Summary
Community viral load (CVL), an aggregation of individual viral loads of HIV-infected persons within a defined community, has been proposed as a useful metric for monitoring HIV treatment uptake and quantifying its impact on transmission. The first publications regarding CVL appeared in 2009 and the metric was subsequently incorporated into the United States National HIV/AIDS Strategy. Although CVL is intuitively appealing, it has several theoretical limitations and biases that require further evaluation. These limitations and biases can be categorized in four areas related to: 1) selection and measurement issues in calculating CVL among HIV-infected persons, 2) the importance of HIV prevalence in determining the potential for ongoing HIV transmission, 3) interpretation of CVL and its impact on ongoing HIV transmission in a community, and 4) the ecological fallacy (ecological bias). These potential issues deserve careful assessment as CVL is being considered as a public health metric to assess the impact of HIV care on prevention.
Community viral load (CVL) is a recently developed metric that has been evaluated in attempts to quantify a population’s exposure to antiretroviral therapy (ART) and assess the effect of treating HIV-infected persons on HIV transmission.1–4 CVL is “an aggregate biological measure of viral load for a particular geographic location or community,”1 typically calculated as the arithmetic mean, geometric mean (the average of the base 10 logarithm), median, or sum of the highest or most recent VL of all reported HIV-infected persons with available viral load measurements in a particular population in a given time period.1,3,4 The central hypothesis underlying the use of CVL is that as ART coverage increases, greater numbers of HIV-infected persons will be virologically suppressed, leading to reduced CVL, and consequently, reduced incidence of HIV infection in the general population.1 CVL has two potential uses: 1) a research measure of the potential for ongoing HIV transmission within a community, and 2) a surveillance metric for monitoring ART uptake and effectiveness. Underlying both of these uses is the assumption that CVL is a key determinant of HIV incidence within a community.
The uptake of this measure has been rapid. Since its introduction in British Columbia2,3 and San Francisco1, CVL has been used in Washington, DC4, Uganda5, and elsewhere.6 Lower CVL was modestly associated with reductions in measures of new HIV diagnoses over time in some studies,1–3 but not in others.4 Although ecological in nature and limited by the use of new HIV diagnoses as a proxy for HIV incidence,7 these associations have been interpreted as being driven by the individual-level effect of ART on HIV transmission.1,3 Motivated by these studies, CVL has been incorporated into requests for proposals from the U.S. National Institutes of Health8 and guidance from the U.S. Centers for Disease Control and Prevention (CDC).9,10 More importantly, the U.S. National HIV/AIDS Strategy has called for reducing CVL as an “innovative solution” that “may help reduce the number of new HIV infections in specific communities that may, in turn, reduce disparities in HIV infection.”11
The concept of CVL, including its incorporation into CDC recommendations and US National HIV/AIDS strategy, has had several positive effects. Foremost, surveillance programs in the U.S., Europe, and elsewhere have become sensitized to the importance of the infectiousness of the HIV-infected individual. Monitoring and surveillance programs have been mobilized to identify persons in and out of care and expand efforts to capture HIV testing, treatment, and viral load data. This increased attention to monitoring will undoubtedly increase the likelihood of truly applying public health principles to the HIV epidemic.12
However, the increased attention to HIV surveillance necessitates a careful examination of the utility of proposed metrics. Although CVL and HIV incidence are plausibly related causally, the data available to estimate CVL and its link to incidence are limited. Some of these limitations have been stated,1,7,13 but the rapid encouragement to implement CVL as a priority measure suggests that some of these limitations may not have been fully appreciated. Here, we address several limitations and potential biases of CVL in four general categories related to: 1) selection and measurement issues in calculating CVL among HIV-infected individuals, 2) the importance of HIV prevalence in determining ongoing HIV transmission, 3) interpretation of CVL and its impact on ongoing transmission in a community, and 4) the ecological fallacy (ecological bias). Each of these issues relates to the use of CVL for estimating the potential for ongoing transmission within a community; the first issue also relates to the use of CVL as a metric for monitoring ART uptake. Our goal is to encourage cautious use of aggregate viral load metrics, and to advocate development of alternative population metrics and study designs.
