Abstract
Metallopolymers combine a processable, versatile organic polymeric skeleton with functional metals, providing multiple functions and methodologies in materials science. Taking advantage of cationic cobaltocenium as the key building block, organogels could be simply switched to hydrogels via a highly efficient ion exchange. With the unique ionic complexion ability, cobaltocenium moieties provide a robust soft substrate for recycling antibiotics from water. The essential polyelectrolyte nature offers the metallopolymer hydrogels to kill multidrug resistant bacteria. The multifunctional characteristics of these hydrogels highlight the potential for metallopolymers in the field of healthcare and environmental treatment.
Hydrogels are water-swelling polymeric networks that could bear extraordinary performance in various aspects, such as good biocompatibility, tunable mechanical strength, and multi-stimuli response1,2,3,4. In the last few decades, various types of hydrogels have been developed, including, but not limited to, organic polymer-crosslinked5, protein-based, peptide-containing6,7, and supramolecular host-guest hydrogels8,9. By inserting functionalities in numerous matrix, hydrogels could be found applications for tissue engineering, drug delivery, matrix chemistry, artificial extracellular materials, to name just a few4,5,10,11.
Metallopolymers combine the synthetic efficiency, processability and versatility of an organic polymer framework with the unique redox, responsive and catalytic properties of inorganic metals, which have attracted great attention recently12,13,14,15,16. However, among the various known hydrogels, metallopolymer-based hydrogels are far less studied9,17,18,19,20. This may be attributed, in part, to concerns such as metal cytotoxicity and the difficulty to integrate metals into gel networks19,21,22,23. There are a few reports on metal ion-based hydrogels18,19,20,24, most of which have focused on chelating bonds25,26,27. As substitutes for metal ions, covalent-bonded (linked via covalent bonds) organometallic moieties are more desirable for hydrogels, which are more chemically stable, compared with non-covalent bonded counterparts. The combination of organometallics and polymer gels not only maintains attributes from polymeric networks, but also develops inherent functionalities from organometallic molecules as unique building blocks20,26,28,29,30. Metallocene-containing polymeric materials have been demonstrated for a variety of applications in the areas of biomedicines, energy storage, catalysts, templating precursors, and magnetic materials13,31,32,33,34,35,36. Given its signature redox chemistry, ferrocene has been used in hydrogels9,17,29, although the hydrophobicity and stability of ferrocene has limited its role mostly as an auxiliary building block for fabricating environmentally-compatible materials33,36. As 18-e analogies of ferrocene, cationic 18-e metallocenium ions (e.g. cobaltocenium) demonstrate several advantages over commonly applied ferrocene, including excellent hydrophilicity, high chemical stability and emerging bioactivities23,37,38,39.
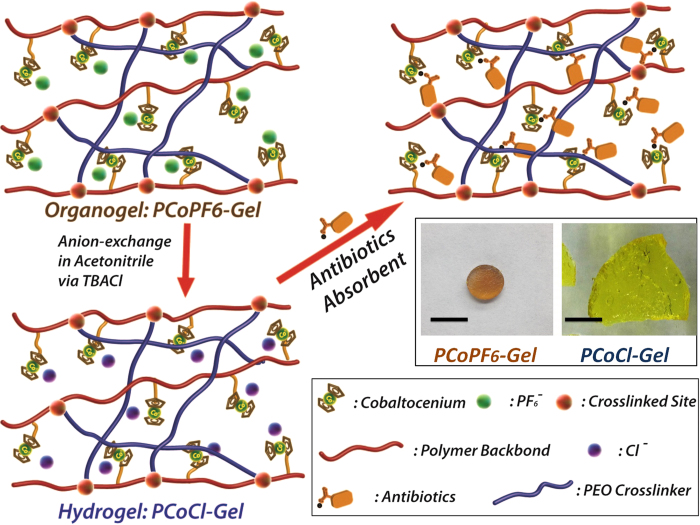
Herein we report the first charged metallocenium-containing organogels and hydrogels, and their emerging applications in recycling antibiotics from water and in killing multidrug resistant bacteria (Fig. 1). Specifically, cationic cobaltocenium-containing polymeric networks exhibit some of most distinguished ion-responsive properties. With different counterions, charged cobaltocenium-containing gels exhibit drastically different physical behaviors, including mechanical properties and water uptake ability. Concurrently, these cationic gels are able to absorb diverse antibiotics from water. Taking advantage of the intrinsic antimicrobial properties of cobaltocenium polyelectrolytes, cobaltocenium-containing hydrogels can be further employed as novel antimicrobial biomaterials.
Figure 1. Illustration of anion-paired (Cl− (purple circles), PF6− (green circles) and antibiotics) cobaltocenium-containing organogels and hydrogels.
Inserted: optical images of two representative gels (Scale Bar: 1 cm).
