Abstract
Objective
To evaluate the effectiveness of conservative management (except drug therapy) for acute Whiplash Associated Disorder (WAD) II.
Design
Systematic review and meta-analysis of Randomised Controlled Trials (RCTs) using a pre-defined protocol. Two independent reviewers searched information sources, decided eligibility of studies, and assessed risk of bias (RoB) of included trials. Data were extracted by one reviewer and checked by the other. A third reviewer mediated any disagreements throughout. Qualitative trial and RoB data were summarised descriptively. Quantitative syntheses were conducted across trials for comparable interventions, outcome measures and assessment points. Meta-analyses compared effect sizes with random effects, using STATA version 12.
Data Sources
PEDro, Medline, Embase, AMED, CINAHL, PsycINFO, and Cochrane Library with manual searching in key journals, reference lists, British National Bibliography for Report Literature, Center for International Rehabilitation Research Information & Exchange, and National Technical Information Service were searched from inception to 15th April 2015. Active researchers in the field were contacted to determine relevant studies.
Eligibility Criteria for Selecting Studies
RCTs evaluating acute (<4 weeks) WADII, any conservative intervention, with outcome measures important to the International Classification of Function, Disability and Health.
Results
Fifteen RCTs all assessed as high RoB (n=1676 participants) across 9 countries were included. Meta-analyses enabled 4 intervention comparisons: conservative versus standard/control, active versus passive, behavioural versus standard/control, and early versus late. Conservative intervention was more effective for pain reduction at 6 months (95%CI: -20.14 to -3.38) and 1-3 years (-25.44 to -3.19), and improvement in cervical mobility in the horizontal plane at <3 months (0.43 to 5.60) compared with standard/control intervention. Active intervention was effective for pain alleviation at 6 months (-17.19 to -3.23) and 1-3 years (-26.39 to -10.08) compared with passive intervention. Behavioural intervention was more effective than standard/control intervention for pain reduction at 6 months (-15.37 to -1.55), and improvement in cervical movement in the coronal (0.93 to 4.38) and horizontal planes at 3-6 months (0.43 to 5.46). For early (<4 days) versus late (>10 days) interventions, there were no statistically significant differences in all outcome measures between interventions at any time.
Conclusions
Conservative and active interventions may be useful for pain reduction in patients with acute WADII. Additionally, cervical horizontal mobility could be improved by conservative intervention. The employment of a behavioural intervention (e.g. act-as-usual, education and self-care including regularly exercise) could have benefits for pain reduction and improvement in cervical movement in the coronal and horizontal planes. The evidence was evaluated as low/very low level according to the Grading of Recommendations Assessment, Development and Evaluation system.
Introduction
Whiplash Associated Disorder (WAD) is a consequence of whiplash injury caused by rapid acceleration-deceleration of the head and neck, leading to bony and soft tissue injuries.[1] Road traffic accidents are the most common cause of whiplash.[2] Over the past 20 years, the incidence of traffic related whiplash has risen in most western countries.[3] Prevalence has been reported as 3/1,000 people in North America and Western Europe,[3] with 300,000 individuals experiencing WAD annually in the UK.[4] 40%- 60% of WAD patients develop chronicity [5–11] with approximately 30% of patients experiencing moderate to severe pain and disability.[12] Systematic reviews report limited effectiveness of chronic WAD management.[13–16] Consequently, effective intervention in the acute stage is required to prevent chronicity.
WAD contributes to a substantial economic burden throughout the industrialised world. Increased direct and indirect costs have been reported, including health care costs, reduced work productivity, lost earning capacity, higher socioeconomic costs and time contributed by caregivers.[17, 18] The annual economic cost related to WAD is estimated as $3.9 billion in the US [19] and €10 billion in Europe.[20] Insurance costs are also high in the western world,[3, 21–25] with the UK described as the ‘whiplash capital of Europe’ by the Association of British Insurers, who estimate that one person in 140 claims for whiplash injury annually.[24] In the UK, the cost of claims has risen from £7 to £14 billion over the past decade.[24]
Although there are five grades of whiplash classification, approximately 93% of patients post whiplash can be classified as WADII.[26] A neck complaint and musculoskeletal sign(s) are characteristic of WADII patients who are commonly managed by physiotherapists.[1] Conservative management (non-invasive treatment) is commonly utilized to manage acute WADII, and mainly focuses on physical treatment in terms of active exercise, manual techniques and physical therapy.[27, 28] Currently, the effectiveness of conservative interventions is still reported as limited in managing acute WADII.[29–38]
Patients with WAD exhibit both physical (e.g. pain and disability) and psychological (e.g. fear of movement, anxiety and depression) problems.[8, 25, 39–44] Currently, the psychological components (e.g. cognitive behavioural therapy and other behavioural approaches) of WADII management are under-explored, and this may be a factor contributing to the limited success of some approaches to management. Some clinical guidelines have suggested psychological strategies in managing chronic WAD II.[27, 28] However, these psychological components are not commonly recommended for management in the acute stage. Effectiveness of conservative management of acute WADII, employing both physical and psychological strategies is therefore important to prevent chronicity. No systematic review to date has specifically addressed this issue. The objective of this study was to evaluate the effectiveness of conservative management for acute WADII in order to summarise what we know about effective management in the acute stage.
Materials and Methods
A systematic review of randomised controlled trials (RCTs) was conducted according to a pre-defined protocol using the method guidelines of the Back Review Group of the Cochrane Collaboration,[45] the Cochrane handbook,[46] and is reported in line with PRISMA.[47]
Eligibility criteria
Table 1 details study eligibility criteria using Population Intervention Comparison Outcome Study Design (PICOS).[47]
Table 1. Eligibility criteria.
| Population | Acute (<4 weeks) WADII (0-II or I-II participants were included when the grade was not classified in study) |
| Intervention | Conservative treatment (inclusive of the range of intervention detailed as part of the search strategy in search stages 3–20, and excluding drug therapy) |
| Comparison | Standard/control intervention |
| Outcome | Clinical relevant outcomes base on the International Classification of Function, Disability and Health (ICF) |
| Study design | Randomised controlled trial |
Information sources and searches
Two independent reviewers (TW/MM) searched:
PEDro, Medline, Embase, AMED, CINAHL, PsycINFO, and Cochrane Library databases from inception to 15th April 2015
Key journals manually, including Spine, Manual Therapy, Physiotherapy, Physical Therapy, Australian Journal of Physiotherapy, Pain, and reference lists
Dissertations and proceedings in the British National Bibliography for Report Literature, Center for International Rehabilitation Research Information & Exchange, Index to Scientific and Technical Proceedings, National Technical Information Service, and System for Information on Grey Literature
Finally, active researchers in the field were contacted to identify other relevant studies.
Examples of Search Strategies. Medline (Ovid) 1946 – 15th April 2015 and Embase 1974 – 15th April 2015
Acute whiplash OR acute whiplash injury* OR acute whiplash associated disorder* OR acute WAD OR acute whiplash associated disorder* II OR acute WAD II OR whiplash associated disorder* OR WAD OR whiplash associated disorder* II OR WAD II, OR whiplash OR whiplash injury* OR whiplash patient* OR whiplash syndrome* OR cervical spine disorder* OR cervical spine injury*
Randomized controlled trial* OR randomised controlled trial* OR randomized clinical trial* OR randomised clinical trial* OR randomized controlled clinical trial* OR randomised controlled clinical trial* OR RCT*
Conservative treatment* OR conservative intervention* OR conservative management* OR conservative approach OR conservative therapy*
1 AND 2
3 AND 4
Physiotherapy OR physical therapy OR physical approach OR physical intervention OR physical management
4 AND 6
Manual therapy OR manipulation OR mobilization OR massage
4 AND 8
Exercise OR exercise therapy OR active intervention* OR active treatment* OR active exercise OR range of motion exercise OR ROM exercise OR strengthening exercise OR stretching exercise OR therapeutic exercise OR endurance exercise OR endurance training OR home exercise OR proprioception exercise
4 AND 10
Electrotherapy OR electrical stimulation OR transcutaneous electrical nerve stimulation OR TENS OR percutaneous electrical nerve stimulation OR PENS OR frequency-modulated electromagnetic neural stimulation OR FREMS OR electromagnetic therapy OR electromagnetic field OR electromagnetic field therapy OR pulse electromagnetic field OR PEMF OR pulse magnetic field OR static magnetic field OR electrical spinal cord stimulation OR SCS OR microcurrent transcutaneous electrical nerve stimulation OR micro-TENS OR high-frequency external muscle stimulation OR external muscle stimulation OR HF OR interferential current OR IFC OR Russian current OR faradic current OR intermittent direct current OR IDC OR galvanic current OR GC OR direct current OR DC OR diadynamic current OR high voltage galvanic current OR HVGC OR microcurrent electrical nerve stimulation OR MENS OR electroacupuncture
4 AND 12
Thermotherapy OR heat OR hot pack ultraviolet OR UV OR infrared radiation OR IR OR infrared therapy OR laser OR laser therapy OR ice OR cold therapy OR ice massage OR ice pack OR contrast bath OR cryotherapy
4 AND 14
Posture OR balance OR traction
4 AND 16
Education OR educational intervention OR patient education OR self-management OR self-management program OR neck school OR whiplash school
4 AND 18
Behaviour approach* OR behaviour therapy* OR behaviour treatment* OR cognitive behaviour OR cognitive therapy* OR cognitive treatment* OR cognitive behaviour approach* OR cognitive behaviour therapy* OR psychological approach* OR psychological aspect*
4 AND 20
Study selection
After searching, the two independent researchers (TW/MM) evaluated identified studies for eligibility by screening 1] title and abstract, then 2] full texts, grading each study as eligible/ not eligible/ might be eligible at each stage.[45] Included studies were agreed by the two reviewers (TW/MM). The third reviewer (AR) mediated in situations of disagreement. Only full articles in English were included.
