Abstract
Skeletal muscle atrophy or wasting accompanies various chronic illnesses and the aging process, thereby reducing muscle function. One of the most important components contributing to effective muscle repair in postnatal organisms, the satellite cells (SCs), have recently become the focus of several studies examining factors participating in the atrophic process. We critically examine here the experimental evidence linking SC function with muscle loss in connection with various diseases as well as aging, and in the subsequent recovery process. Several recent reports have investigated the changes in SCs in terms of their differentiation and proliferative capacity in response to various atrophic stimuli. In this regard, we review the molecular changes within SCs that contribute to their dysfunctional status in atrophy, with the intention of shedding light on novel potential pharmacological targets to counteract the loss of muscle mass.
Keywords: skeletal muscle, stem cells, satellite cells, atrophy, cachexia
Introduction
Skeletal muscle atrophy is characterized by a loss of muscle mass and force, that occurs in response to a variety of pathological and physiological stimuli such as aging, cancer, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), diabetes, AIDS infection, sepsis, burns, muscle disuse, loss of muscle innervation, malnutrition, steroid-induced catabolic stimulation, and different congenital neuromuscular diseases (Bonaldo and Sandri, 2013; Egerman and Glass, 2014; Cohen et al., 2015). Loss in muscle mass is characterized by a decrease in cross-sectional area of muscle fibers, that is primarily the outcome of a preferential increase in intracellular protein degradation over protein synthesis (Bonaldo and Sandri, 2013; Egerman and Glass, 2014; Cohen et al., 2015). In most muscle wasting conditions, the loss of muscle tissue is not homogeneous, displaying differential effects on specific muscle groups and impacting distinct fiber types (Ciciliot et al., 2013). In specific forms of atrophy, such as age-related atrophy (sarcopenia), the reduction in fiber size is also accompanied by a reduction in the number of fibers, further highlighting the heterogeneity of the atrophying process occurring in different muscle wasting conditions (Lexell et al., 1988).
Muscle fibers are post-mitotic syncytia formed by the fusion of several hundreds of myogenic progenitors during pre- and post-natal development (Biressi et al., 2007). A majority of the myonuclei are added to developing fibers during post-natal growth, when there is a dramatic increase in muscle mass (Zhang et al., 1998). Not all myogenic progenitors terminally differentiate into muscle fibers during development, but a fraction of them remains in the adult muscles as a pool of undifferentiated myogenic stem cells. Different cellular types that possess the ability to differentiate into muscle fibers have been identified (Cossu and Biressi, 2005; Peault et al., 2007). Nevertheless, a population of stem cells, called “satellite cells” (SCs), due to the peculiar anatomical location between the fiber sarcolemma and the basal lamina surrounding the fiber, is believed to contribute in a major way to post-natal growth and muscle repair upon injury (Schultz, 1996; Lepper et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). In healthy adult muscles, SCs are largely quiescent, but can be activated by appropriate stimuli in the form of injury or exercise, resulting in regeneration and possibly further growth (hypertrophy) of the muscle tissue (Zammit et al., 2006; Schiaffino and Partridge, 2008). Several studies have investigated the role played by SCs in muscle hypertrophy and conclusions drawn from these studies are equivocal (Blaauw and Reggiani, 2014). A key role for SCs in the hypertrophic response is supported by observations that SCs are consistently activated in hypertrophic models and that their elimination by irradiation severely blunts overload-induced muscle hypertrophy (Rosenblatt et al., 1994). On the contrary, various observations using mutant mice to genetically ablate SCs and induce hypertrophy indicate that SC functionality does not constitute an absolute requirement in initiating muscle hypertrophy, suggesting that the prerequisite nature of their involvement might be limited to later stages in stabilizing the full hypertrophic response on a long-term basis (Amthor et al., 2009; Blaauw et al., 2009; McCarthy et al., 2011; Lee et al., 2012; Fry et al., 2014).
Whereas our knowledge of SC behavior in muscle hypertrophy is rapidly increasing, the role played by SCs during muscle atrophy remains largely enigmatic. Recent reports have generated an active debate in the scientific community on the active participation of SCs in the atrophic response, or whether muscle fiber alterations exclusively account for atrophy. In this manuscript, we review the experimental evidence supporting either view.
Several studies have investigated the effects of atrophic stimuli on muscle fibers and have identified key signaling pathways operating within the fiber that contribute to a decrease in fiber cross-sectional area. These studies highlighted the importance of protein homeostasis in regulating muscle mass, and identified the ubiquitin-proteasome and the autophagy-lysosome machineries as the two most important protolithic systems controlling protein turnover in muscle fibers. Several recent reviews describe the advances in understanding signaling mechanisms controlling the activity of these two systems in skeletal muscle fibers (Sandri, 2013; Schiaffino et al., 2013). In this review, we focus on the signaling pathways triggered in SCs in response to various atrophic stimuli. Moreover, we review the studies that address whether these molecular changes can be suppressed or reversed to induce SCs to increase the mass of atrophied muscles.
Does SC Activity Affect the Loss of Muscle Mass?
Muscle homeostasis depends on a fine balance between catabolism and anabolism. A large body of evidence indicates that alterations in the control of protein turnover modify this balance and play an important role in essentially all forms of muscle atrophy (Sandri, 2008). Nevertheless, the mass of every tissue, including skeletal muscle is not only dependent on protein turnover, but also on cellular turnover (Sartorelli and Fulco, 2004). In keeping with this, muscle fibers are lost in certain forms of muscle wasting (Lexell et al., 1988). Moreover, muscle fibers are multinucleated structures, and it has been proposed that there are distinct “myonuclear domains”, whereby each myonucleus governs the surrounding cytoplasm by producing enough protein to support only a limited portion of the fiber (Mitchell and Pavlath, 2004). Although some studies report a reduction in muscle fiber size without a change in myonuclear number (Wada et al., 2002; Gundersen and Bruusgaard, 2008), several reports have documented a decrease in the number of myonuclei accompanying different forms of atrophy, supporting the hypothesis that myonuclear turnover and a reduction in muscle mass are causally related (Darr and Schultz, 1989; Schmalbruch et al., 1991; Allen et al., 1995, 1996; Day et al., 1995; Hikida et al., 1997). More importantly, this raises the prospect that alterations in myonuclear turnover can stabilize the reduction in muscle mass, at least under certain atrophic conditions.
As myonuclei are postmitotic, the need to replenish lost myonuclei to maintain a constant myonuclear number must come from myogenic progenitors that are able to fuse with the fibers. SCs, which have been reported to contribute to a large fraction of the fiber myonuclei during regeneration and growth, represent a natural candidate for this role (Moss and Leblond, 1971). In keeping with this idea, ablation of SCs in a paired box protein (Pax7)DTR knock-in mouse model revealed a 20–40% loss of muscle mass 2 weeks after intramuscular injection of diphtheria toxin, an effect that persisted for 7 weeks after SC elimination and was exacerbated with strenuous resistance exercise (Sambasivan et al., 2011). Another study demonstrating an essential role for miRNAs in SC quiescence observed mild muscle atrophy within 6 months in uninjured mice that expressed a SC-specific conditional knockout (KO) of the gene Dicer (Cheung et al., 2012). These reports raise the tantalizing possibility that despite a low turnover rate in healthy muscle (Spalding et al., 2005), SCs could have an impact on homeostatic control of muscle mass. In contrast, a study using a genetic approach that allows for long-term depletion of SCs upon tamoxifen intraperitoneal injection in sedentary mice challenges this view (Fry et al., 2015). Despite the low regenerative capacity, these mice do not present signs of atrophy in hind limb muscles 1 month after tamoxifen administration and do not display exacerbated atrophy in 2-year-old mice. Therefore, these findings suggest that skeletal muscles do not necessarily require stem cell participation for tissue maintenance, at least under non-stressful conditions (Fry et al., 2015). Intriguingly, in a recent study, SCs were genetically labeled in adult mice and their fusion to myofibers in the absence of injury was followed throughout the lifespan of the mice. These experiments showed a contribution of SCs to myofibers in all muscles considered, although the extent and timing of their involvement differed in distinct muscles (Keefe et al., 2015). Importantly, the ablation of SCs using a genetic approach similar to that used by Fry et al. (2015) also showed a muscle group-specific response. In corroboration with the Fry et al. (2015) data, limb muscles were not significantly affected 6 months after SC depletion (Keefe et al., 2015). However, the diaphragm and extra ocular muscles displayed smaller fibers after 6 months of depletion of SCs, a decrease that was not exacerbated at later time-points (Keefe et al., 2015). Although a possible explanation for the different results derived from the individual studies could depend on the different nature of the animal models and experimental settings employed, the different types of muscles analyzed, or on the potential stress derived from the intramuscular injection of diphtheria toxin (Sambasivan et al., 2011), further investigations appears to be necessary to clarify the role of SCs in muscle homeostasis.
The discussion on the role played by SCs in the homeostatic maintenance of mass in healthy muscles has broader implications for the study of SC involvement in counteracting muscle mass loss under atrophying stimuli. The long-term SC depletion study conducted by Fry and colleagues, whereby the absence of SCs did not exacerbate sarcopenia in 2-year-old mice, is suggestive of the notion that SCs do not exert a compensatory action to counteract atrophy with age (Fry et al., 2015). Despite the compelling observations made in this study, the conclusions could possibly be limited by the incomplete depletion of SCs obtained in aged mice (an average of ~83%; Fry et al., 2015). Notably, elimination of ~85% of SCs by freeze- or cardiotoxin-mediated injury still results in muscle regeneration (Gayraud-Morel et al., 2007). Therefore, it is particularly relevant that the study from Keefe and colleagues using a mouse strain which allowed for the ablation of >95% of the SCs, obtained results that were similar to those reported by Fry and colleagues. When mice depleted of SCs were analyzed at 20 months of age, the contribution of SCs to myofiber maintenance appeared to be minimal in most hind limb muscles, with the striking exception of extensor digitorum longus (EDL) muscles that displayed a ~15% decline in fiber size (Keefe et al., 2015). Although an age-related reduction in fiber cross-sectional area is apparent at 20 months of age in most muscles, it is mainly after 2 years that a dramatic loss of muscle mass occurs in mice, which correlates with the appearance of an irreversible pre-senescent state in SCs that prevents activation and expansion (Sousa-Victor et al., 2014). The appearance of severe sarcopenia in older mice opens up the possibility for a primary requirement for SC contribution at later stages. Therefore, it would be interesting to extend the ablation studies to mice older than 2 years of age.
Intriguingly, several reports indirectly suggest that SCs may play a role in ameliorating sarcopenia, and that sarcopenia could be at least in part, a consequence of defective SC function. Several changes affecting the SC compartment have been observed in aged muscles (Alway et al., 2014). Strikingly, stem cell function and consequently regenerative potential are severely affected by aging in different tissues, including skeletal muscle (Liu and Rando, 2011). Alterations in the muscle and systemic environment occur during the aging process, thereby contributing to reversible and irreversible changes in SCs (Brack and Rando, 2007; Jang et al., 2011; Sousa-Victor et al., 2015). Moreover, despite the lack of a clear consensus, a reduction in SC number has been reported during aging, and it has been correlated with a reduction in fiber myonuclear content (Brack et al., 2005). Presence of centrally-nucleated fibers and an up-regulation of Myogenic differentiation (MyoD), Myogenin and embryonic Myosin Heavy Chain expression, which are generally considered as hallmarks of fiber regeneration, have also been documented in aging muscle (Edström and Ulfhake, 2005). Notably, a loss of myonuclei with aging in MyoD KO mice was exacerbated compared to wild-type mice. Since SCs display defective differentiation in the absence of MyoD expression, this observation has been interpreted as evidence in favor of a role for SCs in replenishing fiber myonuclei during aging (Brack et al., 2005).
An up-regulation of MyoD expression has also been documented in other forms of atrophy, and is particularly well established in denervation models of atrophy (Legerlotz and Smith, 2008). Upon denervation, SCs proliferate transiently, but do not progress through differentiation and form small immature (embryonic Myosin Heavy Chain+ve) fibers, indicative of an unsuccessful attempt to restore muscle mass and function (Viguie et al., 1997; Borisov et al., 2005; Doppler et al., 2008). As in aging muscle, the increase in MyoD expression observed in denervated muscle could be interpreted as a result of SC activity. Nevertheless, it is notable that muscle fibers also express MyoD at low levels, even in the absence of ongoing regeneration (Hughes et al., 1993). It is therefore possible that the increase in MyoD expression in muscle observed after denervation, and possibly in other models of atrophy as well, could constitute a SC-independent response of the fibers to atrophic stimuli (Koishi et al., 1995). Indeed it has been proposed that the induction of MyoD could represent an attempt by muscle fibers to regain sensitivity to neural activity. In keeping with this idea, MyoD has been shown to regulate the expression of Acetylcholine receptor (AChR), the expression of which is also increased upon denervation (Legerlotz and Smith, 2008). On similar lines, Myogenin is also expressed in fibers in the absence of a regenerative response (Hughes et al., 1993), and can act as an essential mediator of neurogenic atrophy by regulating the expression of Murf1 and Atrogin-1 within the fiber, thereby promoting muscle proteolysis (Moresi et al., 2010).
