Abstract
The risk of cardiovascular events in humans increases in the presence of type 1 or type 2 diabetes mellitus, in large part due to exacerbated atherosclerosis. Genetically engineered mouse models have begun to elucidate cellular and molecular mechanisms responsible for diabetes-exacerbated atherosclerosis. Research on these mouse models has revealed that diabetes independently accelerates initiation and progression of lesions of atherosclerosis and also impairs the regression of lesions following aggressive lipid lowering. Myeloid cell activation in combination with proatherogenic changes allowing for increased monocyte recruitment into arteries of diabetic mice has emerged as an important mediator of the effects of diabetes on the three stages of atherosclerosis. The effects of diabetes on atherosclerosis appear to be dependent on an interplay between glucose and lipids, as well as other factors, and result in increased recruitment of monocytes into both progressing and regressing lesions of atherosclerosis. Importantly, some of the mechanisms revealed by mouse models are now being studied in human subjects. This Perspective highlights new mechanistic findings based on mouse models of diabetes-exacerbated atherosclerosis and discusses the relevance to humans and areas in which more research is urgently needed in order to lessen the burden of macrovascular complications of type 1 and type 2 diabetes mellitus.
Introduction
I was very honored to deliver the Edwin Bierman Award Lecture at the 74th Scientific Sessions of the American Diabetes Association in San Francisco in 2014. Dr. Bierman was one of the outstanding scientists and mentors at the University of Washington, who greatly contributed not only to the fields of diabetes, obesity, dyslipidemia, and atherosclerosis but also to the exceptional scientific environment at the university and beyond.
Research in my laboratory has been devoted to the discovery of mechanisms behind macrovascular complications of diabetes. By taking advantage of new genetically engineered mouse models, we are now at the exciting stage at which some of the mechanisms discovered in mouse models can be studied in human subjects and vice versa.
This Perspective will cover the 2014 Edwin Bierman Award Lecture “Uncomplicating the Macrovascular Complications of Diabetes,” entitled so because of the ability of mouse models to ask targeted mechanistic questions related to the specific stages of the atherosclerotic lesions and to inquire about cell type–specific roles and effects in mediating the effects of diabetes on atherosclerosis. By dissecting the effects of diabetes on macrovascular complications into smaller questions that can be addressed in mice, we are beginning to understand which cell types might be particularly sensitive to hyperglycemia in vivo, that lipids are required, and that myeloid cells play key roles in all stages of atherosclerosis affected by diabetes. Combined with well-planned human studies, mouse models will likely provide important mechanistic insight into diabetic macrovascular complications and reveal new potential treatment and prevention strategies that will be needed in order to avert the expected increase in macrovascular complications as our population with diabetes grows.
Diabetes Promotes Formation of Early Lesions of Atherosclerosis and Progression to Advanced Lesions and Hinders Regression of Atherosclerosis by Stimulating Myeloid Cell Recruitment Into the Artery Wall
Both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are associated with an increased risk of cardiovascular disease (CVD) due primarily to worsened atherosclerosis (1–6). Recent encouraging trends have suggested that CVD is on the decline in subjects with T1DM and T2DM, perhaps due to improved glycemic control and/or statin use (7). A similar reduction in CVD risk has occurred in subjects without diabetes. However, diabetes continues to be associated with a twofold or higher CVD risk as compared with the general population (4,7) and to be a major health issue in subjects with either T1DM or T2DM. The CVD risk in premenopausal women with diabetes is especially high as compared with women without diabetes. Research on the mechanisms responsible for the increased CVD associated with T1DM and T2DM therefore remains a high priority, especially because T2DM is on the rise worldwide.
Lesions of atherosclerosis in susceptible arteries start as accumulations of macrophage foam cells and other immune cells in areas of intimal thickening in humans. These “fatty streak” lesions can be seen in very young children and are believed to be reversible (8). As the lesion progresses, smooth muscle cells become activated and form a fibrous cap covering the fatty streak lesion. Macrophages in the central areas of the lesion undergo apoptosis and death, and as a result, the lesion becomes less stable. Rupture or fissuring of such unstable lesions, followed by thrombosis, are believed to be responsible for most cardiovascular events (8). Data on atherosclerotic lesion morphology in humans with T1DM and T2DM are sparse and are currently limited to small studies. For example, it is unknown if the initiating event in lesion formation in subjects with diabetes is an increased lipoprotein trapping in the artery wall (9,10) or if the initiating insult is due to endothelial cell activation and increased expression of adhesion molecules due to systemic low-grade inflammation or hyperglycemia, for example. Overall, gross lesion morphology appears to be similar in subjects with and without diabetes (11). Postmortem studies on subjects who suffered sudden coronary death have suggested that lesions in subjects with T1DM and T2DM have larger necrotic cores relative to total lesion area as compared with lesions in individuals without diabetes and that macrophages and T cells are more frequent in lesions from individuals with diabetes (12). As necrotic cores are believed to be caused primarily by the death of lesion macrophages, these studies support the hypothesis that both T1DM and T2DM promote atherosclerotic lesions that contain a larger fraction of immune cells relative to other lesion components.