Issues with Selection and Measurement
Non-representative samples of the HIV-infected population
Aggregate viral load measures depend on a person’s diagnosis, engagement in care, measurement of his/her viral load, and reporting of that viral load to a public health authority. In many settings, a significant proportion of HIV-infected persons are not linked to or engaged in care. The HIV care cascade, reflecting HIV diagnosis, linkage to care, retention in care, and appropriate treatment, reflects the incompleteness of HIV care.14–16 In 2010 in the United States, an estimated 1,178,350 persons had HIV infection, but only 61.6% were linked to care and only 40.8% retained in HIV care.16 Consequently, any estimate of the CVL based on persons linked to and/or retained in care will be based on a subset of the HIV-infected population.14–16
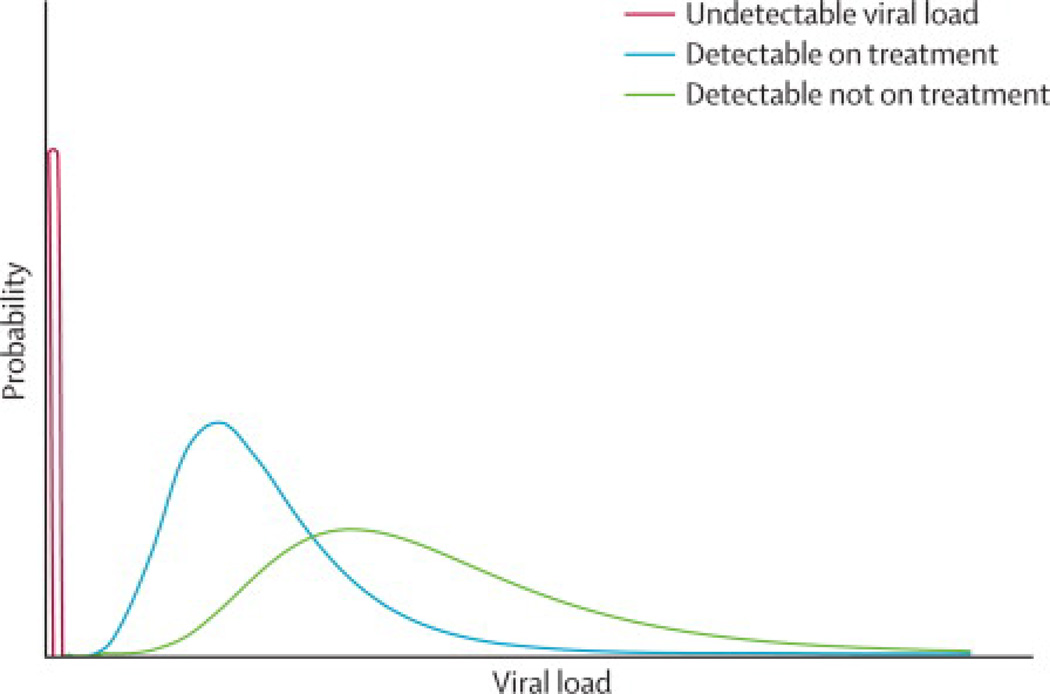
A sample of persons derived only from those in care will be a non-random subset of the total HIV-infected population. An in-care-only population excludes persons with undiagnosed HIV infection and persons with known HIV status who are not engaged in care. The not-in-care population cannot receive the benefits of ART and therefore will have higher viral loads than the in-care population included in the CVL calculation (Figure 1), resulting in a biased estimate of CVL.
Figure 1. Schematic representation of viral load distribution in a hypothetical HIV-infected population.
Any population of HIV-infected persons will consist of individuals with undetectable viral loads (red curve), individuals with detectable viral loads who are receiving treatment (blue curve), and individuals with detectable viral loads who are not receiving treatment (green curve). Diagram provides qualitative representation of expected viral load distribution; actual distribution will be specific to a particular population.
Even if testing and medical care coverage of the HIV-infected population was considerably enhanced, CVL would remain underestimated, because persons with acute and early HIV infection are missed with routine HIV testing in most settings and therefore will not be identified and linked to care.17–21 Acute/early HIV infection, reflecting the first few weeks to months of infection, is associated with enhanced transmissibility,22 due to markedly elevated viral loads23,24 and the characteristics of the initial infecting virus.25 The proportion of ongoing transmission attributable to acute/early HIV infection will vary by setting, depending on the local characteristics of the epidemic. Estimates of the transmission attributable to acute/early HIV have varied,26 but was as high as 38% in one African setting with an established epidemic.27 Detection of acute/early infection requires special efforts, such as pooled testing of ELISA negative specimens with HIV RNA PCR or use of p24 antigen ELISAs in combination with traditional antibody ELISAs.17,19 However, even if these alternative testing approaches were widely used, many, if not most, persons with acute/early infection would remain undetected, because detection requires recently infected persons to actively seek testing. Consequently, even with regular HIV testing and linkage to care, CVL measured through the health system will always underestimate the “true” CVL (i.e. population viral load) because the high viral loads of acute cases are excluded.