Alterations in the myogenic program have also been described in cachexia, a complex metabolic syndrome characterized by a loss of muscle mass, which is initiated by underlying illnesses of different nature, such as cancer, CHF, COPD, CKD, burns, chronic infection and sepsis (Evans et al., 2008; Fearon et al., 2012). Changes in the expression of myogenic factors and impaired differentiation have been reported in cachectic muscles (Coletti et al., 2005; Langen et al., 2006; Schwarzkopf et al., 2006; Penna et al., 2010; Zhang et al., 2010a; Wu et al., 2013). In tumor-bearing mice that recapitulated clinical features of cancer-induced cachexia, as well as in muscle biopsies from patients with pancreatic cancer, a significant expansion of cells expressing high levels of the SC-marker Pax7 was observed (Penna et al., 2010; He et al., 2013). Intriguingly, the majority of the Pax7+ve cells were noted in the interstitium and a fraction of them were reported to express mesenchymal (Pdgfr-α, Sca1) and pericyte (NG2) markers, which are not expressed in the SC lineage (He et al., 2013). These data reveal that cancer-induced atrophy triggers myogenic commitment in multiple stem cell progenitors, in addition to the SC population (He et al., 2013). In stark contrast with other atrophy models (see above), MyoD and Myogenin expression are not significantly induced in tumor-bearing mice compared to controls, and an impaired myogenic program prevents these cells from efficiently fusing with existing myofibers and therefore ameliorating the wasting condition (He et al., 2013). Pax7 has been reported to inhibit differentiation by suppressing MyoD and Myogenin expression (Olguin and Olwin, 2004). Positive or negative modulation of Pax7 expression in cachectic mice impact muscle mass in a negative or positive manner, respectively. Moreover, depletion of Pax7+ve cells further exacerbated muscle loss, directly indicating that stem cells are able to counteract muscle atrophy in cachectic mice. Nevertheless, the persistent expression of Pax7 stalls the myogenic program and limits the functionality of muscle stem cells (He et al., 2013). In line with the idea that SC functionality can compensate for the effects of atrophic stimuli, mdx mouse models of Duchenne muscular dystrophy (DMD) showed a correlation between muscle mass and alterations in regenerative potential. Specifically, during early stages of pathology characterized by effective regeneration, a compensatory hypertrophy has been observed. However, at later stages when regeneration becomes defective, there is a dramatic decrease in muscle mass (Mouisel et al., 2010).
Alterations in SC function, including defective fusion properties, have also been observed in the hind limb suspension model of disuse atrophy (Mitchell and Pavlath, 2004). Conversely, changes in SC numbers are not a consistent finding in disuse atrophy, reflecting the complexity of events occurring in this context (Brooks and Myburgh, 2014). Notably, the decrease in muscle mass accompanying different pathophysiological conditions displays variable changes in SC numbers depending on the type and the severity of the atrophic stimulus (Table 1). Also the time-scale of the analysis appears to play an important role in SC quantification, as highlighted in denervation experiments, in which an initial phase of proliferation is followed by a decrease in numbers of SCs (Viguie et al., 1997). The inconsistent reports on changes in SC numbers, together with the observations that alterations in SC function appear to be a common feature in most atrophic conditions, raises the prospect that rather than absolute numbers, it is the functionality of the SCs that is more relevant in their ability to counteract atrophy. Further studies will be required to confirm this view, and to conclusively impart a non-redundant role for SCs and other muscle resident stem cells in specific atrophic processes.
Table 1.
Satellite cell alterations in different atrophic conditions.
| Condition | Changes in number | Changes in function | Notes | References |
|---|---|---|---|---|
| Aging | ↓ = | Yes | ↓ proliferation ↓ myogenic differentiation |
Snijders et al. (2009) and Alway et al. (2014) |
| Cachexia | ↑ | Yes | ↑ activation/proliferation ↓ differentiation/fusion |
Penna et al. (2010) and He et al. (2013) |
| Denervation | ↑* | Yes | ↑ activation/proliferation ↓ fusion |
Viguie et al. (1997), Borisov et al. (2005) and Doppler et al. (2008) |
| Hind limb suspension | ↓ = ↑ | Yes | ↓ proliferation ↓ differentiation |
Mitchell and Pavlath (2004) Brooks and Myburgh (2014) |
| Glucocorticoids | n.d. | Yes | ↓ proliferation ↓ differentiation |
Dong et al. (2013) |
| DMD | ↑ | Yes | ↓ proliferation ↓ myogenic differentiation/fusion |
Blau et al. (1983) and Delaporte et al. (1984) Jasmin et al. (1984), Melone et al. (1999) and Biressi et al. (2014) |
↑, Increase; ↓, decrease; =, unchanged; n.d., not defined; * initial increase, but reduced in the long-term (Viguie et al., 1997).
Molecular Alterations in SCs During Atrophy
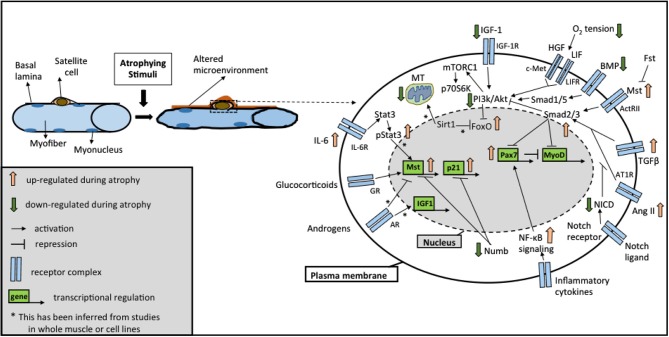
It is increasingly becoming clear that distinct signaling pathways mediate a common outcome of a loss of muscle mass observed in instances of atrophy, sarcopenia, or cachexia (Glass, 2005; Fanzani et al., 2012). While the involvement of these molecular mechanisms has been described in great detail in myofibers in response to atrophic stimuli (Bonaldo and Sandri, 2013; Schiaffino et al., 2013), in this section we review alterations in these pathways occurring in SCs accompanying fiber atrophy. Moreover, we focus on studies reporting functional changes in SCs resulting from alterations in these pathways, which possibly contribute to the loss of muscle mass. Importantly, these pathways extensively modulate one another and coordinate overlapping responses not only in the muscle fiber, but also in the SC compartment (Figure 1). Below, we discuss five major signaling pathways traditionally associated with skeletal muscle atrophy: (a) insulin-like growth factor (IGF)-Akt-FoxO signaling; (b) Transforming Growth Factor Beta (TGFβ) superfamily signaling; (c) Glucocorticoids and androgen signaling; (d) nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling; (e) Sirtuin 1 (Sirt1) signaling and mitochondrial dysfunction. Additionally, we review recent studies involving (f) Notch signaling, a pathway well known to influence SC functionality for a role in regulating muscle mass.
Figure 1.
Overview of the signaling pathways in satellite cells (SCs) during atrophy. Schematic representation of the major signaling pathways implicated in SC biology under different atrophic stimuli (see text for details). Cross-talks between different pathways are highlighted by positive and negative regulation at different levels. Abbreviations: AR, androgen receptor; GR, glucocorticoid receptor; Il-6R, Interleukin-6 receptor; IGF-1R, IGF-1 receptor; LIFR, LIF receptor; AT1R, Angiotensin II receptor 1; ActRII, Activin receptor 2; Fst, Follistatin; Mst, Myostatin; NICD, Notch intracellular domain; MT, mitochondria.
IGF-Akt-FoxO Signaling
A central pathway regulating fiber size is the IGF-Akt pathway (Rommel et al., 2001). Activation of this pathway triggers the activation of mammalian target of Rapamycin (mTOR) that results in the phosphorylation of its targets p70 S6K and 4E-BP1 (Bodine et al., 2001; Pallafacchina et al., 2002; Wullschleger et al., 2006). An additional consequence of these growth- promoting stimuli is the inhibition of the FoxO family of transcription factors that are key in initiating the atrophy program (Sandri et al., 2004). Consistent with this finding, components of the FoxO pathway were found to be increased in skeletal muscle during sepsis, cancer cachexia, and treatment following lipopolysaccharide (LPS), glucocorticoid, and cytokines (Liu et al., 2007; Schmitt et al., 2007; Crossland et al., 2008; Moylan et al., 2008). Additionally, the ability of the FoxO transcription factors to regulate muscle mass during disuse atrophy and following glucocorticoid treatment via the regulation of atrophy-related genes has been well documented (Kamei et al., 2004; Lecker et al., 2004; Sandri et al., 2004; Senf et al., 2010).
Only recently have studies begun to address the influence of IGF1 signaling in the behavior of SCs accompanying the atrophic response in fibers. For instance, in CKD-induced muscle atrophy, SCs displayed a reduction in phosphorylated Akt levels indicative of impaired IGF-1 signaling, and a decrease in activation and myogenic progression (Zhang et al., 2010a). Moreover, both CKD and IGF-1 receptor KO mice developed fibrosis in regenerating muscles, suggesting a decline in SC functionality (Zhang et al., 2010a). Similarly, old dystrophic mdx mice also displayed reduced Akt phosphorylation along with defective regeneration and atrophy (Mouisel et al., 2010). In studies aimed at addressing the role of the FoxO’s in SCs, overexpression of FoxO3 in myogenic progenitors decreased their proliferation (Rathbone et al., 2008). Similarly, abolishing FoxO activity either by injecting dominant negative (DN) FoxO-expressing plasmid into murine muscles or deleting the Foxo3 gene specifically in SCs, resulted in an increase in the proliferation of SCs (Reed et al., 2012; Gopinath et al., 2014). Interestingly, the former study reported a hypertrophic response following global suppression of FoxO activity, which acts as a protective mechanism to suppress atrophy following sepsis and cancer (Reed et al., 2012). However, while FoxO functionality may support atrophy when expressed in the fiber, the expression of a specific isoform, FoxO3, in SCs seems to be a prerequisite for maintaining the regenerative capacity of muscle and might therefore act to protect the muscle from atrophying stimuli (Gopinath et al., 2014). These paradoxical observations suggest that FoxO activity promotes different programs in SCs vs. fibers, and underscores the importance of identifying the biochemical processes that promote atrophy in combination with a SC-focused approach.
TGFβ Superfamily Signaling
Members of the TGFβ superfamily are potent regulators of muscle mass (Goodman and Hornberger, 2014). Canonical TGFβ signaling operates through the Smad signaling cascade. Specifically, the TGF-βs, Activins, and some members of the growth differentiation factor (GDF) subfamilies activate a Smad2/3-dependent signaling cascade, whereas members of the bone morphogenetic protein (BMP) subfamily primarily induce a Smad1/5/8-dependent signaling mechanism (Massagué, 1998). An increasing amount of evidence has implicated Smad2/3 in possessing a catabolic function, while Smads 1/5/8 have an anabolic function (Goodman and Hornberger, 2014). Members of the TGFβ superfamily play an important role in controlling the proliferation and differentiation of myogenic cells, suggesting that the effects exerted on muscle mass are in part achieved by controlling SC function (Kollias and McDermott, 2008).
Myostatin (GDF8), is a member of the TGFβ superfamily and a potent inhibitor of muscle growth, such that various mammalian species bearing Myostatin-null mutations display a hypertrophic response (Kambadur et al., 1997; Lee and McPherron, 2001; Schuelke et al., 2004). Myostatin binding to Activin receptor 2 (ActRII) and Activin receptor-like kinase 4 and 5 (ALK 4/5) activates the Smad2/3-dependent signaling cascade and blocks the Akt-mTOR growth- promoting pathway (Rommel et al., 2001). Systemic administration of Myostatin in adult mice was found to induce profound muscle loss (Zimmers et al., 2002). Moreover, enhanced levels of Myostatin have been observed in a variety of muscle wasting conditions (Gonzalez-Cadavid et al., 1998; Lalani et al., 2000; Yarasheski et al., 2002; McKay et al., 2012). Importantly, Myostatin was suggested to play a role in negatively regulating SC activity by inhibiting myogenic progression (Langley et al., 2002; McCroskery et al., 2003; McFarlane et al., 2008; Trendelenburg et al., 2009). Consistent with the notion that modulation of Myostatin expression is bound to have a significant impact on muscle mass and SC behavior, it was found that inhibition of the ActRIIB pathway stimulated SC proliferative potential and reversed muscle wasting in cancer-bearing mice (Zhou et al., 2010). Moreover, altered levels of Myostatin and its receptor resulted in impaired SC proliferation and differentiation in muscle wasting accompanying liver cirrhosis in a portacaval anastomosis (PCA) rat model (Dasarathy et al., 2004). While it has been suggested that a loss in body weight of the PCA rats compared to sham controls is an overall consequence of specific fiber type atrophy (type 2) and impaired SC functionality, what remains to be determined is a quantitative assessment of SC dysfunction alone that contributed to the atrophy observed in this model.
