Abstract
Background and Aims Flooding can occur at any stage of the life cycle of a plant, but often adaptive responses of plants are only studied at a single developmental stage. It may be anticipated that juvenile plants may respond differently from mature plants, as the amount of stored resources may differ and morphological changes can be constrained. Moreover, different water depths may require different strategies to cope with the flooding stress, the expression of which may also depend on developmental stage. This study investigated whether flooding-induced adventitious root formation and plant growth were affected by flooding depth in Solanum dulcamara plants at different developmental stages.
Methods Juvenile plants without pre-formed adventitious root primordia and mature plants with primordia were subjected to shallow flooding or deep flooding for 5 weeks. Plant growth and the timing of adventitious root formation were monitored during the flooding treatments.
Key Results Adventitious root formation in response to shallow flooding was significantly constrained in juvenile S. dulcamara plants compared with mature plants, and was delayed by deep flooding compared with shallow flooding. Complete submergence suppressed adventitious root formation until up to 2 weeks after shoots restored contact with the atmosphere. Independent of developmental stage, a strong positive correlation was found between adventitious root formation and total biomass accumulation during shallow flooding.
Conclusions The potential to deploy an escape strategy (i.e. adventitious root formation) may change throughout a plant’s life cycle, and is largely dependent on flooding depth. Adaptive responses at a given stage of the life cycle thus do not necessarily predict how the plant responds to flooding in another growth stage. As variation in adventitious root formation also correlates with finally attained biomass, this variation may form the basis for variation in resistance to shallow flooding among plants.
Keywords: Adventitious roots, complete submergence, developmental stage, escape strategy, flooding depth, nightshade, pre-formed root primordia, quiescence strategy, Solanum dulcamara, Solanaceae
INTRODUCTION
Plant adaptation to flooding has been well reviewed over the last decades (Armstrong et al., 1994a; Blom and Voesenek, 1996; Voesenek et al., 2006; Perata et al., 2011). Species may differ in their adaptive responses, explaining a species’ tolerance to this stress, and even within a species, responses may vary among populations depending on the flooding characteristics of the habitat (Lessmann et al., 1997; Lenssen et al., 2004). This variation has been used for breeding purposes, resulting in rice showing a wide variation in adaptive shoot elongation among cultivars that are grown from irrigated to deep-water conditions (Kende et al., 1998; Sauter, 2000).
The potential to respond to the environment may be constrained by the developmental stage of the plant (Novoplansky et al., 1994; Watson et al., 1995; Weinig, 2000; Groeneveld and Voesenek, 2003; Chen et al., 2011). Relatively little is known of such an interaction between developmental stage and flooding responses, i.e. if the optimal strategy to survive flooding changes depending on life stage of the plant, and whether the optimal strategy differs between flooding depths.
Wetland plant species typically display various physiological and morphological adaptations when flooded (Armstrong et al., 1994a; Blom and Voesenek, 1996). Oxygen shortage is the main problem that plants face in these conditions (Colmer, 2003), restraining aerobic respiration and causing a switch to the less efficient anaerobic respiration pathways (Voesenek et al., 2006). Species can employ a quiescence strategy, and stop growing, thereby slowing down energy requirements, to allow carbohydrate reserves that fuel respiration to last longer (Nabben et al., 1999; Almeida et al., 2003; Lee et al., 2009; Striker et al., 2012). By contrast, ‘escapers’ invest in shoot elongation (Akman et al., 2012; Striker et al., 2012), aerenchyma formation and new adventitious roots to improve internal aeration during flooding and alleviate the oxygen shortage (Colmer, 2003). Examples are shoot-elongating species such as deep-water rice and Rumex palustris (Setter and Laureles, 1996; Jackson and Ram, 2003; Voesenek et al., 2006; Jackson, 2008; Chen et al., 2009). These species rapidly elongate their shoots to above the water surface during submergence, thus regaining leaf–air contact. The original root system less easily escapes from the flooding-induced oxygen deficit (Sauter, 2013), but many wetland species develop adventitious roots at the shoot base or on the stem that replace the function of the primary root system, such as water and nutrient uptake, and anchoring (Lorbiecke and Sauter, 1999). Aerenchyma connects these new roots with the shoot, and circumvents potential diffusion barriers such as the shoot–root junction and the taproot (Sauter, 2013). Additionally, underwater photosynthesis may positively contribute to an improved oxygen and carbohydrate status of submerged plants, particularly in wetland species (Mommer and Visser, 2005; Colmer and Pedersen, 2008; Colmer et al., 2011). The coordinated development of these contrasting strategies allows survival in different habitats, i.e. shallow, long-term flooding for the escapers and deep, short-term flooding for the plants adopting a quiescence strategy (Voesenek et al., 2004).
Although different flooding conditions may require different strategies (Manzur et al., 2009), the developmental stage very likely limits which strategy a plant can actually realize. In general, plants at a more mature developmental stage have greater storage of carbon resources (Groeneveld and Voesenek, 2003) that can be exploited for fuelling fermentation or morphological changes (Huber et al., 2012) than those at a young developmental stage. For instance, adult Itea plants survived better and had higher growth rates than juvenile plants, independent of flooding depth (Anderson et al., 2009). On the other hand, young tissues may have higher plasticity than those that are more mature. For instance, the flooding-induced shoot elongation of Rumex palustris was largely dependent on the developmental stage of the leaf, with younger leaves having higher elongation potential (Chen et al., 2009). As selection in natural environments acts on all life stages of a plant species (McGraw and Antonovics, 1983), it is important to consider these potentially differing responses between juvenile and mature plants.
The impact of a given flooding depth depends on plant size as juvenile plants are relatively more deeply submerged than taller mature plants. For terrestrial species, it is crucial to maintain a functional root system during flooding by maintaining oxygen diffusion from the emerging shoot to the submerged root (Sauter, 2013). However, deep flooding leading to complete submergence prevents direct contact with the atmosphere, thus resulting in malfunctioning of the aerenchyma (Voesenek et al., 1993). Some species appear to adjust their strategy depending on flooding depth. For instance, Lotus tenuis switches from an escape to a quiescence strategy by ceasing shoot elongation when completely submerged (Manzur et al., 2009). However, plants at very young age may not be able to respond appropriately when completely submerged as juvenile plants may simply be too small to be able to reach the water surface (Voesenek et al., 1993). Furthermore, the low amount of carbohydrate reserves in juvenile plants may not be sufficient to be invested in either escaping shoot elongation or long-term quiescent metabolism. Consequently, unlike mature plants, plants at an early developmental stage may be unable to invest in either strategy and thus cannot survive prolonged complete submergence.
To study the effect of developmental stage on strategy deployment, we used Solanum dulcamara, a perennial Eurasian species in the family Solanaceae, which is common on wet sites that are regularly flooded and forms a considerable number of adventitious roots in response to flooding (Dawood et al., 2013; Visser et al., 2015). As this species occurs in a broad range of habitats along a hydrological gradient, plants originating from both wet and dry habitats were included to cover the full ecological amplitude of the species.
