Abstract
Derivation of articular chondrocytes from human stem cells would advance our current understanding of chondrogenesis, and accelerate development of new stem cell therapies for cartilage repair. Chondrogenic differentiation of human embryonic stem cells (hESCs) has been studied using supplemental and cell-secreted morphogenetic factors. The use of bioreactors enabled insights into the effects of physical forces and controlled oxygen tension. In this study, we investigated the interactive effects of controlled variation of oxygen tension and chondrocyte-secreted morphogenetic factors on chondrogenic differentiation of hESCs in the embryoid body format (hESC-EB). Transient hypoxic culture (2 weeks at 5 % O2 followed by 1 week at 21 % O2) of hESC-EBs in medium conditioned with primary chondrocytes up-regulated the expression of SOX9 and suppressed pluripotent markers OCT4 and NANOG. Pellets derived from these cells showed significant up-regulation of chondrogenic genes (SOX9, COL2A1, ACAN) and enhanced production of cartilaginous matrix (collagen type II and proteoglycan) as compared to the pellets from hESC-EBs cultured under normoxic conditions. Gene expression profiles corresponded to those associated with native cartilage development, with early expression of N-cadherin (indicator of cell condensation) and late expression of aggrecan (ACAN, indicator of proteoglycan production). When implanted into highly vascularized subcutaneous area in immunocompromised mice for 4 weeks, pellets remained phenotypically stable and consisted of cartilaginous extracellular matrix (ECM), without evidence of dedifferentiation or teratoma formation. Based on these results, we propose that chondrogenesis in hESC can be synergistically enhanced by a control of oxygen tension and morphogenetic factors secreted by chondrocytes.
Keywords: Human embryonic stem cells, Hypoxia, Chondrogenic differentiation, Embryoid body, Morphogenetic factors
Introduction
Cartilage tissue engineering is motivated by the need for effective therapeutic modalities for numerous patients with cartilage injuries and osteoarthritis [1]. A classical approach involves the use of in vitro expanded autologous chondrocytes or their progenitors within a biomaterial scaffold, under conditions inducing the formation of cartilaginous tissue matrix [2]. However, the complications associated with invasive tissue harvesting causing donor site morbidity, chondrocyte dedifferentiation during expansion, and fibrocartilage formation at implant sites are necessitating the search for alternative cell sources for cartilage tissue engineering [3].
Adult stem cells from bone marrow - human mesenchymal stem cells (hMSCs), and fat - human adipose-derived stem cells (hASCs), display varying degrees of proliferation and chondrogenic differentiation [4–6]. The success of functional cartilaginous matrix formation from hMSCs and hASCs has thus far been limited, potentially due to the decrease in capacity of hMSCs for self-renewal, lineage commitment and differentiation with increasing donor age [7]. In vivo, differentiation of adult stem cells is tightly regulated through endochondral ossification leading into both bone and cartilage [8]. To effectively recapitulate chondrogenesis in hMSCs and hASCs in vitro, it is necessary to provide tight control of chondrogenic differentiation and to prevent premature hypertrophy, mineralization and ossification [9].
Alternatively, pluripotent human embryonic stem cells (hESCs) that have the capacity to differentiate into all three germ layers offer an unlimited supply of cells for cartilage tissue engineering. The hESC line H9 was derived from the inner cell mass of a blastocyst in 1998 [10] and has been continuously used in tissue engineering research [11, 12]. Recently, our group derived osteogenic progenitors from this cell line and created tissue-engineered bone using osteoconductive scaffolds and perfusion bioreactors [13]. Similarly, chondrogenic differentiation of the line H9 has been achieved in vitro by using exogenous growth factors and supplements, with cartilage-like tissue development demonstrated in monolayer, pellet and hydrogel cultures [14–16].
Developmental studies indicated that oxygen tension acts as a major regulator of chondrogenic differentiation. In prechondrogenic cells, hypoxia up-regulated the expression of Sox9, a key transcription factor of chondrocyte differentiation [17]. In hESCs, hypoxic culture reduced spontaneous differentiation [18] and improved synthesis of extracellular matrix and biomechanical functionality of engineered cartilage [19]. In separate studies, chondrogenic differentiation of hESCs was induced by co-culture with chondrocytes [20] and chondrocyte-conditioned media [21]. Chondrogenic commitment and in vivo functionality of differentiated cells were demonstrated by the establishment of normal cartilage architecture in osteochondral defects in rat [21].
In the current study, we investigated the interactive effects of hypoxic culture regimes and morphogenetic factors released by primary chondrocytes on the chondrogenesis of in line H9 of hESCs. Priming of H9-EBs with chondrocyte-conditioned medium (CCM) under transient hypoxia regime synergistically enhanced chondrogenesis through up-regulation of SOX9. Differentiation of chondrogenic progenitors derived from hESCs corresponded to native tissue development, as shown by the early expression of lineage commitment and cell condensation markers, and the formation of cartilaginous extracellular matrix (ECM) that was phenotypically stable in vivo.
Materials and Methods
Cell Culture
hESC line H9 (Wicell Research Institute, Madison, WI) was expanded in 6-well plates on mouse embryonic fibroblast (MEF) feeders (Globalstem Rockville, MD). hESC expansion medium was composed of Dulbecco’s minimal essential medium-F12 (DMEM-F12), supplemented with 10 % Knockout™ serum replacement (KSR), 1 mM L-glutamine, 0.1 mM Minimum essential medium (MEM) amino acids, 0.1 mM 2-mercaptoethanol and 4 ng/ml basic fibroblast growth factor (bFGF) (all from Life Technologies, Carlsbad, CA). Cells were passaged every 3–4 days with daily medium changes. Human mesenchymal stem cells (hMSCs) were obtained from Lonza (Walkersville, MD), seeded at a density of 5×103 cells/cm2 and cultured in high glucose DMEM supplemented with 10 % fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin and 1 ng/ml bFGF, with medium changes every 3–4 days.
Chondrocyte-Conditioned Medium (CCM)
Primary chondrocytes were isolated from carpometacarpal joints of 4 to 6 month-old bovine calves as described previously [22]. Chondrocytes were seeded at a high density of 2.5×105 cells/cm2 and cultured in growth medium (high glucose DMEM supplemented with 10 %, FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) for 24 h. Attached cells were rinsed twice with PBS, and incubated in DMEM without FBS for 48 h to obtain the secreted soluble morphogenic factors from chondrocytes. The medium was collected, passed through a 0.2 μm filter, and stored at −80 °C until use. Chondrocyte conditioned medium (CCM) was prepared by diluting the filtered medium (1:1) with fresh DMEM containing 10 % FBS, to get a final concentration of 5 % FBS for EB induction.
Embryoid Body (EB) Formation and Induction
hESCs cultures were incubated in 1 mg/ml collagenase IV (Life Technologies) in Knockout™ DMEM at 37 °C for 5 min. Collagenase IV solution was aspirated and replaced with 2 ml of hESC expansion medium. hESC colonies were removed from wells using cell scrapers, transferred into 50 ml conical tubes and centrifuged at 200×g for 5 min. Supernatant was replaced with CCM. hESC colony fragments were carefully mixed with medium and distributed into Ultra-Low Attachment six well plates (Corning, Tewksbury, MA) to form EBs that were cultured for 3 weeks with medium change every 3–4 days.
Experimental Design
Three studies were conducted in an integrated fashion. The overview of studies 1–3 is shown in supplementary Fig. 1.
-
Study 1
Effects of CCM and hypoxia on gene expression EBs were cultured for 3 weeks under the following three experimental conditions (Fig. 1a): in CCM at 5 % O2 (Group 1), in CCM cultured at 21 % O2 (Group 2), and in control medium at 21 % O2 (control, Group 3). For hypoxic culture (Group 1), EBs were placed inside a Billups-Rothenber modular chamber supplied with low-oxygen gas (5 % O2 + 5 % CO2 + 95 % N2). Humidity in the chamber was maintained by a Petri dish with 20 ml distilled water [23].
