting pik3cg were pooled: 5′-UUGGCAAUUACAAGAGUUU-3′, 5′-CUAUUCAGCUCAGUAAAGU-3′, 5′-GCAACGUGCACGAUGAUGA-3′, and 5′-CUGUGAUCCUGGAAGCGUA-3′. This type of PI3Kγ siRNA pool enters cells without the need for a transfection reagent and is a mixture of four siRNAs, thus providing advantages in both potency and specificity of gene silences. The same volume of scramble siRNA (Accell nontargeting pool; Thermo Fisher Scientific) was used as a negative control. A total 2 μl of active-recombinant PI3Kγ 200 ng or PDE3B 100 ng was injected 30 min before SBI. To prevent possible leakage, the needle was kept in situ for an additional 10 min after completing the injection and then withdrawn slowly over 5 min. After removal of the needle, the burr hole was sealed with bone wax, the incision was sutured, and the mice were allowed to recover.
Brain water content.
Under deep anesthesia, the rat's brain was quickly removed and dissected into six parts on ice: right frontal, left frontal, right parietal, left parietal, cerebellum, and brainstem. The wet weights of tissue samples were measured immediately and dry weights were obtained after drying in an oven at 105°C for 48 h. The percentage of water content in each part was calculated as [(wet weight − dry weight)/wet weight] × 100% (Yamaguchi et al., 2007).
Neurobehavioral tests.
Before animals were killed, each had neurological assessments using the modified Garcia and balance beam tests (Yamaguchi et al., 2007). Tests were performed by an examiner (P.S.) kept blinded to treatment information. The modified Garcia test involves a 21-point sensorimotor assessment that includes seven tests. Each test has a score ranging from 0 to 3, with a maximum score of 21. The tests evaluate spontaneous activity, side stroking, vibrissae touch, limb symmetry, climbing, lateral turning, and forelimb walking. The balance beam test involves a beam held in place by platforms at either side. Rats were observed for behavior and time to reach either platform. Scores ranged from 0 to 5, with 5 being the best.
Western blot assay.
Western blotting was performed as described previously (Ayer et al., 2012; Chen et al., 2013). At each end time point, rats were perfused with cold PBS, pH 7.4, solution delivered via intracardiac injection, followed by dissection of the brain into right frontal, left frontal, right parietal, left parietal, cerebellum, and brainstem. The brain parts were snap frozen in liquid nitrogen and stored at −80°C until use. Protein extraction from whole-cell lysates were obtained by gently homogenizing them in RIPA lysis buffer (Santa Cruz Biotechnology) with further centrifugation at 14,000 × g at 4°C for 30 min. The supernatant was used as whole-cell protein extract, and the protein concentration was determined using a detergent-compatible assay (Bio-Rad). Equal amounts of protein were loaded on an SDS-PAGE gel. After being electrophoresed and transferred to a nitrocellulose membrane, the membrane was blocked and incubated with the primary antibody overnight at 4°C. The primary antibodies were rabbit polyclonal anti-PI3Kγ (1:1000), rabbit polyclonal anti-PI3Kα (1:1000), rabbit polyclonal anti-PI3Kβ (1:1000), rabbit polyclonal anti-PI3Kδ (1:1000), rabbit polyclonal anti-E-selectin (1:1000), goat polyclonal anti-myeloperoxidase (MPO, 1:4000), rabbit polyclonal mast cell tryptase (1:4000), and mouse monoclonal anti-cluster of differentiation 3 (CD3, 1:600) (all from Santa Cruz Biotechnology); monoclonal anti-IL-1β (1:2000; Abcam), goat polyclonal anti-PDE3B (1:500; FabGennix), and rabbit polyclonal anti-phosphor-PDE3B (1:500; FabGennix). For loading control, the same membranes were blotted with primary antibody of goat anti-β-actin (1:8000; Santa Cruz Biotechnology). Nitrocellulose membranes were incubated with appropriate secondary antibodies (1:4000; Santa Cruz Biotechnology) for 1 h at room temperature. Immunoblot bands were further probed with a chemiluminescence reagent kit (ECL Plus; GE Healthcare). The data were analyzed by ImageJ software.
Immunohistochemistry.
Immunohistochemistry was performed as described previously (Chen et al., 2013). Briefly, rats were perfused with ice-cold PBS under deep anesthesia, followed by infusion of 10% formalin 24 h after SBI. The brains were harvested and immersed in 10% formalin at 4°C for 24 h, then in 30% sucrose in PBS until saturation. The brains were cut into 10-μm-thick coronal sections in a cryostat (CM3050S; Leica Microsystems). The sections were incubated overnight at 4°C with the following primary antibodies: rabbit anti-PI3Kγ (1:150), costaining with goat anti-MPO (1:100), mouse monoclonal anti-CD3 (1:100), and goat anti-Von Willebrand factor (1:100) (all from Santa Cruz Biotechnology); goat anti-ionized calcium binding adaptor molecule 1 (IBA1, 1:200; Abcam), followed by incubation with appropriate FITC-conjugated secondary antibodies (Jackson ImmunoResearch). Negative control staining was performed by omitting the primary antibody. The sections were visualized with a fluorescence microscope (Olympus BX51).
Statistics.
Quantitative data are presented as mean ± SEM. One-way ANOVA for multiple comparisons and Student–Newman–Keuls post hoc test were used to determine the differences of brain water content, neurological deficits, and Western blot assay among all groups at each time point. p < 0.05 was considered statistically significant.
Results
All sham-operated rats survived. The overall mortality of SBI was 15.6%. The mortality was not significantly different among the experimental groups (data not shown).
Changes of endogenous PI3Kγ level after SBI
Western blot showed that there were no significant changes in the protein levels of PI3Kα and PI3Kβ within peri-resection brain tissues over 7 d after SBI (Fig. 2A,B). However, there were significant increases in PI3Kγ level at 1, 3, and 7 d after SBI (Fig. 2C). In the absence of significant increases 1 and 3 d after SBI, PI3Kδ also was elevated 7 d later (Fig. 2D). Double immunofluorescence staining showed that PI3Kγ was colocalized with immune cell markers of MPO for neutrophils or CD3 for T-cells at 1 d after SBI (Fig. 2E).
Figure 2.