We focused on flooding-induced adventitious root formation of S. dulcamara plants at two developmental stages under different flooding depths, to investigate how flooding depth and different timing of flooding in the life cycle of plants affect the performance of these plants. We hypothesized that plants in different developmental stages may respond differently to various flooding depths, because juvenile plants may be restrained in the expression of adaptive traits. This would result in a more negative impact of flooding on performance.
MATERIALS AND METHODS
Plant material
In 2012, seeds of Solanum dulcamara were collected at the North Sea island Texel (53° 7′24″N, 4° 47′10″E) and at the more southern coastal location Voorne (51° 51′2″N, 4° 4′29″E). For each of these locations, seeds were sampled in a wetland population at the shores of freshwater dune lakes, and in a dryland population at dry primary sand dunes well above the seasonally flooded dune slacks. The distance between these wet and dry habitats was at least 1 km. Seeds were cleaned, dried at room temperature and then stored at 4 °C.
Experimental design
The effect of developmental stage on flooding response was investigated by comparing juvenile and mature plants. At the start of the experiment, plants at the more mature developmental stage (8-week-old mature plants, mean stem height 53·3±3·1 cm, mean number of adventitious root primordia 19±0·8, n = 4) had already developed a woody stem and had formed visible adventitious root primordia, whereas plants at the juvenile developmental stage (4-week-old juvenile plants, mean stem height 5·9±0·3 cm, n = 4) had not yet developed a woody stem and had no pre-formed root primordia on the stem. These root primordia (from which adventitious roots develop) can only be visually discerned in plants that are older than 6 weeks (Fig. 1; pictures of stems containing either no or several primordia for 4- and 9-week-old S. dulcamara plants, respectively). These primordia stay dormant on the stem until triggered by flooding (Lorbiecke and Sauter, 1999).
Fig. 1.
Images of the stems of (A) 4-week-old and (B) 9-week-old S. dulcamara plants. No adventitious root (AR) primordia were present on the stem of the 4-week-old plant, whereas 6 AR primordia (the white spots indicated by the black arrows) can be discerned upon visual inspection on the stem of the 9-week-old plant. Scale bars: (A) = 0·25 cm; (B) = 0·4 cm.
Both mature and juvenile developmental stages were subjected to control (drained), shallow flooding and deep flooding treatments. Plants under control conditions were given sufficient water to replenish evapotranspiration. In the shallow flooding treatment, the water level was kept at 10 and 3 cm above the soil for mature and juvenile plants, respectively, thus submerging at least 15 % and no more than 50 % of the plant’s height. In deep flooding, water levels were maintained at 75 cm, and all juvenile plants and approx. 50 % of the mature plants were completely submerged at the start of the experiment. In the mature plants that were only partially submerged, the stems extended 0·2–23·5 cm above the water surface at the start of the treatments. These initially fully submerged plants were not prevented from reaching the water surface throughout the experiment. As initial conditions were thus different for mature plants depending on their size, this provided the opportunity to explore the effect of continued contact with the atmosphere in deep flooding on plant performance, versus having to restore this contact by elongation.
For each developmental stage, 150 seeds from each population were sown in seed trays with cells of 3·5 × 3·5 × 4 cm (length × width × depth) filled with commercial sowing compost (Horticoop substrate, Lentse potgrond and Slingerland potgrond, Cuijk, the Netherlands), and subsequently kept at 4 °C in the dark to break dormancy. After 2 weeks, seed trays were transferred to the greenhouse and covered with transparent plastic foil to reduce water loss. Plants were defined to be 0 weeks old at this time point. Two weeks after germination, within each developmental stage, 100 seedlings from each location and habitat combination were selected for homogeneity and transplanted into individual pots of 1·5 L. Nutrient-poor soil consisting of 70 % sand and 30 % clay supplied with 4 g L–1 slow-release fertilizer (Osmocote® Exact Standard, NPK 15–9–12+2 MgO+tracing elements, release time 5–6 months, Everris International B.V., Geldermalsen, the Netherlands) was used as a substrate. One week after potting, 60 mL of nutrient solution (2 g L–1 Kristallon in tap water, Yara International ASA, Vlaardingen, the Netherlands) was added to each pot to avoid nutrient limitation at the onset of the experiment.
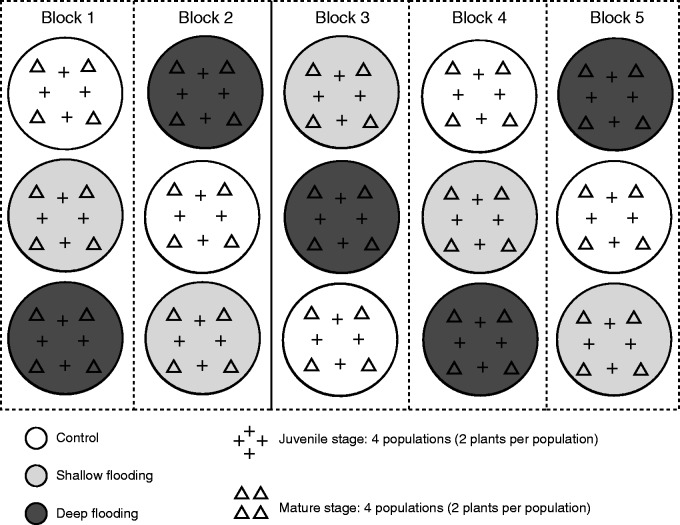
For each developmental stage, ten plants per population were randomly assigned to each treatment and in a block design placed in black containers (height 80 cm, diameter 100 cm). To reduce edge effects of the container, plants were placed 15 cm away from the wall of the container. Fifteen containers were allocated to the three treatments and three containers in a row covering each treatment were subsequently treated as a block (Fig. 2). The experiment was conducted outside in an open non-shaded area for 5 weeks from 7 May to 10 June 2013. The average daily maximum and minimum air temperature were 18·8±0·9 and 8·8±0·5 °C, respectively, and the average daily maximum and minimum relative humidity were 97·6±0·9 and 59·3±2·9 %, respectively. Water temperature was 2 and 4 °C higher in shallow flooding and deep flooding than air temperature, respectively. Throughout the experiment, water was added to or removed from the containers depending on precipitation and/or evaporation, to keep the plants under control well-watered conditions but drained, and to keep the water level constant in the shallow flooding and deep flooding treatments. Juvenile and mature plants were placed together in the same containers. For the shallow flooding treatments, the pots containing juvenile plants were raised by 7·0 cm, so that the water level was 3 cm above soil level. The stagnant flood water remained clear and free of algae during the entire experiment.
Fig. 2.
Schematic representation of the experimental design. Juvenile and mature S. dulcamara plants originating from two habitats in two locations (four populations) were subjected to control, shallow flooding and deep flooding. Two plants per developmental stage per population were randomly allocated to each of the 15 non-transparent containers, resulting in a total of 30 plants per developmental stage per population. The three containers comprising each of the three treatments in a row were treated as a block.
