Abstract
Elevated cardiac triacylglycerol (TAG) content is traditionally equated with cardiolipotoxicity and suggested to be a culprit in cardiac dysfunction. However, previous work demonstrated that myosin heavy-chain–mediated cardiac-specific overexpression of diacylglycerol transferase 1 (MHC-DGAT1), the primary enzyme for TAG synthesis, preserved cardiac function in two lipotoxic mouse models despite maintaining high TAG content. Therefore, we examined whether increased cardiomyocyte TAG levels due to DGAT1 overexpression led to changes in cardiac TAG turnover rates under normoxia and ischemia-reperfusion conditions. MHC-DGAT1 mice had elevated TAG content and synthesis rates, which did not alter cardiac function, substrate oxidation, or myocardial energetics. MHC-DGAT1 hearts had ischemia-induced lipolysis; however, when a physiologic mixture of long-chain fatty acids was provided, enhanced TAG turnover rates were associated with improved functional recovery from low-flow ischemia. Conversely, exogenous supply of palmitate during reperfusion suppressed elevated TAG turnover rates and impaired recovery from ischemia in MHC-DGAT1 hearts. Collectively, this study shows that elevated TAG content, accompanied by enhanced turnover, does not adversely affect cardiac function and, in fact, provides cardioprotection from ischemic stress. In addition, the results highlight the importance of exogenous supply of fatty acids when assessing cardiac lipid metabolism and its relationship with cardiac function.
Introduction
The accumulation of triacylglycerol (TAG) in the heart is often viewed as a pathological condition and is generally associated with an impairment in cardiac function (1). Recent evidence suggests that the TAG pool is not an inert repository of fatty acids but, rather, a dynamic entity that contributes to ∼10% of the total cardiac oxidative metabolism under basal conditions (2–4). In addition, the rate of TAG turnover and its contribution to ATP are increased in conditions of diabetes (5) or decreased in pathological hypertrophy and heart failure (2). Although it appears that endogenous TAG metabolism may be important in maintaining overall cardiac function, particularly during cardiac stress, the precise role in the development and progression of cardiac pathologies awaits further clarification.
Although diabetic and obese hearts present with accumulation of cardiac TAG and alterations in substrate metabolism (6,7), it has not been determined whether defects in endogenous TAG metabolism contribute to impaired function after ischemic events. Alterations in cardiac metabolism during ischemia-reperfusion (I-R) injury are well characterized and include increased rates of fatty acid oxidation (8,9) and uncoupling of glycolysis from glucose oxidation (10,11). Endogenous TAG metabolism as a source of ATP generation in ischemia has generally been discounted, owing to “futile cycling” (TAG–fatty acid cycling), an ATP-dependent process that could energetically compromise the heart (12,13). However, it has been shown that the oxidation of endogenously derived palmitate is significantly increased during the reperfusion period (14), suggesting that cardiac TAG metabolism is active during recovery from ischemia. Although accumulation of lipid droplets after ischemia is associated with greater infarct size (15), the precise cause of the tissue damage is not clear. One possibility is the increased oxygen demand due to elevations in fatty acid oxidation after ischemia. However, a mismatch between the rate of fatty acid uptake and the rate of fatty acid oxidation in the damaged tissue could lead to an imbalance in cytosolic fatty acids that could subsequently lead to an accumulation of toxic signaling lipids such as diacylglycerol (DAG) and/or ceramides.
Toxicity due to increased ceramides has been implicated in the response to cardiac I-R injury (16–18). It is possible that modulation of cardiac TAG metabolism could affect ceramide accumulation and, in turn, the response to ischemic stress. Recent work has shown that myosin heavy-chain–mediated cardiac-specific overexpression of diacylglycerol transferase 1 (MHC-DGAT1), the enzyme responsible for the final step in TAG synthesis, leads to increased TAG content with reductions in the lipid intermediates, DAG and ceramides (19,20). Furthermore, crossing MHC-DGAT1 mice with two mouse models of cardiac lipotoxicity attenuated cardiac dysfunction. Total TAG content was unchanged, but both ceramides and DAG decreased as expected (19,20). These results indicate a cardioprotective role of DGAT1 overexpression, presumably by the reduction of toxic lipid intermediates.
Therefore, we investigated whether increasing TAG synthesis by overexpression of DGAT1 was effective in protecting the heart from ischemic injury. We hypothesized that increasing the synthesis and turnover of cardiac TAG during the reperfusion period would lead to sequestration of fatty acids and the reduction in toxic lipid intermediate formation. Our findings illustrate the dynamic nature of the TAG pool during reperfusion after acute cardiac ischemia. Moreover, our results demonstrate that elevated rates of TAG turnover are associated with improved functional recovery from ischemic stress, in part by redirecting fatty acids away from ceramide synthesis.
Research Design and Methods
Animal Model
Animal studies were approved by the University of Washington Institutional Animal Care and Use Committee. MHC-DGAT1 mice were mated with nontransgenic littermates to produce both study and control (CON) mice. Mice were kept on a 12-h light/dark cycle with water and food ad libitum.
Isolated Heart Perfusion and Nuclear Magnetic Resonance Spectroscopy
Isolated Heart Perfusion
Isolated mouse hearts were perfused in Langendorff mode as previously described (21,22). The perfusate was a modified Krebs-Henseleit (KH) buffer supplemented with 0.4 mmol/L long-chain fatty acids (LCFAs) (bound to 1.2% albumin), 5.5 mmol/L glucose, 1.2 mmol/L lactate, and 50 μU/mL insulin. In a cohort of experiments, the source of fatty acids was a mixture of LCFAs consisting of palmitate (46.3%), oleate (22.2%), palmitoleate (15.4%), linoleate (7.6%), stearate (2.9%), and linolenate (1.0%). In a separate cohort of experiments, the fatty acid source was limited to palmitate (100%). Hearts were equilibrated with the unlabeled (12C) perfusate detailed above for 20 min. The perfusate was then changed to an identical perfusate with uniformly labeled 13C fatty acids (LCFA or palmitate) and 1,6-13C glucose for 45 min to determine substrate preference (21). The I-R protocol consisted of 20 min of baseline equilibration with the unlabeled perfusate, 30 min of low-flow ischemia (1% of baseline), and 55 min of reperfusion with the perfusate containing uniformly labeled 13C fatty acids (LCFA or palmitate) and 1,6-13C glucose.
Dynamic 13C NMR Spectroscopy
13C-labeled fatty acids incorporation into the TAG pool was analyzed by monitoring the resonance of the methylene carbon at ∼31 ppm via 13C nuclear magnetic resonance (NMR) spectroscopy as previously described (5,23,24). Proton-decoupled carbon spectra were acquired on isolated hearts perfused with 13C-labeled fatty acids (either LCFAs or palmitate) over a 60-min period. Peak areas of the methylene resonance of each sequential spectrum were quantified using software from Advanced Chemistry Development Laboratories (ACD Laboratories, Toronto, Ontario, Canada) and corrected for 13C natural abundance.