Several studies have provided insights into mechanisms operating upstream and downstream of Myostatin, thereby identifying diverse signaling pathways that have a shared outcome of a loss of muscle mass. Indeed, it has been shown that Numb, a mediator of asymmetric cell division suppresses Myostatin expression, such that Numb-deficient SCs display impaired proliferation characterized by high levels of p21 and Myostatin (George et al., 2013). A decrease in muscle mass was apparent after developmental deletion of Numb (George et al., 2013). Given these results, it would then be interesting to investigate whether SC-specific Numb conditional KO animals display a reduction in muscle mass, and if the loss is exacerbated in the background of liver cirrhosis, cancer cachexia or other muscle wasting conditions. Intriguingly, lower levels of Numb expression have been reported in muscle biopsies from older men (60–75 years old) than in muscles from younger men (18–25 years old; Carey et al., 2007).
Recent reports have implicated a role for Stat3, a downstream effector of Interleukin 6 (IL-6) in muscle wasting (Muñoz-Cánoves et al., 2013; Zhang et al., 2013). IL-6, an inflammatory cytokine is itself known to be involved in initiating muscle wasting when present systemically and for sustained periods of time (Strassmann et al., 1992; Haddad et al., 2005). Elevated levels of activated Stat3 (p-Stat3) were shown to initiate Myostatin-mediated muscle wasting and inflammation in patients with CKD or diabetes via increase in C/EBP δ levels (Zhang et al., 2013). Consistent with this, muscle-specific deletion of Stat3 and inhibition of CCAAT/enhancer-binding protein gamma (C/EBP δ) expression countered the loss of muscle mass in CKD (Zhang et al., 2013). While this study did not address the status of the Stat3 pathway and the consequences of its modulation within SCs, another study demonstrated that conditional ablation of Stat3 specifically in SCs increased their expansion during regeneration, but compromised their myogenic differentiation and prevented their contribution to regenerating myofibers (Tierney et al., 2014). Intriguingly, transient inhibition of Stat3 function by pharmacological treatment led to an expansion of SCs at a higher rate, and maintained their ability to differentiate into fibers, thus enhancing tissue repair in both aged and dystrophic muscle (Price et al., 2014; Tierney et al., 2014).
Recent findings have proposed a key role for BMPs in controlling muscle mass. Unlike Myostatin signaling, BMP acts through Smad1/5/8, eliciting a hypertrophic response in muscle and involving the Akt pathway, the inhibition of which by Rapamycin attenuates at least partially the BMP-mediated response (Sartori et al., 2013; Winbanks et al., 2013). Inhibition of BMP signaling causes muscle atrophy, abolishes the hypertrophic phenotype of Myostatin-null mice, and exacerbates the effects of denervation and fasting (Sartori et al., 2013; Winbanks et al., 2013). The atrophic response observed after fiber-specific Smad4 conditional ablation suggests that BMPs regulate muscle mass by directly acting on muscle fibers (Sartori et al., 2013). Indeed, the degradation of muscle fiber proteins stimulated by the ubiquitin ligase MUSA1 has been implicated in this process (Sartori et al., 2013; Winbanks et al., 2013). Importantly, the inhibition of BMP signaling not only counteracts the increase in muscle mass in Myostatin-null mice, but also blunts the hypertrophic response induced by Follistatin (Winbanks et al., 2013). Follistatin is a powerful regulator of muscle mass, which exerts its function mainly by inhibiting the action of Myostatin and Activins, both of which are implicated as negative regulators of muscle growth (Link and Nishi, 1997; Souza et al., 2008; Gilson et al., 2009; Lee et al., 2010). Strikingly, the presence of SCs seems to be required for a full Follistatin-dependent hypertrophy, as muscle irradiation which abolishes the proliferative capacity of SCs blunted the effects of Follistatin overexpression on muscle mass (Gilson et al., 2009). A caveat in this set of experiments is that the use of electroporation to introduce Follistatin-expressing plasmids into muscles is accompanied by a regenerative response that almost certainly involves SC participation (Skuk et al., 2013). Nevertheless, since Follistatin-induced hypertrophy is mitigated by both the inhibition of BMP signaling as well as by blocking cell proliferation, it is conceivable that BMP-mediated hypertrophy is dependent on SC activity to a significant extent. Indeed, BMP signaling strongly inhibits the myogenic differentiation program in myogenic precursor cells, and is able to induce features typical of the osteoblast lineage in myogenic cell lines under specific in vitro conditions (Murray et al., 1993; Katagiri et al., 1994). While there is a lack of evidence of the ability of SCs to commit to the osteogenic program in vivo, several observations indicate that BMP signaling plays an important role during muscle regeneration by controlling myogenic progenitor differentiation and positively modulating their proliferative expansion (Lounev et al., 2009; Clever et al., 2010; Friedrichs et al., 2011; Ono et al., 2011). Interfering with normal BMP signaling in vivo leads to smaller regenerated fibers after muscle injury and to smaller muscles during fetal development (Wang et al., 2010; Ono et al., 2011). Together, these observations suggest that BMP signaling occupies a central position in a complex network of signals that control SC biology and muscle mass, besides being implicated in the etiology of atrophy. These observations also support the idea of an active cross-talk between different branches of the TGFβ signaling pathway in the control of muscle mass.
An involvement of SCs in the control of muscle mass is particularly relevant in diseases that continually demand a damage-repair response from the tissue, such as muscular dystrophies. Muscular dystrophies form a group of heterogeneous genetic diseases, often characterized by progressive muscle atrophy (Shin et al., 2013). Little is known about the molecular changes occurring in SCs within the dystrophic environment that prevents them from restoring the growth of the atrophied muscles. Several members of the TGFβ superfamily, including Myostatin, TGFβ1, and TGFβ2 have been implicated in the progression of different forms of muscular dystrophy (Yamazaki et al., 1994; Bogdanovich et al., 2002; Wagner et al., 2002; Andreetta et al., 2006; Onofre-Oliveira et al., 2012; Biressi et al., 2014). Enhanced TGFβ signaling appears to be responsible for the aberrant myogenic program observed in the muscles of mdx mice (Biressi et al., 2014). TGFβ signaling appears to alter SC function also in other diseases, such as Emery-Dreyfuss muscular dystrophy (AD-EDMD), in which mutations in the Lamin A/C genes cause muscle atrophy and necrosis (Sewry et al., 2001). An analysis of SC activity in the Lmna−/− mice revealed that the proliferation rates and kinetics of activation of the SC progeny derived from Lmna−/− muscles were slower and delayed compared to wild type muscles (Cohen et al., 2013). More importantly, Lmna−/− myoblasts displayed elevated levels of Smad2/3 that did not localize to the nucleus in response to TGFβ stimulation. As a result, there was an increase in cell death in myotube cultures, an event that could contribute to wasting (Cohen et al., 2013). Additionally, defective SC behavior, fiber atrophy, and enhanced TGFβ signaling have also been observed in mouse models of Caveolin 3-deficient limb-girdle muscular dystrophy (Ohsawa et al., 2012). Importantly, administration of an inhibitor of TGFβ type I receptor not only ameliorates muscle atrophy, but also restores the number and differentiation potential of SCs, indicating that both TGFβ-dependent reduction in SCs and impaired myoblast differentiation contribute to the cellular mechanism underlying Caveolin 3-deficient muscle atrophy (Ohsawa et al., 2012).
A key modulator of TGFβ signaling in muscle and an effector molecule of the renin-angiotensin system, Angiotensin II (Ang II), has been implicated in muscle degeneration and accumulation of fibrotic tissue in several congenital and acquired muscle disorders (Cohn et al., 2007). In keeping with this, down-regulation of TGFβ signaling by Ang II inhibitors ameliorates muscle wasting in different myopathies (MacDonald and Cohn, 2012). Ang II is also involved in the etiology of cachexia, such that patients with CKD and CHF often have elevated Ang II levels, and treatment with an angiotensin converting enzyme inhibitor can reduce weight loss (Anker et al., 2003). Consistent with this notion, earlier studies showed that the infusion of Ang II in rodents decreased muscle weights by increasing protein degradation, disrupting IGF-1 signaling and inducing apoptosis (Brink et al., 1996). In SCs, Ang II was demonstrated to impair SC proliferation and differentiation by signaling events mediated by one of its receptors, Angiotensin 1 (AT1R), during the course of muscle regeneration (Yoshida et al., 2013). Indeed, by inhibiting AT1R activity in CHF, a condition in which high Ang II levels are present, the observed reduction in SC numbers and muscle weight could be blocked (Yoshida et al., 2013). Intriguingly, an opposite function was ascribed to Ang II Type 2 receptor (AT2R) that is expressed in differentiating SC progeny and is known to promote regeneration (Yoshida et al., 2014). These observations highlight the importance of defining the mechanisms operating downstream of atrophic signals, in order to develop therapeutic approaches that are specific and effective.
Glucocorticoid and Androgen Signaling
Glucocorticoids, either in their synthetic or physiological form, cortisol (in human) and corticosterone (in rodents), have emerged as potent negative regulators of muscle mass (Braun and Marks, 2015). Elevated levels of circulating glucocorticoids have been linked to different atrophic conditions including sepsis, diabetes, and cancer (Braun and Marks, 2015). Glucocorticoids act by binding to glucocorticoid receptors, a family of nuclear receptors, and exerting their function using different mechanisms, including stimulating the expression of muscle-specific E3 ubiquitin ligases in muscle fibers and modulating the function of Akt, Myostatin, and multiple other signaling pathways involved in the control of muscle mass (Braun and Marks, 2015). By stimulating Myostatin expression, glucocorticoids can also decrease SC proliferation and differentiation (Dong et al., 2013).
Importantly, the effects of glucocorticoid administration on muscle mass are counteracted by androgens in both patients and animal models (Creutzberg et al., 2003; Eason et al., 2003). Androgens are efficient positive modulators of muscle mass and function by binding to the androgen receptor, also a member of the nuclear receptor superfamily (Dubois et al., 2012). The androgen receptor is expressed not only in muscle fibers, but also in SCs, in particular in the levator ani muscle, a muscle that is dramatically affected in animal models with a conditional ablation of androgen receptor expression in SCs and fibers (Swift-Gallant and Monks, 2013; Dubois et al., 2014). Several reports suggest that androgens are able to modulate gene expression and function in SCs and myoblast cell lines (Chen et al., 2005). Testosterone, the principal circulating androgen is decreased in the serum of older men, possibly contributing to the SC dysfunction observed in this condition (Yialamas and Hayes, 2003). Intriguingly, the activation of SCs observed in denervated levator ani muscle does not occur in castrated rats, and this correlates with a reduction in myonuclear number accompanying the atrophy observed in denervated muscles of castrated rats (Nnodim, 1999). These observations suggest that at least in specific muscles and under specific atrophic conditions, the control of SC activity by androgens may contribute to fiber homeostasis.
NF-κB Signaling
A large body of evidence has implicated the NF-κB transcription factors as being major modulators of muscle mass (Li et al., 2008; Peterson et al., 2011). When inactive, NF-κB is maintained in the cytoplasm by a class of proteins called IκB. In response to inflammatory cytokines, such as TNFα, IκB is degraded leading to the nuclear translocation of NF-κB and activation of NF-κB-dependent transcription (Li et al., 2008; Peterson et al., 2011). An increase in NF-κB signaling has been reported in the atrophic response associated with different disease conditions. NF-κB signaling has been shown to mediate atrophy by increasing the expression of components of the ubiquitin-proteasome system, and by promoting the atrophic effects of inflammation-related proteins (Li et al., 2008; Peterson et al., 2011). More importantly, an increasing number of observations suggest that NF-κB also regulates SC activity in different atrophic conditions. Specifically, the presence of systemic inflammation accompanying clinical features associated with cachexia led to the discovery of a cytokine-induced up-regulation of NF-κB signaling in the suppression of myogenic gene expression in cell culture (Guttridge et al., 2000). Moreover, a detailed examination of events within muscles from tumor-bearing mice revealed that the induction of NF-κB activity by cachectic serum contributed to Pax7 dysregulation in muscle-resident progenitors, and was followed by a significant decline in muscle mass (He et al., 2013). Although these observations do not distinguish between increases in NF-κB levels in SCs vs. fibers, there is a clear indication that together with functioning as a potent modulator of muscle regeneration and myogenic differentiation, NF-κB signaling promotes atrophy, an effect which is achieved in part by altering the behavior of SCs (Langen et al., 2001; Dogra et al., 2006; Mourkioti et al., 2006; Wang et al., 2007; Bakkar et al., 2008).