To investigate whether juvenile plants can deploy a quiescence strategy in complete submergence, an additional greenhouse experiment was performed. In this experiment, the survival over time of S. dulcamara seedlings completely submerged in either light or dark conditions was monitored. The seeds of a wetland population originating from Voorne were placed on a moist filter paper for germination. After germination seedlings were transferred to 0·5 -L pots filled with nutrient-poor soil consisting of 70 % sand and 30 % clay. Three-week-old seedlings (initial stem height 4·3±0·3 cm, n = 7) were completely submerged in black containers (50 cm high, volume 90 L) filled with tap water. Half of the containers were fully wrapped with black, light-impermeable, plastic sheets and the other half were fully wrapped with transparent plastic sheets. The plastic sheets were positioned 20 cm above the water surface to allow for gas diffusion. For each treatment (light and dark conditions), the survival of 14 plants was scored after 1, 2 and 4 weeks of flooding.
Measurements
Before the onset of the flooding treatments, stem length and leaf number of both the main stem and side branches, the node number and the number of root primordia of the main stem were quantified for each plant. For each developmental stage, ten extra plants per population were harvested to determine the initial biomass.
Throughout the experiment, the date on which the shoot extended above the water surface and the date when the first adventitious root emerged were recorded for each plant. Adventitious roots were considered to be emerged if the root primordium elongated for >2 mm. At harvest, the adventitious roots were separated from the stem and counted. The original roots were carefully washed free of soil with tap water. The length of the main stem and the side branches were measured, and the leaf number and node number on the main stem and side branches were counted. Leaf decay and abscission occurred in both flooding treatments, especially in the deeply flooded plants, and only viable turgescent leaves were included in the measurements. The different plant parts were weighed after drying at 70 °C for 72 h.
Data analysis
Total biomass, stem height and adventitious root number and biomass were log10-transformed to reduce heteroscedasticity. Data were analysed using a mixed model nested analysis of variance (ANOVA), with developmental stage, treatment and location as main effects, and habitat of origin nested within location. To investigate the effects of treatment, location and habitat in each developmental stage, we performed the nested ANOVA for each developmental stage separately followed by a Tukey post-hoc test, with treatment and location as main effects, and habitat of origin nested within location. As juvenile plants did not form any adventitious root in deep flooding, the analysis of adventitious root number and biomass was only performed on plants subjected to shallow flooding. As most adventitious roots of mature plants were produced from the pre-formed root primordia in the experiment, the number of root primordia was added as a covariate to examine the effects of initial root primordia as well as treatment, location and habitat nested within location on adventitious root formation in mature plants. Block was also included in the ANOVA as a random factor. Separate regression analyses were performed to investigate the relationship between adventitious root biomass and initial stem height, adventitious root biomass and total biomass, and adventitious root number and total biomass for each developmental stage in shallow flooding. Regression analysis was also used to investigate the relationship between the time until shoots reached the water surface and the time to form adventitious roots for mature plants in deep flooding. In the experiment investigating whether juvenile plants deploy a quiescence strategy, survival was analysed using a chi-square test, with duration of submergence and light availability as main effects. We also used pairwise chi-square tests to compare the effect of flooding duration for plants within light condition treatments. Significance levels were adjusted to 0·017 and 0·003 instead of 0·05 and 0·01 to take account of performing multiple comparisons. All the analyses were performed in SPSS version 20 (IBM, New York, USA).
RESULTS
Plant performance and biomass allocation were significantly affected by flooding depth
Shallow flooding and deep flooding significantly decreased the total biomass of S. dulcamara in both developmental stages (Fig. 3A, Table 1), compared with control plants. Deep flooding led to substantially lower biomass increment than shallow flooding throughout the experiment. Compared with control plants, juvenile and mature plants subjected to deep flooding had approximately 90 and 60 % lower biomass increment, respectively (Fig. 3A). Final stem height was also negatively affected by both flooding treatments (Fig. 3B). For the mature developmental stage, shallow flooding and deep flooding both resulted in shorter plants, with no clear differences between these flooding depths (Fig. 3B). For the juvenile developmental stage, control plants were only slightly taller than shallowly flooded plants, but almost four times as tall as the deeply flooded plants (although these latter plants still had an average increment of stem height of 5·8 cm) (Fig. 3B, Table 2).
Fig. 3.
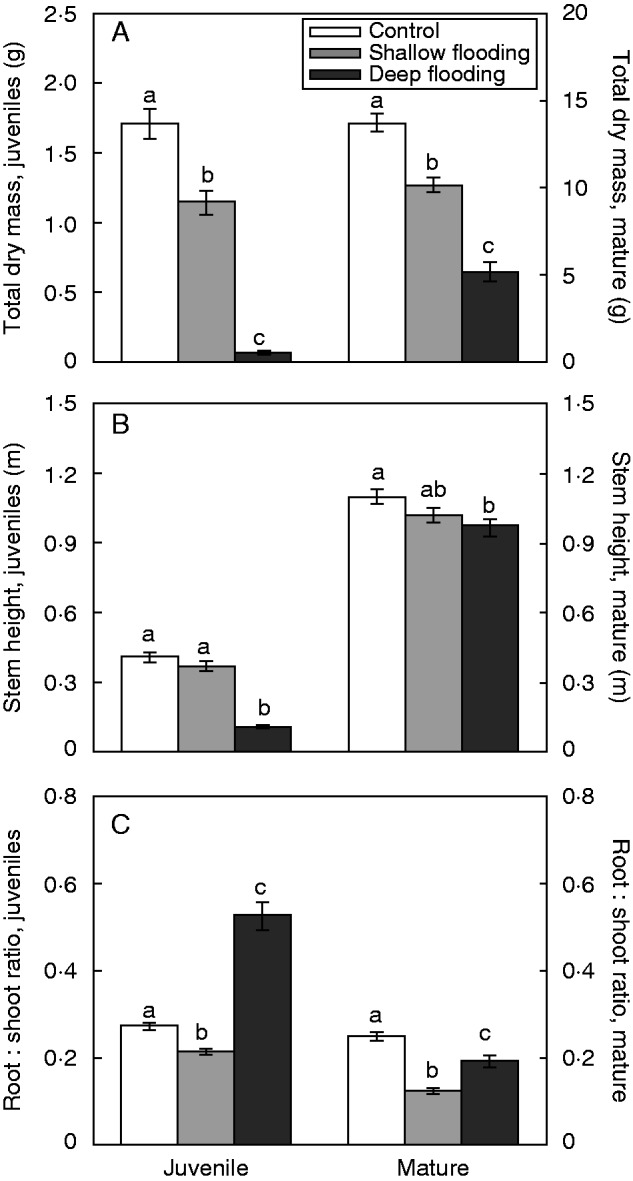
Total biomass (A), stem height (B) and root to shoot ratio (C) of S. dulcamara plants at juvenile and mature developmental stages in control, shallow flooding and deep flooding treatments. Initial stem height and total biomass were 5·93±0·32 and 53·32±0·31 cm, and 0·12±0·01 and 2·91±0·15 g for juvenile and mature plants, respectively. The left y-axis refers to juvenile plants and the right y-axis to mature plants. Means±s.e. (n = 4 populations for each treatment) that do not share a letter are significantly different by a Tukey’s post-hoc test (P ≤ 0·05) within each developmental stage.