31P NMR Spectroscopy
Dynamic changes in cardiac content of phosphocreatine (PCr), ATP, and inorganic phosphate (Pi) were monitored by 31P NMR spectroscopy as previously described (21,22). The PCr-to-ATP ratio was determined by the quotient of the PCr and average ATP areas. The average of the γ-ATP and β-ATP peak areas obtained during baseline equilibration for each heart was calibrated to 10 mmol/L and used as the reference value for all 31P NMR spectra obtained during the ischemia and reperfusion periods. Intracellular pH was estimated based on the relative chemical shift of the Pi and PCr signals.
13C NMR Spectroscopy of Tissue Extracts
Frozen heart tissues perfused with 13C-labeled substrates were extracted with perchloric acid and neutralized by KOH. Proton-decoupled 13C NMR spectra of tissue extracts were obtained on a 14T magnet using TopSpin software (Bruker 600; Billerica, MA). The contributions of each labeled substrate (i.e., 13C fatty acids and 13C glucose) and the total of the unlabeled exogenous and endogenous substrates to oxidative metabolism were determined by modeling the tricarboxylic acid cycle flux using the peak areas of the C3 and C4 13C isotopomers of glutamate (tcaCALC, Dallas, TX).
Analysis of Lipid Extracts
TAG Content
Myocardial lipids were extracted from perfused and nonperfused heart tissues with 2:1 chloroform:methanol. The chloroform layer was dried under a nitrogen stream. The residue was resuspended in 0.5% TritonX/isopropanol. TAG content was measured using a commercially available kit (Wako Chemicals, Richmond, VA). Values were normalized to frozen tissue weight.
Determination of TAG Composition and 13C Enrichment
Neutral lipids were separated from frozen heart tissue using a modified Folch method. In brief, frozen tissue was homogenized in 2:1 chloroform:methanol. After centrifugation, the chloroform layer was removed and injected into a Bond Elut NH2 solid-phase extraction (SPE) column (Agilent Technologies, Santa Clara, CA) (23). The neutral lipid fraction was obtained by rinsing the column with 2:1 chloroform:2-propanol. After drying under nitrogen, the residue was reconstituted in 2.5% H2SO4 in methanol to create fatty acid methyl esters (FAMEs). The samples were analyzed via gas chromatography–mass spectrometry (QP2010; Shimadzu Corporation). Identification of individual FAMEs was confirmed by the use of commercially available standards (Sigma Aldrich, St. Louis, MO). The enrichment of each fatty acid species was calculated by determining the ratio of 13C fragments to 12C fragments and then multiplied by the relative content. This value was used to calibrate the NMR spectra generated in the dynamic 13C NMR spectroscopy experiments to plot the enrichment amount over time. Incorporation rates were calculated by multiplying the enrichment rate by the TAG content.
Detection of Ceramide Species
Ceramide species were detected in lipid extracts from hearts freeze clamped at the end of baseline, ischemia, and reperfusion. In brief, hearts were homogenized in 2:1 chloroform:methanol. The chloroform layer was dried under a nitrogen stream. Lipids were resuspended in methanol and analyzed via liquid chromatography–tandem mass spectrometry (API4000; AB Sciex, Framingham, MA) in positive-ion mode. Ceramide species were confirmed via the use of commercially available standards (Avanti Polar Lipids, Alabaster, AL). N-palmitoyl-d31-D-erythro-sphingosine (C16:d31 ceramide) was used as an internal standard added to all samples before homogenization. The peak area of each ceramide species was normalized to the peak of the internal standard for all samples and then normalized to the mass of frozen tissue.
RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from frozen left ventricular (LV) tissue using the RNeasy kit (Qiagen, Valencia, CA). Omniscript reverse synthase and random hexamers were used for cDNA synthesis according to the manufacturer’s guidelines. Real-time PCR was performed using SYBR green (Bio-Rad, Hercules, CA). Results of mRNA levels were normalized to 18S rRNA levels and reported as fold change over CON.
Transthoracic Echocardiography
Murine transthoracic echocardiography was conducted in 12-month-old mice using a VEVO 770 High-Resolution Imaging System (VisualSonics, Toronto, Canada) machine and a 30-mHz probe as previously described (25). Echocardiography measures were performed while the mice were under anesthesia with 1% isoflurane.
Caspase-3 Activity Assay
Caspase-3 activity was measured in perfused hearts freeze-clamped at the end of baseline, ischemia, and reperfusion for both the LCFA and palmitate perfusion conditions. Frozen hearts were homogenized in 25 mmol/L HEPES (pH = 7.5), 5 mmol/L MgCl2, 1 mmol/L EGTA, and 2% protease inhibitor cocktail as previously described (26). Protein concentration was measured via Lowry Assay. Caspase activity was measured in heart tissue lysates using the Caspase-Glo 3/7 Assay kit (Promega, Madison, WI) per the manufacturer’s instructions. The data presented are the sample luminescence minus the background. Values are shown as relative light units (RLUs × 103).
Data Analysis
Results are expressed as means ± SEM. Statistical analyses were performed using GraphPad Prism software. Comparisons between two groups were made by a two-tailed Student t test. For comparisons made during time course experiments, a repeated-measures ANOVA followed by a Bonferroni post hoc test was used. For group comparisons with multiple conditions, a two-way ANOVA followed by a Bonferroni post hoc test was performed. P < 0.05 was considered statistically significant.
