Abstract
Background
Congestion is the most frequent cause for hospitalization in acute decompensated heart failure (ADHF). Although decongestion is a major goal of acute therapy, it is unclear how the clinical components of congestion (e.g., peripheral edema, orthopnea) contribute to outcomes after discharge or how well decongestion is maintained.
Methods and Results
A post-hoc analysis was performed of 496 patients enrolled in the DOSE-AHF and CARRESS-HF trials during hospitalization with ADHF and clinical congestion. A simple “orthodema” congestion score was generated based on symptoms of orthopnea (≥2 pillows=2 points, <2 pillows=0 points) and peripheral edema (trace=0 points, moderate=1 point, severe=2 points) at baseline, discharge, and 60-day follow-up. Orthodema scores were classified as absent (score of 0), low-grade (score of 1–2), and high-grade (score of 3–4), and the association with death, rehospitalization or unscheduled medical visits through 60 days was assessed. At baseline, 65% of patients had high-grade orthodema and 35% had low-grade orthodema. At discharge, 52% patients were free from orthodema at discharge (score = 0) and these patients had lower 60-day rates of death, rehospitalization, or unscheduled visits (50%) compared to those with low-grade or high-grade orthodema (52% and 68%, respectively, p=0.038). Of the patients without orthodema at discharge, 27% relapsed to low-grade orthodema and 38% to high-grade orthodema at 60-day follow-up.
Conclusions
Increased severity of congestion by a simple orthodema assessment is associated with increased morbidity and mortality. Despite intent to relieve congestion, current therapy often fails to relieve orthodema during hospitalization or to prevent recurrence after discharge.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifiers: NCT00608491, NCT00577135.
Keywords: acute decompensated heart failure, congestion, hospitalization, edema, dyspnea
Patients hospitalized with acute decompensated heart failure (ADHF) are at high risk for readmission, morbidity and mortality.1, 2 The increasing costs and frequency of admissions for ADHF are a focus of patient care efforts and health care reform, necessitating the identification of patients at high risk for future events in order to target therapeutic interventions. The most common symptoms accompanying admission for ADHF are dyspnea on minimal exertion or orthopnea, fatigue and peripheral edema (3). These symptoms reflect an acute or chronic increase in cardiac filling pressures, or ‘congestion’. “Decongestion” is therefore considered a primary goal of acute therapy. However, with current therapy, it is not clear 1) what proportion of patients are relieved of congestion during hospitalization, 2) what proportion remain free of congestion at short-term follow up and 3) which signs and symptoms of congestion best correlate with outcomes post discharge. The ADHF cohorts studied in the NHLBI-sponsored Heart Failure Network trials of Diuretic Optimization Strategy Evaluation (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) provide a unique opportunity to study these relationships given the specificity of entry criteria for baseline congestion and the consistent clinical assessment of congestion status at admission, prior to discharge and through 60 days. We hypothesized that clinical evidence of congestion would be relieved for the majority of patients hospitalized with ADHF and that most patients would remain free from congestion at 60-day follow up. In addition, we hypothesized that residual congestion at hospital discharge would be associated with worse 60-day clinical outcomes.
Methods
Data Source and Study Population
This analysis used data from DOSE-AHF (ClinicalTrials.gov number, NCT00577135) and CARRESS-HF (ClinicalTrials.gov number NCT00608491). The design and results of both trials have been published previously.3–6 Briefly, both trials were prospective, double-blinded, and randomized patients hospitalized with ADHF at 9 regional HF centers to specific decongestion strategies. DOSE-AHF used a 2×2 factorial design to randomize 308 patients to low-dose versus high-dose furosemide therapy and continuous versus intermittent bolus administration of furosemide. CARRESS-HF randomized 188 patients with ADHF and worsened renal function, to stepped pharmacologic therapy or ultrafiltration. Both trials included patients regardless of ejection fraction (EF) and required evidence of congestion prior to entry (detailed below). The same clinical assessment of congestion (jugular venous pressure (JVP), orthopnea and peripheral edema) was made and recorded in both trials at baseline, 24, 48, 72, 96, day 7 or discharge (whichever came first), and day 60 or early termination. All patients were followed after discharge at regular intervals according to trial design and at 60 days for clinical assessments.