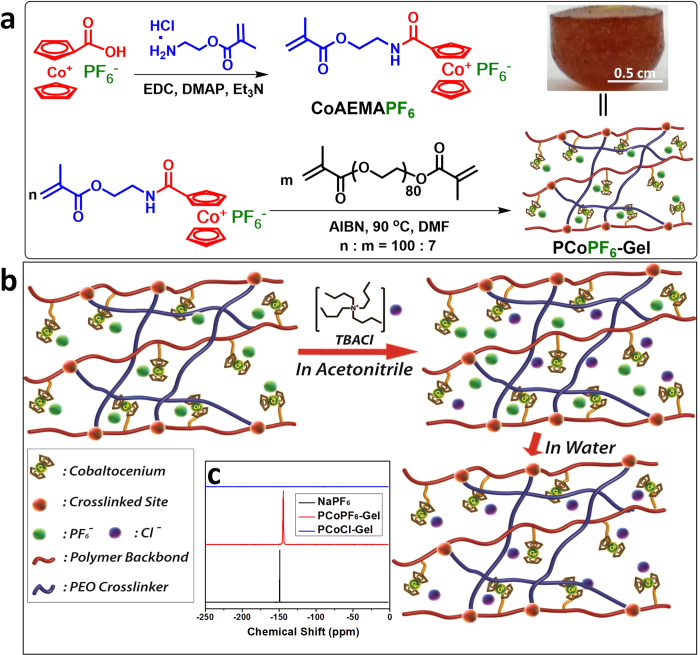
As shown in Fig. 2a, 2-cobaltocenium amidoethyl methacrylate hexafluorophosphate (CoAEMAPF6) was synthesized as a monomer with hexafluorophosphate (PF6ˉ) as the counterion (both 1H and 13C NMR spectra provided in Figures S1 and S2). Cationic cobaltocenium-containing organogels (PCoPF6-Gel) were subsequently prepared by free radical copolymerization of monomer CoAEMAPF6 and a crosslinker, poly(ethylene glycol) dimethacrylate (PEGDMA), in dimethylformamide (DMF). The formation of gel was partially dictated by the ratio of CoAEMAPF6 to PEGDMA. A free-standing gel could be prepared, when the molar fraction of PEGDMA was 7% (When more than 10% of the PEGDMA was used, the gel was very brittle and easy to crack in water, while with less than 5% PEGDMA, the crosslinking was not very effective and the resultant materials would be liquid-like instead of a solid gel.) On the other hand, the concentration of monomer also played a role in generating solid gels, as no gels were obtained at concentrations less than 0.66 g/mL.
Figure 2.
(a) Preparation of cationic cobaltocenium-containing organogels (PCoPF6-Gel) via copolymerization of CoAEMAPF6 and PEGDMA. (b) Preparation of chloride-paired hydrogel (PCoCl-Gel) from the organogel PCoPF6-Gel via ion-exchange with tetrabutylammonium chloride (TBACl). (c) Solid state 31P NMR spectra of organogel PCoPF6-Gel, hydrogel PCoCl-Gel and sodium hexafluorophosphate (NaPF6, as a reference).
In general, compositions of organic gels are usually difficult to be characterized due to the insolubility of their polymeric networks. In the case of PCoPF6-Gel, the compositions could be quantitatively determined using the characteristic UV-vis absorption of cobaltocenium moiety at ~270 nm (Figure S3A)33. After the gels were immersed in water for 3 days, the unreacted monomers could be completely removed. According to the standard calibration curve for monomer CoAEMAPF6 (Figure S3B), the concentration of unreacted monomer was calculated by UV-vis spectroscopy to be 44 μg/mL. The final purified and dried gel had a yield of 56.1%. Based on Equations 1 and 2, the conversion of monomers was 56%, in an excellent agreement with the yield. The weight fraction of cobaltocenium moieties in the gel was 67.3 wt%. The resultant organogel was brownish (Fig. 2a).
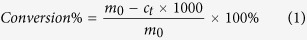 |
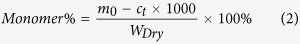 |
(In the equations, conversion represents the percentage of monomers reacted in final gels, m0is the amount of total monomers used before reaction; Ctis the concentration of the diluted solution collected, which was obtained from the UV-vis absorption spectra and the monomer standard curve. Monomer% is the weight percentage of CoAEMAPF6 in final gels. WDry is the final weight of purified and dried PCoPF6-Gel)
According to recent work34,40, cobaltocenium-containing polymers are ion-responsive materials. With different counterions, their corresponding polymers show drastic difference in hydrophobicity. With counterions like PF6− and tetraphenylborate (BPh4−), the cobaltocenium-containing polymers are relatively hydrophobic. In contrast, counterions, such as chloride, bromide and nitrate, result in polymers that are very hydrophilic. Thus we attempted to convert the organogel PCoPF6-Gel into a hydrogel by a recently-developed phase-transfer ion-exchange method (Fig. 2b)34. The anion exchange was done in acetonitrile with the aid of tetrabutylammonium chloride (TBACl), as shown in Scheme S2. All PF6− anions in PCoPF6-Gel were replaced by Cl− to form the hydrogel PCoCl-Gel. As shown by solid state 31P NMR (Fig. 2c), PCoPF6-Gel has a broad phosphorus peak at −144.4 ppm using sodium hexafluorophosphate as a reference with a peak at −149.3 ppm. After ion-exchange, no phosphorus peak was observed in PCoCl-Gel, indicating the complete removal of PF6−anions.
