Abstract
Background and Aims Manganese (Mn) and aluminium (Al) phytotoxicities occur mainly in acid soils. In some plant species, Al alleviates Mn toxicity, but the mechanisms underlying this effect are obscure.
Methods Rice (Oryza sativa) seedlings (11 d old) were grown in nutrient solution containing different concentrations of Mn2+ and Al3+ in short-term (24 h) and long-term (3 weeks) treatments. Measurements were taken of root symplastic sap, root Mn plaques, cell membrane electrical surface potential and Mn activity, root morphology and plant growth.
Key Results In the 3-week treatment, addition of Al resulted in increased root and shoot dry weight for plants under toxic levels of Mn. This was associated with decreased Mn concentration in the shoots and increased Mn concentration in the roots. In the 24-h treatment, addition of Al resulted in decreased Mn accumulation in the root symplasts and in the shoots. This was attributed to higher cell membrane surface electrical potential and lower Mn2+ activity at the cell membrane surface. The increased Mn accumulation in roots from the 3-week treatment was attributed to the formation of Mn plaques, which were probably related to the Al-induced increase in root aerenchyma.
Conclusions The results show that Al alleviated Mn toxicity in rice, and this could be attributed to decreased shoot Mn accumulation resulting from an Al-induced decrease in root symplastic Mn uptake. The decrease in root symplastic Mn uptake resulted from an Al-induced change in cell membrane potential. In addition, Al increased Mn plaques in the roots and changed the binding properties of the cell wall, resulting in accumulation of non-available Mn in roots.
Keywords: Aluminium, manganese toxicity, rice, Oryza sativa, Gouy–Chapman–Stern model, manganese plaques, plasma membrane surface electrical potential, root aerenchyma, root symplast, acid soils.
INTRODUCTION
Manganese (Mn) is an essential element for plants and is involved in regulating several metabolic processes, such as photosynthesis and antioxidant activity (Marschner, 1995a). Nevertheless, an excess of Mn is toxic for most plants (Millaleo et al., 2010). Manganese toxicity is one of the major factors restricting plant growth on poorly drained and acidic soils with high concentrations of readily available Mn2+ (Marschner, 1995b). In plants, Mn tends to accumulate predominantly in shoots rather than roots (Lidon, 2001; Page and Feller, 2005; Page et al., 2006). Therefore, in addition to decreased growth rate, symptoms of Mn toxicity, such as chlorosis and necrotic brown spots, are commonly visible on leaves (Moroni et al., 2003; Rosas et al., 2007; Mora et al., 2009; Führs et al., 2010; Li et al., 2011). The threshold of Mn toxicity and tolerance varies among different plant species and among cultivars and genotypes within a species (Foy et al., 1988; Horst, 1988). In general, rice (Oryza sativa) plants are considered to be tolerant to Mn toxicity (Lidon, 2001). Compared with other grasses, rice plants can accumulate 5–10 times more Mn in the leaves and show milder symptoms of toxicity (Foy et al., 1978). Although Mn toxicity rarely occurs in flooded or paddy rice, this is not the case for upland rice grown on aerobic soils. Acid upland soils may contain toxic levels of both Mn and aluminium (Al) (Nelson, 1983), and as a consequence Al and Mn toxicities may limit the yields of upland rice (Ponnamperuma, 1975).
Aluminium toxicity is generally considered to be the primary limiting factor for plant growth in acid soils (Kochian et al., 2004). Low concentrations (<10 µm) of Al can inhibit root elongation and cause structural and functional damage to roots (Kochian, 1995). Many plants predominately accumulate Al in the roots, while some plants, such as buckwheat (Fagopyrum esculentum) (Ma et al., 1998), tea (Camellia spp.) (Chenery, 1955; Zeng et al., 2013) and plants in the Melastomataceae (e.g. Melastoma malabathricum) (Watanabe et al., 2005), accumulate high concentrations of Al in either roots or leaves.
High concentrations of Al and Mn can co-occur in soils, restricting crop production in acid and high-Mn soils. In previous studies, Australian soils were classified into seven categories covering the range of Al and Mn combinations (Dolling et al., 2001; Yang et al., 2009). Most agricultural soils were designated as Class 2, with a low concentration of available Al and a high Mn concentration. Heavy rainfall can change the concentrations of available Al and Mn, and consequently, change Class 2 soils to Class 3, with moderate Al and very high Mn concentrations (Yang et al., 2009). Although high concentrations of Mn and Al can co-occur in acid soil, the tolerances of plants/cultivars to Al and Mn do not necessarily coincide (Nelson, 1983). This raises questions as to how plants adapt to both Mn and Al toxicity and how Mn interacts with Al in plants.
Previous reports have indicated that Al can reduce Mn accumulation in barley (Hordeum vulgare), atriplex (Atriplex hastata) (Rees and Sidrak, 1961), corn (Zea mays) (Clark, 1977), wheat (Triticum aestivum) (Blair and Taylor, 1997), cowpea (Vigna unguiculata) (Taylor et al., 1998) and soybean (Glycine max) (Yang et al., 2009). Alleviation of the effects of Al on Mn toxicity has been reported for atriplex (Rees and Sidrak, 1961) and soybean (Yang et al., 2009), and a dose-dependent effect of Al on Mn toxicity has been demonstrated in wheat and cowpea (Blair and Taylor, 1997; Taylor et al., 1998). One explanation for this alleviation is that Al may have an antagonistic effect on Mn uptake by plant roots. However, the exact mechanism is still poorly understood. The results of Kopittke et al. (2011a), based on the Gouy–Chapman–Stern (GCS) model, revealed that the effects of cations on Mn nutrition were related to the electrical potential (ψo0) of the root cell membrane (CM) surface. In their study, when cowpea was cultured in solutions containing different Mn concentrations, the root tissue Mn concentration was closely related to the activity of Mn2+ at the outer surface of the CM. This result provided new clues about the interaction between Al and Mn.
The GCS model combines electrostatic theory (Gouy–Chapman theory) with an ion-binding model (Stern model) to calculate ψo0. This could be a useful and powerful tool to explain the Mn–Al interaction in plants. To better understand the effects of Al on Mn toxicity, we explored how Al affected Mn toxicity using Al- and Mn-tolerant rice plants. Our results differ from those reported previously; in particular, the effects of Al on root Mn accumulation in rice completely contrast with those in other plant species.
MATERIALS AND METHODS
Plant material and growth conditions
We used Wuyunjing7, an Al-tolerant cultivar of rice (Oryza sativa var. japonica) (Zhao et al., 2009) in these experiments. The rice seeds were surface-sterilized in 1 % (v:v) sodium hypochlorite solution for 30 min, washed thoroughly with deionized water, and then soaked in deionized water overnight. The seeds were germinated in an incubator at 30 °C for 24 h, and then transferred to a nylon net floating on a 6·5-L plastic pot contained 0·5 mm CaCl2 (pH 4·5). The solution was renewed every 2 d. After 3 days of growth, the seedlings were transferred to light conditions and grown in 0·5 mm CaCl2 (pH 4·5) for a further 2 d. Then, uniform seedlings (5 d old) were transferred to 1·5 L of half-strength Kimura B nutrient solution (pH 4·5) and grown for 6 d. The half-strength Kimura B nutrient solution contained 0·18 mm (NH4)2SO4, 0·27 mm MgSO4·7H2O, 0·09 mm KNO3, 0·18 mm Ca (NO3)2·4H2O, 0·09 mm KH2PO4, 6·7 µm MnCl2·4H2O, 9·4 µm H3BO3, 0·01 µm (NH4)6Mo7O24·4H2O, 0·15 µm ZnSO4·7H2O, 0·16 µm CuSO4·5H2O and 10 µm EDTA-Fe. The solution was renewed every 2 d without aeration. Finally, 11-d-old seedlings were used in experiments with different Al and Mn treatments. The seedlings were grown in a growth room under a 14:10-h light:dark photoperiod with 28 ± 1:25 ± 1 °C, day:night temperatures. The relative humidity was controlled at 65 ± 1 % and the light level was controlled at 375 μmol photon m−2 s−1.