The DOSE-AHF and CARRESS-HF studies were approved by the Heart Failure Network Steering, Protocol Review and Data Safety Monitoring Committees as well as each participating site’s institutional review boards. All patients provided written informed consent.
Symptoms of Congestion
Clinical evidence of congestion (Table 1) was required for entry into both studies. Based on previous studies7–9 peripheral edema, elevated JVP, and orthopnea were included as markers of congestion. Each of these three characteristics was graded separately and then added together for the purpose of assessing their relationship to the combined outcome of death, hospitalization, or unscheduled emergency room or clinic visits through 60 days post-randomization.
Table 1.
Entry criteria and markers of congestion in DOSE and CARRESS.
| DOSE | CARRESS | |
|---|---|---|
| Number of patients | 308 | 188 |
| LVEF | HFrEF and HFpEF | HFrEF and HFpEF |
| Inclusion | Documented HF on loop diuretic > 1 month |
Onset of cardiorenal syndrome after hospitalization for ADHF, or worsening renal function in preceding 12 weeks. |
| Evidence of congestion | 1 sign (rales, peripheral edema, ascites or pulmonary vascular congestion on CXR) AND 1 symptom (dyspnea, orthopnea or edema) |
2 of the following:
|
| Moderate or severe peripheral edema |
79% | 89% |
| JVP>8cmH20 | 81% | 97% |
| ≥ 2 pillow orthopnea | 78% | 82% |
LVEF = Left ventricular ejection fraction; HFrEF = Heart failure with reduced ejection fraction HFpEF = heart failure with preserved ejection fraction; HF = heart failure; ADHF = acute decompensated heart failure; CXR = chest x-ray; JVP= jugular venous pressure
JVP did not correlate with subsequent outcomes in this analysis (Supplemental Figure 1) and is not a part of patient symptom burden. Furthermore, the reliability of JVP assessment in general care settings has been called into question.10 As inclusion of JVP did not provide added predictive value, it was not used in the present investigation. Rather, for this analysis we combined the two resting symptoms of congestion that were systematically recorded: peripheral edema and orthopnea. Edema was categorized as trace/mild (0 points), moderate (1 point), or severe (2 points). Orthopnea was defined as present if the patient needed at least 2 pillows to breathe comfortably (2 points) or absent (0 points). The Orthodema Score was then generated by the sum of the individual orthopnea and edema scores (Table 2). A total score of 1 represents the presence of moderate edema without orthopnea. A score of 2 indicates the presence of orthopnea or severe peripheral edema, but not both. Scores of 1–2 represent low-grade congestion. High-grade congestion includes orthopnea and edema, with a score of 3 for orthopnea plus moderate edema, and a score of 4 if orthopnea is accompanied by severe edema.
Table 2.
Orthodema Scores
| Mild edema, no orthopnea | 0 | No congestion |
| Moderate edema, no orthopnea | 1 | Low-grade orthodema/congestion |
| Severe edema OR orthopnea | 2 | |
| Moderate edema AND orthopnea | 3 | High-grade orthodema/congestion |
| Severe edema AND orthopnea | 4 |
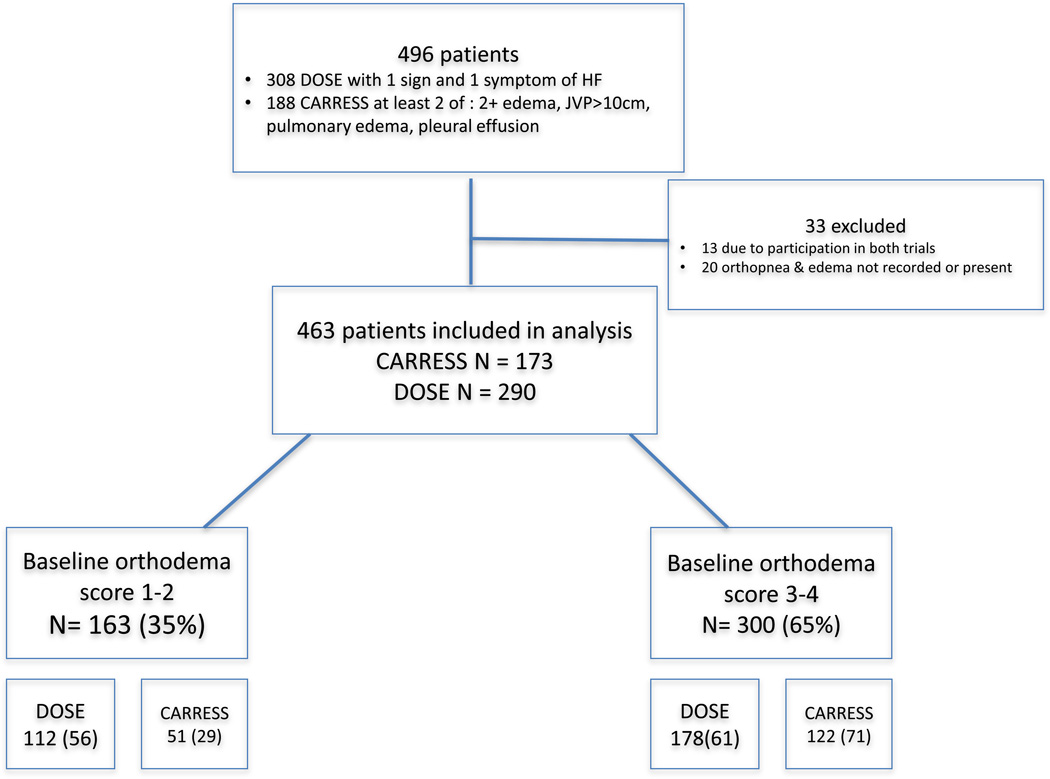