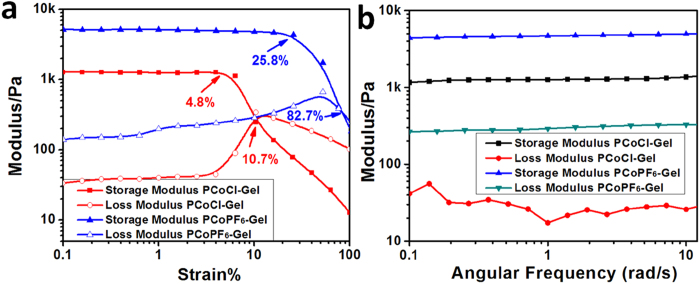
After ion-exchange, the light yellow PCoCl-Gel (Fig. 1) is hydrophilic that can have a water content as high as 96.4%, approximately 27 times of its dry gel weight. While for PCoPF6-Gel, the water content was 80% (only four times of the weight of dry gel), which probably also had a significant contribution from PEO crosslinkers due to the ability of hydrophilic PEO to retain water in gels. The structure of these two gels were also characterized by Field-Emission Scanning Electron Microscopy (FE-SEM). As shown in Figure S7, the chloride gel (PCoCl-Gel) was porous, indicating its potential to hold more water, while PCoPF6-Gel appeared much more compact. The drastic change in their morphologies, hydrophilicity and mechanical strength was mainly attributed to the unique ion-dependent solubilities of cobaltocenium-containing polymers. Furthermore, the mechanical properties of PCoCl-Gel and PCoPF6-Gel are significantly different. Figure. 3a illustrates the oscillatory strain results for both hydrogels. In comparison with PCoPF6-Gel, PCoCl-Gel exhibits a narrower linear viscoelastic region (LVR, about 0 ~ 25.8% changed to about 0 ~ 4.8%) and a lower critical strain (82.7% dropped to 10.7%), demonstrating a shortened elastic response range. A frequency sweep experiment was performed to compare the stiffness of both hydrogels (Fig. 3b). The storage modulus of both hydrogels was much higher than their loss modulus, and all of the values were relatively independent from frequency, which showed that both hydrogels behaved as a true gel over the frequency tested (range from 0.1 to 10 rad/s). Notably, PCoCl-Gel showed a weaker and more viscous gel nature than that of PCoPF6-Gel. Due to the same crosslinking degree for both gels, such difference in their mechanical properties was possibly resulted from the stronger electrostatic repulsive interactions in PCoCl-Gel than those in PCoPF6-Gel, which led to a looser network, as well as the increasing solubility of the more hydrophilic and ionic polymer networks in PCoCl-Gel, thus leading to lower stiffness.
Figure 3. Mechanical properties of PCoCl-Gel; and PCoPF6-Gel:
(a) The curves of shear storage modulus and shear loss modulus vs. strain for PCoCl-Gel and PCoPF6-Gel performed by amplitude sweep under the frequency of 10 rad/s. (b) The shear storage modulus and shear loss modulus with different frequency performed by a frequency sweep under the strain at 0.2%.
Antibiotic-contamination is an urgent challenge in public health and environmental science, as it has deleteriously enhanced the evolution of bacterial resistance to commonly-used antibiotics such as β-lactam antibiotics41,42. Current techniques to remove β-lactam antibiotics are primarily based on oxidation methods (such as ozonation and fenton-oxidation)43,44, nanofiltration45,46, and photocatalytic reactions46,47. However, these techniques usually introduce new oxidizing chemicals into water and thus require special facilities44,48. On the other hand, adsorption (e.g. the use of activated carbons) is a more convenient and ‘clean’ strategy for removal of β-lactam antibiotics48,49,50. However, current adsorption materials are usually only suitable for antibiotics with high concentrations in the range of 50 mg/L to 200 mg/L. With some specific procedures (e.g. a combination of ozonation and ion-exchange columns), only a few of them could remove antibiotics with concentrations below 10 mg/L50,51,52. Recently we showed that cobaltocenium-containing polymers can form bioconjugates with β-lactam antibiotics (e.g. penicillin-G, amoxicillin, ampicillin and cefazolin) via ion-pairing between cationic metal centers and carboxylate anions23. Given the relatively strong ion-pairing capabilities of cobaltocenium, cationic PCoCl-Gel could be a promising novel material to remove antibiotics.
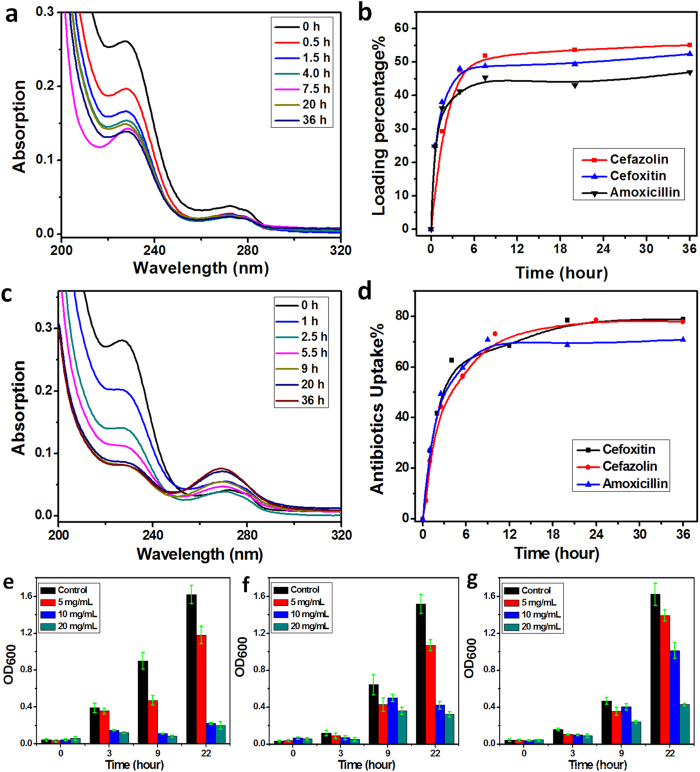
As shown in UV-vis spectra (Fig. 4c and Figure S4), after mixing dry PCoCl-Gel with different β-lactam antibiotics (amoxicillin, cefazolin and cefoxitin) in water (10 mg/L), ~75% of antibiotics were absorbed with a final concentration at ~2 mg/L (Fig. 4d). Compared with other adsorption techniques, such as ion-exchange columns and activated carbon with ~3 g/L absorbents for antibiotics at 50 ~ 200 mg/L in DI water (Resistivity: 18.2 MΩ × cm)44,48,49,53,54, PCoCl-Gel showed a better capacity to remove β-lactam antibiotics (1.5 g/L gel could efficiently treat with antibiotics waste at a concentration of 3 ~ 10 mg/L). After immersing PCoCl-Gel in the solution of antibiotics at a higher concentration (2 g/L), about 47% of the antibiotics were removed (Fig. 4a,b and S5), indicating that 1.0 mg PCoCl-Gel could load 0.42 mg antibiotics (based on theoretical calculation, see supporting information). Theoretically 1.0 mg PCoCl-Gel could absorb 0.60 ~ 0.80 mg antibiotics, assuming that the molar ratio of cobaltocenium to antibiotics is 1:1. The ability to remove antibiotics from aqueous solution by cobaltocenium moieties might be contributed by the ionic binding and coordination effects between cationic cobaltocenium and anionic antibiotics. Similar to techniques used in industry44,48,49,53,54, we performed an experiment in tap water (Resistivity: 5.7 Ω × cm) under the same conditions as above (4.5 mg gel in 3 mL tap water with 10 mg/L cefazolin antibiotics). The result showed that in the first 12 hours, the drug uptake could remove 44.5% antibiotics (Figure S6), which was lower than the one in DI water (~75%).
