Abstract
Temperate forests provide favorable conditions for carbonate bedrock weathering as the soil CO2 partial pressure is high and soil water is regularly available. As a result of weathering, abiotic CO2 can be released and contribute to the soil CO2 efflux. We used the distinct isotopic signature of the abiotic CO2 to estimate its contribution to the total soil CO2 efflux. Soil cores were sampled from forests on dolomite and limestone and were incubated under the exclusion of atmospheric CO2. Efflux and isotopic signatures of CO2 were repeatedly measured of cores containing the whole mineral soil and bedrock material (heterotrophic respiration + CO2 from weathering) and of cores containing only the mineral top-soil layer (A-horizon; heterotrophic respiration). An aliquot of the cores were let dry out during incubation to assess effects of soil moisture. Although the δ13C values of the CO2 efflux from the dolomite soil cores were within a narrow range (A-horizon −26.2 ± 0.1 ‰; whole soil profile wet −25.8 ± 0.1 ‰; whole soil profile dry −25.5 ± 0.1 ‰) the CO2 efflux from the separated A-horizons was significantly depleted in 13C when compared to the whole soil profiles (p = 0.015). The abiotic contribution to the total CO2 efflux from the dolomite soil cores was 2.0 ± 0.5 % under wet and 3.4 ± 0.5 % under dry conditions. No abiotic CO2 efflux was traceable from the limestone soil cores. An overall low contribution of CO2 from weathering was affirmed by the amount and 13C signature of the leached dissolved inorganic carbon (DIC) and the radiocarbon signature of the soil CO2 efflux in the field. Together, our data point towards no more than 1–2 % contribution of abiotic CO2 to the growing season soil CO2 efflux in the field.
Electronic supplementary material
The online version of this article (doi:10.1007/s10533-015-0097-0) contains supplementary material, which is available to authorized users.
Keywords: Soil respiration, Carbonate weathering, 13C, Temperate forest
Introduction
The CO2 efflux from forest soils is a major component of the global C cycle. It primarily consists of two biological components, i.e. heterotrophic respiration from decomposers and autotrophic respiration from plant roots and interacting rhizosphere microorganisms (Högberg et al. 2001). Aside these biological sources, a minor abiotic fraction of the total soil CO2 efflux can be released during carbonate weathering and subsequent outgassing from soil water. Because weathering of carbonate bedrock proceeds at comparably low rates and because most of the released C is considered to be leached out of the soil, the abiotic component of the soil CO2 efflux is generally presumed as marginal (Kuzyakov 2006). Accordingly, the abiotic component of the soil CO2 efflux is generally neglected in partitioning approaches and forest C budgeting (e.g. Davidson et al. 2002; Giardina and Ryan 2002; Reichstein et al. 2005). A growing number of studies, however, report high abiotic contributions (10–60 %) to the overall CO2 efflux from arable and natural soils in different environments (Čatera and Ogrinc 2011; Emmerich 2003; Inglima et al. 2009; Kowalski et al. 2008; Plestenjak et al. 2012; Ramnarine et al. 2012; Serrano-Ortiz et al. 2010; Stevenson and Verburg 2006; Tamir et al. 2011). Considering that carbonate rock outcrops cover approximately 15 % of the total continental surface area (Amiotte Suchet et al. 2003; Meybeck 1987), an accurate estimate of the soil CO2 efflux associated with carbonate weathering is a prerequisite for the understanding and quantification of ecosystem C dynamics in these regions.
Carbonate weathering is predominately controlled by water availability and CO2 partial pressure in the soil. Therefore, weathering rates and the contribution of carbonate weathering to the soil CO2 efflux will vary with ecosystem productivity, climate, as well as soil and bedrock properties. The succession of wet and dry periods can cause significant CO2 uptake and release due to carbonate dissolution and precipitation in arid and semi-arid environments (reviewed in Serrano-Ortiz et al. 2010) whereas carbonate precipitation plays a negligible role in the humid temperate zone. The type of carbonate bedrock (dolomite vs. limestone) influences the production of abiotic CO2 as the dissolution rates and weathering intensity are lower for dolomite (Chou et al. 1989; Morse and Arvidson 2002; Pokrovsky et al. 2005) and also vary with morphology and microbial colonization (Davis et al. 2007). Carbonate dissolution based on CO2 dissolution and formation and dissociation of carbonic acid is commonly considered as a net CO2 sink, and can be expressed as:
| 1 |
| 2 |
In more temperate humid regions, the majority of the end products of carbonate weathering, the base cations (Ca2+, Mg2+) and the dissolved inorganic carbon (DIC), are transported from the soils into ground waters and rivers (Fig. 1a) (Ciais et al. 2008; Szramek et al. 2007). Because CO2 is consumed during carbonate dissolution, carbonate weathering can become a significant temporal sink of atmospheric or biogenic soil CO2 on regional and global scales (Beaulieu et al. 2012; Gombert 2002; Liu and Zhao 1999). Abiotic CO2 release due to carbonate precipitation (the reverse reaction of Eq. 1) is less significant in temperate forest soils because soil water is mostly in contact with soil surfaces, carbonate minerals, and soil air. Under specific conditions, however, variations in soil CO2 partial pressure, moisture, temperature, or pH can shift the equilibrium conditions towards degassing of CO2 and can thereby generate a transient abiotic soil CO2 efflux component (Fig. 1a). This abiotic efflux can consist of atmospheric CO2 which had entered the soil in the liquid phase already (atmospheric CO2 diluted in rainfall) and/or of CO2 from carbonate dissolution products.
Fig. 1.
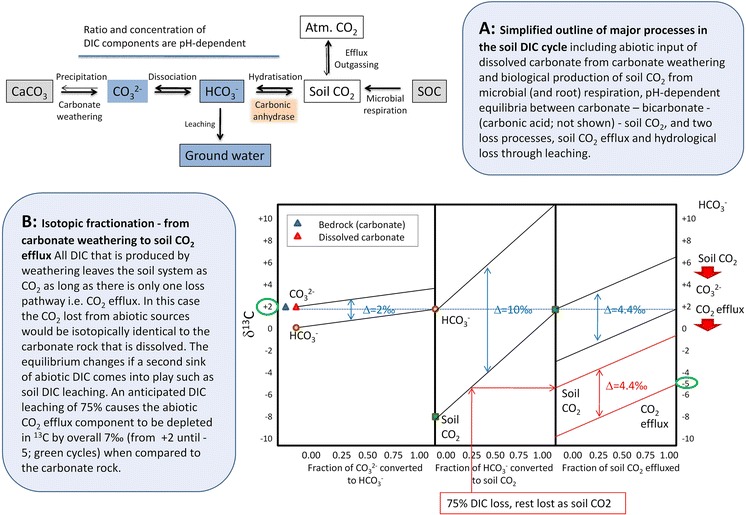
Simplified scheme of the soil DIC cycle (a) and conceptual overview of isotope fractionation from carbonate rock to abiotic soil CO2 efflux (b)
A number of pH dependent exchange reactions determine the DIC equilibrium in the soil solution (Fig. 1a). In temperate forests, the production and release of organic acids by plant roots and microbes (Attiwill and Adams 1993; van Hees et al. 2005) or the proton input by nitrification, oxidation of organic sulfur and acid rain can foster the dissolution of carbonate and CO2 degassing from the soil solution. Enzymes such as carbonic anhydrase which catalyze the conversion between CO2 and HCO3− in soil solution (Fig. 1a) can positively affect carbonate dissolution rates and abiotic CO2 release from the soil solution (Liu et al. 2005; Wingate et al. 2009). Considering the overall rates of geochemical weathering and the climatic preconditions in the temperate zone, the abiotic contribution to the total soil CO2 efflux should nevertheless be small (Serrano-Ortiz et al. 2010). However, reliable quantitative assessments of the abiotic CO2 efflux component from temperate forest soil are rare.
The CO2 efflux from forest soil on carbonate bedrock consists of the following components which differ in their isotopic signature: (I) heterotrophic respiration, (II) autotrophic respiration, (III) abiotic CO2 from weathering, and (IV) atmospheric CO2 which entered the soil by convection, diffusion or rainwater. The isotopic signature of the heterotrophic respiration is in the range of that of the SOM which is decomposed (δ13C between ~−24 and −30 ‰ for C3 plants) but can deviate by several per mill due to the preferential mineralization of specific substrates (Formánek and Ambus 2004; Werth and Kuzyakov 2010). The isotopic signature of autotrophic respiration is in a similar range but can vary for instance with weather conditions which affect the isotope discrimination during photosynthesis (Ekblad and Högberg 2001). The carbonate source material has a distinct isotopic signature with δ13C values close to zero whereas the δ13C of atmospheric air is close to −8 ‰. Due to its strong signal, the abiotic flux component from carbonate weathering influences the isotopic signature of the total soil CO2 efflux even at low contribution. In this study, we use the distinct carbon isotopic signal of abiotic CO2 to estimate its contribution to the total soil CO2 efflux. As the overall field soil CO2 efflux consists of four components, partitioning becomes complex and quantification of a minor component such as the abiotic efflux is hardly feasible. In order to constrain the number of potential CO2 sources, we incubated soil cores without plants under the exclusion of atmospheric CO2 in the laboratory. Intact soil cores were collected in forests on dolomite and limestone bedrock. We measured CO2 and its isotopic signature from cores containing solely the litter and upper mineral soil layer (heterotrophic respiration) and from cores containing the whole soil profiles plus bedrock material (heterotrophic respiration + abiotic CO2 from weathering). We hypothesized that (I) the contribution of abiotic CO2 would be low because carbonate dissolution is a comparatively slow process and because most of the abiotic C would be leached out of the soil. To assess the effects of soil moisture on abiotic CO2 release, we let half of the dolomite soil cores dry out during incubation while soil moisture in the other cores was held at field capacity. We hypothesized (II) that the relative contribution of abiotic CO2 to the soil CO2 efflux would be higher under the wet treatment because of higher abundance of dissociated carbonic acid in the soil. We further hypothesized that (III) the contribution of abiotic CO2 is higher in the limestone soil because calcite dissolution proceeds faster than dolomite dissolution (Chou et al. 1989; Liu et al. 2005; Morse and Arvidson 2002; Pokrovsky et al. 2005). In a parallel experiment we measured soil CO2 concentrations and soil CO2 efflux as well as their isotopic signature at the dolomite field site throughout the seasons in 2012/2013.