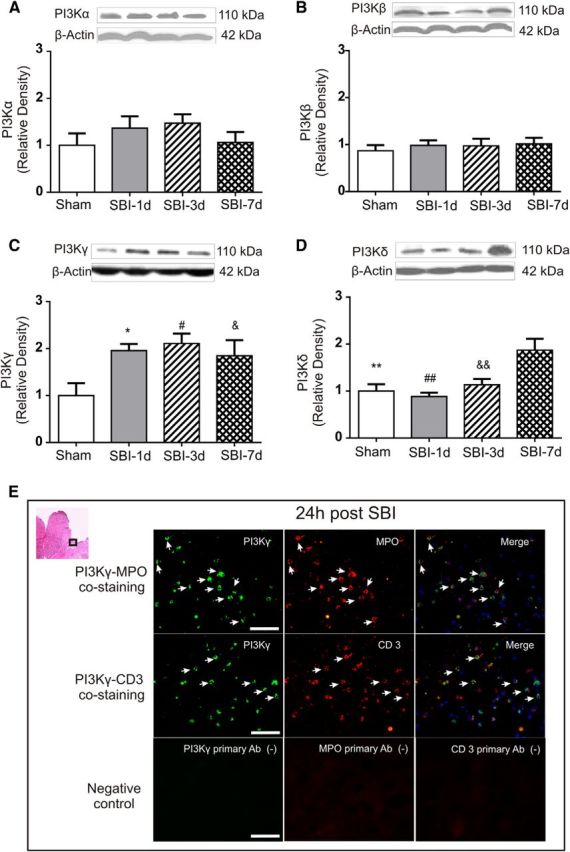
Time course of PI3K isoforms changes within peri-resection brain tissues after SBI. Although there were no significant changes in PI3Kα (p = 0.419) and PI3Kβ (p = 0.856) over 7 d (A, B), PI3Kγ levels increased at 1, 3, and 7 d after SBI (C) and PI3Kδ level increased 7 d after SBI (D) (n = 5/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. C, *p = 0.036 versus sham; #p = 0.026 versus sham, &p = 0.027 versus sham. D, **p = 0.004 versus SBI-7d; ##p = 0.002 versus SBI-7d; &&p = 0.005 versus SBI-7d. Representative immunohistochemistry microphotographs of PI3Kγ staining with MPO or CD3 revealed that PI3Kγ expression was colocalized with immune cells including infiltrated neutrophils (MPO) and T-cells (CD 3) 1 d after SBI (E). Negative control staining without the primary antibody (Ab) did not show detectable labeling (E). Arrows indicate cells with positive staining. Scale bar, 50 μm.
Changes of endogenous PDE3B level after SBI
Although total PDE3B levels remained relative stable, there was a tendency toward increased PDE3B phosphorylation (p-PDE3B) within peri-resection brain tissues 1 d after SBI (p > 0.05; Fig. 3).
Figure 3.
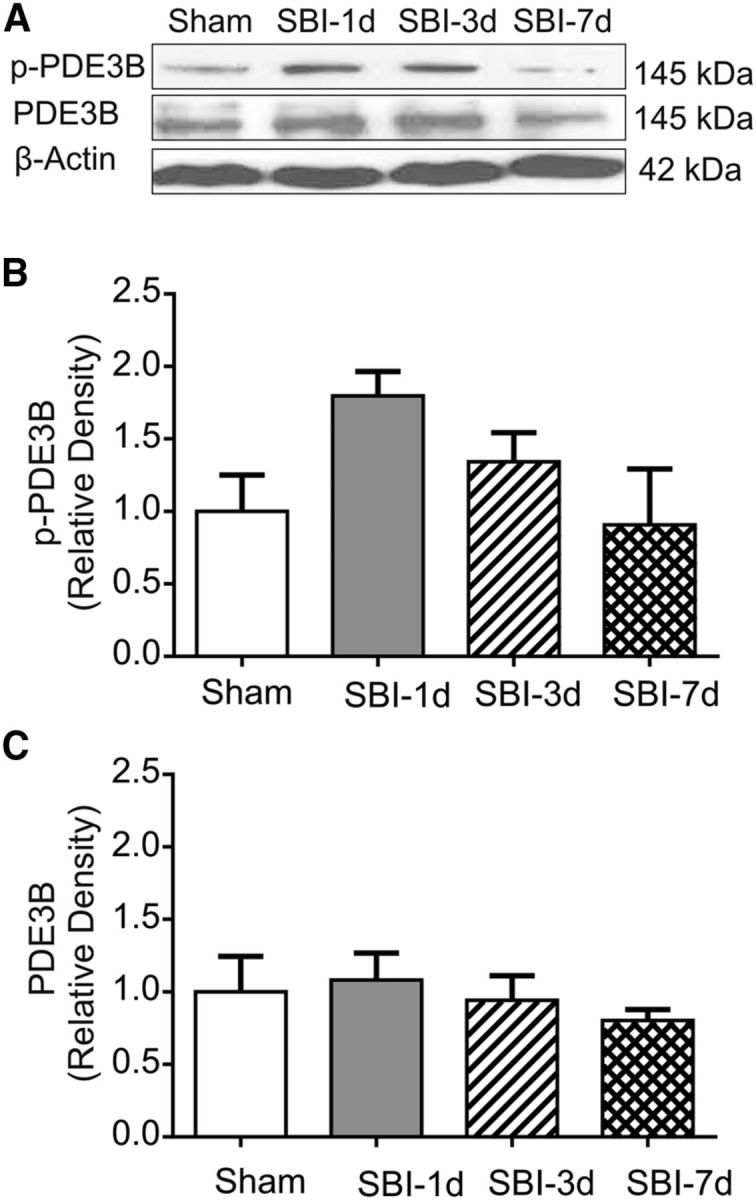
Temporal changes of PDE3B within peri-resection brain tissues after SBI. Western blot showed that there was a tendency toward increase in PDE3B phosphorylation 1 and 3 d after SBI (p = 0.197), although the total PDE3B levels remained relatively stable compared with shams (p = 0.38) (n = 4/group). One-way ANOVA showed that the differences were not statistically significant. A, B, p = 0.197; A, C, p = 0.38.
Effects of AS252424 and human recombinant active-PI3Kγ protein on brain edema and neurological impairments after SBI
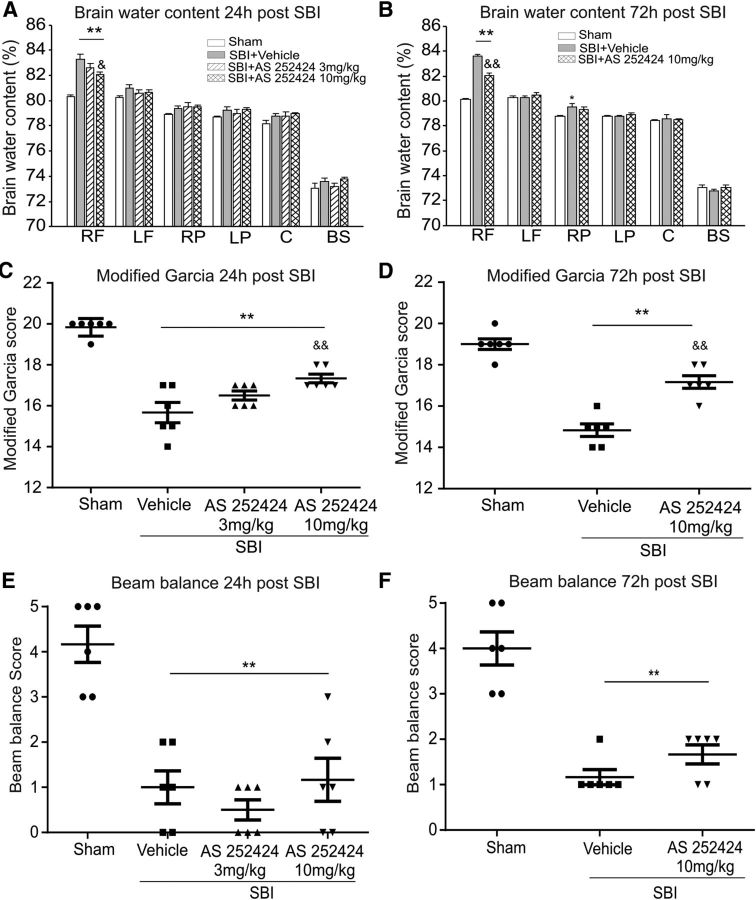
Twenty-four and 72 h after surgery, SBI animals had higher brain water content within peri-resection tissues and neurological deficits compared with the shams (Fig. 4). Compared with animals treated with vehicle, PI3Kγ inhibitor AS252424 at a dose of 10 mg/kg reduced brain edema and improved neurological performance (the Garcia test), but not the balance beam 24 and 72 h after SBI (Fig. 4).