Table 1.
The results of nested ANOVAs for the effects of stage, treatment, location and habitat (location) on total biomass, stem height and root to shoot ratio of S. dulcamara; degrees of freedom (d.f.) and F values and their significance are presented
|
F-values |
||||
|---|---|---|---|---|
| d.f. | Total dry mass | Stem height | Root/shoot ratio | |
| Stage | 1 | 2642·334*** | 5·414* | 152·045*** |
| Treatment | 2 | 610·385*** | 231·959*** | 79·731*** |
| Stage × treatment | 2 | 149·124*** | 210·992*** | 58·418*** |
| Location | 1 | 0·264ns | 1·712ns | 0·575ns |
| Stage × location | 1 | 0·569ns | 10·168** | 2·768* |
| Treatment × location | 2 | 3·929** | 3·403* | 4·08** |
| Stage × treatment × location | 2 | 3·954** | 5·574** | 4·182** |
| Habitat (location) | 2 | 2·619* | 3·531* | 1·756ns |
| Stage × habitat (location) | 2 | 3·037* | 0·236ns | 0·148ns |
| Treatment × habitat (location) | 4 | 2·511** | 0·409ns | 1·096ns |
| Stage × treatment × habitat (location) | 4 | 1·001ns | 0·527ns | 0·432ns |
| Block | 4 | 1·901ns | 0·750ns | 1·375ns |
***P < 0·001, **0·001<P<0·01, *0·01<P<0·05, nsP>0·05. Significant differences at P<0·05 are highlighted in bold.
Table 2.
The results of nested ANOVAs for treatment, location and habitat (location) on total biomass, stem height and root to shoot ratio of S. dulcamara in mature and juvenile stages; degrees of freedom (d.f.) and F values and their significance are presented
|
F-values |
||||
|---|---|---|---|---|
| d.f. | Total dry mass | Stem height | Root/shoot ratio | |
| Mature stage | ||||
| Treatment | 2 | 81·692*** | 6·610*** | 37·782*** |
| Location | 1 | 0·030ns | 4·232* | 0·894ns |
| Treatment × location | 2 | 0·044ns | 1·664ns | 0·395ns |
| Habitat (location) | 2 | 4·849** | 17·085*** | 1·019ns |
| Treatment × habitat (location) | 4 | 3·267** | 0·219ns | 2·006ns |
| Block | 4 | 2·657* | 2·694* | 0·419ns |
| Juvenile stage | ||||
| Treatment | 2 | 664·365*** | 247·447*** | 78·861*** |
| Location | 1 | 0·786ns | 0ns | 1·914ns |
| Treatment × location | 2 | 7·660*** | 8·531*** | 5·275** |
| Habitat (location) | 2 | 0·915ns | 2·182ns | 0·938ns |
| Treatment × habitat (location) | 4 | 0·325ns | 0·240ns | 0·396ns |
| Block | 4 | 0·390ns | 0·236ns | 1·581ns |
***P<0·001, **0·001<P<0·01, *0·01<P<0·05, nsP>0·05. Significant differences at P<0·05 are highlighted in bold.
Flooding also affected biomass allocation patterns. Root to shoot ratio (adventitious roots were excluded) was reduced by flooding except for the completely submerged juvenile plants (Fig. 3C). Generally, mature flooded plants had a significantly lower root to shoot ratio than those under drained control conditions (Fig. 3C, Table 2). However, in mature plants, shoot growth was less limited by shallow flooding than by deep flooding, resulting in a smaller reduction of the root to shoot ratio in plants subjected to deep flooding than in those subjected to shallow flooding (Fig. 3C). Surprisingly, the root to shoot ratio of juvenile plants was substantially increased by deep flooding (Fig. 3C), which was probably due to the reduced shoot biomass, resulting from leaf abscission under water, rather than an increase in root biomass.
Adventitious root formation was affected by flooding depth and developmental stage
Mature plants formed adventitious roots under both flooding conditions, whereas juvenile plants only produced adventitious roots if subjected to shallow flooding (Fig. 4A, B). None of the juvenile plants subjected to deep flooding formed adventitious roots throughout the experiment. The timing of the onset of adventitious root formation differed significantly between juvenile and mature plants subjected to shallow flooding; under these conditions mature plants started to form adventitious roots about 20 d earlier (Fig. 4C). For completely submerged mature plants in deep flooding, the onset of adventitious root formation also depended on the time when the shoot extended above water surface. Plants that were extending above the water surface at the start of deep flooding formed adventitious roots on average 9 d after the onset of flooding (Fig. 4D). However, plants that were entirely under water at the start of flooding produced the first adventitious roots up to 30 d after the onset of flooding, which was 15–20 d after they reached the water surface (Fig. 4D).
Fig. 4.
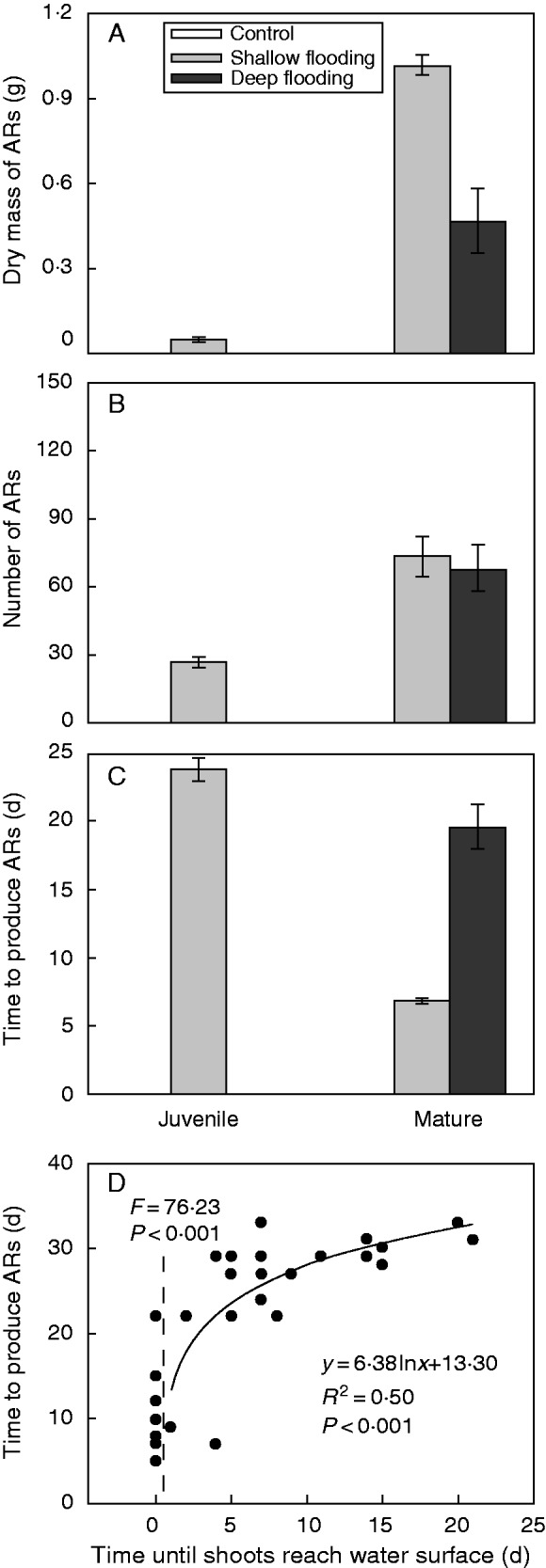
Adventitious root (AR) biomass (A), number of adventitious roots (B), time to produce adventitious roots (C) of juvenile and mature S. dulcamara plants in control, shallow flooding and deep flooding treatments, and the relationship between the time to produce adventitious roots and the time until shoots reach the water surface (D) of mature plants in deep flooding.
