Abstract
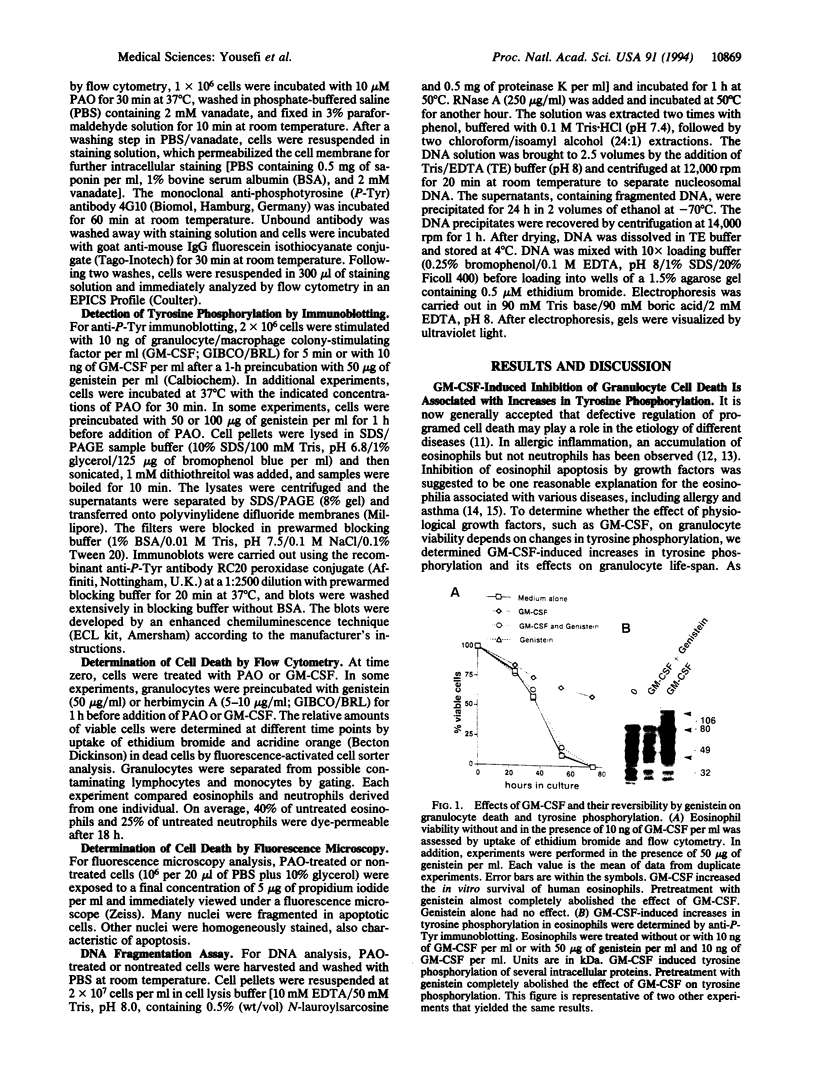
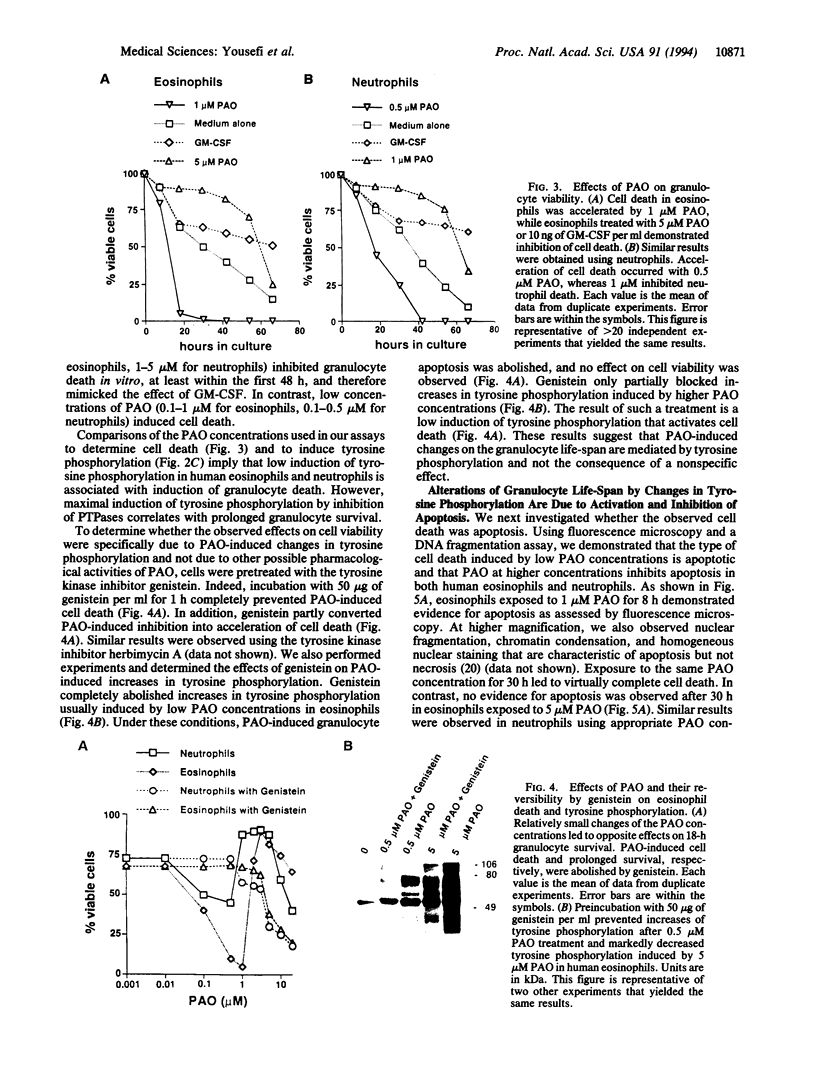
Early signaling events that control the process of programmed cell death are largely unknown. Tyrosine phosphorylation plays a major role in transmembrane signal transduction through most cell surface receptors. Granulocyte/macrophage colony-stimulating factor (GM-CSF), a cytokine released by activated T cells, has been shown to increase tyrosine phosphorylation in several cells and to inhibit granulocyte cell death in vitro. In this study, we demonstrate that the effect of GM-CSF on granulocyte cell death can be blocked by the tyrosine kinase inhibitor genistein, suggesting that increases in tyrosine phosphorylation are essential to inhibit cell death. To analyze the role of tyrosine phosphorylation for the regulation of granulocyte cell death more precisely, we increased levels of tyrosine phosphorylation using the protein-tyrosine phosphatase inhibitor phenylarsine oxide (PAO). Similar to GM-CSF, treatment of the cells with PAO was followed by high increases in tyrosine phosphorylation and inhibition of programmed cell death in human eosinophils and neutrophils. Strikingly, at low concentrations of the inhibitor and low induction of tyrosine phosphorylation, acceleration of apoptosis was observed. Genistein and herbimycin A reversed the effects of PAO on tyrosine phosphorylation and granulocyte apoptosis. These results suggest that programmed eosinophil and neutrophil death is regulated by early events of signal transduction pathways such as tyrosine phosphorylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergamaschi G., Rosti V., Danova M., Ponchio L., Lucotti C., Cazzola M. Inhibitors of tyrosine phosphorylation induce apoptosis in human leukemic cell lines. Leukemia. 1993 Dec;7(12):2012–2018. [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales P., Minami Y., Luong E., Klausner R. D., Samelson L. E. Tyrosine phosphorylation in T cells is regulated by phosphatase activity: studies with phenylarsine oxide. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9255–9259. doi: 10.1073/pnas.87.23.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson L. E., Rotello R. J. Apoptosis: a different type of cell death. FASEB J. 1992 Apr;6(7):2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Hansel T. T., De Vries I. J., Iff T., Rihs S., Wandzilak M., Betz S., Blaser K., Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991 Dec 15;145(1-2):105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- Harnett M., Rigley K. The role of G-proteins versus protein tyrosine kinases in the regulation of lymphocyte activation. Immunol Today. 1992 Dec;13(12):482–486. doi: 10.1016/0167-5699(92)90022-Y. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Manabe A., Yi T., Kumagai M., Campana D. Use of stroma-supported cultures of leukemic cells to assess antileukemic drugs. I. Cytotoxicity of interferon alpha in acute lymphoblastic leukemia. Leukemia. 1993 Dec;7(12):1990–1995. [PubMed] [Google Scholar]
