Abstract
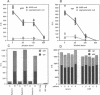
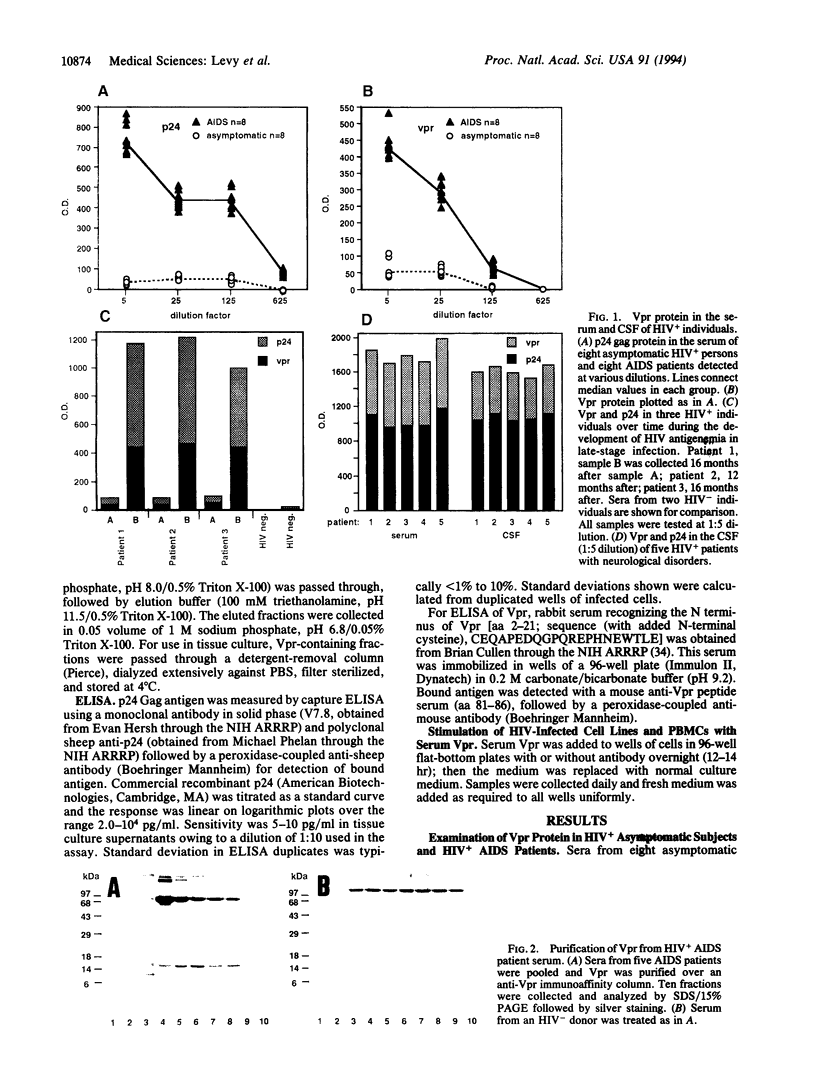
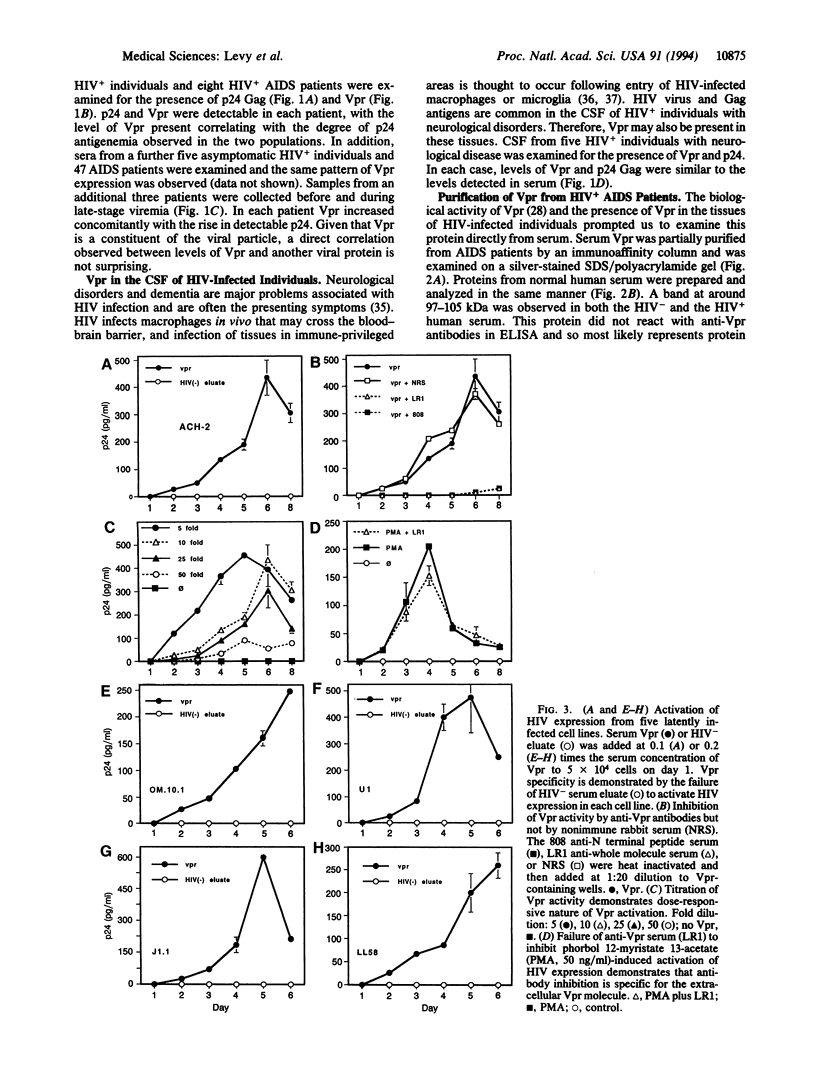
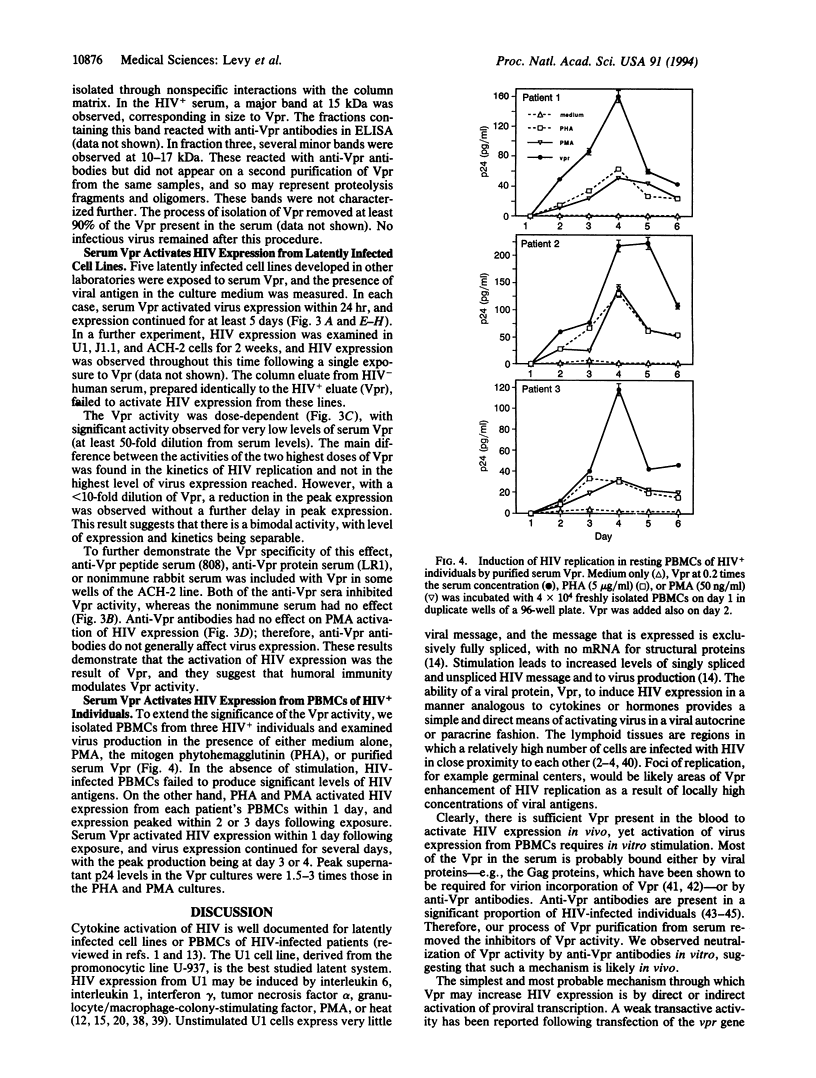
In human immunodeficiency virus (HIV)-positive individuals, the vast majority of infected peripheral blood cells and lymph node cells may be latently or nonproductively infected. The vpr open reading frame of HIV-1 encodes a 15-kDa virion-associated protein, Vpr. The vpr gene has been shown to increase virus replication in T cells and monocyte/macrophages in vitro. We have previously reported that vpr expression in various tumor lines leads to growth inhibition and differentiation, indicating that Vpr may function as a regulator of cellular permissiveness to HIV replication. Here we show that Vpr protein is present in significant amounts in the serum of AIDS patients. Purified serum Vpr activated virus expression from five latently infected cell lines, U1, OM.10.1, ACH-2, J1.1, and LL58. Serum Vpr also activated virus expression from resting peripheral blood mononuclear cells of HIV-infected individuals. Together, these findings implicate serum Vpr in the activation of HIV replication in vivo and in the control of latency. Anti-Vpr antibodies inhibited Vpr activity, suggesting that humoral immunity modulates Vpr activity in vivo. These results have broad implications for the virus life cycle and for the prospective control of HIV replication and pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balotta C., Lusso P., Crowley R., Gallo R. C., Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1993 Jul;67(7):4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Folks T. M. Mechanisms of HIV-1 latency. AIDS. 1992 Jan;6(1):3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- Biswas P., Poli G., Kinter A. L., Justement J. S., Stanley S. K., Maury W. J., Bressler P., Orenstein J. M., Fauci A. S. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992 Sep 1;176(3):739–750. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera S. T., Perez V. L., Wu B. Y., Nabel G. J., Folks T. M. Oscillation of the human immunodeficiency virus surface receptor is regulated by the state of viral activation in a CD4+ cell model of chronic infection. J Virol. 1991 Sep;65(9):4645–4653. doi: 10.1128/jvi.65.9.4645-4653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera S. T., Roberts B. D., Leung K., Nabel G. J., Folks T. M. Tumor necrosis factor receptor expression and signal transduction in HIV-1-infected cells. AIDS. 1993 Jul;7(7):911–918. doi: 10.1097/00002030-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. I., Koyanagi Y., Horiuchi S., Hazeki O., Ui M., Kitano K., Golde D. W., Takada K., Yamamoto N. cAMP stimulates human immunodeficiency virus (HIV-1) from latently infected cells of monocyte-macrophage lineage: synergism with TNF-alpha. Virology. 1993 May;194(1):345–349. doi: 10.1006/viro.1993.1265. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Dehni G., Sodroski J. G., Haseltine W. A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990 Jun;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Jalinoos Y., Proulx J., Sodroski J. G., Haseltine W. A. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3(1):11–18. [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Beneke J., Till M., Wolinsky S., Ribas J. L., Burke A., Haase A. T. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Garrett E. D., Tiley L. S., Cullen B. R. Rev activates expression of the human immunodeficiency virus type 1 vif and vpr gene products. J Virol. 1991 Mar;65(3):1653–1657. doi: 10.1128/jvi.65.3.1653-1657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras-Masse H., Ameisen J. C., Boutillon C., Gesquière J. C., Vian S., Neyrinck J. L., Drobecq H., Capron A., Tartar A. A synthetic protein corresponding to the entire vpr gene product from the human immunodeficiency virus HIV-1 is recognized by antibodies from HIV-infected patients. Int J Pept Protein Res. 1990 Sep;36(3):219–226. doi: 10.1111/j.1399-3011.