Results
Cardiac-Specific Overexpression of DGAT1 Increases Endogenous TAG Turnover Without Affecting Cardiac Function or Substrate Metabolism
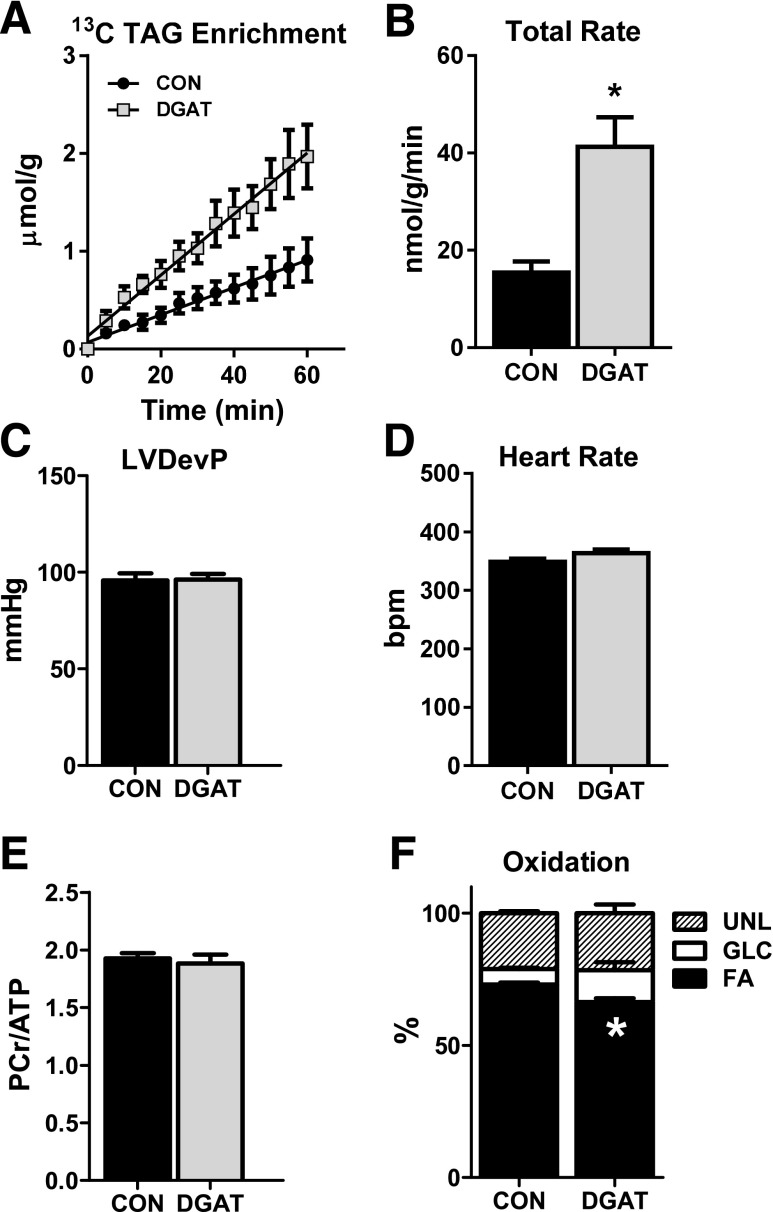
For evaluation of the effect of DGAT1 overexpression on cardiac function, metabolism, and myocardial energetics, isolated perfused heart experiments were performed in conjunction with NMR spectroscopy. With use of dynamic 13C NMR spectroscopy, the incorporation of 13C-labeled LCFAs into the TAG pool was monitored (Fig. 1A). The enrichment of 13C LCFAs into the TAG pool was linear and did not reach a plateau after 60 min (Fig. 1B). Gas chromatography–mass spectrometry analysis of lipid extracts from MHC-DGAT1 and CON hearts revealed ∼2.4-fold increase in the incorporation rate of 13C LCFAs into the TAG pool, confirming elevated synthesis of TAGs via DGAT1 overexpression (Fig. 1C). Greater TAG synthesis was attributed to increased esterification of palmitate and oleate, consistent with the concentrations of these fatty acids in the perfusate (Fig. 1D). TAG content of nonperfused MHC-DGAT1 hearts was ∼2.5-fold higher than CON, consistent with previous reports (19,20); however, since the TAG content did not change significantly during the perfusion period (Fig. 1E), the incorporation rates are reflective of TAG turnover rates. Despite the increased cardiac TAG turnover rates, the ∼2.5 fold increase in the expression of the DGAT1 gene did not significantly alter the expression of the DGAT2 isoform or drastically alter the expression of other genes involved in lipid metabolism (Fig. 1F and G). Increased DGAT1 expression was associated with a significant increase in two isoforms of stearoyl CoA desaturase (SCD1 and SCD2) (Fig. 1F). The upregulation of SCD isoforms may be a consequence of increased TAG muscle content, which has been reported in both pathological and physiological conditions in humans (27,28), rodents (29,30), and cell models (31), although the significance in the heart has not been elucidated.
Figure 1.
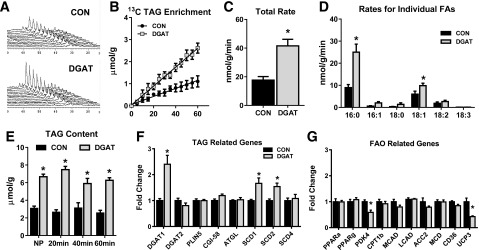
The effect of DGAT1 overexpression on cardiac TAG synthesis, content, and metabolic gene expression. A: Representative spectra from dynamic 13C NMR spectroscopy in isolated hearts perfused with a mixture of 13C LCFAs. B: Enrichment of 13C fatty acids into the TAG pool in lipid extracts from isolated perfused hearts (n = 3–4). C: Total incorporation rate of LCFAs into the TAG pool in isolated perfused hearts (n = 4–6). D: Incorporation rates for individual fatty acids (FAs) into the TAG pool. Rates for palmitate (16:0), palmitoleate (16:1), stearate (18:0), oleate (18:1), linoleate (18:2), and linolenate (18:3) are shown (n = 4–6 for each). E: TAG content measured in lipid extracts from nonperfused hearts (NP) and in isolated hearts perfused for 20, 40, or 60 min. Content is normalized to frozen heart tissue weight (n = 4–5). F and G: Expression of genes related to the TAG pathway and lipid metabolism in CON and MHC-DGAT1 (DGAT) hearts assessed via RT-PCR. *P < 0.05 vs. CON.
The increased TAG turnover in MHC-DGAT1 hearts did not adversely affect isolated heart function, substrate metabolism, or myocardial energetics (Fig. 2A–C), suggesting that the decreased gene expression of PDK4 and UCP3 did not lead to alterations in the metabolic phenotype. Furthermore, cardiac-specific overexpression of DGAT1 did not significantly affect the heart weight normalized to tibia length or cardiac function and morphometry, assessed by echocardiography, in mice up to 12 months of age (Fig. 2D–H). Overall, these results demonstrate that overexpression of DGAT1 enhances cardiac TAG turnover without negatively affecting function, substrate metabolism, or myocardial energetics. Thus, unlike increased cardiac TAG associated with high-fat diets (32), DGAT1-mediated increased TAG did not alter glucose use.
Figure 2.
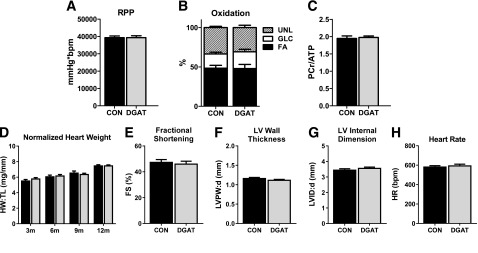
DGAT1 overexpression does not adversely affect cardiac function or energetics. A: RPP (the product of LVDevP and heart rate) measured in Langendorff isolated perfused hearts (n = 28–32). B: Percent oxidation of fatty acids (FA), glucose (GLC), and unlabeled substrates (UNL) (lactate, glycogen, and TAG) in extracts from isolated perfused hearts analyzed by 13C NMR spectroscopy (n = 7 each group). C: PCr-to-ATP ratio (PCr/ATP) measured by 31P NMR spectroscopy in isolated perfused hearts (n = 8–9). D: Heart weight normalized to tibia length (HW:TL) in CON and MHC-DGAT1 (DGAT) mice at 3 months (n = 11–12), 6 months (n = 5–7), 9 months (n = 4 each), and 12 months (n = 8 each) of age. E–H: Fractional shortening (FS), LV posterior wall thickness at diastole (LVPW:d), LV internal dimension at diastole (LVID:d), and heart rate (HR) in 12-month-old CON and DGAT mice assessed by echocardiography (n = 3–4).