-
Study 2
Effects of hypoxic culture duration on EB induction EBs were cultured in CCM for 3 weeks under the following four conditions (Fig. 1b): 3 weeks at 5 % O2 (hypoxia, Group 1 as in Study 1), 2 weeks at 5 % O2 followed by 1 week at 21 % O2 (transient hypoxia, Group 4), 1 week at 5 % O2 followed by 2 weeks at 21 % O2 (transient hypoxia, Group 5) and 3 weeks at 21 % O2 with no hypoxic exposure (normoxia, Group 2 as in Study 1). At the end of the experiment, EBs were collected to assay gene expression.
-
Study 3
Chondrogenic differentiation of progenitor cells Chondrogenic differentiation potential of hESCs was compared for EBs derived at transient hypoxia (Group 4) and normoxia (Group 2) (Fig. 1c). After 3 weeks of induction, the EBs were dissociated into single cells [24], counted, and 2×105 cells were used to prepare chondrogenic pellets. The pellets were cultured for 6 weeks at 21 % O2 in chondrogenic medium (ChondM), composed of high glucose DMEM supplemented with 10 ng/ml TGF-β3 (Peprotech, Rocky Hill, NJ), 5 μg/ml proline, 1 % ITS+ (BD Biosciences), 100 nM dexamethasone (Sigma-Aldrich, St. Louis, MO), 50 μg/ml ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO), 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin, with medium changes twice a week. Control pellets were prepared from 2×105 hMSCs and cultured in parallel (Fig. 1c). Pellets were collected weekly to assay gene expression, and at the end of experiment for biochemical analyses and histology.
Fig. 1.
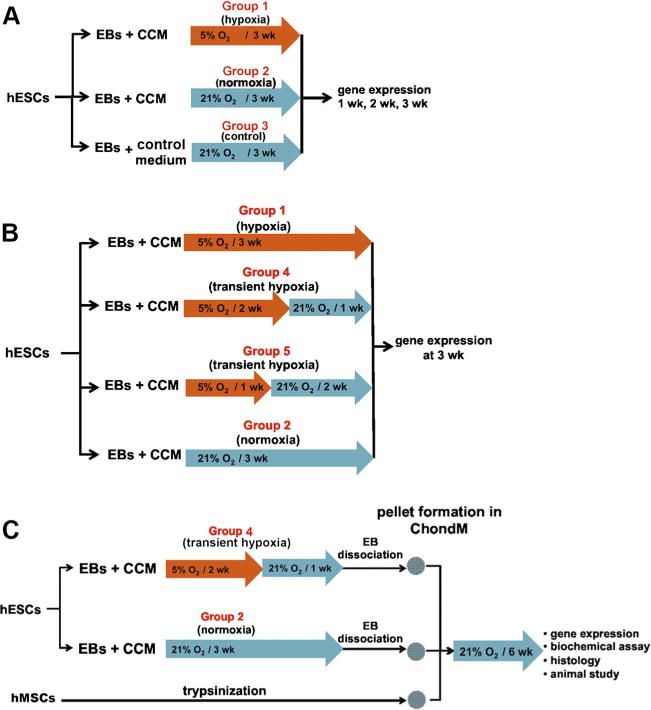
Experimental design. a Study 1: Effects of conditioned media and hypoxia on EBs induction. EBs were prepared from hESCs and cultured in CCM or growth medium for 3 weeks under 5 % O2 or 21 % O2 to determine the effects of media and oxygen levels on gene expression every week as indicated in Group 1, 2 and 3. b Study 2: Effects of hypoxic culture period on EBs induction. EBs were induced in CCM with different hypoxic (5 % O2) exposure periods: 3 weeks (hypoxia, Group 1), 2 weeks (transient hypoxia, Group 4), 1 week (transient hypoxia, Group 5) and no exposure (normoxia, Group 2). Gene expression was determined at the end of the experiment on week 3. c Study 3: Chondrogenic differentiation of EB-induced cells. Pellet culture was used to assess the potential for cartilage tissue formation. Transient hypoxia EBs (Group 4) and normoxia EBs (Group 2) were dissociated into single cells, pelleted and cultured in ChondM for 6 weeks. hMSC pellets were used as control
Embryoid Body and Pellet Dissociation
The EBs and chondrogenic pellets (at week 1) were dissociated into single cells [24]. Briefly, EBs or pellets were collected, washed in PBS and incubated in 0.2 % collagenase type I (Gibco) in PBS containing 20 % FCS for 1 h at 37 °C. The cell suspension was centrifuged at 200×g for 5 min, and the pellet was incubated in 0.25 % trypsin for 5 min at 37 °C. An equal volume of DMEM supplemented with 10 % FBS was added to quench the enzymatic digestion. Clumped cells were dissociated by resuspending through a 20G needle, washed with DMEM and resuspended in ChondM for chondrogenic differentiation in Study 3. Cells from dissociated pellets were washed with PBS and used for flow cytometric analysis.
Flow Cytometry
Cell from dissociated chondrogenic pellets (at week 1), and the remaining suspended cells that did not form the pellets were collected separately, rinsed with PBS, counted and resuspended in staining buffer (1 % BSA in PBS). 2×105 cells were stained with N-cadherin monoclonal antibody conjugated with DyLight®488 (clone EPR1792Y, Abcam, Cambridge, MA) at 4 °C for 30 min in dark, washed and fixed in 4 % paraformaldehyde at 4 °C for 15 min. Analysis was performed on the BD FACSCalibur™. Rabbit IgG isotype antibody (clone ab153686, Abcam, Cambridge, MA) was used as control.
Real-time
PCR Total RNAwas extracted from embryoid bodies and chondrogenic pellets using RNeasy® Mini Kit, and the DNA was removed by RNase-Free DNase Set according to the manufacturer’s instructions (QIAGEN, Valencia, CA). Total RNA was quantified using NanoDrop™ Spectrophotometer (Thermo Scientific, Wilmington, DE). 10 ng of RNA was used for reverse transcription with High Capacity cDNA Reverse Transcription Kit (Life Technologies/Applied Biosystems®). Gene expression was evaluated using the StepOnePlus™ Real-Time PCR system (Life Technologies/Applied Biosystems®).
The following TaqMan® Gene Expression Assays (Life Technologies/Applied Biosystems®) were used for detection of transcription factors: SOX9 (Hs01001343_g1), RUNX2 (Hs00231692_m1), FOXA2 (Hs00232764_m1), PAX6 (Hs00240871_m1), OCT4 (Hs01654807_s1) and NANOG (Hs02387400_g1), extracellular matrix components: COL1A1 (Hs01076780_g1), COL2A1 (Hs00264051_m1), COL10A1 (Hs00166657_m1) and ACAN (Hs00153936_m1), and cell adhesion molecule: CDH2 encoding N-cadherin (Hs00983056_m1). Gene expression values were normalized to ACTB (Hs99999903_m1) and expressed as 2−ΔCt [25]. All reactions were performed in triplicate, using 4 independent samples for each time point.
Histology, Immunohistochemistry and Immunofluorescence
After 6 weeks of culture, pellets were washed with PBS, fixed in 4 % paraformaldehyde overnight at 4 °C, transferred to 70 % ethanol, encapsulated in HistoGel™ (Thermo Scientific), embedded in paraffin and sectioned at 5 μm. The sections were stained with Alcian Blue to detect glycosaminoglycanes (GAG) using standard procedures. The presence of collagens type I and type II was evaluated by immunohistochemistry. Pellet sections were rehydrated, and the antigen retrieval was performed by incubation in heated 0.01 M citrate buffer (pH 6.0) for 15 min. Endogenous peroxidase was blocked by immersing the sections in 0.3 % H2O2/methanol for 30 min at room temperature. The sections were then incubated with blocking serum (Vectastain ABC, Burlingame, CA) for 30 min at room temperature, rinsed three times for 5 min in PBS, incubated overnight at 4 °C with 1:100 mouse anti-human IgG collagen type I or type II monoclonal antibody (Millipore, Billerica, MA), washed and incubated for 30 min with biotinylated secondary antibody. For signal detection, Vectastain ABC Kit with peroxidase and DAB Peroxidase Substrate Kit were used by following the manufacturer’s protocol (Vectastain ABC, Burlingame, CA).