For all Al–Mn interaction experiments, four seedlings were grown in each 1·5 L of nutrient solution. To identify a suitable Al concentration for experiments on the Al–Mn interaction, 11-d-old seedlings were grown in half-strength Kimura B nutrient solutions containing 0, 200, 500 or 1000 µm Al for 3 weeks. Based on the results of this preliminary experiment, 200 µm Al was chosen for further experiments. For the Al–Mn interaction experiments, 11-d-old seedlings were grown in half-strength Kimura B nutrient solution containing 6·7, 200, 500 or 1000 µm Mn in the presence or absence of 200 µm Al for 24 h (short term) or 3 weeks (long term). These solutions were renewed every 2 d without aeration. At the end of the long-term experiments, we measured rice growth, Mn concentration, root Mn plaques, root aerenchyma, root diameter and root volume. At the end of the short-term experiments, we determined Mn concentrations in the root symplast, whole roots and whole shoots. The Al and Mn were supplied as AlCl3 and MnCl2, respectively. The initial pH of each solution was adjusted to 4·5 using 1 m NaOH or HCl. All experiments were conducted with three replicates, and each experiment was performed at least twice.
Extraction of root symplastic sap
The roots were washed with deionized water and gently blotted dry, and then cut into pieces. The pieces were placed in an ice-cool filter unit. The root symplastic sap was collected by centrifugation according to Klug et al. (2010) and Xia et al. (2010). Briefly, roots that were freshly cut from the shoot base of four seedlings were placed in a filter unit with a pore size of 0·45 µm (Ultrafree-MC, Millipore, Billerica, MA, USA). The nutrient solution adhering to the surface of the roots was removed by centrifugation at 60 g for 2 min. Apoplastic solutions were removed by centrifugation at 3000 g for 15 min at 4 °C. The roots were then frozen at −80 °C overnight. The root symplastic sap solution was obtained by thawing the samples at room temperature and then centrifuging them at 21 600g for 10 min. The residues (i.e. whole root) were washed three times with deionized water and then oven-dried at 75 °C to constant weight. The Mn concentrations in root symplastic sap and whole roots were determined by inductively coupled plasma mass spectrometry (ICP-MS) using an Agilent 7700x instrument (Agilent, Santa Clara, CA, USA), as described below.
Extraction and observation of root Mn plaques
Roots were washed twice with deionized water to remove any extraneous precipitate, and then cut from each plant at the shoot base. Root Mn plaques on the root surfaces were extracted using the dithionite citrate bicarbonate (DCB) technique (Taylor and Crowder, 1983; Crowder and Coltman, 1993), which was modified as follows: all root segments from each plant were agitated in a solution containing 40 mL of 0·3 m Na2C6H5O7•3H2O and 5 mL of 1 m NaHCO3 for 3 h. At the start of the experiment, 3 g of Na2S2O4 was added to the solution. The extractant was then decanted into a 100-mL volumetric flask and the roots were washed twice with deionized water, which was also decanted into the 100-mL volumetric flask. The resulting solution was made up to 100 mL with deionized water. We prepared DCB control solutions without roots as described above, but substituting an equimolar concentration of Na2SO3 for Na2S2O4 (with respect to Na). After roots had been extracted, they were oven-dried at 75 °C to constant weight. The dry masses of roots and shoots were measured and recorded. The Mn concentrations of Mn plaques were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Optima 8000, Perkin Elmer) after appropriate dilution. The Mn concentrations of roots after extraction were determined as described below.
To detect Mn plaques by microscopy, roots were harvested after treatments and cut into 5-mm pieces, and then quick-frozen and freeze-dried. After coating with gold, root segments were observed under a scanning electron microscope (SEM-3000N, Hitachi, Tokyo, Japan) and analysed by energy-dispersive X-ray spectrometry (EDX) (EDX-250, Horiba, Kyoto, Japan).
Observation of aerenchyma and measurement of root diameter and volume
Newly formed adventitious roots, ∼30 mm long, were collected after the long-term experiment. Fresh samples were cut into 5-mm pieces using a razor blade to observe aerenchyma by SEM using the method of David and Olga (2000). Then, the root samples were viewed and photographed under an SEM (3000N, Hitachi).
To measure root diameter and volume, whole roots were washed with deionized water and then scanned using a root scanner (Perfection V700 Photo, Seiko Epson, Nagano, Japan). Root diameter and volume were calculated using the WinRHIZO analysis system (Regent, Quebec, Canada).
Computation of CM surface electrical potential and Mn activity at CM surface
Values for the CM surface electrical potential (ψo0) and Mn2+ activity at the CM surface ({Mn2+}o0) were calculated using a computer program for the GCS model (Kinraide et al., 1998; Kopittke et al., 2014). This computer program was a kind gift from Professor T. B. Kinraide and Dr Peng Wang. Briefly, the GCS model consists of the Gouy–Chapman portion and the Stern portion. The Gouy–Chapman portion of the model refers to electrostatic theory pertaining to charged surfaces in contact with solutions. As regards the Stern portion, the CM was considered to contain negatively charged (R–) and neutral (P0) sites to which ions (IZ) may bind. A converging calculation between the Gouy–Chapman and Stern portions allows the calculation of ψo0 (Kinraide and Wang, 2010; Tatulian, 1999). The ψo0 values calculated from the GCS model allow the calculation of ion IZ at the CM surface ({IZ}o0) by the Nernst equation ({IZ}o0 = {IZ}b exp[–ZFψo0/(RT)]), where F, R and T are the Faraday constant, the gas constant and temperature, respectively (Wang et al., 2011). For details of the calculation of ψo0, refer to Kinraide (1994) and Kinraide et al. (1998).
Rice harvest and Mn analysis
At the end of the long-term experiment, the roots were rinsed briefly with deionized water and blotted dry. The roots and shoots were then harvested separately. The longest root and the longest shoot on each seedling were measured with a ruler. The roots and shoots were oven-dried at 75 °C to constant weight and then weighed and ground. Then, ground samples (100–500 mg) were digested with 5 mL of 5:1 (v:v) concentrated nitric acid:perchloric acid. After complete digestion, the samples were diluted to 50 mL using deionized water and the Mn concentrations were determined by ICP-AES (Optima 8000, Perkin Elmer). At the end of the short-term experiment, roots were harvested and their symplastic sap was collected, and then the roots were digested in 0·5 mL of ultra-trace concentrated nitric acid. After appropriate dilution, the Mn concentrations in the samples were determined by ICP-MS using an Agilent 7700x instrument.
