Abstract
Although hemoglobin S (HbS) and hemoglobin C (HbC) are well known to protect against severe Plasmodium falciparum malaria, conclusive evidence on their role against infection has not yet been obtained. Here we show, in 2 populations from Burkina Faso (2007–2008), that HbS is associated with a 70% reduction of harboring P. falciparum parasitemia at the heterozygous state (odds ratio [OR] for AS vs AA, 0.27; 95% confidence interval [CI], .11–.66; P = .004). There is no evidence of protection for HbC in the heterozygous state (OR for AC vs AA, 1.49; 95% CI, .69–3.21; P = .31), whereas protection even higher than that observed with AS is observed in the homozygous and double heterozygous states (OR for CC + SC vs AA, 0.04; 95% CI, .01–.29; P = .002). The abnormal display of parasite-adhesive molecules on the surface of HbS and HbC infected erythrocytes, disrupting the pathogenic process of sequestration, might displace the parasite from the deep to the peripheral circulation, promoting its elimination at the spleen level.
Keywords: Burkina Faso, Fulani, hemoglobin C, hemoglobin S, Plasmodium falciparum, infection
Sickle hemoglobin (hemoglobin S [HbS]) results from a single-nucleotide polymorphism (SNP; rs334) of the HBB gene encoding the β-globin chain, leading to an aminoacidic substitution from glutamic acid to valine (sixth codon: GAG → GTG). Individuals with the SS genotype have sickle cell anemia, a highly lethal condition [1].
Nevertheless, HbS maintains a high frequency in sub-Saharan Africa, probably owing to the survival advantage against malaria of individuals with the AS genotype. There is much epidemiological evidence that HbS protects against severe [2–8] and uncomplicated [9–12] Plasmodium falciparum malaria in heterozygosis. Furthermore, multiple studies have shown lower parasite densities during symptomatic malaria in subjects with the AS genotype than for those with AA [13–15]. Similar evidence is not available for protection against infection. A systematic review [16] pointed out that the role of the AS genotype on P. falciparum infection has been investigated mainly through single cross-sectional surveys of the prevalence of parasitemia, yielding conflicting results. Some studies found reduced prevalence in subjects with AS compared with AA [17–20], but others showed a similar [21–30] or even an increased prevalence [31, 32]. The review [16] found only 2 longitudinal studies of the incidence of parasitemia [33, 34], reporting similar rates in subjects with AA and those with AS. More recently, however, a longitudinal study conducted in Uganda showed that children with the AS genotype had a lower number of new strains detected per person-year than those with the AA genotype and that the effect of HbS was greater in older children [35]. A second longitudinal study conducted in Mali showed that children with the AS genotype remained smear-negative for a longer time than those with the AA genotype [36].
Hemoglobin C (HbC) results from a SNP (rs33930165) of the HBB gene leading to an aminoacidic substitution from glutamic acid to lysine (sixth codon: GAG → AAG). Individuals with the AC genotype are asymptomatic, those with the CC genotype have mild hemolytic anemia, and SC double heterozygotes have moderate sickle cell disease [1].
HbC is known to protect against severe malaria in an additive way, with CC homozygotes showing a much higher degree of protection than subjects with the AC genotype [2, 3, 6, 8]. There is not as much evidence of protection conferred by HbC against uncomplicated malaria: CC homozygotes shows protection in 1 study [2], and subjects with AC show protection in case-control [2, 37] but not perspective studies [11, 12]. The role of HbC in parasitemia has been addressed by 6 cross-sectional studies [21, 23, 25, 31, 38] and 1 longitudinal study [12], none of which provided evidence for protection in subjects with the AC genotype [16]. A reduced prevalence of P. falciparum infection was observed in subjects with CC or SC genotypes in only 1 cross-sectional study conducted in Ghana [25], but differences did not reach statistical significance.
Our study aimed to provide new insights into the protective role of HbS and HbC against P. falciparum infection. We conducted 5 cross-sectional surveys in rural villages of Burkina Faso inhabited by the Fulani, Mossi, and Rimaibe communities. The Fulani were reported elsewhere to be less infected with P. falciparum and to have a different genetic background from their neighbors, whereas the Mossi and Rimaibe show comparable susceptibility to infection, are genetically similar [39, 40], and will be thus grouped together as non-Fulani.
METHODS
Ethics Statement
The study received approval from the ethical committees of the Ministry of Health of Burkina Faso and the University of Oxford. Study subjects or their guardians gave written informed consent for participation.
Study Area and Populations
The study was carried out in 4 rural villages of Burkina Faso, northeast (Barkoumbilen and Barkoundouba) and east (Bassy and Zanga) of Ouagadougou. Malaria transmission is hyperendemic and seasonal, with a rainy season from June to October. The entomological inoculation rates, estimated at about 100–200 infective bites per person per year, are similar across villages [39].
Study Design and Epidemiological Surveys
The study had a repeated cross-sectional design: 5 surveys were carried out, at the beginning (August) and end (November/December) of the 2007 and 2008 high malaria transmission seasons and in the middle of the intervening dry low transmission season (March 2008). For each survey a team of physicians examined participants for clinical signs, measured axillary body temperature, and prepared blood slides for malaria diagnosis from finger pricks. Subjects exhibiting fever (temperature, ≥37.5°C) were treated presumptively with artemether-lumefantrine (Coartem) according to manufacturer's dosage recommendations. No treatment was offered to carriers of asymptomatic parasitemia. During the first survey when a subject entered the study, a 2-mL venous blood sample was collected in ethylenediaminetetraacetic acid tubes for DNA extraction.
