Abstract
The blood calcium concentration during fetal life is tightly regulated within a narrow range by highly interactive homeostatic mechanisms that include transport of calcium across the placenta and fluxes in and out of bone; the mechanisms of this regulation are poorly understood. Our findings that endochondral bone-specific PTH/PTHrP receptor (PPR) knockout (KO) mice showed significant reduction of fetal blood calcium concentration compared with that of control littermates at embryonic day 18.5 led us to focus on bone as a possibly major determinant of fetal calcium homeostasis. We found that the fetal calcium concentration of Runx2 KO mice was significantly higher than that of control littermates, suggesting that calcium flux into bone had a considerable influence on the circulating calcium concentration. Moreover, Runx2:PTH double mutant fetuses showed calcium levels similar to those of Runx2 KO mice, suggesting that part of the fetal hypocalcemia in PTH KO mice was caused by the increment of the mineralized bone mass allowed by the formation of osteoblasts. Finally, Rank:PTH double mutant mice had a blood calcium concentration even lower than that of the either Rank KO or PTH KO mice alone at embryonic day 18.5. These observations in our genetic models suggest that PTH/PTHrP receptor signaling in bones has a significant role of the regulation of fetal blood calcium concentration and that both placental transport and osteoclast activation contribute to PTH's hypercalcemic action. They also show that PTH-independent deposition of calcium in bone is the major controller of fetal blood calcium level.
The blood calcium concentration is tightly regulated within a narrow range by highly interactive homeostatic mechanisms. PTH regulates blood calcium concentrations through actions on the kidney and bone (1). PTH is secreted from the parathyroid gland, which senses small reductions in blood calcium levels (2). PTH directly acts on bone to stimulate calcium mobilization into the systemic circulation. In addition, PTH acts on the kidney to increase the blood calcium level by increasing distal tubular calcium reabsorption and producing a proximal tubular 1α-hydroxylation of vitamin D, which in turn increases calcium absorption from intestine and increases bone resorption (3). These actions of PTH eventually result in an increase in the blood calcium, which in turn reduces the release of PTH from the parathyroid gland to complete the cycle of these calcemic events. Postnatally, calcium primarily enters the system through the digestive system and is excreted from the kidney. In addition to this systemic role in maintenance of calcium levels in body fluids, PTH also modulates local events such as accumulation of bone mass (4). On the other hand, during fetal life, the regulation of calcium homeostasis is much less completely understood. Calcium is actively transported from the mother's blood to the fetus across the placenta, which plays a significant role in maintaining fetal calcium homeostasis (5). As the skeletal system develops in fetal life, calcium is rapidly deposited in bone. The roles of fetal bone, kidney, intestine, and other organs in maintaining fetal calcium homeostasis are topics of current study (6).
PTH regulates blood calcium in fetal life as well as in postnatal life. The genetic loss of PTH causes severe hypocalcemia in fetuses, at least partly through decreased net flux of calcium across the placenta (7). PTH also modulates local events such as fetal bone formation (8). The relative of PTH, PTHrP also regulates fetal blood calcium and does so at least in part through activation of a receptor distinct from the PTH/PTHrP receptor (PPR) in the placenta (9). Loss of the PPR appears to cause more severe fetal hypocalcemia than either loss of PTH or PTHrP alone (7, 9); these levels were comparable with those caused by loss of both PTH and PTHrP (10). Although the placenta is clearly an important target organ for both PTH and PTHrP, the observation that PPR knockout (KO) mice are severely hypocalcemic despite increased placental calcium transport (9) suggests that other target organs of the PPR are vital for fetal calcium homeostasis. PTH regulates calcium handling by the fetal kidney, as well (5). For example, parathyroidectomy in fetal sheep caused increased renal clearance of calcium but no change in total excreted calcium (presumably because of the low filtered load) (11). However, because fetal urine contributes to amniotic fluid that is swallowed by the fetus, this recycling of renal excretory products may lessen the role of the renal response to PTH in regulating blood calcium (5).