Statistical analysis
All data were subjected to one-way analysis of variance after testing for normality and homogeneity. Multiple comparisons among treatments were performed using Tukey’s multiple range test at the 0·05 probability level. All these analyses were performed using SAS 9.1 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Rice growth
We conducted a preliminary Al toxicity experiment to identify a suitable Al concentration for research on the Mn–Al interaction. As shown in Supplementary Data Table S1, there was no significant difference in the root dry weights among all Al concentrations, but Al significantly inhibited root elongation at treatment concentrations of 500 and 1000 µm Al but not with the treatment concentration of 200 µm Al. The shoot dry weight and shoot height were lower in plants treated with 1000 µm Al than in control plants (0 µm Al), and shoot dry weight was significantly decreased by 500 µm Al. However, 200 µm Al had little effect on shoot height and shoot dry weight. Therefore, 200 µm Al (18·8 µm {A13+}o0) was used in the following Mn–Al interaction experiments.
In the absence of Al, 500 and 1000 µm Mn significantly decreased root length relative to that of plants grown with 6·7 µm Mn (Fig. 1A). However, there was no significant difference in root length among all Mn concentrations in the presence of Al (Fig. 1A). Shoot height and dry weights of roots and shoots significantly decreased with increasing Mn concentrations in the absence of Al (Fig. 1B–D). However, addition of Al markedly alleviated this inhibition, because all of these parameters were increased by Al addition at 500 and 1000 µm Mn (Fig. 1B–D). These results indicated that Al addition alleviated the toxic effects of Mn on rice growth. The photographs of the seedlings in the various treatments illustrate the alleviating effects of Al on Mn toxicity (Fig. 1E, F). Rice growth was inhibited by high Mn concentrations and old leaves were nearly wilting (Fig. 1E), but addition of Al markedly improved growth of rice and the old leaves at 500 and 1000 µm Mn (Fig. 1F).
Fig. 1.
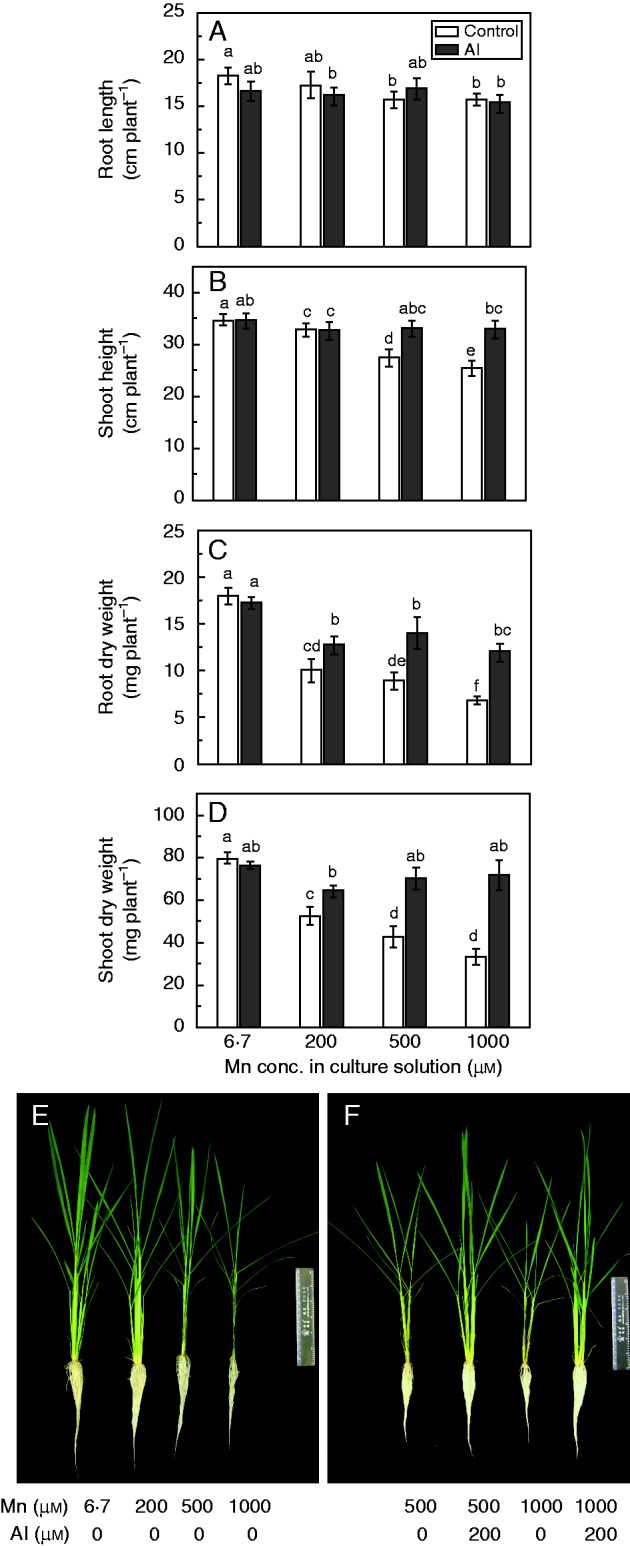
Effects of Al on root length (A), shoot height (B), root dry weight (C) and shoot dry weight (D) of rice under Mn toxicity in a long-term experiment. (E) Toxicity symptoms of rice under Mn toxicity. (F) Ameliorating effect of Al on rice Mn toxicity. Rice seedlings (11 d old) were grown in nutrient solution with 6·7, 200, 500 or 1000 µm Mn in the presence or absence of 200 µm Al for 3 weeks. Values are mean ± s.e. (n = 3 replicates, each comprising four seedlings). Different letters in A–D show significant differences among treatments (P < 0·05; Tukey’s test).
Rice Mn accumulation
After the long-term Al–Mn treatments (3 weeks), both root and shoot Mn accumulation significantly increased with increasing Mn concentrations, regardless of whether Al was present or not (Fig. 2A, B). At 6·7 µm Mn, addition of Al did not significantly affect Mn accumulation in roots and shoots (Fig. 2A, B). At 200, 500 and 1000 µm Mn, Al significantly decreased Mn accumulation in shoots (Fig. 2B) but increased Mn accumulation in roots (Fig. 2A).
Fig. 2.