Parasitological Diagnosis
Plasmodium falciparum asexual parasitemia was microscopically diagnosed. Blood slides with thick and thin blood smears were prepared and stained with Giemsa stain according to standard procedures and read independently by 2 skilled microscopists. The Plasmodium species was identified on the thin blood smear. Readers examined 100 microscopic fields (corresponding to 0.25 µL of blood) from the thick blood smear, parasite counts were converted to numbers of parasites per microliter of blood (assuming a standard count of 8000/μL), and the mean density from 2 readings was used. A third reader was involved when the 2 readers disagreed about positivity or when estimated densities differed by >30%. In these cases, the mean of the 2 closest density readings was used.
Microscopic diagnosis was confirmed on DNA samples by polymerase chain reaction amplification of P. falciparum EBA175 and TRAP sequences (Supplementary Methods). Across all surveys, P. falciparum accounted for 91.4% of malaria infections, followed by P. malariae (2.1%) and P. ovale (0.2%); 6.3% were mixed infections. Of the P. falciparum infections, 4.5% were symptomatic (parasitemia associated with fever).
DNA Extraction and HBB Genotyping
Genomic DNA was extracted from whole blood using the Nucleon BACC2 Kit. The rs334 (A → HbA/T → HbS) and rs33930165 (G → HbA /A → HbC) SNPs at the HBB locus were genotyped using the Sequenom MassArray System [41]. Genotypes at the 2 SNPs were combined to obtain the HBB genotypes: HbAA (AA + GG), HbAC (AA + AG), HbAS (AT + GG), HbCC (AA + AA), HbSC (AT + AG), and HbSS (TT + GG). Polymerase chain reaction [42] and sequencing (BMR Genomics) were used to validate genotype calls for the rare genotypes CC, SC, and SS (expected frequency, <1%). The genotyping assays had 97% and 98% success rates respectively, resulting in a complete HBB genotype for 95% of recruited subjects. Genotypes were in Hardy–Weinberg equilibrium within each population.
Statistical Analysis
Statistical analysis was conducted both at the single survey level as well as longitudinally, and outcome data were compared between subjects with AC, AS, CC, SC, and AA (reference) genotypes. At each of the 5 cross-sectional surveys (n = 1162, n = 1595, n = 1281, n = 1554, and n = 1524, respectively), we compared the prevalence of asymptomatic parasitemia. In longitudinal analyses we included all infection events, regardless of whether or not parasitemia was associated with fever. Among subjects who participated in all surveys (n = 481), we compared the proportion infected at least once, the total number of infections, and the mean parasite density over 5 measurements. Finally, starting from the first survey (n = 1162), we compared the proportion of subjects who remained uninfected over the 5 measurements.
Prevalences were compared using a maximum likelihood estimate of the odds ratios (ORs) and 95% confidence intervals (CIs) within each ethnic group and a Mantel–Haenszel χ2 test stratifying by ethnicity in the overall population. The proportions of individuals infected at least once were compared using logistic regression, the numbers of infections using Poisson regression, and the mean parasite densities using linear regression after logarithmic transformation. The proportions of subjects who remain uninfected over time were compared using survival analysis based on lifetime tables and log-rank test for the equality of survival functions, and the cumulative probabilities of infection were compared using Poisson regression. All regression models included age (in years) as a covariate. Ethnicity (Fulani or non-Fulani group) was included as a covariate when the analysis was conducted in the overall population. Two-sided P values were reported, with differences considered significant at P ≤ .05. All analyses were carried out with the statistical software package Stata/IC 10.0 (StataCorp LP, College Station, Texas).
RESULTS
Frequencies of HbS and HbC
The study included a total of 2206 subjects. The frequencies of HbS and HbC (Table 1) are consistent with previous data in the study populations [2, 43]. The frequency of HbS was 4%, irrespectively of ethnicity, sex, or age group, and the frequency of HbC was lower in Fulani (5%) than in non-Fulani (12%), as reported elsewhere [43]. Because ethnicity [39] and age [44] play a major role in immunity to infection, we adjusted all genotype-phenotype association analyses for these 2 factors.
Table 1.