For human nuclei immunofluorescence staining, the rehydrated sections were permeabilized with 0.2 % Triton X-100 in PBS for 45 min, blocked with normal goat serum for 1 h at room temperature and incubated overnight at 4 °C with 1:100 anti-human nuclei antibody (clone 235–1, Millipore, Temecula, CA). The sections were washed, incubated with goat anti-mouse IgG secondary antibody conjugated with FITC (Santa Cruz Biotechnology, Dallas, TX) for 30 min, and counterstained with DAPI.
Biochemical Analyses
Chondrogenic pellets (n=4) were harvested every week and digested for 16 h at 56 °C in PBS containing 20 μl/ml papain (Sigma-Aldrich, St. Louis, MO), 0.5 mg/ml proteinase K (Fisher Scientific, Pittsburgh, PA), 1 mM iodoacetamide and 10 mg/ml pepstatin-A (Sigma Aldrich). Aliquots of digested pellets were analyzed for the glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue dye binding (DMMB) assay [26]. GAG content was normalized to DNA content that was quantified using PicoGreen assay (Life Technologies/Applied Biosystems®) following the manufacturer’s protocol.
Western Blot Analysis
Chondrogenic pellets were washed in PBS and homogenized in 1 μg/ml pepsin in 150 mM NaCl, 1 % Triton X-100, 0.1 % SDS, 0.5 M acetic acid and protease inhibitor cocktail (Sigma Aldrich) overnight at 4 °C with gentle swirling. The digested mixture was neutralized by addition of NaOH [27–29]. Tissue lysate was centrifuged for 10 min at 12,000×g and 4 °C. The supernatant was transferred to a new tube and the total protein concentration was quantified using Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). 15 μg of protein was separated for all groups by 10 % SDS-PAGE and transferred to PVDF membrane using standard protocol. Purified human collagen type II (Millipore, Billerica, MA) was loaded into the gel as a positive control. The membrane was blocked for 12 h with 5 % skim milk in TBST (2 mM Tris, 50 mM NaCl and 0.1 % Tween 20) and incubated for 12 h at 4 °C with 1 μg mouse anti-human IgG collagen type II or 1:500 mouse anti-human IgG actin monoclonal antibody (Millipore). The membrane was then washed and incubated with 1:1000 of goat anti-mouse IgG conjugated with alkaline phosphatase antibody for 1 h, and washed with TBST three times for 5 min. For signal detection, 1-Step™ NBT/BCIP solution was added to the membrane and incubated for 5–15 min until color developed. Collagen type II and actin protein band intensities were quantified using ImageJ software (National Institutes of Health).
Evaluation of Chondrogenic Pellets in vivo
Six weeks hESCs- and hMSCs-chondrogenic pellets were transplanted subcutaneously into 6-week old immunocompromised (SCID-Beige) mice, following a protocol approved by the Institutional Animal Care And Use Committee (IACUC). Mice (n=3) were anesthetized using an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg). The hESC-derived pellet was implanted on the left dorsal pouch of a mouse, and the hMSC-derived pellet was implanted on the right dorsal pouch of the same mouse. The pellets were harvested 4 weeks after implantation, fixed in 4 % paraformaldehyde at 4 °C and processed for histology.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software (La Jolla, CA). Data were expressed as the average ± SD of n=3–4 samples per group and time point. The differences in gene expression and GAG content between the groups were evaluated using two-way ANOVA, followed by Bonferroni post-test. p<0.05, 0.01 and 0.001 were considered statistically significant.
Results
Synergistic Effects of Morphogenetic Factors and Hypoxia on Chondrogenic Induction
Chondrogenesis was induced by culturing EBs in CCM at normoxia and hypoxia, with growth medium serving as control (Study 1, Fig. 1a). At week 2, real- time PCR revealed that CCM (Group 2) up-regulated SOX9 expression in EBs compared to growth medium (Group 3) (Fig. 2a). The effect of CCM was augmented under hypoxic conditions (Group 1), as evidenced by further up-regulation of SOX9 relatively to the normoxic cultures (Group 2). The kinetics of SOX9 expression in CCM at hypoxia and normoxia were similar, with transient up-regulation at 2 weeks, and a 3-fold higher expression levels of SOX9 in hypoxic than in normoxic cultures (p<0.001) (Fig. 2a).
Fig. 2.
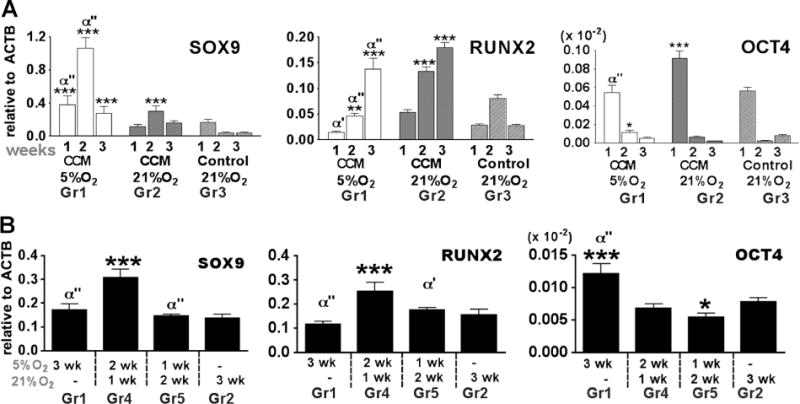
Gene expression in EBs cultured in conditioned medium (CCM) under hypoxic conditions. a Real-time PCR showed expression profiles of transcription factors and extracellular matrix over 3 weeks time course of EB induction (study 1). *,**,*** denote significant differences, compared with control medium group (group 3) at the same time point, where p<0.05, 0.01 and 0.001, respectively. α, α’, α” denote significant differences, compared with 3 weeks 21 % O2 (group 2) at the same time point, where p<0.05, 0.01 and 0.001, respectively. b EBs were cultured in CCM with different exposure periods of 5 % O2 for 3 weeks (study 2). *,**,*** denote significant differences, compared with group 2. α, α’, α” denote significant differences, compared with group 4. Gene expression levels were normalized to ACTB. Data show average ± SD of 4 samples
CCM also significantly induced osteogenic transcription factor RUNX2 (Fig. 2a). Unlike SOX9 expression, RUNX2 was higher at normoxic (Group 2) than hypoxic (Group 1) culture conditions and peaked at week 3 of culture, consistent with the known role of oxygen in bone development. A significant decrease in the expression of OCT4, a pluripotent transcription factor, was detected over time in all groups (Fig. 2a). Gene expression data are presented as boxplots in supplementary Fig. 2a.
The Effects of Transient Hypoxia Regime on Gene Expression
In Study 2 (Fig. 1b), we investigated the effects of the timing and duration of hypoxic culture on gene expression, to identify a regime of hypoxia-normoxia that is optimal for chondrogenic induction of hESC-EBs. The regime of transient hypoxia (Group 4), with 2 weeks of culture under 5 % O2 followed by 1 week of culture under 21 % O2 significantly up-regulated SOX9 (p<0.001) compared to normoxia condition (Group 2) (Fig. 2b). Varying the hypoxic exposure period also affected RUNX2 expression, which was significantly increased by shorter (2 or 1 week) periods of hypoxia. We found that constant hypoxic condition possibly inhibited RUNX2 expression. RUNX2 was slightly decreased but not significantly different (p<0.05) in Group 1 compared to Group 2 (Fig. 2b). At 3 weeks culture, OCT4 expression was very low in every chondrogenic induction condition, the magnitude of gene expression was 10−4 compared to β-actin gene (Fig. 2b). In particular, both transient hypoxic groups (Group 4 and Group 5) showed significant decreases of OCT4 compared to long-term hypoxic condition (Group 1). Gene expression data are presented as boxplots in supplemental Fig. 2b.