The number of pre-formed root primordia significantly affected adventitious root number and biomass (Table 3). Mature plants in deep flooding had much lower adventitious root biomass and almost the same number of adventitious roots compared with plants in shallow flooding (Fig. 4A, B, Table 3), indicating that not root initiation but rather root development was negatively affected by deep flooding.
Table 3.
The results of nested ANOVA for the effect of treatment, location, habitat (location) and initial root primordia on the biomass of adventitious roots (AR dry mass) and number of adventitious root (AR number) of S. dulcamara from mature developmental stage; degrees of freedom (d.f.) and F values and their significance are presented
|
F-values |
|||
|---|---|---|---|
| d.f. | AR dry mass | AR number | |
| Treatments | 1 | 25·124*** | 0·181ns |
| Location | 2 | 0·659ns | 0·037ns |
| Treatment × location | 1 | 2·416ns | 0·78ns |
| Habitat (location) | 1 | 0·117ns | 1·283ns |
| Treatment × habitat (location) | 2 | 1·502ns | 1·166ns |
| Root primordia | 1 | 10·342** | 16·909*** |
| Location × root primordia | 1 | 1·438ns | 0·399ns |
| Root primordia × habitat (location) | 2 | 0·317ns | 0·184ns |
| Block | 4 | 1·307ns | 0·546ns |
***P<0·001, **0·001<P<0·01, *0·01<P<0·05, nsP>0·05. Significant differences at P<0·05 are highlighted in bold.
Plant biomass after shallow flooding correlated with adventitious root formation
Stem height at the onset of the experiment had only a very small effect on adventitious root formation (Fig. 5), suggesting that within a given developmental stage, adventitious root formation did not depend on initial size of the plant at the start of the treatments. However, the final biomass of the adventitious roots was positively correlated with the biomass of the entire plant after treatment in both developmental stages (Fig. 6A, B). Similarly, the number of adventitious roots was positively correlated with total biomass of the plants (Fig. 6C, D), which might reflect a positive effect of adventitious roots on plant performance during flooding.
Fig. 5.
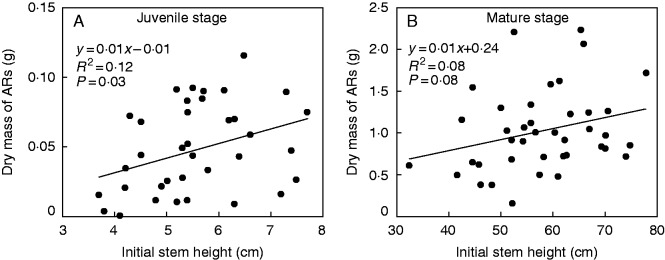
The relationship between initial stem height and biomass of adventitious roots (ARs) in juvenile (A) and mature S. dulcamara plants (B) subjected to shallow flooding.
Fig. 6.
The relationship between the biomass of adventitious roots (ARs) and total biomass of juvenile (A) and mature S. dulcamara plants (B), and the relationship between the number of adventitious roots and total biomass of juvenile (C) and mature plants (D) subjected to shallow flooding.
Habitat and location of populations had only limited effects on flooding responses
In mature plants, a significant interaction was found between the effects of treatment and habitat nested within location on total biomass (Tables 1 and 2), as plants from the dry habitat accumulated 55 % more total biomass than those from the wet habitat in Voorne in the deep flooding treatment (Supplementary Data Fig. S1). In juvenile plants, treatment and location interacted significantly in determining total biomass, stem height and root to shoot ratio (Tables 1 and 2). Plants originating from Voorne had approx. 40 % less total biomass and 22 % shorter stems in the shallow flooding treatment, but 30 % more biomass and 26 % taller stems in the deep flooding treatment than those originating from Texel (Supplementary Data Fig. S2). The plants originating from Voorne also had approx. 30 % smaller root to shoot ratio than those originating from Texel in the deep flooding treatment (Supplementary Data Fig. S2).
Light availability was important for plant survival in complete submergence
To investigate if juvenile plants possessed an alternative strategy for surviving complete submergence, i.e. a quiescence strategy, we tested if these plants could withstand submergence in complete darkness. Plants completely submerged in the light had 100 % survival after 4 weeks of flooding, whereas the survival rate of plants completely submerged in the dark decreased to approx. 20 % within 2 weeks of flooding (Fig. 7). After 4 weeks of flooding, all plants subjected to complete submergence in the dark died (P < 0·01, Fig. 7). This suggested that underwater photosynthesis was needed to supply the plants with carbohydrates and/or oxygen, and no true quiescence strategy was present.
Fig. 7.
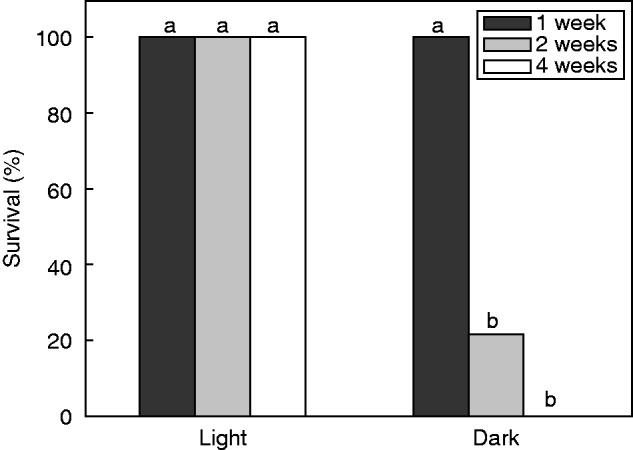
The survival percentage of juvenile plants completely submerged in light and in dark at the end of the first, second and fourth week. Different letters indicate significant differences at P = 0·017, n = 14 (significance levels were adjusted to 0·017 and 0·003 instead of 0·05 and 0·01 to take account of performing multiple comparisons).