As congestion was a prerequisite for study entry, patients with an orthodema score of 0 (similar to the overall analysis population) were excluded from the present study (Figure 1). Orthodema scores were described at baseline, discharge and at 60-day follow-up. If a hospitalization for heart failure occurred in the 60-day follow-up period, patients were assigned the worst orthodema score of 4. This occurred in 85 instances.
Figure 1.
Study patient population
Abbreviation: Sx: symptom; HF = heart failure.
Outcomes
The primary clinical outcome analyzed was the time to the composite of death, rehospitalization and/or unscheduled urgent clinic or emergency room visit by 60 days.
Statistical Analysis
Patients with an orthodema score of 1–2 were compared to patients with an orthodema score of 3–4 for baseline characteristics, presented as medians (25th, 75th percentiles) and compared with Wilcoxon rank sum tests for continuous variables. Categorical variables were presented as percentages and compared with chi-squared tests. Baseline variables included clinical covariates of age, sex, systolic blood pressure (SBP), medications, heart rate, history of ischemic cardiomyopathy, diabetes mellitus, EF, current smoking, and body mass index (BMI).
Characteristics of patients who demonstrated relief of congestion (orthodema score of 0) at discharge were compared to those with low-grade congestion (scores of 1–2) and those with high-grade congestion (scores of 3–4) and presented as medians (25th, 75th percentiles). Wilcoxon rank sum tests were used for comparison of continuous variables, and chi-square tests were used for comparison of categorical variables.
Logistic regression models were used to analyze the association between orthodema scores at baseline or at discharge and the composite clinical outcome of death, rehospitalization or unscheduled emergency room or clinic visit. Models were not adjusted for baseline characteristics as many contribute to congestion and doing so would diminish the practical utility of the orthodema score. No imputation or carry forward was used to account for missing data.
Weight changes in pounds were presented as mean values. A general linear model was used to compare the baseline congestion scores of 1–2 to 3–4 with respect to percentage weight loss at Day 7 or discharge. Pairwise testing was performed to detect differences between the orthodema score groups. Length of stay was expressed in mean number of days.
A P-value of 0.05 was considered statistically significant. SAS version 9.2 (Cary, NC) was used for all analyses.
Results
Congestion Status at Baseline
Of the 496 patients enrolled in the DOSE-AHF and CARRESS-HF trials from March 2008 to January 2012, 13 were excluded due to overlapping enrollment in both trials and an additional 20 were excluded because baseline values for edema or orthopnea were either missing, or summed to an orthodema score of 0 (Figure 1). High-grade orthodema (scores 3–4) was present in 65% of patients at enrollment. These subjects had lower use of angiotensin-converting-enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), higher BMI, worse renal function and lower hemoglobin compared to those with low-grade orthodema (scores 1–2). No significant differences were observed for age, EF, etiology of HF, number of prior cardiovascular (CV) or HF hospitalizations, or pre-hospital furosemide dose (Table 3).