Figure 4. Treatment of antibiotics in water by cobaltocenium-containing hydrogels:
PCoCl-Gel (dry weight: 4.5 mg) as an absorbent for β-lactam antibiotics: amoxicillin, cefazolin and cefoxitin; (a) UV-vis absorption curves of 2 mL, 2 g/L amoxicillin under different time intervals; (b) UV-vis absorption data for time-dependent antibiotics-uptake (2 mL, 2 g/L) of PCoCl-Gel; (c) UV-vis absorption curves of 10 mg/L amoxicillin under different time intervals; (d) UV-vis absorption data for time-dependent antibiotics-uptake under a low concentration (3 mL, 10 mg/L) of PCoCl-Gel. Inhibition of cobaltocenium-containing hydrogels (PCoCl-Gel) was observed against (e) Gram-negative E-coli; (f) Gram-positive S. aureus; and (g) HA-MRSA under different concentrations by standard solution micro-broth measurement.
Cobaltocenium-containing methacrylate polymers have been reported to inhibit the growth of bacteria without any obvious toxicity toward human cells in in vitro and in vivo studies23. Cationic cobaltocenium-containing hydrogels may also have the similar ability to disrupt negative charged cell walls of bacteria. Thus we tested the antimicrobial activities of cobaltocenium-containing hydrogels against various bacteria, including drug-resistant strains. This unique property showed additional advantage over other kinds of absorbents for antibiotics. It could enable practical utilization of such hydrogels in the environment considering the existence of drug-resistant bacteria in antibiotics-contaminated water41,42. The antimicrobial efficacies of hydrogels are shown in Fig. 4e,f,g. The hydrogel PCoCl-Gel (at a concentration of 20 mg/mL) showed inhibition of the growth for Gram-negative E-coli (90% inhibition, Fig. 4e), Gram-positive S. aureus (80% inhibition; Fig. 4f), and hospital-acquired methicillin-resistant S. aureus (HA-MRSA, 80% inhibition, Fig. 4g)23,55. These data indicated that cobaltocenium-containing hydrogel can not only absorb antibiotics in contaminated water, but also inhibit the growth of drug-resistant bacteria in their environment. The antimicrobial ability of hydrogels originates from cationic cobaltocenium-containing macromolecules in the gels. Due to the much lower solubility of the gels compared to homopolymers, the concentration of inhibition increased about ~1000 times.
In conclusion, we report the preparation and application of novel ion-responsive metallopolymer gels. Using an ion-exchange technique, the gels showed a transition from organogels to hydrogels. In the presence of different counterions, these gels showed strikingly different mechanical, water-absorbing, and optical properties. Due to the ability of cobaltocenium moieties to bind β-lactam antibiotics, these hydrogels were additionally capable of absorbing antibiotics from contaminated water. Furthermore, these cationic hydrogels can not only efficiently remove antibiotics in water, but also inhibit the growth of different bacteria, including drug-resistant strains. As a class of novel polymeric gels, these cobaltocenium-containing hydrogels could open up new avenues for diverse applications, especially in the areas of biomedicines and environmental treatment.
Methods
Materials
2-Aminoethyl methacrylate hydrochloride (90%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC-HCl, 98%), 4-(dimethylamino) pyridine and tetrabutylammonium chloride (TBACl) were purchased from Aldrich. Polyethylene glycol dimethacrylate (PEGDMA) (M.W. 3,400 g/mol) was purchased from VWR. N,N-Dimethylformamide (DMF) was dried and freshly distilled. Water was from Thermo Scientific Nanopure with ion conductivity at 18.2 MΩ. Staphylococcus aureus and Escherichia coli strains were purchased from ATCC: HA-MRSA (ATCCBAA-29213), and MSSA (ATCCBAA-1718), and E-coli (ATCC-25922). All other chemicals were from commercial sources and used as received.