Sirt1 and Mitochondrial Dysfunction
Mitochondrial function and metabolism is crucial for SC activation, proliferation, and for efficient muscle regeneration (Jash and Adhya, 2012; Rodgers et al., 2014). A reduction in mitochondrial mass, increased damage to mitochondrial DNA, and increased levels of reactive oxygen species (ROS) produced by the existing mitochondria were observed with age (Minet and Gaster, 2012; Wang et al., 2013). Sirt1 is a potent regulator of mitochondrial metabolism, displaying altered expression in tumor-bearing animals and contributing to reduced regeneration in muscle wasting (Toledo et al., 2011). Intriguingly, an increase in Sirt1function is one of the many outcomes of caloric restriction, that also include an increase in SC proliferation, an increase in mitochondrial abundance and an enhancement of the regenerative capacities of muscles (Lee et al., 1998; Cohen et al., 2004; Cerletti et al., 2012; McKiernan et al., 2012). These observations support a possible role for Sirt1 in reducing muscle loss that occurs with aging. Indeed, Sirt1 overexpression has been reported to block fasting and denervation-induced fiber atrophy by reducing FoxO activity (Lee and Goldberg, 2013). Moreover, Sirt1 controls the transcription of peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC1α), which in turn induces mitochondrial biogenesis and regulates peroxisome proliferator-activated receptors gamma (PPARδ), a positive regulator of SC proliferation (Amat et al., 2009; Angione et al., 2011). Consistent with this observation, over-expression of Sirt1 increases the proliferation of myogenic progenitors (Rathbone et al., 2009). Moreover, as a consequence of ablating Sirt1 in SCs, myogenic progenitors undergo premature differentiation, thereby negatively affecting muscle regeneration and growth (Ryall et al., 2015). Although additional mechanistic studies are required, this body of evidence suggests that the Sirt1-PGC1α axis could ameliorate the loss of muscle mass at least in part by improving mitochondrial function and the regenerative potential of SCs.
Notch Signaling
The importance of Notch signaling during muscle development and regeneration has been well established (Luo et al., 2005; Vasyutina et al., 2007a; Mourikis and Tajbakhsh, 2014). Notch signaling plays an important role in maintaining quiescence, proliferation, homing and self-renewal capabilities of SCs (Conboy and Rando, 2002; Bjornson et al., 2012; Bröhl et al., 2012; Mourikis et al., 2012b; Wen et al., 2012; Gopinath et al., 2014). Notch signaling is essential to maintain muscle progenitors during fetal development, to promote their expansion, and to generate SCs (Vasyutina et al., 2007b; Mourikis et al., 2012a). Indeed, developmental inactivation of the Notch transcriptional complex by the selective ablation of the Notch regulators, recombination signal binding protein J (RBP-J) or mastermind-like (MAML1), specifically in the myogenic compartment resulted in the reduction of muscle mass (Vasyutina et al., 2007b; Lin et al., 2013).
Aging muscles consist of SCs with decreased regenerative potential, which in turn is an outcome of reduced Notch signaling, and can be restored by exposure to a young systemic environment (Conboy et al., 2003, 2005). Intriguingly, it was also reported that the consequences of Vitamin D depletion in aged rats exacerbated the muscle loss associated with aging and was accompanied by reduced Notch activity in these muscles (Domingues-Faria et al., 2014). At this point it remains to be established whether Vitamin D exerts its effects on skeletal muscle through the inhibition of the Notch signaling pathway. Additionally, it remains to be demonstrated whether enhancing Notch function in the Vitamin D-depleted aged rats display possible beneficial effects on muscle mass, in addition to the involvement of SCs in this context. Intriguingly, the observation that Notch activation was able to abrogate the inhibitory effects of the cachectic factor Ang ll on proliferation of myogenic progenitors in culture raises the possibility of using positive modulators of Notch signaling to boost SC proliferation and counteract muscle loss (Yoshida et al., 2013).
However, recent literature suggests that the outcome of manipulating Notch activity has been met with incongruous results. Using a Notch reporter mouse, it was demonstrated that SCs isolated from mdx mice displayed reduced activation of Notch signaling, and that constitutive Notch activation could at least in part rescue the self-renewal deficit observed in mdx SCs, without ameliorating muscle pathology associated with these dystrophic mice (Jiang et al., 2014). On the other hand, a recent study observed that acute manipulation of Notch signaling by the injection of activators or inhibitors did not affect muscle mass or maximal force in mdx mice, as well as mice that were double-deficient for Utrophin and Dystrophin (Church et al., 2014). These observations suggest that while SCs display a stage-specific requirement for Notch signaling, dystrophic muscles present a complex environment with SCs at different stages of progression and consequently exhibit different responses to Notch manipulation that may not contribute to a net increase in muscle weight. These studies underscore the importance of not only investigating specific signaling pathways in the context of specific forms of atrophy, but also addressing the efficacy of manipulating a particular signaling mechanism for therapeutic purposes.
Reversal of Atrophy: A Role for SCs?
The primary objective of obtaining a comprehensive understanding of the mechanistic processes underlying atrophy in a manner that discerns molecular changes in SCs from those in fibers, is to address whether manipulating these signaling mechanisms can enhance the participation of SCs in restoring muscle mass. In this section, we review those studies in which SC activity has been modulated to expand their functionality in muscle wasting accompanying chronic diseases. Broadly, the focus of these interventions has been signaling pathways that modulate SC proliferation, differentiation, senescence and survival.
In muscle wasting accompanying COPD, patients experience decreased oxygen saturation level (hypoxemia) that can elicit hypoxic responses in tissues (Wüst and Degens, 2007). This includes an impairment in anabolic pathways, decrease in food intake by the induction of leptin, and muscle disuse that are directly responsible for a loss in muscle mass (Wüst and Degens, 2007). In studies aimed to increase the protein synthesis pathway in SCs under conditions of hypoxia-induced atrophy, a regimen of alternating treatments with hepatocyte growth factor (HGF) and leukemia inhibitory factor (LIF) not only increased SC proliferation, but also increased the cross sectional area of fibers and total muscle weight (Hauerslev et al., 2014). Corroborating these results in normoxic mice, this growth factor treatment promoted SC proliferation and increased the weight of the tibialis anterior muscle in mice deleted for Myostatin expression (Hauerslev et al., 2014). These results further highlight the potential of exploring Myostatin regulation in SCs for therapeutic purposes in combatting atrophy.
Strategies aimed at enhancing SC proliferation to increase their participation in muscle mass restoration have been particularly useful in limb-girdle muscular dystrophy type 1B (LGMD1B) and AD-EDMD forms of dystrophies. Under these conditions, increased levels of lamina-associated polypepetide alpha (Lap2α), a protein that interacts with Lamin A/C and phosphorylated Rb has been implicated in reducing SC proliferation (Mancini et al., 1994; Ozaki et al., 1994). Since the Lap2α−/− cells are hyperproliferative due to a defect in cell cycle exit, the authors created a Lmna−/− Lap2α−/− double KO mice to enhance SC proliferation, thereby resulting in an increase in the fusion index and overall muscle size of the double KOs (Cohen et al., 2013). This study not only offers valuable insights into the etiology of laminopathies but also provides alternate strategies using SCs for therapeutic intervention.
Contrary to the atrophic conditions discussed above, the increase in SC numbers in the interstitium in cancer-induced cachexia prompted the use of mutant mouse models to inactivate Pax7 expression in tumor-bearing mice (He et al., 2013). Not only did this cause a reversal of wasting by promoting cell differentiation and fusion with injured fibers, but also demonstrated that impaired myogenic progression by sustained Pax7 expression was the primary cause for muscle wasting in these mice, and offered the attractive possibility of gene therapy approaches to modulate Pax7 expression (He et al., 2013). In particular, the identification of regulators that repress Pax7 expression to limit its window of action and allow for myogenic progression during the course of normal regeneration can be potentially exploited for therapeutic purposes. Indeed, the activation of the Polycomb Repressive Complex 2 (PRC2) by p38α kinase results in the formation of repressive chromatin on the Pax7 locus, thereby providing an additional interventional target that could be explored in a tumor-promoting milieu (Palacios et al., 2010).
In disuse atrophy, the focus of various interventional studies have been on regulators that enhance SC functionality and increase muscle growth, especially in the subsequent recovery phase. In a recent study, E3 ubiquitin ligase tripartite motif-containing 32 (TRIM32) was demonstrated to be essential for the selective regrowth of Type 2 fast fibers after hind limb suspension-induced atrophy (Kudryashova et al., 2012). TRIM32-deficient myoblasts displayed impaired differentiation, and elevated levels of senescence-associated β-galactosidase (β-gal; Kudryashova et al., 2012). Premature senescence of SCs was also demonstrated to be the underlying cause for the pathogenic features associated with limb-girdle muscular dystrophy 2H (LGMD2H), that arise from mutations in TRIM32 (Saccone et al., 2008; Cossée et al., 2009). These studies suggested that unlike other E3 ubiquitin ligases that promote atrophy, TRIM32 might possess a unique function of preventing premature senescence in SCs, thereby enhancing muscle growth. Although these speculations support the idea of an involvement of SCs, more investigations are required to explore the mechanisms by which TRIM32 could promote regrowth after atrophy. Indeed, the extent to which SCs are required in the process of recovery from hind limb suspension is still unclear. Ablation studies indicate that muscle mass recovery after hind limb suspension could also occur in the absence of SCs (Jackson et al., 2012). Nevertheless, during the recovery phase of soleus muscle mass upon its reloading, the decrease in myonuclear content derived from hind limb unloading was restored to control levels, suggesting that myogenic precursor cells can proliferate and fuse with myofibers during the recovery process (Mitchell and Pavlath, 2001). Moreover, after an initial phase of muscle regrowth, inhibiting the proliferation of muscle precursor cells by irradiation prevented a full recovery (Mitchell and Pavlath, 2001). As such, it would be crucial to quantify the relative contributions of SC-mediated and myofiber-mediated processes, in addition to investigating molecular events that distinguish between SC-intrinsic and fiber-intrinsic processes during muscle recovery.
IGF-1 is another factor that has been shown to modulate SC activity and has been reported to be beneficial in promoting regrowth after muscle unloading. In a study aimed to investigate the effects of IGF-1 overexpression on the recovery of muscle size during ambulation after cast immobilization, it was observed that viral-mediated IGF-1 transfer to skeletal muscle enhanced regeneration (Stevens-Lapsley et al., 2010). Intriguingly, IGF-1 overexpression did not protect against cast immobilization-induced muscle atrophy, indicating that there are different mechanisms regulating muscle mass during unloading and reloading (Stevens-Lapsley et al., 2010). Delivery of IGF-1 into muscle and muscle-specific overexpression of IGF-1 were also beneficial in ameliorating sarcopenia and stimulating recovery in immobilized old muscles (Barton-Davis et al., 1998; Chakravarthy et al., 2000; Musarò et al., 2001). Moreover, increased levels of IGF-1 in muscle has been shown to control SC activity and reduce muscle wasting in different genetic disorders, including muscular dystrophy and amyotrophic lateral sclerosis (ALS; Barberi et al., 2009).
A crucial aspect underlying the enhancement of SC functionality for cellular therapy is the investigation of mechanisms that specifically promote cell survival without affecting proliferation, in order to avoid the risk of cancer-promoting effects. A growth factor-derived engineered protein, Magic-Factor 1 (or Met-Activating Genetically Improved Chimeric Factor 1), has been developed to elicit the activation of the Akt survival pathway, but not the mitogenic ERK pathway. Magic-Factor 1 decreased the expression of Myostatin and apoptotic markers in myogenic cells in vitro. Consistent with these observations, Magic-Factor 1 promoted survival and differentiation (Cassano et al., 2008). Moreover, transgenic mice expressing muscle-specific Magic-factor 1 displayed hypertrophic fast twitch fibers with increased endurance to exercise, in addition to partially rescuing the degeneration observed in α-sarcoglycan KO mice (Cassano et al., 2008). Thus, tissue-specific engineered proteins hold potent clinical applications for ameliorating muscle wasting associated with dystrophies.
In addition to modulating specific signaling pathways, exercise and electrical stimulation appear to be promising therapeutic approaches in countering atrophy. Exercise has been proven to be effective in reducing muscle loss and in mobilizing SCs in aging muscles (Snijders et al., 2009). Concomitant to the increase in SC content, an up-regulation of myogenic regulatory factors and a reduction in Myostatin expression have been observed (Snijders et al., 2009). The beneficial effects of exercise have also been reported in disuse atrophy occurring with immobilization, and correlate with an increase in IGF-1 and Myogenin, and a decrease in Myostatin levels (Adams et al., 2007). Recent reports have demonstrated that the decrease in muscle size and SC number occurring during hind limb unloading could also be attenuated by electrical stimulation (Zhang et al., 2010b; Guo et al., 2012; Dirks et al., 2014). Although a causal relationship between SC activation and an attenuation in atrophy remain to be fully established, it seems that electrical stimulation can modify SC activity and prove beneficial to other forms of atrophy such as sarcopenia (Kern et al., 2014).