Materials and methods
Site description
The dolomite site was located at 910 m a.s.l. on a north–north-east slope of a mountain in the Northern Limestone Alps, close to Achenkirch, Austria (47°34′ 50″N; 11°38′ 21″E). Mean annual air temperature and precipitation were 5.7 °C and 1480 mm (1987–2007), respectively. The 125 year old forest was dominated by Norway spruce (Picea abies), with interspersed European beech (Fagus sylvatica) and silver fir (Abies alba). The soils were a mosaic of shallow Chromic Cambisols and Rendzic Leptosols (FAO 1998). The bedrock was composed of dolomite (Upper Triassic Hauptdolomit Formation). Mull was the dominant humus form with an average thickness of 1–3 cm. A-horizons showed a strong, small-scale variability in thickness reaching from 10 cm up to 40 cm. The C-horizon consists of fine-grained, angular dolomite gravel and reached down (20–40 cm) to the solid bedrock. Between the A and C-horizons a 5–10 cm-thick transitional A/C-horizon was characterised by a mixture of mineral soil and dolomite gravel. Root density was highest in the O and A-horizons and few roots were found down to a depth of 60 cm. Organic C stocks were estimated to be ~10 t ha−1 in the organic layer and ~120 t ha−1 in the mineral soil (Schindlbacher et al. 2010).
The limestone site was located on a south–south-west slope of the Hochschwab massif in the Northern Limestone Alps, Austria (47°34′02″N; 15°02′19″E). Mean annual air temperature was between 4 and 5 °C. Mean annual precipitation was 1450 mm. The dominant tree species in the montane region (800–1400 m) were Norway spruce and European larch (Piceaabies and Larix decidua). The soils were LepticHistosols (FAO 1998) formed on limestone (Middle Triassic Wettersteinkalk Formation). The O-horizon depth at the sampling site was 1–4 cm. The A-horizons depth varied between 10 and 20 cm. As at the dolomite site, the C-horizon material consisted of fine gravel (20–50 cm deep).
Soil column sampling and treatment
At the dolomite site, soil was sampled at five randomly distributed locations in late November 2011. From each location a pair of columns containing whole soil profile and an additional column containing only the A-horizon was sampled for incubation. Sampling was performed as little destructive as possible. A Plexiglass cylinder (20 cm diameter × 60 cm length for whole soil profiles; 20 cm diameter × 30 cm length for A-horizons) was pushed into the soil after cutting the roots around the cylinder edge with a knife. This procedure worked well until larger stones in the C-horizon blocked the insertion of the cylinder. Cylinders containing the undisturbed soil were then taken out and the last part of the C-horizon was filled from below with a shovel. Five cores with whole soil profiles as well as five A-horizon cores were watered to field capacity. No water was added to the remaining five cores with whole soil profiles. Accordingly, at the dolomite site, three different sets of cores were incubated; “wet” (whole profile), “dry” (whole profile) and the separated “A-horizon” with a replication of five columns each.
After the dolomite soil incubation was finished, we sampled (same procedure) cores from four randomly distributed locations at the limestone site. We took four whole-profile cores and four cores containing only A-horizons in mid-October 2012. Cores from the limestone site were incubated at the original water content and the corresponding set of cores for limestone soil were “whole profile” and “A-horizon” with a replication of four cores each. Soil moisture of the limestone cores was kept constant at the original water content by periodical watering as described above until day 45 of the incubation. At day 45, soil moisture of all cores from the limestone site was increased to field capacity and kept at this level until the end of incubation.
Incubation and sampling
Soil cores were incubated in complete darkness at a temperature of 20 ± 1 °C. For CO2 measurements (Fig. 2), soil columns were closed at the top and bottom and attached to the flushing system. In our attempt to expel all atmospheric CO2 from the soil columns, CO2-free air was pumped through each soil column from top to bottom at a flow rate of 10 L h−1 during the first week. After a week the bottom exhaust was closed and only the headspace of the soil column was flushed for further 2 weeks (acclimation period). Using adjustable flow meters, the flow of CO2-free air through the headspace (~10–15 cm height) of each soil column was regulated manually to a rate at which the column CO2 headspace concentration stabilized at 380–400 ppm. The CO2-free air was produced from ambient air which was compressed and blown through two consecutive columns (12 × 100 cm each) filled with sodalime granulate.
Fig. 2.
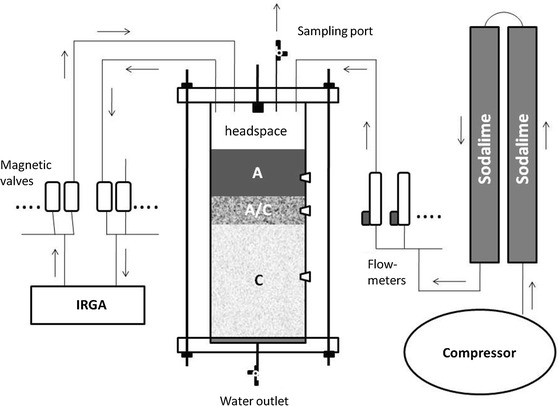
Schematic drawing of the incubation system (arrows indicate the direction of air-flow). Ambient air was compressed and pumped through sodalime-columns to scrub ambient CO2. Flow rates to the soil column headspace were regulated in a way that headspace CO2 concentrations ranged between 380 and 400 ppm. The flushing-air left the soil column headspace through an outlet which was also used as sampling port for isotopic analyses. Two benches of magnetic valves (inlet, outlet) allowed to switch between individual soil columns (n = 15) for CO2 concentration measurements with an IRGA. Water was added through a spray valve at the top of the column headspace and leaching water was collected from an outlet at the bottom of the soil column. At each soil horizon, a septum was installed into the column wall to allow direct sampling of soil–air with a syringe
CO2 concentrations in the soil-column headspaces were measured with an infrared gas analyzer (IRGA) (EGM-4, PP-Systems, Amesbury). A control unit of 30 magnetic valves allowed switching between column headspaces for CO2 concentration measurements in a completely closed system (Fig. 2). For the determination of CO2 efflux rates, the headspace-flush was interrupted and after a 1 min equilibration time the CO2 concentration in the chamber headspace was measured every 30 s throughout 3 min. The CO2 efflux was derived from the linear concentration increase over time.
CO2 concentrations of soil air were measured directly from septa installed in the column wall at three depths (Fig. 2). A short (~20 cm) tubing (inner diameter 4 mm) was attached to the inlet of a second IRGA. The tubing ended in a 5 cm-long syringe needle which was directly inserted into the septa/soil. After ~5 s, the IRGA showed a steady value of the soil–air CO2 concentration.
Air samples for isotopic analyses were obtained from both, the soil column headspace and from the three soil horizons of each core. The headspace sampling port which simultaneously served as the outlet of the flushing air was equipped with a stopcock and a three-way Luer lock. For sampling, a syringe (25 mL) was attached and the stopcock and needle volume were flushed with headspace air by using the Luer lock. For isotopic analysis, 12 mL of headspace air were injected into 12 mL glass vials (Exetainer, Labco Ltd, High Wycombe, UK) containing CO2-free air. While injecting the sample, we inserted a second needle to allow outflow of air in order to avoid overpressurizing the vials. The second needle was removed shortly before the full sample was injected thereby leaving a minimal overpressure in the vial. Soil air samples from the soil horizons were obtained through three septa which were installed into the column wall. A syringe needle was inserted directly into the soil and 6 mL of soil air were sampled with a 25 mL syringe. Before sampling, the syringe needle space was flushed with another 4 mL of soil air as described above with a Luer lock system. Soil air was injected into a 12 mL vial as described above for the headspace sampling.
The carbon isotope ratios of the CO2 were then analyzed by continuous-flow isotope-ratio mass spectrometry (IRMS) on a Delta V Advantage Mass Spectrometer coupled to a GasBench system (Thermo Fisher Scientific, Bremen, Germany). CO2 efflux and isotopic signatures were determined every 2–3 weeks throughout 166 days incubation of the dolomite soil and 60 days incubation of the limestone soil.