Characterization
1H NMR (400 MHz) spectra were recorded on a Varian Mercury 400 spectrometer with tetramethylsilane (TMS) as an internal reference. 19F NMR (376 MHz) spectra were recorded on a Varian Mercury 400 spectrometer with CHF3 as an internal reference standard. A 500 MHz Bruker Avance III-HD spectrometer with a microprocessor-controlled gradient unit was used for 31P Solid State NMR spectra. UV-vis spectra were recorded on a Shimadzu UV 2450 spectrophotometer. Mechanical measurement was performed on Discovery HR-3, hybrid rheometer (TA Instrument). The images of gels were recorded by Field-Emission Scanning Electron Microscopy (FE-SEM, Zeiss UltraPlus). The samples were firstly coated with gold using Denton Dest II Sputter Coater for 45 s and then observed by SEM. Gels were freeze-dried before using for SEM.
Synthesis of 2-cobaltoceniumamidoethyl methacrylate hexafluorophosphate (CoAEMAPF6)
CoAEMAPF6 was synthesized based on an amidation reaction. Cobaltocenium carboxylic acid hexafluorophosphate (2 g, 5.29 mmol), 2-aminoethyl methacrylate hydrochloride (0.94 g, 5.68 mmol), and 4-(dimethylamino)pyridine (0.13 g, 1.06 mmol) were dissolved in 20 mL dichloromethane (DCM) and the solution was cooled to 0 oC. EDC-HCl (1.1 g, 5.74 mmol) was slowly added into the above solution. Then, dry triethylamine (1.6 g, 15.8 mmol) was added into reaction. The reaction was stirred for 5 hours at room temperature. Then, solution was extracted by aqueous saturated sodium hexafluorophosphate solution three times to remove unreacted chemicals. The organic phase was collected, condensed and precipitated into diethyl ether. Yellow solids were collected and dried under vacuum overnight. Yield: 1.6 g, 58%. 1H NMR (Figure S1) (CD3COCD3, δ, ppm): 8.30 (broad, NHCH2, 1H), 6.42 (t, Cp, 2H), 6.22 (m, CH2C, 1H), 6.10 (t, Cp, 2H), 5.92 (s, Cp, 5H), 5.62 (m, CH2C, 1H), 4.42 (m, OCH2CH2NH, 2H), 3.66 (m, OCH2CH2NH, 2H), 1.94 (m, CH3CCO, 3H). 13C NMR (Figure S2) (CD3COCD3, δ, ppm): 138 (CH2CCO), 126 (CH2CCO), 83–86 (Cp ring), 62 (COOCH2), 39 (CH2CH2NH), 18 (CH3CCO). Mass spectrum: theoretical m/z: 344.07; found m/z: 344.00.
Synthesis of cobaltocenium polymer gels (PCoPF6-Gel)
PCoPF6-Gel was synthesized via polymerization of PEGDMA and CoAEMAPF6. CoAEMAPF6 (0.3 g, 6.17 × 10−1 mol) and PEGDMA (145 mg, 4.26 × 10−2 mmol) were dissolved in 0.35 mL DMF in a test tube. AIBN solution (0.1 mL, 3 mg/mL, 1.83 × 10−3 mmol) was then added into the above solution. The solution was purged by nitrogen gas for 30 minutes and then placed in an oil bath under 90 oC for 6 hours. The solid gel was then collected by immersing in 100 mL acetone for three days (change acetone every day) to remove unreacted monomers and crosslinkers.
Characterization of PCoPF6-Gel compositions
The compositions of PCoPF6-Gel were characterized by measuring the UV-vis absorption of cobaltocenium moieties at ~270 nm. PCoPF6-Gel was prepared according to the above procedure using the following materials (0.1 g CoAEMAPF6, 48 mg PEGDMA, 0.1 mg AIBN and 0.15 mL DMF). After 6 hours, all materials in a test tube were collected and immersed in 15 mL nanopure (deionized) water for 24 h, and then the water was collected and another 15 mL nanopure water. This procedure was repeated in triplicate to remove unbound monomers from the gels, as shown in Scheme S1. In total, 45 mL of water solution was collected, then diluted to 50 mL. A 1 mL solution was then taken and diluted 20 times for UV-vis measurement. A standard procedure was repeated one more time. This procedure was aimed to remove monomers from the gels, as shown in Scheme S1. Totally 45 mL water solution was collected and diluted to 50 mL. 1 mL solution was then taken and diluted 20 times for UV-vis measurement. A standard curve (Figure S3B) for CoAEMAPF6 was established using a series of monomer solution (67 μg/mL, 50 μg/mL, 25 μg/mL, 12.5 μg/mL and 6.3 μg/mL). The compositions of the PCoPF6-Gel were calculated according to equations 1 and 2.
Ion-exchange of PCoPF6-Gel
Ion-exchange of the PCoPF6-Gel was performed according to Scheme S2. PCoPF6-Gel (0.1 g) was immersed in 20 mL acetonitrile with 0.1 g TBACl salt. After 24 hours, acetonitrile solvent was replaced by 20 mL fresh TBACl acetonitrile solution (0.1 g TBACl salt). The procedure was repeated one more time to ensure complete ion-exchange. After three ion-exchanges, the resultant gel was then immersed in 40 mL water for three successive times to remove acetonitrile and TBACl residuals. The swollen gel was then collected and dried for later use. The chloride-paired gel (PCoCl-Gel) was characterized by 31P Solid-State NMR (Fig. 2c) to ensure the complete removal of PF6 anions.