Another promising approach to ameliorate loss of muscle mass is cell therapy. Transplantation of SCs and other cellular types capable of myogenic differentiation have been shown to improve muscle phenotypes associated with different primary genetic diseases, especially in muscular dystrophies. In most of these studies, the rational of the cell therapy approach involves using cells to carry therapeutic genes into myofibers (Partridge and Davies, 1995). After transplantation, healthy donor-derived or patient-derived corrected cells fuse with existing myofibers and correct for the absence of expression from the mutated gene. The efficacy of this approach lies in the ability of the transplanted cells to not only differentiate into myofibers, but also to replenish the reservoir of stem cells and thereby sustain the repair process. Several studies have been exploring the potential of other stem cells besides SCs for therapeutic purposes (Peault et al., 2007). A complete and exhaustive discussion of these studies is beyond the scope of the present report and the readers are directed to comprehensive reviews that extensively describe cell transplantation approaches in primary genetic myopathies (Skuk and Tremblay, 2003; Price et al., 2007; Quattrocelli et al., 2010; Tedesco et al., 2010; Meng et al., 2011; Meregalli et al., 2013; Sirabella et al., 2013). We focus in this paragraph on acquired muscle wasting conditions. Recent observations suggest that a cell transplantation approach could be useful in reducing muscle loss after hind limb suspension and denervation (Plowman et al., 2014; Kim et al., 2015). Importantly, it has been reported that transplantation of SCs attached to their myofiber coupled with muscle injury could prevent the loss of muscle mass associated with aging (Hall et al., 2010). Intriguingly, transplantation in the absence of injury did not result in a similar increase in muscle mass (Hall et al., 2010). The interpretation of this observation is confounded by the high rate of lethality of donor cells generally associated with transplantation (Beauchamp et al., 1999). Nevertheless it supports the hypothesis that the authors of this study propose that unknown factors produced during injury can signal to fiber-associated SCs, and promote their long-term engraftment and hypertrophic function (Hall et al., 2010). In these set of experiments, fiber-associated SCs were transplanted into muscles of young mice and an increase in muscle mass was observed in 2-year-old mice (Hall et al., 2010). However, it remains to be tested whether heterologous transplantation of young fiber-associated SCs into injured muscles of old mice would also be beneficial in ameliorating sarcopenia. A large body of evidence indicates that during aging, local and systemic environments undergo profound changes that bear a negative impact on muscle precursor cell activity and regenerative potential (Gopinath and Rando, 2008), thus proving to be a major hurdle in transplantation approaches in aged muscles. Intriguingly, a recent study demonstrated that inhibiting p38α and p38β signaling transiently in myogenic progenitors isolated from aged mice, in conjunction with culture on soft hydrogel substrates rejuvenates their regenerative potential and increases muscle strength upon transplantation into damaged muscles of aged mice (Cosgrove et al., 2014). Although it is not clearly understood as to how these mechanisms enhance the regenerative capacity of old SCs, these observations suggest that the detrimental effects of an aging muscle microenvironment can be circumvented. Changes in the local and systemic environments are not a feature of aging alone, but are also profoundly altered in other chronic muscle wasting conditions, such as cancer cachexia (He et al., 2013). Future studies will be required to evaluate the efficacy of cell transplantation approaches in ameliorating muscle loss under various atrophic stimuli.
Concluding Remarks
In conclusion, it is becoming increasingly evident that understanding the basic molecular mechanisms underlying various forms of atrophy is crucial to develop defined parameters and describe distinct features that categorize different forms of atrophy. It is also possible that different forms of atrophy could co-exist, such as disuse atrophy that accompanies aging coupled to bedridden conditions, as a result of chronic debilitating illnesses. Moreover, aging is a risk factor for many pathologies impacting muscle mass (Evans, 1995). Such multiple cause-related atrophies warrant the use of combinatorial therapies that selectively target the signaling pathway or pathways involved in specific atrophic processes. It has also become significantly clear that SC-focused studies expand the approaches that can be used to counter muscle loss. In general, despite a large body of evidence in favor of a role for SCs at least in some forms of atrophy, there have been reports that have restricted and thereby defined the window of SC action, or have evaluated SC functionality quantitatively rather than qualitatively. As such, these studies may have contributed to the “quasi-status” of SC involvement in atrophy. This, in addition to a knowledge of the molecular and cellular changes in SCs accompanying atrophy offer a powerful tool in being able to manipulate this compartment pharmacologically to increase muscle mass. In this context, SC-specific gene ablation studies in mice would provide a promising avenue to uncover novel signaling networks and thereby adopt a more focused approach towards tackling muscle wasting diseases in humans. These studies are currently being limited by difficulties in achieving a complete ablation of genes in a SC-specific manner, and the development of highly efficient strategies will be key in obtaining an accurate assessment of the requirement of the candidate genes during atrophy. A corollary to this approach is a comprehensive understanding of the systemic factors and the molecular milieu constituting the SC niche in the context of various atrophic conditions. In providing the means to modulate and eventually enhance SC function, this area of study renders itself to alternate therapeutic strategies such as nutritional interventions, other than exercise or pharmacological-based solutions (Alway et al., 2014). This is especially relevant in aging individuals with unrelated muscle wasting pathologies, wherein there are considerable limitations during the course of treatment. This shift in focus of examining molecular events during atrophy from fiber to SCs can potentially further be exploited even in scenarios that have reported limited involvement of the SC compartment in restoring muscle mass. A critical discussion of the cellular and molecular events operating in the atrophying muscles and SCs will contribute to the field for further studies investigating novel approaches to ameliorate muscle wasting diseases.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Satyajit Rath (National Institute of Immunology, Delhi), Atul Butte (Stanford University, California) and Antoine de Morrée (Stanford University, California), for stimulating discussions. This work was supported by the Telethon and Provincia autonoma di Trento—Italy (Grant n. TCP13007) to SB, and by the Department of Biotechnology, India (BT/MB/INDO-US/HIPC/05/2013-14) to SDG. We apologize to the authors of publications that we could not cite due to space restriction.
References
- Adams G. R., Haddad F., Bodell P. W., Tran P. D., Baldwin K. M. (2007). Combined isometric, concentric and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J. Appl. Physiol. (1985) 103, 1644–1654. 10.1152/japplphysiol.00669.2007 [DOI] [PubMed] [Google Scholar]
- Allen D. L., Monke S. R., Talmadge R. J., Roy R. R., Edgerton V. R. (1995). Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. (1985) 78, 1969–1976. [DOI] [PubMed] [Google Scholar]
- Allen D. L., Yasui W., Tanaka T., Ohira Y., Nagaoka S., Sekiguchi C., et al. (1996). Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J. Appl. Physiol. (1985) 81, 145–151. [DOI] [PubMed] [Google Scholar]
- Alway S., Myers M., Mohamed J. (2014). Regulation of satellite cell function in sarcopenia. Front. Aging Neurosci. 6:246. 10.3389/fnagi.2014.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat R., Planavila A., Chen S. L., Iglesias R., Giralt M., Villarroya F. (2009). SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284, 21872–21880. 10.1074/jbc.m109.022749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H., Otto A., Vulin A., Rochat A., Dumonceaux J., Garcia L., et al. (2009). Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc. Natl. Acad. Sci. U S A 106, 7479–7484. 10.1073/pnas.0811129106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreetta F., Bernasconi P., Baggi F., Ferro P., Oliva L., Arnoldi E., et al. (2006). Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. J. Neuroimmunol. 175, 77–86. 10.1016/j.jneuroim.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Angione A. R., Jiang C., Pan D., Wang Y. X., Kuang S. (2011). PPARδ regulates satellite cell proliferation and skeletal muscle regeneration. Skelet. Muscle 1:33. 10.1186/2044-5040-1-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker S. D., Negassa A., Coats A. J., Afzal R., Poole-Wilson P. A., Cohn J. N., et al. (2003). Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 361, 1077–1083. 10.1016/s0140-6736(03)12892-9 [DOI] [PubMed] [Google Scholar]
- Bakkar N., Wang J., Ladner K. J., Wang H., Dahlman J. M., Carathers M., et al. (2008). IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J. Cell Biol. 180, 787–802. 10.1083/jcb.200707179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi L., Dobrowolny G., Pelosi L., Giacinti C., Musarò A. (2009). Muscle involvement and IGF-1 signaling in genetic disorders: new therapeutic approaches. Endocr. Dev. 14, 29–37. 10.1159/000207474 [DOI] [PubMed] [Google Scholar]
- Barton-Davis E. R., Shoturma D. I., Musaro A., Rosenthal N., Sweeney H. L. (1998). Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. U S A 95, 15603–15607. 10.1073/pnas.95.26.15603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J. R., Morgan J. E., Pagel C. N., Partridge T. A. (1999). Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 144, 1113–1122. 10.1083/jcb.144.6.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S., Miyabara E. H., Gopinath S. D., Carlig P. M., Rando T. A. (2014). A Wnt-TGFβ2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci. Transl. Med. 6:267ra176. 10.1126/scitranslmed.3008411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biressi S., Molinaro M., Cossu G. (2007). Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 308, 281–293. 10.1016/j.ydbio.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Bjornson C. R. R., Cheung T. H., Liu L., Tripathi P. V., Steeper K. M., Rando T. A. (2012). Notch signaling is necessary to maintain quiescence in adult stem cells. Stem Cells 30, 232–242. 10.1002/stem.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw B., Canato M., Agatea L., Toniolo L., Mammucari C., Masiero E., et al. (2009). Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 23, 3896–3905. 10.1096/fj.09-131870 [DOI] [PubMed] [Google Scholar]
- Blaauw B., Reggiani C. (2014). The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 35, 3–10. 10.1007/s10974-014-9376-y [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C., Pavlath G. K. (1983). Defective myoblasts identified in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. U S A 80, 4856–4860. 10.1073/pnas.80.15.4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine S. C., Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., et al. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019. 10.1038/ncb1101-1014 [DOI] [PubMed] [Google Scholar]
- Bogdanovich S., Krag T. O., Barton E. R., Morris L. D., Whittemore L.-A., Ahima R. S., et al. (2002). Functional improvement of dystrophic muscle by myostatin blockade. Nature 420, 418–421. 10.1038/nature01154 [DOI] [PubMed] [Google Scholar]
- Bonaldo P., Sandri M. (2013). Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39. 10.1242/dmm.010389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov A. B., Dedkov E. I., Carlson B. M. (2005). Abortive myogenesis in denervated skeletal muscle: differentiative properties of satellite cells, their migration and block of terminal differentiation. Anat. Embryol. (Berl) 209, 269–279. 10.1007/s00429-004-0429-7 [DOI] [PubMed] [Google Scholar]
- Brack A. S., Bildsoe H., Hughes S. M. (2005). Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 118, 4813–4821. 10.1242/jcs.02602 [DOI] [PubMed] [Google Scholar]
- Brack A. S., Rando T. A. (2007). Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 3, 226–237. 10.1007/s12015-007-9000-2 [DOI] [PubMed] [Google Scholar]
- Braun T. P., Marks D. L. (2015). The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 6:12. 10.3389/fphys.2015.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink M., Wellen J., Delafontaine P. (1996). Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J. Clin. Invest. 97, 2509–2516. 10.1172/jci118698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröhl D., Vasyutina E., Czajkowski M. T., Griger J., Rassek C., Rahn H. P., et al. (2012). Colonization of the satellite cell niche by skeletal muscle progenitor cells depends on Notch signals. Dev. Cell 23, 469–481. 10.1016/j.devcel.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Brooks N. E., Myburgh K. H. (2014). Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 5:99. 10.3389/fphys.2014.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey K. A., Farnfield M. M., Tarquinio S. D., Cameron-Smith D. (2007). Impaired expression of Notch signaling genes in aged human skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 62, 9–17. 10.1093/gerona/62.1.9 [DOI] [PubMed] [Google Scholar]