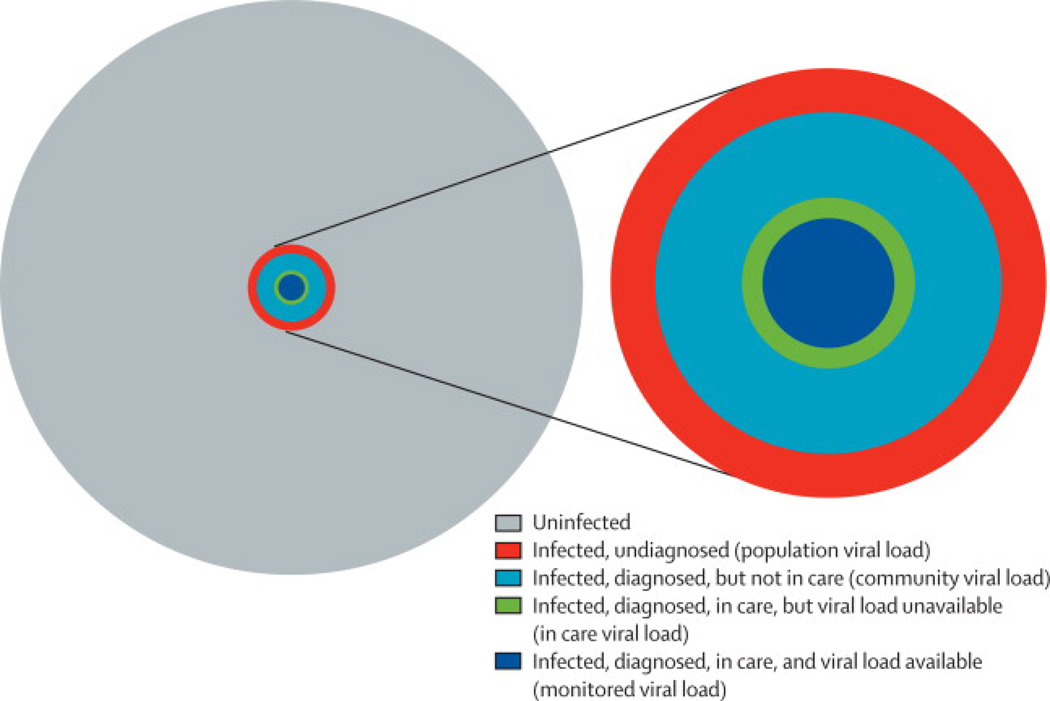
In its recent guidance for monitoring CVL, the CDC has acknowledged the issue of selection bias.9,10 The CDC has proposed a series of aggregate viral load measures reflecting different levels of data availability in the HIV care cascade, including “population viral load”, “community viral load”, “in-care viral load”, and “monitored viral load” (Figure 2). (Note: We have used the term community viral load to broadly represent these aggregate viral load measures, except where explicitly stated in the text.) Although the definition of these measures partially addresses the issue of selection bias due to limited availability of data for persons not engaged in care, the magnitude of the bias and the utility of these various measures are not directly addressed. Furthermore, none of these proposed measures accounts for the relative size of the HIV-infected population (i.e., the prevalence), as discussed in the next section.
Figure 2. Relationship between population subgroups and CDC-defined aggregate measures of viral load.
In the set of concentric circles representing an entire hypothetical population (gray) and various population sub-groups (each a different color), subpopulations represented by a given, smaller circle belong to all groups represented by larger, surrounding circles. The size of each circle is proportional to the size of a given sub-group, assuming 15% HIV prevalence (hypothetical), 79% of cases diagnosed,14 50% of diagnosed cases in care,14 and 75% of cases in care with viral loads available (hypothetical). For each CDC-defined viral load metric listed on the right side of the figure, the origin of the corresponding arrow indicates the sub-population (including all smaller sub-populations) contributing to the metric. Notably, none of the proposed metrics accounts for the size of the uninfected population (i.e., 1 – prevalence).