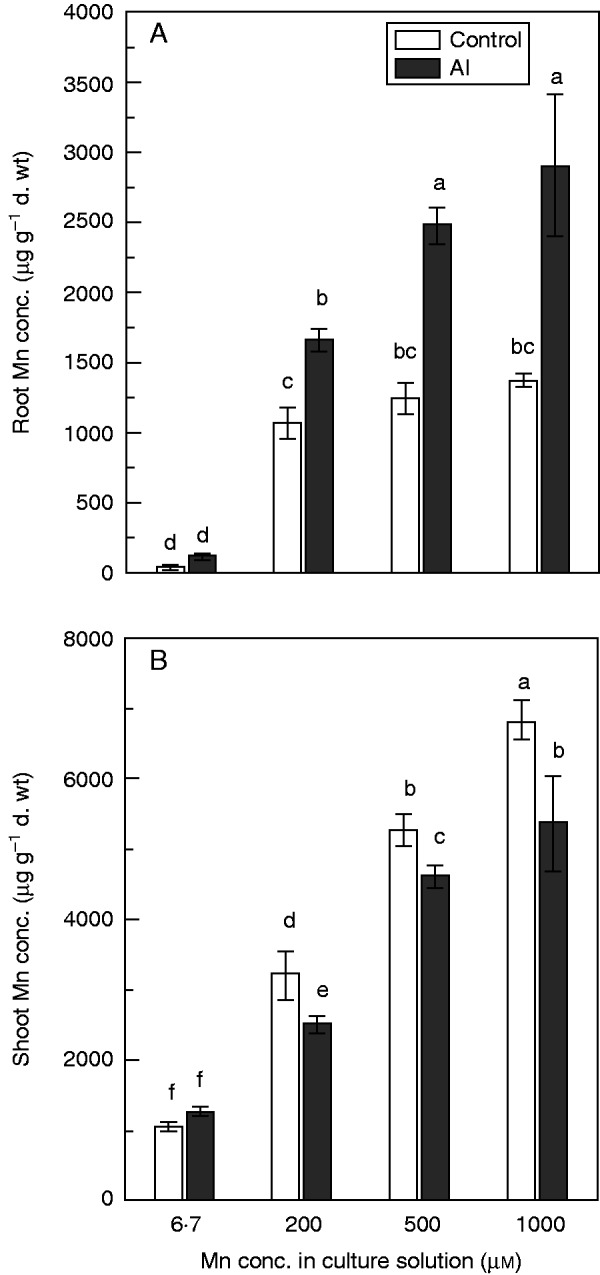
Effects of Al on Mn accumulation in roots (A) and shoots (B) of rice under Mn toxicity in a long-term experiment. Rice seedlings (11 d old) were grown in nutrient solution with 6·7, 200, 500 or 1000 µm Mn in the presence or absence of 200 µm Al for 3 weeks. Values are mean ± s.e. (n = 3 replicates, each comprising four seedlings). Different letters show significant differences among treatments (P < 0·05; Tukey’s test).
After the short-term Al–Mn treatments (24 h), Mn concentration in the root symplast, whole roots and whole shoots also gradually increased with increasing Mn in all treatments (Fig. 3A–C). At 6·7 µm Mn, addition of Al did not significantly affect the Mn concentrations in the root symplast (Fig. 3A), whole roots (Fig. 3B) or whole shoots (Fig. 3C). At 200, 500 and 1000 µm Mn, addition of Al significantly reduced the Mn concentration in the root symplast (Fig. 3A) and whole shoots (Fig. 3C), consistent with the results of the long-term experiment (Fig. 2B). In roots, the Mn concentrations were significantly reduced by addition of Al at 1000 µm Mn, but not at 200 or 500 µm Mn (Fig. 3B). These results suggested that Al inhibited Mn uptake into the root symplast, resulting in less Mn being transported to shoots.
Fig. 3.
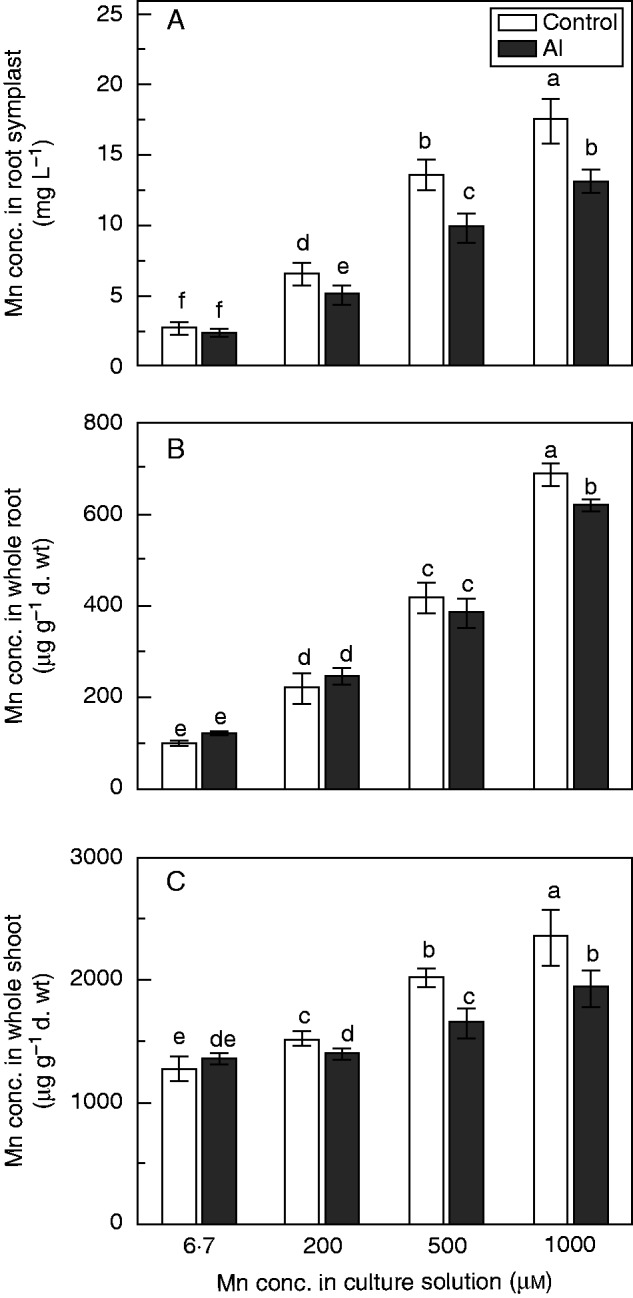
Concentrations of Mn in root symplast (A), whole root (B) and whole shoot (C) after short-term treatment. Rice seedlings (11 d old) were grown in nutrient solution with 6·7, 200, 500 or 1000 µm Mn in the presence or absence of 200 µm Al for 24 h. Values are mean ± s.e. (n = 3 replicates, each comprising four seedlings). Different letters show significant differences among treatments (P < 0·05, Tukey’s test).
Cell membrane surface electrical potential and Mn activity in the bulk solution and at the cell membrane surface
At the same Al concentration, the value of ψo0 gradually became more positive with increasing Mn concentration (Table 1). Addition of Al drastically changed the ψo0 values from negative to positive (Table 1). The values of {Mn2+}o0 and Mn2+ activity in bulk solution ({Mn2+}b) increased with increasing Mn supply, while addition of Al drastically decreased {Mn2+}o0 but barely affected {Mn2+}b (Table 1).
Table 1.
Calculated surface electrical potential at outer surface of the plasma membrane and Mn activity in bulk solution and at the cell membrane surface
| Al (µm) | Mn (µm) | ψo0 (mV) | {Mn2+}b (µm) | {Mn2+}0o (µm) |
|---|---|---|---|---|
| 0 | 6·7 | –30·4 | 5·1 | 55·0 |
| 200 | 6·7 | 7·9 | 5·1 | 2·8 |
| 0 | 200 | –26·7 | 152·0 | 1212·9 |
| 200 | 200 | 8·2 | 150·0 | 79·0 |
| 0 | 500 | –22·8 | 370·6 | 2183·2 |
| 200 | 500 | 8·6 | 365·6 | 187·6 |
| 0 | 1000 | –18·5 | 715·8 | 3010·0 |
| 200 | 1000 | 9·1 | 707·3 | 349·2 |
ψo0, surface electrical potential at outer surface of plasma membrane; {Mn2+}b, Mn activity in bulk solution; {Mn2+}o0, Mn activity at cell membrane surface.