Characteristics of Study Subjects and Frequency of HbS and HbC
| Factor | Subjects by HBB Genotype, No. |
Frequency (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| AA | AC | AS | CC | SC | Total | HbS | HbC | |
| Ethnicity | ||||||||
| Fulani | 708 | 80 | 80 | 0 | 2 | 870 | 0.05 (.03–.06) | 0.05 (.03–.06) |
| Non-Fulani | 930 | 282 | 98 | 15 | 11 | 1336 | 0.04 (.03–.05) | 0.12 (.10–.14) |
| Sex | ||||||||
| Male | 722 | 166 | 84 | 6 | 6 | 984 | 0.05 (.03–.06) | 0.09 (.08–.11) |
| Female | 916 | 196 | 94 | 9 | 7 | 1222 | 0.04 (.03–.05) | 0.09 (.07–.11) |
| Age group, ya | ||||||||
| ≤5 | 321 | 71 | 30 | 4 | 1 | 427 | 0.04 (.02–.06) | 0.09 (.07–.12) |
| >5 ≤ 10 | 319 | 64 | 27 | 3 | 2 | 415 | 0.03 (.02–.05) | 0.09 (.06–.11) |
| >10 ≤ 20 | 449 | 100 | 53 | 4 | 6 | 612 | 0.05 (.03–.07) | 0.09 (.07–.12) |
| >20 | 549 | 127 | 68 | 4 | 4 | 752 | 0.05 (.03–.06) | 0.09 (.07–.11) |
| Total | 1638 | 362 | 178 | 15 | 13 | 2206 | 0.04 (.04–.05) | 0.09 (.08–.10) |
Abbreviations: CI, confidence interval; HbC, hemoglobin C; HbS, hemoglobin S.
a Based on age at the first survey.
Proportion of P. falciparum–Positive Slides
During 5 surveys conducted from August 2007 to December 2008, we performed 7008 microscopic diagnoses of malaria in asymptomatic subjects (2792 in Fulani and 4216 in non-Fulani subjects). The percentage of P. falciparum–positive slides varied according to HBB genotype: compared with that for subjects with the AA genotype, percentages were lower for subjects with the AS, CC, or SC genotype, in both ethnic groups, whereas for subjects with the AC genotype the percentage was similar (non-Fulani) or even higher (Fulani) than for AA (Table 2).
Table 2.
Proportion of Plasmodium falciparum–Positive Blood Slides According to HBB Genotype
| HBB Genotype by Ethnicity | Subjects, No. |
P. falciparum Positive, % | ||
|---|---|---|---|---|
| P. falciparum Negative | P. falciparum Positive | Total | ||
| Fulani | ||||
| AA | 1734 | 530 | 2264 | 23.4 |
| AC | 183 | 76 | 259 | 29.3 |
| AS | 209 | 55 | 264 | 20.8 |
| SC | 4 | 1 | 5 | 20.0 |
| Total | 2130 | 662 | 2792 | 23.7 |
| Non-Fulani | ||||
| AA | 1566 | 1386 | 2952 | 47.0 |
| AC | 489 | 425 | 914 | 46.5 |
| AS | 159 | 103 | 262 | 39.3 |
| CC | 29 | 20 | 49 | 40.8 |
| SC | 30 | 9 | 39 | 23.1 |
| Total | 2273 | 1943 | 4216 | 46.1 |
Cross-sectional Analysis: Prevalence of Asymptomatic P. falciparum Parasitemia
We therefore stratified the sample by survey to investigate the association of HbS and HbC with the prevalence of P. falciparum asymptomatic infection. The results of the statistical comparison among HBB genotypes are shown in Table 3. The data do not convey any consistent pattern of association with prevalence of infection across surveys or populations. Indeed, in both Fulani and non-Fulani groups, the AC genotype, compared with the wild-type AA genotype, shows similar prevalences (OR ≈ 1) in some surveys and even higher prevalences (OR > 1) in others (difference close to significance in the overall population for the fourth survey). On the contrary, in both populations the AS genotype shows similar prevalences in some surveys but lower prevalences (OR < 1) in others (significant difference in the overall population for the first and fifth surveys). The CC genotype is present only in the non-Fulani group and shows similar prevalences in some surveys and lower prevalences (OR << 1) in others, although the difference never reached statistical significance. Finally, for surveys in which comparison was possible in both populations, the SC genotype showed lower prevalences (OR << 1) than the AA genotype (significant difference in the overall population for the fourth survey).
Table 3.
Statistical Comparison of the Prevalence of P. falciparum Asymptomatic Infection According to HBB Genotype at Each of 5 Cross-sectional Surveys
| HBB Genotype by Survey | Fulani |
Non-Fulani |
Overall |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | OR (95% CI) | P Valueb | |
| AC | ||||||
| 1 | 1.06 (.58–1.92) | .85 | 0.87 (.61–1.23) | .43 | 0.91 (.68–1.24) | .56 |
| 2 | 1.44 (.79–2.62) | .23 | 0.90 (.65–1.24) | .52 | 0.99 (.75–1.32) | .97 |
| 3 | 1.27 (.56–2.84) | .56 | 0.83 (.54–1.27) | .39 | 0.90 (.62–1.31) | .59 |
| 4 | 1.73 (.94–3.20) | .94 | 1.23 (.89–1.71) | .89 | 1.32 (.99–1.76) | .06 |
| 5 | 1.15 (.58–2.27) | .70 | 1.03 (.75–1.42) | .87 | 1.05 (.78–1.40) | .75 |
| AS | ||||||
| 1 | 0.48 (.21–1.09) | .07 | 0.51 (.26–1.00) | .05 | 0.50 (.29–.84) | .01 |
| 2 | 0.87 (.47–1.61) | .66 | 1.13 (.68–1.87) | .63 | 1.02 (.69–1.50) | .93 |
| 3 | 1.33 (.59–2.99) | .49 | 1.03 (1.53–1.99) | .93 | 1.14 (.68–1.89) | .63 |
| 4 | 1.12 (.61–2.08) | .71 | 0.64 (.36–1.13) | .12 | 0.83 (.54–1.25) | .37 |
| 5 | 0.85 (.43–1.70) | .65 | 0.52 (.29–.95) | .03 | 0.64 (.41–1.01) | .05 |
| CC | ||||||
| 1 | … | … | 1.17 (.19–7.06) | .86 | … | … |
| 2 | … | … | 0.45 (.14–1.47) | .17 | … | … |
| 3 | … | … | 1.13 (.29–4.32) | .86 | … | … |
| 4 | … | … | 1.18 (.37–3.75) | .78 | … | … |
| 5 | … | … | 0.57 (.14–2.30) | .42 | … | … |
| SC | ||||||
| 1 | 1.61 (.10–26.17) | .73 | 0.19 (.02–1.77) | .10 | 0.40 (.09–1.88) | .23 |
| 2 | … | 0.50 (.12–2.03) | .33 | … | … | |
| 3 | … | 1.00 (.20–5.04) | >.99 | … | … | |
| 4 | 0.00 (Nc) | .55 | 0.09 (.01–.75) | .01 | 0.09 (.01–.73) | .00 |
| 5 | 0.00 (Nc) | .46 | 0.46 (.09–2.37) | .34 | 0.39 (.07–2.02) | .24 |
“…” indicates there were no CC subjects in the Fulani ethnic group.