We also collected drainage water to estimate the flux and isotopic ratio of dissolved inorganic carbon (DIC). This analysis was restricted to the dolomite soil and to the “wet” and “A-horizon” cores. We simulated rainfall events by slowly adding larger quantities (400 mL for whole profiles; 200 mL for A-horizons) of water, equivalent to ~12 and ~6 mm of rainfall. Drainage water was collected 4 h after irrigation from the bottom outlet of the column. We collected 5 mL with a syringe and pressed 3 mL through a Teflon filter (0.45 µm mesh size) attached to the syringe. The first 2 mL were used to flush the needle; the third ml of filtered soil water was injected into an evacuated 12 mL vial containing ~1 µL concentrated phosphoric acid. Concentrations and isotopic signatures of the evolving CO2 were measured as described above by GasBench-IRMS. DIC was sampled less frequently than CO2 with in total four sampling dates throughout the 166 day incubation.
Soil and bedrock analysis
After incubation, soil columns were disaggregated and the dry weight, stone content, water content, pH, carbonate content, contents of organic C (Corg) and total N as well as the isotopic signature of the Corg of each soil horizon were determined. Dry weight and gravimetric water content were determined after drying ~50 g of sieved soil at 105 °C for 12 h. Volumetric stone content was estimated by dividing the horizon specific mass of stones larger than 2 mm by the density of dolomite (2.9 g cm−3) and limestone (2.7 g cm−3) respectively. Soil pH was measured potentiometrically according to ISO 10390 (www.iso.ch). For determination of the carbonate content, ground soil samples were treated with a strong acid (10 % HCl). The volume of the carbon dioxide produced was measured by using a calcimeter (Scheibler unit), and was compared with the volume of carbon dioxide produced by pure carbonate (ISO 10693; www.iso.ch). Total C and N contents of the soil horizons were determined with a LECO CN-2000 dry combustion analyzer (www.leco.org). Organic C content was assessed by correcting total soil C by carbonate contents (ISO 10694; www.iso.ch). The isotopic signature of Corg from the different soil horizons was determined after decarbonatization with a Flash EA elemental analyzer coupled via ConFlo III interface to a DeltaPlus IRMS system (Thermo Fisher Scientific, Bremen, Germany). For pre-treatment aliquots of finely ground soil (100 mg) were treated with 1 mL 2 M HCl at room temperature until the full decarbonisation of the samples and subsequently dried in a drying oven at 60° C for 2 days.
Dolomite and calcite of the carbonate bedrock material were reacted with phosphoric acid at 90 and 72 °C, respectively, and analysed using an automated continuous-flow DeltaPlusXL isotope ratio mass spectrometer at the University of Innsbruck. Calibration of dolomite samples was accomplished using a dolomite standard, whose isotopic composition was previously determined using classical offline preparation (provided by T. Vennemann, Lausanne). Calibration of calcite samples was based on NBS19, CO1 and CO8 reference materials. Results are reported with respect to the VPDB scale, and the long-term analytical uncertainties at the 1σ level is equal 0.07 for δ13C (Spötl&Vennemann Spötl and Vennemann 2003).
Field measurements
Soil CO2 efflux, soil air CO2 concentrations and the corresponding isotopic signatures were assessed in the field at the dolomite site in 2012/2013. Measurements were performed once during spring (16 May), summer (09 July), autumn (08 October), and winter (26 February). Three soil pits were equipped with stainless steel capillary tubes (inner diameter 1 mm) attached to perforated 4 cm-long Teflon tubes (inner diameter 4 mm, inserted into the side walls of the soil pits) to assess the CO2 concentrations within the soil profiles. Capillary tubes were installed in the A-horizon, the A/C-horizon and the C-horizon, and the pits thereafter carefully filled with horizon-specific soil material. Soil CO2 concentrations were assessed by directly connecting the IRGA to the steel capillaries. Samples for isotopic analyses were taken with a syringe and transferred into 12 mL vials as described above. Surface soil CO2 efflux during the snow-free season was estimated from closed dynamic chamber measurements as described by Schindlbacher et al. (2009) (one chamber per soil profile) and by a CO2 concentration gradient method applied during winter (Schindlbacher et al. 2014). To assess the isotopicsignature of soil respired CO2 during the snow free season, the Keeling plotapproach was used (Keeling 1958). The interceptof a linear regression of δ13C of sampled CO2versus 1/[CO2] provided an estimate of δ13C ofsoil-respired CO2 (where [CO2] was the CO2 concentrationin %). During snow cover, the isotopic composition of the soil CO2 efflux was estimated from the δ13C along the CO2 gradient in the snow cover according to Bowling et al. (2009) and Davidson (1995).
Data analysis and estimate of the abiotic CO2 efflux
Treatment effects on isotopic signatures of the soil CO2 efflux and soil CO2 concentration were statistically tested by one-way repeated measures ANOVA (procedure GLM, SAS Institute Inc., Cary, NC, USA). The fractional contribution of CO2 derived from weathering (f) to overall soil CO2 efflux from the incubated soil columns was calculated following the two-pool mixing model:
| 3 |
where δ13Ctotal is the C isotope signature (‰) of the CO2 efflux from the whole soil profile, δ13Cmicrobial is the C isotope signature (‰) of the CO2 efflux from the separately incubated A-horizons, and δ13Ccarbonate is the C isotope signature (‰) of CO2 originating from carbonate (dolomite, limestone) weathering. The mixing model applied assumes that the abiotic contribution to the CO2 efflux from A-horizon cores is zero or negligible. This assumption was challenged as some of the A-horizon cores contained up to 200 mg carbonate g−1 dw (see results; Table 1). Nonetheless, we found strong evidence that the CO2 efflux from the A-horizon cores contained no or only negligible amounts of abiotic CO2. There was no relationship between the δ13C of the CO2 efflux and the carbonate content of the separately incubated A-horizons (Fig. S1) ranging from 0 to 200 mg g−1 dry weight. Furthermore, the absolute amount of carbonate in the upper layer of the mineral soil (A-horizon) was almost two orders lower when compared to the amount of carbonate in the deeper soil layers. Therefore, the insignificant contribution of abiotic CO2 to the soil CO2 efflux of the A-horizon cores was neglected in our mixing model. Alternatively, we run the same calculations with the isotope signatures of soil organic matter (Corg, weighted for the whole profile) as proxy for δ13Cmicrobial. The results that we obtained that way were very similar and in some cases contributions from carbonate weathering were even lower (data not shown).
Table 1.
Properties of dolomite (mean ± SE; n = 5) and limestone soil cores (mean ± SE; n = 4) and the corresponding separately incubated A-horizons. All parameters were assessed after disaggregation of the columns after finishing the incubation
| Bedrock | Dolomite | Limestone | ||||
|---|---|---|---|---|---|---|
| Hori-zon | Whole profile wet | Whole profile dry | A-horizon | Whole-profile | A-horizon | |
| Depth (cm) | FF | 1.1 (0.4) | 1.5 (0.4) | 1.0 (0.2) | 1.9 (0.3) | 0.40 (0.1) |
| A | 13.1 (1.1) | 12.9 (0.8) | 6.3 (0.4) | 10.9 (1.5) | 10.8 (1.3) | |
| A/C | 6.5 (6.5) | 5.9 (0.5) | 7.9 (1.8) | |||
| C | 16.8 (1.1) | 16.1 (1.5) | 15.8 (1.8) | |||
| Dry weight (g) forest floor and soil <2 mm | FF | 20 (9) | 41 (13) | 29 (5) | 18 (4) | 18 (4) |
| A | 910 (132) | 1024 (213) | 363 (36) | 508 (81) | 533 (202) | |
| A/C | 578 (66) | 623 (30) | 2207 (1016) | |||
| C | 1508 (136) | 1338 (288) | 4777 (589) | |||
| Stones >2 mm (vol%) | FF | |||||
| A | 6.6 (2.5) | 4.2 (1.6) | 0.7 (0.3) | 0.9 (0.3) | 1.1 (0.5) | |
| A/C | 23.7 (2.4) | 23.8 (6.0) | 18.2 (3.6) | |||