All pH values were adjusted to 4·5 at the start of experiments. All solutions were 1/2 Kimura B nutrient solution containing various concentrations of Al and Mn.
Root Mn plaques
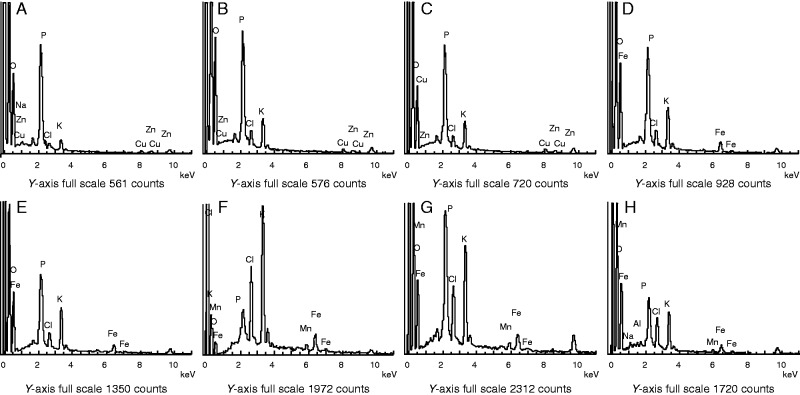
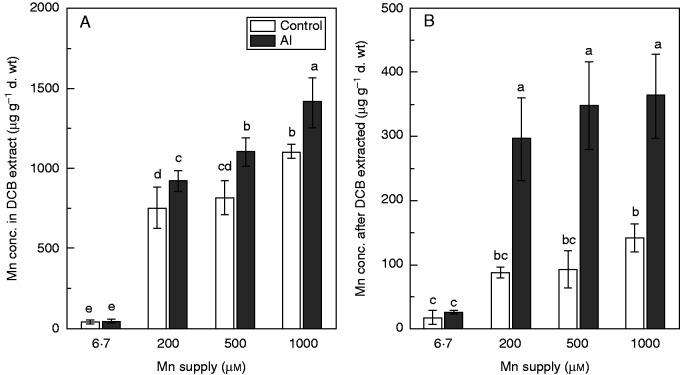
We detected root Mn plaques using EDX. The EDX spectra showed that Mn signals had two peaks, one around 0·5 keV and the other is around 6·0 keV (Fig. 4F–H). Since the first one could not easily be differentiated from other element peaks, the second peak was usually used for Mn analysis (Batty et al., 2002). This peak was absent from all Mn treatments in the absence of Al (Fig. 4A–D) and from the 6·7 µm Mn treatment in the presence of Al (Fig. 4E). However, Mn signals intensified when Al was added to solutions containing 200, 500 or 1000 µm Mn (Fig. 4F–H). The Mn plaques in roots were quantified using the DCB extraction technique. The Mn concentration in the DCB extract from roots and in roots after DCB extraction gradually increased with increasing Mn supply (Fig. 5A, B). Addition of Al significantly increased the DCB-extracted Mn concentrations in roots (Fig. 5A), consistent with the EDX results (Fig. 4). Root Mn concentrations after DCB extraction were higher in the presence of Al than in its absence. These results indicated that Mn plaques in roots were enhanced by Al addition under Mn toxicity.
Fig. 4.
Energy-dispersive X-ray analysis (EDX) of precipitates formed in roots of rice in hydroponic culture supplemented with Mn and Al or Mn only in a long-term experiment. The y-axis shows the counts, which refer to the number of X-rays received and processed by the detector. The counts principally represent the intensity of the elements in the surface of root cell. Rice seedlings (11 d old) were grown in nutrient solution with 6·7 (A, E), 200 (B, F), 500 (C, G) or 1000 (D, H) µm Mn in the absence (A–D) or presence (E–H) of 200 µm Al for 3 weeks.
Fig. 5.
Manganese concentrations in dithionite citrate bicarbonate extract (A) and root (B) after long-term treatment with Mn and Al or Mn only. Rice seedlings (11 d old) were grown in nutrient solution with 6·7, 200, 500 or 1000 µm Mn in the presence or absence of 200 µm Al for 3 weeks. Values are mean ± s.e. (n = 3 replicates, each comprising four seedlings). Different letters show significant differences among treatments (P < 0·05, Tukey’s test).
Root aerenchyma, diameter and volume
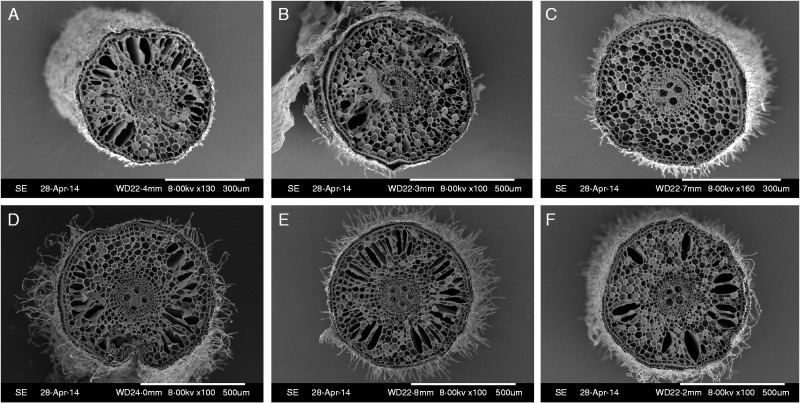
We observed roots using SEM to identify changes in root aerenchyma. There were fewer gas spaces in roots in the absence of Al (Fig. 6A–C) than in the presence of Al (Fig. 6D–F). Moreover, Mn toxicity significantly decreased the average diameter and volume of roots, while addition of Al increased these parameters (Fig. 7). These results indicated that Al facilitated the formation of more root aerenchyma and thicker, larger roots under Mn toxicity.
Fig. 6.
Effects of Al and Mn supply on aerenchyma formation in long-term experiment. Rice seedlings (11 d old) were grown in nutrient solution containing 6·7 (A, D), 500 (B, E) or 1000 (C, F) µm Mn without (A–C) or with (D–F) 200 µm Al for 3 weeks. After the 3-week treatment, newly formed adventitious roots were cut transversely 10 mm behind the root tip.
Fig. 7.