DISCUSSION
The risk of flooding events will increase in the near future due to global warming (IPCC, 2007). Economic losses may therefore become substantially higher as flooding causes high mortality and considerable damage to many crop species (Normile, 2008; Bailey-Serres et al., 2012). However, wild plant species occurring in flood-prone habitats survive flooding much better than crop species, due to the ability to show morphological and anatomical acclimations, e.g. shoot elongation, adventitious rooting and aerenchyma formation (Visser et al., 1996; Chen et al., 2009). Studying these adaptive responses of natural wetland species may provide knowledge that can help to introduce flooding tolerance into crops. Here, we show that adventitious root development induced by flooding is constrained at the juvenile stage of Solanum dulcamara plants, and that deep flooding delays adventitious root formation compared with shallower flooding. These results indicate that it is important to study the impact of environmental stress and a plant’s adaptive responses at different stages of the life cycle, as responses at a given developmental stage do not necessarily predict how the plant responds in another growth stage.
Developmental stage affects the timing of adventitious root emergence
The timing of emergence of adventitious roots varies among species but is generally quick, from within 12 h in deep-water rice (Lorbiecke and Sauter, 1999) to 1–2 d in sunflower (Wample and Reid, 1975, 1978), tomato (Vidoz et al., 2010) and Rumex species (Visser et al., 1996). These adventitious roots develop from new primordia that are rapidly formed in response to flooding (Vidoz et al., 2010), or from pre-formed primordia that are present on the stem and remain dormant until triggered by flooding (Lorbiecke and Sauter, 1999). The latter is the case in mature S. dulcamara plants, which typically show numerous root primordia on the woody base of the stem (Dawood et al., 2013). In juvenile plants of this species, however, root primordia still need to be developed upon flooding, potentially explaining the strong delay in adventitious root formation observed in our experiment. Mechanisms of outgrowth of adventitious roots (referred to as nodal or crown roots in Poaceae species) are well studied in rice (Steffens and Sauter, 2005, 2009; Steffens et al., 2006). In this species, genes involved in adventitious root formation were earlier expressed upon flooding when root primordia were on older stem nodes, leading to faster root outgrowth (Lorbiecke and Sauter, 1999). A similar mechanism may be an additional reason for the difference in timing of adventitious root development between juvenile and mature plants in S. dulcamara. Furthermore, the outgrowth of adventitious roots in juvenile plants may have been more constrained by carbohydrate limitation than in mature plants. A positive relationship between carbohydrate content and adventitious root development was shown by Druege et al. (2004) for cuttings of Pelargonium, and improved adventitious rooting capacity by carbohydrate supply was found in cuttings of Eucalyptus saligna Smith and Eucalyptus globulus Labill (Corrêa et al., 2005), suggesting that accumulation of carbohydrate reserves at later life stages may have accelerated adventitious root formation in our experiment.
In shallowly flooded S. dulcamara, adventitious root formation started 7 d after the onset of flooding in the current study, which is 5 d later than in previous experiments on this species (Dawood et al., 2013). This relative delay in the formation of adventitious roots might seem surprising, as Dawood et al. (2013) also used mature plants in their study. However, floodwater temperature was substantially lower in the current experiment (12·9 vs. 20 °C), which may have slowed down metabolism and growth rate (van Eck et al., 2005). Moreover, higher oxygen solubility in cold water (van Eck et al., 2005) may also have delayed the signal of oxygen deficit in the current experiment. Genotypic differences in rate of outgrowth can also not be excluded, although observations on plants from a larger set of populations did not reveal substantial differences in the timing of development of adventitious roots (Q. Zhang, pers. observ.). These results indicate that different mechanisms such as developmental stage and water temperature can contribute to variation in adventitious root formation. Whether such variation has ecological consequences for a species when confronted with environmental stress needs further attention.
It is surprising that plants originating from dry habitats displayed similar adventitious root formation in response to flooding to those originating from wet habitats. As habitat effect was not a primary question in this study, only two habitat pairs were included, and existing but relatively weak habitat effects may have been masked by this small number of replicates or by local selection pressures other than soil moisture. Alternatively, the lack of habitat effects may also indicate a generally high level of plasticity in this species, enabling S. dulcamara to perform well in a wide range of habitats, independent of eventual local selection pressures. To evaluate whichever reason explains the low level of habitat differentiation found in this experiment, it would be interesting to perform a wider comparison of population pairs originating from dry and wet habitats.
Complete submergence suppresses adventitious root formation
Complete submergence, resulting in stronger oxygen deficit than partial submergence (Armstrong et al., 1994b; Rijnders et al., 2000; Mommer and Visser, 2005), may cause severe stress even in flood-tolerant terrestrial plants. This stress may be largely alleviated in clear water, where light levels remain sufficiently high to enable underwater photosynthesis (Mommer et al., 2006; Pedersen et al., 2013). Still, the formation of adventitious roots in our experiment was suppressed in S. dulcamara plants when fully submerged in clear water. This inhibition was relieved after shoots extended above the water surface in the course of the experiment, which is a similar response to that shown by fully submerged Rumex maritimus and R. palustris plants (van der Sman et al., 1993). The most obvious reason for this inhibition of root outgrowth is the assumed low aerobic respiratory rate and thus low ATP production in completely submerged plants due to limited diffusion of oxygen into the plant, causing processes demanding high energy availability, such as cell division, to cease. Also, the conversion of starch into sugars that fuel respiration may be hampered at low oxygen concentrations (Perata et al., 1997), which in turn inhibits growth. Some species, however, such as Meionectes brownii and Cotula coronopifolia, can produce adventitious roots both under partial flooding and during complete submergence (Rich et al., 2012). In those species, maintenance of underwater photosynthesis may have contributed to a high endogenous oxygen concentration during day time, facilitating energy generation through aerobic respiration. Solanum dulcamara plants, however, lost most submerged leaves in our experiment, thus constraining their capacity for underwater photosynthesis. One may expect then that after the shoot restores contact with the atmosphere, the renewed oxygen supply to the adventitious root primordia would result in immediate outgrowth. This was not the case, as adventitious roots still took up to 2–3 weeks to emerge. This may indicate that the production of adventitious roots is under regulatory control and actively inhibited, rather than just constrained by oxygen shortage.