Data collection process
Data were extracted by one researcher (TW) and checked by a second (MM). Trial authors were contacted for additional data when data were missing or ambiguous.
Data items
Trial authors, countries, study design, stage of whiplash patients, WAD classification, sample size, interventions, study setting, power calculations, outcome measures, follow-up period, loss of follow-up, intention to treat and main results were extracted for each trial. Data relating to key outcome measures including pain, disability, function, patient satisfaction, social impact, and physical impairment based on the International Classification of Functioning, Disability and Health were extracted.[48]
Risk of bias (RoB) in individual trials
Training and a pilot of RoB assessment was carried out by the two reviewers (TW/MM). The two reviewers evaluated RoB for each included trial independently. The Cochrane RoB assessment tool, which is informed by empirical evidence, was utilised to assess the internal validity / risk of bias.[47, 49, 50] The third reviewer (AR) mediated situations of disagreement following discussion. Each component of RoB was reported in terms of unclear, low or high risk of bias in tabular form.[49, 50] The Kappa Measure of Agreement was utilised to assess the agreement between the two reviewers using SPSS version 21.
Summary measures
Risk of bias was assessed using the Cochrane RoB assessment tool.[49, 50] The recommendation for overall RoB was in line with the Cochrane handbook.[46, 49, 50] Quantitative data analysis was conducted in situations of comparability of interventions, outcome measures and assessment points across trails. Meta-analyses compared effect sizes with random effects as the primary analyses and were conducted using STATA software version 12.[51]
Synthesis of results
Standardised mean difference (SMD) and standard error of SMD were calculated for meta-analyses. The results of meta-analyses were graphically demonstrated in forest plots. Summary statistics including 95% Confident Interval (CI), p-value, and heterogeneity (I2) were tabulated.
Risk of bias across studies
RoB assessment across studies was tabulated. The criteria of judgement for overall RoB followed recommendations in the Cochrane Handbook.[50] A consensus in overall potential risk of bias was explored to evaluate the level of evidence. As the number of studies in each meta-analysis was less than 10, evaluation of publication bias using Funnel plots was not helpful.[52, 53]
Results
Study selection
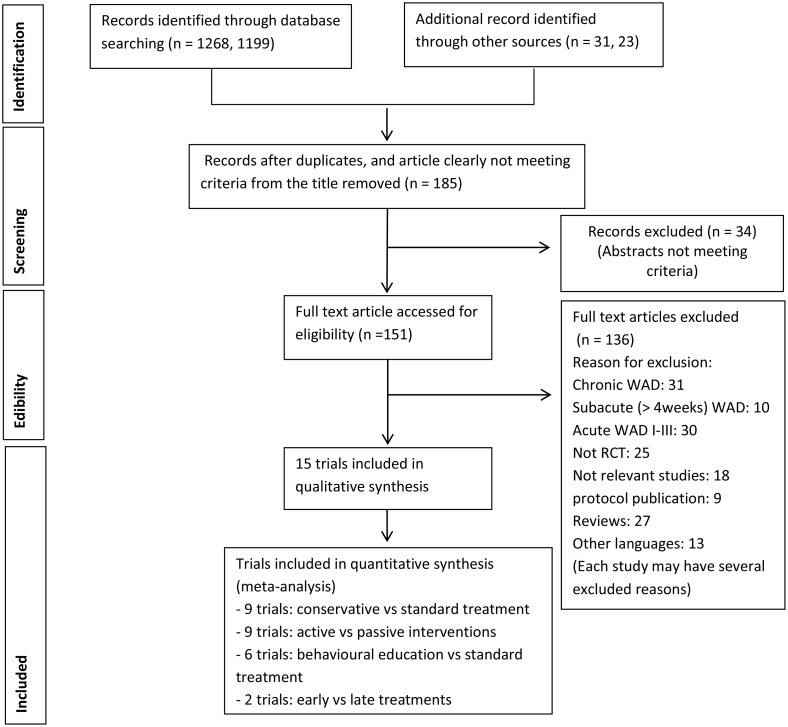
15 RCTs (n = 1676 participants, 9 countries) were included. The process of study selection is detailed in Fig 1.
Fig 1. Study selection flow diagram.
Study characteristics
Trial characteristics are summarised in Table 2. A range of conservative treatments were employed across included trials (see Table 2 for detail of interventions). Interventions could be grouped to inform analyses into conservative (broad group), active, passive and behavioural interventions.
Table 2. Summary results from the 15 included trials.