Expression of Lineage Markers During Chondrogenic Differentiation in Pellet Cultures
In Study 3 (Fig. 1c), the potential for cartilage tissue formation was evaluated in pellet cultures. EBs induced by transient hypoxia (Group 4) and normoxia (Group 2) were dissociated into single cells, pelleted and cultured for 6 weeks at 21 % O2. SOX9 expression in pellets made from transient hypoxia EBs increased over time until week 4, followed by a gradual decrease (Fig. 3a). The SOX9 expression of the pellets made from normoxia EBs gradually increased at a slower rate and reached a comparable level at 6 weeks as the transient hypoxia EBs at 4 weeks. In comparison, pellets made of hMSCs expressed high SOX9 level after 1 week and the peak of expression after 3 weeks, followed by a significant down-regulation at weeks 4 and 5 (Fig. 3a). RUNX2 expression profiles were similar for pellets derived from transient hypoxia and normoxia EBs, showing a slow increase over 6 weeks of culture. The expression levels during the first 3 weeks were low compared to the pellets derived from hMSCs (Fig. 3b).
Fig. 3.
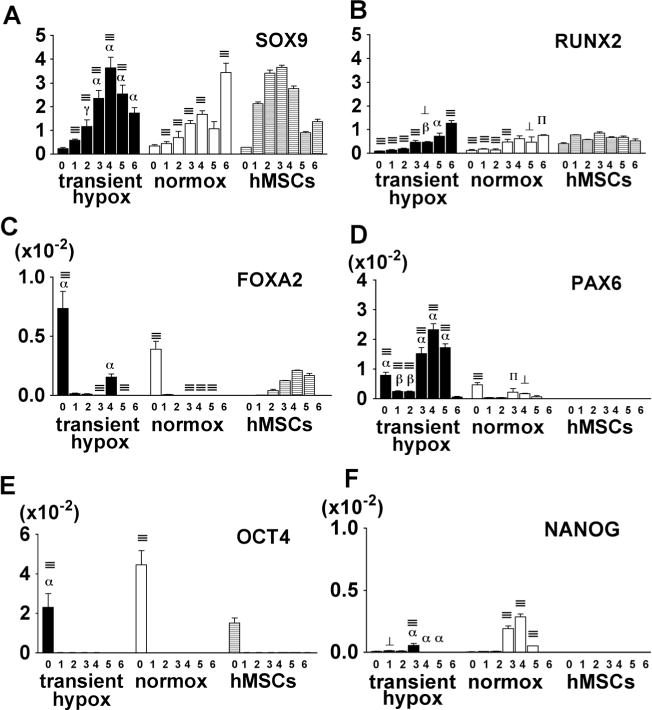
Transcription factor expression of chondrogenic pellets. hESCs-derived chondrogenic pellets in transient hypoxia (Group 4) and normoxia (Group 2) were collected weekly and analyzed for the expression of chondrogenic - SOX9 (a), osteogenic - RUNX2 (b), endodermal - FOX2 (c), ectodermal - PAX6 (d) and pluripotent - OCT4 (e) and NANOG (f) transcription factors. Gene expression levels were normalized with ACTB. Data show average ± SD of 3–4 samples. ⊥, ∏, ≡ denotes significant differences compared with hMSCs at the same time point, where p<0.05, 0.01 and 0.001, respectively. γ, β, α denote significant differences compared with normoxia, where p<0.05, 0.01 and 0.001, respectively
Expression of transcription factors involved in endodermal (FOXA2) and ectodermal (PAX6) lineage differentiation was monitored to determine the nonspecific lineage induction. FOXA2 expression in pellets prepared from EBs was detected only at the initiation of culture (Fig. 3c). Pellets derived from transient hypoxia EBs expressed increasing levels of PAX6 until week 4, while pellets derived from normoxia EBs showed low expression of PAX6 (Fig. 3d). We could not detect PAX6 in hMSCs pellets.
To test for the loss of pluripotency, OCT4 and NANOG were monitored over the time of chondrogenic differentiation. After week 1, we did not detect OCT4 in pellets derived from either transient hypoxia EBs, normoxia EBs or hMSCs (Fig. 3e). Transient expression of NANOG was detected at weeks 3 and 4 of pellet culture, with higher expression levels in pellets derived from normoxia EBs than transient hypoxia EBs (Fig. 3f). We could not detect NANOG expression in hMSC pellets (Fig. 3f).
Cartilaginous Matrix Production in Chondrogenic Pellets Derived from transient hypoxia EBs
We analyzed gene expression of collagen type I, type II and type X to evaluate the progression of chondrogenic differentiation in pellet cultures (Study 3). Chondrogenic pellets derived from transient hypoxia EBs (Group 4) progressively increased the expression of COL2A1 that is encoding for collagen type II, a key component of cartilage matrix and a marker of chondrocyte maturation (Fig. 4a). At the end of 6 weeks, COL2A1 levels were significantly higher compared to normoxia EBs- (Group 2) and hMSC-derived pellets. In contrast, pellets derived from normoxia EBs (Group 2) increased the COL2A1 expression at a slower amount. Chondrogenic pellets from hMSCs expressed COL2A1 from week 2 onward, at constant levels throughout the time course of cultivation.
Fig. 4.
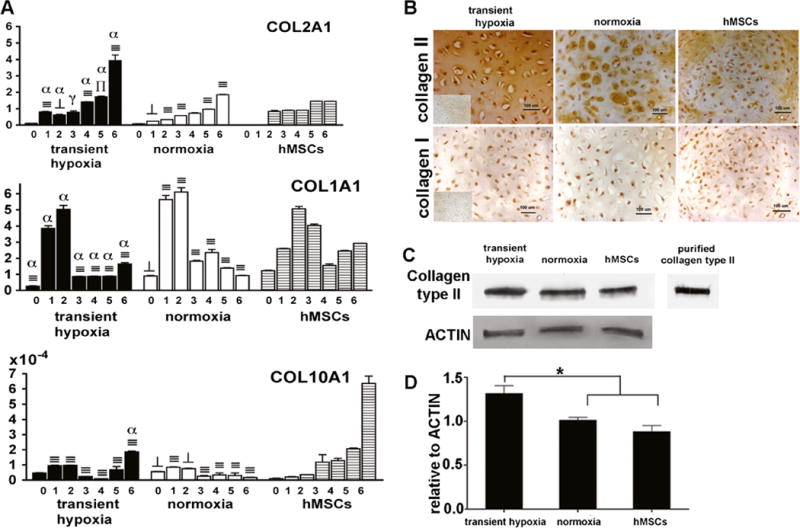
Extracellular matrix production in chondrogenic pellets. a Pellets derived from transient hypoxia EBs (Group 4), normoxia EBs (Group 2) and hMSCs were analyzed for gene expression of collagen type II, I and X. Gene expression levels were normalized with ACTB. Data show average ± SD of 4 samples. ⊥, ∏, ≡ denotes significant differences compared with hMSCs at the same time point, where p<0.05, 0.01 and 0.001, respectively. γ, β, α denote significant differences compared with normoxia, where p < 0.05, 0.01 and 0.001, respectively. b Immunohistochemistry showed localization of type II and type I collagen staining in 6 week pellets. Inserts show negative staining controls. c Bands specific for collagen type II protein were detected in pellet matrix using Western blot. Purified human collagen type II was used as positive control. d Relative intensities of collagen type II protein levels normalized to actin levels. Data show average ± SD (n=3).* denotes statistically significant differences (p<0.05)
Interestingly, we found a transient increase in COL1A1 expression, indicating a switch in profiles from collagen type I to type II in all pellet groups. In the first 2 weeks of culture, EB-derived pellets exhibited similar or significantly higher levels of COL1A1 compared to hMSC pellets, followed by a marked reduction by 70 % in both transient hypoxia-(Group 4) and normoxia EB (Group 2) pellets and by 50 % in hMSC pellets. COL10A1 that is encoding for collagen type X, the hypertrophic marker, showed only minimal, significantly lower expression in both EB-derived pellet groups compared to hMSCs, however it gradually increased in hMSC pellets.