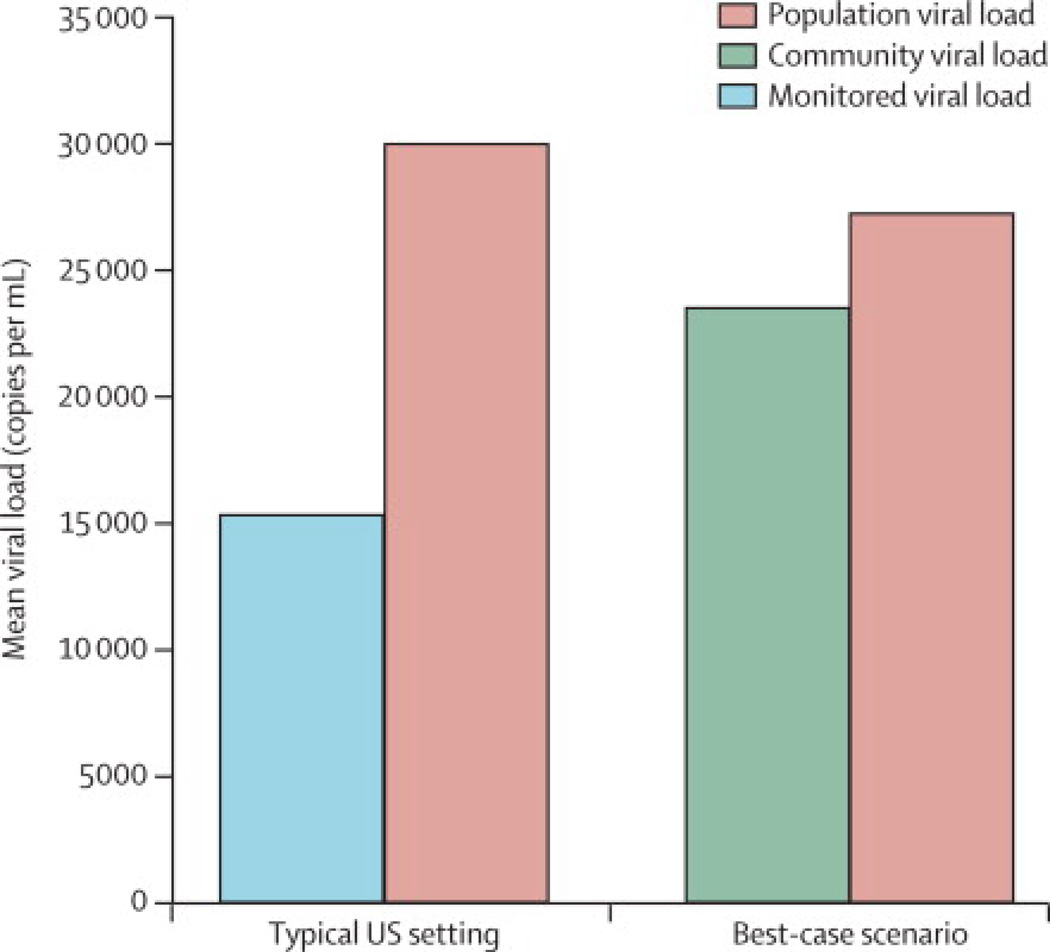
To demonstrate the potential impact of selection bias related to the HIV care cascade and acute/early HIV infection, we conducted a simple exercise using plausible estimates of the proportion of the HIV-infected population and mean viral loads present at each step of the cascade (Figure 3). Based on CDC estimates of the HIV care cascade, the measured CVL (i.e. monitored viral load) in a typical US setting may underestimate the true population viral load by about 50%. In a best-case scenario, such as in San Francisco, where greater proportions of the HIV-infected population are aware of their infection status and in care (and thus captured by CVL measures), the underestimation may be smaller, approximately 15%.
Figure 3. Estimated mean values of true population viral load, community viral load, and monitored viral load as defined by CDC.
Population viral load = mean viral load among all HIV-infected individuals (theoretical; currently unobserved). Monitored viral load = estimated mean viral load as measurable in settings with VL data available only for persons in care. Community viral load = estimated mean viral load for persons in and out of care, excluding undiagnosed, as measured in a best-case setting (based on San Francisco data). A detailed description of the methods used in generating the figure is contained in the supplemental material. Briefly, figure assumptions and calculations were based on published estimates of the proportions of HIV-infected populations in key subgroups along the HIV care cascade (ref [1] for San Francisco, ref [14] for the US overall), proportions of infection-unaware persons with acute infection [18, 19], and mean viral loads in each sub-group [1, 18, 20].