| C | 49.5 (2.8) | 49.7 (2.7) | 50.8 (13.8) | |||
| Water content (mass%; post-incubation) | FF | 62.7 (3.2) | 33.1 (4.9) | 72.6 (1.5) | 72.7 (0.9) | 74.5 (4.3) |
| A | 61.9 (2.2) | 48.5 (4.1) | 65.7 (1.9) | 70.6 (1.8) | 70.6 (3.0) | |
| A/C | 42.2 (2.4) | 36.3 (1.9) | 35.3 (6.9) | |||
| C | 21.5 (3.6) | 15.0 (3.0) | 25.6 (2.2) | |||
| pH | FF | 5.9 (0.4) | 5.8 (0.3) | 5.5 (0.4) | 5.8 (0.5) | 5.9 (0.4) |
| A | 6.8 (0.2) | 6.7 (0.3) | 6.9 (0.1) | 6.4 (0.5) | 6.2 (0.5) | |
| A/C | 7.3 (0.0) | 7.2 (0.1) | 7.1 (0.1) | |||
| C | 7.4 (0.1) | 7.6 (0.1) | 7.4 (0.0) | |||
| CaMg(CO3)2 (dolomite) CaCO3 (limestone) (mg g−1 dw) | FF | |||||
| A | 105 (18) | 84 (28) | 121 (44) | 197 (81) | 173 (91) | |
| A/C | 528 (50) | 414 (97) | 722 (48) | |||
| C | 817 (54) | 821 (39) | 780 (68) | |||
| Corg (mg g−1 dw) | FF | 344 (15) | 293 (40) | 367 (14) | 413 (23) | 398 (31) |
| A | 172 (23) | 177 (13) | 162 (11) | 272 (40) | 296 (42) | |
| A/C | 61 (8) | 72 (5) | 58 (11) | |||
| C | 21 (3) | 22 (4) | 24 (5) | |||
| Isotopic signature Corg (δ13C ‰) | FF | −28.21 (0.37) | −28.60 (0.27) | −28.85 (0.36) | −28.46 (0.11) | −28.42 (0.09) |
| A | −26.30 (0.16) | −26.26 (0.02) | −26.38 (0.06) | −27.08 (0.17) | −27.28 (0.20) | |
| A/C | −24.97 (0.19) | −25.34 (0.22) | −25.60 (0.37) | |||
| C | −24.06 (0.40) | −24.44 (1.24) | −25.20 (0.71) | |||
| Bedrock C (δ13C ‰) | ||||||
| CaMg(CO3)2 (Dolomite) | +2.92 (0.04) | (0.04) | ||||
| CaCO3 (Limestone) | +2.12 (0.04) | |||||
| N tot (mg g−1 dw) | FF | 16.3 (0.6) | 15.2 (1.7) | 15.9 (1.0) | 22.3 (0.6) | 21.4 (1.0) |
| A | 11.1 (1.1) | 11.7 (1.1) | 10.5 (0.7) | 17.2 (2.3) | 18.5 (0.7) | |
| A/C | 4.4 (0.6) | 5.0 (0.4) | 3.3 (0.9) | |||
| C | 0.7 (0.3) | 0.8 (0.3) | 0.6 (0.1) | |||
The isotopic signature of the CO2 from carbonate weathering (δ13Ccarbonate) was not directly measured but estimated from isotopic measurements of bedrock carbonate (dolomite, limestone). To account for potential isotope fractionation during the weathering process and during the transformation from HCO3− to gaseous CO2 we assumed steady state conditions in an open system (isotopic equilibrium conditions) during our soil CO2 measurements (Fig. 1b). Under such (e.g. in well-drained soils) there is a C isotope equilibrium effect between Ca carbonate and bicarbonate (~1–2 ‰ 13C depletion of bicarbonate relative to Ca carbonate) and between bicarbonate and soil CO2 (~10 ‰ 13C depletion of soil CO2 relative to bicarbonate, Fig. 1b) (Amundson et al. 1998; Cerling 1984; Emrich and Vogel 1970; Halas et al. 1997; Mook et al. 1974; Nordt et al. 1998). These equilibrium isotope effects are additive and slightly temperature-dependent, i.e. the lower the temperature the larger the equilibrium isotope effect (Halas et al. 1997; Myrttinen et al. 2012). Moreover, soil CO2 is enriched by 13C by up to ~4 ‰ relative to soil CO2 efflux due to diffusional isotope fractionation during CO2 escape from the soil (Cerling et al. 1991), but this fractionation is lower when CO2 efflux is triggered by advective instead of diffusive soil gas transport (Kayler et al. 2010). In an open system the fraction of weathered (i.e. dissolved) carbonate that is emitted as CO2 from the soil determines whether the effluxed CO2 reflects the isotopic composition of the carbonate or not (Hendy 1971). We would expect the same isotopic signature of both the carbonate bedrock and soil abiotic CO2 efflux if all C released through weathering is emitted from soils in the form of CO2 (Fig. 1b). If a larger fraction of DIC is lost through hydrological pathways (e.g. leaching of 75 % of the bicarbonate produced and only 25 % is converted to soil CO2), as was anticipated for the studied forest soil, then soil CO2 should be 13C-depleted (by approximately −5 ‰) relative to carbonate (+2 ‰), resulting in an isotopic offset of about −7 ‰ relative to carbonate rock which was taken into account in the isotopic mixing model (Eq. 3). In some cases, e.g. glacial settings, carbonate weathering does not occur under steady state (equilibrium) conditions. In this case kinetic isotope fractionation with up to 17 ‰ enrichment in 13C (Skidmore et al. 2004) may occur which causes DIC and soil CO2 efflux to become intermittently 13C-depleted relative to the carbonate bedrock, until equilibrium conditions are reached. Kinetic isotope fractionation during carbonate dissolution may be expected to occur when soil water-carbonate contact times are short, e.g. shortly after rainfall events but can be excluded in our experimental setup.
Results
Soil properties
Carbonate contents sharply increased from litter (zero) to ~800 mg g−1 in the C-horizon soil fraction. The stone content increased with depth and was roughly 50 % of the C-horizon volume in the incubated cores (Table 1). The carbonate content of the separately incubated A-horizons showed high spatial variability ranging from 10 to 200 mg g−1 in the cores from the dolomite site and from 0 to 280 mg g−1 in the cores from the limestone site. Isotope signatures (δ13C) of Corg significantly increased (linear regression; p < 0.0005) with soil depth at both sites from −28 ‰ in the litter layer to −25 ‰ in the C-horizon (Table 1). Bedrock material showed δ13C signatures of +2.9 ‰ for dolomite and +2.1 ‰ for limestone.
Watering to field capacity initially increased the weight of the dolomite soil cores by 400–600 g (Fig. 3). Soil moisture and the weight of the soil cores, were kept constant during the wet treatment, whereas in the dry treatment, the soil cores gradually dried out and lost weight (Fig. 3). At the end of the incubation period soil moisture contents of the organic layers and A-horizons were significantly lower in the dry treatment cores (33 and 48 mass%) than in the wet treatment cores (both 63 mass%) (Table 1).
Fig. 3.
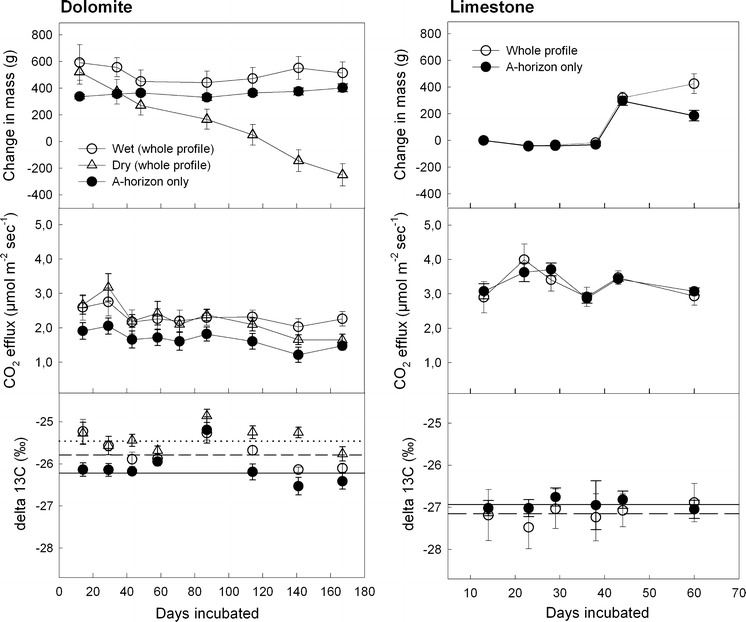
Soil CO2 efflux and its isotopic signature from dolomite (left panel) and limestone (right panel) cores (mean ± SE; Dolomite n = 5; Limestone n = 4). Temporal changes in soil-core mass (upper panel) reflect changes in soil moisture. A set of complete dolomite-soil profiles was initially watered and incubated at near field capacity (Wet open circles) whereas a second set was allowed to dry out (Dry triangles). A third set contained solely A-horizons (full circles) but no dolomite gravel. Limestone-soil was incubated in sets of whole soil profiles (open circles) and A-horizons only (full circles) which were all watered at incubation day 45. Lines in the lowermost panel indicate means over all sampling dates except day 86 (Dolomite: Wet dashed; Dry dotted; A-horizon full; Limestone: whole profile dashed; A-horizon full). At day 86 leaky seals of vial caps likely biased the δ13C measurements
Soil CO2 efflux and soil CO2 concentrations
While the CO2 efflux from the limestone soil cores and the separately incubated A-horizons was in a similar range, the CO2 efflux of the dolomite soil cores was continuously higher than that of the separately incubated A-horizons (Fig. 3). This can be explained by the lower amount of top-soil which was incubated for the dolomite site (Table 1). As the deeper layer of the A-horizon at the dolomite site already contained stones, we only incubated the uppermost layer. The CO2 efflux from dolomite soil showed a slightly decreasing trend throughout the 166 day incubation period (Fig. 3). The CO2 efflux was similar under wet and dry treatment during the first 86 days of incubation. Dry treatment efflux rates decreased more pronouncedly during the latter part of incubation (day 113–166; Fig. 3). The effect of drying became more evident in soil CO2 concentrations which gradually decreased in the A-horizon from the beginning onwards (Fig. 4). CO2 concentrations in the deeper soil horizons of the dry treatment remained relatively constant until day 86 of the incubation but dropped significantly afterwards (Fig. 4). Watering of the limestone soil cores did neither affect the CO2 efflux from whole limestone soil cores nor from the respective A-horizons.
Fig. 4.