At the end of chondrogenic differentiation, pellets were evaluated for the presence of type II and type I collagens. Pellets derived from transient hypoxia EBs (Group 4) stained homogeneously for collagen type II in the extracellular matrix space (Fig. 4b), whereas pellets derived from normoxia EBs (Group 2) exhibited positive staining only in the matrix adjacent to the cells. hMSC pellets exhibited a weaker and less homogenous staining in the pericellular space as compared to pellets derived from transient hypoxia EBs. We were not able to detect collagen type I in any of the pellet groups (Fig. 4b).
Abundance of collagen type II together with the loss of collagen type I corresponded to high COL2A1 and low COL1A1 gene expression levels at late stages of chondrogenic differentiation (5–6 weeks, Fig. 4a). We further confirmed the gene expression and immunohistochemistry data by Western blot analysis for collagen type II. Bands with molecular weight of 145 kDa corresponding to type II collagen were detected in pellets made from transient hypoxia EBs (Group 4), normoxia EBs (Group 2) and hMSCs (Fig. 4c). Semi-quantitative evaluation of the protein band intensities showed a higher amount (p<0.05) of collagen type II relative to actin in pellets derived from transient hypoxia EBs than in either normoxia EB- pellets or hMSC-pellets (Fig. 4d).
Cell Condensation and Proteoglycan Production in Chondrogenic Pellets
The first stage of chondrogenesis is cellular condensation, with cell-cell interactions mediated by N-cadherin [30]. hMSCs consistently formed pellets within 24 h, in contrast to EB-derived progenitors where some of the cells did not adhere to pellets but remained in suspension, and were washed away during medium changes. Dissociated cells from transient hypoxia EBs (Group 4) formed spherical pellets within 4 days, whereas the cells isolated from normoxia EBs (Group 2) aggregated only after 12 days.
Corresponding to the above findings, real-time PCR showed a temporal gene expression pattern for CDH2 encoding N-cadherin, and ACAN encoding chondroitin sulfate proteoglycan core-protein in maturing chondrogenic pellets (Fig. 5a). Pellets made from EBs in both induction regimes exhibited the highest CDH2 expression at start of culture and week 1, followed by a gradual decrease (Fig. 5a). These expression levels early in culture were significantly lower compared to the levels found in hMSC pellets at the same time points. However, in pellets derived from transient hypoxia EBs (Group 4) and hMSCs, high expression of CDH2 was maintained through week 2, and then decreased. In contrast, CDH2 expression decreased already after week 1 in pellets made from normoxia EBs (Group 2). ACAN expression increased progressively with time in culture and reached significantly higher levels in pellets made from transient hypoxia EBs (Group 4) compared to either normoxia EBs (Group 2) or hMSCs at all time points (Fig. 5a).
Fig. 5.
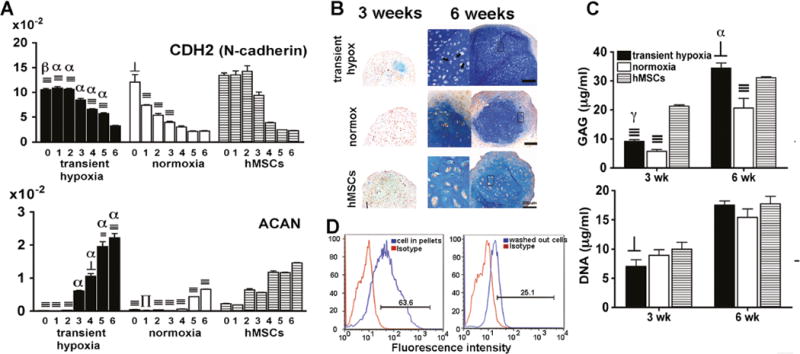
Cell condensation and proteoglycan production. a Pellets derived from transient hypoxia EBs (Group 4), normoxia EBs (Group 2) and hMSCs were analyzed for gene expression for N-cadherin and ACAN. Gene expression levels were normalized with ACTB. Data show average ± SD of four samples. ⊥, ∏, ≡ denote significant differences compared with hMSCs within the same time point, where p<0.05, 0.01 and 0.001, respectively. γ, β, α denote significant differences compared with normoxia, where p<0.05, 0.01 and 0.001, respectively. b Alcian blue staining at 3 and 6 weeks of chondrogenic pellets. Arrows indicate lacunae inside 40× inserts. c GAG and DNA contents at 3 and 6 weeks of chondrogenic pellets. Data show average±SD of four samples. ⊥ and≡denote significant differences compared with hMSCs within the same time point, where p<0.05 and 0.001, respectively. γ, β, α denote significant difference compared with normoxia, where p<0.05, 0.01 and 0.001, respectively. d Flow cytometric analysis of pellets derived from transient hypoxia EBs (Group 4). Cells of chondrogenic pellets and washed out cells at week 1 were stained with N-cadherin antibody. Percentages of N-cadherin positive cells with respect to isotype control are indicated in each figure
Gene expression data were confirmed by Alcian blue staining, which showed some glycosaminoglycan (GAG) deposition in pellets from transient hypoxia EBs (Group 4) and hMSCs by 3 weeks (Fig. 5b). Interestingly, the pellets derived from transient hypoxia EBs showed discrete regions strongly staining for GAG, whereas hMSC pellets exhibited weak but homogeneous staining for GAG. By week 6, abundant and homogenous GAG staining was observed in pellets from transient hypoxia EBs (Group 4), while hMSC pellets stained less strongly and pellets from normoxia EBs (Group 2) stained less homogeneously (Fig. 5b). The appearance of chondrocytes in lacunar spaces surrounded by dense ECM was clearly seen in pellets made from transient hypoxia EBs (Fig. 5b, indicated by arrows). Biochemical analysis confirmed the significant differences in GAG deposition observed histologically and DNA content increased from 3 to 6 weeks in all groups (Fig. 5c).
To further evaluate the N-cadherin mediated cell condensation of EB-derived chondrogenic progenitors, pellets cultured under transient hypoxia (Group 4) and the cells remaining in suspension were analyzed by flow cytometry at week 1. About 60 % of the cell population in the pellets expressed N-cadherin, in contrast to only 25 % of the cells that remained in suspension (Fig. 5d).
Model of Chondrogenic Differentiation of hESCs
Data collected for the time-dependent changes in the expression of ECM genes, cell adhesion molecules, and spatial organization of cartilaginous ECM are consistent with the following model for in vitro chondrogenic differentiation of hESCs-progenitors in EB and pellet cultures (Fig. 6). hESCs-progenitors with chondrogenic potential were induced in EB cultures in the presence of morphogenetic factors and controlled low oxygen tension regime (Fig. 6a). EBs were dissociated (Fig. 6b) and formed pellets that compacted within 1 week from chondrogenic progenitors expressing N-cadherin (Fig. 6c). The pellets started to grow in size, due to the newly secreted ECM progressively separating the neighboring cells, starting from the center of pellets and towards the periphery (Fig. 6d). Finally, mature pellets formed containing lacunar structures and dense extracellular matrix in both the pericellular space and distributed homogenously throughout the pellet (Fig. 6e–f).
Fig. 6.