In this manuscript we focus on bone as a possibly major determinant of calcium homeostasis in the fetus and target organ for action of PTH, PTHrP, and the PPR. Previous studies have suggested that increased activation of PTH receptors in fetal bone raises blood calcium. Fetuses with hyperparathyroidism caused by ablation of the calcium-sensing receptor are hypercalcemic and have skeletons with diminished ash weight. An increase in levels of deoxypyridinoline in amniotic fluid of these mice suggests that increased bone resorption might contribute to the hypercalcemia of these mice (12). Further, in fetal mice missing the PTH receptor, expression of a constitutively active PTH receptor only in osteoblastic cells increased blood calcium (13). Here, we examine the role of PTH action on bone in normal fetal calcium homeostasis. We show that the PTH-independent deposition of calcium in bone is a major determinant of fetal blood calcium. PTH increases fetal bone resorption, in addition to its actions on net placental calcium flux and renal tubules to maintain fetal blood calcium despite the enormous movement of calcium into bone.
Materials and Methods
Mice
Collagen IIα1 promoter-Cre (Col2-Cre) transgenic mice (14), and Runt-related gene 2/core binding factor 1 (Runx2) (+/−) (15), receptor activator of nuclear factor κB (RANK) (+/−) (16), PTH (+/−) (8), and PTHrP (+/−) (17) have been described. To generate Col2-Cre:PPRfl/fl mice, we crossed Col2-Cre:PPRfl/f+ mice to PPRfl/fl mice and then used the Col2-Cre:PPRfl/fl and PPRfl/fl progeny littermates in all the experiments described here. The Col2-Cre:PPRfl/fl mice are from mixed 129/Sv and C57BL/6J backgrounds. Double KOs of PTH and Runx2 were created by mating heterozygous (PTH+/− and Runx2+/−) mice to generate double heterozygotes (ie, PTH+/−:Runx2+/−) mice. Double KOs of PTHrP and Runx2 were created by mating heterozygous (PTHrP+/− and Runx2+/−) mice to generate double heterozygotes (ie, PTHrP+/−:Runx2+/−) mice. Double KOs of PTH and Rank were created by mating heterozygous (Rank+/− and PTH+/−) mice to generate double heterozygotes (ie, Rank+/−:PTH+/−). These mice were maintained on a mixed genetic background with contributions from C57BL/6, 129/SvJ, and Black Swiss strain. Littermates were used as controls for all experiments. Mice of both genders were used in each experiments. All mice were given a standard chow diet and water. Genomic DNA was isolated from tail clips to genotype mice by standard methods described previously (18). Genotyping of Cre transgenic mice was performed by PCR using primers detecting the Cre sequence (19). These studies were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital. Mice were maintained in a virus- and parasite-free barrier facility and exposed to a 12-hour light, 12-hour dark cycle.
Biochemical parameter
Blood ionized calcium was measured with a Chiron Diagnostics model 634 Ca2+/pH analyzer as described previously (19). Serum total calcium level was measured by using the Calcium CPC LiquiColor test kit (Stanbio Laboratory).
Histology and LacZ staining
Five-micron sections were deparaffinized, rehydrated in a graded ethanol series, and transferred to distilled water. For morphological assessment of the growth plate, sections were stained with hematoxylin and eosin or 1% methyl green, then dehydrated and mounted. Cre activity was assessed using Rosa26-R (R26R) reporter mice: Cre transgenic mice were crossed with R26R mice. Embryos were collected, fixed and stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal), as described previously (20).
Statistics
Statistically significant differences between groups were evaluated by one-way ANOVA. P <.05 was considered to be statistically significant.