How do these findings on human arteries correlate with findings in mouse models of diabetes-exacerbated atherosclerosis? One important hurdle to overcome when developing mouse models of diabetes-accelerated atherosclerosis was that induction of diabetes in mice often leads to severe hypercholesterolemia and hypertriglyceridemia as compared with nondiabetic controls. The hypertriglyceridemia in diabetic mice is primarily due to reduced peripheral lipolysis by lipoprotein lipase and is likely to be due to reduced insulin action (13). Because hyperlipidemia is such a strong promoter of atherosclerosis, diabetes-induced hyperlipidemia makes it difficult to study the effects of diabetes per se on the artery wall. The first study to demonstrate that diabetes promotes atherosclerosis in mice even in the absence of significant changes in plasma lipid levels was conducted by Renee LeBoeuf’s group in the 1990s (14). In this study, BALB/c mice made diabetic using multiple low-dose injections of the β-cell toxin streptozotocin (STZ) were fed a high-fat diet. The diabetic mice developed small atherosclerotic lesions in the aortic sinus without marked changes in plasma lipid levels as compared with their nondiabetic controls fed the same diet.
Another model of diabetes-induced atherosclerosis that more closely resembles T1DM and that exhibits lesions in the aorta and brachiocephalic artery as well as the aortic sinus was developed several years later (15). In this model, LDL receptor–deficient mice (Ldlr−/− mice) were crossed with mice that express a viral glycoprotein transgene under control of the rat insulin promoter. These mice develop insulitis followed by β-cell loss and diabetes when infected with lymphocytic choriomeningitis virus (LCMV), due primarily to CD8+ T-cell infiltration of the islets (15,16). The diabetic mice become hyperglycemic and exhibit elevated levels of glycated hemoglobin, and they require exogenous insulin treatment to avoid weight loss and ketonuria (15). Indeed, the loss of β-cells in this model is almost complete, and it has been shown that these mice develop autoantibodies to glutamic acid decarboxylase (17), thus resembling human T1DM. In this model of diabetes-accelerated atherosclerosis, diabetic mice exhibit accelerated formation of early fatty streak lesions (15,18), as well as progression to advanced lesions characterized by intraplaque hemorrhage in the absence of significant diabetes-induced hyperlipidemia (15,19), as shown in Fig. 1A and B. The effect of diabetes on lesion progression is independent of the effects on lesion initiation, but also affects lesion areas containing macrophages (19).
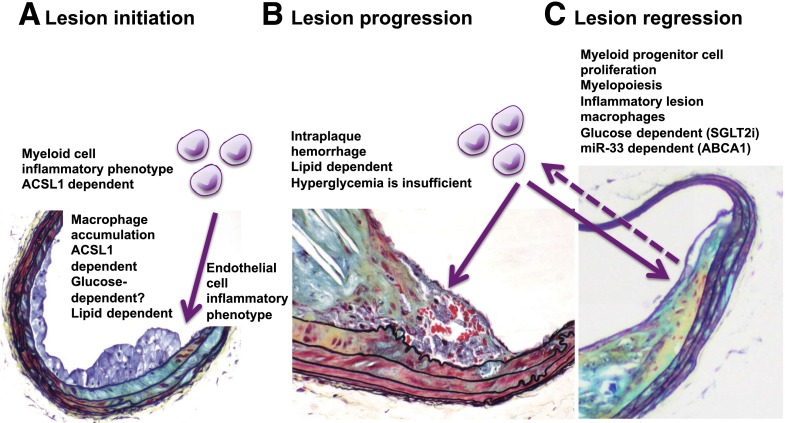
Figure 1.
Diabetes promotes atherosclerotic lesion initiation and progression and inhibits lesion regression. A: Diabetes promotes early atherosclerotic lesion formation by stimulating monocyte recruitment to the artery wall. This is likely to be due partly to an increased inflammatory phenotype of myeloid cells dependent on ACSL1 induction and partly to increased expression of adhesion molecules and chemokines by endothelial cells. The accumulation of macrophages is lipid dependent. It is still not known if lesion initiation is dependent on hyperglycemia or other factors associated with the diabetic environment. B: Diabetes promotes progression of lesions of atherosclerosis to advanced lesions characterized by intraplaque hemorrhage. This effect of diabetes can be prevented by aggressive lipid lowering, even in the presence of severe hyperglycemia. C: Diabetes prevents effective lesion regression in response to lipid lowering. This effect of diabetes is due to maintained recruitment of monocytes into lesions under conditions at which these cells leave the lesions in nondiabetic mice. The maintained accumulation of macrophages in diabetic regression models is dependent on hyperglycemia, which promotes myelopoiesis, an inflammatory lesion macrophage phenotype, and miR-33–mediated reduction of the cholesterol exporter ABCA1. A is modified and reproduced with permission from Renard et al. (15). B is reproduced with permission from Johansson et al. (19). SGLT2i, SGLT2 inhibitor.
Other mouse models of diabetes have shown similar results on atherosclerosis (20). In addition, the virally induced model exhibits loss of sympathetic neurons in the pancreas during the autoimmune attack (21), similar to what occurs in humans. This model therefore exhibits at least two different complications of diabetes. Other advantages of this virally induced model of diabetes-accelerated atherosclerosis is that diabetes can be induced at will by LCMV injection and that littermates lacking the glycoprotein transgene can be used as LCMV-injected controls that do not develop diabetes.