Abbreviations: CI, confidence interval; Nc, not computed; OR, odds ratio.
a Maximum likelihood estimation of the OR.
b P values determined with Mantel–Haenszel χ2 test.
Longitudinal Analysis of Repeated Cross-sectional Data: Odds of P. falciparum Infection, Number of Infections, and Mean Parasite Counts Over 5 Measurements
The prevalence of P. falciparum parasitemia at a given moment in a given population can vary with the measurement itself as well as with many environmental (eg, transmission level) or parasite (eg, strains with different abilities to establish infection) factors other than host factors. Therefore, it might not be the most appropriate phenotype to evaluate the role of human genetic variants in protection from infection, which is why we used what we believe to be a more robust phenotype indicating susceptibility/resistance: the odds of being infected with P. falciparum at least once during 5 surveys.
We included in the analysis individuals included in all 5 surveys (n = 481). We defined as not infected those subjects who never had a diagnosis of P. falciparum parasitemia, and as infected those who had P. falciparum parasitemia diagnosed at least once (Table 4). In both the Fulani (n = 186) and non-Fulani (n = 295) groups, we observed that the odds of infection for subjects with the AC genotype is comparable to that for those with AA (OR in the overall population, 1.49; 95% CI, .69–3.21; P = .31). This is not the case for subjects with AS, who showed much lower odds than those with AA in both ethnic groups. In the overall population, with adjustment for ethnicity and age, we indeed observed a 70% reduction in the odds of infection for carriers of HbS (OR, 0.27; 95% CI, .11–.66; P = .004).
Table 4.
Proportion of Subjects Infected With Plasmodium falciparum at Least Once Over 5 Surveys According to HBB Genotype and Statistical Comparison
| HBB Genotype by Ethnicity | Subjects, No.a |
OR (95% CI)b | P Valuec | ||
|---|---|---|---|---|---|
| P. falciparum Negative | P. falciparum Positive | P. falciparum Positive , % | |||
| Fulani | |||||
| AA | 49 | 97 | 66.4 | … | … |
| AC | 9 | 14 | 60.9 | 1.13 (.36–3.58) | .83 |
| AS | 11 | 6 | 35.3 | 0.32 (.09–1.06) | .06 |
| Non-Fulani | |||||
| AA | 21 | 181 | 89.6 | … | … |
| AC | 6 | 63 | 91.3 | 1.8 (.62–5.22) | .28 |
| AS | 6 | 12 | 66.7 | 0.24 (.07–.82) | .02 |
| CC | 1 | 1 | 50 | 0.11 (.00–3.40) | .21 |
| SC | 2 | 1 | 33.3 | 0.02 (.00–.28) | .003 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a The P. falciparum–negative group includes subjects not infected with P. falciparum over 5 cross-sectional surveys; the P. falciparum–positive group, subjects infected with P. falciparum at least once over 5 cross-sectional surveys.
b ORs were adjusted for age group and ethnicity.
c P values based on logistic regression for the statistical comparison of odds, where the wild-type AA genotype is used as the reference group.
In the non-Fulani group, we could also observe that individuals with CC or SC genotypes show lower odds of infection than those with AA, although the comparison reaches statistical significance only for SC. It must be noted that these genotype groups are very small. If we combine CC and SC genotypes in a unique group of individuals with double-mutant hemoglobin, these show >90% reduction in the odds of infection (OR for CC + SC vs AA, 0.04; 95% CI, .01–.29; P = .002). It seems therefore that HbS is sufficient in single copy to protect from infection, whereas HbC must be associated with either another copy of HbC or with HbS for protection to be achieved. This is similar to what was observed with respect to the protection conferred against severe malaria, where HbC has an additive effect, and individuals with the CC genotype show a much higher degree of protection than those with AC [2].