Results
Conditional PPR ablation in bone and cartilage causes hypocalcemia
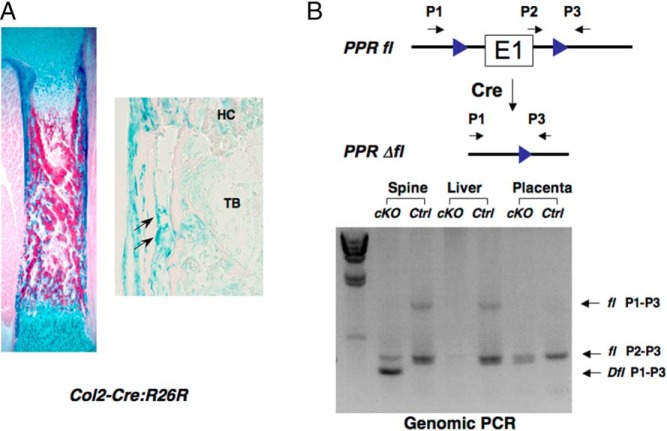
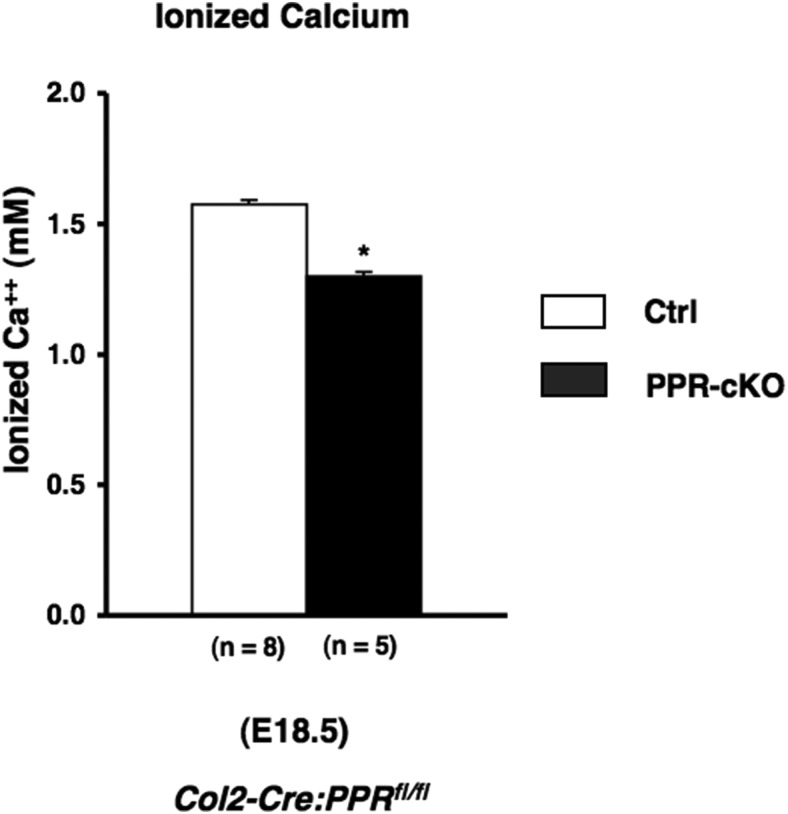
In order to examine the role of bone as a target organ in PPR regulation of fetal calcium homeostasis, we generated mutant mice in which the PPR was disrupted in cartilage and bone. Transgenic mice in which Cre recombinase is driven by a collagen IIα1 promoter (Col2-Cre) were crossed to mice in which the essential E1 exon of the PPR gene was flanked by loxP sequences (floxed PPR) (21). As the collagen IIα1 promoter is active in common progenitor cells of chondrocytes and osteoblasts in endochondral bones, this system can remove target genes both in the endochondral chondrocytes and adjacent osteoblasts. To test Cre specificity, we crossed Col2-Cre transgenic mice to Rosa26; Cre reporter mice (18) that express LacZ upon Cre activation. As shown in Figure 1A, the LacZ-expressing cells assessed by using X-gal staining were seen in chondrocytes, cortical bones, and trabecular bones, indicating that the ROSA gene was activated both in chondrocytes and osteoblasts in the endochondral bones. Recently, Ono et al have used more sensitive reporter than β-galactosidase to show that Col2-Cre marks most osteoblasts (22). We then used these Col2-Cre transgenic mice to delete the PPR gene from osteoblasts in the endochondral bones, through mating with the floxed PPR mice (Col2-Cre:PPRfl/fl). DNA recombination of the PPR gene in the Col2-Cre:PPRfl/fl mice was seen in the spine, but not in placenta or liver, showing specific gene ablation in skeletal tissues (Figure 1B). The bones of these mice, demonstrated at embryonic day (E)15.5 and E17.5 previously (21), resemble those of the universal PPR KO (21), in that they are shorter than wild-type (WT) because of their growth plate phenotype, and the cortical bone of the tibia at E18.5 was thicker than that found in WT tibia (Supplemental Figure 1). We found significant reduction of the fetal blood calcium concentration in Col2-Cre:PPRfl/fl mice at E18.5 compared with that of control littermates (Figure 2). Because this finding probably underestimates the role of PPR in osteoblasts, osteocytes, and hypertrophic chondrocytes, because of less than complete gene ablation in endochondral bones and likely continued expression of the PPR in the skull, this result suggests that PPR signaling in endochondral bones has a significant role of the regulation of blood calcium concentration in fetal life.