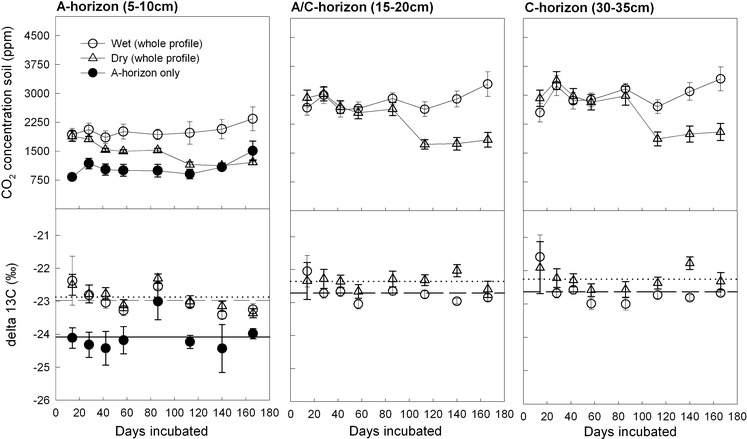
CO2 concentrations and isotopic signatures (mean ± SE, n = 5) of soil air collected in the A, A/C, and C horizons of the incubated dolomite soil cores. Centimeter values in brackets indicate the depth of the sampling point. For wet (open circles) and dry (triangles) treatments, complete soil profiles were incubated. A set of A-horizon only cores (full circles) was incubated for comparison. Lines in the lower panel indicate means over all sampling days except day 86 when leaky seals biased the δ13C measurements (Wet dashed; Dry dotted; A-horizon full)
Isotopic signature and CO2 efflux partitioning
The isotopic signature of soil CO2 efflux varied within a narrow range throughout the incubations of both the dolomite and limestone soil (Fig. 3). At day 86, δ13C values were unusually high for all sets of cores of the dolomite soil incubation, suggesting influx of atmospheric CO2 due to leaky sealing of the Exetainer vials. We therefore rejected this date for statistical analysis of the δ13C values of soil CO2 efflux and soil CO2. Average δ13C values throughout the dolomite soil incubation were −26.2 ± 0.1 ‰ for the separately incubated A-horizons, −25.8 ± 0.1 ‰ for the wet soil cores, and −25.5 ± 0.1 ‰ for the dry soil cores (p = 0.015, repeated measures ANOVA). Applying the two-pool mixing model (Eq. 3) we calculated an average abiotic contribution of 2.0 ± 0.5 % to the total soil CO2 efflux from the wet dolomite soil cores. The mean abiotic contribution to the dry treatment CO2 efflux was 3.4 ± 0.5 % when calculated for the whole incubation period. The estimated abiotic contribution to the total soil CO2 efflux was lower during the first phase of drying (until day 57; mean contribution 2.8 ± 0.6 %) than during the phase during which soil moisture was at lowest levels (day 58–166; mean contribution 4.3 ± 0.8 %). δ13C values of the CO2 efflux from the wet cores decreased with incubation time (linear regression, p < 0.05) whereas δ13C from dry cores and the separated A-horizons did not show a clear temporal trend. Generally, soil CO2 efflux was 2–3 ‰ depleted when compared with soil air CO2, pointing to kinetic isotope fractionation during soil CO2 efflux (Figs. 3, 4). The soil CO2 in the A-horizons of the whole dolomite soil cores was significantly 13C enriched compared to that in separately incubated A-horizons (p = 0.0013, repeated measures ANOVA) (Fig. 4). In dolomite soil cores, the δ13C values of soil CO2 in the A/C and C-horizons were slightly higher than in the A-horizons (Fig. 4). Moisture treatment (wet, dry) had no significant effect on the isotopic signature of soil CO2 in any horizon.
The isotopic signature of the soil CO2 efflux of limestone soil cores showed higher spatial variability when compared to that of the dolomite soil cores (Fig. 3) but the mean δ13C signatures of soil CO2 efflux were nearly identical for separately incubated A-horizons (−26.9 ± 0.1 ‰) and whole limestone soil cores (−27.2 ± 0.1 ‰). Due to the insignificant (p = 0.63, repeated measures ANOVA) isotopic differences, an abiotic contribution to the total soil CO2 efflux of limestone soil cores could not be detected. There was also no clear temporal pattern regarding the isotopic signature of limestone soil CO2 efflux throughout the 60 day incubation period. Mean δ13C values of CO2 in the A-horizons were similar between whole soil cores and separated A-horizons and were also similar to the δ13C values of soil CO2 in the A/C and C horizons (Fig. 5). Watering of limestone soil cores at day 45 did not affect the isotopic signature of the CO2 efflux or the soil CO2.
Fig. 5.
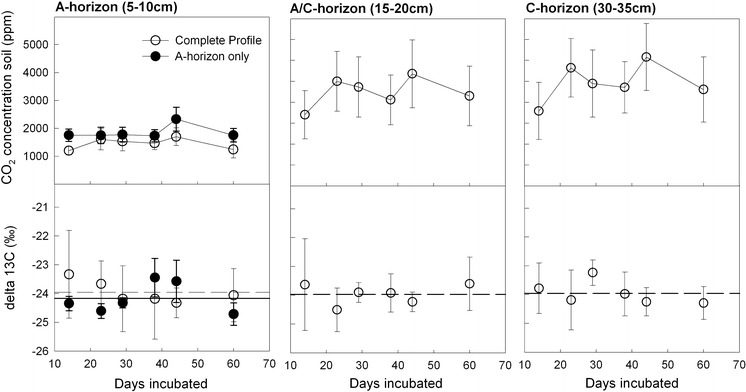
CO2 concentrations and isotopic signatures (mean ± SE, n = 4) of soil air sampled from the A, A/C, and C horizons of the incubated limestone soil cores. Centimeter values in brackets indicate the depth of the sampling point. Soil was incubated in sets of complete profiles (open circle) and A-horizons only (full circles). Lines in the lower panel indicate means over all sampling days (Complete soil profile dashed; A-horizon full)
Dissolved inorganic carbon (DIC)
DIC concentrations in drainage water from whole soil cores were higher (average over all 4 dates: 30.7 ± 0.7 mg L−1) than from separated top-soil (14.7 ± 0.9 mg L−1) (Table 2). Drainage water DIC from the whole soil profile cores was more 13C enriched (−15.2 ± 0.1 ‰) than drainage water from the top-soil cores (−17.7 ± 0.5 ‰) (Table 2).
Table 2.
Dissolved inorganic carbon (DIC) concentration and isotopic signature in drainage water of dolomite soil cores
| Days incubated | DIC (sample ppm CO2) | DIC (mg/L) | δ13C (‰) | |||
|---|---|---|---|---|---|---|
| Whole profile | A-horizon | Whole profile | A-horizon | Whole profile | A-horizon | |
| 43 | 5613 (230) | 2693 (1302) | 30.9 (1.3) | 14.8 (3.9) | −15.14 (0.58) | −17.99 (0.38) |
| 114 | 5211 (58) | 2288 (1550) | 28.7 (0.3) | 12.6 (3.6) | −15.09 (0.32) | −17.90 (0.42) |
| 141 | 5941 (280) | 3098 (1715) | 32.7 (1.5) | 17.4 (8.7) | −14.98 (0.35) | −18.60 (0.54) |
| 167 | 5540 (297) | 2579 (1445) | 30.5 (1.6) | 14.8 (5.4) | −15.56 (0.38) | −16.20 (0.22) |
Drainage water was collected from the wet treatment of whole soil profile cores (Whole profile) and from separately incubated A-horizons 1 h after water addition
Field measurements
The in situ soil CO2 efflux showed the typical seasonal pattern with highest flux rates during summer and lowest flux rates during winter (Table 3). The summertime isotopic signature of the field soil CO2 efflux was very similar to that of the soil cores in the laboratory (dolomite cores) (Table 3; Fig. 3). However, the average isotopic signatures of the field soil CO2 efflux varied substantially throughout seasons, i.e. between −24.7 ‰ in spring and −27.7 ‰ in winter. Summertime field soil CO2 concentrations were in all soil horizons approximately twice as high as in the incubation study (Table 3). The δ13Cvalues of soil CO2 were close to those in the incubation study. During spring, soil CO2 in the A-horizon was most depleted in 13C and became more 13C enriched with increasing soil depth (Table 3). This pattern reversed during the other seasons during which soil CO2 in the A-horizon was most 13C enriched and CO2 in the C-horizon was most depleted. Wintertime δ13C values of soil CO2 were generally less negative when compared with those of the warmer seasons.
Table 3.