Table 3.
Characteristics by baseline Orthodema score.
| Baseline Orthodema Score 1 or 2 | Baseline Orthodema Score 3 or 4 | P-Value for Any Difference |
|
|---|---|---|---|
| Age | |||
| N | 163 | 300 | 0.54 |
| Median (25th, 75th) | 69 ( 59, 78) | 68 ( 57, 78) | |
| Mean +/− SD | 68 +/− 13 | 67+/− 14 | |
| Sex, N (%) | |||
| Male | 118 (72) | 222 (74) | 0.71 |
| Female | 45 (28) | 78 (26) | |
| SBP | |||
| N | 163 | 298 | 0.44 |
| Median (25th, 75th) | 113 (104,125) | 116 (103,130) | |
| Mean +/− SD | 117+/− 19 | 119+/− 19 | |
| HR | |||
| N | 162 | 300 | 0.55 |
| Median (25th, 75th) | 75 ( 68, 83) | 76 ( 68, 86) | |
| Mean +/− SD | 77+/− 14 | 78+/− 17 | |
| ACE-inhibitor at baseline, N (%) | |||
| Yes | 68 (42) | 112 (37) | 0.36 |
| No | 95 (58) | 188 (63) | |
| ARB at baseline, N (%) | |||
| Yes | 38 (23) | 52 (17) | 0.12 |
| No | 125 (77) | 248 (83) | |
| ACE/ARB at baseline, N (%) | |||
| Yes | 103 (63) | 157 (52) | 0.024 |
| No | 60 (37) | 143 (48) | |
| Beta blocker at baseline, N (%) | |||
| Yes | 137 (84) | 240 (80) | 0.28 |
| No | 26 (16) | 60 (20) | |
| Aldosterone antagonist at baseline, N (%) | |||
| Yes | 41 (25) | 80 (27) | 0.73 |
| No | 122 (75) | 220 (73) | |
| Calcium channel blocker at baseline, N (%) | |||
| Yes | 27 (17) | 52 (17) | 0.83 |
| No | 136 (83) | 248 (83) | |
| BMI | |||
| N | 155 | 285 | 0.023 |
| Median (25th, 75th) | 31 (26, 37) | 33 (28, 40) | |
| Mean +/− SD | 32+/− 8 | 35+/− 10 | |
| PVD, N (%) | |||
| Yes | 28 (17) | 51 (17) | 0.96 |
| No | 135 (83) | 249 (83) | |
| Diabetes, N (%) | |||
| Yes | 85 (52) | 176 (59) | 0.18 |
| No | 78 (48) | 124 (41) | |
| Gout, N (%) | |||
| Yes | 37 (23) | 66 (22) | 0.86 |
| No | 126 (77) | 234 (78) | |
| Smoking history, N (%) | |||
| Current | 21 (13) | 34 (11) | 1.0 |
| Quit < 6 months | 8 (5) | 10 (3) | |
| Quit >= 6 months | 77 (47) | 158 (53) | |
| Never | 57 (35) | 98 (33) | |
| LVEF | |||
| N | 162 | 293 | 0.84 |
| Median (25th, 75th) | 31 ( 20, 55) | 30 ( 20, 55) | |
| Mean +/− SD | 36+/− 18 | 36+/− 18 | |
| HF Etiology | |||
| Ischemic | 102 (63) | 164 (55) | 1.0 |
| Non-Ischemic | 61 (37) | 136 (45) | |
| Number CV hosp past 12 mo | |||
| N | 161 | 295 | 0.26 |
| Median (25th, 75th) | 1 (1, 3) | 2 (1,3) | |
| Mean +/− SD | 2+/− 2 | 2+/− 2 | |
| Number HF hosp past 12 mo | 161 | 295 | 0.15 |
| N | 161 | 295 | 0.15 |