Figure 4.
Effect of AS 252424 on brain water content, modified Garcia test, and balance beam test 24 and 72 h after SBI. Compared with the vehicle-treated SBI animals, AS252424 at dose of 10 mg/kg significantly reduced brain water content in peri-resection brain tissues (A, B) and improved the modified Garcia neurological function (C, D), but not the performance in balance beam test (E, F) (n = 6/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. A, **p < 0.001 versus sham; &p = 0.019 versus SBI + vehicle. B, **p < 0.001 versus sham; &&p < 0.001 versus SBI + vehicle. C, **p < 0.001 versus sham; &&p = 0.003 versus SBI + vehicle. D, **p < 0.001 versus sham; &&p < 0.001 versus SBI + vehicle. E, **p < 0.001 versus sham. F, **p < 0.001 versus sham. RF, Right frontal lobe; LF, left frontal lobe; RP, right parietal lobe; LP, left parietal lobe; C, cerebellum; BS, brainstem.
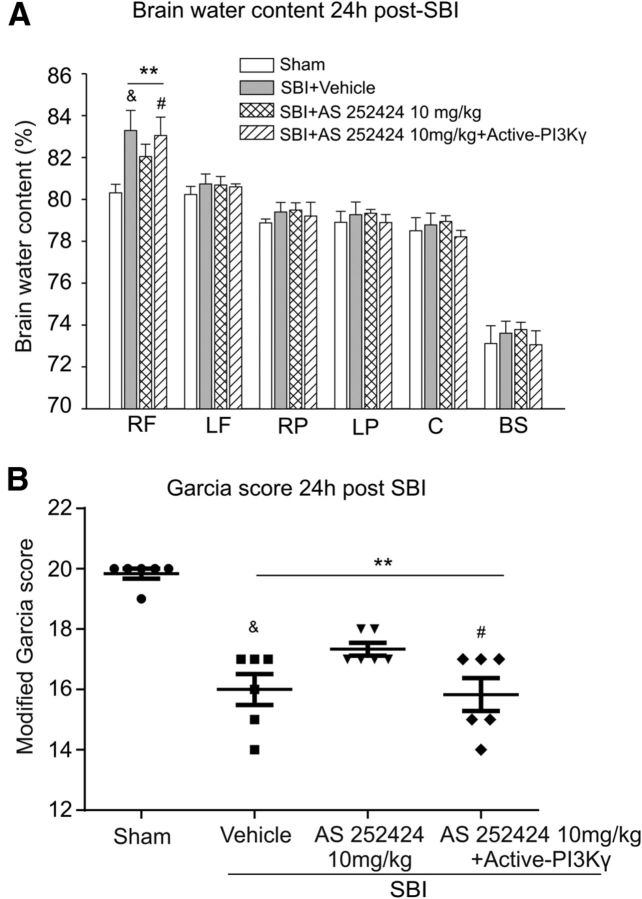
Human recombinant active-PI3Kγ protein, administered intracerebroventricularly 30 min before surgery, countered the beneficial effect of the PI3Kγ inhibitor AS252424 on brain water content and neurological outcome at 24 h after SBI (Fig. 5).
Figure 5.
Effect of human recombinant active-PI3Kγ protein 24 h after surgery. The protective effects of AS252424 against brain edema (A) and neurological deficit (B) were significantly reversed by human recombinant active-PI3Kγ 24 h after SBI (n = 6/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. A, **p < 0.001 versus sham; &p = 0.024 versus SBI + AS 252424 10 mg/kg; #p = 0.049 versus SBI + AS 252424 10 mg/kg. B, **p < 0.001 versus sham; &p = 0.029 versus SBI + AS 252424 10 mg/kg; #p = 0.04 versus SBI + AS 252424 10 mg/kg. RF, Right frontal lobe; LF, left frontal lobe; RP, right parietal lobe; LP, left parietal lobe; C, cerebellum; BS, brainstem.
Effects of AS252424 and human recombinant active-PI3Kγ protein on neuroinflammation 24 h after SBI
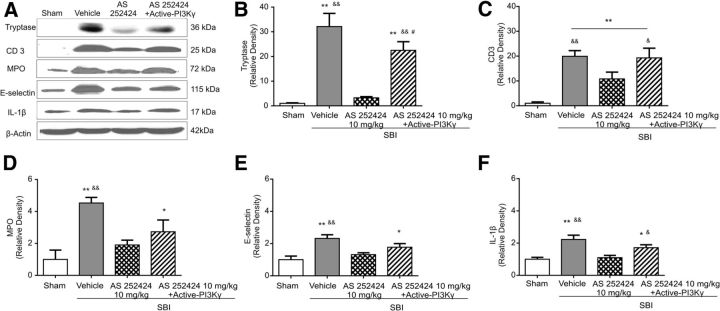
Western blot assay showed significantly higher protein levels of MPO, CD3, mast cell tryptase, E-selectin, and IL-1β in peri-resection brain tissues after SBI (Fig. 6). Selective inhibition of PI3Kγ by AS252424 (10 mg/kg) reduced the expression of those inflammatory markers (Fig. 6). Human recombinant active-PI3Kγ protein partially abolished the anti-inflammation effect of AS252424 (Fig. 6).
Figure 6.