The third stage of atherosclerosis negatively affected by diabetes is lesion regression in response to aggressive lipid lowering (Fig. 1C). Thus, in two diabetes mouse models, the STZ model and the Akita model, diabetes prevents efficient lesion regression by promoting monocyte recruitment into regressing lesions, whereas under nondiabetic conditions, new monocytes do not enter regressing lesions to the same extent (22,23). The STZ and Akita models of diabetes exhibit hyperglycemia and in this respect are reflective of both T1DM and T2DM. They are not ideal models of human T1DM or T2DM, although some studies suggest that a low-dose STZ protocol of diabetes induction results in generation of autoantibodies (24). There is currently no good mouse model of T2DM-accelerated atherosclerosis. An atherosclerotic mouse model with T2DM, characterized by β-cell dysfunction, hyperglycemia, insulin resistance, and lipid levels in the moderate (300–500 mg/dL) range is greatly needed to advance the field. Of the mouse models analyzed to date, the virally induced transgenic model most closely resembles T1DM in humans (16).
Together, the careful analyses of different stages of atherosclerosis in these mouse models have demonstrated that diabetes promotes three different phases of atherosclerosis without significant changes in plasma lipid levels and that the effects of diabetes are associated with increased recruitment and/or activation of myeloid cells. However, it is important to bear in mind that the presence of atherogenic lipoproteins is required in all animal studies for diabetes to promote atherosclerosis. These findings appear to be consistent with the human postmortem data (12) discussed above.
Diabetes Is Associated With Increased Myeloid Cell Activation
Many human studies have demonstrated that T1DM and T2DM are associated with increased systemic inflammation (25–27). The term inflammation is often used to mean that various cytokines and other inflammatory biomarkers are elevated in plasma. Thus, plasma interleukin 6 (IL-6) and monocyte chemoattractant protein 1 (CCL2) are often elevated in subjects with T2DM and have been shown to be predictive risk factors for cardiovascular events and death in subjects with T2DM (25) or overweight/obese subjects (28). Other inflammatory mediators elevated in plasma in T2DM subjects include the chemokines CXCL1, CXCL10 (26), and CX3CL1 (29). Plasma chemokines and cytokines are derived partly from immune cells. Thus, IL-6 release is elevated from CD14+ monocytes isolated from subjects with T1DM as compared with control subjects (30). Furthermore, studies have identified many cytokines and inflammatory mediators as being elevated in peripheral blood mononuclear cells from subjects with T1DM, including IL-6, IL-1β, genes in the IL-1β pathway, and genes involved in eicosanoid production (31–33). However, there is a plethora of proinflammatory pathways that act in different tissues and cell types, and these pathways are likely to have different effects on circulating cells, on lesion cells, and even on different stages of atherosclerosis. Adipose tissue and liver inflammation associated with accumulation of leukocytes in these tissues in the metabolic syndrome/obesity or T2DM (34) are likely to affect the artery wall through indirect mechanisms, whereas increased activation of leukocytes in lesions of atherosclerosis can have more direct autocrine or paracrine effects.
What is the role of the increased inflammatory mediators in CVD associated with diabetes in humans? Two clinical trials designed to test the role of inflammatory pathways in CVD in subjects with and without T2DM are ongoing, and a third trial has been recently terminated.
The Cardiovascular Inflammation Reduction Trial (CIRT) is a randomized clinical trial investigating whether low-dose methotrexate treatment reduces heart attacks, strokes, or death in people with T2DM or metabolic syndrome who have already had a myocardial infarction or multiple coronary blockages. The study compares methotrexate (5–20 mg weekly), a drug currently in use for the treatment of arthritis and other rheumatic conditions, to placebo (35,36). The mechanisms of action of methotrexate to suppress inflammatory processes are complex and not fully understood but are believed to involve the release of adenosine and inhibition of polyamines (37). This clinical trial aims to recruit 7,000 individuals and has an estimated completion date in 2018.
The other ongoing clinical trial, Cardiovascular Risk Reduction Study (Reduction in Recurrent Major CV Disease Events), also known as CANTOS, will test the hypothesis that treatment with canakinumab (a human monoclonal anti-human IL-1β antibody, already used in the treatment of some autoimmune diseases) of patients with myocardial infarction at least 1 month prior to study entry and elevated C-reactive protein (as a marker of inflammation) will prevent recurrent cardiovascular events. The expected enrollment is 10,000 subjects with and without T2DM (38). This trial has an estimated completion date in the spring of 2017. Although this study contains subjects with T2DM, the primary outcome measure in these T2DM subjects is insulin secretion rate. However, the T2DM subjects are part of the main study with the primary outcome of major adverse cardiovascular event (cardiovascular death, nonfatal myocardial infarction, and stroke). A confounding factor might be that if canakinumab improves HbA1c levels, as has been suggested by a study on T2DM subjects treated with canakinumab as an add-on to metformin (39), the improvement in cardiovascular outcomes in these subjects might be due partly to improved glycemic control.
A third study, which included some subjects with T2DM, on the effect of a nonspecific secretory phospholipase A2 (sPLA2) inhibitor (varespladib) on CVD was terminated in 2012 (40). This study demonstrated no protective effect on a composite of cardiovascular mortality, nonfatal myocardial infarction, nonfatal stroke, and unstable angina (40). The effects of sPLA2 inhibitors are likely to be complex because of the large number of sPLA2 isoforms and multiple effects of these enzymes on downstream eicosanoids. In this context, it is interesting to note that the risk of CVD is increased across a range of chronic inflammatory disorders and that the cardiovascular risk is associated with severity of inflammation (41).