It is also interesting to note that the protection seen with AS, CC, and SC genotypes in the non-Fulani group brings the odds of infection to levels comparable to that of individuals with wild-type AA in the Fulani group. The observation that the odds of infection is lower in the Fulani for any HBB genotype, together with the observation that HbS and HbC are present at comparable and lower frequencies, respectively, further confirms that mutant hemoglobins are not responsible for the lower susceptibility to malaria observed in this ethnic group [44]. Comparable results were obtained applying a standard repeated measure approach, using data from the 5 surveys and a mixed logistic regression model including a random effect describing the statistical dependency between measurements from the same individual (Supplementary Table 1).
In the same group of subjects, we also looked at the number of infections measured over 5 surveys according to HBB genotype (data not shown). We observed that the numbers of infections are comparable for subjects with AC and those with AA in both Fulani and non-Fulani groups and in the overall population (incidence rate ratio [IRR], 1.08; 95% CI, .92–1.27; P = .33). However, the numbers of infections are lower for AS than for AA in both ethnic groups, although this difference reached statistical significance only in the overall population (IRR, 0.72; 95% CI, .53–.98; P = .04). In the non-Fulani group, there was a nonsignificant reduction in the number of infections with the CC (IRR, 0.46; 95% CI, .11–1.85; P = .27) and SC (IRR, 0.48; 95% CI, .18–1.28; P = .14) genotypes and also when the 2 genotypes are grouped (IRR, 0.47; 95% CI, .21–1.06; P = .07).
Finally, we compared the mean parasite density observed during the 5 surveys (ie, the mean of 5 trophozoite counts) among HBB genotypes (data not shown). In both study populations, no difference in mean parasite density (log-transformed parasite count per microliter) was observed between AC and AA genotypes (OR, 1.07; 95% CI, .91–1.25; P = .42 in the overall population). A nonsignificant reduction in mean parasite density was observed in both Fulani and non-Fulani groups when comparing AS and AA genotypes, and this difference reached statistical significance in the overall population (OR, 0.78; 95% CI, .62–.99; P = .04). A reduction was observed among the non-Fulani for the CC (OR, 0.48; 95% CI, .18–1.27; P = .14) and SC (OR, 0.39; 95% CI, .18–.87; P = .02) genotypes, although the difference was significant only for SC. A significant protective effect was observed in the combined group of double-mutant individuals (OR, 0.43; 95% CI, .23–.79; P = .007).
Survival Analysis of Repeated Cross-sectional Data: Proportion of Subjects Who Remain P. falciparum Negative
We finally investigated whether HbS and HbC affect the proportion of subjects who remain uninfected over surveys. We included in this analysis all individuals present at the first survey (n = 1163) and followed them up until the end of the study, looking at the first time they became infected or whether they stayed clear of infection.
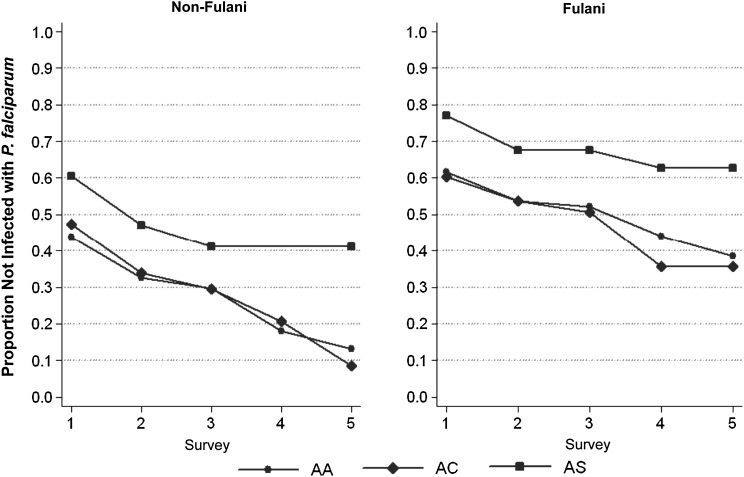
The lifetime table resulting from this analysis is shown in Supplementary Table 2. Because only the first survey contributed information for subjects with CC and SC genotypes, we excluded these individuals from statistical analysis. Survival curves for subjects AA, AC, and AS genotypes are shown in Figure 1, and the results of the log-rank test in Table 5. In both Fulani (n = 401) and non-Fulani (n = 761) groups, we observed that the proportion of individuals with the AC genotype who remain uninfected is always comparable to that of those with AA (hazard ratio, 1.02; 95% CI, .86–1.23; P = .75 in the overall population with adjustment for ethnicity and age). On the contrary the proportion of uninfected individuals is always higher for AS than for AA. In the overall population, subjects carrying the HbS mutation show a lower cumulative probability of becoming infected (hazard ratio, 0.68; 95% CI, .47–.97; P = .03 with adjustment for ethnicity and age). Similar results have been obtained in longitudinal studies conducted in Uganda [35] and Mali [36].
Figure 1.
Lifetime curves showing proportions of individuals who remain uninfected with Plasmodium falciparum across 5 cross-sectional surveys, according to HBB genotype; results are displayed separately for Fulani (right) and non-Fulani (left) groups.
Table 5.