| Median (25th, 75th) | 1.0 ( 0.0, 2.0) | 1.0 ( 1.0, 2.0) | |
| Mean +/− SD | 1.5+/− 1.5 | 1.7+/− 1.7 | |
| BUN | 163 | 300 | 0.38 |
| N | 163 | 300 | 0.38 |
| Median (25th, 75th) | 38 (25, 56) | 40 (25, 58) | |
| Mean +/− SD | 42+/− 23 | 44.4+/− 24.6 | |
| Local Lab Creatinine | |||
| N | 163 | 298 | 0.017 |
| Median (25th, 75th) | 1.7 (1.2, 2.1) | 1.8 (1.3, 2.3) | |
| Mean +/− SD | 1.69+/− 0.59 | 1.85+/− 0.63 | |
| GFR | |||
| N | 163 | 298 | 0.085 |
| Median (25th, 75th) | 42 (32, 59) | 40 (29, 57) | |
| Mean +/− SD | 49.5+/− 25.6 | 45.2+/− 21.1 | |
| Hemoglobin | |||
| N | 160 | 296 | 0.02 |
| Median (25th, 75th) | 11.7 ( 10.3, 12.8) | 11.1 (9.8, 12.5) | |
| Mean +/− SD | 11.6 +/− 1.8 | 11.2 +/− 1.9 | |
| Pre-Hospital/Qualifying Furosemide Dose | |||
| N | 163 | 300 | 0.83 |
| Median (25th, 75th) | 120 (80, 160) | 120 (80, 160) | |
| Mean +/− SD | 133+/− 89 | 139+/− 101 |
SBP= systolic blood pressure; HR = heart rate; ACE inhibitor = angiotensin converting enzyme inhibitor; ARB= angiotensin receptor blocker; BMI = body mass index; PVD = peripheral vascular disease; LVEF = left ventricular ejection fraction; HF = heart failure; CV = cardiovascular; Hosp = hospitalizations; BUN = blood urea nitrogen; GFR = glomerular filtration rate
Congestion Status at Discharge
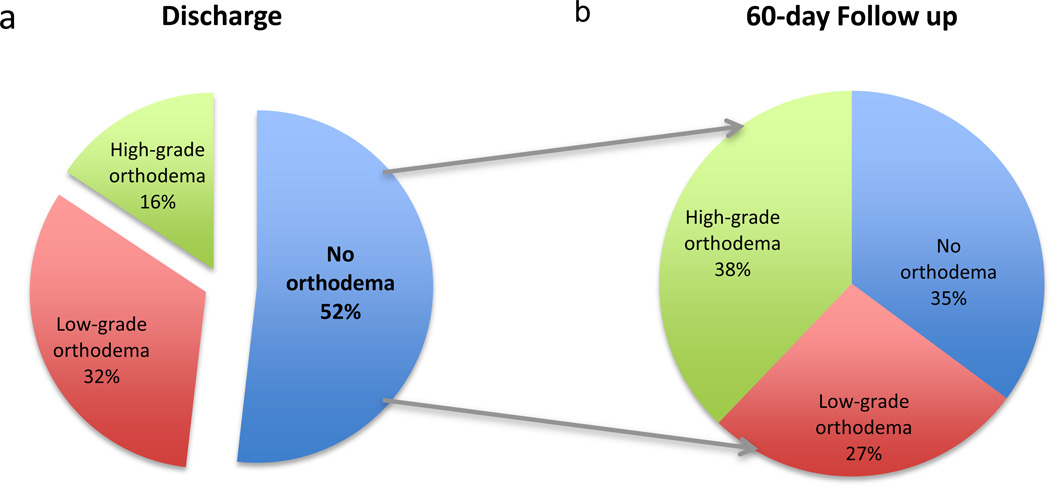
Orthodema scores at all three time points (baseline, discharge and 60-day follow-up) were available in 357 patients. Following treatment with diuretic therapy and/or ultrafiltration, an orthodema score of 0 was achieved in 52% of patients at discharge; the remaining patients had scores of 1–2 (32%) or scores 3–4 (16%) as shown in Figure 2a. Compared with patients who achieved successful decongestion by discharge (orthodema score of 0), patients who remained congested at discharge (orthodema scores of 1–4) had higher BMI (median of 34.1 vs. 30.5; p<0.0001) were more likely to be diabetic (62% vs. 51%; p=0.02) and had higher BUN levels (41 vs. 37.5; p=0.03). None of these factors were significantly worse in patients with more congestion at baseline. No between-group differences were noted in age, gender, SBP, heart rate, other comorbidity profile, EF, HF etiology or hemoglobin levels when comparing the patients with low or high-grade orthodema with those without orthodema at discharge.