The issue of selection bias could be addressed with a population-based survey in a clearly defined target population of all persons in a community, including those with and without known HIV infection and measurement of viral loads in infected persons. Such a random sample would overcome the biased sample associated with engagement in care and would provide a more accurate estimation of the distribution of the viral loads in the community. However, a survey-based approach is unlikely to be used routinely for surveillance purposes because of the expense, especially in low prevalence settings, where extremely large samples would be needed. Furthermore, acute HIV cases will be missed with routine HIV tests, unless testing is completed using HIV RNA testing (e.g. pooling).19 Finally, even if selection bias is substantially reduced, several challenges with CVL remain, as described below.
Sampling concerns within persons - Instability of viral loads within persons over time
Viral loads are dynamic within persons over time. While the goal of treatment is long-term suppression, some treated persons experience intermittent28 or long-term virological failure.29 CVL calculation has typically used a single viral load from each patient collected during the course of a given calendar year.1,3,4 Some persons have multiple viral loads, others one, and some none. Whether single or multiple viral loads are available, the time at which the available or selected viral load was measured could influence the accuracy and appropriateness of the CVL measure, both for predicting ongoing transmission and monitoring ART uptake. For example, any viral load measurement recorded while a person is on treatment may underestimate his/her overall contribution to CVL in the course of a given year if he/she subsequently drops out of care. Similar issues arise for those entering care, as viral loads before entering care, which will not routinely contribute to aggregate viral load measurements, will be higher on average than those recorded after entering care and initiating ART.
The Uninfected Population and HIV Transmission - Prevalence is important
Any population (or “community”) for which we wish to understand HIV transmission will comprise both HIV-infected and HIV-uninfected persons. HIV incidence will depend not only on the viral loads of infected persons, but also on the relative sizes of the infected and uninfected populations (i.e. HIV prevalence), and the rates and patterns of contact between them. To date, CVL measures have not considered the impact of HIV prevalence within the communities under observation.1–4
Consider two communities with the same population size but markedly different prevalence of HIV infection. In Community A, the prevalence of HIV infection is 5%. In Community B, the prevalence of HIV infection is 0.1%. Assume that in each community the mean CVL is 2000 copies/ml and is measured perfectly among all infected people. Also, assume that average contact rates are the same. Despite the equal CVL across communities, the HIV incidence rate will be substantially greater in the community with higher prevalence because the incidence rate is approximately equal to the product of the prevalence of infection, the average contact rate, and per-contact transmission probability.30
CVL interpretation – Estimating the potential for ongoing transmission in a community
Within any given population, the distribution of viral loads is likely to be multimodal (Figure 1). Among those in care, many persons will have achieved virological suppression, which will be reflected by a large peak below the limits of detection. The viral loads of the remaining persons in care will tend to be log-normally distributed.31 For persons out of care, the distribution is expected to be broader with a tail extending far to the right reflecting persons with especially high viral loads, including those with acute/early HIV infection. The shape of this composite distribution (Figure 1) presents a problem for any single summary measure of viral loads, including the arithmetic mean, geometric mean, median, or sum.