Effect of AS252424 and human recombinant active-PI3Kγ protein on neuroinflammation associated with SBI 24 h after surgery. Representative images are shown of Western blot assay for mast cell tryptase, CD3, MPO, E-selectin, and IL-1β level within peri-resection brain tissues (A). Compared with the vehicle-treated SBI, AS 252424 significantly reduced levels of mast cell tryptase (B), T-cell associated with CD 3 level (C), neutrophils associated MPO level (D), cell-adhesion molecule E-selectin (E), and IL-1β cytokine (F) elevation by SBI. Human recombinant active-PI3Kγ partially reversed the protective effect of AS252424 against neuroinflammation (n = 6/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. B, **p < 0.001 versus sham; &&p < 0.001 versus SBI + AS252424 10 mg/kg; #p = 0.016 versus SBI + vehicle. C, **p < 0.001 versus sham; &&p = 0.003 versus SBI + AS252424 10 mg/kg; &p = 0.019 versus SBI + AS252424 10 mg/kg. D, *p = 0.023 versus sham; **p < 0.001 versus sham; &&p = 0.004 versus SBI + AS252424 10 mg/kg. E, *p = 0.039 versus sham; **p = 0.002 versus sham; &&p = 0.008 versus SBI + AS252424 10 mg/kg. F, *p = 0.029 versus sham; **p < 0.001 versus sham; &p = 0.026 versus SBI + AS252424 10 mg/kg; &&p = 0.001 versus SBI + AS252424 10 mg/kg.
Role of downstream enzyme PDE3B in PI3Kγ-mediated injury 24 h after SBI
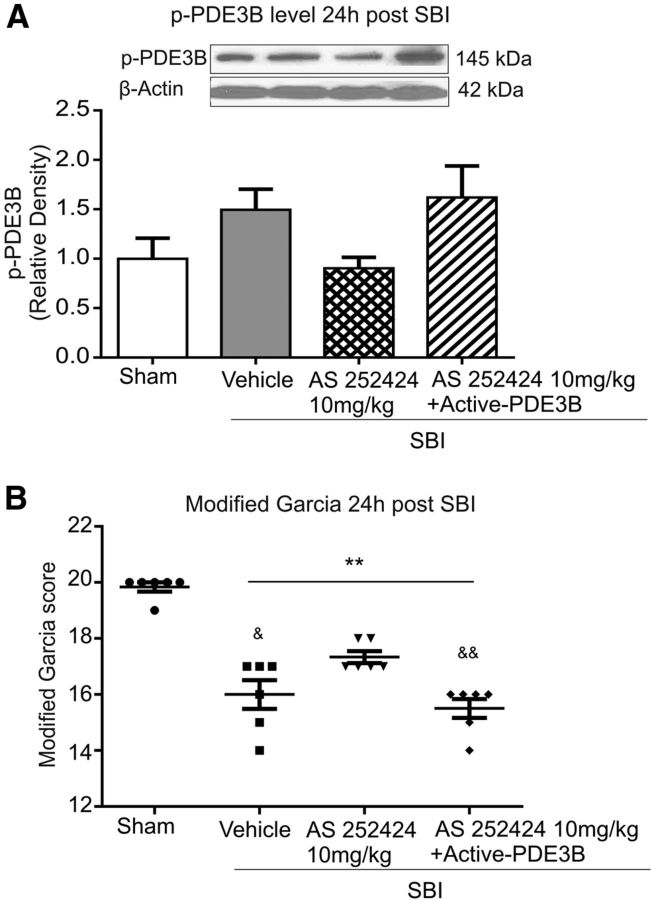
Compared with vehicle-treated SBI, PI3Kγ inhibitor AS252424 (10 mg/kg) administration had no significant effect on PDE3B phosphorylation, even though a tendency to decrease was shown 24 h after SBI (Fig. 7A). Without significantly increasing p-PDE3B level in peri-resection brain tissues (Fig. 7A), exogenous human recombinant active-PDE3B protein reversed the effects of AS252424 in the Garcia score at 24 h after SBI (Fig. 7B).
Figure 7.
Effect of exogenous human recombinant active-PDE3B at 24 h after surgery. A, Western blot showed a trend toward reduction in PED3B phosphorylation by AS252424, which tended to be reversed by active-PDE3B administration. However, these differences did not reach statistical significance (p = 0.085). B, The protective effects of AS252424 on neurological deficit were significantly reversed by active PDE3B (n = 6/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used.**p < 0.001 versus sham; &p = 0.011 versus SBI + AS252424 10 mg/kg; &&p = 0.003 versus SBI + AS252424 10 mg/kg.
Effects of AS605240 on brain edema and neurological impairments after SBI
SBI animals treated by the selective PI3Kγ inhibitor AS605240 at a dose of 10 mg/kg demonstrated similar results of significant reduction in brain water content in peri-resection brain tissues and improvement in the Garcia neurological performance at 24 h after SBI compared with vehicle-treated SBI (Fig. 8).
Figure 8.
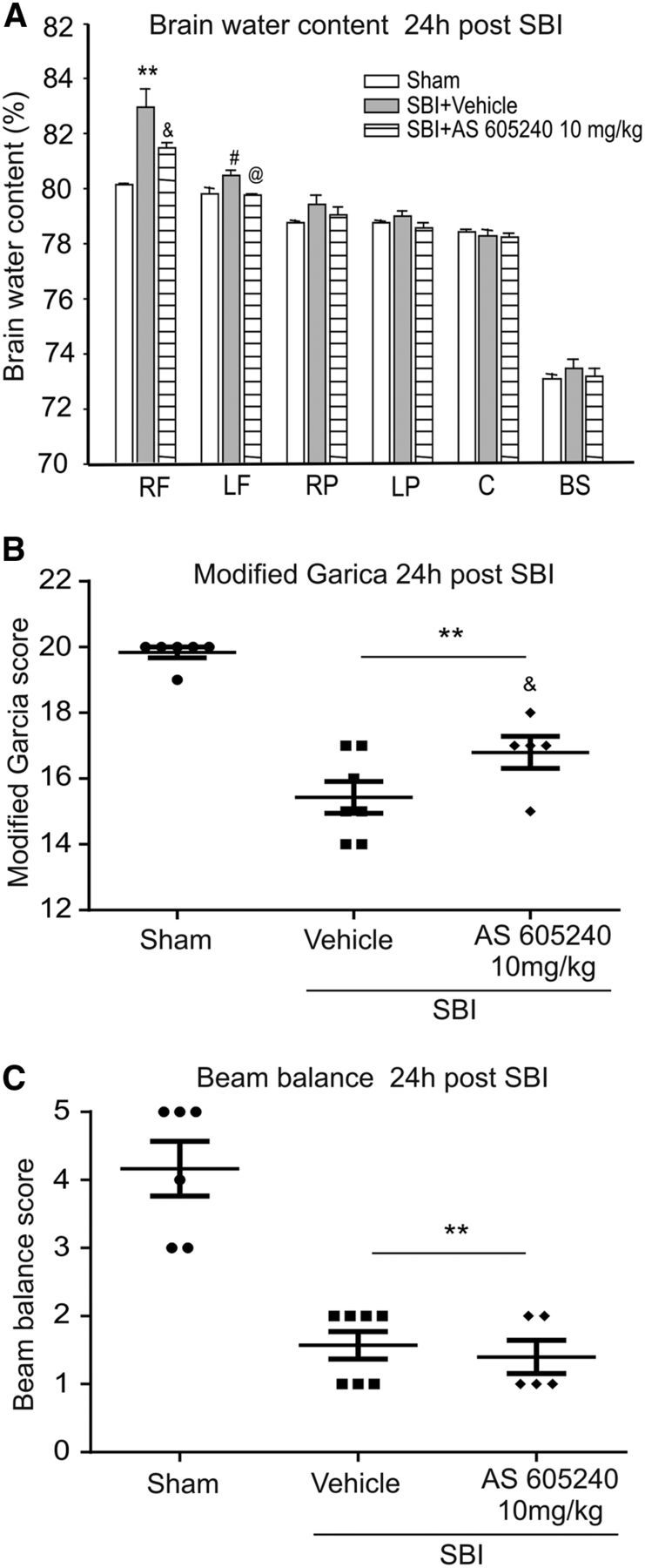
Effect of AS605240 on brain water edema, the modified Garcia test, and balance beam test 24 h after SBI. Compared with the vehicle-treated SBI animals, AS605240 significantly reduced brain water content in peri-resection brain tissues (A) and improved the modified Garcia neurological function (B), but not the performance in balance beam test (C) (n = 6 in sham, n = 7 in SBI + vehicle, n = 5 in SBI + AS605240). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. A, **p = 0.001 versus sham; #p = 0.022 versus sham; &p = 0.038 versus SBI + vehicle. @p = 0.044 versus SBI + vehicle. B, **p < 0.001 versus sham; &p = 0.035 versus SBI + vehicle. D, **p < 0.001 versus sham. RF, Right frontal lobe; LF, left frontal lobe; RP, right parietal lobe; LP, left parietal lobe; C, cerebellum; BS, brainstem.