Figure 1.
Conditional ablation of PPR in chondrocytes and endochondral osteoblasts. A, Tissue-specific activity of Cre recombinase. Col2-Cre:R26R doubly transgenic, E17.5 embryo was stained with X-gal to visualize Cre activity (left panel). Chondrocytes, the bone collar, and the primary spongiosa are stained blue, representing lacZ activity. Right panel showed higher magnifications of a section stained by X-gal. Arrows indicate single LacZ-positive cells. HC, hypertrophic cartilage; TB, trabeculae. B, A diagram showing the PPR conditional and deletion alleles and locations of primers for detecting Cre-mediated recombination. Floxed PPR allele and Cre transgene detected by genomic PCR from spine, liver, and placenta of genomic DNA. Arrows show positions and directions of PCR primers used for detecting products of recombination.
Figure 2.
Conditional PPR ablation in bone and cartilage causes hypocalcemia. Blood ionized calcium in fetuses (E18.5) obtained from and Col2-Cre:PPRfl/fl and PPRfl/fl control mice. The number of observations for each genotype is indicated within parentheses. Statistical significance: *, P < .05, significantly different from PPRfl/fl control mice.
Critical role of bone in fetal calcium homeostasis
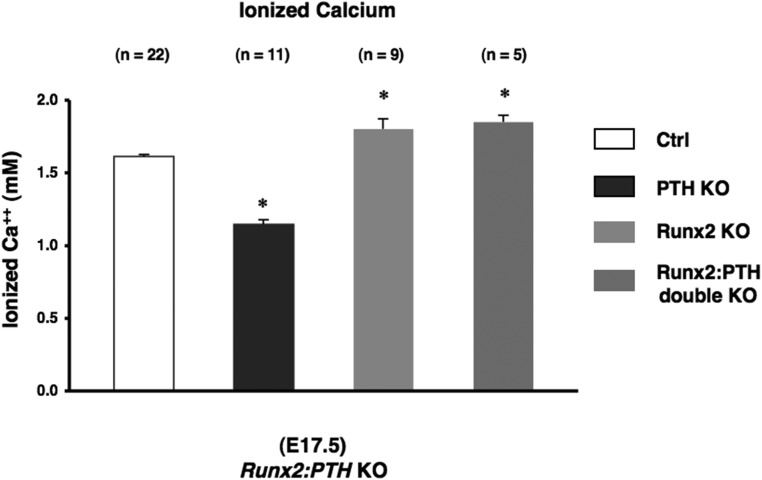
Next, to determine the role of PTH signaling in fetal bone on the levels of fetal blood calcium, we analyzed the fetal calcium levels of PTH KO mice, Runx2 KO, and Runx2:PTH double KO mutant mice at E17.5 (Figure 3). PTH KO fetuses had a reduced blood calcium concentration compared with their control littermates (Figure 3). Miao et al (8) previously showed that newborn PTH KO mice have less trabecular bone and more cortical bone than WT, with a decrease in both osteoblasts and osteoclasts in the long bones of these mice and a decrease in the mineralization of hypertrophic cartilage. The decrease in blood calcium that we note at E18.5 presumably reflects a net decreased flux of calcium across the placenta (7) possible effects of PTH on renal handling of calcium, and the net effects of the decrease in osteoblast and osteoclast activity in these mice. The decreased ash weight of these mice (Simmonds et al [7] and Supplemental Figure 2) presumably reflects the decreased net placental calcium flux into the fetus and possibly the decreased osteoblast activity in these mice, despite the decreased number of osteoclasts.
Figure 3.