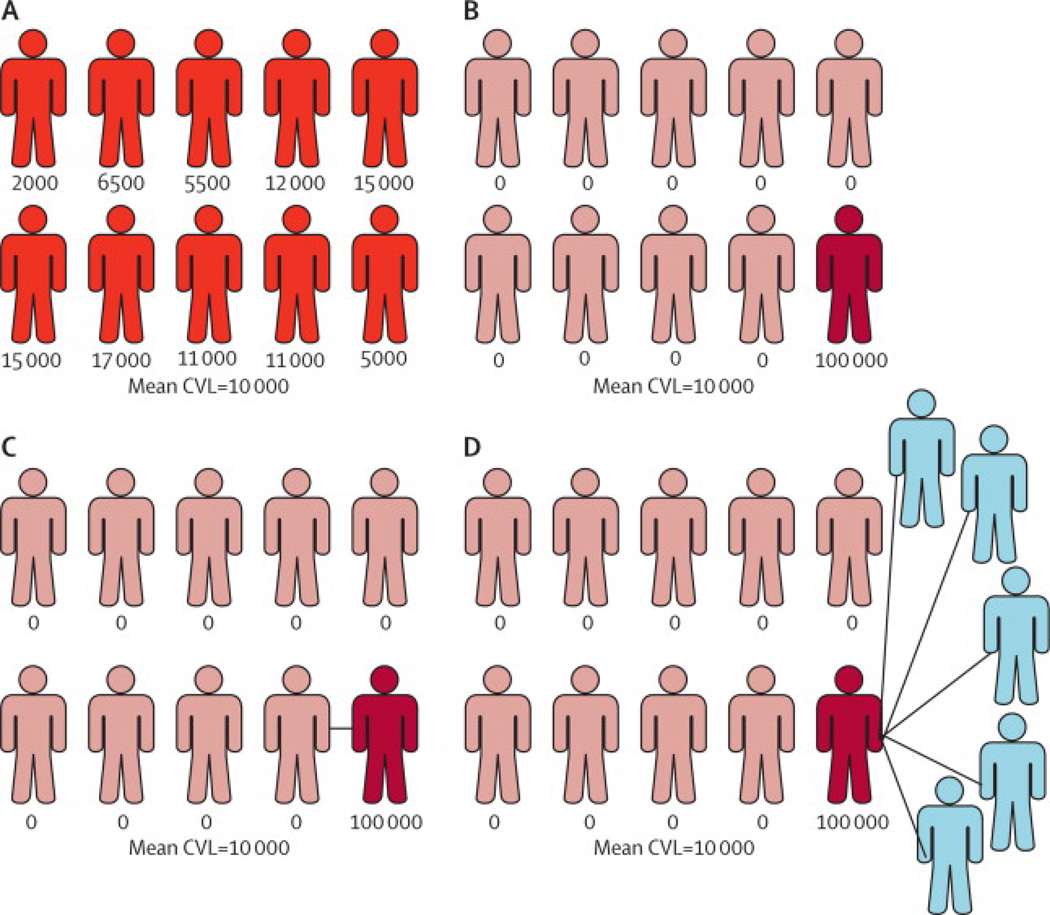
An aggregate viral load metric is a suboptimal measure of the potential for ongoing transmission in a community, even if all viral loads in a community are available. Consider two, extreme examples of populations with identical HIV prevalence and 10 HIV-infected persons. In each population, the viral loads for all HIV-infected persons are known and the mean CVL is 10,000 copies/ml (Figure 4). In Population A, the 10 HIV-infected persons have viral loads ranging from 2,000 to 20,000 with a mean of 10,000 copies/ml. In Population B, 9 of the 10 HIV-infected persons have undetectable viral loads and the tenth person has a viral load of 100,000 copies/ml. Although the CVL based on the mean or sum is identical, the likelihood of ongoing transmission in these communities is likely not the same.
Figure 4. Representation of populations with identical prevalence and community viral loads, but different potential for ongoing transmission.
A) Viral loads distributed between 2000 and 20,000; B) Viral loads <50 in 9 persons and 100,000 in 10th person; C) Population B with person with high viral load in monogamous relationship with HIV-infected, suppressed person; D) Population B with person with high viral load with multiple uninfected partners.
In addition to his/her viral load, the number of secondary transmissions attributable to any person depends on his/her sexual behavior and the properties of his/her sexual network. In Population B above (Figure 4), if the one person with the viral load of 100,000 copies/ml is monogamous with an HIV-infected partner (Figure 4, C), the probability of secondary transmissions will be considerably lower than if s/he has multiple HIV-uninfected partners (Figure 4, D). Similar variations can be considered with Population A.
The sexual networks of these two populations further affect the likelihood of ongoing transmission in the community. If the sexual networks are densely connected between HIV-infected and -uninfected persons, transmission is facilitated.32 But, if the networks are predominated by pairs of stable couples with few inter-couple links, ongoing transmission will be restricted.33 Overall, transmission is critically determined by a combination of several factors including the sexual behavior, network positions and viral loads of the HIV-infected persons in a population. In other words, in communities with similar mean viral loads, substantial variation in transmission patterns is not just possible, but expected. Consequently, the relationship between CVL and HIV incidence will vary across settings, reducing the utility of CVL as a universal measure of the likelihood of ongoing transmission within a community.
CVL and the ecological fallacy
In epidemiology, ecological data are useful for assessing group-level effects that occur above and beyond the expected contribution of individual-level effects. For example, for many infectious diseases, the group-level effects may be greater than expected based on individual data, due to interdependence of transmission events.34 However, using ecological data alone to determine individual-level causality is problematic.30 The attribution of causal effects at the individual level based on group-level observations is referred to as the ecological fallacy.30
Most studies relating CVL to measures of HIV transmission have noted the limitation of using ecological data, but have still interpreted the observed ecological associations as being driven solely by the individual-level effects of ART on HIV transmission.1,3 However, reduced HIV diagnosis rates in areas with lower CVL cannot be attributed with certainty to reduced transmission from persons with lower viral loads.