| Studies | Countries | N | WAD | Design | Intervention 1 | Intervention 2 | Intervention 3 | Outcome Measures | Follow-up period | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Aigner et al. 2006 [29] | Austria | 53 | II | Parallel RCT with single blind | Collar and laser acupuncture | Collar and placebo laser acupuncture | - | -CROM,Subjective symptoms (neck pain, dizziness, paresthesia and tinnitus),Sick leave | 3 weeks (Clinically)8-12 Months (Postal) | No significant difference between interventions in all outcome measures |
| Bonk et al. 2000 [30] | Germany | 147 | 0-II | Parallel RCT | Active therapy (active mobilization and exercise) | Collar therapy | Control | -Subjective symptoms (such as pain, stiffness), CROM | 3 months | No significant difference between interventions at 3 months |
| Borchgrev-ink et al. 1998 [31] | Norway | 201 | 0-II | Parallel RCT with single blind | Act-as-usual | Immobilisati-on | - | -Subjective symptoms using questionnaire, Pain (VAS), CROM, Shoulder movement, Sick leave | 6 months | I1>I2 significantly in improvement neck pain (p<0.01), pain during daily activities (p< 0.05), headaches (p<0.01), painful regions (p<0.01), and memory and concentration problems (p<0.001) at 6 months. ROM of neck and shoulder did not differ between interventions. |
| Conforti and Fachinetti 2013 [54] | Italy | 135 | I-II | Parallel RCT with single blind | HPLT | PT (manual therapy, passive and active exercise) | - | -Pain (VAS), The date of return to work | 6 weeks | I1 > I2significantly improved in both pain (p = 0.005) and the date of return to work (p<0.001) |
| Dehner et al. 2006 [55] | Germany | 70 | II | Parallel RCT | 2 days with collar + standard PT after a weeks | 10 days with collar + standard PT after a weeks | - | -Pain (VAS), Disability (VAS), CROM | 6 months | No significant difference between interventions in all outcome measures |
| Dehner et al. 2009 [32] | Germany | 90 | II | Parallel RCT | Active physical therapy | Passive physical therapy | Act as usual | -Pain (VAS), CROM, Period of disability/ sickness costs | 2 months | - Pain improvement: I1>I2>I3 significantly- CROM: I1 = I2 = I3- Period of disability: I1 = I2<I3 |
| Ferrari et al. 2005 [33] | Canada | 112 | I-II | Parallel RCT with single blind | Education pamphlet | Control group | - | -The number of recovery | 3 months | No significant difference between interventions |
| Foley-Nolan et al. 1992 [34] | Ireland | 40 | 0-II | Parallel RCT with single blind | PEMT + collar + active exercise | Placebo + collar + active exercise | - | -Pain (VAS), CROM, Number of analgesics | 3 months | I1>I 2 significantly improved in terms of pain (VAS) at 2 and 4 weeks but no significance at 12 weeks. For the CROM, I1>I2 significantly at 3 months (p<0.001). |
| Jull et al. 2013 [56] | Australia | 101 | II | Parallel RCT with single blind | Multiprofess-ional intervention | Usual care | - | -Pain (VAS), NDI, IES, PFActS-C, GHQ 28, CROM, Craniocervical flexor test, Balance, Cervical proprioception, PPT, HPT, CPT, Sympathetic vasoconstrictor response, Types and dosage of medications | 12 months | No significant difference between interventions in all outcome measures but the recovery of pain and disability between baseline, 6 and 12 months has significant differences in both interventions. |
| Ottosson et al. 2007 [35] | Sweden | 127 | I-II | Parallel RCT with unblind | Educational programme + self-care (exercise for relaxation and postural control) | Standard Rx.(basic medications) | - | -Self-reported recovery, SF-36, SMFA, Pain and mental distress (VAS), Sick leave | 12 months | I1>I2 significantly in terms of self-reported recovery (p<0.03) but no significant difference in other outcomes between interventions |
| Picelli et al. 2011 [36] | Italy | 18 | I-II | Parallel RCT with single blind | Neck fascia manipulation | Neck mobilization exercise + stretching | - | -CROM, Pain (VAS), NDI, PPT | 2 weeks | CROM: I1>I2 significantly (p<0.01) but other outcome measures, no significant differences between interventions. |
| Rosenfeld et al. 2003 [37][Rosenfeld et al. 2006 reporting same trail][57] | Sweden | 102 | 0-II | Parallel RCT with single blind | Active mobilization within 96 hours or Active mobilization 14 days | Standard Rx. (rest, collar and gradual self-mobil)within 96 hrs or Standard Rx. (rest, collar and gradual self-mobil) 14 days | - | -Pain (VAS), CROM, Sick leave | 3 years | Pain and sick leave I1<I2 significantly (p<0.05) but no significance in CROM (p = 0.06–0.08) |
| Schnabel et al. 2004 [58] | Germany | 200 | I-II | Parallel RCT with un- blind | Mobilisation exercise | Collar therapy | - | -Pain (VAS), Disability (VAS) | 6 weeks | I1>I2 significantly in improving pain and disability (p<0.05) |
| Scholten-Peerters et al. 2006 [59] | Netherlands | 80 | I-II | Parallel RCT with single blind | Education and exercise by PTs | Education by GPs | - | -Pain (VAS), Functional recovery (VAS), SF-36, CROM, TSK, PCI, NDI, Disability in housekeeping and social activities (VAS) | 13 months | At 12 weeks, I1>I2 significantly for CROM improvement but in long term I2>I1 significantly in terms of functional recovery, coping, and physical functioning. |
| Vassiliou et al. 2006 [38] | Germany | 200 | I-II | Parallel RCT with unblind | PT + active exercise | Standard Rx (drugs + soft collar) | - | -Pain and disability (NRS), Number of days with oral medication, The period of soft collar | 6 months | I1>I2 significantly improved in terms of pain intensity and disability. Other outcomes had been reported but no compare using statistic procedure. |
CPT: Cold Pain Threshold, CROM: Cervical Range of Motion, GHQ 28: General Health Questionnaire, HPLT: High Power Laser Therapy, HPT: Hot Pain Threshold, I: Intervention, IES: Impact of Events Scale, NDI: Neck Disability Index, NRS: Numeric Rating Scale, PCI: Pain Coping Inventory, PFActS-C: Pictorial Fear of Activity Scale-Cervical, PPT: Pressure Pain Thresholds, PT: Physiotherapy, RCT: Randomised Controlled Trail, Rx: Treatment, SMFA: Short Musculoskeletal Function Assessment, SF-36: Functional Health Status (Short Form 36), TSK: Tampa Scale for Kinesiophobia, VAS: Visual Analogue Scale
Risk of bias within and across trials
Agreement of RoB assessment between two reviewers was very good (Kappa 0.87).[60] The RoB of individual trials is detailed in Table 3. All trials were assessed as high RoB for a range of reasons including: inadequate allocation concealment, selective outcome reporting, no intention to treat analysis, no specification of primary outcome, no specification primary endpoint, no reporting statistical analysis, no reporting reasons of drop-out, difference in loss to follow up between groups, and no reporting of some outcome measures or/and information.
Table 3. Summary of risk of bias assessment [61].
| Studies | Components of risk of bias | Summary risk of bias | Overall RoB | Comments, high risk components | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5a | 5b | 6 | ||||
| Aigner et al. 2006 [29] | U | U | U | L | H | H | H | High (3) Unclear (3) Low (1) | H | Three high component: 5a, 5b, 6 (5a: No primary outcomes pre-specified, 5b: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Bonk et al. 2000 [30] | U | U | H | L | H | N/A | H | High (3) Unclear (2) Low (1) N/A (1) | H | Three high component: 3, 5a, 6 (3: Unblind assessors, 5a: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Borchgrevink et al. 1998 [31] | U | U | L | H | U | N/A | H | High (2) Unclear (3) Low (1) N/A (1) | H | Two high component: 4, 6 (4: loss follow-up >16 without stating of which group, 6: No primary endpoint specified and No ITT reported) |
| Conforti and Fachinetti 2013 [54] | U | U | L | L | H | N/A | H | High (2) Unclear (2) Low (2) N/A (1) | H | Two high component: 5a, 6 (5a: No primary outcomes pre-specified, 6: No primary endpoint specified, No ITT reported and No statistical analysis reported) |
| Dehner et al. 2006 [55] | L | L | U | L | H | N/A | H | High (2) Unclear (1) Low (3) N/A (1) | H | Two high component: 5a, 6 (5a: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Dehner et al. 2009 [32] | L | L | U | U | H | N/A | H | High (2) Unclear (2) Low (2) N/A (1) | H | Two high component: 5a, 6 (5a: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Ferrari et al. 2005 [33] | L | L | L | H | L | N/A | H | High (2) Unclear (1) Low (4) N/A (1) | H | Two high component: 4, 6 (4: reasons of drop-out were not reported, 6: No primary endpoint specified and No ITT reported) |
| Foley-Nolan et al. 1992 [34] | U | U | U | L | H | N/A | H | High (2) Unclear (3) Low (1) N/A (1) | H | Two high component: 5a, 6 (5a: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Jull et al. 2013 [56] | L | L | L | U | L | L | H | High (1) Unclear (1) Low (5) | H | One high component: 6 (No ITT and Pain (VAS) has reported in significant difference between group at baseline) |
| Ottosson et al. 2007 [35] | L | L | H | L | N/A | L | H | High (2) Low (4) N/A (1) | H | Two high component: 3, 6 (3: Unblind, 6: No primary endpoint specified) |
| Picelli et al. 2011 [36] | L | U | L | L | H | N/A | H | High (2) Unclear (2) Low (3) N/A (1) | H | Two high component: 5a, 6 (5a: P-value did not report in NDI and PPT, 6: No primary endpoint specified and No ITT reported) |
| Rosenfeld et al. 2003 [37][Rosenfeld et al. 2006 reporting same trail][57] | L | L | L | H | H | H | H | High (4) Low (3) | H | Four high component: 4, 5a, 5b, 6 (4: drop-out difference between groups, 5a: No primary outcomes pre-specified, 5b: No primary outcomes pre-specified, Reporting sick leave but have not stated, 6: No primary endpoint specified and No ITT reported) |
| Schnabel et al. 2004 [58] | U | U | U | H | H | N/A | H | High (3) Unclear (3) N/A (1) | H | Three high component: 4, 5a, 6 (4: Loss of follow-up: A = 36%, B = 15%, 5a: No primary outcomes pre-specified, 6: No primary endpoint specified and No ITT reported) |
| Scholten-Peeters et al. 2006 [59][Scholten-Peeters et al. 2003 trial protocol][62] | L | L | L | L | L | L | H | High (1) Low (6) | H | One high component: 6 (No primary endpoint specified) |
| Vassiliou et al. 2006 [38] | L | L | L | L | L | N/A | H | High (1) Low (4) N/A (5) | H | One high component: 6(No primary endpoint specified) |
1 = Sequence generation, 2 = Allocation concealment, 3 = Blinding of participants, personnel and assessors, 4 = Incomplete outcome data, 5a = Selecting outcome reporting (Short term), 5b = Selecting outcome reporting (Long term), 6 = Other potential threats to validity, L = low risk of bias, H = high risk of bias, U = unclear risk of bias, N/A = not applicable
Results of individual trials
Based on the comparability of interventions, outcome measures and assessment points across trials, meta-analyses were conducted on 4 intervention comparisons (please see Table 2 for details of specific interventions): conservative [any non-invasive intervention] versus standard/control (9 RCTs, n = 1182 participants),[29–31, 33–35, 37, 38, 58] active [activities from health professional suggestion to improve symptoms or reduce suffering from illness] versus passive [any intervention which use other people, equipment or other things to reduce symptoms or illness] (9 RCTs, n = 1145 participants),[30–32, 35–38, 58, 59] behavioural [strategies to promote useful behaviour to improve symptoms or reduce illness] versus standard/control (6 RCTs, n = 987 participants),[30, 31, 33, 35, 38, 58] and early [< 1 week] versus late [>2 week] (2 RCTs, n = 172 participants).[37, 55] A summary of the meta-analyses is detailed in Table 4.