The outcomes of the CIRT and CANTOS trials will therefore be very informative. Furthermore, testing the effect of anti-inflammatory therapies in subjects with T1DM would be of great interest. However, because of the many diverse inflammatory pathways and mechanisms, it is possible that more targeted treatment strategies have to be developed in order to minimize side effects and effectively prevent diabetes-induced inflammation without hindering the ability of treated patients to respond to infection.
Mouse models might provide important mechanistic insight into this area. How does diabetes promote inflammatory properties of myeloid cells in mouse models? Elegant studies demonstrate that diabetes is associated with increased myelopoiesis in mouse models of atherosclerotic lesion regression (23). These studies identified the damage-associated molecular pattern molecules S100A8 and S100A9 in neutrophils and their interaction with the receptor for advanced glycation end products as mediators of myelopoiesis associated with diabetes. It is therefore possible that diabetes results in activation of neutrophils and subsequent myelopoiesis and increased levels of circulating monocytes with an inflammatory phenotype. Increased plasma S100A8/S100A9 levels and gene expression in peripheral blood mononuclear cells have been identified in subjects with T1DM (42,43) and T2DM (44), and these proteins are also significantly associated with CVD in subjects with T1DM (23) as well as in subjects without diabetes (45). The usefulness of S100A8 and S100A9 inhibitors as therapeutic targets is still unknown and is complicated by the fact that these proteins exert proinflammatory effects in dendritic cells (46).
Another marker and mediator of the inflammatory phenotype of myeloid cells associated with T1DM is the enzyme acyl-CoA synthetase 1 (ACSL1). ACSL1 catalyzes the linking of acyl-CoA moieties to free fatty acids, thus enabling fatty acids to enter many different fates in the cells. ACSL1 is induced in myeloid cells in diabetic mice and in CD14+ monocytes in a small cohort of human subjects with T1DM as compared with control subjects (47). Myeloid cell–targeted ACSL1 deficiency protected macrophages and monocytes from the inflammatory effects of diabetes and Ldlr−/− mice from virally induced diabetes-mediated atherogenesis (47). Interestingly, myeloid cell ACSL1 deficiency had no effect on inflammation or atherosclerosis in nondiabetic mice, suggesting that its induction in diabetes is part of an inflammatory pathway stimulated by diabetes.
ACSL1 is induced by several inflammatory ligands in myeloid cells, such as lipopolysaccharide, Gram negative bacteria, interferon-γ, and tumor necrosis factor-α, but not by peroxisome proliferator–activated receptor (PPAR)-α and -γ agonists (48,49), in contrast to adipose tissue, liver, and heart in which ACSL1 is a PPAR target gene (50–52). This important difference in the regulation of ACSL1 might indicate divergent functions of ACSL1 in most tissues as compared with myeloid cells. Indeed, whereas ACSL1 has important functions in mediating the fatty acid oxidation in adipose tissue, liver, and heart (53–55), it does not have the same role in inflammatory myeloid cells (47), which rely heavily on glycolysis for energy.
Furthermore, ACSL1 has emerged as a marker of a “metabolically activated” macrophage phenotype. In a recent study by Kratz et al. (56), ACSL1 was induced in adipose tissue macrophages from fat-fed mice, concomitant with induction of inflammatory mediators. Plasma membrane–associated ACSL1 was also induced in metabolically activated macrophages exposed to high levels of palmitate, glucose, and insulin in vitro (56). Other cell surface proteins induced in these macrophages were the cholesterol export protein ABCA1, the fatty acid transporter CD36, and perilipin-2, a protein that coats intracellular lipid droplets. Furthermore, ACSL1 is induced in thioglycollate-elicited macrophages by fat feeding in C57Bl/6 (Ldlr+/+) mice, but not in macrophages from Ldlr−/− mice fed the same diet (57). Macrophages from fat-fed Ldlr−/− mice are much more lipid loaded than macrophages from fat-fed C57Bl/6 mice; macrophages from Ldlr−/− mice had a fourfold increase in total cholesterol and increases in almost all major lipid classes as compared with macrophages from C57Bl/6 mice fed the same diet (57).
Thus, it is unlikely that ACSL1 induction is merely a reflection of myeloid cell lipid loading. Instead, it is possible that ACSL1 induction in myeloid cells in the diabetic environment is a marker and mediator of metabolically activated myeloid cells. Another interesting possibility is that ACSL1 might be translocated to the plasma membrane in activated macrophages (47,56), where it might act on distinct pools of fatty acids. The enzyme ACSL1 thus fits into the category of proteins involved in immunometabolism (58).
In further support of the important role of inflammatory pathway activation in diabetes-accelerated atherosclerosis in mice are recent studies on a cell-permeable peptide corresponding to the Janus kinase/STAT (signal transducer and activator of transcription) inhibitory region of the suppressor of cytokine signaling protein, which inhibits STAT 1 and 3. This peptide was demonstrated to impair both systemic inflammation, measured as levels of circulating Ly6Chi monocytes and splenic expression of cytokines, and atherosclerosis in apolipoprotein E–deficient (Apoe−/−) STZ-diabetic mice without affecting metabolic parameters (59). These results further emphasize that inflammation plays an important role in diabetes-accelerated atherosclerosis, at least in mouse models. The clinical trials discussed above are likely to provide the first information on the role of inflammation in CVD associated with T2DM in humans.