Improved Functional Recovery After Ischemic Stress in DGAT Hearts
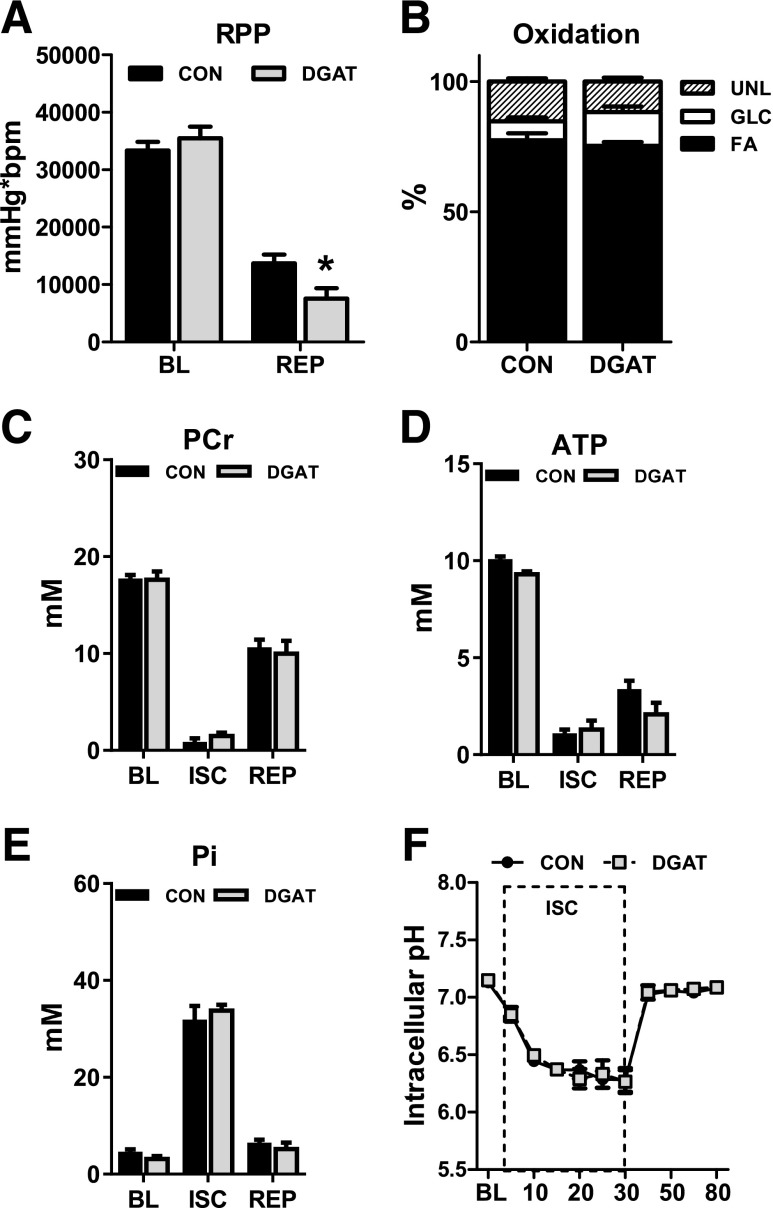
For determination of whether endogenous TAG metabolism is important during acute cardiac stress, isolated hearts from CON and MHC-DGAT1 were subjected to an I-R protocol using the Langendorff preparation in conjunction with multinuclear NMR spectroscopy. After 30 min of low-flow ischemia, MHC-DGAT1 hearts demonstrated a significant improvement in LV developed pressure (LVDevP) during the reperfusion period (Fig. 3A). Heart rates did not differ from CON (Fig. 3B). As a result, rate pressure product (RPP) was also significantly improved with no changes in LV end-diastolic pressure (Fig. 3C and D). The improved function during reperfusion was not associated with a better recovery of high-energy phosphate content (Fig. 3E–G) or changes in intracellular pH (Fig. 3H).
Figure 3.
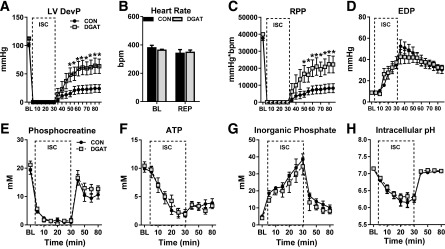
Improved recovery after low-flow ischemia in MHC-DGAT1 hearts. A–D: LVDevP, heart rate, RPP, and LV end diastolic pressure (EDP) in perfused hearts during 30 min of low-flow ischemia (ISC) (1% of baseline [BL]) followed by 55 min of reperfusion (REP). Hearts were perfused with a mixed substrate buffer consisting of 5.5 mmol/L glucose, 0.4 mmol/L LCFAs, 1.2 mmol/L lactate, and 50 μU/mL insulin (n = 7–8 at each time point). E–G: PCr, ATP, and Pi content measured with 31P NMR spectroscopy in isolated perfused hearts (n = 5 for each group at each time point). H: Intracellular pH assessed by 31P NMR spectroscopy in isolated perfused hearts, calculated by the chemical shift between Pi and PCr (n = 5 each group). *P < 0.05 vs. CON.
Enhanced TAG Turnover During Reperfusion Enhances Function
Consistent with previous reports (3,4,14), TAG content in CON hearts remained relatively constant during I-R (Fig. 4A). Interestingly, TAG content in MHC-DGAT1 hearts was depleted by 50% of baseline levels during ischemia and remained similar to CON during reperfusion (Fig. 4A). TAG incorporation rates during reperfusion were reduced by ∼25% compared with baseline values in both CON and DGAT hearts. Despite this, MHC-DGAT1 hearts maintained an ∼2.2-fold higher TAG incorporation rate, primarily via increased palmitate and oleate incorporation (Fig. 4B and C). Fatty acid oxidation during reperfusion was elevated by ∼40% in both CON and MHC-DGAT1 hearts (compared with Fig. 2B), but no significant differences in substrate oxidation were observed between the groups (Fig. 4D). These data show that endogenous TAG metabolism is mildly inhibited during reperfusion after ischemic stress, consistent with increased oxidation of exogenous fatty acids. However, the dynamic nature of endogenous TAG metabolism persists during reperfusion, as a significant movement of fatty acids into the TAG pool is still observed. Importantly, maintaining elevated rates of TAG turnover during reperfusion is associated with a significant improvement in functional recovery after acute cardiac ischemia.
Figure 4.