Table 4. Summary meta-analyses.
| Meta-analyses | I2 (%) | 95% Confidence Interval (CI) | p-value |
|---|---|---|---|
| Conservative vs standard/control interventions | |||
| Pain intensity (VAS) | |||
| at <3 months | 70.0 | -12.90, 2.19 | 0.164 |
| at 6 months | 63.8 | -20.14, -3.38 | 0.005* |
| at 1–3 years | 0.0 | -25.44, -3.19 | 0.012* |
| CROM in sagittal plane | |||
| at 6 months | 10.9 | -3.61, 9.17 | 0.394 |
| at 3 years | 60.5 | -7.78, 27.69 | 0.271 |
| CROM in coronal plane | |||
| at <3 months | 64.2 | -5.78, 6.48 | 0.911 |
| at 6 months | 0.0 | -1.89, 6.42 | 0.285 |
| at 3 years | 64.1 | -5.83, 16.99 | 0.338 |
| CROM in horizontal plane | |||
| at < 3 months | 0.0 | 0.43, 5.60 | 0.022* |
| at 6 months | 55.1 | -16.04, 8.48 | 0.545 |
| at 3 years | 19.0 | -6.80, 16.51 | 0.415 |
| Sick leave (days) | 0.0 | -39.02, 3.83 | 0.107 |
| Active vs passive interventions | |||
| Pain intensity (VAS) | |||
| at <3 months | 76.3 | -10.51, 6.07 | 0.599 |
| at 6 months | 56.9 | -17.19, -3.23 | 0.004* |
| at 1–3 years | 0.0 | -26.39, -10.08 | < 0.001* |
| CROM in sagittal plane | |||
| at <3 months | 80.6 | -17.73, 8.69 | 0.452 |
| at 6 months | 0.0 | -1.69, 8.44 | 0.192 |
| at 3 years | 60.5 | -7.78, 27.69 | 0.271 |
| CROM in coronal plane | |||
| at <3 months | 82.2 | -6.94, 6.15 | 0.905 |
| at 6 months | 0.0 | -1.13, 5.99 | 0.180 |
| at 3 years | 64.1 | -6.83, 16.99 | 0.338 |
| CROM in horizontal plane | |||
| at <3 months | 85.2 | -8.96, 12.35 | 0.755 |
| at 6 months | 69.4 | -11.28, 12.81 | 0.892 |
| at 3 years | 19.0 | -6.80, 16.51 | 0.415 |
| Sick leave (days) | 0.0 | -39.02, 3.83 | 0.107 |
| Behavioural vs standard/control interventions | |||
| Pain intensity (VAS) | |||
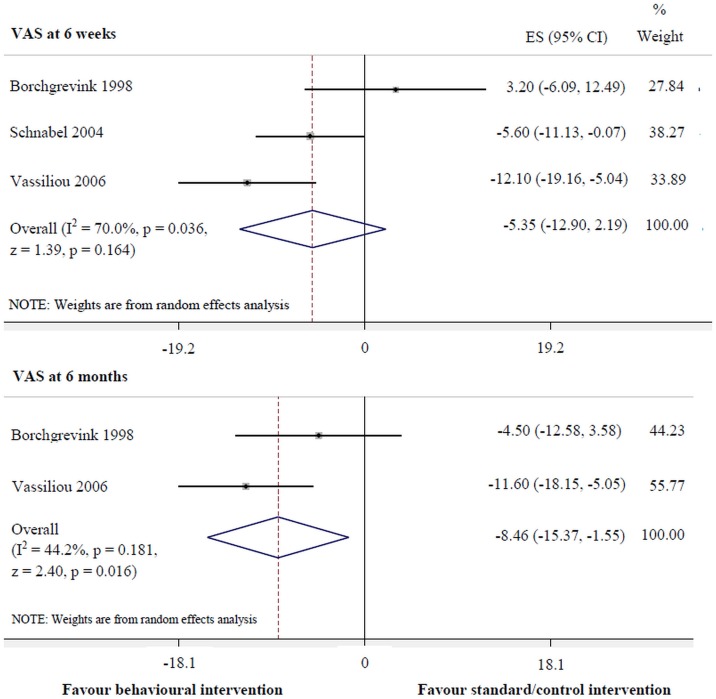
| at 6 weeks | 70.0 | -12.90, 2.19 | 0.164 |
| at 6 months | 44.2 | -15.37, -1.55 | 0.016* |
| CROM in coronal plane | |||
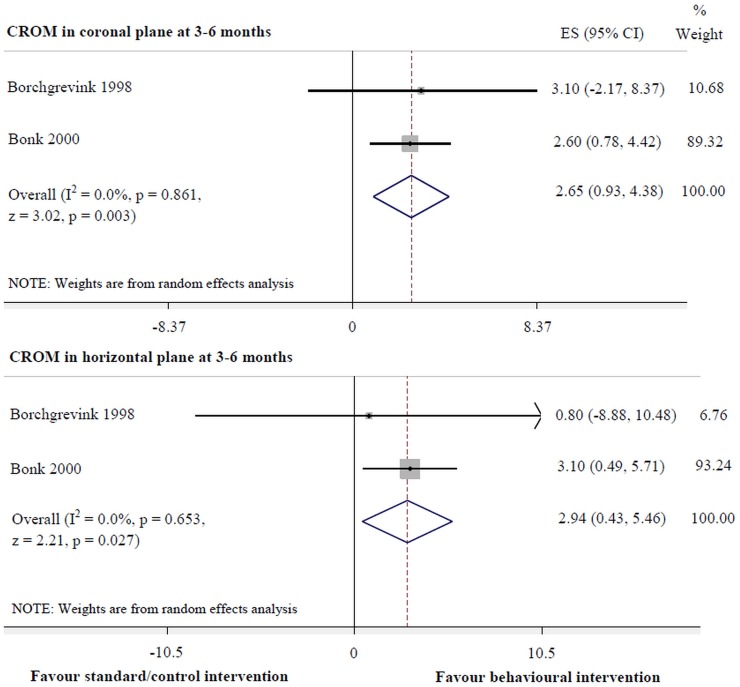
| at 3–6 months | 0.0 | 0.93, 4.38 | 0.003* |
| CROM in horizontal plane | |||
| at 3–6 months | 0.0 | 0.43, 5.46 | 0.027* |
| Early vs late interventions | |||