Although ecological associations between CVL and new HIV diagnoses may be partially driven by reduced biological infectiousness of those on treatment, these associations are also determined by additional factors at both the individual and population levels. Improved treatment services may be paired with other HIV prevention efforts that may have a population-level effect. For example, the population benefits of increased ART use among injection drug users cannot easily be dissociated from the beneficial impact of needle exchange and other programs targeted to this population.3,35,36 The ecological level of these analyses without data to link to individual transmission events is insufficient to determine whether viral suppression is responsible for reduced incidence, even if selection bias in CVL measurements were adequately addressed and an appropriate summary of the distribution was used.
Alternative metrics for consideration
Many of the shortcomings of CVL are due to the limitations of routinely available public health data. Surveillance data vary in quality, depending on the data sources and the completeness of data capture. Many alternative metrics will be limited similarly. Nonetheless, improvements may be possible, even if a perfect measure is unavailable.
One alternative measure is the proportion of persons in the entire population with a viral load greater than a pre-specified threshold, such as any detectable viral load (“detectable viral load proportion”).37 Other examples of this type of proportion include the proportion of persons with VL >400 or proportion of persons with VL >1000. The rationale for this measure has three key components: 1) transmission will occur almost exclusively in persons with measurable viral loads, 2) the HIV infection prevalence is incorporated into the measure directly, and 3) persons not in care can be assumed to have a detectable viral load. This third point is critical, but requires that the community have a reasonably valid estimate of the HIV infection prevalence in the community. Reasonable prevalence estimates will be available in some settings, but not others.
From a surveillance perspective, a combination of metrics may be necessary to obtain a complete description of the status of the HIV epidemic in a community. Considering treatment for an individual’s own benefit and treatment for the potential prevention of transmission will require monitoring the full cascade of HIV testing, diagnosis, engagement in care, retention in care, and viral suppression. Incorporating aspects of these stages of the care cascade into a composite metric may provide a useful measure for enhanced monitoring.
Conclusions
We have entered a new and exciting phase of HIV prevention. The potential for HIV treatment to prevent HIV transmission is substantial and promising. As the impact of broader and earlier ART use on HIV incidence in a population undergoes further study, the value of CVL as a routine measure of a community’s ART uptake and potential for ongoing transmission will be examined further. However, in practice, few areas currently have sufficient engagement and retention in care to estimate population-level aggregate viral load measures accurately and meaningfully. Even with adequate coverage across the HIV care cascade, aggregate viral load measures are imperfect metrics of ART uptake due to issues related to acute/early HIV infection and intra-individual viral load variations. The use of CVL as a measure of potential ongoing transmission in a community is complicated by those same issues, as well as those related to HIV prevalence, sexual behavior, sexual networks, and the ecological fallacy. The relative strengths and weaknesses of CVL and other proposed metrics for monitoring the impact of ART on the HIV epidemic must be addressed in future studies including observational studies, community randomized trials, and mathematical modeling.
Supplementary Material
Acknowledgments
We wish to thank the following people for comments on earlier drafts of this paper: Adaora Adimora, Till Bärnighausen, David Burns, Stephen Cole, Anna Barry Cope, Jeffrey Eaton, Christophe Fraser, Jesus M. Garcia Calleja, Kristen Hampton, Vicki Mobley, Habib O. Ramadhani, James Shelton, Nalyn Siripong, and David Wohl.
Financial Support: This work was supported by the National Institutes of Health, NIAID R01AI083059 (WCM, KAP); NIDDK R37DK049381 (MSC); NIAID T32 AI007001 (MKS). The funding sources had no involvement in the development of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ roles
William C. Miller conceived of the idea for the paper, wrote the initial draft, and contributed to subsequent editing of the paper. Kimberly A. Powers, M. Kumi Smith, and Myron S. Cohen contributed intellectually to the development of the paper’s content, edited the drafts for intellectual content, and approved the final version.
References
- 1.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castel AD, Befus M, Willis S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012;26:345–353. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 5.Jain V, Liegler T, Chamie G, et al. CROI. Seattle, WA: 2012. Mar 5–8, Assessment of community viral load using a fingerprick-based blood collection method: Uganda. 2012, 2012; [Google Scholar]
- 6.Henard S, Jeanmaire E, Nguyen Y, et al. Is total community viral load a robust predictive marker of the efficacy of the TasP strategy? Journal of Acquired Immune Deficiency Syndromes. 2012 doi: 10.1097/QAI.0b013e318263a111. [DOI] [PubMed] [Google Scholar]
- 7.Smith MK, Powers KA, Muessig KE, Miller WC, Cohen MS. HIV treatment as prevention: the utility and limitations of ecological observation. PLoS Med. 2012;9:e1001260. doi: 10.1371/journal.pmed.1001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. [Accessed May 31, 2012];Seek, Test, Treat, and Retain: Addressing HIV among Vulnerable Populations (R01), RFA-DA-11-001. 2010 http://grants.nih.gov/grants/guide/rfa-files/RFA-DA-11-001.html.