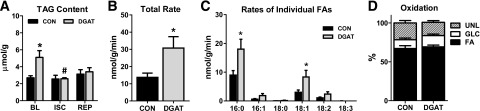
Increased TAG turnover during reperfusion in MHC-DGAT1 hearts. A: TAG content measured in lipid extracts from perfused hearts freeze-clamped at the end of baseline (BL), ischemia (ISC), or reperfusion (REP) (n = 3–8 per group at each time point). B: Total incorporation rate of LCFAs into the TAG pool during reperfusion (n = 6–7). C: Incorporation rates of the individual fatty acids into the TAG pool during reperfusion in isolated perfused hearts. Rates for palmitate (16:0), palmitoleate (16:1), stearate (18:0), oleate (18:1), linoleate (18:2), and linolenate (18:3) are shown (n = 6–7 for each). D: Percent oxidation of fatty acids (FA), glucose (GLC), and unlabeled substrates (UNL) (lactate, glycogen, and TAG) during reperfusion in extracts from isolated perfused hearts analyzed by 13C NMR spectroscopy (n = 6–7). *P < 0.05 vs. CON; #P < 0.05 vs. MHC-DGAT1 (DGAT) at BL.
Palmitate Impairs Recovery in DGAT Hearts Subjected to Ischemic Stress
In the aforementioned I-R experiments using a mixture of LCFAs, the incorporation rate of palmitate and oleate accounted for ∼85% of the total in both CON and MHC-DGAT1 hearts. Since palmitate has been implicated as a cytotoxic lipid (31,33), it is possible that the higher rate of palmitate incorporation into the TAG pool during reperfusion was responsible for the facilitated recovery in MHC-DGAT1 hearts. Therefore, the isolated heart perfusion experiments were repeated using palmitate as the sole source of fatty acids. Similar to hearts perfused with mixed LCFAs, the linear enrichment of 13C palmitate into the TAG pool, as well as the incorporation rate, was elevated in MHC-DGAT1 hearts compared with CON (Fig. 5A and B). Cardiac function and myocardial energetics were similar between MHC-DGAT1 and CON hearts (Fig. 5C–E). The enhanced endogenous TAG metabolism in MHC-DGAT1 hearts was associated with an ∼10% decrease in the oxidation of exogenous palmitate (P < 0.05) (Fig. 5F), although fatty acid oxidation in both groups was ∼40–50% higher with palmitate compared with LCFA condition (Fig. 2B).
Figure 5.
Effect of palmitate on TAG metabolism. A: Enrichment of 13C palmitate into the neutral lipid fraction in lipid extracts from isolated perfused hearts (n = 4 each group). B: Incorporation rate of 13C palmitate into the TAG pool in isolated perfused hearts (n = 7–9). C and D: LVDevP and heart rate measured in Langendorff isolated perfused hearts. Hearts were perfused with a mixed substrate buffer consisting of 5.5 mmol/L glucose, 0.4 mmol/L palmitate, 1.2 mmol/L lactate, and 50 μU/mL insulin (n = 31–32). E: PCr-to-ATP ratio (PCr/ATP) measured by 31P NMR spectroscopy in isolated perfused hearts (n = 10–12). F: Percent oxidation of fatty acids (FA), glucose (GLC), and unlabeled substrates (UNL) (lactate, glycogen, and TAG) in extracts from isolated perfused hearts analyzed by 13C NMR spectroscopy (n = 3 each group). *P < 0.05 vs. CON.
The I-R protocol was repeated with the expectations that MHC-DGAT1 hearts would also demonstrate improved recovery. However, surprisingly, functional recovery after 30 min of low-flow ischemia was impaired in MHC-DGAT1 hearts (Fig. 6A). Fatty acid and glucose oxidation during the reperfusion period was not different in CON and MHC-DGAT1 hearts (Fig. 6B), with no changes in PCr, ATP, or Pi during ischemia or reperfusion (Fig. 6C–E). Intracellular pH remained similar between the groups (Fig. 6F).
Figure 6.
Palmitate impairs functional recovery in MHC-DGAT1 hearts. A: RPP measured at the end of baseline equilibration (BL) and at the end of reperfusion (REP) in isolated perfused hearts from CON and MHC-DGAT1 (DGAT) hearts. Hearts were perfused with a mixed substrate buffer consisting of 5.5 mmol/L glucose, 0.4 mmol/L palmitate, 1.2 mmol/L lactate, and 50 μU/mL insulin during the entire protocol (n = 7 each group). B: Percent oxidation of fatty acids (FA), glucose (GLC), and unlabeled substrates (UNL) (lactate, glycogen, and TAG) during reperfusion in extracts from isolated perfused hearts analyzed by 13C NMR spectroscopy (n = 3–4). C–E: PCr, ATP, and Pi content measured with 31P NMR spectroscopy in isolated perfused hearts at the end of baseline equilibration, end of ischemia (ISC), and end of reperfusion (n = 4 each group at each time point). F: Intracellular pH assessed by 31P NMR spectroscopy in isolated perfused hearts, calculated by the chemical shift between Pi and PCr (n = 4 each group). *P < 0.05 vs. CON.
Impaired TAG Synthesis Leads to Ceramide Accumulation in Hearts Perfused With Palmitate
In contrast with what was observed in nonischemic hearts, the elevated rates of palmitate incorporation into the TAG pool in MHC-DGAT1 hearts were suppressed to levels similar to CON hearts during the reperfusion period (Fig. 7A). MHC-DGAT1 hearts remained vulnerable to the ischemia-induced lipolysis observed in the previous LCFA perfusion experiments, as TAG content at the end of I-R was similar in both CON and MHC-DGAT1 hearts, despite ∼2.5-fold increase at baseline (Fig. 7B). The lower functional recovery in MHC-DGAT1 hearts perfused with palmitate was associated with a significant increase in caspase-3 activity (Fig. 7C). In addition, there was a significant increase in total ceramide content, specifically, C16 ceramide and its precursor C16 dihydroceramide, during the reperfusion period in MHC-DGAT1 hearts perfused with palmitate (Fig. 7D–F). Notably, the increase of caspase-3 activity, total ceramides, or C16 ceramide did not occur in MHC-DGAT1 hearts perfused with the mixture of LCFAs (Fig. 7C–E). These data show that palmitate, but not a physiologic mixture of LCFA, leads to an accumulation of ceramides during the reperfusion period, which is exacerbated in MHC-DGAT1 hearts that are vulnerable to ischemia-induced lipolysis.
Figure 7.