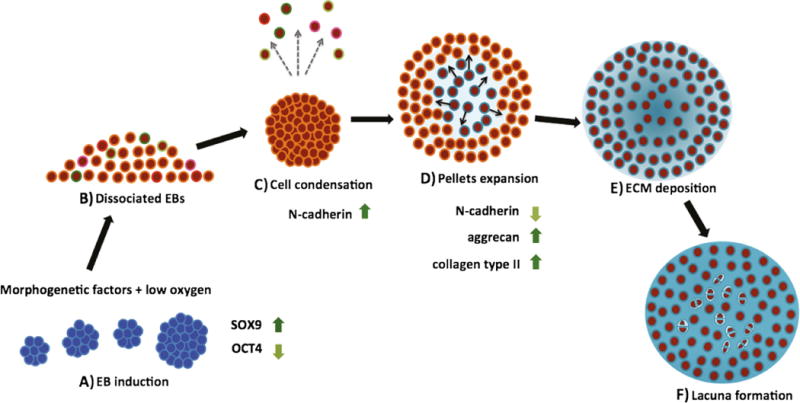
Model of chondrogenesis. a Embryoid bodies (EBs) were induced for 3 weeks by morphogenetic factors in chondrocyte-conditioned medium, under controlled oxygen tension (transient hypoxia regime). Induced EBs showed up-regulation of SOX9 and down-regulation of OCT4. b Chondrogenic progenitors mixed with non-chondrogenic progenitors are obtained after induction. c Chondrogenic progenitors expressing N-cadherin expression undergo cell condensation, while other cell populations are washed away with medium changes. d The spherical pellets increase in size as ECM production continues, resulting in increased intercellular spaces, starting at the middle of pellet and extending to the periphery. The progression of ECM synthesis occurs with a concomitant decrease in the expression of cell adhesion molecules. e ECM occupies entire intercellular spaces of chondrogenic pellets. The progenitor cells differentiate to chondrocytes. f After cell division, daughter cells located at the same area as parent cells secrete matrix to separate each other and form enclosed structures (lacunae)
Phenotypic Stability and in vivo Maturation of hESCs-derived Chondrogenic Pellets
To investigate the phenotypic stability of transient hypoxia EB-derived cartilaginous pellets, the 6-week pellets were subcutaneously implanted into immunocompromised mice and examined 4 weeks following implantation. We could not detect any signs of dedifferentiation and teratoma formation in explanted pellets (Fig. 7). The markers of embryonic germ layers ectoderm (Nestin), mesoderm (Vimentin) and endoderm (Alpha-fetal protein) were not detected in the pellets (Fig. 7a). Capillaries formed outside the pellets and stained with CD31 antibody specific for mouse vascular endothelial cells, but not inside the pellets. Anti-human nuclei staining confirmed that the chondrogenic pellets contained cells of human origin. Importantly, transient hypoxia EB-derived pellets exhibited cartilaginous tissue, as shown by the positive staining of collagen type II and GAG, which was more intense compared to the hMSC-derived explanted pellets. Collagen type I staining was not detected in any of the pellets, and weak collagen type X staining was detected in hMSCs-derived pellets (Fig. 7b). Representative bone markers, such as ALP and ostepontin, were not observed (Fig. 7c).
Fig. 7.
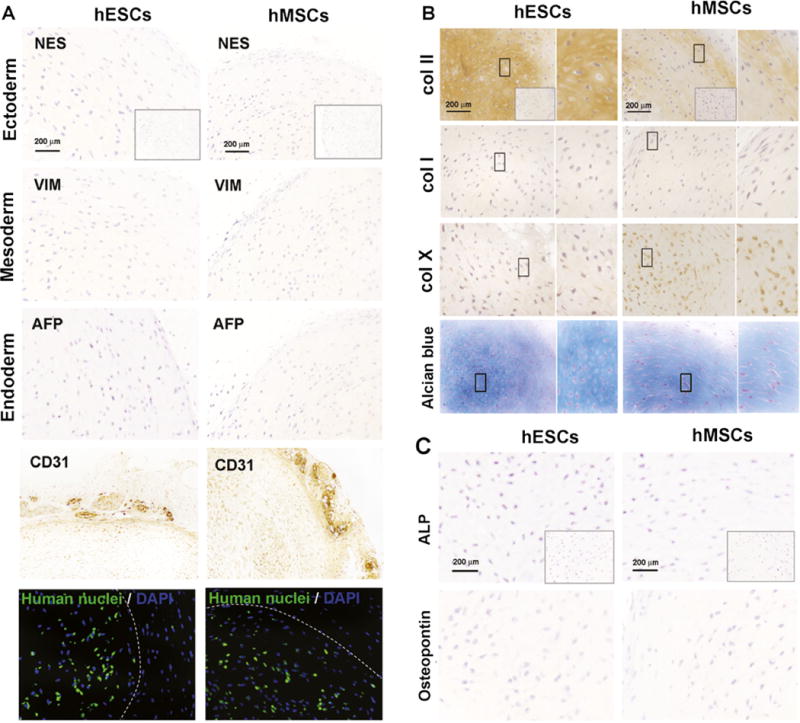
Phenotypic stability of chondrogenic pellets implanted in ectopic location. a Identification of three germ layers by immunohistochemistry. Nestin (NES) indicates ectoderm, Vimentin (VIM) indicates mesoderm and Alpha-fetoprotein (AFP) indicates endoderm. Inserts show negative staining controls. Vascular endothelia cells were stained with CD31 antibody. Immunofluorescence staining confirmed the human origin of chondrogenic pellets (green). The dashed line shows the outline of the pellets. b Immunohistochemistry of collagen and Alcian blue staining for GAG. Rectangular boxes indicate 60 × magnified regions. Insert boxes show negative staining of immunohistochemistry. c Immunohistochemistry of bone markers alkaline phosphatase (ALP) and osteopontin. Inserts show negative staining control.
Discussion
hESCs are an effectively unlimited source of cells that can be differentiated into any specialized lineage from all three germ layers, and thus have a great research and therapeutic potential, assuming we can address the challenges associated with their controlled differentiation into desired lineages, and avoid tumorogenicity resulting from the presence of undifferentiatend cells.
We propose a novel approach for enhancing chondrogenic differentiation of hESCs that combines two factors previously shown to be necessary for the development and maintenance of healthy cartilage: controlled oxygen tension and morphogenetic factors secreted by chondrocytes [31]. In our experiments, chondrogenic progenitors were induced during 3 weeks of EB culture, a critical period during which the mesodermal precursors develop under various induction regimes [32–34].
hESC-EBs cultured in CCM under transient hypoxia (5 % O2 for 2 weeks, followed by 21 % O2 for 1 week, Group 4) exhibited the highest expression of SOX9 (Fig. 2), and chondrogenic progenitors dissociated from these EBs displayed excellent chondrogenic differentiation in subsequent pellet cultures (Figs. 3, 4 and 5). These data indicate that both CCM and transient hypoxia stimulate chondrogenic commitment of hESCs in EB culture (Fig. 2ab), and that lineage specific differentiation depends not only on cell-secreted factors, but also on oxygen levels as previously reported for osteogenic differentiation [35, 36]. The transient hypoxia had stronger effect on priming the chondrogenic progenitors in EBs when maintained for 2 weeks compared to only 1 week (Fig. 2). A possible explanation is that at early stages of development, it is beneficial to have a longer period of hypoxia to promote the expression of cartilaginous genes, while at later stages the transfer to normoxic, energy-rich conditions enhances the production and functional assembly of cartilaginous matrix [23].
We decided to use hypoxic conditions only during EB-culture and not during the pellet culture, based on the results from the study by Koay et al. that had two phases, embryoid body (EB) differentiation for 3 weeks followed by a scaffoldless tissue engineering strategy called self-assembly (for 4 weeks) [19]. During each phase, hypoxic conditions (2 % O2) or normoxic conditions (20 % O2) were applied. The hESCs differentiated under normoxic conditions during EB culture made constructs that were not as matrix rich as compared to the cells differentiated under hypoxic conditions during EB culture, which produced dense, collagen-rich constructs. In the “hypoxic EB” groups, collagen I and collagen II accounted for a greater proportion of the total collagen and the proportion of collagen II to total collagen increased about twofold. In addition, the GAG content was the highest in the group with hypoxic EB culture and normoxic self-assembled constructs. Also normoxic self-assembled constructs outperformed hypoxic self-assembled ones in tensile and compressive biomechanical characteristics.