Essential role of bone in fetal calcium homeostasis. Blood ionized calcium in fetuses (E17.5) obtained from Runx2, PTH, and Runx2:PTH double KO mice. The number of observations for each genotype is indicated within parentheses. Statistical significance: *, P < .05, significantly different from control mice.
To dissect out the role of bone in the hypocalcemia of PTH KO mice, we first examined the blood calcium of Runx2 KO mice. Runx2 KO bones have no osteoblasts or bone matrix; alizarin Red (which binds to calcium) fails to detect any mineralized matrix in these bones (15). In the Runx KO mice, we observed that the fetal calcium concentration was significantly higher than that of control littermates, demonstrating that calcium flux into bone had a considerable influence on the circulating calcium concentration In addition, Runx2:PTH double mutant fetuses showed calcium levels similar to those of Runx2 KO mice. This double mutant mouse had higher blood calcium than the PTH null mutant mouse alone (Figure 3). This finding suggested that the bone in mice with normal Runx2 draws calcium from the bloodstream, lowering the level of blood calcium; some transfer of calcium into bone occurs even in the absence of PTH. Next, to determine the requirement for osteoblasts in the actions of PTHrP to maintain fetal calcium homeostasis, we analyzed fetal calcium levels of Runx2:PTHrP double mutant mice at E17.5 (Supplemental Figure 3). Runx2:PTHrP double mutant fetuses exhibited calcium levels similar to those of Runx2-null mice. These observations, that deletion of either PTH or PTHrP does not lower blood calcium in mice without osteoblasts, provide in vivo evidence that bone is a major target of PPR signaling in regulation of blood calcium concentration.
Role of osteoclasts in mediating effects of PTH on fetal blood calcium
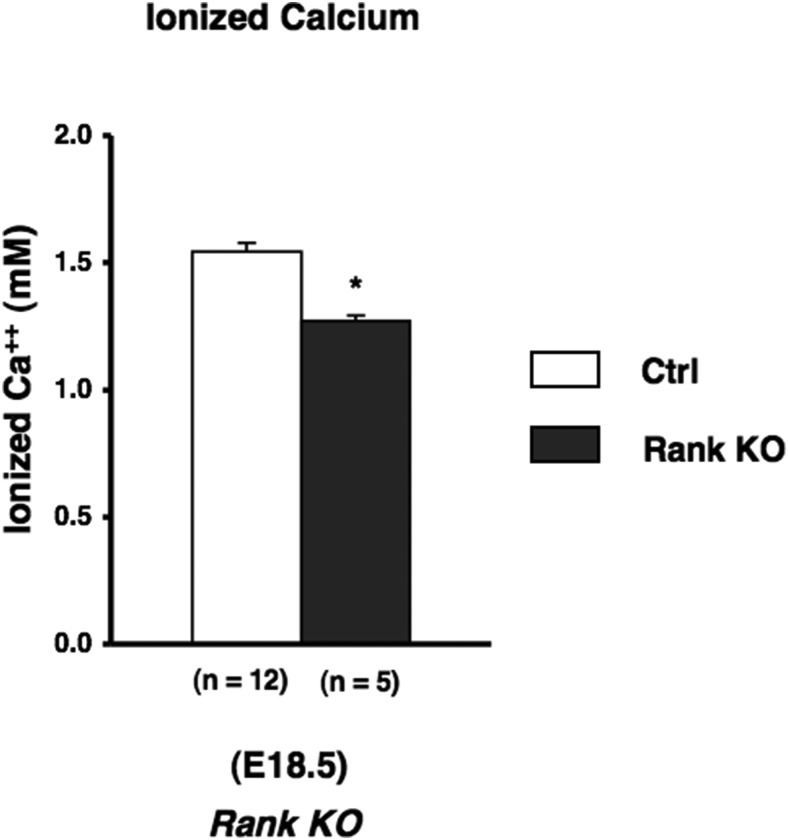
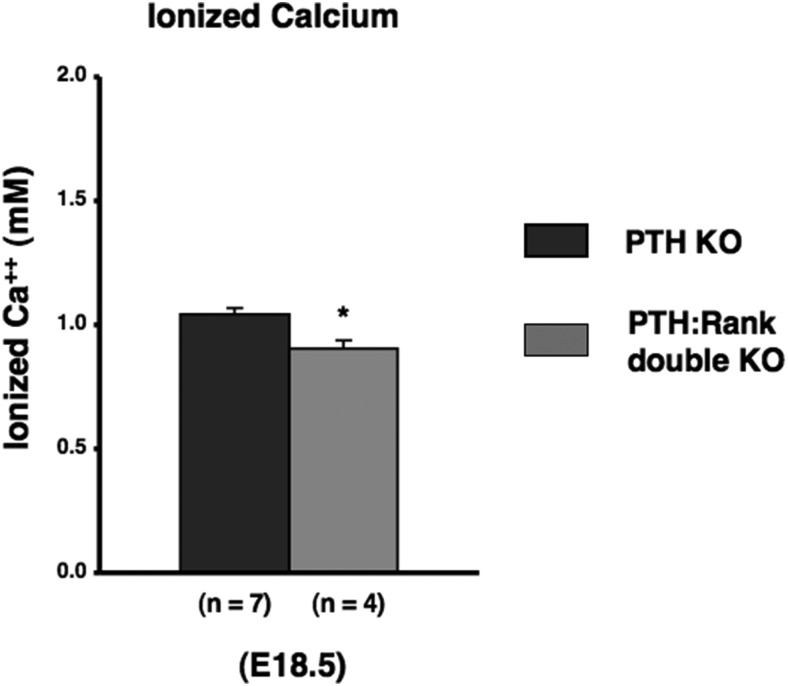