- McColl S. R., DiPersio J. F., Caon A. C., Ho P., Naccache P. H. Involvement of tyrosine kinases in the activation of human peripheral blood neutrophils by granulocyte-macrophage colony-stimulating factor. Blood. 1991 Oct 1;78(7):1842–1852. [PubMed] [Google Scholar]
- Ong C. J., Chui D., Teh H. S., Marth J. D. Thymic CD45 tyrosine phosphatase regulates apoptosis and MHC-restricted negative selection. J Immunol. 1994 Apr 15;152(8):3793–3805. [PubMed] [Google Scholar]
- Otani H., Erdos M., Leonard W. J. Tyrosine kinase(s) regulate apoptosis and bcl-2 expression in a growth factor-dependent cell line. J Biol Chem. 1993 Oct 25;268(30):22733–22736. [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Stern M., Meagher L., Savill J., Haslett C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J Immunol. 1992 Jun 1;148(11):3543–3549. [PubMed] [Google Scholar]
- Stover D. R., Charbonneau H., Tonks N. K., Walsh K. A. Protein-tyrosine-phosphatase CD45 is phosphorylated transiently on tyrosine upon activation of Jurkat T cells. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7704–7707. doi: 10.1073/pnas.88.17.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Sun L., Spry C. J. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991 Aug;85(2):312–316. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H. Yin and yang of phosphorylation in cytokine signaling. Science. 1993 Oct 15;262(5132):376–377. doi: 10.1126/science.7692598. [DOI] [PubMed] [Google Scholar]
- Walker C., Virchow J. C., Jr, Bruijnzeel P. L., Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991 Mar 15;146(6):1829–1835. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Ohta S., Tominaga K., Miura Y., Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991 Nov 15;78(10):2542–2547. [PubMed] [Google Scholar]
- Yao X. R., Scott D. W. Antisense oligodeoxynucleotides to the blk tyrosine kinase prevent anti-mu-chain-mediated growth inhibition and apoptosis in a B-cell lymphoma. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7946–7950. doi: 10.1073/pnas.90.17.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. Y., Horvitz H. R. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990 Mar;138(1):33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]