Measurement of water uptake for PCoPF6-Gel and PCoCl-Gel
Water uptake by PCoPF6-Gel and PCoCl-Gel followed the same procedure. The weight of dried gels was first measured. After immersion in water for 24 hours, the weight of wet gels were then measured. The percent water uptake was calculated according to Equation 3 and data from Table S1:
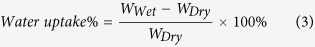 |
Equation 3,WWet means the weight of wet gel and WDry is the weight of dry gel.
Antibiotic contaminant removal by cobaltocenium-containing hydrogels
Four antibiotic sodium salts, including penicillin-G, amoxicillin, ampicillin, cefazolin, were tested following the same procedure. PCoCl-Gel (dry weight = 4.5 mg) was immersed into 2 mL aliquots of aqueous antibiotic (2 mg/mL). At certain time intervals (0.0 h, 0.5 h, 1.5 h, 4.0 h, 7.5 h, 20.0 h, 36.0 h), 10 μL solution was removed and then diluted 20 times to measure UV-vis absorption of antibiotic in the solution at the following wavelengths: 230 nm for amoxicillin, 266 nm for ampicillin, and 230 nm for cefazolin. The concentration of antibiotic was obtained according to their respective standard curve profiles. The standard curves for each antibiotic were established by measuring UV-vis absorption for a series of solutions with different concentrations (1.56 μg/mL, 3.12 μg/mL, 6.3 μg/mL, 12.5 μg/mL, 25 μg/mL and 50 μg/mL). The removal of antibiotic at low concentrations (10 μg/mL) was also performed in DI water. PCoCl-Gel (dry weight = 4.5 mg) was immersed in 3 mL antibiotic aqueous solution (10 μg/mL). Samples were also taken at certain time intervals (0.0 h, 1.0 h, 2.5 h, 5.5 h, 9.0 h, 20.0 h, 36.0 h; see Fig. 4). The removal of antibiotic at low concentrations (10 μg/mL) was also performed in tap water (Resistivity: 5.7 Ω × cm).
Mechanical tests for PCoPF6-Gel and PCoCl-Gel
The samples were measured using a Discovery HR-3, hybrid rheometer (TA Instrument) with a parallel-plate geometry. The hydrogel was cut into a circular disk with a thickness of 2 mm and a diameter of 25 mm. The gap between the two plates was accurately set by controlling the normal force. Oscillation amplitude measurement was performed to find the linear viscoelastic region. Frequency was 10.0 rad/s and strain was from 0.1% ~ 100.0%. Then, oscillatory frequency measurements were performed in the linear viscoelastic regime at 25 oC. The strain was kept at 0.2% while the frequency was changed from 0.1 to 100 rad/s. The shear storage modulus (G’) and the shear loss modulus (G”) were then measured.
Antimicrobial evaluation for PCoCl-Gel
The antimicrobial evaluation for PCoCl-Gel followed a procedure similar to those published in previous reports23,55,56. For these bacteria growing on agar plates, a single colony was inoculated in 5 mL Tryptic Soy broth (TSB) at 37 °C for 24 hours, shaking at 190 rpm/min. All bacteria were grown to an optical density of about l.00 (OD600 = ~1.00) for further use. A series of TSB medium solutions containing different concentrations of PCoCl-Gel (5, 10 and 20 mg/mL, dried weight) were prepared in test tubes. Then, 5 μL bacteria (O.D. = 1.00) was incubated in each test tube with a starting O.D. = ~0.05. The culture solution, without gels, was used as the control. The cultured solutions were incubated at 37 oC. Bacterial growth was detected at OD600 and was compared to controls of TSB without gels. The percent inhibition was calculated, according Equation 4, which was determined by UV-vis at OD600. All assays were carried out in duplicate using the same assay plate.
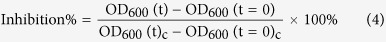 |
OD600 (t = 0) indicates the initial OD600 value, and OD600 (t) is the OD600 value for cells after incubation with gels for t hours. OD600 (t = 0)c is the initial OD600 value and OD600 (t)c is the OD600 value for control samples after incubation for t hours.
Additional Information
How to cite this article: Zhang, J. et al. Anion-Responsive Metallopolymer Hydrogels for Healthcare Applications. Sci. Rep. 5, 11914; doi: 10.1038/srep11914 (2015).
Supplementary Material
Acknowledgments
Support from the National Science Foundation (CHE-1151479 and DMR-1206072) is acknowledged.
Footnotes
The authors declare no competing financial interests.
Author Contributions C.T. and J.Z. designed the experiments. J.Z., J.Y., A.W., Y.Y., Y.Q. and P.P. synthesized the materials. Y.C. and A.W.D. evaluated antimicrobial properties. J.Z. and C.T. analyzed the data. J.Z., C.T., A.W.D. and Q.W. wrote the manuscript. All authors contributed to writing the manuscript.