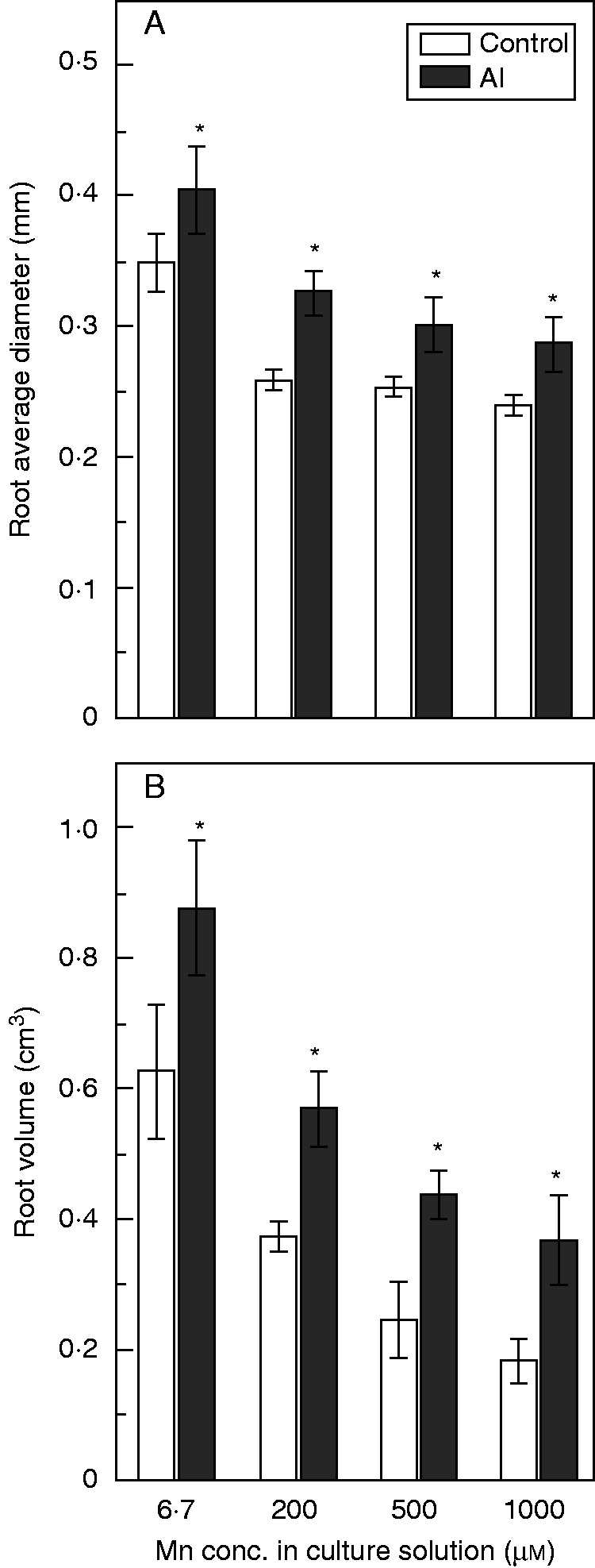
Root average diameter (A) and volume (B) after long-term treatment with Mn plus Al or Mn only. Rice seedlings (11 d old) were grown in nutrient solution containing 6·7, 200, 500 or 1000 µm Mn without or with 200 µm Al for 3 weeks. Values are mean ± s.e. (n = 12 replicates). Asterisks above columns indicate statistically significant differences between control and Al treatment (P < 0·05; independent samples t-test).
DISCUSSION
In preliminary experiments, we identified 200 µm Al as an appropriate concentration to determine the effects of Al on Mn toxicity in rice, because of its weak toxicity to rice growth. Shen et al. (2006) reported that the Al concentration range in solutions of acid soils from southern China was 100–200 µm. These soils also contain high concentrations of total Mn (763 mgkg–1 soil) and exchangeable Mn (196 mg kg–1 soil) (Chinese National Soil Survey Office, 1998). Given that the amount of exchangeable Mn varies depending on the total Mn content and waterlogging, it was reasonable to use 200 µm Al to interact with 6·7, 200, 500 and 1000 µm Mn in these Al–Mn interaction experiments.
Our results show that Al alleviated Mn toxicity to rice seedlings, consistent with previous reports for barley and atriplex (Rees and Sidrak, 1961), corn (Clark, 1977), wheat (Blair and Taylor, 1997), cowpea (Taylor et al., 1998) and soybean (Yang et al., 2009). Our data show that Al addition decreased Mn accumulation in the root symplast, whole roots and shoots in a 24-h treatment and in shoots in a 3-week treatment, which resulted in alleviation of Mn toxicity. Rice grew much better at 500 and 1000 µm Mn supply in the presence of Al than plants grown at 200 µm Mn supply in the absence of Al in the long-term treatment (Fig. 1D). However, shoot Mn accumulation in the former was higher than in the latter (Fig. 2B). This might be involved in the effects of Al on Mn internal detoxication, such as Mn distribution between apoplast and symplast or old and new leaves. These processes could be regulated by OsYLS6 and OsNRAMP3 under different Mn environments (Sasaki et al., 2011; Yamaji et al., 2013). This is a topic needing further investigation.
In previous studies it has been postulated that antagonistic effects of Al on Mn, or non-specific competition between Al and Mn, may be responsible for reduced Mn accumulation in plants. However, these ideas have not been tested using detailed and deep analyses. In this study we used the GCS model, which is based on classical electrostatic theory (Gouy–Chapman theory) (Kinraide, 1994; Kinraide et al., 1998), to explain how Al reduced Mn accumulation in rice plants. We observed that Al decreased Mn accumulation in the root symplast and the whole root in a short-term Al treatment, resulting in less Mn being transported to the shoots.
Generally, Al is considered to be toxic for plant growth, especially root growth. However, Al has been shown to alleviate H+ (Kinraide, et al., 1992; Kinraide, 1993) and Cu2+ toxicity (Wang et al., 2008; Kopittke et al., 2011b). In our previous reports we showed that Al enhanced the growth of Lespedeza bicolor (Chen et al., 2010) and rice (Zhao et al., 2013) in the presence of ammonium, which can be phytotoxic at high concentrations (Britto and Kronzucker, 2002). It has been suggested that the alleviation of Cu2+ and H+ toxicity resulted from the Al-induced decrease in the negativity of ψo0, which corresponded to reduced Cu2+ or H+ activity at the CM surface (Kinraide, 1993; Kopittke et al., 2011b; Wang et al., 2012). In the present study we calculated that Al drastically increased the positivity of ψo0, resulting in lower {Mn2+}o0 (Table 1). This may have contributed to the decreased Mn concentrations in the root symplast in the short-term Al treatment (Fig. 3A) and consequently less Mn being transported to the shoots (Fig. 3C). The roots are the main site of Al toxicity, while the symptoms of Mn toxicity are mainly observed in the shoots (stunted growth, and chlorosis and necrotic lesions in the leaves) (Kochian et al., 2004). Thus, the toxicity of Mn to plants generally results from excess Mn accumulation in the leaves (Foy et al., 1978, 1988; Wissemeier et al., 1992; Migocka and Klobus, 2007). In rice, the roots are much less sensitive to Mn toxicity than are the shoots (Nelson, 1983; Lidon, 2001). Given the delay in the translocation of Mn from the roots to the shoots, Al may have repressed root Mn uptake in the short-term experiment. This would prevent excess Mn accumulation in shoots, thereby alleviating Mn toxicity.