Statistical Comparison of Survival Curves According to HBB Genotype
| HBB Genotype by Ethnicity | Infections Observed, No. | Infections Expected, No. | P Valuea |
|---|---|---|---|
| Fulani | |||
| AA | 159 | 152.1 | … |
| AC | 29 | 29.2 | .66 |
| AS | 12 | 18.9 | .047 |
| Non-Fulani | |||
| AA | 391 | 381.8 | … |
| AC | 121 | 121.6 | .93 |
| AS | 20 | 29.2 | .02 |
a P values were determined with the log-rank test for equality of survival curves, with the wild-type AA genotype used as the reference group.
Modification of HbS Protective Effect by Age Group and Ethnicity
Gong and colleagues [35] observed in their study that the protective effect of HbS increased with age. We therefore stratified our sample into subjects aged ≤10 years and those aged >10 years to investigate whether age modifies the effect of HbS on the odds of infection, number of infections, mean parasite count, and cumulative probability of infection (Supplementary Table 3). For each phenotype analyzed, we observed no protective effect of HbS in the younger group but a significant effect in the older group. We also observed that in subjects aged >10 years the protective effect of HbS (except with respect to mean parasite counts) seemed greater in the Fulani than in the non-Fulani group, although there is no statistical evidence of effect modification (data not shown).
DISCUSSION
This study was intended to elucidate the role of HbS and HbC in susceptibility to P. falciparum infection. One important challenge encountered in such studies is defining an infection phenotype for association analysis. Indeed, the point prevalence of P. falciparum parasitemia might not be a robust phenotype, because it can vary with both measurement and epidemiological factors. Other phenotypes might prove more useful, such as the odds of infection, the number of infections, the mean parasite density over repeated measurements, and the proportion of subject who remain clear of infection over time. These can be based on a series of cross-sectional surveys, as in our study, or, better, from cohort studies.
We actually observed that individuals who are heterozygotes for HbS show lower prevalences of parasitemia than AA homozygotes in some but not every survey, and not always in both study populations. This observation reflects discrepancies among results of studies based on single cross-sectional surveys [16]. In longitudinal analysis, however, AS heterozygotes show lower odds of infection than AA homozygotes, fewer infections, lower mean parasite density, and a higher proportion of subjects who remain clear of infection over time, in both Fulani and non-Fulani groups, suggesting a protective role of HbS against the establishment of P. falciparum parasitemia. These results are consistent with those obtained by Billo and colleagues [36], who observed that a protective role of HbS against infection could be observed only when the data were analyzed longitudinally and not when the same data were analyzed using a cross-sectional approach.
By stratifying the sample by age group, we were able to observe that the protective effect of HbS becomes apparent in subjects aged >10 years, consistently with a recent report by Gong and colleagues [35]. Furthermore, this protective effect is greater in the less susceptible and more immune reactive Fulani population [39, 45]. These 2 observations suggest that the role of HbS is magnified by concomitant immunity of the host. The fact that the effect of HbS is greater at older ages could also indicate, as suggested elsewhere [35], that the mechanism of protection involves acquired immunity. However, because the Fulani are less susceptible to infection on a different genetic basis than HbS, the fact that the effect of HbS is greater in this ethnic group suggests that the mechanism of protection is amplified by, and not only mediated by, the host immune response, either innate or acquired.
With respect to HbC, our data do not provide evidence for protection against any of the phenotypes analyzed at the heterozygote state. Nevertheless, they suggest a protective effect of HbC in a double-mutant state, that is, in CC homozygotes and SC double heterozygotes. The level of protection observed is similar if not higher to that observed in subjects with the AS genotype. However, given the low frequencies of these genotypes (<1%), our study sample size is too small to draw solid conclusions.
Our results suggest a scenario made of consistent experimental and epidemiological observations. The abnormal display of parasite-adhesive molecules (ie, PfEMP1) on the surface of the HbS and HbC infected erythrocytes [46, 47], disrupting the pathogenic process of sequestration, might displace the parasite from the deep to the peripheral circulation, hence promoting its elimination at the spleen level. It has been proposed [48] that the increased phagocytosis of the HbS and HbC infected erythrocytes in the spleen may also result in improved antigen presentation, which could explain an increase of the protective effect against infection with age, as well as the higher immune response to variant surface antigens observed in individuals carrying those hemoglobinopathies [13, 49, 50]. A protective role of HbS and HbC against infection per se is coherent with the observation that both mutant hemoglobins protect against all syndromes of severe malaria (namely, cerebral malaria and severe malarial anemia) across diverse populations [8, 16].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the entire staff of physicians, nurses, and laboratory technicians as well as the data management staff at Centre National of Research and Formation sur le Paludisme for their invaluable skilled work. Among the members of the MalariaGEN Consortium Resource Centre, we thank Anna Jeffreys, Kate Rowlands, Christina Hubbart, Rachel Craik, and Angela Green for the great effort put into DNA samples handling and genotyping and Jennifer Shelton for assembling results of molecular diagnosis of parasitemia. We also thank Jantina De Vries and Miguel SanJoaquin at the MalariaGEN Consortium Resource Centre for useful discussions on ethical aspects of the study and field study implementation, respectively.