Field CO2 data (mean ± SE, n = 3) throughout the seasons 2012/13 (spring 16.05.2012, summer 09.07.2012, autumn 08.10.2012, winter 26.02.2013)
| Horizon | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| CO2 efflux (µmol m−2 s−1) | ||||
| 2.43 (0.07) | 4.84 (0.97) | 2.33 (0.53) | 0.33 (0.04) | |
| CO2 efflux (δ 13C ‰) | ||||
| −24.68 (0.62) | −25.62 (0.23) | −26.25 (0.08) | −27.71 (0.02) | |
| CO2 concentration in soil (ppm) | ||||
| A | 1431 (249) | 4004 (1026) | 3319 (288) | 1430 (234) |
| A/C | 2439 (479) | 6313 (1674) | 4986 (399) | 1476 (541) |
| C | 3909 (581) | 8800 (1439) | 7055 (778) | 2143 (576) |
| Isotopic signature of soil CO2 (δ 13C ‰) | ||||
| A | −22.39 (0.54) | −21.86 (0.78) | −21.36 (0.80) | −18.98 (0.77) |
| A/C | −21.19 (0.97) | −23.02 (0.54) | −22.59 (0.50) | −19.73 (1.21) |
| C | −19.30 (0.37) | −23.72 (0.31) | −23.16 (0.29) | −20.59 (0.84) |
Soil CO2 efflux was estimated from closed dynamic chamber measurements during the snow-free season and by a snow-CO2 gradient method during winter. Isotopic signature of the CO2 efflux were derived from Keeling-plots. Soil CO2 concentrations and δ13C values were determined from soil air sampled directly out of the soil profile at different depths (A-horizon 8 ± 1 cm; A/C-horizon 20 ± 3 cm; C-horizon 38 ± 4 cm)
Discussion
Our results point toward a minimal contribution of carbonate weathering to the overall soil CO2 efflux. Source partitioning using intact soil cores in the laboratory indicated a ~2–3 % contribution of CO2 from dolomite weathering, whereas a contribution of abiotic CO2 from limestone weathering was not detectable at all. These estimates include a 7 ‰ equilibrium isotope effect on soil CO2 caused by DIC leaching (see Fig. 1b) and would be lower without accounting for the 13C depletion inferred by this process. We incubated intact soil cores in the laboratory to constrain the CO2 sources to heterotrophic CO2 and CO2 originating from carbonate weathering products. Although tree roots in the cores likely continued to respire at lower rates during the first incubation stage, substantial CO2 contribution from the cut-off roots was unlikely during the latter part of the long-term incubation (60 and 166 days, respectively). Autotrophic respiration can make up to 50 % of the total soil CO2 efflux at the dolomite site (Schindlbacher et al. 2009). If the weathering rate is similar as in the lab, then the contribution of weathering derived CO2 in the field would be about 1 %, given a 50 % contribution of autotrophic respiration to soil CO2 efflux. As autotrophic respiration increases the CO2 partial pressure by up to 2–3 times in the A/C and C-horizon (Table 3) it likely contributes to carbonate weathering during the growing season. Accordingly, our incubation data indicate a realistic abiotic CO2 contribution between 1 and 2 % to total soil CO2 efflux at the forest growing on dolomite bedrock. As mentioned in the method section, we found considerable amounts of carbonate in some A-horizon cores which were supposed to exhibit only heterotrophic soil respiration. Although we could not find any sign for significant abiotic CO2 production in A-horizon cores, a minimal abiotic CO2 release could have occurred. Accordingly, the abiotic contribution to the soil CO2 efflux would be slightly underestimated. Our estimates, however, hold some uncertainty in the reverse direction as well. As we compared A-horizon cores with whole soil profile cores, we implied that the heterotrophic respiration from all cores has the same isotopic signature. It turned out, however, that the δ13C values of the organic C became less negative with increasing soil depth (Table 1). If heterotrophic respiration in deeper soil layers was enriched in13C and contributed significantly to the soil CO2 efflux, then the preconditions of our mass balance (Eq. 3) had been violated and the abiotic contribution was overestimated. The difference in absolute CO2 efflux between A-horizon cores and whole soil profile cores (Fig. 3) suggests that most of the CO2 was produced in the A-horizon. However, a smaller part of the CO2 efflux from the whole soil profile cores originated from deeper layers and could have influenced (13C enriched) the isotope signature of the headspace CO2. Therefore the estimated 1–2 % CO2 from carbonate weathering should rather be seen as the upper limit for the abiotic contribution to the total soil CO2 efflux.
Our DIC data further constrain the potential contribution of abiotic CO2 from carbonate weathering. Drainage water DIC concentrations from the dolomite soil cores were around 30 mg L−1 in our lab experiment. Considering seepage of about 1000 mm year−1 at the dolomite site (Feichtinger et al. 2002), the annual export of DIC would be around ~0.3 t C ha−1 year−1. This value is within the range of DIC export in other similar forests in Europe (Kindler et al. 2011) and fits well with catchment data of the Inn river of which our dolomite site is part of. The weathering intensity in the Inn river catchment was estimated at 60 meq HCO3− km−2 s−1 (corresponding to 0.23 t C ha−1 year−1) at a mean deep percolation rate of 750 mm (Szramek et al. 2007). Furthermore, our drainage water δ13C values were similar to those of other carbonate soils throughout Europe (~−15 ‰) (Kindler et al. 2011) suggesting that the majority of the DIC was of biogenic origin. In comparison to the annual soil respiration of ~7 t C ha−1 year−1 at the dolomite site (Schindlbacher et al. 2014), our roughly estimated DIC export of ~0.3 t C ha−1 year−1 makes less than 5 % of the annual soil CO2 efflux. Considered that only a minor fraction of these 5 % was abiotic (δ13C ~−15 ‰), and taking into account that most abiotic C is percolated, the contribution of abiotic C to the soil CO2 efflux must be minimal.
Minimal abiotic contribution to the soil CO2 efflux was supported by radiocarbon data which was assessed in a previous study at the dolomite site (Schindlbacher et al. 2012). Given that carbonate has a radiocarbon signature of −1000 ‰, even small amounts of CO2 released from this source have a strong impact on the radiocarbon signature of soil CO2 efflux. The radiocarbon signatures of the latter ranged between 21 and 76 ‰ (mean 54 ‰) at three sampling dates in the growing season of 2009, indicating that CO2 from dolomite weathering comprised on average not more than 1–1.5 % of the total soil CO2 efflux at this site.
Our incubation data suggest that the relative contribution of abiotic C is higher under drier conditions. We hypothesized (II) that wetter conditions foster carbonate dissolution and thereby increase the abiotic efflux-share whereas dryer conditions reduce carbonate weathering rates and the corresponding efflux. During our drying experiment, however, the following observations were made. Soil dried out very slowly because of low evaporation and lack of plant water use. During the first phase of the incubation only the litter layer and the very top-soil dried out whereas the larger part of the A-horizon as well as the deeper horizons remained moist. During the latter part of the incubation, the A-horizon had significantly dried out whereas the deeper horizons were still moist. Therefore, heterotrophic respiration in the SOM-rich upper soil layer was more affected by drying than carbonate weathering in the deeper and wetter soil horizons. Accordingly, the share of the abiotic contribution to the decreasing total soil CO2 efflux became larger. The pattern of soil moisture can be similar at the field site with driest litter layer and top-soil and comparatively wet sub-soil (Schindlbacher et al. 2012). Similar to our study, abiotic CO2 efflux in a Mediterranean shrubland commenced at low but steady rates, whereas decreasing soil moisture mostly affected the heterotrophic respiration of the dried out top-soil (Inglima et al. 2009).
Our lab data were generally in good agreement with the field measurements. Lab flux rates and soil CO2 concentrations were roughly half as high in the field during summer because of missing autotrophic respiration in the soil cores. The isotopic signature of the summertime field soil CO2 efflux and concentrations were also coherent with the lab data. An exception was the A-horizon where δ13C values of field soil CO2 were less negative than in the lab. This however was not surprising as the laboratory incubation was made under exclusion of atmospheric CO2 (δ13C values ~−8 ‰) which is considered to diffuse into the uppermost soil layers in the field. The seasonal variation in the isotopic signature of the soil CO2 efflux and CO2 concentrations in the field (Table 3) can have several reasons, one of them being variations in the contribution of abiotic CO2 from weathering. The dissolution rate of carbonate minerals is negatively related to temperature (Langmuir 1997) as well as the solubility of CO2 in water. Therefore, the relative contribution of abiotic CO2 to the soil CO2 efflux could be higher during the cold season. Indeed, we found less negative δ13C values of the soil CO2 during winter, which might be a hint in this direction. Similar patterns were observed by Carmi et al. (2013) who measured the δ13C values of the soil CO2 in a carbonate containing pine forest soil. Another reason for the less negative δ13C values of soil CO2 during winter could be higher mixing with atmospheric CO2, which occurs under lower soil respiration rates (Cerling 1984). The inverse pattern of δ13C values of soil CO2 in spring with less negative values in the deeper soil may be another indication for a potentially higher abiotic contribution. During spring, deeper soil layers are still cold but the CO2 partial pressure is already twice as high as during winter. Therefore enhanced carbonate weathering may have contributed to this atypical distribution of δ13C values throughout the soil profile. However, this is speculative because seasonal variations in autotrophic respiration and its isotopic signature could have influenced the isotopic signature of the field soil CO2 as well (Ekblad and Högberg 2001). The seasonal variation in the isotopic signature of the soil CO2 in the field suggests that our incubation based estimates of the abiotic CO2 efflux apply under growing season conditions whereas the relative abiotic contribution to the cold season soil CO2 efflux could be higher. As the wintertime soil CO2 efflux at our sites is in a range of ~10 % of the annual soil CO2 efflux (Schindlbacher et al. 2014), the effect on the annual C budget would, however, be small. At both of our field sites, the A-horizons showed small-scale variations in thickness (10–50 cm depth). CO2 efflux from soil with deep A-horizons is generally higher than from soil with shallow A-horizons (Schindlbacher, unpublished data). Accordingly, the relative contribution of abiotic CO2 will likely show high spatial variation in the field. Such small-scale variations in the abiotic contribution and hence in the isotopic signal of the soil CO2 efflux are relevant if natural abundance methods or radiocarbon studies are applied to forest soils on carbonate bedrock.