Figure 2.
(a) Congestion status at discharge
(b) For patients relieved of congestion at discharge, congestion status at 60-day follow-up
Weight change
Weight recordings were available at baseline and discharge for 426 patients. Overall, no significant difference in weight change was observed for patients with baseline orthodema scores of 1–2 versus 3–4 (p=0.33). For patients who achieved decongestion (score 0), the average weight loss from baseline to discharge was 13.5 pounds. Patients with low-grade orthodema (scores 1–2) at baseline who attained decongestion at discharge lost a mean of 12.2 pounds, compared to a loss of 8.8 pounds in patients for whom low-grade orthodema persisted at discharge (Table 4). The pattern of greater observed weight loss corresponding to decongestion was not consistent however. Comparable weight loss occurred even for patients with persistent orthodema at discharge. Of 282 patients with high-grade orthodema at baseline, 68 patients were discharged with persistent high-grade orthodema despite a weight loss of 14.2 pounds. Comparatively, the patients with high-grade orthodema at baseline who were successfully decongested and free of orthodema at discharge (N=123) lost a similar average of 14.6 pounds. After adjustment for baseline weight, the baseline orthodema score did not predict day 7/discharge absolute or relative weight loss.
Table 4.
Observed weight loss (in pounds) according to baseline and discharge orthodema scores
| Discharge Orthodema score | |||
|---|---|---|---|
| Baseline Orthodema Score |
0 | 1/2 | 3/4 |
| 1/2 | 12.2 ± 9.1 (N=93) | 8.8 ± 9.5 (N=50) | * |
| 3/4 | 14.6 ± 14.5 (N=123) | 12.6 ± 11.5 (N=91) | 14.2 ± 14.1 (N=68) |
22.7 ± N/A (N=1)
Re-Congestion After Discharge
Of the 185 patients who were free from congestion at discharge, 35% remained free from congestion, 27% regressed to low-grade orthodema (scores of 1–2) and 38% regressed to high-grade orthodema (scores of 3–4), indicating recurrence of congestion at 60-day follow up (Figure 2b). Compared with patients who maintained a decongested status, patients who experienced high-grade recongestion at 60 days were less likely to be on ACE inhibitors/ARBs and aldosterone antagonists, had worse renal function and lower hemoglobin levels. There were no between-group differences in EF, HF etiology, the number of CV or HF admissions in the prior year or pre-hospital furosemide dose.
Congestion in Relation to Outcomes
Patients with more congestion at baseline (orthodema scores 3–4) had longer length of stay than patients with baseline orthodema scores 1–2 (mean 8.9 day vs. 7.1 days) and at each time point after admission were more likely to still be in hospital (p=0.004). The presence of orthopnea at baseline or discharge was also associated with worse post-discharge outcomes. Orthopnea was present at baseline in 83% of patients, who had an event rate of 57% compared to 30% in the patients without baseline orthopnea (Supplemental Figure 2). While baseline edema severity did not predict outcomes, persistent edema at discharge was associated with worse post-discharge outcomes (Supplemental Figure 2b). The 60-day rates of death, readmission, or unplanned clinic visit were 51, 55, and 71% in those with no edema, moderate, and severe edema, respectively, at discharge. As mentioned previously, there was no association between JVP and outcome either at baseline or at discharge (Supplemental Figure 1).