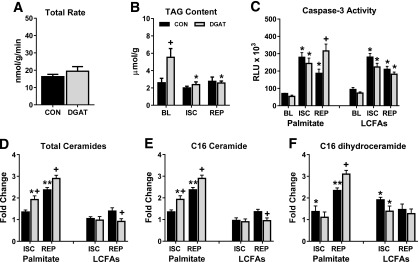
TAG incorporation, lipolysis, and ceramide accumulation in MHC-DGAT1 hearts perfused with palmitate. A: Incorporation rate of palmitate into the TAG pool during the reperfusion period in isolated perfused hearts (n = 6–7). B: TAG content measured in lipid extracts from perfused hearts freeze-clamped at the end of baseline (BL), ischemia (ISC), or reperfusion (REP) (n = 5–8 per group at each time point). C: Caspase-3 activity measured in tissue lysates from isolated hearts perfused with palmitate or LCFAs at the end of baseline, ischemia, and reperfusion. Values shown are relative light units (RLU × 103; n = 3–6 at each time point). D–F: Total abundance of ceramides, palmitoyl-ceramide (C16 ceramide), and palmitoyl-dihydroceramide (C16 dihydroceramide) in isolated hearts perfused with palmitate or LCFAs at the end of ISC and REP. Values are expressed as fold change relative to CON hearts at baseline (n = 4–5 at each time point). *P < 0.05 vs. baseline; **P < 0.05 vs. ischemia; +P < 0.05 vs. CON at same time point.
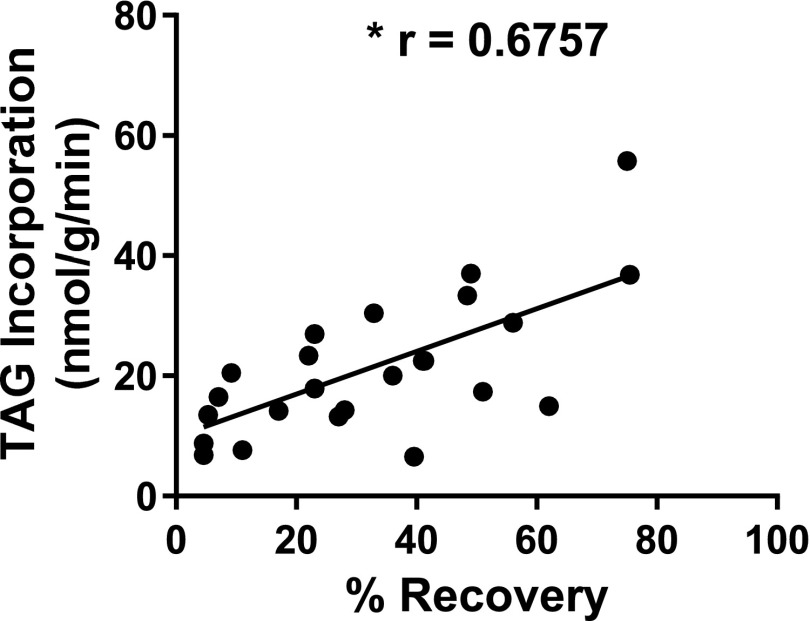
TAG Turnover Correlates With Recovery From Ischemia
For confirmation of the relationship of TAG metabolism and recovery from ischemia, a correlation analysis was performed from the data obtained from both CON and MHC-DGAT1 hearts. As shown in Fig. 8, the rate of TAG incorporation during reperfusion is strongly correlated (r = 0.6757) with the percent recovery of LVDevP. These results demonstrate that enhanced TAG turnover may be a critical factor in determining the recovery of cardiac function from ischemic stress.
Figure 8.
TAG incorporation rates are strongly correlated with recovery in MHC-DGAT1 hearts. Pearson correlation for TAG incorporation versus percent recovery of LVDevP for both CON and DGAT hearts (n = 25).
Discussion
The current study reveals several key findings with regard to endogenous cardiac TAG metabolism under both baseline (i.e., normoxia) and I-R conditions. First, increased TAG content and turnover as a result of DGAT1 overexpression do not adversely affect cardiac function, energetics, or the oxidation of exogenous substrates. Second, DGAT1 overexpression significantly increased the incorporation rates of various LCFAs into the TAG pool. Third, our data show that maintaining elevated TAG turnover rates during reperfusion after acute ischemia is cardioprotective, in part, by sequestering fatty acids into the TAG pool and reducing the accumulation of ceramides. Last, we show that when palmitate is the sole source of exogenous fatty acids during the reperfusion period, there are deleterious effects on recovery from ischemia in DGAT1 transgenic hearts. All told, our findings demonstrate an important role of endogenous cardiac TAG metabolism in determining outcomes of cardiac stress. Moreover, they imply that stored TAG is beneficial in I-R and that greater fatty acid oxidation is not necessarily harmful.
The prevailing dogma is that increased accumulation of cardiac TAG content is associated with cardiac dysfunction and is a hallmark of cardiac lipotoxicity. Our current work as well as previous work (19,20) clearly shows that increasing TAG content by greater than twofold via DGAT1 overexpression does not impair cardiac function in mice and does not, on its own, induce a lipotoxic phenotype. In fact, the combined results of the DGAT1 transgenic studies are comparable with the skeletal muscle phenotype in athletes and exercise-trained rodents where an upregulation of DGAT1 leads to increased TAG content, while other lipid intermediates, namely ceramides, are reduced (19,27,34,35). In contrast, a recent report showed that cardiac-specific deletion of DGAT1 in mice led to an accumulation of toxic lipids (i.e., DAG and ceramides) and reduced cardiac function, which was associated with increased mortality (36). Overall, these findings suggest that elevated TAG content does not contribute to an unfavorable phenotype if accompanied by an enhanced turnover of the TAG pool and a reduced accumulation of potentially toxic lipid intermediates such as DAG and ceramides.
Several previous studies have utilized the novel method of dynamic 13C NMR spectroscopy to understand the movement of fatty acids into the TAG pool in both the normal and diseased heart (2,5,24). Our findings extend the previous work by examining the movement of several LCFAs, differing in chain length and degree of saturation, into the cardiac TAG pool. Our results suggest that the exogenous supply of various LCFAs, if provided simultaneously, is incorporated into the TAG pool in a concentration-dependent manner. Moreover, upregulation of DGAT1 generally leads to expected increases in incorporation rates for all fatty acids.
Recently, several mouse models have been developed to elucidate the consequences of altered TAG metabolism on cardiac function, particularly via the primary enzyme of TAG lipolysis, adipose triglyceride lipase (ATGL). In general, the studies show that deletion of ATGL leads to a cardiac lipotoxic phenotype (37,38), while overexpression of ATGL is beneficial during pressure overload or type 1 diabetes (39,40). These findings are similar to the reports in DGAT1 mouse models where overexpression is beneficial and deletion is not (19,20,36). However, reports from ATGL mice yield paradoxical observations with regard to substrate oxidation in that both the overexpression and the deletion models have decreased fatty acid oxidation (38,41). Here, we show that overexpression of DGAT1 has minimal to no effect on substrate preference, as the percentage of acetyl CoA arising from fatty acids is similar in both CON and MHC-DGAT1 hearts during baseline and reperfusion conditions. Previous studies have reported a greater than 10-fold difference between the rate of oxidation of exogenous palmitate and the rate of incorporation or oxidation of endogenous palmitate (2,14,23,42), which becomes more discrepant during the reperfusion condition (14). Results of the current study are consistent with these observations. Thus, it is not surprising that we did not observe a significant difference in substrate selection by increasing DGAT activity. Nevertheless, the conclusions of the combined studies suggest that upregulation of either DGAT1 or ATGL has the potential to alleviate dysfunction associated with cardiac stress.