| Pain intensity (VAS) | |||
| at 6 months | 79.2 | -25.74, 18.21 | 0.737 |
| at 3 years | 0.0 | -12.51, 9.85 | 0.816 |
| CROM in sagittal plane | |||
| at 6 months | 51.3 | -13.16, 17.02 | 0.802 |
| at 3 years | 60.5 | -11.35, 24.04 | 0.482 |
| CROM in coronal plane | |||
| at 6 months | 27.0 | -8.93, 6.92 | 0.803 |
| at 3 years | 64.0 | -12.95, 9.96 | 0.799 |
| CROM in horizontal plane | |||
| at 6 months | 75.9 | -20.66, 27.31 | 0.786 |
| at 3 years | 19.1 | -4.86, 20.25 | 0.230 |
| Sick leave (days) | 0.0 | -13.37, 28.40 | 0.481 |
* Statistical significance (p < 0.05)
Synthesis of results
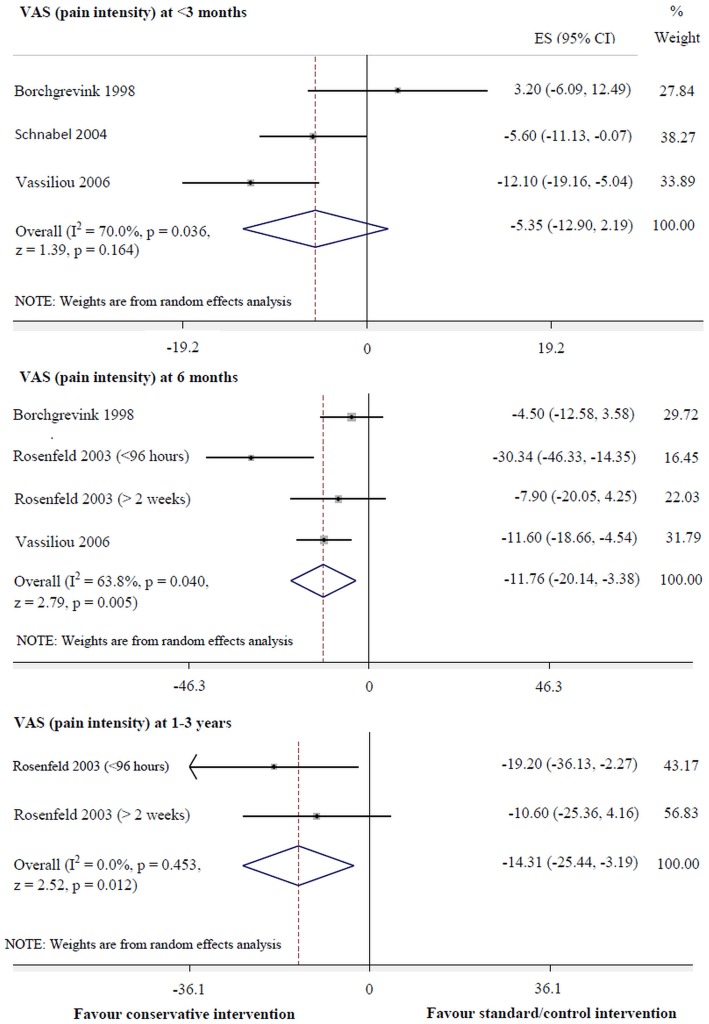
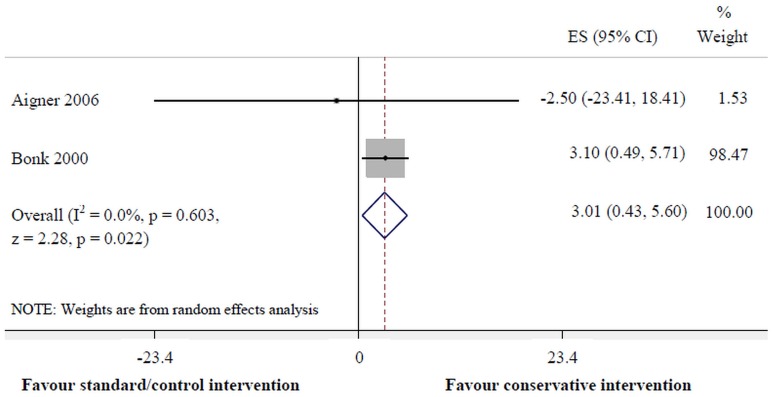
Conservative intervention was more effective for pain reduction than standard/control intervention at 6 months (95% CI: -20.14 to -3.38, p = 0.005, I2 = 63.8%) and 1–3 years (-25.44 to -3.19, p = 0.012, I2 = 0.0%) (Fig 2). Moreover, conservative intervention was superior to the standard/control intervention for improvement of cervical mobility in the horizontal plane at <3months (0.43 to 5.60, p = 0.022, I2 = 0.0%) (Fig 3). However, there were no significant differences between interventions for pain reduction at <3months, other follow-up periods in the horizontal plane of Cervical Range of Motion (CROM), nor any follow-up periods in terms of other planes of CROM, including the number of days of sick leave.
Fig 2. Conservative versus standard/control interventions for VAS (pain intensity).
Fig 3. Conservative versus standard/control interventions for cervical horizontal movement at <3 months.
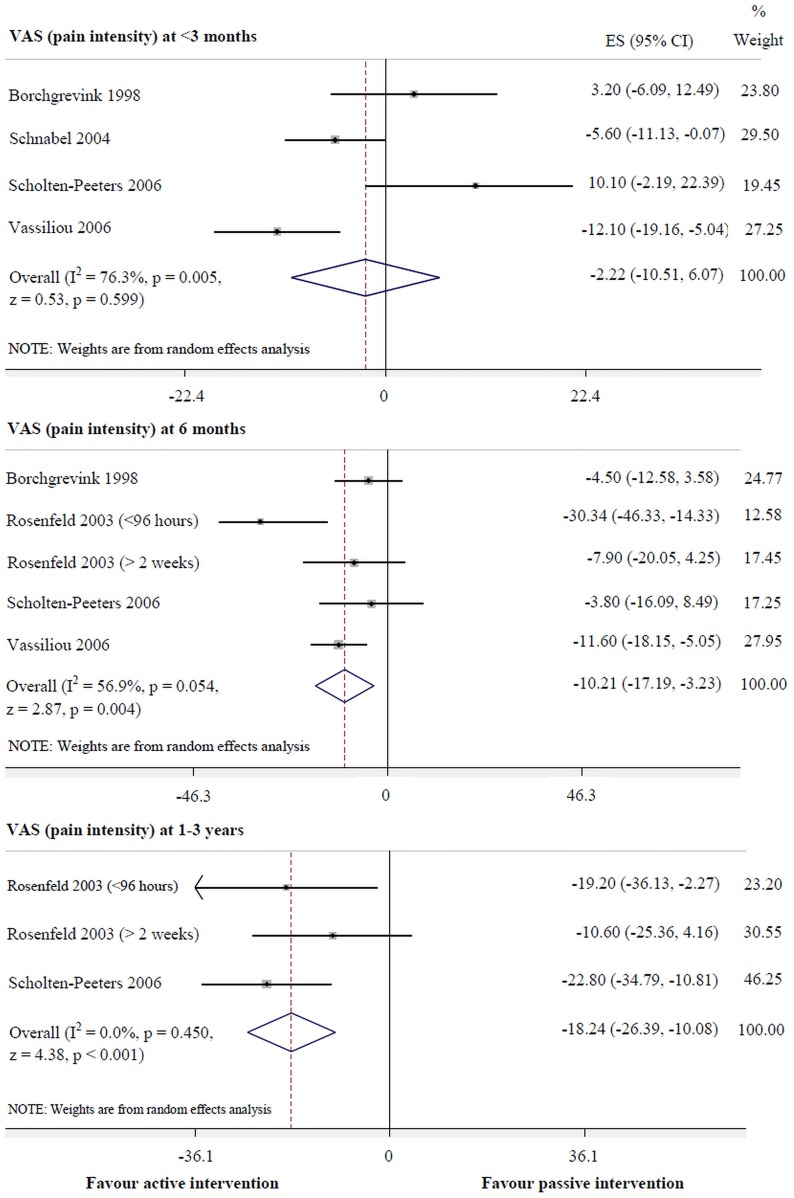
Active intervention was more effective than passive intervention for pain reduction at 6 months (-17.19 to -3.23, p = 0.004, I2 = 56.9%) and 1–3 years (-26.39 to -10.08, p<0.001, I2 = 0.0%) (Fig 4). However, there was no significant difference in pain reduction at <3 months. Also, improvement of cervical mobility and days of sick leave were not significantly different between interventions.
Fig 4. Active versus passive interventions for VAS (pain intensity).