To assess the role of osteoclastic bone resorption in regulation of calcium concentration in fetal mice, we analyzed fetal calcium levels in RANK KO mice at E18.5. These mice make no osteoclasts; we found no tartrate-resistant acidic phosphatase-positive multinuclear cells in RANK KO mice (Supplemental Figure 4). In addition, the long bones of these mice have an expanded hypertrophic layer with an accumulation of chondrocytes (Supplemental Figures 5–7), as previously reported in postnatal RANK KO mice (16, 23). This finding is consistent with a role for osteoclasts in limiting the extent of the hypertrophic region. However, the fetal tibia in RANK KO mice does not exhibit the enormous accumulation of osteoblastic cells reported for postnatal RANK KO mice (16). The RANK KO bones exhibited mineralization of cortical, trabecular, and cartilage areas by von Kossa analysis (Supplemental Figure 6). Ablation of Rank reduced the fetal calcium level (Figure 4), but this effect was less than the lowering of blood calcium in PTH KO mice. To assess the relative contribution of osteoclastic bone resorption as a mediator of the effects of PTH on fetal calcium levels, we analyzed fetal calcium levels of Rank:PTH double mutant mice at E18.5. The bones of these mice resembled those of the RANK KO mice (Supplemental Figures 4–7). Rank:PTH double mutant had a blood calcium concentration even lower than that of the PTH KO mice alone (Figure 5). This result suggests that the action of PTH to stimulate osteoclastogenesis and osteoclast activity cannot explain all the effects of PTH on fetal blood calcium. Simmonds et al (7) postulated that PTH may decrease the flux of calcium back from the fetus to the mother in their attempt to explain fetal calcium homeostasis; the present data are consistent with this postulate. Further, this result means that PTH is not the only determinant of osteoclastic activity in fetal life, because, even without PTH, RANK action contributes to fetal calcium homeostasis.
Figure 4.
Contribution of osteoclastic activity in fetal calcium concentration. Blood ionized calcium in fetuses (E18.5) obtained from Rank KO mice. The number of observations for each genotype is indicated within parentheses. Statistical significance: *, P < .05, significantly different from control mice.
Figure 5.
Osteoclastic activity in fetal calcium concentration in hypocalcemia of PTH null mice. Blood ionized calcium in fetuses at E18.5 obtained from Rank:PTH double KO mice. Statistical significance: *, P < .05, significantly different from the value obtained in PTH null mice.