Author contributions. V. D. M., Y. K., E. C. B., F. V., A. D., and I. N. conducted field and laboratory activities; C. B., F. S., and P. A. performed laboratory activities; Y. K. coordinated clinical activities; V. D. M., S. B. S., and D. M. designed and coordinated the study; V. D. M. and N. S. analyzed the data; V. D. M. and D. M. wrote the manuscript; K. A. R. coordinated DNA sample handling and genotyping and general support to the study from the MalariaGEN Consortium Resource Centre; and the MalariaGEN Consortium provided support and resources for the study, handled the DNA samples, and performed the genotyping.
Members of the MalariaGEN Consortium are listed at http://www.malariagen.net/resource/1.
Financial support. The field, laboratory, and genotyping activities were funded through the MalariaGEN Project. The MalariaGEN Project is supported by the Wellcome Trust (grant WT077383/Z/05/Z) and the Bill & Melinda Gates Foundation through the Foundation of the National Institutes of Health (grant 566) as part of the Grand Challenges in Global Health Initiative. This research was supported by the Medical Research Council (grant G0600718). The Wellcome Trust also provides core awards to Wellcome Trust Centre for Human Genetics (grant 090532/Z/09/Z) and the Wellcome Trust Sanger Institute (grant 098051). V. D. M. was funded by fellowships from Istituto Pasteur-Fondazione Cenci Bolognetti (Borse Italia 2008) and Evimalar (European Community's Seventh Framework Programme; grant agreement 242095); F. V. was funded by the Italian Malaria Network, sponsored by Compagnia di San Paolo, Turin, Italy; and N. S. was funded by Wellcome Trust (grant 091924) and Fundação Para a Ciência e Tecnologia (through project Pest-OE/MAT/UI0006/2011).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376:2018–31. [DOI] [PubMed] [Google Scholar]
- 2.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 2001; 414:305–8. [DOI] [PubMed] [Google Scholar]
- 3.Mockenhaupt FP, Ehrhardt S, Cramer JP, et al. Hemoglobin C and resistance to severe malaria in Ghanaian children. J Infect Dis 2004; 190:1006–9. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman H, Usen S, Jallow M, Sisay-Joof F, Pinder M, Kwiatkowski DP. A comparison of case-control and family-based association methods: the example of sickle-cell and malaria. Ann Hum Genet 2005; 69(pt 5):559–65. [DOI] [PubMed] [Google Scholar]
- 5.Williams TN, Mwangi TW, Wambua S, et al. Negative epistasis between the malaria-protective effects of α+-thalassemia and the sickle cell trait. Nat Genet 2005; 37:1253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May J, Evans J, Timmann C, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA 2007; 297:2220–6. [DOI] [PubMed] [Google Scholar]
- 7.Jallow M, Teo YY, Small KS, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet 2009; 41:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockett KA, Clarke GM, Fitzpatrick K, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet 2014; 46:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh S, Dorsey G, Rosenthal PJ. Host polymorphisms and the incidence of malaria in Ugandan children. Am J Trop Med Hyg 2004; 71:750–3. [PubMed] [Google Scholar]
- 10.Clark TD, Greenhouse B, Njama-Meya D, et al. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis 2008; 198:393–400. [DOI] [PubMed] [Google Scholar]
- 11.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis 2008; 198:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreuels B, Kreuzberg C, Kobbe R, et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood 2010; 115:4551–8. [DOI] [PubMed] [Google Scholar]
- 13.Marsh K, Otoo L, Hayes R, Carson D, Greenwood B. Antibodies to blood stage antigens of plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg 1989; 83:293–303. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Zeid YA, Theander TG, Abdulhadi NH, et al. Modulation of the cellular immune response during Plasmodium falciparum infections in sickle cell trait individuals. Clin Exp Immunol 1992; 88:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aidoo M, Terlouw DJ, Kolczak MS, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet 2002; 359:1311–2. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison A. Protection afforded by sickle-cell trait against subertian malarial infection. Br Med J 1954; 1:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colbourne MJ, Edington GM. Sickling and malaria in the Gold Coast. Br Med J 1956; 1:784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol 1979; 73:161–72. [DOI] [PubMed] [Google Scholar]
- 20.Ntoumi F, Mercereau-Puijalon O, Ossari S, et al. Plasmodium falciparum: sickle-cell trait is associated with higher prevalence of multiple infections in Gabonese children with asymptomatic infections. Exp Parasitol 1997; 87:39–46. [DOI] [PubMed] [Google Scholar]
- 21.Edington GM, Lang WN. Relationship between haemoglobin C and S and malaria in Ghana. Br Med J 1957; 2:143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson G. Significance of haemoglobins S and C in Ghana. Br Med J 1962; 1:682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labie D, Richin C, Pagnier J, Gentilini M, Nagel RL. Hemoglobins S and C in Upper Volta. Hum Genet 1984; 65:300–2. [DOI] [PubMed] [Google Scholar]