Behavioural intervention was more effective for pain reduction at 6 months (-15.37 to -1.55, p = 0.016, I2 = 44.2%) (Fig 5) and improvement of cervical mobility in the coronal (0.93 to 4.38, p = 0.003, I2 = 0.0%) and horizontal planes (0.43 to 5.46, p = 0.027, I2 = 0.0%) at 3–6 months, compared with the standard/control intervention (Fig 6). However, there was no significant difference between interventions for pain reduction at 6 weeks.
Fig 5. Behavioural versus standard/control interventions for VAS (pain intensity).
Fig 6. Behavioural versus standard/control interventions for cervical movement.
There were no significant differences between early and late interventions for pain reduction, CROM, and days of sick leave at any follow-up period.
Discussion
Summary of evidence
15 RCTs with high RoB were included. Some trials [30, 31, 34] may be high risk of bias owing to poor reporting as published prior to the CONSORT reporting guidelines.[63, 64] Although trial reporting in terms of primary outcome, sample calculation, random sequence generation and allocation concealment significantly improved between 2000 and 2006,[65] the quality of reporting blinding, and descriptions of approach, exclusion, treatment and missing data is still frequently inadequate,[65–67] contributing in 2010 to the revised CONSORT statement.[68, 69] In this systematic review, only three trials were published after 2010.[36, 54, 56] Due to the high RoB across all trials, confidence in findings is reduced.
The meta-analyses findings are more powerful and reliable than individual trials because of minimised biases from the individual trials.[70] The results of the meta-analyses were influenced by individual trials demonstrating conflicting conclusions. For example, the meta-analysis demonstrated that conservative intervention was more effective than standard/control intervention for pain reduction long term, despite some trials reporting negative finding.[35, 37] Another example is that some trials [32, 38, 58] found the active intervention was more effective than the passive short term, but there was no effect demonstrated in the meta-analysis.
The level of heterogeneity was evaluated to determine the credibility of the evidence.[71] For example, in the meta-analyses demonstrating an effect for pain reduction at 6 months, the heterogeneity ranged from moderate (I2 = 44.2%, behavioural intervention) to substantial (I2 = 63.8%, conservative intervention; I2 = 56.9%, active intervention), and this was acceptable overall.
Although this systematic review identified some interventions demonstrating an effect, the size of the effect was not clinically significant. The minimal clinical significant differences in improvement of pain intensity (VAS) and CROM are at least 20 millimetres [72] and 10°,[73] respectively. Currently, therefore, there is no evidence of an effective intervention for acute WADII management. However, conservative intervention (non-invasive treatment inclusive of both physical and psychological components such as active mobilisation exercise, manual techniques, physical agents, multimodal therapy, behavioural approaches, and education, except for drug therapy) seems to be a useful intervention for acute WADII management in terms of pain reduction in the medium (95% CI: -20.14 to -3.38, p = 0.005, I2 = 63.8%) to long term (95%CI: -25.44 to -3.19, p = 0.012, I2 = 0.0%), and improvement of cervical mobility in the horizontal plane in the short term (95%CI: 0.43 to 5.60, p = 0.022, I2 = 0.0%) compared with standard/control intervention.
From these findings, there are two interesting interventions for acute WADII management worthy of further consideration. Firstly, active intervention (involving range of movement, mobilising exercises, and strengthening of the neck and scapular muscles) is strong recommended from whiplash guidelines [27, 28] and may be useful for pain reduction medium (95%CI: -17.19 to -3.23, p = 0.004) to long term (95%CI: -26.39 to -10.08, p = <0.001). Secondly, behavioural intervention (e.g. act-as-usual, education and self-care including regularly exercise) may be effective for pain reduction medium term (95%CI: -15.37 to -1.55, p = 0.016) and improvement of cervical mobility in the coronal (95%CI: 0.93 to 4.38, p = 0.003) and horizontal planes (95%CI: 0.43 to 5.46, p = 0.27) short-medium term. The combination of the two into an active behavioural intervention may be a good strategy to manage acute WADII.
Strengths
This study’s strengths are its design and specific focus to acute WADII using a pre-defined protocol and attention to potential sources of bias such as: a minimisation of errors from searching, using two independent reviewers, decreased publication bias through searching both published and unpublished trials, assessment of RoB using two independent reviewers, and data extraction using two reviewers.
Limitations
This study’s limitations include the small number of trials identified and their high RoB. Furthermore, effectiveness for the outcome of NDI which is a key outcome measure [11, 74] with high validity and reliability [75]could not be calculated in a meta-analysis due to an insufficient number of trials evaluating this outcome.
According to GRADE (the Grading of Recommendations Assessment, Development and Evaluation system),[76] the evidence reviewed in this study is low/very low (low in conservative and active interventions, very low in behavioural intervention). Consequently, an adequately powered, low risk of bias, and well-reported trial to evaluate effectiveness of acute WADII management is warranted to enable confidence in evidence for clinical practitioners, health policy-makers and researchers.
Conclusions
This rigorous systematic review found that conservative and active interventions may be useful for pain reduction in acute WADII management in the medium-long term. Additionally, improvement of cervical movement in the horizontal plane short term could be promoted by the employment of a conservative intervention. The employment of a behavioural intervention (e.g. act-as-usual, education and self-care including regularly exercise) may be an effective treatment in reducing pain and improving cervical mobility in patients with acute WADII in the short-medium term. Finally, there was no significantly difference between treating in early (<1 week) and late (2 weeks) interventions. The level of evidence from this systematic review is evaluated as low/very low level according to GRADE.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study has not received specific grants from any funding agency in the public, commercial or profit sectors.
References
- 1. Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, et al. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining "whiplash" and its management. Spine. 1995;20(8 Suppl):1s–73s. Epub 1995/04/15. . [PubMed] [Google Scholar]
- 2. Cassidy JD, Carroll LJ, Côté P, Lemstra M, Berglund A, Nygren Å. Effect of Eliminating Compensation for Pain and Suffering on the Outcome of Insurance Claims for Whiplash Injury. New England Journal of Medicine. 2000;342(16):1179–86. 10.1056/NEJM200004203421606 . [DOI] [PubMed] [Google Scholar]
- 3. Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Cote P, Guzman J, et al. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S52–9. Epub 2008/02/07. 10.1097/BRS.0b013e3181643ece . [DOI] [PubMed] [Google Scholar]
- 4.Burton K, editor Treatment guideline: is there a need? Whiplash Conference; 2003 6th-8th May Bath. Bristol: Lyons Davidson Solicitors; 2003.