Interplay Between Glucose and Lipids in Diabetes-Accelerated Atherosclerosis
Improved metabolic control has long-term beneficial effects on cardiovascular events in young subjects with T1DM, and these protective effects correlate with improved HbA1c levels (60). The effects of glucose-lowering on CVD associated with T2DM are less obvious (61). Blood glucose lowering by metformin reduced cardiovascular outcomes in overweight subjects with T2DM in the UK Prospective Diabetes Study as compared with diet treatment alone (62). It is unclear whether this effect was due to blood glucose lowering or to other potentially cardioprotective effects by metformin, such as direct effects on macrophages or the liver or other systemic, including lipid lowering, effects in subjects with T2DM (63–67). Several risk factors contribute to CVD, and whether hyperglycemia directly and universally contributes to CVD associated with diabetes is still a matter of debate.
Mouse models have demonstrated that hyperglycemia in the presence of atherogenic lipoproteins promotes certain aspects of diabetes-exacerbated atherosclerosis but that it is not sufficient to promote advanced atherosclerosis (19,68). For example, treating diabetic mice with sodium–glucose cotransporter 2 (SGLT2) inhibitors to reduce blood glucose, without confounding effects of insulin on lipid metabolism and other parameters, has demonstrated reduced myelopoiesis, reduced S100A9 expression in neutrophils, reduced accumulation of monocytes in regressing lesions of atherosclerosis, and reduced expression of inflammatory cytokines in lesion macrophages (23). In another study, an SGLT2 inhibitor prevented systemic inflammation, measured as plasma levels of IL-6, tumor necrosis factor-α, and CCL2, in diabetic mice, as well as reduced plasma cholesterol and triglyceride levels (69). These effects are most likely due to the reduced glucose levels, although it is possible that secondary effects contribute to the beneficial effects. SGLT2 inhibitors are now approved for use in human subjects with T2DM, and it will be interesting to evaluate their effects on CVD.
If glucose indeed exacerbates atherosclerosis, what is the culprit cell type, what is the mechanism, and what is the interplay between glucose and lipids? Interestingly, hyperglycemia likely contributes to increased hepatic production of VLDL in human subjects (70), an effect that might be explained by the increased transcription of apolipoprotein CIII (APOC3) in response to glucose (71). There is strong emerging genetic evidence that loss-of-function mutations of APOC3 in humans protect against CVD in subjects without diabetes (72,73). Thus, hyperglycemia could contribute to increased VLDL levels and CVD by inducing APOC3 expression in the liver. Another recent example of interplay between glucose and lipids is provided by microRNA 33 (miR-33). Inhibition of miR-33 by an antisense oligonucleotide (anti–miR-33) prevented the detrimental effects of diabetes on lesion regression, monocytosis, monocyte recruitment to lesions, and the inflammatory lesion macrophage phenotype despite persistent hyperglycemia in mice treated with anti–miR-33 (74). Thus, all the effects of hyperglycemia in a similar model (23) were prevented by anti–miR-33.
In addition, anti–miR-33 treatment resulted in elevated HDL levels in diabetic mice, concomitant with increased hepatic expression of ABCA1, consistent with previous studies (75). Anti–miR-33 also restored the lower levels of ABCA1 in bone marrow progenitor cells in diabetic mice. Thus, anti–miR-33 is likely to act by increasing cholesterol efflux from bone marrow myeloid precursor cells and perhaps by raising HDL, which can have anti-inflammatory effects in myeloid cells (76,77). On the other hand, anti–miR-33 did not affect macrophage expression of Acsl1 (74), suggesting that miR-33 and ACSL1 act via different pathways.
Together, these findings suggest that the stimulatory effects of hyperglycemia on myelopoiesis can be prevented by stimulation of cholesterol efflux from bone marrow cells and/or perhaps by anti-inflammatory effects mediated by ABCA1 or HDL. In other mouse models, myelopoiesis is induced by fat feeding and adipose tissue inflammation through a mechanism independent of hyperglycemia (78). It is therefore possible that diabetes-induced myelopoiesis is, in part, lipid dependent.
We evaluated the effect of glucose flux in macrophages by a more direct and cell type–specific approach by knocking down the housekeeping glucose transporter GLUT1 by small interfering RNA and by overexpressing the same transporter in myeloid cells in vivo under control of the myeloid cell CD68 promoter (79). Knockdown of GLUT1 results in reduced glycolysis in these cells, whereas overexpression promotes glycolysis. GLUT1 and several other proteins involved in glycolysis and export of lactate are stimulated by inflammatory mediators in macrophages (80–82). Knockdown of glycolytic proteins or inhibition of glycolysis prevents the macrophage from mounting a full inflammatory response (79,82–84). Downregulation of GLUT1 also prevents myelopoiesis in response to cholesterol loading in cells lacking the cholesterol export proteins ABCA1 and ABCG1 (85), hinting at another interesting connection between HDL and glucose metabolism in myeloid cells.