We observed that Al increased Mn accumulation in roots after the 3-week Al and Mn treatments (Fig. 2A). This result contrasts with those reported for other plant species (Rees and Sidrak, 1961; Clark, 1977; Blair and Taylor, 1997; Taylor et al., 1998; Yang et al., 2009). It is difficult to explain this based on the decreased Mn accumulation in the root symplast and whole roots in the short-term Al–Mn treatments (Fig. 3). There was an increase in Mn plaques in the Al treatments (Figs 4 and 5A), consistent with the Al-induced increase in Mn accumulation in roots. Because Mn plaques carry variable charge, they have greater capacity for adsorbing cation and P. In line with this, we also detected P, K and Na in Mn plaques (Batty et al., 2002; Chen et al., 2006). The development of root structure (aerenchyma, and root diameter and volume) was also improved by Al under Mn toxicity (Figs 6 and 7). The improved root structure may have played a role in the increased Mn accumulation in roots in the long-term Al treatment. A similar effect was reported for silicon (Horiguchi and Morita, 1987). That is, silicon promoted the development of root and root aerenchyma and improved the oxidizing power of the root, allowing more Mn to be oxidized on the root surface (Horiguchi and Morita, 1987; Luiz et al., 2010). Our results also showed that root Mn concentrations after DCB extraction were higher in Al–Mn treatments than in Mn-only treatments (Fig. 5B), suggesting that the increased Mn accumulation in roots could be attributed not only to the increase in Mn plaques, but also to increased Mn binding in the roots. Several previous studies have reported that Al increased cell wall pectin and hemicellulose (Chang et al., 1999; Tabuchi and Matsumoto, 2001; Tabuchi et al., 2004; Yang et al., 2008), which are primary Al-binding components (Kochian et al., 2005; Horst et al., 2010). We also observed that Al increased the amounts of these cell wall components in rice (Wang et al., 2015), suggesting that Al-induced increase in pectin and hemicellulose might result in increased Mn binding in the roots. Therefore, increases in both Mn plaques and Mn binding could lead to more unavailable Mn being accumulated in the roots. Together with the Al-induced decrease in root symplastic Mn uptake, these changes resulted in less Mn being transported to shoots, thereby alleviating Mn toxicity in rice.
In conclusion, our results show that Al alleviated Mn toxicity in rice. This was attributed to decreased shoot Mn accumulation resulting from an Al-induced decrease in root symplastic Mn uptake. The decrease in root symplastic Mn uptake resulted from an Al-induced change in cell membrane potential. Also, Al increased Mn plaques in the roots and changed the binding properties of the cell wall, resulting in accumulation of unavailable Mn in roots. To our knowledge, this is the first report that Al can increase Mn accumulation in roots.
SUPPLEMENTARY DATA
Supplementary data are available at www.aob.oxfordjournals.org and consist of Table S1: effects of aluminium at different concentrations on rice growth in the 3-week (long-term) experiment.
ACKNOWLEDGEMENTS
This work was supported by the National Key Basic Research Program of China (No. 2014CB441000), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB15030000) and the Natural Science Foundation of China (No. 41 025 005).
LITERATURE CITED
- Batty LC, Baker AJM, Wheeler BD. 2002. Aluminium and phosphate uptake by Phragmites australis: the role of Fe, Mn and Al root plaques. Annals of Botany 89: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LM, Taylor GJ. 1997. The nature of interaction between aluminum and manganese on growth and metal accumulation in Triticum aestivum. Environmental and Experimental Botany 37: 25–37. [Google Scholar]
- Britto DT, Kronzucker HJ. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159: 567–584. [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. 1999. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell & Environment 22: 1009–1017. [Google Scholar]
- Chen RF, Shen RF, Gu P, Dong XY, Du CW, Ma JF. 2006. Response of rice (Oryza sativa) with root surface iron plaque under aluminium stress. Annals of Botany 98: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZC, Zhao XQ, Shen RF. 2010. The alleviating effect of ammonium on aluminum toxicity in Lespedeza bicolor results in decreased aluminum-induced malate secretion from roots compared with nitrate. Plant and Soil 337: 389–398. [Google Scholar]
- Chenery EM. 1955. A preliminary study of aluminium and the tea bush. Plant and Soil 6: 174–200. [Google Scholar]
- Chinese National Soil Survey Office. 1998. Soil of China [in Chinese]. Beijing: Chinese Agriculture Press, 95–96, 950. [Google Scholar]
- Clark RB. 1977. Effect of aluminum on growth and mineral elements of Al-tolerant and Al-intolerant corn. Plant and Soil 47: 653–662. [Google Scholar]
- Crowder AA, Coltman DW. 1993. Formation of manganese oxide plaque on rice roots in solution culture under varying pH and manganese (Mn2+) concentration conditions. Journal of Plant Nutrition 16: 589–599. [Google Scholar]
- David JL, Olga NB. 2000. Root cell ultrastructure in developing aerenchyma tissue of three wetland species. Annals of Botany 86: 641–646. [Google Scholar]
- Dolling PJ, Moody P, Noble A, et al. 2001. Soil acidity and acidification in Australia. National Land & Water Resources Audit Project Report. Canberra, Australia. [Google Scholar]
- Foy CD, Chaney RL, White MC. 1978. The physiology of metal toxicity in plants. Annual Review of Plant Physiology 29: 511–566. [Google Scholar]
- Foy CD, Scott BJ, Fisher JA. 1988. Genetic differences in plant tolerance to manganese toxicity. In: Graham R, Hannam R, Uren N, eds. Manganese in soils and plants. Dordrecht, Netherlands: Springer Kluwer Academic Publishers, 293–307. [Google Scholar]
- Führs H, Behrens C, Gallien S, et al. 2010. Physiological and proteomic characterization of manganese sensitivity and tolerance in rice (Oryza sativa) in comparison with barley (Hordeum vulgare). Annals of Botany 105: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi T, Morita S. 1987. Mechanism of manganese toxicity and tolerance of plants VI. Effect of silicon on alleviation of manganese toxicity of barley. Journal of Plant Nutrition 10: 2299–2310. [Google Scholar]
- Horst WJ. 1988. The physiology of manganese toxicity. In: Graham R, Hannam R, Uren N, eds. Manganese in soils and plants. Dordrecht, Netherlands: Springer Kluwer Academic Publishers, 175–188. [Google Scholar]
- Horst WJ, Wang YX, Eticha D. 2010. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany 106: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. 1993. Aluminum enhancement of plant growth in acid rooting media. A case of reciprocal alleviation of toxicity by two toxic cations. Physiologia Plantarum 88: 619–625. [DOI] [PubMed] [Google Scholar]
- Kinraide TB. 1994. Use of a Gouy-Chapman-Stern model for membrane-surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiology 106: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Wang P. 2010. The surface charge density of plant cell membranes (σ): an attempt to resolve conflicting values for intrinsic Journal of Experimental Botany 61: 2507–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. 1992. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiology 99: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB, Yermiyahu U, Rytwo G. 1998. Computation of surface electrical potentials of plant cell membranes. Correspondence to published zeta potentials from diverse plant sources. Plant Physiology 118: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug B, Horst WJ. 2010. Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum). New Phytologist 187: 380–391. [DOI] [PubMed] [Google Scholar]