- Cassano M., Biressi S., Finan A., Benedetti L., Omes C., Boratto R., et al. (2008). Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS One 3:e3223. 10.1371/journal.pone.0003223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti M., Jang Y. C., Finley L. W. S., Haigis M. C., Wagers A. J. (2012). Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell 10, 515–519. 10.1016/j.stem.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy M. V., Davis B. S., Booth F. W. (2000). IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J. Appl. Physiol. (1985) 89, 1365–1379. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zajac J. D., MacLean H. E. (2005). Androgen regulation of satellite cell function. J. Endocrinol. 186, 21–31. 10.1677/joe.1.05976 [DOI] [PubMed] [Google Scholar]
- Cheung T. H., Quach N. L., Charville G. W., Liu L., Park L., Edalati A., et al. (2012). Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482, 524–528. 10.1038/nature10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J. E., Trieu J., Chee A., Naim T., Gehrig S. M., Lamon S., et al. (2014). Alterations in Notch signaling skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne Muscular dystrophy. Exp. Physiol. 99, 675–687. 10.1113/expphysiol.2013.077255 [DOI] [PubMed] [Google Scholar]
- Ciciliot S., Rossi A., Dyar K. A., Blaauw B., Schiaffino S. (2013). Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 45, 2191–2199. 10.1016/j.biocel.2013.05.016 [DOI] [PubMed] [Google Scholar]
- Clever J. L., Sakai Y., Wang R. A., Schneider D. B. (2010). Inefficient skeletal muscle repair in inhibitor of differentiation knockout mice suggests a crucial role for BMP signaling during adult muscle regeneration. Am. J. Physiol. Cell Physiol. 298, C1087–C1099. 10.1152/ajpcell.00388.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T. V., Gnocchi V. F., Cohen J. E., Aditi P., Liu H., Ellis J. A., et al. (2013). Defective skeletal muscle growth in lamin A/C-deficient mice is rescued by loss of lap2α. Hum. Mol. Genet. 22, 2852–2869. 10.1093/hmg/ddt135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. Y., Miller C., Bitterman K. J., Wall N. R., Hekking B., Kessler B., et al. (2004). Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392. 10.1126/science.1099196 [DOI] [PubMed] [Google Scholar]
- Cohen S., Nathan J. A., Goldberg A. L. (2015). Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74. 10.1038/nrd4467 [DOI] [PubMed] [Google Scholar]
- Cohn R. D., van Erp C., Habashi J. P., Soleimani A. A., Klein E. C., Lisi M. T., et al. (2007). Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210. 10.1038/nm1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti D., Moresi V., Adamo S., Molinaro M., Sassoon D. (2005). Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43, 120–128. 10.1002/gene.20160 [DOI] [PubMed] [Google Scholar]
- Conboy I. M., Conboy M. J., Smythe G. M., Rando T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577. 10.1126/science.1087573 [DOI] [PubMed] [Google Scholar]
- Conboy I. M., Conboy M. J., Wagers A. J., Girma E. R., Weissman I. L., Rando T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- Conboy I. M., Rando T. A. (2002). The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409. 10.1016/s1534-5807(02)00254-x [DOI] [PubMed] [Google Scholar]
- Cosgrove B. D., Gilbert P. M., Porpiglia E., Mourkioti F., Lee S. P., Corbel S. Y., et al. (2014). Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264. 10.1038/nm.3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossée M., Lagier-Tourenne C., Seguela C., Mohr M., Leturcq F., Gundesli H., et al. (2009). Use of SNP array analysis to identify a novel TRIM32 mutation in limb-girdle muscular dystrophy type 2H. Neuromuscul. Disord. 19, 255–260. 10.1016/j.nmd.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Cossu G., Biressi S. (2005). Satellite cells, myoblasts and other occasional myogenic progenitors: possible origin, phenotypic features and role in muscle regeneration. Semin. Dev. Biol. 16, 623–631. 10.1016/j.semcdb.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Creutzberg E. C., Wouters E. F., Mostert R., Pluymers R. J., Schols A. M. (2003). A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest 124, 1733–1742. 10.1378/chest.124.5.1733 [DOI] [PubMed] [Google Scholar]
- Crossland H., Constantin-Teodosiu D., Gardiner S. M., Constantin D., Greenhaff P. L. (2008). A potential role for Akt / FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J. Physiol. 22, 5589–5600. 10.1113/jphysiol.2008.160150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr K. C., Schultz E. (1989). Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J. Appl. Physiol. (1985) 67, 1827–1834. [DOI] [PubMed] [Google Scholar]
- Dasarathy S., Dodig M., Muc S. M., Kalhan S. C., McCullough A. J. (2004). Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1124–G1130. 10.1152/ajpgi.00202.2004 [DOI] [PubMed] [Google Scholar]
- Day M. K., Allen D. L., Mohajerani L., Greenisen M. C., Roy R. R., Edgerton V. R. (1995). Adaptations of human skeletal muscle fibers to spaceflight. J. Gravit. Physiol. 2, P47–P50. [PubMed] [Google Scholar]
- Delaporte C., Dehaupas M., Fardeau M. (1984). Comparison between the growth pattern of cell cultures from normal and Duchenne dystrophy muscle. J. Neurol. Sci. 64, 149–160. 10.1016/0022-510x(84)90033-9 [DOI] [PubMed] [Google Scholar]
- Dirks M. L., Wall B. T., Snijders T., Ottenbros C. L., Verdijk L. B., van Loon L. J. (2014). Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. (Oxf) 210, 628–641. 10.1111/apha.12200 [DOI] [PubMed] [Google Scholar]
- Dogra C., Changotra H., Mohan S., Kumar A. (2006). Tumor necrosis factor-like weak inducer of apoptosis inhibits skeletal myogenesis through sustained activation of nuclear factor-kappaB and degradation of MyoD protein. J. Biol. Chem. 281, 10327–10336. 10.1074/jbc.m511131200 [DOI] [PubMed] [Google Scholar]
- Domingues-Faria C., Chanet A., Salles J., Berry A., Giraudet C., Patrac V., et al. (2014). Vitamin D deficiency down-regulates Notch pathway contributing to skeletal muscle atrophy in old wistar rats. Nutr. Metab. (Lond) 11:47. 10.1186/1743-7075-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Pan J. S., Zhang L. (2013). Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction. PLoS One 8:e58554. 10.1371/journal.pone.0058554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler K., Mittelbronn M., Bornemann A. (2008). Myogenesis in human denervated muscle biopsies. Muscle Nerve 37, 79–83. 10.1002/mus.20902 [DOI] [PubMed] [Google Scholar]
- Dubois V., Laurent M., Boonen S., Vanderschueren D., Claessens F. (2012). Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci. 69, 1651–1667. 10.1007/s00018-011-0883-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois V., Laurent M. R., Sinnesael M., Cielen N., Helsen C., Clinckemalie L., et al. (2014). A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 28, 2979–2994. 10.1096/fj.14-249748 [DOI] [PubMed] [Google Scholar]
- Eason J. M., Dodd S. L., Powers S. K. (2003). Use of anabolic steroids to attenuate the effects of glucocorticoids on the rat diaphragm. Phys. Ther. 83, 29–36. [PubMed] [Google Scholar]
- Edström E., Ulfhake B. (2005). Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 4, 65–77. 10.1111/j.1474-9728.2005.00145.x [DOI] [PubMed] [Google Scholar]
- Egerman M. A., Glass D. J. (2014). Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 49, 59–68. 10.3109/10409238.2013.857291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. J. (1995). What is sarcopenia? J. Gerontol. A Biol. Sci. Med. Sci. 50, 5–8. 10.1093/gerona/50A.Special_Issue.5 [DOI] [PubMed] [Google Scholar]
- Evans W. J., Morley J. E., Argilés J., Bales C., Baracos V., Guttridge D., et al. (2008). Cachexia: a new definition. Clin. Nutr. 27, 793–799. 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- Fanzani A., Conraads V. M., Penna F., Martinet W. (2012). Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J. Cachexia Sarcopenia Muscle 3, 163–179. 10.1007/s13539-012-0074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon K. C. H., Glass D. J., Guttridge D. C. (2012). Cancer cachexia: mediators, signaling and metabolic pathways. Cell Metab. 16, 153–166. 10.1016/j.cmet.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Friedrichs M., Wirsdöerfer F., Flohé S. B., Schneider S., Wuelling M., Vortkamp A. (2011). BMP signaling balances proliferation and differentiation of muscle satellite cell descendants. BMC Cell Biol. 12:26. 10.1186/1471-2121-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. S., Lee J. D., Jackson J. R., Kirby T. J., Stasko S. A., Liu H., et al. (2014). Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 28, 1654–1665. 10.1096/fj.13-239426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. S., Lee J. D., Mula J., Kirby T. J., Jackson J. R., Liu F., et al. (2015). Inducible depletion of satellite cells in adult , sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21, 76–80. 10.1038/nm.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayraud-Morel B., Chrétien F., Flamant P., Gomès D., Zammit P. S., Tajbakhsh S. (2007). A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 312, 13–28. 10.1016/j.ydbio.2007.08.059 [DOI] [PubMed] [Google Scholar]
- George R. M., Biressi S., Beres B. J., Rogers E., Mulia A. K., Allen R. E., et al. (2013). Numb-deficient satellite cells have regeneration and proliferation defects. Proc. Natl. Acad. Sci. U S A 110, 18549–18554. 10.1073/pnas.1311628110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson H., Schakman O., Kalista S., Lause P., Tsuchida K., Thissen J. P. (2009). Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am. J. Physiol. Endocrinol. Metab. 297, E157–E164. 10.1152/ajpendo.00193.2009 [DOI] [PubMed] [Google Scholar]
- Glass D. (2005). Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 37, 1974–1984. 10.1016/j.biocel.2005.04.018 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cadavid N. F., Taylor W. E., Yarasheski K., Sinha-Hikim I., Ma K., Ezzat S., et al. (1998). Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc. Natl. Acad. Sci. U S A 95, 14938–14943. 10.1073/pnas.95.25.14938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. A., Hornberger T. A. (2014). New roles for Smad signaling and phosphatidic acid in the regulation of skeletal muscle mass. F1000Prime Rep. 6:20. 10.12703/P6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S. D., Rando T. A. (2008). Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7, 590–598. 10.1111/j.1474-9726.2008.00399.x [DOI] [PubMed] [Google Scholar]
- Gopinath S. D., Webb A. E., Brunet A., Rando T. A. (2014). FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports 2, 414–426. 10.1016/j.stemcr.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K., Bruusgaard J. C. (2008). Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J. Physiol. 586, 2675–2681. 10.1113/jphysiol.2008.154369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B. S., Cheung K. K., Yeung S. S., Zhang B. T., Yeung E. W. (2012). Electrical stimulation influences satellite cell proliferation and apoptosis in unloading-induced muscle atrophy in mice. PLoS One 7:e30348. 10.1371/journal.pone.0030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge D. C., Mayo M. W., Madrid L. V., Wang C. Y., Baldwin A. S., Jr. (2000). NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289, 2363–2366. 10.1126/science.289.5488.2363 [DOI] [PubMed] [Google Scholar]
- Haddad F., Zaldivar F., Cooper D. M., Adams G. R. (2005). IL-6-induced skeletal muscle atrophy IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. (1985) 98, 911–917. 10.1152/japplphysiol.01026.2004 [DOI] [PubMed] [Google Scholar]
- Hall J. K., Banks G. B., Chamberlain J. S., Olwin B. B. (2010). Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci. Transl. Med. 2:57ra83. 10.1126/scitranslmed.3001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauerslev S., Vissing J., Krag T. O. (2014). Muscle atrophy reversed by growth factor activation of satellite cells in a mouse muscle atrophy model. PLoS One 9:e100594. 10.1371/journal.pone.0100594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W. A., Berardi E., Cardillo V. M., Acharyya S., Aulino P., Thomas-Ahner J., et al. (2013). NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J. Clin. Invest. 123, 4821–4835. 10.1172/jci68523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida R. S., Van Nostran S., Murray J. D., Staron R. S., Gordon S. E., Kraemer W. J. (1997). Myonuclear loss in atrophied soleus muscle fibers. Anat. Rec. 247, 350–354. [DOI] [PubMed] [Google Scholar]
- Hughes S. M., Taylor J. M., Tapscott S. J., Gurley C. M., Carter W. J., Peterson C. A. (1993). Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 118, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Jackson J. R., Mula J., Kirby T. J., Fry C. S., Lee J. D., Ubele M. F., et al. (2012). Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am. J. Physiol. Cell Physiol. 303, C854–C861. 10.1152/ajpcell.00207.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y. C., Sinha M., Cerletti M., Dall’Osso C., Wagers A. J. (2011). Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb. Symp. Quant. Biol. 76, 101–111. 10.1101/sqb.2011.76.010652 [DOI] [PubMed] [Google Scholar]
- Jash S., Adhya S. (2012). Induction of muscle regeneration by RNA-mediated mitochondrial restoration. FASEB J. 26, 4187–4197. 10.1096/fj.11-203232 [DOI] [PubMed] [Google Scholar]
- Jasmin G., Tautu C., Vanasse M., Brochu P., Simoneau R. (1984). Impaired muscle differentiation in explant cultures of Duchenne muscular dystrophy. Lab. Invest. 50, 197–207. 6694359 [Google Scholar]
- Jiang C., Wen Y., Kuroday K., Hannon K., Rudnicki M. A., Kuang S. (2014). Notch signaling deficiency underlies age-dependent depletion of satellite cells in muscular dystrophy. Dis. Model. Mech. 7, 997–1004. 10.1242/dmm.015917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambadur R., Sharma M., Smith T. P. L., Bass J. J. (1997). Mutations in myostatin (GDF8) in double-muscled belgian blue and piedmontese cattle. Genome Res. 7, 910–915. [DOI] [PubMed] [Google Scholar]
- Kamei Y., Miura S., Suzuki M., Kai Y., Mizukami J., Mochida K., et al. (2004). Metabolism and Bioenergetics: Skeletal Muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass , down-regulated Type I (slow twitch/red muscle) fiber genes and impaired glycemic control. J. Biol. Chem. 279, 41114–41123. 10.1074/jbc.M400674200 [DOI] [PubMed] [Google Scholar]
- Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., et al. (1994). Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1755–1766. 10.1083/jcb.127.6.1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe A. C., Lawson J. A., Flygare S. D., Fox Z. D., Colasanto M. P., Mathew S. J., et al. (2015). Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 6:7087. 10.1038/ncomms8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern H., Barberi L., Löfler S., Sbardella S., Burggraf S., Fruhmann H., et al. (2014). Electrical stimulation counteracts muscle decline in seniors. Front. Aging Neurosci. 6:189. 10.3389/fnagi.2014.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J., Kim Y. M., Kim Z. H., Heo S. H., Kim S. M., Hwang J. W., et al. (2015). Mesenchymal stem cells suppress muscle atrophy induced by Hindlimb suspension. J. Stem Cell Res. Ther. 5:266 10.4172/2157-7633.1000266 [DOI] [Google Scholar]
- Koishi K., Zhang M., McLennan I. S., Harris A. J. (1995). MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev. Dyn. 202, 244–254. 10.1002/aja.1002020304 [DOI] [PubMed] [Google Scholar]
- Kollias H. D., McDermott J. C. (2008). Transforming growth factor-beta and myostatin signaling in skeletal muscle. J. Appl. Physiol. (1985) 104, 579–587. 10.1152/japplphysiol.01091.2007 [DOI] [PubMed] [Google Scholar]
- Kudryashova E., Kramerova I., Spencer M. J. (2012). Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J. Clin. Invest. 122, 1764–1776. 10.1172/JCI59581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani R., Bhasin S., Byhower F., Tarnuzzer R., Grant M., Shen R., et al. (2000). Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 167, 417–428. 10.1677/joe.0.1670417 [DOI] [PubMed] [Google Scholar]
- Langen R. C., Schols A. M., Kelders M. C. J., van der Velden J. L., Wouters E. F., Janssen-Heininger Y. M. (2006). Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 35, 689–696. 10.1165/rcmb.2006-0103oc [DOI] [PubMed] [Google Scholar]
- Langen R. C., Schols A. M., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M. (2001). Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 15, 1169–1180. 10.1096/fj.00-0463 [DOI] [PubMed] [Google Scholar]
- Langley B., Thomas M., Bishop A., Sharma M., Gilmour S., Kambadur R. (2002). Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277, 49831–49840. 10.1074/jbc.m204291200 [DOI] [PubMed] [Google Scholar]
- Lecker S. H., Jagoe R. T., Gilbert A., Gomes M., Baracos V., Bailey J., et al. (2004). Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51. 10.1096/fj.03-0610com [DOI] [PubMed] [Google Scholar]
- Lee C. M., Aspnes L. E., Chung S. S., Weindruch R., Aiken J. M. (1998). Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann. N Y Acad. Sci. 854, 182–191. 10.1111/j.1749-6632.1998.tb09901.x [DOI] [PubMed] [Google Scholar]
- Lee D., Goldberg A. L. (2013). SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 288, 30515–30526. 10.1074/jbc.M113.489716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Huynh T. V., Lee Y. S., Sebald S. M., Wilcox-Adelman S. A., Iwamori N., et al. (2012). Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc. Natl. Acad. Sci. U S A 109, E2353–E2360. 10.1073/pnas.1206410109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Lee Y. S., Zimmers T. A., Soleimani A., Matzuk M. M., Tsuchida K., et al. (2010). Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 24, 1998–2008. 10.1210/me.2010-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., McPherron A. C. (2001). Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. U S A 98, 9306–9311. 10.1073/pnas.151270098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerlotz K., Smith H. K. (2008). Role of MyoD in denervated, disused and exercised muscle. Muscle Nerve 38, 1087–1100. 10.1002/mus.21087 [DOI] [PubMed] [Google Scholar]
- Lepper C., Partridge T. A., Fan C. M. (2011). An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646. 10.1242/dev.067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J., Taylor C. C., Sjöström M. (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 84, 275–294. 10.1016/0022-510X(88)90132-3 [DOI] [PubMed] [Google Scholar]
- Li H., Malhotra S., Kumar A. (2008). Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. (Berl) 86, 1113–1126. 10.1007/s00109-008-0373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Shen H., Jin B., Gu Y., Chen Z., Cao C., et al. (2013). Blockade of Notch signaling in muscle stem cells causes muscular dystrophic phenotype and impaired regeneration. Stem Cells 31, 823–828. 10.1002/stem.1319 [DOI] [PubMed] [Google Scholar]
- Link B. A., Nishi R. (1997). Opposing effects of activin A and follistatin on developing skeletal muscle cells. Exp. Cell Res. 233, 350–362. 10.1006/excr.1997.3575 [DOI] [PubMed] [Google Scholar]
- Liu L., Rando T. A. (2011). Manifestations and mechanisms of stem cell aging. J. Cell Biol. 193, 257–266. 10.1083/jcb.201010131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yang Z., Liu C., Wang R., Tien P., Dale R., et al. (2007). Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Thera. 14, 945–952. 10.1038/sj.cgt.7701091 [DOI] [PubMed] [Google Scholar]
- Lounev V. Y., Ramachandran R., Wosczyna M. N., Yamamoto M., Maidment A. D. A., Shore E. M., et al. (2009). Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 91, 652–663. 10.2106/jbjs.h.01177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Renault V. M., Rando T. A. (2005). The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 16, 612–622. 10.1016/j.semcdb.2005.07.002 [DOI] [PubMed] [Google Scholar]
- MacDonald E. M., Cohn R. D. (2012). TGFη signaling: its role in fibrosis formation and myopathies. Curr. Opin. Rheumatol. 24, 628–634. 10.1097/bor.0b013e328358df34 [DOI] [PubMed] [Google Scholar]
- Mancini M. A., Shan B., Nickerson J. A., Penman S., Lee W. H. (1994). The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. U S A 91, 418–422. 10.1073/pnas.91.1.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791. 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- McCarthy J. J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A. B., et al. (2011). Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666. 10.1242/dev.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S., Thomas M., Maxwell L., Sharma M., Kambadur R. (2003). Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135–1147. 10.1083/jcb.200207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C., Hennebry A., Thomas M., Plummer E., Ling N., Sharma M., et al. (2008). Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp. Cell Res. 314, 317–329. 10.1016/j.yexcr.2007.09.012 [DOI] [PubMed] [Google Scholar]
- McKay B. R., Ogborn D. I., Bellamy L. M., Tarnopolsky M. A., Parise G. (2012). Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 26, 2509–2521. 10.1096/fj.11-198663 [DOI] [PubMed] [Google Scholar]
- McKiernan S. H., Colman R. J., Aiken E., Evans T. D., Beasley T. M., Aiken J. M., et al. (2012). Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp. Gerontol. 47, 229–236. 10.1016/j.exger.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M. A., Peluso G., Petillo O., Galderisi U., Cotrufo R. (1999). Defective growth in vitro of Duchenne Muscular Dystrophy myoblasts: the molecular and biochemical basis. J. Cell. Biochem. 76, 118–132. [DOI] [PubMed] [Google Scholar]
- Meng J., Muntoni F., Morgan J. E. (2011). Stem cells to treat muscular dystrophies–where are we? Neuromuscul. Disord. 21, 4–12. 10.1016/j.nmd.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Meregalli M., Farini A., Belicchi M., Parolini D., Cassinelli L., Razini P., et al. (2013). Perspectives of stem cell therapy in Duchenne muscular dystrophy. FEBS J. 280, 4251–4262. 10.1111/febs.12083 [DOI] [PubMed] [Google Scholar]
- Minet A. D., Gaster M. (2012). Cultured senescent myoblasts derived from human vastus lateralis exhibit normal mitochondrial ATP synthesis capacities with correlating concomitant ROS production while whole cell ATP production is decreased. Biogerontology 13, 277–285. 10.1007/s10522-012-9372-9 [DOI] [PubMed] [Google Scholar]
- Mitchell P. O., Pavlath G. K. (2001). A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am. J. Physiol. Cell Physiol. 281, C1706–C1715. [DOI] [PubMed] [Google Scholar]
- Mitchell P. O., Pavlath G. K. (2004). Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am. J. Physiol. Cell Physiol. 287, C1753–C1762. 10.1152/ajpcell.00292.2004 [DOI] [PubMed] [Google Scholar]
- Moresi V., Williams A. H., Meadows E., Flynn J. M., Potthoff M. J., McAnally J., et al. (2010). Myogenin and Class II HDACs control neurogenic muscle Atrophy by inducing E3 Ubiquitin Ligases. Cell 143, 35–45. 10.1016/j.cell.2010.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. (1971). Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 170, 421–435. 10.1002/ar.1091700405 [DOI] [PubMed] [Google Scholar]
- Mouisel E., Vignaud A., Hourdé C., Butler-Browne G., Ferry A. (2010). Muscle weakness and atrophy are associated with decreased regenerative capacity and changes in mTOR signaling in skeletal muscles of venerable (18–24-month-old) dystrophic mdx mice. Muscle Nerve 41, 809–818. 10.1002/mus.21624 [DOI] [PubMed] [Google Scholar]
- Mourikis P., Gopalakrishnan S., Sambasivan R., Tajbakhsh S. (2012a). Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development 139, 4536–4548. 10.1242/dev.084756 [DOI] [PubMed] [Google Scholar]
- Mourikis P., Sambasivan R., Castel D., Rocheteau P., Bizzarro V., Tajbaksh S. (2012b). A critical requirement for Notch signaling in maintenance of the quiescent skeletal muscle stem cell fate. Stem Cells 30, 243–252. 10.1002/stem.775 [DOI] [PubMed] [Google Scholar]
- Mourikis P., Tajbakhsh S. (2014). Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev. Biol. 14:2. 10.1186/1471-213x-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourkioti F., Kratsios P., Luedde T., Song Y.-H., Delafontaine P., Adami R., et al. (2006). Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass and promotes regeneration. J. Clin. Invest. 116, 2945–2954. 10.1172/jci28721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan J. S., Smith J. D., Chambers M. A., McLoughlin T. J., Reid M. B. (2008). TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am. J. Physiol. Cell Physiol. 295, C986–C993. 10.1152/ajpcell.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Cánoves P., Scheele C., Pedersen B. K., Serrano A. L. (2013). Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 280, 4131–4148. 10.1111/febs.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. M., Lawson J. A., Mathew S. J., Hutcheson D. A., Kardon G. (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637. 10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S. S., Murray E. J., Glackin C. A., Urist M. R. (1993). Bone morphogenetic protein inhibits differentiation and affects expression of helix-loop-helix regulatory molecules in myoblastic cells. J. Cell. Biochem. 53, 51–60. 10.1002/jcb.240530107 [DOI] [PubMed] [Google Scholar]
- Musarò A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., et al. (2001). Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195–200. 10.1038/84839 [DOI] [PubMed] [Google Scholar]
- Nnodim J. O. (1999). Quantitative study of the effects of denervation and castration on the levator ani muscle of the rat. Anat. Rec. 255, 324–333. [DOI] [PubMed] [Google Scholar]
- Ohsawa Y., Okada T., Nishimatsu S.-I., Ishizaki M., Suga T., Fujino M., et al. (2012). An inhibitor of transforming growth factor beta type I receptor ameliorates muscle atrophy in a mouse model of caveolin 3-deficient muscular dystrophy. Lab. Invest. 92, 1100–1114. 10.1038/labinvest.2012.78 [DOI] [PubMed] [Google Scholar]
- Olguin H. C., Olwin B. B. (2004). Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev. Biol. 275, 375–388. 10.1016/j.ydbio.2004.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Calhabeu F., Morgan J. E., Katagiri T., Amthor H., Zammit P. S. (2011). BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 18, 222–234. 10.1038/cdd.2010.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofre-Oliveira P. C. G., Santos A. L. F., Martins P. M., Ayub-Guerrieri D., Vainzof M. (2012). Differential expression of genes involved in the degeneration and regeneration pathways in mouse models for muscular dystrophies. Neuromolecular Med. 14, 74–83. 10.1007/s12017-012-8172-3 [DOI] [PubMed] [Google Scholar]
- Ozaki T., Saijo M., Murakami K., Enomoto H., Taya Y., Sakiyama S. (1994). Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene 9, 2649–2653. [PubMed] [Google Scholar]
- Palacios D., Mozzetta C., Consalvi S., Caretti G., Saccone V., Proserpio V., et al. (2010). TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7, 455–469. 10.1016/j.stem.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallafacchina G., Calabria E., Serrano A. L., Kalhovde J. M., Schiaffino S. (2002). A protein kinase B-dependent and rapamycin- sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. U S A 99, 9213–9218. 10.1073/pnas.142166599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A., Davies K. E. (1995). Myoblast-based gene therapies. Br. Med. Bull. 51, 123–137. [DOI] [PubMed] [Google Scholar]
- Peault B., Rudnicki M., Torrente Y., Cossu G., Tremblay J. P., Partridge T., et al. (2007). Stem and progenitor cells in skeletal muscle development, maintenance and therapy. Mol. Ther. 15, 867–877. 10.1038/mt.sj.6300145 [DOI] [PubMed] [Google Scholar]
- Penna F., Costamagna D., Fanzani A., Bonelli G., Baccino F. M., Costelli P. (2010). Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS One 5:e13604. 10.1371/journal.pone.0013604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. M., Bakkar N., Guttridge D. C. (2011). NF-κB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 96, 85–119. 10.1016/b978-0-12-385940-2.00004-8 [DOI] [PubMed] [Google Scholar]
- Plowman E. K., Bijangi-Vishehsaraei K., Halum S., Cates D., Hanenberg H., Domer A. S., et al. (2014). Autologous myoblasts attenuate atrophy and improve tongue force in a denervated tongue model: a pilot study. Laryngoscope 124, E20–E26. 10.1002/lary.24352 [DOI] [PubMed] [Google Scholar]