Conversely, a recent study demonstrated that overexpression of GLUT1 in a macrophage cell line is sufficient for increasing the inflammatory activation of these cells (86). Is it therefore possible that hyperglycemia promotes inflammation and atherosclerosis by stimulating glycolysis in myeloid cells? We addressed this question in Ldlr−/− mice with GLUT1 overexpression targeted to myeloid cells (79). However, we observed no effect on cytokine production by GLUT1-overexpressing myeloid cells, no myelopoiesis, and no increased atherosclerosis at three different arterial sites. Thus, increased glucose flux in myeloid cells is not sufficient to explain the effects of diabetes on these cells. It is likely that hyperglycemia affects other cell types involved in atherogenesis directly or indirectly. For example, the endothelial cell is likely to be sensitive to glucose and to express proatherosclerotic adhesion molecules and chemokines under hyperglycemic conditions, resulting in increased monocyte recruitment (87). Another possibility is that glucose produces a more atherogenic lipid profile by affecting the liver, as discussed above.
Increased oxidative stress in vascular cells is often put forward as a mechanism whereby hyperglycemia could exert proatherosclerotic effects. Indeed, lack of the enzyme glutathione peroxidase 1, which mediates peroxide breakdown and other antioxidant processes, accelerates atherosclerosis in diabetic Apoe−/− mice (88), whereas knockout of NADPH oxidase 1, involved in superoxide production, protects against atherosclerosis in diabetic Apoe−/− mice (89). Interestingly, overexpression or knockdown of glyoxalase 1, an enzyme that detoxifies reactive carbonyls, does not affect atherosclerosis in nondiabetic or diabetic Apoe−/− mice (90,91). There is therefore evidence that reactive oxygen species and perhaps other oxidative processes contribute to atherosclerosis in diabetes. The cell type most vulnerable to oxidative stress is unknown, but the endothelial cell is again a strong contender. Other possible proatherosclerotic mediators of the effects of increased glucose include RAGE (the receptor for advanced glycation end products) (92–94) and protein kinase Cβ activation (95).
Thus, further studies are needed to gain a better understanding of the effects of glucose and hyperglycemia in cells involved in atherosclerosis in mouse models and in human subjects and the relative role of hyperglycemia, inflammation, and lipid abnormalities in CVD progression.
Finally, it is important to consider that hyperglycemia and elevated HbA1c levels could be markers of CVD risk rather than glucose being a mediator. For example, improved HbA1c levels are strongly associated with a long-term beneficial effect on kidney disease (96), which is, in turn, a significant risk factor for cardiovascular mortality (97). Moreover, hyperglycemia is due to reduced insulin action as well as insulin-independent hepatic glucose production (98). Reduced insulin action in vascular cells has been proposed to contribute to atherosclerosis in diabetes. For example, lack of insulin receptors in endothelial cells promotes atherosclerosis in Apoe−/− mice (99), whereas the lack of insulin receptors in myeloid cells has different effects depending on the mouse model (100–102). There are many other possible cardiovascular risk factors that could be associated with elevated HbA1c levels in diabetes.
Conclusion and Future Perspective
Have the mechanistic studies performed so far in genetically engineered mouse models combined with human studies “uncomplicated” the macrovascular complications of diabetes? First, these studies have shown us that diabetes exacerbates several major stages of atherosclerosis by activating myeloid cells. It is still unclear to what extent hyperglycemia directly exacerbates atherosclerosis, but mouse models have started to generate important mechanistic insight. Second, mouse studies have shown that there are important interactions between glucose metabolism in myeloid cells and HDL, fatty acids, and other lipids. The increased inflammation likely to play a part in driving CVD associated with both T1DM and T2DM could therefore be due to several factors associated with suboptimally maintained diabetes. These include altered HDL function; elevated triglycerides, which are a hallmark of T2DM and suboptimally controlled T1DM (103,104); lack of insulin or reduced insulin action; and renal disease. In addition, factors that promote atherosclerosis in the population without diabetes, such as dyslipidemia, hypertension, and smoking, also enhance atherosclerosis in subjects with diabetes. It is possible that the relative contributions of various risk factors differ in CVD associated with T1DM versus T2DM (6) and that lipid abnormalities are relatively more important than glucose in T2DM (105). Thus, multipronged approaches or individualized treatment strategies are likely to be the way of the future in the treatment and prevention of macrovascular complications of diabetes.
Article Information
Acknowledgments. The author thanks members of her laboratory who have made many important contributions over the years.
Funding. Research reported in this article was supported by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers R01HL062887, P01HL092969, and R01HL097365 and by the American Heart Association, grant number 14GRNT20410033 (to K.E.B.). It was also supported by the Diabetes Research Center (P30DK017047) and the Nutrition Obesity Center (P30DK035816) at the University of Washington.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. Projects unrelated to this article in the author’s laboratory are funded by Novo Nordisk A/S.