- 9.Centers for Disease Control and Prevention. [Accessed May 31, 2012];Using Viral Load Data to Monitor HIV Burden and Treatment Outcomes in the United States. 2012 http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/viral_load.htm.
- 10.Centers for Disease Control and Prevention. [Accessed May 31, 2012];Guidance on Community Viral Load: A Family of Measures, Definitions, and Method for Calculation. 2011 http://www.ct.gov/dph/lib/dph/aids_and_chronic/surveillance/statewide/community_viralload_guidance.pdf.
- 11.National HIV/AIDS Strategy for the United States. [Accessed May 31, 2012];2010 http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf.
- 12.Frieden TR, Das-Douglas M, Kellerman SE, Henning KJ. Applying public health principles to the HIV epidemic. The New England journal of medicine. 2005;353:2397–2402. doi: 10.1056/NEJMsb053133. [DOI] [PubMed] [Google Scholar]
- 13.Shelton JD, Cohen M, Barnhart M, Hallett T. Is antiretroviral therapy modifying the HIV epidemic? Lancet. 2010;376:1824–1825. doi: 10.1016/S0140-6736(10)62163-0. author reply 1825. [DOI] [PubMed] [Google Scholar]
- 14.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment--United States. MMWR. Morbidity and mortality weekly report. 2011;60:1618–1623. [PubMed] [Google Scholar]
- 17.Fiscus SA, Pilcher CD, Miller WC, et al. Rapid, real-time detection of acute HIV infection in patients in Africa. The Journal of infectious diseases. 2007;195:416–424. doi: 10.1086/510755. [DOI] [PubMed] [Google Scholar]
- 18.Patel P, Klausner JD, Bacon OM, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006;42:75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. The New England journal of medicine. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 20.Priddy FH, Pilcher CD, Moore RH, et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J Acquir Immune Defic Syndr. 2007;44:196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 21.Kelley CF, Barbour JD, Hecht FM. The relation between symptoms, viral load, and viral load set point in primary HIV infection. J Acquir Immune Defic Syndr. 2007;45:445–448. doi: 10.1097/QAI.0b013e318074ef6e. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. Journal of Infectious Diseases. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 23.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 24.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma ZM, Stone M, Piatak M, Jr, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. The New England journal of medicine. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Percus JK, Percus OE, Di Mascio M. The amplitudes of viral blips in HIV-1 infected patients treated with antiretroviral therapy are power-law distributed. J Theor Biol. 2009;257:454–459. doi: 10.1016/j.jtbi.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Hull MW, Lima VD, Hogg RS, Harrigan PR, Montaner JS. Epidemiology of treatment failure: a focus on recent trends. Curr Opin HIV AIDS. 2009;4:467–473. doi: 10.1097/COH.0b013e328331d353. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3 ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 31.Chu H, Gange SJ, Li X, et al. The effect of HAART on HIV RNA trajectory among treatment-naïve men and women: a segmental Bernoulli/lognormal random effects model with left censoring. Epidemiology. 2010;21(Suppl 4):S25–S34. doi: 10.1097/EDE.0b013e3181ce9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. The Journal of infectious diseases. 2005;191(Suppl 1):S42–S54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 33.Reniers G, Watkins S. Polygyny and the spread of HIV in sub-Saharan Africa: a case of benign concurrency. AIDS. 2010;24:299–307. doi: 10.1097/QAD.0b013e328333af03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koopman JS, Longini IM., Jr The ecological effects of individual exposures and nonlinear disease dynamics in populations. Am J Public Health. 1994;84:836–842. doi: 10.2105/ajph.84.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr T, Small W, Buchner C, et al. Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health. 2010;100:1449–1453. doi: 10.2105/AJPH.2009.178467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr T, Tyndall M, Li K, Montaner J, Wood E. Safer injection facility use and syringe sharing in injection drug users. Lancet. 2005;366:316–318. doi: 10.1016/S0140-6736(05)66475-6. [DOI] [PubMed] [Google Scholar]
- 37.Kelley CF, Rosenberg ES, O'Hara BM, et al. Measuring population transmission potential: an alternative metric of transmission risk in men who have sex with men (MSM) in the USA. Paper presented at: XIX International AIDS Conference; July 22–27, 2012; Washington, D.C.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.