Our hypothesis (III) that the abiotic CO2 efflux is higher in the limestone soil could not be confirmed as well. While we already operated close to the detection limits of our experimental setup regarding the abiotic CO2 contribution from dolomite cores, we did not find evidence for an abiotic contribution to the soil CO2 efflux from the limestone cores. Due to the higher spatial variability of δ13C values of the soil CO2 efflux and the lower number of limestone cores, a minimal abiotic contribution to the soil CO2 efflux can, however, not be excluded. Generally, limestone dissolution is considered to occur at faster rates as dolomite dissolution (Chou et al. 1989; Morse and Arvidson 2002; Pokrovsky et al. 2005). Actual site specific weathering rates also depend on the degree of rock surface fracturing and probably also on microbial rock surface colonization (Davis et al. 2007). Site specific soil properties such as porosity and soil density might affect the transport and release of abiotic C which is produced predominately in the deeper soil layers and thereby also control the contribution to the total soil CO2 efflux. Therefore, a simple relationship between dissolution rates of the various carbonate bedrock and the abiotic soil CO2 efflux seems rather unlikely.
Conclusions
Our lab incubation indicated only minimal abiotic contributions to the soil CO2 efflux at the dolomite site whereas an abiotic contribution was not detectable at the limestone site. This is in agreement with the radiocarbon signature of the soil CO2 efflux and with geochemical weathering rates and the expected downward leaching of most of the weathering products in moist temperate environments. Seasonal variations in the isotopic signature of the CO2 in the field soil indicate that our incubation data apply under growing season conditions whereas the abiotic flux component could be higher during winter. The overall low contribution of abiotic CO2 to the soil CO2 efflux may be negligible in most C budgeting and biotic source partitioning approaches where the abiotic efflux should largely fall within the uncertainty range of the methods applied. Our data suggest that the abiotic contribution to soil CO2 efflux varies in space, time, and with environmental conditions. Such variations would influence the isotopic signal of the soil CO2 efflux and therefore could bias isotopic studies if not accounted for.
Electronic supplementary material
Relationship between the isotopic signature of the soil air CO2 and the inorganic and organic C concentration of the A-horizon only cores (a, b, dolomite; c, d, limestone) (JPEG 83 kb)
Acknowledgments
The study was funded by the Austrian Science Fund FWF (Project P23222-B17). We thank two reviewers for their constructive comments.
Footnotes
Responsible Editor: E. Veldkamp.
References
- Amiotte Suchet P, Probst J-L, Ludwig W. Worldwide distribution of continental rock lithology: implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob Biogeochem Cycles. 2003;17:1038. doi: 10.1029/2002GB001891. [DOI] [Google Scholar]
- Amundson R, Stern L, Baisden T, Wang Y. The isotopic composition of soil and soil-respired CO2. Geoderma. 1998;82:83–114. doi: 10.1016/S0016-7061(97)00098-0. [DOI] [Google Scholar]
- Attiwill PM, Adams MA. Nutrient cycling in forests. New Phytol. 1993;124:561–582. doi: 10.1111/j.1469-8137.1993.tb03847.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Godderis Y, Donnadieu Y, Labat D, Roelandt C. High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nature Clim Change. 2012;2:346–349. doi: 10.1038/nclimate1419. [DOI] [Google Scholar]
- Bowling DR, Massman WJ, Schaeffer SM, Burns SP, Monson RK, Williams MW. Biological and physical influences on the carbon isotope content of CO2 in a subalpine forest snowpack, Niwot Ridge, Colorado. Biogeochemistry. 2009;95:37–59. doi: 10.1007/s10533-008-9233-4. [DOI] [Google Scholar]
- Carmi I, Yakir D, Yechieli Y, Kronfeld J, Stiller M. Variations in soil CO2 concentrations and isotopic values in a semi-arid region due to biotic and abiotic processes in the unsaturated zone. Radiocarbon. 2013;55:932–942. doi: 10.2458/azu_js_rc.55.16236. [DOI] [Google Scholar]
- Čatera M, Ogrinc N. Soil respiration rates and in natural beech forest (Fagus sylvatica L.) in relation to stand structure. Isot Environ Health Stud. 2011;47:221–237. doi: 10.1080/10256016.2011.578214. [DOI] [PubMed] [Google Scholar]
- Cerling TE. The stabile isotopic composition of modern soil carbonate and its relation to climate. Earth Planet Sci Lett. 1984;71:229–240. doi: 10.1016/0012-821X(84)90089-X. [DOI] [Google Scholar]
- Cerling TE, Solomon DK, Quade J, Bowman JR. On the isotopic composition of carbon in soil carbon dioxide. Geochim Cosmochim Acta. 1991;55:3403–3405. doi: 10.1016/0016-7037(91)90498-T. [DOI] [Google Scholar]
- Chou L, Garrels RM, Wollast R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chemical Geology. 1989;78:269–282. doi: 10.1016/0009-2541(89)90063-6. [DOI] [Google Scholar]
- Ciais P, Borges AV, Abril G, Meybeck M, Folberth G, Hauglustaine D, Janssens IA. The impact of lateral carbon fluxes on the European carbon balance. Biogeosciences. 2008;5:1259–1271. doi: 10.5194/bg-5-1259-2008. [DOI] [Google Scholar]
- Davidson GR. The stable isotopic composition and measurement of carbon in soil CO2. Geochim Cosmochim Acta. 1995;59:2485–2489. doi: 10.1016/0016-7037(95)00143-3. [DOI] [Google Scholar]
- Davidson EA, Savage K, Bolstad P, Clark DA, Curtis PS, Ellsworth DS, Hanson PJ, Law BE, Luo Y, Pregitzer KS, Randolph JC, Zak D. Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agric For Meteorol. 2002;113:39–51. doi: 10.1016/S0168-1923(02)00101-6. [DOI] [Google Scholar]
- Davis KJ, Nealson KH, Lüttge A. Calcite and dolomite dissolution rates in the context of microbe–mineral surface interactions. Geobiology. 2007;5:191–205. doi: 10.1111/j.1472-4669.2007.00112.x. [DOI] [Google Scholar]
- Ekblad A, Högberg P. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia. 2001;127:305–308. doi: 10.1007/s004420100667. [DOI] [PubMed] [Google Scholar]
- Emmerich WE. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric For Meteorol. 2003;116:91–102. doi: 10.1016/S0168-1923(02)00231-9. [DOI] [Google Scholar]
- Emrich K, Vogel JC. Carbon isotope fractionation during precipitation of calcium carbonate. Earth Planet Sci Lett. 1970;8:363–371. doi: 10.1016/0012-821X(70)90109-3. [DOI] [Google Scholar]
- FAO . World Reference Base for Soil Resources. Rome: Food and Agriculture Organization of the United Nations; 1998. [Google Scholar]
- Feichtinger F, Smidt S, Klaghofer E. Water and nitrate fluxes at a forest site in the North Tyrolean Limestone Alps. Environ Sci Pollut Res. 2002;9:31–36. doi: 10.1007/BF02987475. [DOI] [PubMed] [Google Scholar]
- Formánek P, Ambus P. Assessing the use of δ13C natural abundance in separation of root and microbial respiration in a Danish beech (Fagus sylvatica L.) forest. Rapid Commun Mass Spectrom. 2004;18:897–902. doi: 10.1002/rcm.1424. [DOI] [PubMed] [Google Scholar]
- Giardina CP, Ryan MG. Total belowground carbon allocation in a fast-growing eucalyptus plantation estimated using a carbon balance approach. Ecosystems. 2002;5:487–499. doi: 10.1007/s10021-002-0130-8. [DOI] [Google Scholar]
- Gombert P. Role of karstic dissolution in global carbon cycle. Global Planet Change. 2002;33:177–184. doi: 10.1016/S0921-8181(02)00069-3. [DOI] [Google Scholar]
- Halas S, Szaran J, Niezgoda H. Experimental determination of carbon isotope equilibrium fractionation between dissolved carbonate and carbon dioxide. Geochim Cosmochim Acta. 1997;61:2691–2695. doi: 10.1016/S0016-7037(97)00107-5. [DOI] [Google Scholar]
- Hendy CH. The isotopic geochemistry of speleothems—I. The calculation of the effects of different modes of formation on the isotopic composition of speleothems and their applicability as palaeoclimatic indicators. Geochim Cosmochim Acta. 1971;35:801–824. doi: 10.1016/0016-7037(71)90127-X. [DOI] [Google Scholar]
- Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottoson-Lövenius M, Read DJ. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature. 2001;411:789–792. doi: 10.1038/35081058. [DOI] [PubMed] [Google Scholar]
- Inglima I, Alberti G, Bertolini T, Vaccari FP, Gioli B, Miglietta F, Cotrufo MF, Peressotti A. Precipitation pulses enhance respiration of Mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux. Glob Change Biol. 2009;15:1289–1301. doi: 10.1111/j.1365-2486.2008.01793.x. [DOI] [Google Scholar]
- Kayler ZE, Sulzman EW, Rugh WD, Mix AC, Bond BJ. Characterizing the impact of diffusive and advective soil gas transport on the measurement and interpretation of the isotopic signal of soil respiration. Soil Biol Biochem. 2010;42:435–444. doi: 10.1016/j.soilbio.2009.11.022. [DOI] [Google Scholar]
- Keeling CD. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim Cosmochim Acta. 1958;13:322–334. doi: 10.1016/0016-7037(58)90033-4. [DOI] [Google Scholar]
- Kindler R, Siemens JAN, Kaiser K, Walmsley DC, Bernhofer C, Buchmann N, Cellier P, Eugster W, Gleixner G, Grünwald T, Heim A, Ibrom A, Jones SK, Jones M, Klumpp K, Kutsch W, Larsen KS, Lehuger S, Loubet B, McKenzie R, Moors E, Osborne B, Pilegaard KIM, Rebmann C, Saunders M, Schmidt MWI, Schrumpf M, Seyfferth J, Skiba UTE, Soussana J-F, Sutton MA, Tefs C, Vowinckel B, Zeeman MJ, Kaupenjohann M. Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob Change Biol. 2011;17:1167–1185. doi: 10.1111/j.1365-2486.2010.02282.x. [DOI] [Google Scholar]
- Kowalski AS, Serrano-Ortiz P, Janssens IA, Sánchez-Moral S, Cuezva S, Domingo F, Were A, Alados-Arboledas L. Can tower flux research neglect geochemical CO2 exchange. Agric For Meteorol. 2008;148:1045–1054. doi: 10.1016/j.agrformet.2008.02.004. [DOI] [Google Scholar]
- Kuzyakov Y. Sources of CO2 from soil and review of partitioning methods. Soil Biol Biochem. 2006;38:425–448. doi: 10.1016/j.soilbio.2005.08.020. [DOI] [Google Scholar]
- Langmuir D. Aqueous environmental geology. Cardiff: Prentice Hall; 1997. [Google Scholar]
- Liu Z, Zhao J. Contribution of carbonate rock weathering to the atmospheric CO2 sink. Environ Geol. 1999;39:1053–1058. doi: 10.1007/s002549900072. [DOI] [Google Scholar]
- Liu Z, Yuan D, Dreybrodt W. Comparative study of dissolution rate-determining mechanisms of limestone and dolomite. Environ Geol. 2005;49:274–279. doi: 10.1007/s00254-005-0086-z. [DOI] [Google Scholar]
- Meybeck M. Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci. 1987;287:401–428. doi: 10.2475/ajs.287.5.401. [DOI] [Google Scholar]
- Mook WG, Bommerso JC, Staverma WH. Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet. Sci. Lett. 1974;22:169–176. doi: 10.1016/0012-821X(74)90078-8. [DOI] [Google Scholar]
- Morse JW, Arvidson RS. The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci Rev. 2002;58:51–84. doi: 10.1016/S0012-8252(01)00083-6. [DOI] [Google Scholar]
- Myrttinen A, Becker V, Barth JAC. A review of methods used for equilibrium isotope fractionation investigations between dissolved inorganic carbon and CO2. Earth-Sci Rev. 2012;115:192–199. doi: 10.1016/j.earscirev.2012.08.004. [DOI] [Google Scholar]
- Nordt LC, Hallmark CT, Wilding LP, Boutton TW. Quantifying pedogenic carbonate accumulations using stable carbon isotopes. Geoderma. 1998;82:115–136. doi: 10.1016/S0016-7061(97)00099-2. [DOI] [Google Scholar]
- Plestenjak G, Eler K, Vodnik D, Ferlan M, Čater M, Kanduč T, Simončič P, Ogrinc N. Sources of soil CO2 in calcareous grassland with woody plant encroachment. J Soils Sediments. 2012;12:1327–1338. doi: 10.1007/s11368-012-0564-3. [DOI] [Google Scholar]
- Pokrovsky OS, Golubev SV, Schott J. Dissolution kinetics of calcite, dolomite and magnesite at 25 & #xB0;C and 0 to 50 atm pCO2. Chemical Geology. 2005;217:239–255. doi: 10.1016/j.chemgeo.2004.12.012. [DOI] [Google Scholar]
- Ramnarine R, Wagner-Riddle C, Dunfield KE, Voroney RP. Contributions of carbonates to soil CO2 emissions. Can J Soil Sci. 2012;92:599–607. doi: 10.4141/cjss2011-025. [DOI] [Google Scholar]
- Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P, Bernhofer C, Buchmann N, Gilmanov T, Granier A, Grünwald T, Havránková K, Ilvesniemi H, Janous D, Knohl A, Laurila T, Lohila A, Loustau D, Matteucci G, Meyers T, Miglietta F, Ourcival J-M, Pumpanen J, Rambal S, Rotenberg E, Sanz M, Tenhunen J, Seufert G, Vaccari F, Vesala T, Yakir D, Valentini R. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Change Biol. 2005;11:1424–1439. doi: 10.1111/j.1365-2486.2005.001002.x. [DOI] [Google Scholar]
- Schindlbacher A, Zechmeister-Boltenstern S, Jandl R. Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Glob Change Biol. 2009;15:901–913. doi: 10.1111/j.1365-2486.2008.01757.x. [DOI] [Google Scholar]
- Schindlbacher A, De Gonzalo C, Díaz-Pinés E, Gorría P, Matthews B, Inclán R, Zechmeister-Boltenstern S, Rubio A, Jandl R. Temperature sensitivity of forest soil organic matter decomposition along two elevation gradients. J Geophys Res. 2010;115:G03018. [Google Scholar]
- Schindlbacher A, Wunderlich S, Borken W, Kitzler B, Zechmeister-Boltenstern S, Jandl R. Soil respiration under climate change: prolonged summer drought offsets soil warming effects. Glob Change Biol. 2012;18:2270–2279. doi: 10.1111/j.1365-2486.2012.02696.x. [DOI] [Google Scholar]
- Schindlbacher A, Jandl R, Schindlbacher S. Natural variations in snow cover do not affect the annual soil CO2 efflux from a mid-elevation temperate forest. Glob Change Biol. 2014;20:622–632. doi: 10.1111/gcb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Ortiz P, Roland M, Sanchez-Moral S, Janssens IA, Domingo F, Goddéis Y, Kowalski AS. Hidden, abiotic CO2 flows and gaseous reservoirs in the terrestrial carbon cycle: review and perspectives. Agric For Meteorol. 2010;150:321–329. doi: 10.1016/j.agrformet.2010.01.002. [DOI] [Google Scholar]
- Skidmore M, Sharp M, Tranter M. Kinetic isotopic fractionation during carbonate dissolution in laboratory experiments: implications for detection of microbial CO2 signatures using δ 13C-DIC. Geochim Cosmochim Acta. 2004;68:4309–4317. doi: 10.1016/j.gca.2003.09.024. [DOI] [Google Scholar]
- Spötl C, Vennemann TW. Continuous-flow isotope ratio mass spectrometric analysis of carbonate minerals. Rapid Commun Mass Spectrom. 2003;17:1004–1006. doi: 10.1002/rcm.1010. [DOI] [PubMed] [Google Scholar]
- Stevenson BA, Verburg PSJ. Effluxed CO2-13C from sterilized and unsterilized treatments of calcareous soil. Soil Biol Biochem. 2006;38:1727–1733. doi: 10.1016/j.soilbio.2005.11.028. [DOI] [Google Scholar]
- Szramek K, McIntosh JC, Williams EL, Kanduc T, Ogrinc N, Walter LM. Relative weathering intensity of calcite versus dolomite in carbonate-bearing temperate zone watersheds: carbonate geochemistry and fluxes from catchments within the St. Lawrence and Danube river basins. Geochem Geophys Geosyst. 2007;8:Q04002. doi: 10.1029/2006GC001337. [DOI] [Google Scholar]
- Tamir G, Shenker M, Heller H, Bloom PR, Fine P, Bar-Tal A. Can soil sarbonate dissolution lead to overestimation of soil respiration? Soil Sci Soc Am J. 2011;75:1414–1422. doi: 10.2136/sssaj2010.0396. [DOI] [Google Scholar]
- van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundström US. The carbon we do not see—the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol Biochem. 2005;37:1–13. doi: 10.1016/j.soilbio.2004.06.010. [DOI] [Google Scholar]
- Werth M, Kuzyakov Y. 13C fractionation at the root-microorganisms-soil interface: a review and outlook for partitioning studies. Soil Biol Biochem. 2010;42:1372–1384. doi: 10.1016/j.soilbio.2010.04.009. [DOI] [Google Scholar]
- Wingate L, Jrm Ogée, Cuntz M, Genty B, Reiter I, Seibt U, Yakir D, Maseyk K, Pendall EG, Barbour MM, Mortazavi B, Rg Burlett, Peylin P, Miller J, Mencuccini M, Shim JH, Hunt J, Grace J. The impact of soil microorganisms on the global budget of δ18O in atmospheric CO2. Proc Natl Acad Sci. 2009;106:22411–22415. doi: 10.1073/pnas.0905210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between the isotopic signature of the soil air CO2 and the inorganic and organic C concentration of the A-horizon only cores (a, b, dolomite; c, d, limestone) (JPEG 83 kb)