Prior Presentation. The Edwin Bierman Award Lecture was delivered at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 3.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 4.Taylor KS, Heneghan CJ, Farmer AJ, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care 2013;36:2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group . Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 9.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock LR, King VL. Proteoglycan mediated lipoprotein retention: a mechanism of diabetic atherosclerosis. Rev Endocr Metab Disord 2008;9:289–300 [DOI] [PubMed] [Google Scholar]
- 11.Strandness DE Jr, Priest RE, Gibbons GE. Combined clinical and pathologic study of diabetic and nondiabetic peripheral arterial disease. Diabetes 1964;13:366–372 [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Burke AP, Kolodgie F. Morphological characteristics of coronary atherosclerosis in diabetes mellitus. Can J Cardiol 2006;22(Suppl. B):81B–84B [DOI] [PMC free article] [PubMed]
- 13.Willecke F, Scerbo D, Nagareddy P, et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler Thromb Vasc Biol 2015;35:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest 1996;97:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renard CB, Kramer F, Johansson F, et al. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 2004;114:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Belle TL, Taylor P, von Herrath MG. Mouse models for type 1 diabetes. Drug Discov Today Dis Models 2009;6:41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz A, Dyrberg T, Hagopian W, Homann D, von Herrath M, Oldstone MB. Neither B lymphocytes nor antibodies directed against self antigens of the islets of Langerhans are required for development of virus-induced autoimmune diabetes. J Immunol 2000;165:5945–5953 [DOI] [PubMed] [Google Scholar]
- 18.Lamharzi N, Renard CB, Kramer F, et al. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes 2004;53:3217–3225 [DOI] [PubMed] [Google Scholar]
- 19.Johansson F, Kramer F, Barnhart S, et al. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A 2008;105:2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bornfeldt KE. 2013 Russell Ross Memorial Lecture in vascular biology: cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taborsky GJ Jr, Mei Q, Bornfeldt KE, Hackney DJ, Mundinger TO. The p75 neurotrophin receptor is required for the major loss of sympathetic nerves from islets under autoimmune attack. Diabetes 2014;63:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parathath S, Grauer L, Huang LS, et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes 2011;60:1759–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elias D, Prigozin H, Polak N, Rapoport M, Lohse AW, Cohen IR. Autoimmune diabetes induced by the beta-cell toxin STZ. Immunity to the 60-kDa heat shock protein and to insulin. Diabetes 1994;43:992–998 [DOI] [PubMed] [Google Scholar]
- 25.Lowe G, Woodward M, Hillis G, et al. Circulating inflammatory markers and the risk of vascular complications and mortality in people with type 2 diabetes and cardiovascular disease or risk factors: the ADVANCE study. Diabetes 2014;63:1115–1123 [DOI] [PubMed] [Google Scholar]
- 26.Sajadi SM, Khoramdelazad H, Hassanshahi G, et al. Plasma levels of CXCL1 (GRO-alpha) and CXCL10 (IP-10) are elevated in type 2 diabetic patients: evidence for the involvement of inflammation and angiogenesis/angiostasis in this disease state. Clin Lab 2013;59:133–137 [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Larsson A, Vessby J, Vessby B, Berne C. Type 1 diabetes is associated with increased cyclooxygenase- and cytokine-mediated inflammation. Diabetes Care 2005;28:1371–1375 [DOI] [PubMed] [Google Scholar]
- 28.Piemonti L, Calori G, Lattuada G, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care 2009;32:2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah R, Hinkle CC, Ferguson JF, et al. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 2011;60:1512–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 2006;55:774–779 [DOI] [PubMed] [Google Scholar]
- 31.Levy H, Wang X, Kaldunski M, et al. Transcriptional signatures as a disease-specific and predictive inflammatory biomarker for type 1 diabetes. Genes Immun 2012;13:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradshaw EM, Raddassi K, Elyaman W, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 2009;183:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanter JE, Bornfeldt KE. Inflammation and diabetes-accelerated atherosclerosis: myeloid cell mediators. Trends Endocrinol Metab 2013;24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 2014;41:36–48 [DOI] [PubMed] [Google Scholar]
- 35.Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207, e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM. Closing the loop on inflammation and atherothrombosis: why perform the CIRT and CANTOS trials? Trans Am Clin Climatol Assoc 2013;124:174–190 [PMC free article] [PubMed] [Google Scholar]
- 37.Chan ES, Cronstein BN. Methotrexate—how does it really work? Nat Rev Rheumatol 2010;6:175–178 [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J 2011;162:597–605 [DOI] [PubMed] [Google Scholar]
- 39.Hensen J, Howard CP, Walter V, Thuren T. Impact of interleukin-1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab 2013;39:524–531 [DOI] [PubMed] [Google Scholar]
- 40.Nicholls SJ, Kastelein JJ, Schwartz GG, et al.; VISTA-16 Investigators . Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA 2014;311:252–262 [DOI] [PubMed] [Google Scholar]
- 41.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 2014;130:837–844 [DOI] [PubMed] [Google Scholar]
- 42.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes 2004;53:1979–1986 [DOI] [PubMed] [Google Scholar]
- 43.Jin Y, Sharma A, Carey C, et al. The expression of inflammatory genes is upregulated in peripheral blood of patients with type 1 diabetes. Diabetes Care 2013;36:2794–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortega FJ, Sabater M, Moreno-Navarrete JM, et al. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur J Endocrinol 2012;167:569–578 [DOI] [PubMed] [Google Scholar]
- 45.Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 2006;113:2278–2284 [DOI] [PubMed] [Google Scholar]
- 46.Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 2012;32:223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A 2012;109:E715–E724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubinow KB, Wall VZ, Nelson J, et al. Acyl-CoA synthetase 1 is induced by Gram-negative bacteria and lipopolysaccharide and is required for phospholipid turnover in stimulated macrophages. J Biol Chem 2013;288:9957–9970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YL, Morales-Rosado J, Ray J, et al. Toll-like receptor agonists promote prolonged triglyceride storage in macrophages. J Biol Chem 2014;289:3001–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem 1997;272:28210–28217 [DOI] [PubMed] [Google Scholar]
- 51.Durgan DJ, Smith JK, Hotze MA, et al. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol Heart Circ Physiol 2006;290:H2480–H2497 [DOI] [PubMed] [Google Scholar]