- Kochian LV. 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 46: 237–260. [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology 55: 459–493. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Piñeros MA, Hoekenga OA. 2005. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant and Soil 274: 175–195. [Google Scholar]
- Kopittke PM, Blamey FPC, Wang P, Menzies NW. 2011a. Calculated activity of Mn2+ at the outer surface of the root cell plasma membrane governs Mn nutrition of cowpea seedlings. Journal of Experimental Botany 62: 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Kinraide TB, Wang P, Blamey FPC, Reichman SM, Menzies NW. 2011b. Alleviation of Cu and Pb rhizotoxicities in cowpea (Vigna unguiculata) as related to ion activities at root-cell plasma membrane surface. Environmental Science & Technology 45: 4966–4973. [DOI] [PubMed] [Google Scholar]
- Kopittke PM, Wang P, Menzies NW, Naidu R, Kinraide TB. 2014. A web-accessible computer program for calculating electrical potentials and ion activities at cell-membrane surfaces. Plant and Soil 375: 35–46. [Google Scholar]
- Li P, Song AL, Li ZJ, Fan FL, Liang YC. 2011. Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant and Soil 354: 407–419. [Google Scholar]
- Lidon FC. 2001. Tolerance of rice to excess manganese in the early stages of vegetative growth. Characterisation of manganese accumulation. Journal of Plant Physiology 158: 1341–1348. [Google Scholar]
- Luiz AZJ, Renildes LFF, Júlio CLN, Gaspar HK, Vinícius TÁ. 2010. Rice grown in nutrient solution with doses of manganese and silicon. Revista Brasileira de Ciência do Solo 34: 1629–1639. [Google Scholar]
- Ma JF, Hiradate S, Matsumoto H. 1998. High aluminum resistance in buckwheat. II. Oxalic acid detoxifies aluminum internally. Plant Physiology 117: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. 1995a. Functions of mineral nutrients: micronutrients. In: Marschner H, ed. Mineral nutrition of higher plants, 2nd edn London: Academic Press, 313–404. [Google Scholar]
- Marschner H. 1995b. Adaptation of plants to adverse chemical soil conditions. In: Marschner H, ed. Mineral nutrition of higher plants, 2nd edn London: Academic Press, 596–680. [Google Scholar]
- Migocka M, Klobus G. 2007. The properties of the Mn, Ni and Pb transport operating at plasma membranes of cucumber roots. Physiologia Plantarum 129: 578–587. [Google Scholar]
- Millaleo R, Reyes DM, Ivanov AG, María LM, Alberdi M. 2010. Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. Journal of Soil Science and Plant Nutrition 10: 470–481. [Google Scholar]
- Mora de la M, Rosas A, Ribera A, Rengel Z. 2009. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: involvement of antioxidative enzymes and root exudation of carboxylates. Plant and Soil 320: 79–89. [Google Scholar]
- Moroni JS, Scott BJ, Wratten N. 2003. Differential tolerance of high manganese among rapeseed genotypes. Plant and Soil 253: 507–519. [Google Scholar]
- Nelson LE. 1983. Tolerances of 20 rice cultivars to excess Al and Mn. Agronomy Journal 75: 134–138. [Google Scholar]
- Page V, Feller U. 2005. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Annals of Botany 96: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page V, Weisskopf L, Feller U. 2006. Heavy metals in white lupin: uptake, root-to-shoot transfer and redistribution within the plant. New Phytologist 171: 329–341. [DOI] [PubMed] [Google Scholar]
- Ponnamperuma FN. 1975. Growth-limiting factors of aerobic soils. In: Major research in upland rice. Los Baños, Philippines: International Rice Research Institute, 40–43. [Google Scholar]
- Rees WJ, Sidrak GH. 1961. Inter-relationship of aluminium and manganese toxicities towards plants. Plant and Soil 14: 101–117. [Google Scholar]
- Rosas A, Rengel Z, María LM. 2007. Manganese supply and pH influence growth, carboxylate exudation and peroxidase activity of ryegrass and white clover. Journal of Plant Nutrition 30: 253–270. [Google Scholar]
- Sasaki A, Yamaji N, Xia JX, Ma JF. 2011. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiology 157: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RF, Chen RF, Ma JF. 2006. Buckwheat accumulates aluminum in leaves but not in seeds. Plant and Soil 284: 265–271. [Google Scholar]
- Tabuchi A, Matsumoto H. 2001. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiologia Plantarum 112: 353–358. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Kikui S, Matsumoto H. 2004. Differential effects of aluminium on osmotic potential and sugar accumulation in the root cells of Al-resistant and Al-sensitive wheat. Physiology Plantarum 120: 106–112. [DOI] [PubMed] [Google Scholar]
- Tatulian SA. 1999. Surface electrostatics of biological membranes and ion binding. In: Sørensen TS, ed. Surface chemistry and electrochemistry of membranes. New York: Marcel Dekker, 871–922. [Google Scholar]
- Taylor GJ, Crowder AA. 1983. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. American Journal of Botany 70: 1254–1257. [Google Scholar]
- Taylor GJ, Blarney FPC, Edwards DG. 1998. Antagonistic and synergistic interactions between aluminum and manganese on growth of Vigna unguiculata at low ionic strength. Physiology Plantarum 104: 183–194. [Google Scholar]
- Wang P, Zhou DM, Kinraide TB, et al. 2008. Cell membrane surface potential (ψ0) plays a dominant role in the phytotoxicity of copper and arsenate. Plant Physiology 148: 2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Kinraide TB, Zhou D, Kopittke PM, Peijnenburg WJGM. 2011. Plasma membrane surface potential: dual effects upon ion uptake and toxicity. Plant Physiology 155: 808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, De Schamphelaere KAC, Kopittke PM, Zhou DM, Peijnenburg WJGM, Lock K. 2012. Development of an electrostatic model predicting copper toxicity to plants. Journal of Experimental Botany 63: 659–668. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao XQ, Chen RF, et al. 2015. Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant, Cell & Environment, in press. doi: 10.1111/pce.12490. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Jansen S, Osaki M. 2005. The beneficial effect of aluminium and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytologist 165: 773–780. [DOI] [PubMed] [Google Scholar]
- Wissemeier AH, Diening A, Hergenröder A, Horst WJ, Mix-Wagner G. 1992. Callose formation as parameter for assessing genotypical plant tolerance of aluminium and manganese. Plant and Soil 146: 67–75. [Google Scholar]
- Xia JX, Yamaji N, Kasai T, Ma JF. 2010. Plasma membrane-localized transporter for aluminum in rice. Proceedings of the National Academy of Sciences of the United States of America 107: 18381–18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. 2008. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology 146: 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, You JF, Xu MY, Yang ZM. 2009. Interaction between aluminum toxicity and manganese toxicity in soybean (Glycine max). Plant and Soil 319: 277–289. [Google Scholar]
- Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF. 2013. A node-based switch for preferential distribution of manganese in rice. Nature Communications 4: 2442. [DOI] [PubMed] [Google Scholar]
- Zeng QL, Chen RF, Zhao XQ, et al. 2013. Aluminum could be transported via phloem in Camellia oleifera Abel. Tree Physiology 33: 96–105. [DOI] [PubMed] [Google Scholar]
- Zhao XQ, Shen RF, Sun QB. 2009. Ammonium under solution culture alleviates aluminum toxicity in rice and reduces aluminum accumulation in roots compared with nitrate. Plant and Soil 315: 107–121. [Google Scholar]
- Zhao XQ, Guo SW, Shinmachi F, et al. 2013. Aluminium tolerance in rice is antagonistic with nitrate preference and synergistic with ammonium preference. Annals of Botany 111: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.