- Price F. D., Kuroda K., Rudnicki M. A. (2007). Stem cell based therapies to treat muscular dystrophy. Biochim. Biophys. Acta 1772, 272–283. 10.1016/j.bbadis.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Price F. D., von Maltzahn J., Bentzinger C. F., Dumont N. A., Yin H., Chang N. C., et al. (2014). Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181. 10.1038/nm.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocelli M., Cassano M., Crippa S., Perini I., Sampaolesi M. (2010). Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ. 17, 1222–1229. 10.1038/cdd.2009.160 [DOI] [PubMed] [Google Scholar]
- Rathbone C. R., Booth F. W., Lees S. J. (2008). FoxO3a preferentially induces p27Kip1 expression while impairing muscle precursor cell-cycle progression. Muscle Nerve 37, 84–89. 10.1002/mus.20897 [DOI] [PubMed] [Google Scholar]
- Rathbone C. R., Booth F. W., Lees S. J. (2009). Sirt1 increases skeletal muscle precursor cell proliferation. Eur. J. Cell Biol. 88, 35–44. 10.1016/j.ejcb.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. A., Sandesara P. B., Senf S. M., Judge A. R. (2012). Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J. 26, 987–1000. 10.1096/fj.11-189977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. T., King K. Y., Brett J. O., Cromie M. J., Charville G. W., Maguire K. K., et al. (2014). mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 509, 393–396. 10.1038/nature13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel C., Bodine S. C., Clarke B. A., Rossman R., Nunez L., Stitt T. N., et al. (2001). Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt /GSK3 pathways. Nat. Cell Biol. 3, 1009–1013. 10.1038/ncb1101-1009 [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. D., Yong D., Parry D. J. (1994). Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17, 608–613. 10.1002/mus.880170607 [DOI] [PubMed] [Google Scholar]
- Ryall J. G., Dell’Orso S., Derfoul A., Juan A., Zare H., Feng X., et al. (2015). The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory Epigenetics in skeletal muscle stem cells. Cell Stem Cell 16, 171–183. 10.1016/j.stem.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone V., Palmieri M., Passamano L., Piluso G., Meroni G., Politano L., et al. (2008). Mutations that impair interaction properties of TRIM32 associated with Limb-Girdle muscular Dystrophy 2H. Hum. Mutat. 29, 240–247. 10.1002/humu.20633 [DOI] [PubMed] [Google Scholar]
- Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., et al. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 4333–4333. 10.1242/dev.073601 [DOI] [PubMed] [Google Scholar]
- Sandri M. (2008). Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 23, 160–170. 10.1152/physiol.00041.2007 [DOI] [PubMed] [Google Scholar]
- Sandri M. (2013). Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 45, 2121–2129. 10.1016/j.biocel.2013.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Walsh K., et al. (2004). Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117, 399–412. 10.1016/s0092-8674(04)00400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Fulco M. (2004). Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci. STKE 2004:re11. 10.1126/stke.2442004re11 [DOI] [PubMed] [Google Scholar]
- Sartori R., Schirwis E., Blaauw B., Bortolanza S., Zhao J., Enzo E., et al. (2013). BMP signaling controls muscle mass. Nat. Genet. 45, 1309–1318. 10.1038/ng.2772 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314. 10.1111/febs.12253 [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Partridge T. (2008). Skeletal Muscle Repair and Regeneration. Netherlands: Springer. [Google Scholar]
- Schmalbruch H., al-Amood W. S., Lewis D. M. (1991). Morphology of long-term denervated rat soleus muscle and the effect of chronic electrical stimulation. J. Physiol. 441, 233–241. 10.1113/jphysiol.1991.sp018748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt T. L., Martignoni M. E., Bachmann J., Fechtner K., Friess H., Kinscherf R., et al. (2007). Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 85, 647–654. 10.1007/s00109-007-0206-1 [DOI] [PubMed] [Google Scholar]
- Schuelke M., Wagner K. R., Stolz L. E., Hübner C., Riebel T., Kömen W., et al. (2004). Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 350, 2682–2688. 10.1056/nejmoa040933 [DOI] [PubMed] [Google Scholar]
- Schultz E. (1996). Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 175, 84–94. 10.1006/dbio.1996.0097 [DOI] [PubMed] [Google Scholar]
- Schwarzkopf M., Coletti D., Sassoon D., Marazzi G. (2006). Muscle cachexia is regulated by a p53-PW1/Peg3-dependent pathway. Genes Dev. 20, 3440–3452. 10.1101/gad.412606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf S. M., Dodd S. L., Judge A. R. (2010). FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol. 298, C38–C45. 10.1152/ajpcell.00315.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewry C. A., Brown S. C., Mercuri E., Bonne G., Feng L., Camici G., et al. (2001). Skeletal muscle pathology in autosomal dominant Emery-Dreifuss muscular dystrophy with lamin A/C mutations. Neuropathol. Appl. Neurobiol. 27, 281–290. 10.1046/j.0305-1846.2001.00323.x [DOI] [PubMed] [Google Scholar]
- Shin J., Tajrishi M. M., Ogura Y., Kumar A. (2013). Wasting mechanisms in muscular dystrophy. Int. J. Biochem. Cell Biol. 45, 2266–2279. 10.1016/j.biocel.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirabella D., De Angelis L., Berghella L. (2013). Sources for skeletal muscle repair: from satellite cells to reprogramming. J. Cachexia Sarcopenia Muscle 4, 125–136. 10.1007/s13539-012-0098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D., Goulet M., Tremblay J. P. (2013). Electroporation as a method to induce myofiber regeneration and increase the engraftment of myogenic cells in skeletal muscles of primates. J. Neuropathol. Exp. Neurol. 72, 723–734. 10.1097/nen.0b013e31829bac22 [DOI] [PubMed] [Google Scholar]
- Skuk D., Tremblay J. P. (2003). Cell therapies for inherited myopathies. Curr. Opin. Rheumatol. 15, 723–729. 10.1097/00002281-200311000-00007 [DOI] [PubMed] [Google Scholar]
- Snijders T., Verdijk L. B., van Loon L. J. (2009). The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res. Rev. 8, 328–338. 10.1016/j.arr.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P., García-Prat L., Serrano A. L., Perdiguero E., Muñoz-Cánoves P. (2015). Muscle stem cell aging: regulation and rejuvenation. Trends Endocrinol. Metab. 26, 287–296. 10.1016/j.tem.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., et al. (2014). Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321. 10.1038/nature13013 [DOI] [PubMed] [Google Scholar]
- Souza T. A., Chen X., Guo Y., Sava P., Zhang J., Hill J. J., et al. (2008). Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol. Endocrinol. 22, 2689–2702. 10.1210/me.2008-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K. L., Bhardwaj R. D., Buchholz B. A., Druid H., Frisén J. (2005). Retrospective birth dating of cells in humans. Cell 122, 133–143. 10.1016/j.cell.2005.04.028 [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley J. E., Ye F., Liu M., Borst S. E., Conover C., Yarasheski K. E., et al. (2010). Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am. J. Physiol. Endocrinol. Metab. 299, E730–E740. 10.1152/ajpendo.00230.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann G., Fong M., Kenney J. S., Jacob C. (1992). Evidence for the involvement of Interleukin 6 in experimental cancer cachexia. J. Clin. Invest. 89, 1681–1684. 10.1172/jci115767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift-Gallant A., Monks D. A. (2013). Androgen receptor expression in satellite cells of the neonatal levator ani of the rat. Dev. Neurobiol. 73, 448–454. 10.1002/dneu.22066 [DOI] [PubMed] [Google Scholar]
- Tedesco F. S., Dellavalle A., Diaz-Manera J., Messina G., Cossu G. (2010). Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 120, 11–19. 10.1172/jci40373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M. T., Aydogdu T., Sala D., Malecova B., Gatto S., Puri P. L., et al. (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 20, 1182–1186. 10.1038/nm.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo M., Busquets S., Ametller E., Lopez-Soriano F. J., Argiles J. M. (2011). Sirtuin 1 in skeletal muscle of cachectic tumour-bearing rats: a role in impaired regeneration? J. Cachexia Sarcopenia Muscle 2, 57–62. 10.1007/s13539-011-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg A. U., Meyer A., Rohner D., Boyle J., Hatakeyama S., Glass D. J. (2009). Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 296, C1258–C1270. 10.1152/ajpcell.00105.2009 [DOI] [PubMed] [Google Scholar]
- Vasyutina E., Lenhard D. C., Birchmeier C. (2007a). Notch function in myogenesis. Cell Cycle 6, 1451–1454. 10.4161/cc.6.12.4372 [DOI] [PubMed] [Google Scholar]
- Vasyutina E., Lenhard D. C., Wende H., Erdmann B., Epstein J. A., Birchemier C. (2007b). RBP-J (Rbpsuh)is essential to maintain muscle progenitor cells and to generate satellite cells. Proc. Natl. Acad. Sci. U S A 104, 4443–4448. 10.1073/pnas.0610647104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguie C. A., Lu D. X., Huang S. K., Rengen H., Carlson B. M. (1997). Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat. Rec. 248, 346–354. [DOI] [PubMed] [Google Scholar]
- Wada K. I., Takahashi H., Katsuta S., Soya H. (2002). No decrease in myonuclear number after long-term denervation in mature mice. Am. J. Physiol. Cell Physiol. 283, C484–C488. 10.1152/ajpcell.00025.2002 [DOI] [PubMed] [Google Scholar]
- Wagner K. R., McPherron A. C., Winik N., Lee S. J. (2002). Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann. Neurol. 52, 832–836. 10.1002/ana.10385 [DOI] [PubMed] [Google Scholar]
- Wang H., Hertlein E., Bakkar N., Sun H., Acharyya S., Wang J., et al. (2007). NF-B regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of Myofibrillar genes. Mol. Cell. Biol. 27, 4374–4387. 10.1128/mcb.02020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Noulet F., Edom-Vovard F., Tozer S., Le Grand F., Duprez D. (2010). Bmp signaling at the tips of skeletal muscles regulates the number of fetal muscle progenitors and satellite cells during development. Dev. Cell 18, 643–654. 10.1016/j.devcel.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Wang X., Pickrell A. M., Rossi S. G., Pinto M., Dillon L. M., Hida A., et al. (2013). Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool. Hum. Mol. Genet. 22, 3976–3986. 10.1093/hmg/ddt251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Bi P., Liu W., Asakura A., Keller C., Kuang S. (2012). Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 32, 2300–2311. 10.1128/mcb.06753-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winbanks C. E., Chen J. L., Qian H., Liu Y., Bernardo B. C., Beyer C., et al. (2013). The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J. Cell Biol. 203, 345–357. 10.1083/jcb.201211134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Walters T. J., Rathbone C. R. (2013). Skeletal muscle satellite cell activation following cutaneous burn in rats. Burns 39, 736–744. 10.1016/j.burns.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M. N. (2006). Review TOR signaling in growth and metabolism. Cell 124, 471–484. 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Wüst R. C., Degens H. (2007). Factors contributing to muscle wasting and dysfunction in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2, 289–300. [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Minota S., Sakurai H., Miyazono K., Yamada A., Kanazawa I., et al. (1994). Expression of transforming growth factor-beta 1 and its relation to endomysial fibrosis in progressive muscular dystrophy. Am. J. Pathol. 144, 221–226. [PMC free article] [PubMed] [Google Scholar]
- Yarasheski K. E., Bhasin S., Sinha-Hikim I., Pak-Loduca J., Gonzalez-Cadavid N. F. (2002). Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J. Nutr. Health Aging 6, 343–348. [PubMed] [Google Scholar]
- Yialamas M. A., Hayes F. J. (2003). Androgens and the ageing male and female. Best Pract. Res. Clin. Endocrinol. Metab. 17, 223–236. 10.1016/s1521-690x(03)00018-6 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Galvez S., Tiwari S., Rezk B. M., Semprun-Prieto L., Higashi Y., et al. (2013). Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J. Biol. Chem. 288, 23823–23832. 10.1074/jbc.m112.449074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Huq T. S., Delafontaine P. (2014). Angiotensin Type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J. Biol. Chem. 289, 26239–26248. 10.1074/jbc.m114.585521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P. S., Partridge T. A., Yablonka-Reuveni Z. (2006). The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54, 1177–1191. 10.1369/jhc.6r6995.2006 [DOI] [PubMed] [Google Scholar]
- Zhang M., Koishi K., McLennan I. S. (1998). Skeletal muscle fibre types: detection methods and embryonic determinants. Histol. Histopathol. 13, 201–207. [DOI] [PubMed] [Google Scholar]
- Zhang L., Pan J., Dong Y., Tweardy D. J., Dong Y., Garibotto G., et al. (2013). Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 18, 368–379. 10.1016/j.cmet.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang X. H., Wang H., Du J., Mitch W. E. (2010a). Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 21, 419–427. 10.1681/asn.2009060571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. T., Yeung S. S., Liu Y., Wang H. H., Wan Y. M., Ling S. K., et al. (2010b). The effects of low frequency electrical stimulation on satellite cell activity in rat skeletal muscle during hindlimb suspension. BMC Cell Biol. 11:87 10.1186/1471-2121-11-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang J. L., Lu J., Song Y., Kwak K. S., Jiao Q., et al. (2010). Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543. 10.1016/j.cell.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Zimmers T. A., Davies M. V., Koniaris L. G., Haynes P., Esquela A. F., Tomkinson K. N., et al. (2002). Induction of cachexia in mice by systemically administered myostatin. Science 296, 1486–1488. 10.1126/science.1069525 [DOI] [PubMed] [Google Scholar]