- 24.Aluoch JR. Higher resistance to Plasmodium falciparum infection in patients with homozygous sickle cell disease in western Kenya. Trop Med Int Health 1997; 2:568–71. [DOI] [PubMed] [Google Scholar]
- 25.Danquah I, Ziniel P, Eggelte TA, Ehrhardt S, Mockenhaupt FP. Influence of haemoglobins S and C on predominantly asymptomatic Plasmodium infections in northern Ghana. Trans R Soc Trop Med Hyg 2010; 104:713–9. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein SC, Bowman JE, Kaptue Noche L. Population studies in Cameroon: hemoglobin S, glucose-6-phosphate dehydrogenase deficiency and falciparum malaria. Hum Hered 1980; 30:251–8. [DOI] [PubMed] [Google Scholar]
- 27.Willcox MC, Beckman L. Haemoglobin variants, beta-thalassaemia and G-6-PD types in Liberia. Hum Hered 1981; 31:339–47. [DOI] [PubMed] [Google Scholar]
- 28.Motulsky A, Vandepitte J, Fraser G. Population genetic studies in the Congo. I. Glucose-6-phosphate dehydrogenase deficiency, hemoglobin S, and malaria. Am J Hum Genet 1966; 18:514–37. [PMC free article] [PubMed] [Google Scholar]
- 29.Bienzle U, Guggenmoos-Holzmann I, Luzzatto L. Plasmodium falciparum malaria and human red cells. I. A genetic and clinical study in children. Int J Epidemiol 1981; 10:9–15. [DOI] [PubMed] [Google Scholar]
- 30.Jeremiah Z, Jeremiah T, Emelike FO. Frequencies of some human genetic markers and their association with Plasmodium falciparum malaria in the Niger Delta, Nigeria. J Vector Borne Dis 2010; 47:11–6. [PubMed] [Google Scholar]
- 31.Ringelhann B, Hathorn MK, Jilly P, Grant F, Parniczky G. A new look at the protection of hemoglobin AS and AC genotypes against Plasmodium falciparum infection: a census tract approach. Am J Hum Genet 1976; 28:270–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Allen SJ, Bennett S, Riley EM, et al. Morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in children with sickle cell trait in The Gambia. Trans R Soc Trop Med Hyg 1992; 86:494–8. [DOI] [PubMed] [Google Scholar]
- 33.Le Hesran JY, Personne I, Personne P, et al. Longitudinal study of Plasmodium falciparum infection and immune responses in infants with or without the sickle cell trait. Int J Epidemiol 1999; 28:793–8. [DOI] [PubMed] [Google Scholar]
- 34.Stirnadel HA, Stöckle M, Felger I, Smith T, Tanner M, Beck HP. Malaria infection and morbidity in infants in relation to genetic polymorphisms in Tanzania. Trop Med Int Health 1999; 4:187–93. [DOI] [PubMed] [Google Scholar]
- 35.Gong L, Maiteki-Sebuguzi C, Rosenthal PJ, et al. Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood 2012; 119:3808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billo M, Johnson ES, Doumbia SO, et al. Sickle cell trait protects against Plasmodium falciparum infection. Am J Epidemiol 2012; 176(suppl 7):S175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willcox M, Björkman A, Brohult J, Pehrson PO, Rombo L, Bengtsson E. A case-control study in northern Liberia of Plasmodium falciparum malaria in haemoglobin S and beta-thalassaemia traits. Ann Trop Med Parasitol 1983; 77:239–46. [DOI] [PubMed] [Google Scholar]
- 38.Storey J, Fleming AF, Cornille-Brøgger R, Molineaux L, Matsushima T, Kagan I. Abnormal haemoglobins in the Sudan savanna of Nigeria. IV. Malaria, immunoglobulins and antimalarial antibodies in haemoglobin AC individuals. Ann Trop Med Parasitol 1979; 73:311–5. [DOI] [PubMed] [Google Scholar]
- 39.Modiano D, Petrarca V, Sirima BS, et al. Different response to Plasmodium falciparum malaria in West African sympatric ethnic groups. Proc Natl Acad Sci U S A 1996; 93:13206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lulli P, Mangano VD, Onori A, et al. HLA-DRB1 and -DQB1 loci in three West African ethnic groups: genetic relationship with sub-Saharan African and European populations. Hum Immunol 2009; 70:903–9. [DOI] [PubMed] [Google Scholar]
- 41.Ross P, Hall L, Smirnov I, Haff L. High level multiplex genotyping by MALDI-TOF mass spectrometry. Nat Biotechnol 1998; 16:1347–51. [DOI] [PubMed] [Google Scholar]
- 42.Gouagna LC, Bancone G, Yao F, et al. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat Genet 2010; 42:328–31. [DOI] [PubMed] [Google Scholar]
- 43.Modiano D, Luoni G, Sirima BS, et al. The lower susceptibility to Plasmodium falciparum malaria of Fulani of Burkina Faso (West Africa) is associated with low frequencies of classic malaria-resistance genes. Trans R Soc Trop Med Hyg 2001; 95:149–52. [DOI] [PubMed] [Google Scholar]
- 44.Langhorne J, Ndungu FM, Sponaas A, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol 2008; 9:725–32. [DOI] [PubMed] [Google Scholar]
- 45.Torcia MG, Santarlasci V, Cosmi L, et al. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairhurst RM, Baruch DI, Brittain NJ, et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 2005; 435:1117–21. [DOI] [PubMed] [Google Scholar]
- 47.Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A 2008; 105:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong L, Parikh S, Rosenthal PJ, Greenhouse B. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar J 2013; 12:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verra F, Simpore J, Warimwe GM, et al. Haemoglobin C and S role in acquired immunity against Plasmodium falciparum malaria. PLoS One 2007; 2:e978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJF. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis 2005; 191:1631–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.