- 5. Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58(3):283–307. Epub 1994/09/01. . [DOI] [PubMed] [Google Scholar]
- 6. Radanov BP, Sturzenegger M, Di Stefano G. Long-term outcome after whiplash injury. A 2-year follow-up considering features of injury mechanism and somatic, radiologic, and psychosocial findings. Medicine. 1995;74(5):281–97. Epub 1995/09/01. . [DOI] [PubMed] [Google Scholar]
- 7. Scholten-Peeters GG, Verhagen AP, Bekkering GE, van der Windt DA, Barnsley L, Oostendorp RA, et al. Prognostic factors of whiplash-associated disorders: a systematic review of prospective cohort studies. Pain. 2003;104(1–2):303–22. Epub 2003/07/12. . [DOI] [PubMed] [Google Scholar]
- 8. Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Physical and psychological factors predict outcome following whiplash injury. Pain. 2005;114(1–2):141–8. Epub 2005/03/01. 10.1016/j.pain.2004.12.005 . [DOI] [PubMed] [Google Scholar]
- 9. Carroll LJ, Hurwitz EL, Cote P, Hogg-Johnson S, Carragee EJ, Nordin M, et al. Research priorities and methodological implications: the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(4 Suppl):S214–20. Epub 2008/02/07. 10.1097/BRS.0b013e318164462c . [DOI] [PubMed] [Google Scholar]
- 10. Kamper SJ, Rebbeck TJ, Maher CG, McAuley JH, Sterling M. Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain. 2008;138(3):617–29. Epub 2008/04/15. 10.1016/j.pain.2008.02.019 . [DOI] [PubMed] [Google Scholar]
- 11. Merrick D, Stalnacke BM. Five years post whiplash injury: Symptoms and psychological factors in recovered versus non-recovered. BMC research notes. 2010;3:190 Epub 2010/07/16. 10.1186/1756-0500-3-190 ; PubMed Central PMCID: PMCPmc2912943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jull GA, Sterling M, Curatolo M, Carroll L, Hodges P. Toward lessening the rate of transition of acute whiplash to a chronic disorder. Spine. 2011;36(25 Suppl):S173–4. Epub 2011/12/30. 10.1097/BRS.0b013e31823883e6 . [DOI] [PubMed] [Google Scholar]
- 13. Jull G, Sterling M, Kenardy J, Beller E. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash?—A preliminary RCT. Pain. 2007;129(1–2):28–34. [DOI] [PubMed] [Google Scholar]
- 14. Stewart MJ, Maher CG, Refshauge KM, Herbert RD, Bogduk N, Nicholas M. Randomized controlled trial of exercise for chronic whiplash-associated disorders. Pain. 2007;128(1–2):59–68. [DOI] [PubMed] [Google Scholar]
- 15. Verhagen AP, Scholten-Peeters GGGM, Van Wijngaarden S, De Bie RA, Bierma-Zeinstra SMA. Conservative treatments for whiplash. Cochrane Database of Systematic Reviews. 2007;(2):CD003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michaleff ZA, Maher CG, Lin CW, Rebbeck T, Jull G, Latimer J, et al. Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial. Lancet. 2014. Epub 2014/04/08. 10.1016/s0140-6736(14)60457-8 . [DOI] [PubMed] [Google Scholar]
- 17. Leth-Petersen S, Rotger GP. Long-term labour-market performance of whiplash claimants. Journal of health economics. 2009;28(5):996–1011. Epub 2009/08/18. 10.1016/j.jhealeco.2009.06.013 . [DOI] [PubMed] [Google Scholar]
- 18. Jennum P, Kjellberg J, Ibsen R, Bendix T. Health, social, and economic consequences of neck injuries: a controlled national study evaluating societal effects on patients and their partners. Spine. 2013;38(5):449–57. Epub 2012/12/15. 10.1097/BRS.0b013e3182819203 . [DOI] [PubMed] [Google Scholar]
- 19. Eck JC, Hodges SD, Humphreys SC. Whiplash: a review of a commonly misunderstood injury. The American journal of medicine. 2001;110(8):651–6. Epub 2001/05/31. . [DOI] [PubMed] [Google Scholar]
- 20. Galasko CSB, Murray P, Stephenson W. Incidence of whiplash-associated disorder. British Columbia Medical Journal. 2002;44(5). Epub 240. [Google Scholar]
- 21. Buitenhuis J, de Jong PJ, Jaspers JP, Groothoff JW. Work disability after whiplash: a prospective cohort study. Spine. 2009;34(3):262–7. Epub 2009/01/17. 10.1097/BRS.0b013e3181913d07 . [DOI] [PubMed] [Google Scholar]
- 22. Cote P, Hogg-Johnson S, Cassidy JD, Carroll L, Frank JW, Bombardier C. Early aggressive care and delayed recovery from whiplash: isolated finding or reproducible result? Arthritis and rheumatism. 2007;57(5):861–8. Epub 2007/05/29. 10.1002/art.22775 . [DOI] [PubMed] [Google Scholar]
- 23. Chappuis G, Soltermann B. Number and cost of claims linked to minor cervical trauma in Europe: results from the comparative study by CEA, AREDOC and CEREDOC. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(10):1350–7. Epub 2008/08/16. 10.1007/s00586-008-0732-8 ; PubMed Central PMCID: PMCPmc2556470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooney H. Insurance companies are reeling from the number of claims being made by people who say they have whiplash injuries 2012 [updated 1st February; cited 2013 October]. Available from: http://www.csp.org.uk/frontline/article/what%E2%80%99s-driving-rise-whiplash-injuries.
- 25. Barnsley L. Whiplash after motor vehicle crashes. Bmj. 2013;347:f5966 Epub 2013/10/08. 10.1136/bmj.f5966 . [DOI] [PubMed] [Google Scholar]
- 26. Sterling M. A proposed new classification system for whiplash associated disorders-implications for assessment and management. MANUAL THERAPY. 2004;9(2):60. [DOI] [PubMed] [Google Scholar]
- 27. Moore A, Jackson A, Jordan J, Hammersley S, Hill J, Mercer C, et al. Clinical guidelines for the physiotherapy management of Whiplash Associated Disorder. London: Chartered Society of Physiotherapy; 2005. [Google Scholar]
- 28. TRACsa. Clinical guidelines for best practice management of acute and chronic whiplash associated disorders: Clinical resource guide. Adelaide: TRACsa: Trauma and Injury Rocovery; 2008. [Google Scholar]
- 29. Aigner N, Fialka C, Radda C, Vecsei V. Adjuvant laser acupuncture in the treatment of whiplash injuries: a prospective, randomized placebo-controlled trial. Wiener Klinische Wochenschrift. 2006;118(3–4):95–9. . [DOI] [PubMed] [Google Scholar]
- 30. Bonk AD, Ferrari R, Giebel GD, Edelmann M, Huser R. Prospective, randomized, controlled study of activity versus collar, and the natural history for whiplash injury, in Germany. Journal of Musculoskeletal Pain. 2000;8(1–2):123–32. [Google Scholar]
- 31. Borchgrevink GE, Kaasa A, McDonagh D, Stiles TC, Haraldseth O, Lereim I. Acute treatment of whiplash neck sprain injuries: A randomized trial of treatment during the first 14 days after a car accident. Spine. 1998;23(1):25–31. [DOI] [PubMed] [Google Scholar]
- 32. Dehner C, Elbel M, Strobel P, Scheich M, Schneider F, Krischak G, et al. Grade II whiplash injuries to the neck: what is the benefit for patients treated by different physical therapy modalities? Patient safety in surgery. 2009;3(1):2 Epub 2009/01/20. 10.1186/1754-9493-3-2 ; PubMed Central PMCID: PMCPmc2635353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrari R, Rowe BH, Majumdar SR, Cassidy JD, Blitz S, Wright SC, et al. Simple educational intervention to improve the recovery from acute whiplash: Results of a randomized, controlled trial. Academic Emergency Medicine. 2005;12(8):699–706. [DOI] [PubMed] [Google Scholar]
- 34. Foley-Nolan D, Moore K, Codd M, Barry C, O'Connor P, Coughlan RJ. Low energy high frequency pulsed electromagnetic therapy for acute whiplash injuries. A double blind randomized controlled study. Scandinavian Journal of Rehabilitation Medicine. 1992;24(1):51–9. . [PubMed] [Google Scholar]
- 35. Ottosson C, Pettersson H, Johansson SE, Nyren O, Ponzer S. Recovery after minor traffic injuries: a randomized controlled trial. PLoS clinical trials. 2007;2(3):e14 Epub 2007/03/24. 10.1371/journal.pctr.0020014 ; PubMed Central PMCID: PMCPmc1829405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Picelli A, Ledro G, Turrina A, Stecco C, Santilli V, Smania N. Effects of myofascial technique in patients with subacute whiplash associated disorders: A pilot study. European Journal of Physical and Rehabilitation Medicine. 2011;47(4):561–8. . [PubMed] [Google Scholar]
- 37. Rosenfeld M, Seferiadis A, Carlsson J, Gunnarsson R. Active intervention in patients with whiplash-associated disorders improves long-term prognosis: a randomized controlled clinical trial. Spine. 2003;28(22):2491–8. Epub 2003/11/19. 10.1097/01.brs.0000090822.96814.13 . [DOI] [PubMed] [Google Scholar]
- 38. Vassiliou T, Kaluza G, Putzke C, Wulf H, Schnabel M. Physical therapy and active exercises—An adequate treatment for prevention of late whiplash syndrome?. Randomized controlled trial in 200 patients. Pain. 2006;124(1–2):69–76. [DOI] [PubMed] [Google Scholar]
- 39. Myran R, Zwart JA, Kvistad KA, Folvik M, Lydersen S, Ro M, et al. Clinical characteristics, pain, and disability in relation to alar ligament MRI findings. Spine. 2011;36(13):E862–7. Epub 2011/02/04. 10.1097/BRS.0b013e3181ff1dde . [DOI] [PubMed] [Google Scholar]
- 40. Nijs J, Inghelbrecht E, Daenen L, Hachimi-Idrissi S, Hens L, Willems B, et al. Long-term functioning following whiplash injury: the role of social support and personality traits. Clinical rheumatology. 2011;30(7):927–35. Epub 2011/02/18. 10.1007/s10067-011-1712-7 . [DOI] [PubMed] [Google Scholar]
- 41. Buitenhuis J, de Jong PJ. Fear avoidance and illness beliefs in post-traumatic neck pain. Spine. 2011;36(25 Suppl):S238–43. Epub 2011/10/25. 10.1097/BRS.0b013e3182388400 . [DOI] [PubMed] [Google Scholar]
- 42. Sterling M. Physiotherapy management of whiplash-associated disorders (WAD). Journal of physiotherapy. 2014;60(1):5–12. Epub 2014/05/27. 10.1016/j.jphys.2013.12.004 . [DOI] [PubMed] [Google Scholar]
- 43. Sterling M, Chadwick BJ. Psychologic processes in daily life with chronic whiplash: relations of posttraumatic stress symptoms and fear-of-pain to hourly pain and uptime. The Clinical journal of pain. 2010;26(7):573–82. Epub 2010/07/20. 10.1097/AJP.0b013e3181e5c25e . [DOI] [PubMed] [Google Scholar]
- 44. Sterling M, McLean SA, Sullivan MJ, Elliott JM, Buitenhuis J, Kamper SJ. Potential processes involved in the initiation and maintenance of whiplash-associated disorders: discussion paper 3. Spine. 2011;36(25 Suppl):S322–9. Epub 2011/12/30. 10.1097/BRS.0b013e318238853f . [DOI] [PubMed] [Google Scholar]
- 45. Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34(18):1929–41. Epub 2009/08/15. 10.1097/BRS.0b013e3181b1c99f . [DOI] [PubMed] [Google Scholar]
- 46.Higgins JPT, Green S. Cochrane handbook for systematics reviews of intervention version 5.1.0.: The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org/.