From our data, it is clear that maintaining elevated TAG cycling via DGAT1 overexpression during the reperfusion period is cardioprotective. A greater flux of fatty acids into the TAG pool was achieved primarily by palmitate and oleate in MHC-DGAT1 hearts perfused with a physiologic mixture of LCFAs. This enhanced movement of fatty acids into the TAG pool appears to be an important determinant of myocardial function after ischemia, which could be related to the diversion of fatty acids from other lipids pathways, such as de novo ceramide synthesis. Indeed, when only palmitate was provided and the flux of fatty acids into the TAG pool was reduced in MHC-DGAT1 hearts, the cardioprotective phenotype was lost and, in fact, reversed. The reduced movement of palmitate into the TAG pool during the reperfusion period combined with the amount of palmitate lost during ischemia-induced lipolysis likely represented a palmitate overload condition in MHC-DGAT1 hearts and led to significantly increased ceramide content, specifically, C16:0 ceramide. The accumulation of ceramides during I-R has previously been noted in cardiac tissue (16–18,43,44) and is attenuated in hearts exposed to ischemic preconditioning in both in vivo and ex vivo models (16,43,44). Previous work has shown that palmitoyl (C16:0) and stearoyl (C18:0) have the highest abundance both at baseline and in I-R conditions (16). Here, we specifically implicate a role of C16:0 ceramide in determining the functional outcome of ischemic stress. We suspect that palmitate released from the TAG pool during ischemia-induced lipolysis in MHC-DGAT1 hearts is, in part, contributing to the increased C16:0 ceramide, which is further elevated during reperfusion with palmitate.
A surprising finding from our studies is the accelerated lipolysis of the TAG pool in MHC-DGAT1 hearts during the ischemic period. Active lipolysis during ischemia has been repeatedly observed without measurable changes in TAG content (12,13,45). However, previous studies have generally discounted the role of endogenous lipid metabolism in cardiac I-R owing to the energetic inefficiency of TAG lipolysis/synthesis (i.e., futile cycling) (12,13,45). However, it should be noted that these studies were performed without the presence of fatty acids in the perfusate and were limited to glycerol released from the heart as the measure of TAG metabolism. In support of previous findings, we did not observe a significant change in TAG content in CON hearts after 30 min of ischemia. However, in MHC-DGAT1 hearts, a significant decrease in TAG content was observed, consistent with accelerated ischemia-induced lipolysis. Previous reports have shown that elevated TAG content in diabetic hearts is also particularly vulnerable to lipolysis during isolated heart perfusions when the exogenous concentration is lower than adapted levels for the condition, indicating greater reliance on the endogenous stores for oxidative metabolism (4,5,14). In this regard, the accelerated lipolysis during ischemia may be an indicator that the DGAT transgenic heart likewise has a greater dependence on endogenously derived fatty acids for metabolism, especially during instances when nutrient supply and oxygen are low. As our data show, this phenomenon presents a double-edged sword depending upon the rate of incorporation of exogenous fatty acids during the reperfusion period.
Our data also present a cautionary tale regarding the exogenous supply of fatty acids, especially in the isolated perfused heart model. When a nonphysiologic palmitate-only-containing perfusate was used, the cardioprotective phenotype from ischemia in MHC-DGAT1 hearts was completely eliminated. This finding highlights the importance of the exogenous supply to the contracting heart and supports previous findings made in cell culture models implicating palmitate as a cytotoxic lipid (31,33). Since many previous isolated heart perfusion studies relied on the use of palmitate as the source of exogenous fatty acids, it is interesting to speculate how the observations may have differed if a physiologic mixture of fatty acids had been used. This detail could be particularly salient in studies involving diabetic hearts. For example, several reports state that diabetic hearts demonstrate reduced function during reperfusion after ischemia when palmitate is the fatty acid provided (46,47). However, infusion of Intralipid, an emulsion high in unsaturated fatty acids, has been associated with improved recovery from ischemia (48,49). Whether Intralipid supply would be an appropriate strategy to improve outcomes in ischemic diabetic hearts is not currently known. However, the importance of unsaturated fatty acids, particularly polyunsaturated fatty acids, has been noted in reducing the risk of coronary heart disease in both humans without diabetes and humans with type 2 diabetes (50,51).
In summary, our data highlight the dynamic nature of endogenous cardiac TAG metabolism and indicate a novel role of enhanced TAG turnover in modulating the response to I-R injury. The movement of fatty acids into the TAG pool during reperfusion does not redirect fatty acids away from fatty acid oxidation but, rather, leads to the attenuation of ceramide production. Finally, the supply of exogenous fatty acids to the stressed heart may be extremely important in determining functional outcomes and deserves additional consideration in experimental design. Taken together, these findings implicate a therapeutic potential of DGAT1 activation after an ischemic event, particularly in obese patients and patients with diabetes with increased cardiac TAG content that may be vulnerable to ischemia-induced lipolysis.
Article Information
Acknowledgments. The authors thank Dale Whittington and Ross F. Lawrence of the University of Washington Mass Spectrometry Core for technical assistance. The authors also thank Lorena Garcia-Menendez of the Mitochondria and Metabolism Center, University of Washington, for assistance with mouse echocardiography.
Funding. This work was supported by grants from the American Heart Association (14SDG18590020 to S.C.K.) and National Institutes of Health (NIH) Heart, Lung, and Blood Institute (HL73029 to I.J.G. and HL110349 and HL118989 to R.T.) and by an NIH Biomedical Research Support Shared Instrumentation grant (S10RR029021 to 14T High Resolution Imaging [HRIM] Core Facility).
Duality of Interest. No potential conflicts of interests relevant to this article were reported.
Author Contributions. S.C.K. and R.T. designed the research. S.C.K. performed the research. L.L. and I.J.G. contributed the mouse models. S.C.K. and R.T. analyzed data and wrote the manuscript. L.L. and I.J.G. reviewed and edited the manuscript. S.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
S.C.K. and R.T. jointly directed this work.