References
- Haque M. A., Kurokawa T. & Gong J. P. Super tough double network hydrogels and their application as biomaterials. Polymer 53, 1805–1822 (2012). [Google Scholar]
- Li P. et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 10, 149–156 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339–343 (2010). [DOI] [PubMed] [Google Scholar]
- Appel E. A., del Barrio J., Loh X. J. & Scherman O. A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 41, 6195–6214 (2012). [DOI] [PubMed] [Google Scholar]
- Kopecek J. Hydrogel biomaterials: A smart future? Biomaterials 28, 5185–5192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiday J. et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. 99, 9996–10001 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano S., Williams R. J., Jayawarna V. & Ulijn R. V. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J. Am. Chem. Soc. 128, 1070–1071 (2006). [DOI] [PubMed] [Google Scholar]
- Appel E. A. et al. Ultrahigh-Water-Content Supramolecular Hydrogels Exhibiting Multistimuli Responsiveness. J. Am. Chem. Soc. 134, 11767–11773 (2012). [DOI] [PubMed] [Google Scholar]
- Nakahata M., Takashima Y. & Harada A. Redox-Responsive Macroscopic Gel Assembly Based on Discrete Dual Interactions. Angew. Chem. Int. Ed. 53, 3617–3621 (2014). [DOI] [PubMed] [Google Scholar]
- Lee K. Y. & Mooney D. J. Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1879 (2001). [DOI] [PubMed] [Google Scholar]
- Schexnailder P. & Schmidt G. Nanocomposite polymer hydrogels. Colloid. Polym. Sci. 287, 1–11 (2009). [Google Scholar]
- Zhou J., Whittell G. R. & Manners I. Metalloblock Copolymers: New Functional Nanomaterials. Macromolecules 47, 3529–3543 (2014). [Google Scholar]
- Chadha P. & Ragogna P. J. Side chain Co(I) polymers featuring acrylate functionalized neutral 18 electron CpCo(C4R4) (R = Ph, Me) units, Chem. Commun. 47, 5301–5303 (2011). [DOI] [PubMed] [Google Scholar]
- Ma Y., Dong W.-F., Hempenius M. A., Mohwald H. & Julius Vancso G. Redox-controlled molecular permeability of composite-wall microcapsules. Nat. Mater. 5, 724–729 (2006). [DOI] [PubMed] [Google Scholar]
- Burnworth M. et al. Optically healable supramolecular polymers. Nature 472, 334–337 (2011). [DOI] [PubMed] [Google Scholar]
- Jäkle F. Advances in the Synthesis of Organoborane Polymers for Optical, Electronic, and Sensory Applications. Chem. Rev. 110, 3985–4022 (2010). [DOI] [PubMed] [Google Scholar]
- Lakshmi N. V., Mandal D., Ghosh S. & Prasad E. Multi-Stimuli-Responsive Organometallic Gels Based on Ferrocene-Linked Poly(Aryl Ether) Dendrons: Reversible Redox Switching and Pb2+-Ion Sensing. Chem. Eur. J. 20, 9002–9011 (2014). [DOI] [PubMed] [Google Scholar]
- Weng W. G., Beck J. B., Jamieson A. M. & Rowan S. J. Understanding the mechanism of gelation and stimuli-responsive nature of a class of metallo-supramolecular gels. J. Am. Chem. Soc. 128, 11663–11672 (2006). [DOI] [PubMed] [Google Scholar]
- Piepenbrock M. O. M., Lloyd G. O., Clarke N. & Steed J. W. Metal- and Anion-Binding Supramolecular Gels. Chem. Rev. 110, 1960–2004 (2010). [DOI] [PubMed] [Google Scholar]
- Whittell G. R., Hager M. D., Schubert U. S. & Manners I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 10, 176–188 (2011). [DOI] [PubMed] [Google Scholar]
- Gasser G., Ott I. & Metzler-Nolte N. Organometallic Anticancer Compounds. J. Med. Chem. 54, 3–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger C. G., Metzler-Nolte N. & Dyson P. J. Challenges and Opportunities in the Development of Organometallic Anticancer Drugs. Organometallics 31, 5677–5685 (2012). [Google Scholar]
- Zhang J. et al. Antimicrobial Metallopolymers and Their Bioconjugates with Conventional Antibiotics against Multidrug-Resistant Bacteria. J. Am. Chem. Soc. 136, 4873–4876 (2014). [DOI] [PubMed] [Google Scholar]
- Bode S. et al. Self-healing metallopolymers based on cadmium bis(terpyridine) complex containing polymer networks. Polym. Chem. 4, 4966–4973 (2013). [Google Scholar]
- Po C., Ke Z. H., Tam A. Y. Y., Chow H. F. & Yam V. W. W. A Platinum(II) Terpyridine Metallogel with an L-Valine-Modified Alkynyl Ligand: Interplay of Pt center dot center dot center dot Pt, pi-pi and Hydrogen-Bonding Interactions. Chem. Eur. J. 19, 15735–15744 (2013). [DOI] [PubMed] [Google Scholar]
- Jochum F. D., Brassinne J., Fustin C. A. & Gohy J. F. Metallo-supramolecular hydrogels based on copolymers bearing terpyridine side-chain ligands. Soft Matter 9, 2314–2320 (2013). [Google Scholar]
- Svobodova H., Nonappa, Lahtinen M., Wimmer Z. & Kolehmainen E. A steroid-based gelator of A(LS)(2) type: tuning gel properties by metal coordination. Soft Matter 8, 7840–7847 (2012). [Google Scholar]
- Terech P. & Weiss R. G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 97, 3133–3159 (1997). [DOI] [PubMed] [Google Scholar]
- Hempenius M. A., Cirmi C., Lo Savio F., Song J. & Vancso G. J. Poly(ferrocenylsilane) Gels and Hydrogels with Redox-Controlled Actuation. Macromol. Rapid Commun. 31, 772–783 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Nanostructured Metal/Carbon Composites from Heterobimetallic Block Copolymers with Controlled Magnetic Properties. Chem. Mater. 26, 3185–3190 (2014). [Google Scholar]