Effects of PI3Kγ siRNA on PI3Kγ levels and outcomes 24 h after SBI
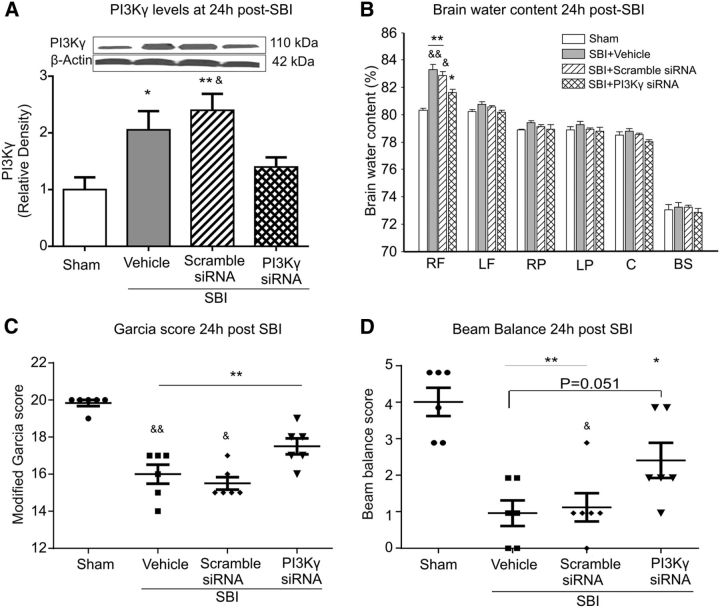
Twenty-four hours after SBI, Western blot showed that the PI3Kγ protein levels had increased in peri-resection brain tissue (Fig. 9A). Compared with the vehicle-treated and scramble siRNA-treated SBI, PI3Kγ siRNA reduced PI3Kγ levels (Fig. 9A). In addition, PI3Kγ siRNA treatment attenuated brain edema in peri-resection brain tissues (Fig. 9B) and improved neurological function (Fig. 9C,D). Double immunofluorescence staining showed PI3Kγ expression in brain endothelia and microglia cells in sham-operated and SBI animals at 24 h after injury, which were suppressed by PI3Kγ siRNA intracerebroventricular injection (Fig. 10).
Figure 9.
Effect of PI3Kγ siRNA on brain water content and neurological score 24 h after SBI. Compared with vehicle- or scramble siRNA-treated SBI animals, PI3Kγ siRNA significantly reduced PI3Kγ levels in peri-resection brain tissue (A). PI3K siRNA-treated SBI animals were associated with significantly less brain water content (B) and a better neurological function (C, D) (n = 6/group). One-way ANOVA followed by Student–Newman–Keuls post hoc tests were used. A, *p = 0.028 versus sham; **p = 0.005 versus sham; &p = 0.043 versus SBI + PI3Kγ siRNA. B, *p = 0.003 versus sham; **p < 0.001 versus sham; &p = 0.005 versus SBI + PI3Kγ siRNA; &&p = 0.001 versus SBI + PI3Kγ siRNA. C, **p < 0.001 versus sham; &p = 0.003 versus SBI + PI3Kγ siRNA; &&p < 0.001 versus SBI + PI3Kγ siRNA. D, *p = 0.001 versus sham; **p < 0.001 versus sham; &p = 0.036 versus SBI + PI3Kγ siRNA. RF, Right frontal lobe; LF, left frontal lobe; RP, right parietal lobe; LP, left parietal lobe; C, cerebellum; BS, brainstem.
Figure 10.
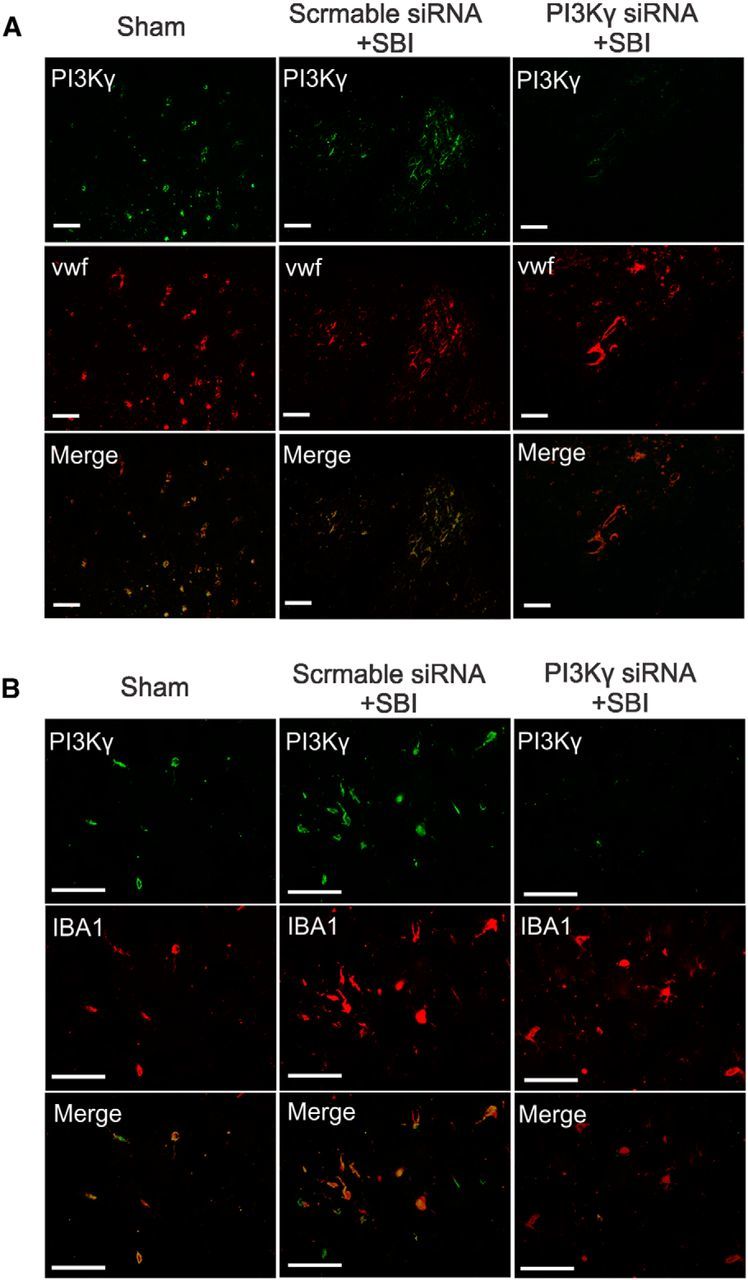
Effects of PI3Kγ siRNA on brain resident cells. Representative immunohistochemistry microphotographs of PI3Kγ staining with endothelia cells (Von Willebrand factor; A) and microglia (IBA1; B) 24 h hours after SBI. Sham- and scramble siRNA-pretreated SBI rats expressed PI3Kγ in brain endothelia and microglia within the ipsilateral frontal lobe. PI3Kγ siRNA intracerebroventricular injection suppressed endothelia and microglia PI3Kγ expression after SBI. Scale bar, 50 μm.