Discussion
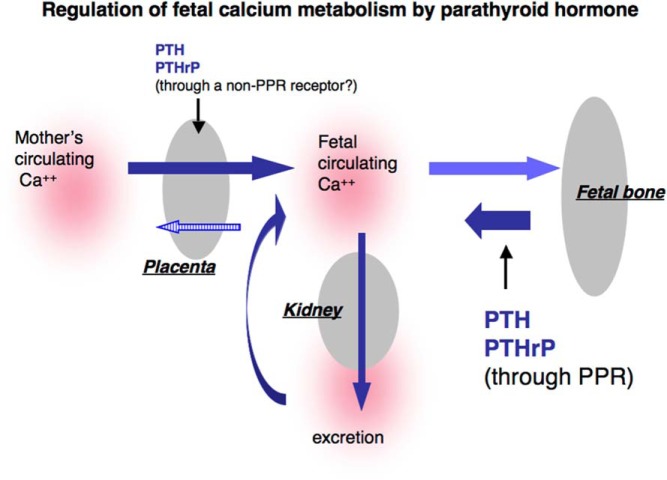
In the present study, we explore the role of bone in fetal calcium homeostasis and the role of bone in mediating the actions of PTH and PTHrP. We demonstrate that activation of the PPR expressed in bone, plays an essential role in calcium homeostasis in fetal mice, because mice missing these receptors have low levels of blood calcium. This result suggests that actions of the PPR in bone tend to increase blood calcium. In this way, the actions of PTH on fetal bone may be synergizing with those of PTH on the placenta, which also act to increase fetal blood calcium (7). The considerably lower blood calcium found in the PTH KO mouse than in the conditional KO of the PPR in bone may well result from the actions of PTH on the placenta as well as on the bone. The lower than normal ash weight of bone from the PTH KO mouse (Figure 6) is consistent with an important role for placental transport of calcium to allow normal mineralization of bone, as well as, perhaps, an important role for PTH in enhancing bone formation by stimulating osteoblast activity.
Figure 6.
Regulation of fetal calcium homeostasis by PTH and PTHrP.
Runx2 is a key transcription factor required for osteoblast differentiation and bone formation (15, 24). The absence of osteoblast differentiation in Runx2 KO mice leads to little skeletal mineralization (15). The Runx2 KO mutants had higher blood calcium than WT mice. This finding supports the idea that bone formation drives calcium from the bloodstream into bone and that, in the absence of osteoblasts, fetal blood calcium rises. Further, the Runx2:PTH double mutants had a considerably higher level of blood calcium than the PTH KO alone (Figure 3). With little calcium moving into bone, PTH appears to have little impact on blood calcium. Neither the loss of PTH or of PTHrP had important effects on the fetal blood calcium concentration in Runx2 KO mice, which were deficient in bone (Figure 3). It is also possible that in this setting, with a higher than normal fetal blood calcium, the ability of PTH and PTHrP to pump calcium across the placenta is more limited than normal. Further, Simmonds et al (7), in studies of PTH action on the placenta, have suggested that PTH might normally decrease backflux of calcium from the fetus to the mother across the placenta. In the current studies of Runx2 KO mice, the already high level of fetal blood calcium might increase this backflux of calcium to the mother, but we have no current methods for measuring this flux. It is also possible that the high blood calcium in the Runx2 KO mouse leads to increases in urinary calcium; this would be expected to somewhat lower the blood calcium. Further, it is possible that PTH and/or PTHrP have actions on the fetal kidney to increase reabsorption of calcium; KO of either ligand might increase urine/amniotic fluid calcium through this action. Complicating matters further, phosphate levels, which we did not measure, might vary in the mice that we have analyzed and might influence the blood calcium levels. In any case, the findings here show that the capacity of PTH and PPR signaling to maintain fetal blood calcium may only occur in the context of the capacity of the fetus to develop a mineralized skeleton
In postnatal bones, a prominent action of PTH on bones involves the stimulation of bone resorption via activation of osteoclasts (25, 26). PTH regulates osteoclastic differentiation and function through the RANK/RANK ligand (RANKL)/OPG pathway. We hypothesized that the reduction of fetal blood calcium concentration by ablating PPR signaling in bone is most likely caused by a reduction in osteoclastic activity through the RANK/RANKL pathway. As expected, ablation of RANK reduced the fetal blood calcium concentration and did so about as much as the bone-specific KO of the PPR did (Figures 2 and 4). The considerably lower blood calcium in the PTH KO mice then mandates that some of the action of PTH must be through mechanisms other than stimulation of bone resorption, such as placental calcium transport or renal reabsorption of calcium. Furthermore, PTH cannot be the sole stimulator of RANK action in fetal life, because the double PTH:RANK KO has a lower blood calcium than the PTH KO alone (Figure 5). The stimulators of RANKL production in hypertrophic chondrocytes are possible contributors to this PTH-independent activation of RANK (27).