- 47. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 Epub 2009/07/22. 10.1371/journal.pmed.1000097 ; PubMed Central PMCID: PMCPmc2707599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Organization WH. International Classification of Functioning, Disability and Health: ICF. 2001.
- 49. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928 Epub 2011/10/20. 10.1136/bmj.d5928 ; PubMed Central PMCID: PMCPmc3196245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions 2011. [Google Scholar]
- 51. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010;1(2):97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 52. Schmid CH, Lau J, McIntosh MW, Cappelleri JC. An empirical study of the effect of the control rate as a predictor of treatment efficacy in meta-analysis of clinical trials. Statistics in medicine. 1998;17(17):1923–42. Epub 1998/10/20. . [DOI] [PubMed] [Google Scholar]
- 53. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333(7568):597–600. Epub 2006/09/16. 10.1136/bmj.333.7568.597 ; PubMed Central PMCID: PMCPmc1570006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conforti M, Fachinetti GP. High power laser therapy treatment compared to simple segmental physical rehabilitation in whiplash injuries (1 degrees and 2 degrees grade of the Quebec Task Force classification) involving muscles and ligaments. Muscles, ligaments and tendons journal. 2013;3(2):106–11. Epub 2013/07/28. ; PubMed Central PMCID: PMCPmc3711700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dehner C, Hartwig E, Strobel P, Scheich M, Schneider F, Elbel M, et al. Comparison of the Relative Benefits of 2 Versus 10 Days of Soft Collar Cervical Immobilization After Acute Whiplash Injury. Archives of Physical Medicine and Rehabilitation. 2006;87(11):1423–7. [DOI] [PubMed] [Google Scholar]
- 56. Jull G, Kenardy J, Hendrikz J, Cohen M, Sterling M. Management of acute whiplash: A randomized controlled trial of multidisciplinary stratified treatments. Pain. 2013;154(9):1798–806. 10.1016/j.pain.2013.05.041 [DOI] [PubMed] [Google Scholar]
- 57. Rosenfeld M, Seferiadis A, Gunnarsson R. Active involvement and intervention in patients exposed to whiplash trauma in automobile crashes reduces costs: A randomized, controlled clinical trial and health economic evaluation. Spine. 2006;31(16):1799–804. [DOI] [PubMed] [Google Scholar]
- 58. Schnabel M, Ferrari R, Vassiliou T, Kaluza G. Randomised, controlled outcome study of active mobilisation compared with collar therapy for whiplash injury. Emergency Medicine Journal. 2004;21(3):306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scholten-Peeters GGM, Neeleman-Van Der Steen CWM, Van Der Windt DAWM, Hendriks EJM, Verhagen AP, Oostendorp RAB. Education by general practitioners or education and exercises by physiotherapists for patients with whiplash-associated disorders? A randomized clinical trial. Spine. 2006;31(7):723–31. [DOI] [PubMed] [Google Scholar]
- 60. Peat J. Health science resaerch: a handbook of quantitative methods. Sydney: Allen & Unwin; 2001. [Google Scholar]
- 61. Rushton A, Wright C, Heneghan N, Eveleigh G, Calvert M, Freemantle N. Physiotherapy rehabilitation for whiplash associated disorder II: a systematic review and meta-analysis of randomised controlled trials. BMJ open. 2011;1(2):e000265 Epub 2011/11/22. 10.1136/bmjopen-2011-000265 ; PubMed Central PMCID: PMCPmc3221298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scholten-Peeters GGM, Verhagen AP, Neeleman-Van der Steen CWM, Hurkmans JCAM, Wams RWA, Oostendorp RAB. Randomized clinical trial of conservative treatment for patients with whiplash-associated disorders: Considerations for the design and dynamic treatment protocol. Journal of Manipulative and Physiological Therapeutics. 2003;26(7):412–20. [DOI] [PubMed] [Google Scholar]
- 63. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. The Lancet. 2001;357(9263):1191–4. 10.1016/S0140-6736(00)04337-3. [DOI] [PubMed] [Google Scholar]
- 64. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. Jama. 1996;276(8):637–9. Epub 1996/08/28. . [DOI] [PubMed] [Google Scholar]
- 65. Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in PubMed. Bmj. 2010;340:c723 Epub 2010/03/25. 10.1136/bmj.c723 ; PubMed Central PMCID: PMCPmc2844941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? Bmj. 2008;336(7659):1472–4. Epub 2008/06/28. 10.1136/bmj.39590.732037.47 ; PubMed Central PMCID: PMCPmc2440840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. Bmj. 2010;340:c2697 Epub 2010/06/16. 10.1136/bmj.c2697 ; PubMed Central PMCID: PMCPmc2885592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Bmj. 2010;340:c869 Epub 2010/03/25. 10.1136/bmj.c869 ; PubMed Central PMCID: PMCPmc2844943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. WALKER E, HERNANDEZ AV, KATTAN MW. Meta-analysis: Its strengths and limitations. Cleveland Clinic Journal of Medicine. 2008;75(6):431–9. [DOI] [PubMed] [Google Scholar]
- 71. Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions 2011. [Google Scholar]
- 72. Oostendorp RAB, Rutten GM, Dommerholt J, Nijhuis-van der Sanden MW, Harting J. Guideline-based development and practice test of quality indicators for physiotherapy care in patients with neck pain. Journal of Evaluation in Clinical Practice. 2013;19(6):1044–53. 10.1111/jep.12025 [DOI] [PubMed] [Google Scholar]
- 73. de Koning CH, van den Heuvel SP, Staal JB, Smits-Engelsman BC, Hendriks EJ. Clinimetric evaluation of active range of motion measures in patients with non-specific neck pain: a systematic review. Eur Spine J. 2008;17(7):905–21. Epub 2008/04/23. 10.1007/s00586-008-0656-3 ; PubMed Central PMCID: PMCPmc2443270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miettinen T, Leino E, Airaksinen O, Lindgren KA. The possibility to use simple validated questionnaires to predict long-term health problems after whiplash injury. Spine. 2004;29(3):E47–51. Epub 2004/01/31. . [DOI] [PubMed] [Google Scholar]
- 75. MacDermid JC, Walton DM, Avery S, Blanchard A, Etruw E, McAlpine C, et al. Measurement properties of the neck disability index: a systematic review. The Journal of orthopaedic and sports physical therapy. 2009;39(5):400–17. Epub 2009/06/13. 10.2519/jospt.2009.2930 . [DOI] [PubMed] [Google Scholar]
- 76. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. Epub 2008/04/26. 10.1136/bmj.39489.470347.AD ; PubMed Central PMCID: PMCPmc2335261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.