Discussion
In the present study, we have made the following observations: (1) endogenous PI3Kγ levels were significantly elevated in peri-resection brain tissues at 1 d and up to 7 d after corticotomy in a rat SBI model; (2) there was a tendency toward increase of endogenous PDE3B phosphorlation, a downstream enzyme of PI3Kγ proinflammation signaling pathways, in the peri-resection brain tissues at 1 d after SBI; (3) the selective PI3Kγ inhibitor AS252424 at a dose of 10 mg/kg significantly attenuated SBI-induced brain edema and neurological deficits 24 and 72 h after SBI; (4) the improved outcomes by the PI3Kγ inhibitor AS252424 were associated with less immune cell infiltration, activation, and cytokine release in peri-resection brain tissues 24 h after SBI; (5) exogenous human recombinant active-PI3Kγ protein partially offset the neuroprotection provided by the selective PI3Kγ inhibitor AS252424 24 h after SBI; (6) exogenous human recombinant active-PDE3B reversed the benefits of AS252424 on neurological outcomes at 24 h after SBI; (7) another selective PI3Kγ inhibitor, AS605240, at a dose of 10 mg/kg produced a similar protective effect against brain edema and neurological deficit 24 h after SBI; and (8) PI3Kγ siRNA decreased the PI3Kγ level, reduced brain edema, and improved neurological outcomes 24 h after SBI. These results demonstrate that PI3Kγ and its downstream enzyme, PDE3B, are involved in neuroinflammation in adjacent resection brain tissues after SBI.
PI3Kγ upregulation within 24 h was reported previously in a mouse model of focal cerebral ischemia (Jin et al., 2010b). Genetic ablation of the PI3Kγ gene resulted in less ischemia-induced microglia activation and blood–brain barrier (BBB) disruption in mice (Jin et al., 2010b; Jin et al., 2011). In the present study, there were significant increases in the PI3Kγ protein level at 1 d and up to 7 d after SBI. PI3Kγ was localized in immune cells such as neutrophils and T cells. The pattern of PI3Kγ elevation conforms to the time course of inflammatory cells recruitment after brain injury (Ling et al., 2003; Jin et al., 2010a; Rhodes, 2011), suggesting PI3Kγ involvement in neuroinflammation after SBI. In addition to PI3Kγ in class IB of PI3K, there are three members in subclass IA of PI3K, PI3Kα, PI3Kβ, and PI3Kδ, which are activated by receptor tyrosine kinases (Barberis and Hirsch, 2008). Ubiquitously expressed PI3Kα and PI3Kβ are vital for cell growth, division, and survival (Venable et al., 2010) and genetic ablations of each resulted in early embryonic lethality (Bi et al., 1999; Bi et al., 2002). PI3Kδ, also mainly expressed in hematopoietic cells, cooperates with PI3Kγ in immune-mediated inflammation with a complex epistatic interaction with activities critical during the process of inflammatory diseases (Liu et al., 2007; Rommel et al., 2007; Cushing et al., 2012). In the absence of changes in PI3Kα and PI3Kβ, PI3Kδ was the only PI3K class IA isoform significantly increased within peri-resection brain tissues 7 d after corticotomy. Future studies are needed to investigate the specific role of PI3Kδ in neuroinflammation after SBI.
Accumulation of leukocytes into the injured brain area is crucial to the extent of inflammation and secondary brain damage (Jin et al., 2010a; Ma et al., 2011; Rhodes, 2011). We found there were higher levels of MPO, CD3, and mast cell tryptase in the peri-resection tissues of SBI animals than shams, suggesting that the increased neutrophil, T-cell infiltration, and mast cell degranulation occurred after SBI. It was accompanied by increases in proinflammatory cytokine levels of IL-1β. Given the central role of PI3Kγ in regulating chemotaxis of leukocytes, as well as immune cell activation (Rückle et al., 2006), inhibition of the common signaling pathway of PI3Kγ is expected to provide comprehensive anti-inflammation effects and thus benefit overall outcome after SBI. The fact that its expression is restricted mainly to the hematopoietic system, although not exclusively, ensures overall benefits in the absence of severe side effects (Hirsch et al., 2000). PI3Kγ knock-out mice had significantly less infarct volumes and better neurological scores 24 h after focal ischemic stroke (Jin et al., 2011). PI3Kγ inhibition prevented β-amyloid1-40 peptide-induced cognitive deficits and synaptic dysfunction in mice (Passos et al., 2010). In agreement with these observations, we found that PI3Kγ inhibitor reduced neuroinflammation, leading to better outcomes at 24 and 72 h after SBI. Due to our model limitations that brain edema and neurological deficits gradually recede over 14 d, we did not evaluate the long-term effects of PI3Kγ inhibitor. However, preclinical studies have demonstrated that daily administration of PI3Kγ inhibitors benefit systemic inflammatory diseases for up to 1 month without evident side effects (Barber et al., 2005; Camps et al., 2005; Martin et al., 2010). Our findings complement earlier reports demonstrating impaired leukocyte trafficking to inflamed venues of rodents in which PI3Kγ activation was blocked using a pharmacological or genetic approach (Barber et al., 2005; Camps et al., 2005; Ferrandi et al., 2007). The absence of PI3Kγ activity impaired lipid product accumulation at the leading edge and actin rearrangement in leukocytes (Rickert et al., 2000) and stable adhesion to the endothelial wall (Smith et al., 2006), thus hampering their migration and infiltration. Conversely, endothelial PI3Kγ blockade reduced E-selectin-mediated attachment and increased neutrophil rolling velocity (Puri et al., 2005; Ghigo et al., 2010). PI3Kγ expresses in rodent brain endothelial cells and microglia (Jin et al., 2010a,b). PI3Kγ deficiency mice have a lower E-selectin level after focal brain ischemia (Jin et al., 2011). Consistent with this, in our study, PI3Kγ inhibitor diminished E-selectin elevation after SBI. Drug delivered through the CSF compartment could penetrate into the brain parenchyma and circulate into blood along CSF flow tracks (Kuo and Smith, 2014). Therefore, intracerebroventricular PI3Kγ siRNA administration suppressed PI3Kγ expression in brain endothelial cells and microglia, which could further reduce endothelial E-selectin-mediated leukocyte adhesion. To some extent, PI3Kγ siRNA may also suppress leukocyte PI3Kγ expression and associated chemotaxis.