Taken together, the observations in our genetic models suggest that both placental transport and osteoclast activation contribute to PTH's role in fetal calcium homeostasis (Figure 6). But they also show that the calcium reservoir in bone, which is facilitated by the bone-forming actions of PTH, is the major controller of the level of fetal blood calcium. Without bone, PTH is unable to optimally regulate fetal blood calcium.
Acknowledgments
Author contributions: T.H., T.K., and H.M.K. designed the study; T.H. and H.M.K. conducted the study; T.H. and T.K. collected the data; T.H., S.N., and T.K. analyzed the data; T.H., T.K., S.N., A.C.K., D.G., and H.M.K. interpreted the data; T.H., T.K., S.N., A.C.K., D.G., and H.M.K. drafted the manuscript; and T.H. and H.M.K. take responsibility for the integrity of the data analysis.
This work was supported by the National Institutes of Health Grant DK 56246.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Col2-Cre
- collagen IIα1 promoter-Cre
- E
- embryonic day
- KO
- knockout
- PPR
- PTH/PTHrP receptor
- RANK
- receptor activator of nuclear factor κB
- R26R
- Rosa26-R
- Runx2
- Runt-related gene 2/core binding factor 1
- WT
- wild-type
- X-Gal
- 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside.
References
- 1. Potts JT, Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Ann NY Acad Sci. 2007;1117:196–208. [DOI] [PubMed] [Google Scholar]
- 2. Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. [DOI] [PubMed] [Google Scholar]
- 3. Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. In: Melmed S, Polonsky K, Larsen R, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12th ed Philadelphia, PA: Elsevier Saunders; 2011:1237–1304. [Google Scholar]
- 4. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–887. [DOI] [PubMed] [Google Scholar]
- 6. Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev. 2014;94:1143–1218. [DOI] [PubMed] [Google Scholar]
- 7. Simmonds CS, Karsenty G, Karaplis AC, Kovacs CS. Parathyroid hormone regulates fetal-placental mineral homeostasis. J Bone Miner Res. 2010;25:594–605. [DOI] [PubMed] [Google Scholar]
- 8. Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002;109:1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci USA. 1996;93:15233–15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovacs CS, Chafe LL, Fudge NJ, Friel JK, Manley NR. PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology. 2001;42:4983–4993. [DOI] [PubMed] [Google Scholar]
- 11. MacIsaac RJ, Horne RS, Caple IW, Martin TJ, Wintour EM. Effects of thyroparathyroidectomy, parathyroid hormone, and PTHrP on kidneys of ovine fetuses. Am J Physiol. 1993;264:37–44. [DOI] [PubMed] [Google Scholar]
- 12. Kovacs CS, Ho-Pao CL, Hunzelman JL, et al. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. J Clin Invest. 1998;101:2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soegiarto DW, Kiachopoulos S, Schipani E, Jüppner H, Erben RG, Lanske B. Partial rescue of PTH/PTHrP receptor knockout mice by targeted expression of the Jansen transgene. Endocrinology. 2001;142:5303–5310. [DOI] [PubMed] [Google Scholar]
- 14. Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- 15. Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Sarosi I, Yan XQ, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karaplis AC, Luz A, Glowacki J, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. [DOI] [PubMed] [Google Scholar]
- 18. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. [DOI] [PubMed] [Google Scholar]
- 19. Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM. Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc Natl Acad Sci USA. 2011;108:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA. 1998;95:13030–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi T, Chung UI, Schipani E, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. [DOI] [PubMed] [Google Scholar]
- 22. Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. [DOI] [PubMed] [Google Scholar]
- 25. Nissenson R, Jüppner H. Parathyroid hormone. In: Rosen CJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7th ed Washington, DC: ASBMR Press; 2008:123–127. [Google Scholar]
- 26. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. [DOI] [PubMed] [Google Scholar]
- 27. Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]