Juvenile plants fail to employ an alternative strategy in complete submergence
Juvenile plants survived complete submergence without developing adventitious roots, indicating that these plants may deploy an alternative strategy, i.e. quiescence (Bailey-Serres and Voesenek, 2008). This would require juvenile plants to also be able to survive complete submergence without photosynthesis, i.e. in turbid water or in the dark. However, juvenile plants showed as much as 80 % mortality after 2 weeks of complete submergence in the dark, as opposed to no mortality at all in the light. Apparently, these juvenile plants did not have sufficient carbohydrate stores, or could not access these reserves, to survive 2 weeks of flooding-induced anaerobiosis, and largely depended on underwater photosynthesis to maintain basic physiological functions. This indicates that lack of adventitious root formation in these young plants is not a sign of the proposed quiescence strategy.
Adventitious roots improve plant growth during shallow flooding
Developing abundant adventitious roots may result in superior plant growth during flooding, as severing adventitious roots largely reduced plant growth under these conditions (Tsukahara and Kozlowski, 1985; Javier, 1987; Rich et al., 2012). In our experiment, juvenile and mature S. dulcamara plants subjected to shallow flooding showed a positive correlation between adventitious roots (number and biomass) and total plant biomass. This could be explained by two opposing hypotheses. First, large plants may have accumulated more resources and thereby were able to produce more adventitious roots. Alternatively, plants may have profited from forming more adventitious roots and were thus able to accumulate a higher biomass. The first hypothesis is partly confirmed, since initial stem height, as an indicator of plant biomass, did affect adventitious root biomass after the flooding period, but this effect was relatively small, explaining just 10 % of the variation and being only significant for mature plants. The biomass produced during the flooding period, however, correlated much more strongly with the number and biomass of roots formed (explaining >65 % of the variation), suggesting that plant growth during flooding directly benefited from the development of large numbers of adventitious roots. This was also shown in experiments where the number of adventitious roots was manipulated by root removal. In sunflower, the initially decreased shoot growth rate in response to flooding partly recovered in plants with normal adventitious root development, and remained low in plants whose adventitious roots were removed. Severing adventitious roots also negatively affected plant growth in two legume species (Javier, 1987) and in two herbaceous wetland species (Rich et al., 2012). Comparisons of closely related species differing in adventitious root formation showed a correlation between the number of adventitious roots and the position of the species in a natural flooding gradient (for Rumex; Visser et al., 1996), or the maintenance of high plant growth rates (in woody Hakea species; Poot and Lambers, 2003). In our experiment, we showed that even within a single species, natural variation in adventitious root formation may be associated with plant performance under soil flooding.
CONCLUSIONS
Our results show that juvenile plants delayed the flooding-induced plastic response of adventitious root formation more than mature plants, probably due to the absence of already pre-formed adventitious root primordia. Root production may have further been delayed by a lower amount of stored carbohydrates, suggesting that the potential to express essential responses necessary to withstand flooding changes throughout the life cycle of a plant. A specific adaptive response expressed at any given developmental stage does thus not necessarily predict to what extent a plant is adapted to flooding throughout its life-time and the nature of responses at other developmental stages. The fact that juvenile plants responded more slowly and suffered strongly reduced performance under deep flooding indicates that even S. dulcamara plants growing in permanently flooded lake shores may require drained conditions during germination and juvenile development. Moreover, flooding depth and the capacity and timing of restored shoot contact with the atmosphere greatly influenced the formation of adventitious roots, indicating that delayed contact with the atmosphere further delays root outgrowth, even after contact with the atmosphere is regained. Furthermore, our data show that the natural variation in adventitious root formation within a species is likely to contribute to the flooding resistance of the plant. Further experiments would need to focus on the nature of this relationship.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: total biomass of mature S. dulcamara plants originating from wet and dry habitats in control, shallow flooding and deep flooding treatments. Figure S2: total biomass, root to shoot ratio and stem height of juvenile S. dulcamara plants originating from control, shallow flooding and deep flooding treatments.
ACKNOWLEDGEMENTS
We are grateful to Tijn Raaijmakers for performing the survival experiment. We also appreciate the assistance of technicians and students at the department, and Gerard van der Weerden and co-workers at the Nijmegen Experimental Garden. This experiment is part of the B’sweet collaborative research initiative of the Departments of Experimental Plant Ecology and Molecular Plant Physiology of the Radboud University Nijmegen. Q.Z. was supported by China Scholarship Council grant number 201206510011.
LITERATURE CITED
- Akman M, Bhikharie AV, McLean EH, et al. 2012. Wait or escape? Contrasting submergence tolerance strategies of Rorippa amphibia, Rorippa sylvestris and their hybrid. Annals of Botany 109: 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AM, Vriezen WH, van der Straeten D. 2003. Molecular and physiological mechanisms of flooding avoidance and tolerance in rice. Russian Journal of Plant Physiology 50: 743–751. [Google Scholar]
- Anderson JT, Landi AA, Marks PL. 2009. Limited flooding tolerance of juveniles restricts the distribution of adults in an understory shrub (Itea virginica; Iteaceae). American Journal of Botany 96: 1603–1611. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Brandle R, Jackson MB. 1994a. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: 307–358. [Google Scholar]
- Armstrong W, Strange ME, Cringle S, Beckett PM. 1994b. Microelectrode and modelling study of oxygen distribution in roots. Annals of Botany 74: 287–299. [Google Scholar]
- Bailey-Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. The Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E. 2012. Waterproofing crops: effective flooding survival strategies. Plant Physiology 160: 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom CWPM, Voesenek LACJ. 1996. Flooding: the survival strategies of plants. Trends in Ecology & Evolution 11: 290–295. [DOI] [PubMed] [Google Scholar]
- Chen X, Huber H, de Kroon H, et al. 2009. Intraspecific variation in the magnitude and pattern of flooding-induced shoot elongation in Rumex palustris. Annals of Botany 104: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Visser EJW, de Kroon H, et al. 2011. Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytologist 190: 409–420. [DOI] [PubMed] [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26: 17–36. [Google Scholar]
- Colmer TD, Pedersen O. 2008. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177: 918–926. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Winkel A, Pedersen O. 2011. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants: plr030, doi:10·1093/aobpla/plr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa LdR, Paim DC, Schwambach J, Fett-Neto AG. 2005. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regulation 45: 63–73. [Google Scholar]
- Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJW. 2013. Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants: plt058, doi: 10·1093/aobpla/plt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druege U, Zerche S, Kadner R. 2004. Nitrogen- and storage-affected carbohydrate partitioning in high-light-adapted Pelargonium cuttings in relation to survival and adventitious root formation under low light. Annals of Botany 94: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld HW, Voesenek LACJ. 2003. Submergence-induced petiole elongation in Rumex palustris is controlled by developmental stage and storage compounds. Plant and Soil 253: 115–123. [Google Scholar]
- Huber H, Chen X, Hendriks M, et al. 2012. Plasticity as a plastic response: how submergence-induced leaf elongation in Rumex palustris depends on light and nutrient availability in its early life stage. New Phytologist 194: 572–582. [DOI] [PubMed] [Google Scholar]
- IPCC. 2007. IPCC, 2007: Climate change 2007: The physical science basis. In: Solomon S, Qin D, Manning M, et al. eds. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Jackson MB. 2008. Ethylene-promoted elongation: an adaptation to submergence stress. Annals of Botany 101: 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Ram PC. 2003. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Annals of Botany 91: 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier RR. 1987. Effects of adventitious root removal on the growth of flooded tropical pasture legumes. Transactions of the National Academy of Science and Technology 9: 443–449. [Google Scholar]
- Kende H, van der Knaap E, Cho H-T. 1998. Deepwater rice: a model plant to study stem elongation. Plant Physiology 118: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-W, Chen P-W, Lu C-A, Chen S, Ho T-HD, Yu S-M. 2009. Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Science Signaling 2: ra61, doi: 10.1126/scisignal.2000333. [DOI] [PubMed] [Google Scholar]
- Lenssen JPM, van Kleunen M, Fischer M, de Kroon H. 2004. Local adaptation of the clonal plant Ranunculus reptans to flooding along a small-scale gradient. Journal of Ecology 92: 696–706. [Google Scholar]
- Lessmann JM, Mendelssohn IA, Hester MW, McKee KL. 1997. Population variation in growth response to flooding of three marsh grasses. Ecological Engineering 8: 31–47. [Google Scholar]
- Lorbiecke R, Sauter M. 1999. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiology 119: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur ME, Grimoldi AA, Insausti P, Striker GG. 2009. Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Annals of Botany 104: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw JB, Antonovics J. 1983. Experimental ecology of Dryas Octopetala ecotypes: I. Ecotypic differentiation and life-cycle stages of selection. Journal of Ecology 71: 879–897. [Google Scholar]
- Mommer L, Visser EJW. 2005. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany 96: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Visser EJW. 2006. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. Journal of Experimental Botany 57: 283–290. [DOI] [PubMed] [Google Scholar]
- Nabben RHM, Blom CWPM, Voesenek LACJ. 1999. Resistance to complete submergence in Rumex species with different life histories: the influence of plant size and light. New Phytologist 144: 313–321. [Google Scholar]
- Normile D. 2008. Reinventing rice to feed the world. Science 321: 330–333. [DOI] [PubMed] [Google Scholar]
- Novoplansky A, Cohen D, Sachs T. 1994. Responses of an annual plant to temporal changes in light envrionment: an interplay between plasticity and determination. Oikos 69: 437–446. [Google Scholar]
- Pedersen O, Colmer TD, Sand-Jensen K. 2013. Underwater photosynthesis of submerged plants – recent advances and methods. Frontiers in Plant Science 4: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. 1997. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany 79: 49–56. [Google Scholar]
- Perata P, Armstrong W, Voesenek LACJ. 2011. Plants and flooding stress. New Phytologist 190: 269–273. [DOI] [PubMed] [Google Scholar]
- Poot P, Lambers H. 2003. Growth responses to waterlogging and drainage of woody Hakea (Proteaceae) seedlings, originating from contrasting habitats in south-western Australia. Plant and Soil 253: 57–70. [Google Scholar]
- Rich SM, Ludwig M, Colmer TD. 2012. Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Annals of Botany 110: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders JGHM, Armstrong W, Darwent MJ, Blom CWPM, Voesenek LACJ. 2000. The role of oxygen in submergence-induced petiole elongation in Rumex palustris: in situ measurements of oxygen in petioles of intact plants using micro-electrodes. New Phytologist 147: 497–504. [DOI] [PubMed] [Google Scholar]
- Sauter M. 2000. Rice in deep water: ‘How to take heed against a sea of troubles’. Naturwissenschaften 87: 289–303. [DOI] [PubMed] [Google Scholar]
- Sauter M. 2013. Root responses to flooding. Current Opinion in Plant Biology 16: 282–286. [DOI] [PubMed] [Google Scholar]
- Setter TL, Laureles EV. 1996. The beneficial effect of reduced elongation growth on submergence tolerance of rice. Journal of Experimental Botany 47: 1551–1559. [Google Scholar]
- Steffens B, Sauter M. 2005. Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiology 139: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Sauter M. 2009. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. Plant Cell 21: 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Wang JX, Sauter M. 2006. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223: 604–612. [DOI] [PubMed] [Google Scholar]
- Striker GG, Izaguirre RF, Manzur ME, Grimoldi AA. 2012. Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biology 14: 50–55. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Kozlowski TT. 1985. Importance of adventitious roots to growth of flooded Platanus occidentalis seedlings. Plant and Soil 88: 123–132. [Google Scholar]
- van der Sman AJM, Blom CWPM, Barendse GWM. 1993. Flooding resistance and shoot elongation in relation to developmental stage and environmental conditions in Rumex maritimus L. and Rumex palustris Sm. New Phytologist 125: 73–84. [DOI] [PubMed] [Google Scholar]
- van Eck WHJM, Lenssen JPM, Rengelink RHJ, Blom CWPM, de Kroon H. 2005. Water temperature instead of acclimation stage and oxygen concentration determines responses to winter floods. Aquatic Botany 81: 253–264. [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. 2010. Hormonal interplay during adventitious root formation in flooded tomato plants. The Plant Journal 63: 551–562. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Blom CWPM, Voesenek LACJ. 1996. Flooding-induced adventitious rooting in Rumex: morphology and development in an ecological perspective. Acta Botanica Neerlandica 45: 17–28. [Google Scholar]
- Visser EJW, Zhang Q, De Gruyter F, Martens S, Huber H. 2015. Shade affects responses to drought and flooding-acclimation to multiple stresses in bittersweet (Solanum dulcamara L.). Plant Biology, in press, doi:10·1111/plb.12304. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Van Oorschot FJMM, Smits AJM, Blom CWPM. 1993. The role of flooding resistance in the establishment of Rumex seedlings in river flood plains. Functional Ecology 7: 105–114. [Google Scholar]
- Voesenek LACJ, Rijnders JHGM, Peeters AJM, van de Steeg HM, de Kroon H. 2004. Plant hormones regulate fast shoot elongation under water: from genes to communities. Ecology 85: 16–27. [Google Scholar]
- Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. 2006. How plants cope with complete submergence. New Phytologist 170: 213–226. [DOI] [PubMed] [Google Scholar]
- Wample RL, Reid DM. 1975. Effect of aeration on the flood-induced formation of adventitious roots and other changes in sunflower (Helianthus annuus L.). Planta 127: 263–270. [DOI] [PubMed] [Google Scholar]
- Wample RL, Reid DM. 1978. Control of adventitious root production and hypocotyl hypertrophy of sunflower (Helianthus annuus) in response to flooding. Physiologia Plantarum 44: 351–358. [Google Scholar]
- Watson MA, Geber MA, Jones CS. 1995. Ontogenetic contingency and the expression of plant plasticity. Trends in Ecology & Evolution 10: 474–475. [DOI] [PubMed] [Google Scholar]
- Weinig C. 2000. Plasticity versus canalization: population differences in the timing of shade-avoidance responses. Evolution 54: 441–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.