Brain mast cells located mainly in meninges and several perivascular brain structures responded and degranulated to a multitude of physical/chemical challenges (Dimitriadou et al., 1990; Zhuang et al., 1996). By releasing potent preformed vasoactive and inflammatory mediators, including proteases and cytokines, that then act on cerebral vessels, mast cells initiate and promote breakdown of BBB and secondary inflammatory responses after focal brain ischemia (Strbian et al., 2007; Stokely and Orr, 2008; Jin et al., 2009). After SBI, increased tryptase in the brain region surrounding resection implicated the participation of mast cell activation and degranulation in initiating and promoting pathological processes associated with SBI. AS252424 significantly reduced tryptase release after SBI. Our findings echo previous studies in experimental systemic anaphylaxis showing that PI3Kγ inhibition reduced mast cell degranulation and that PI3Kγ deficiency animals were resistant to passive systemic anaphylaxis (Laffargue et al., 2002). PI3Kγ inhibitor or PI3Kγ knock-out could interrupt the degranulation amplification in mast cells (Laffargue et al., 2002).
Notably, neurobehavioral benefits were not evident in the balance beam test. Given that the balance beam test examines the overall performance of a task, rats can compensate for deficits using the uninjured body side, thus reducing the sensitivity to reveal unilateral brain damage. In the mouse model of intracerebral hemorrhage, the balance beam test showed a limited ability to identify the sensorimotor deficits compared with the modified Garcia neuroscore (Krafft et al., 2014). Nevertheless, either pharmacological PI3Kγ inhibition or siRNA-mediated PI3Kγ reduction significantly improved composite neurological function evaluated by the modified Garcia test after SBI.
When exogenous human recombinant active-PI3Kγ was administrated, it reversed the protective effect of selective PI3Kγ inhibitor against neuroinflammation, brain edema, and neurological deficit in SBI, confirming the detrimental role of PI3Kγ in SBI pathological progression.
Stimulatory effects of PI3Kγ on PDE3B activity have been documented in several types of cells through kinase-independent protein-protein direct interaction and downstream protein kinase B/Akt-dependent phosphorylation of PDE3B (Patrucco et al., 2004; Baragli et al., 2011; Degerman et al., 2011; Perino et al., 2011; Schmidt et al., 2013). Active-PDE3B degrades the phosphodiester bond in cAMP, which could further activate a variety of immune cell functions (Serezani et al., 2008; Schmidt et al., 2013). Selective PI3Kγ inhibitor was able to suppress microglial phagocytosis, possibly through preventing Akt-dependent PDE3B phosphorylation (Schmidt et al., 2013). Along with PI3Kγ elevation in the present study, there was a tendency toward increase in PDE3B phosphorylation within peri-resection brain tissues at 24 h after SBI. SBI animals treated with the selective PI3Kγ inhibitor AS252424 had lower levels of phosphorylated PDE3B. The administration of human recombinant active PDE3B reversed the protective effect of AS252424 against SBI-induced neurological deficit. These results suggested that the proinflammation effects of PI3Kγ may be at least in part through PDE3B activation.
In conclusion, signaling through PI3Kγ appears to be a common platform controlling diverse immune modulation after SBI. Our study underscores the suppression of multimodal inflammatory cells achieved by selective PI3Kγ inhibition in the setting of SBI. Local administration of human recombinant active-PI3Kγ and active-PDE3B proteins partially reversed the neuroprotective effects associated with pharmacological inhibition of PI3Kγ. Selective PI3Kγ inhibitor may offer pleiotropic anti-inflammation effects in patients undergoing neurosurgery and may thus prove beneficial to postoperative outcomes.
Footnotes
This work was supported by the National Institutes of Health (Grants NS084921, NS082184, and NS43338 to J.H.Z.).
The authors declare no competing financial interests.
References
- Ayer RE, Jafarian N, Chen W, Applegate RL, 2nd, Colohan AR, Zhang JH. Preoperative mucosal tolerance to brain antigens and a neuroprotective immune response following surgical brain injury. J Neurosurg. 2012;116:246–253. doi: 10.3171/2011.8.JNS11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baragli A, Ghè C, Arnoletti E, Granata R, Ghigo E, Muccioli G. Acylated and unacylated ghrelin attenuate isoproterenol-induced lipolysis in isolated rat visceral adipocytes through activation of phosphoinositide 3-kinase gamma and phosphodiesterase 3B. Biochim Biophys Acta. 2011;1811:386–396. doi: 10.1016/j.bbalip.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Rückle T, Schwarz MK, Rodríguez S, Martinez-A C, Balomenos D, Rommel C, Carrera AC. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase gamma to fight inflammation and more. Thromb Haemost. 2008;99:279–285. doi: 10.1160/TH07-10-0632. [DOI] [PubMed] [Google Scholar]
- Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169–172. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- Camps M, Rückle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Françon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Chen S, Ma Q, Krafft PR, Hu Q, Rolland W, 2nd, Sherchan P, Zhang J, Tang J, Zhang JH. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis. 2013;58:296–307. doi: 10.1016/j.nbd.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing TD, Metz DP, Whittington DA, McGee LR. PI3Kdelta and PI3Kgamma as targets for autoimmune and inflammatory diseases. J Med Chem. 2012;55:8559–8581. doi: 10.1021/jm300847w. [DOI] [PubMed] [Google Scholar]
- Degerman E, Ahmad F, Chung YW, Guirguis E, Omar B, Stenson L, Manganiello V. From PDE3B to the regulation of energy homeostasis. Curr Opin Pharmacol. 2011;11:676–682. doi: 10.1016/j.coph.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-U. [DOI] [PubMed] [Google Scholar]
- Ferrandi C, Ardissone V, Ferro P, Rückle T, Zaratin P, Ammannati E, Hauben E, Rommel C, Cirillo R. Phosphoinositide 3-kinase gamma inhibition plays a crucial role in early steps of inflammation by blocking neutrophil recruitment. J Pharmacol Exp Ther. 2007;322:923–930. doi: 10.1124/jpet.107.123026. [DOI] [PubMed] [Google Scholar]
- Ghigo A, Damilano F, Braccini L, Hirsch E. PI3K inhibition in inflammation: Toward tailored therapies for specific diseases. BioEssays. 2010;32:185–196. doi: 10.1002/bies.200900150. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost. 2006;95:29–35. [PubMed] [Google Scholar]
- Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res. 2008;1215:218–224. doi: 10.1016/j.brainres.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010a;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yu S, Song Z, Quillin JW, Deasis DP, Penninger JM, Nanda A, Granger DN, Li G. Phosphoinositide 3-kinase-gamma expression is upregulated in brain microglia and contributes to ischemia-induced microglial activation in acute experimental stroke. Biochem Biophys Res Comm. 2010b;399:458–464. doi: 10.1016/j.bbrc.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, Granger DN, Li G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- Kim DI, Kim SR, Kim HJ, Lee SJ, Lee HB, Park SJ, Im MJ, Lee YC. PI3K-gamma inhibition ameliorates acute lung injury through regulation of IkappaBalpha/NF-kappaB pathway and innate immune responses. J Clin Immunol. 2012;32:340–351. doi: 10.1007/s10875-011-9628-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Ueki K, Okazaki Y, Iwane A, Kubota N, Ohsugi M, Awazawa M, Kobayashi M, Sasako T, Kaneko K, Suzuki M, Nishikawa Y, Hara K, Yoshimura K, Koshima I, Goyama S, Murakami K, Sasaki J, Nagai R, Kurokawa M, et al. Blockade of class IB phosphoinositide-3 kinase ameliorates obesity-induced inflammation and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:5753–5758. doi: 10.1073/pnas.1016430108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafft PR, McBride DW, Lekic T, Rolland WB, Mansell CE, Ma Q, Tang J, Zhang JH. Correlation between subacute sensorimotor deficits and brain edema in two mouse models of intracerebral hemorrhage. Behav Brain Res. 2014;264:151–160. doi: 10.1016/j.bbr.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Smith MT. Theoretical and practical applications of the intracerebroventricular route for CSF sampling and drug administration in CNS drug discovery research: a mini review. J Neurosci Methods. 2014;233:166–171. doi: 10.1016/j.jneumeth.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/S1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Ling C, Sandor M, Fabry Z. In situ processing and distribution of intracerebrally injected OVA in the CNS. J Neuroimmunol. 2003;141:90–98. doi: 10.1016/S0165-5728(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414:228–232. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen PH, Raman SK, Boyle K, el-Beheiry H. Early postoperative complications following neurosurgical procedures. Can J Anaesth. 1999;46:7–14. doi: 10.1007/BF03012507. [DOI] [PubMed] [Google Scholar]
- Martin EL, Souza DG, Fagundes CT, Amaral FA, Assenzio B, Puntorieri V, Del Sorbo L, Fanelli V, Bosco M, Delsedime L, Pinho JF, Lemos VS, Souto FO, Alves-Filho JC, Cunha FQ, Slutsky AS, Rückle T, Hirsch E, Teixeira MM, Ranieri VM. Phosphoinositide-3 kinase gamma activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Resp Crit Care Med. 2010;182:762–773. doi: 10.1164/rccm.201001-0088OC. [DOI] [PubMed] [Google Scholar]
- Passos GF, Figueiredo CP, Prediger RD, Silva KA, Siqueira JM, Duarte FS, Leal PC, Medeiros R, Calixto JB. Involvement of phosphoinositide 3-kinase gamma in the neuro-inflammatory response and cognitive impairments induced by beta-amyloid 1–40 peptide in mice. Brain Behav Immun. 2010;24:493–501. doi: 10.1016/j.bbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, Baillie GS, Damilano F, Dunlop AJ, Pawson C, Walser R, Levi R, Altruda F, Silengo L, Langeberg LK, Neubauer G, Heymans S, Lembo G, Wymann MP, Wetzker R, Houslay MD, et al. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110gamma. Mol Cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, Burgat-Charvillon F, Valognes D, Camps M, Chabert C, Gillieron C, Françon B, Perrin D, Leroy D, Gretener D, Nichols A, Vitte PA, Carboni S, Rommel C, Schwarz MK, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase gamma. J Med Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- Puri KD, Doggett TA, Huang CY, Douangpanya J, Hayflick JS, Turner M, Penninger J, Diacovo TG. The role of endothelial PI3Kgamma activity in neutrophil trafficking. Blood. 2005;106:150–157. doi: 10.1182/blood-2005-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. Peripheral immune cells in the pathology of traumatic brain injury? Curr Opin Crit Care. 2011;17:122–130. doi: 10.1097/MCC.0b013e3283447948. [DOI] [PubMed] [Google Scholar]
- Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–473. doi: 10.1016/S0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- Rückle T, Schwarz MK, Rommel C. PI3Kgamma inhibition: towards an ‘aspirin of the 21st century’? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schneble N, Müller JP, Bauer R, Perino A, Marone R, Rybalkin SD, Wymann MP, Hirsch E, Wetzker R. Phosphoinositide 3-kinase gamma mediates microglial phagocytosis via lipid kinase-independent control of cAMP. Neuroscience. 2013;233:44–53. doi: 10.1016/j.neuroscience.2012.12.036. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006;80:1491–1499. doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- Smith LD, Hickman ES, Parry RV, Westwick J, Ward SG. PI3Kgamma is the dominant isoform involved in migratory responses of human T lymphocytes: effects of ex vivo maintenance and limitations of non-viral delivery of siRNA. Cell Signal. 2007;19:2528–2539. doi: 10.1016/j.cellsig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Beskonakli E, Kaptanoglu E, Okutan O, Ak F, Taskin Y. Transcortical-transventricular approach in colloid cysts of the third ventricle: surgical experience with 26 cases. Neurosurg Rev. 2004;27:89–92. doi: 10.1007/s10143-003-0309-2. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Orr EL. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- Strbian D, Tatlisumak T, Ramadan UA, Lindsberg PJ. Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:795–802. doi: 10.1038/sj.jcbfm.9600387. [DOI] [PubMed] [Google Scholar]
- Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol. 2008;84:814–823. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dop WA, Marengo S, te Velde AA, Ciraolo E, Franco I, ten Kate FJ, Boeckxstaens GE, Hardwick JC, Hommes DW, Hirsch E, van den Brink GR. The absence of functional PI3Kgamma prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunol Lett. 2010;131:33–39. doi: 10.1016/j.imlet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Venable JD, Ameriks MK, Blevitt JM, Thurmond RL, Fung-Leung WP. Phosphoinositide 3-kinase gamma (PI3Kgamma) inhibitors for the treatment of inflammation and autoimmune disease. Recent Pat Inflamm Allergy Drug Discov. 2010;4:1–15. doi: 10.2174/187221310789895603. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61:1067–1075. doi: 10.1227/01.neu.0000303203.07866.18. discussion 1075–1066. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO%3B2-4. [DOI] [PubMed] [Google Scholar]