- 52.Rakhshandehroo M, Hooiveld G, Müller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One 2009;4:e6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li LO, Ellis JM, Paich HA, et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J Biol Chem 2009;284:27816–27826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis JM, Li LO, Wu PC, et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab 2010;12:53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellis JM, Mentock SM, Depetrillo MA, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol 2011;31:1252–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kratz M, Coats BR, Hisert KB, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab 2014;20:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012;151:138–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol 2011;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Recio C, Oguiza A, Lazaro I, Mallavia B, Egido J, Gomez-Guerrero C. Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice. Arterioscler Thromb Vasc Biol 2014;34:1953–1960 [DOI] [PubMed] [Google Scholar]
- 60.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet 2014;383:2008–2017 [DOI] [PubMed] [Google Scholar]
- 62.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 63.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes 2015;64:2028–2041 [DOI] [PubMed]
- 64.Forouzandeh F, Salazar G, Patrushev N, et al. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc 2014;3:e001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riksen NP, Tack CJ. The cardiovascular effects of metformin: lost in translation? Curr Opin Lipidol 2014;25:446–451 [DOI] [PubMed] [Google Scholar]
- 66.Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism 2011;60:1278–1284 [DOI] [PubMed] [Google Scholar]
- 67.Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 2004;53:159–164 [DOI] [PubMed] [Google Scholar]
- 68.Reaven P, Merat S, Casanada F, Sutphin M, Palinski W. Effect of streptozotocin-induced hyperglycemia on lipid profiles, formation of advanced glycation endproducts in lesions, and extent of atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 1997;17:2250–2256 [DOI] [PubMed] [Google Scholar]
- 69.Tahara A, Kurosaki E, Yokono M, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol 2013;715:246–255 [DOI] [PubMed] [Google Scholar]
- 70.Adiels M, Borén J, Caslake MJ, et al. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 2005;25:1697–1703 [DOI] [PubMed] [Google Scholar]
- 71.Caron S, Verrijken A, Mertens I, et al. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol 2011;31:513–519 [DOI] [PubMed] [Google Scholar]
- 72.Crosby J, Peloso GM, Auer PL, et al.; TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute . Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41 [DOI] [PubMed] [Google Scholar]
- 74.Distel E, Barrett TJ, Chung K, et al. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res 2014;115:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest 2011;121:2921–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014;15:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki M, Pritchard DK, Becker L, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation 2010;122:1919–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014;19:821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishizawa T, Kanter JE, Kramer F, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Reports 2014;7:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010;115:4742–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013;493:346–355 [DOI] [PubMed] [Google Scholar]
- 82.Tan Z, Xie N, Banerjee S, et al. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem 2015;290:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruiz-García A, Monsalve E, Novellasdemunt L, et al. Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem 2011;286:19247–19258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013;496:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gautier EL, Westerterp M, Bhagwat N, et al. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med 2013;210:339–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem 2014;289:7884–7896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab 2011;14:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis P, Stefanovic N, Pete J, et al. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation 2007;115:2178–2187 [DOI] [PubMed] [Google Scholar]
- 89.Gray SP, Di Marco E, Okabe J, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013;127:1888–1902 [DOI] [PubMed] [Google Scholar]
- 90.Geoffrion M, Du X, Irshad Z, et al. . Differential effects of glyoxalase 1 overexpression on diabetic atherosclerosis and renal dysfunction in streptozotocin-treated, apolipoprotein E-deficient mice. Physiol Rep; 2014;2:e12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hanssen NM, Brouwers O, Gijbels MJ, et al. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE-/- mice with or without diabetes. Cardiovasc Res 2014;104:160–170 [DOI] [PubMed] [Google Scholar]
- 92.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med 2009;11:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koulis C, Kanellakis P, Pickering RJ, et al. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond) 2014;127:485–497 [DOI] [PubMed] [Google Scholar]
- 94.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 2008;57:2461–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong L, Shen X, Lin L, et al. PKCβ promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol 2013;33:1779–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.DCCT/EDIC Research Group . Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2014;2:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Velde M, Matsushita K, Coresh J, et al.; Chronic Kidney Disease Prognosis Consortium . Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–1352 [DOI] [PubMed] [Google Scholar]
- 98.Schwartz MW, Seeley RJ, Tschöp MH, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 2013;503:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rask-Madsen C, Li Q, Freund B, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 2010;11:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumgartl J, Baudler S, Scherner M, et al. Myeloid lineage cell-restricted insulin resistance protects apolipoprotein E-deficient mice against atherosclerosis [published correction appears in Cell Metab 2006;3:469]. Cell Metab 2006;3:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Han S, Liang CP, DeVries-Seimon T, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab 2006;3:257–266 [DOI] [PubMed] [Google Scholar]
- 102.Liang CP, Han S, Senokuchi T, Tall AR. The macrophage at the crossroads of insulin resistance and atherosclerosis. Circ Res 2007;100:1546–1555 [DOI] [PubMed] [Google Scholar]
- 103.Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol 2011;31:1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller M, Stone NJ, Ballantyne C, et al.; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–2333 [DOI] [PubMed] [Google Scholar]
- 105.Ståhlman M, Fagerberg B, Adiels M, et al. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: impact on small HDL particles. Biochim Biophys Acta 2013;1831:1609–1617 [DOI] [PubMed]