References
- 1.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta 2010;1801:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol 2008;44:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 1991;266:8162–8170 [PubMed] [Google Scholar]
- 4.Saddik M, Lopaschuk GD. Triacylglycerol turnover in isolated working hearts of acutely diabetic rats. Can J Physiol Pharmacol 1994;72:1110–1119 [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab 2006;290:E448–E455 [DOI] [PubMed] [Google Scholar]
- 6.Lopaschuk GD. Metabolic abnormalities in the diabetic heart. Heart Fail Rev 2002;7:149–159 [DOI] [PubMed] [Google Scholar]
- 7.Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S. Myocardial substrate metabolism in obesity. Int J Obes (Lond) 2013;37:972–979 [DOI] [PubMed] [Google Scholar]
- 8.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 1995;270:17513–17520 [DOI] [PubMed] [Google Scholar]
- 9.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 2002;39:718–725 [DOI] [PubMed] [Google Scholar]
- 10.Liu B, el Alaoui-Talibi Z, Clanachan AS, Schulz R, Lopaschuk GD. Uncoupling of contractile function from mitochondrial TCA cycle activity and MVO2 during reperfusion of ischemic hearts. Am J Physiol 1996;270:H72–H80 [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther 1993;264:135–144 [PubMed] [Google Scholar]
- 12.Schoonderwoerd K, Broekhoven-Schokker S, Hülsmann WC, Stam H. Enhanced lipolysis of myocardial triglycerides during low-flow ischemia and anoxia in the isolated rat heart. Basic Res Cardiol 1989;84:165–173 [DOI] [PubMed] [Google Scholar]
- 13.van Bilsen M, van der Vusse GJ, Willemsen PH, Coumans WA, Roemen TH, Reneman RS. Lipid alterations in isolated, working rat hearts during ischemia and reperfusion: its relation to myocardial damage. Circ Res 1989;64:304–314 [DOI] [PubMed] [Google Scholar]
- 14.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover during reperfusion of isolated rat hearts subjected to a transient period of global ischemia. J Biol Chem 1992;267:3825–3831 [PubMed] [Google Scholar]
- 15.Perman JC, Boström P, Lindbom M, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest 2011;121:2625–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beresewicz A, Dobrzyń A, Górski J. Accumulation of specific ceramides in ischemic/reperfused rat heart; effect of ischemic preconditioning. J Physiol Pharmacol 2002;53:371–382 [PubMed] [Google Scholar]
- 17.Bielawska AE, Shapiro JP, Jiang L, et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol 1997;151:1257–1263 [PMC free article] [PubMed] [Google Scholar]
- 18.Cordis GA, Yoshida T, Das DK. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J Pharm Biomed Anal 1998;16:1189–1193 [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem 2009;284:36312–36323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Yu S, Khan RS, et al. Diacylglycerol acyl transferase 1 overexpression detoxifies cardiac lipids in PPARγ transgenic mice. J Lipid Res 2012;53:1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 2012;111:728–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolwicz SC Jr, Tian R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. J Vis Exp. 31 August 2010. [Epub ahead of print]. DOI: 10.3791/2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carley AN, Bi J, Wang X, et al. Multiphasic triacylglycerol dynamics in the intact heart during acute in vivo overexpression of CD36. J Lipid Res 2013;54:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Donnell JM, Alpert NM, White LT, Lewandowski ED. Coupling of mitochondrial fatty acid uptake to oxidative flux in the intact heart. Biophys J 2002;82:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Menendez L, Karamanlidis G, Kolwicz S, Tian R. Substrain specific response to cardiac pressure overload in C57BL/6 mice. Am J Physiol Heart Circ Physiol 2013;305:H397–H402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu D, Li C, Chen Y, et al. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem 2004;279:48434–48442 [DOI] [PubMed] [Google Scholar]
- 27.Amati F, Dubé JJ, Alvarez-Carnero E, et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 2011;60:2588–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulver MW, Berggren JR, Carper MJ, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2005;2:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobrzyn P, Dobrzyn A, Miyazaki M, Ntambi JM. Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. J Lipid Res 2010;51:2202–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogowski MP, Flowers MT, Stamatikos AD, Ntambi JM, Paton CM. SCD1 activity in muscle increases triglyceride PUFA content, exercise capacity, and PPARδ expression in mice. J Lipid Res 2013;54:2636–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003;100:3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005;54:3530–3540 [DOI] [PubMed] [Google Scholar]
- 33.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem 2001;276:14890–14895 [DOI] [PubMed] [Google Scholar]
- 34.Ikeda S, Miyazaki H, Nakatani T, et al. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun 2002;296:395–400 [DOI] [PubMed] [Google Scholar]
- 35.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 2007;117:1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Trent CM, Fang X, et al. Cardiomyocyte-specific loss of diacylglycerol acyltransferase 1 (DGAT1) reproduces the abnormalities in lipids found in severe heart failure. J Biol Chem 2014;289:29881–29891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med 2011;17:1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kienesberger PC, Pulinilkunnil T, Nagendran J, et al. Early structural and metabolic cardiac remodelling in response to inducible adipose triglyceride lipase ablation. Cardiovasc Res 2013;99:442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulinilkunnil T, Kienesberger PC, Nagendran J, Sharma N, Young ME, Dyck JR. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes (Lond) 2014;38:205–215 [DOI] [PubMed] [Google Scholar]
- 40.Pulinilkunnil T, Kienesberger PC, Nagendran J, et al. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 2013;62:1464–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kienesberger PC, Pulinilkunnil T, Sung MM, et al. Myocardial ATGL overexpression decreases the reliance on fatty acid oxidation and protects against pressure overload-induced cardiac dysfunction. Mol Cell Biol 2012;32:740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lahey R, Wang X, Carley AN, Lewandowski ED. Dietary fat supply to failing hearts determines dynamic lipid signaling for nuclear receptor activation and oxidation of stored triglyceride. Circulation 2014;130:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argaud L, Prigent AF, Chalabreysse L, Loufouat J, Lagarde M, Ovize M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol 2004;286:H246–H251 [DOI] [PubMed] [Google Scholar]
- 44.Der P, Cui J, Das DK. Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol 2006;40:313–320 [DOI] [PubMed] [Google Scholar]
- 45.de Groot MJ, Coumans WA, Willemsen PH, van der Vusse GJ. Substrate-induced changes in the lipid content of ischemic and reperfused myocardium. Its relation to hemodynamic recovery. Circ Res 1993;72:176–186 [DOI] [PubMed] [Google Scholar]
- 46.Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: the sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res 1997;34:113–120 [DOI] [PubMed] [Google Scholar]
- 47.Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res 1997;34:104–112 [DOI] [PubMed] [Google Scholar]
- 48.Li J, Iorga A, Sharma S, et al. Intralipid, a clinically safe compound, protects the heart against ischemia-reperfusion injury more efficiently than cyclosporine-A. Anesthesiology 2012;117:836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahman S, Li J, Bopassa JC, et al. Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology 2011;115:242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009;48:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virtanen JK, Mursu J, Voutilainen S, Uusitupa M, Tuomainen TP. Serum omega-3 polyunsaturated fatty acids and risk of incident type 2 diabetes in men: the Kuopio Ischemic Heart Disease Risk Factor study. Diabetes Care 2014;37:189–196 [DOI] [PubMed] [Google Scholar]