The general conclusion of this study was that hypoxic EB culture outperforms normoxic EB culture, while normoxic self-assembly outperforms hypoxic self-assembly, which is one of the reasons why we adopted the hypoxic EB culture followed by the normoxic pellet culture protocol.
In addition, other studies that used chondrocytes alone [37] as well as co-culture of chondrocytes and MSCs [38] show the enhanced cartilage formation with hypoxia expanded cells, but decreased chondrogenesis within hypoxic pellet cultures.
Our hypothesis was about testing the different regimens of hypoxia during EB culture i.e., early steps of chondrogenesis in combination with morphogenetic factors, which is why we used hypoxia only for EB culture and normoxia for pellet culture. We believe this approach brings novelty and avoids repetition of already documented findings.
The observed effect on the COL2A1 expression in the later normoxic stage of culture confirms that the conditions (transient hypoxia and morphogenetic factors) used in pre-differentiation stage of culture induced differentiation towards chondrogenic phenotype. One way to explain this detected effect is that the protocol of transient hypoxia during early chondrogenesis followed by normoxia is similar to native chondrogenesis. No matter what the location or stage of development, chondrocytes in growth cartilage are arranged in morphologically distinct zones [39], with stratification that reflects the temporal gradients in cell differentiation [40], as well as differences in oxygen tensions [41]. The zones of the cartilage component in the growth plate are reserve, proliferative and hypertrophic zone: the reserve zone has low oxygen tension (analogous to our EB-induction regimen) while proliferative zone exhibits the highest oxygen concentration and is analogous to the normoxia regimen present in the pellet structure in our study. Our hypothesis can be presented as that the progenitors subjected to the hypoxic conditions similar to the reserve zone will eventually differentiate into cells with highly expressed chondrogenic markers analogous to the chondrocytes in proliferative, normoxic zone.
The up-regulation of SOX9 in the transient hypoxia EBs (Group 4), our best induction regime to obtain chondrogenic progenitors, coincided with strong down-regulation of OCT4, suggesting that EB culture conditions suppress the population of pluripotent stem cells that could differentiate into other lineages and form teratomas in vivo (Figs. 2 and 7). Loss of pluripotency was further evidenced by the decrease and eventual disappearance of OCT4 and NANOG expression during chondrogenic differentiation in pellet cultures (Fig. 3). Expression of SOX9 in hESC-pellets lagged behind the expression in hMSC pellets, indicating that hMSCs differentiated into chondrogenic lineage sooner than hESCs-progenitors dissociated from EB cultures. However, chondrogenic differentiation potential of hMSCs was limited when compared to hESCs-progenitors induced under transient hypoxia, based on the measured overall expression levels of SOX9, ACAN and COL2A1 in pellet cultures (Fig. 3, 4 and 5).
The expression of FOXA2, a key transcription factor of gut formation that would indicate the presence of endodermal lineages, was suppressed in hESC pellets already during the first week of culture (Fig. 3). However, the expression of PAX6, a transcription factor in the formation of eye and sensory organs that would indicate the presence of ectodermal lineages, decreased more slowly (by week 6) and it was higher in hypoxic than normoxic cultures, consistent with the role of low oxygen tensions in neurogenic induction [42]. Therefore, an optimal balance between soluble biochemical cues and oxygen tension on chondrogenic differentiation of hESCs remains to be determined.
Efficient isolation of chondrogenic progenitors from EBs remains a challenge. Scarce information about surface markers limits the use of fluorescence activated or magnetic cell sorting for the selection of chondrogenic progenitors. We thus chose to dissociate EBs into single cells and use the whole single cell population for the preparation of pellets. The cells underwent self-selection to form aggregates and initiate the first step of chondrogenic differentiation - the cell condensation. Formation of pellets also helped the formation of more homogenous cell aggregates compared to the heterogeneous EBs, which are characterized by gastrula-like tissues with varying local concentrations of morphogenic factors [43–45]. At 6 weeks, chondrogenic pellets derived from transient hypoxia EBs (Group 4) exhibited an excellent homogeneity, which could partially be attributed to the loss of unspecified cells remaining in cell suspension and washed away during medium changes in pellet cultures. These discordant cells can separate from chondrogenic progenitors, enter apoptotic pathway and leave the aggregate [46].
Immune staining of collagen type II and Western blot analysis confirmed that induction in transient hypoxia (Group 4) enhanced hESCs to enter chondrogenic lineage as compared to induction in normoxia (Group 2). It is possible that hypoxia and chondrocyte-secreted morphogenetic factors augmented the chondrogenic differentiation of hESC-progenitors by promoting collagen II gene expression and allowing procollagen type II to undergo post-translational modification before being secreted [47]. Collagen type II was also detected in pellets derived from normoxia EBs and hMSCs, but was less completely secreted into pericellular spaces than in transient hypoxia EBs (Fig. 4b). Up-regulation of collagen-remodeling enzymes, e.g., P4HA1, P4HA2, and PLOD2 was reported in tumors under low oxygen tension, via the hypoxic inducible factor 1 alpha (HIF-1A) pathway [48, 49].
Our model of chondrogenic differentiation of hESCs in EBs and pellet culture system corresponds to N-cadherin-mediated cell condensation of hMSCs. Cell condensation is directed in part by cell-cell interactions mediated by N-cadherin, as demonstrated using neutralizing antibody to block biological activity of N-cadherin in vitro in the hMSC culture and in vivo for the limb bud formation [50]. We found time-dependent expression of CDH2 encoding for N-cadherin and differential proportion of N-cadherin positive cells in transient hypoxia EB-derived pellets as compared to the cells remaining in cell suspension during the first week of pellet cultures. We thus suggest that N-cadherin presenting cells played an important role in chondrogenic pellet formation and tissue homogeneity. Nonetheless, the dissociated cells that express N-cadherin were not conclusively shown to be chondrogenic progenitors. FGF receptors and N-CAM on cell surfaces have also been involved in mesenchymal condensation [30, 51]. Further studies of adhesion proteins could be a powerful tool to identify the markers of chondrogenic progenitors and help develop improved cell selection/sorting protocols.
We propose that the sequence of hESCs-derived chondrogenic pellet formation starts with the aggregation of N-cadherin presenting cells that have chondrogenic differentiation potential. It is possible that the cells induced under normoxia (Group 2) contained smaller number of chondrogenic progenitors and/or N-cadherin presenting cells, and therefore had lower ability for condensation and phenotypic maturation in comparison to cells induced under transient hypoxia EBs regime (Group 4).
Tumor formation is a major safety concern in the use of pluripotent hESCs. Therefore, we set to evaluate the stability of hESCs (transient hypoxia EB)-derived chondrogenic pellets, and their resistance to vascular invasion and hypertrophic differentiation in an ectopic environment. Assessment of explanted chondrogenic pellets revealed excellent homogeneity and abundance of cartilaginous ECM without evidence of dedifferentiation or teratoma formation (Fig. 7). In addition, hESCs-derived chondrogenic pellets did not undergo hypertrophy and mineralization, as evidenced by the lack of the late-stage chondrocyte hypertrophic marker, collagen X, and early markers of noncollagenous bone matrix alkaline phosphatase and osteopontin [52]. Vascular invasion during chondrogenesis is essential for endochondral ossification [53]. The hESCs-derived pellets showed ectopic stability of cartilage tissue after 4 weeks of implantation into a highly vascularized region, and were surrounded by capillaries but not vascularized.