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Hattori N., Michaels F., Fargnoli K., Marcon L., Gallo R. C., Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Lang S. M., Weeger M., Stahl-Hennig C., Coulibaly C., Hunsmann G., Müller J., Müller-Hermelink H., Fuchs D., Wachter H., Daniel M. M. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993 Feb;67(2):902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. N., Fernandes L. S., Williams W. V., Weiner D. B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993 Feb 26;72(4):541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. L., Spearman P., Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993 Nov;67(11):6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia B. A., Price R. W. The acquired immunodeficiency syndrome dementia complex as the presenting or sole manifestation of human immunodeficiency virus infection. Arch Neurol. 1987 Jan;44(1):65–69. doi: 10.1001/archneur.1987.00520130051017. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Shibata R., Kiyomasu T., Higuchi I., Kishida Y., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989 Sep;63(9):4110–4114. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Butini L., Pizzo P. A., Schnittman S. M., Kotler D. P., Fauci A. S. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Paxton W., Connor R. I., Landau N. R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J Virol. 1993 Dec;67(12):7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez V. L., Rowe T., Justement J. S., Butera S. T., June C. H., Folks T. M. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991 Nov 1;147(9):3145–3148. [PubMed] [Google Scholar]
- Poli G., Bressler P., Kinter A., Duh E., Timmer W. C., Rabson A., Justement J. S., Stanley S., Fauci A. S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990 Jul 1;172(1):151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Reiss P., Lange J. M., de Ronde A., de Wolf F., Dekker J., Danner S. A., Debouck C., Goudsmit J. Antibody response to viral proteins U (vpu) and R (vpr) in HIV-1-infected individuals. J Acquir Immune Defic Syndr. 1990;3(2):115–122. [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Shibata R., Adachi A., Sakai H., Ishimoto A., Miura T., Hayami M. Mutational analysis of simian immunodeficiency virus from African green monkeys and human immunodeficiency virus type 2. J Med Primatol. 1990;19(3-4):217–225. [PubMed] [Google Scholar]
- Shibata R., Miura T., Hayami M., Ogawa K., Sakai H., Kiyomasu T., Ishimoto A., Adachi A. Mutational analysis of the human immunodeficiency virus type 2 (HIV-2) genome in relation to HIV-1 and simian immunodeficiency virus SIV (AGM). J Virol. 1990 Feb;64(2):742–747. doi: 10.1128/jvi.64.2.742-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S. K., Bressler P. B., Poli G., Fauci A. S. Heat shock induction of HIV production from chronically infected promonocytic and T cell lines. J Immunol. 1990 Aug 15;145(4):1120–1126. [PubMed] [Google Scholar]
- Stanley S. K., Kessler S. W., Justement J. S., Schnittman S. M., Greenhouse J. J., Brown C. C., Musongela L., Musey K., Kapita B., Fauci A. S. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992 Jul 15;149(2):689–697. [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Chanda P. K., Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987 Spring;3(1):33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Yu X. F., Matsuda M., Essex M., Lee T. H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990 Nov;64(11):5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Matsuda Z., Matsuda M., Essex M., Lee T. H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]