- Ren L., Hardy C. G. & Tang C. Synthesis and Solution Self-Assembly of Side-Chain Cobaltocenium-Containing Block Copolymers. J. Am. Chem. Soc. 132, 8874–8875 (2010). [DOI] [PubMed] [Google Scholar]
- Ren L., Zhang J., Hardy C., Ma S. & Tang C. Cobaltocenium-Containing Block Copolymers: Ring-Opening Metathesis Polymerization, Self-Assembly and Precursors for Template Synthesis of Inorganic Nanoparticles. Macromol. Rapid Commun. 33, 510–516 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang J., Ren L., Hardy C. & Tang C. Cobaltocenium-Containing Methacrylate Homopolymers, Block Copolymers, and Heterobimetallic Polymers via RAFT Polymerization. Macromolecules 45, 6857–6863 (2012). [Google Scholar]
- Zhang J. et al. Charged Metallopolymers as Universal Precursors for Versatile Cobalt Materials. Angew. Chem. Int. Ed. 52, 13387–13391 (2013). [DOI] [PubMed] [Google Scholar]
- Whittell G. R. & Manners I. Metallopolymers: New multifunctional materials. Adv. Mater. 19, 3439–3468 (2007). [Google Scholar]
- Hardy C. G., Zhang J., Yan Y., Ren L. & Tang C. Metallopolymers with Transition Metals in the Side-Chain by Living and Controlled Polymerization Techniques. Prog. Polym. Sci. 39, 1742–1796 (2014). [Google Scholar]
- Mayer U. F. J., Gilroy J. B., O’Hare D. & Manners I. Ring-Opening Polymerization of 19-Electron [2]Cobaltocenophanes: A Route to High-Molecular-Weight, Water-Soluble Polycobaltocenium Polyelectrolytes. J. Am. Chem. Soc. 131, 10382–10383 (2009). [DOI] [PubMed] [Google Scholar]
- Qiu H. B., Gilroy J. B. & Manners I. DNA-induced chirality in water-soluble poly(cobaltoceniumethylene). Chem. Commun. 49, 42–44 (2012). [DOI] [PubMed] [Google Scholar]
- Ren L. et al. Preparation of cationic cobaltocenium polymers and block copolymers by “living” ring-opening metathesis polymerization. Chem. Sci. 3, 580–583 (2012). [Google Scholar]
- Zhang J., Pellechia P. J., Hayat J., Hardy C. G. & Tang C. Quantitative and Qualitative Counterion Exchange in Cationic Metallocene Polyelectrolytes. Macromolecules 46, 1618–1624 (2013). [Google Scholar]
- Engler A. C. et al. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 7, 201–222 (2012). [Google Scholar]
- Nederberg F. et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 3, 409–414 (2011). [DOI] [PubMed] [Google Scholar]
- Rozas O., Contreras D., Mondaca M. A., Perez-Moya M. & Mansilla H. D. Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J. Hazard. Mater. 177, 1025–1030 (2010). [DOI] [PubMed] [Google Scholar]
- Homem V. & Santos L. Degradation and removal methods of antibiotics from aqueous matrices - A review. J. Environ. Manage. 92, 2304–2347 (2011). [DOI] [PubMed] [Google Scholar]
- Radjenovic J., Petrovic M., Ventura F. & Barcelo D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 42, 3601–3610 (2008). [DOI] [PubMed] [Google Scholar]
- Augugliaro V. et al. Degradation of lincomycin in aqueous medium: Coupling of solar photocatalysis and membrane separation. Sol. Energy 79, 402–408 (2005). [Google Scholar]
- Klauson D., Babkina J., Stepanova K., Krichevskaya M. & Preis S. Aqueous photocatalytic oxidation of amoxicillin. Catal. Today 151, 39–45 (2010). [Google Scholar]
- Putra E. K., Pranowo R., Sunarso J., Indraswati N. & Ismadji S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 43, 2419–2430 (2009). [DOI] [PubMed] [Google Scholar]
- Rivera-Utrilla J., Prados-Joya G., Sanchez-Polo M., Ferro-Garcia M. A. & Bautista-Toledo I. Removal of nitroimidazole antibiotics from aqueous solution by adsorption/bioadsorption on activated carbon. J. Hazard. Mater. 170, 298–305 (2009). [DOI] [PubMed] [Google Scholar]
- Choi K. J., Kim S. G. & Kim S. H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 151, 38–43 (2008). [DOI] [PubMed] [Google Scholar]
- Choi K. J., Son H. J. & Kim S. H. Ionic treatment for removal of sulfonamide and tetracycline classes of antibiotic. Sci. Total Environ. 387, 247–256 (2007). [DOI] [PubMed] [Google Scholar]
- Adams C., Wang Y., Loftin K. & Meyer M. Removal of antibiotics from surface and distilled water in conventional water treatment processes. J. Environ. Eng. 128, 253–260 (2002). [Google Scholar]
- Kim S. H., Shon H. K. & Ngo H. H. Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J. Ind. Eng. Chem. 16, 344–349 (2010). [Google Scholar]
- Mendez-Diaz J. D. et al. Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase. J. Colloid Interface Sci. 345, 481–490 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang X. L., Soontornworajit B., Zhang Z. Y., Chen N. C. & Wang Y. Enhanced Loading and Controlled Release of Antibiotics Using Nucleic Acids As an Antibiotic-Binding Effector in Hydrogels. Biomacromolecules 13, 2202–2210 (2012). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Broad-Spectrum Antimicrobial and Biofilm-Disrupting Hydrogels: Stereocomplex-Driven Supramolecular Assemblies. Adv. Mater. 52, 674–678 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.