In summary, we demonstrate phenotypically stable differentiation of hESCs into chondrogenic cells, by coordinated application of transient hypoxia and soluble morphogenetic factors secreted by primary chondrocytes. These cells, derived without the use of laborious cell sorting, differentiated into homogenous, well-developed cartilaginous pellets exhibiting lacunar structures and strong positive staining of cartilage matrix components. Our findings expand the current understanding of chondrogenic differentiation by control of oxygen tension and suggest that transient hypoxia reinforced the chondrogenic effect of regulatory factors secreted by primary chondrocytes.
Supplementary Material
Acknowledgments
We gratefully acknowledge research funding received from the NIH (grants DE016525, EB002520 and AR061988 to GVN), New York Stem Cell Foundation (NYSCF-Helmsley Investigator award to DM; grant CU09-3055 to GVN), the Ministry of Education and Science of Serbia (grants ON174028 and III41007 to IG) and Royal Thai Graduate Fellowship (to SY). We thank Dr. Jenny Yuan and Chandhanarat Chandhanayingyong for their help with animal studies.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12015-015-9584-x) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors declare no conflict of interest.
Contributor Information
Supansa Yodmuang, Department of Biomedical Engineering, Columbia University, 622 West 168th Street, Vanderbilt Clinic, 12th floor, Room VC12-234, New York, NY 10032, USA.
Darja Marolt, The New York Stem Cell Foundation Research Institute, New York, NY, USA.
Ivan Marcos-Campos, Department of Biomedical Engineering, Columbia University, 622 West 168th Street, Vanderbilt Clinic, 12th floor, Room VC12-234, New York, NY 10032, USA.
Ivana Gadjanski, Center for Bioengineering-BioIRC, Belgrade Metropolitan, University, Kragujevac, Serbia.
Gordana Vunjak-Novakovic, Email: gv2131@columbia.edu, Department of Biomedical Engineering, Columbia University, 622 West 168th Street, Vanderbilt Clinic, 12th floor, Room VC12-234, New York, NY 10032, USA.
References
- 1.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Research and Therapy. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. International Journal of Experimental Pathology. 2012;93(6):389–400. doi: 10.1111/j.1365-2613.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, et al. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnology and Bioengineering. 2004;88(3):379–391. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 5.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells and Development. 2009;18(7):969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 6.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nature Protocols. 2010;5(7):1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harbor Perspectives in Biology. 2013;5(1):a008334. doi: 10.1101/cshperspect.a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis and Rheumatism. 2006;54(10):3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 10.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 11.Sa S, McCloskey KE. Stage-specific cardiomyocyte differentiation method for h7 and h9 human embryonic stem cells. Stem Cell Reviews. 2012;8(4):1120–1128. doi: 10.1007/s12015-012-9403-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circulation Research. 2009;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, et al. Engineering bone tissue from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8705–8709. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nature Biotechnology. 2010;28(11):1187–1194. doi: 10.1038/nbt.1683. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis and Rheumatism. 2009;60(12):3686–3692. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- 16.Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, et al. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31(27):6968–6980. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 17.Amarilio R, Viukov SV, Sharir A, Eshkar-Oren I, Johnson RS, Zelzer E. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134(21):3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 18.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(13):4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis and Cartilage. 2008;16(12):1450–1456. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One. 2008;3(6):e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(52):20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima EG, Grace Chao PH, Ateshian GA, Bal BS, Cook JL, Vunjak-Novakovic G, et al. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008;29(32):4292–4299. doi: 10.1016/j.biomaterials.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yodmuang S, Gadjanski I, Chao PH, Vunjak-Novakovic G. Transient hypoxia improves matrix properties in tissue engineered cartilage. Journal of Orthopaedic Research. 2013;31(4):544–553. doi: 10.1002/jor.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 27.Bruckner P, Horler I, Mendler M, Houze Y, Winterhalter KH, Eich-Bender SG, et al. Induction and prevention of chondrocyte hypertrophy in culture. Journal of Cell Biology. 1989;109(5):2537–2545. doi: 10.1083/jcb.109.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, Ito Y, Chappel J, Andrews NW, Teitelbaum SL, Ross FP. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteblast secretion. Developmental Cell. 2008;14(6):914–925. doi: 10.1016/j.devcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Experimental Cell Research. 1994;215(2):354–362. doi: 10.1006/excr.1994.1352. [DOI] [PubMed] [Google Scholar]
- 31.Meier S, Solursh M, Vaerewyck S. Modulation of extracellular matrix production by conditioned medium. American Zoologist. 1973;13:1051–1060. [Google Scholar]
- 32.Keller GM. In vitro differentiation of embryonic stem cells. Current Opinion in Cell Biology. 1995;7(6):862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 33.Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mechanisms of Development. 2000;92(2):193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 34.Vats A, Bielby RC, Tolley N, Dickinson SC, Boccaccini AR, Hollander AP, et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Engineering. 2006;12(6):1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin S, Sheyn D, Ben-David S, Oh A, Kallai I, Li N, et al. Oxygenated environment enhances both stem cell survival and osteogenic differentiation. Tissue Engineering Part A. 2013;19(5–6):748–758. doi: 10.1089/ten.TEA.2012.0298. [DOI] [PubMed] [Google Scholar]
- 36.Muller J, Benz K, Ahlers M, Gaissmaier C, Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplantation. 2011;20(10):1589–1602. doi: 10.3727/096368910X564094. [DOI] [PubMed] [Google Scholar]
- 37.Egli RJ, Bastian JD, Ganz R, Hofstetter W, Leunig M. Hypoxic expansion promotes the chondrogenic potential of articular chondrocytes. Journal of Orthopaedic Research. 2008;26(7):977–985. doi: 10.1002/jor.20603. [DOI] [PubMed] [Google Scholar]
- 38.Meretoja VV, Dahlin RL, Wright S, Kasper FK, Mikos AG. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials. 2013;34(17):4266–4273. doi: 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Gadjanski I, Spiller K, Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Reviews. 2012;8(3):863–881. doi: 10.1007/s12015-011-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brighton CT. Epiphyseal bone formation. In: Newton CD, Nunamaker DM, editors. Textbook of Small Animal Orthopaedics. Lippincott Williams & Wilkins; 1985. pp. 50–55. [Google Scholar]
- 42.Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Molecular Neurobiology. 2005;31(1–3):231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]
- 43.Van Winkle AP, Gates ID, Kallos MS. Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells, Tissues, Organs. 2012;196(1):34–47. doi: 10.1159/000330691. [DOI] [PubMed] [Google Scholar]
- 44.Wilson JL, Suri S, Singh A, Rivet CA, Lu H, McDevitt TC. Single-cell analysis of embryoid body heterogeneity using microfluidic trapping array. Biomedical Microdevices. 2014;16(1):79–90. doi: 10.1007/s10544-013-9807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stachelscheid H, Wulf-Goldenberg A, Eckert K, Jensen J, Edsbagge J, Bjorquist P, et al. Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(9):729–741. doi: 10.1002/term.1467. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita A, Krawetz R, Rancourt DE. Loss of discordant cells during micro-mass differentiation of embryonic stem cells into the chondrocyte lineage. Cell Death and Differentiation. 2009;16(2):278–286. doi: 10.1038/cdd.2008.149. [DOI] [PubMed] [Google Scholar]
- 47.Schipani E. Posttranslational modifications of collagens as targets of hypoxia and Hif-1alpha in endochondral bone development. Annals of the New York Academy of Sciences. 2010;1192:317–321. doi: 10.1111/j.1749-6632.2009.05236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. Journal of Biological Chemistry. 2013;288(15):10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Eisinger-Mathason TS, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3(10):1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120(1):177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 51.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes and Development. 2002;16(12):1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 52.Butler WT. The nature and significance of osteopontin. Connective Tissue Research. 1989;23(2–3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 53.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. Journal of Cellular Biochemistry. 2006;97(1):33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