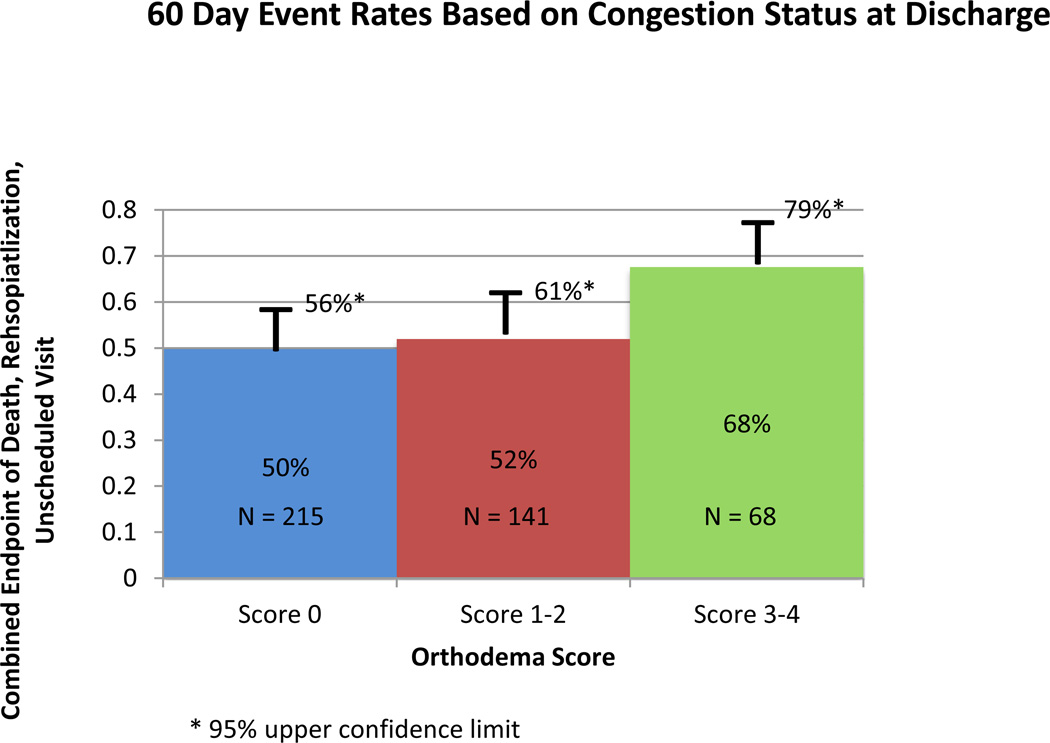
High-grade orthodema at discharge was associated with worse outcomes (Figure 3): patients with an orthodema score of 0 had an event rate of 50% as compared to 52% for patients with scores of 1–2 and 68% with scores of 3–4 (p=0.038).
Figure 3.
60-day Event rates based on discharge orthodema score to represent congestion. P=0.038
Discussion
In the present analysis of patients undergoing therapy to relieve congestion, orthodema persisted in nearly half of subjects at hospital discharge. Even among patients achieving clinical decongestion, only one-third remained free from congestion at 60-day follow-up with the remaining two-thirds demonstrating relapse of orthopnea and/or edema. Though event rates were high regardless of discharge congestion status, the presence of orthodema was associated with even worse outcomes.
Identifying Congestion
There is increasing recognition of the contribution of clinical congestion not only to the symptomatic burden of patients but also to the national burden of re-hospitalizations and to the progression of renal and liver dysfunction with cardiac cachexia.11–16 Multiple scores have been proposed to help identify and track congestion after HF admission.
JVP has been included in many of these scores, in part due to its recognized role in outpatient triage. The presence of jugular venous distention (JVD) in a stable ambulatory population was shown by Dries et. al. 7 to predict worse outcomes in the landmark Study of Left Ventricular Dysfunction (SOLVD) trial of ACE inhibition. For hospitalized patients in the ESCAPE trial, Drazner et. al. 17 showed that HF physician estimates of JVP correlated with invasively measured right atrial pressure, which is a strong predictor of rehospitalization in studies including hemodynamic parameters.18 In clinical practice however, significant inter-observer and intra-observer variability of clinician-assessed JVD have been noted. In the current analysis, the estimated level of elevation of JVP, whether measured at admission or discharge, was unrelated to subsequent outcome which may underscore the challenges associated with its use as either a criterion or endpoint for effective therapy in AHF trials. In fact, specific JVP appraisal is no longer a core data element of the assessment in Heart Failure Network trials of ADHF.
Unlike JVD, orthopnea and edema are perceived by patients, for whom they often contribute substantially to their symptom burden. When consistently assessed, all three have been shown to reflect invasively measured filling pressures. Orthopnea correlates with high pulmonary capillary wedge pressures7 and peripheral edema correlates with high right atrial pressures.19 Orthopnea may be particularly important not only as a marker of high filling pressures but also as a contributor to sleep-disordered breathing, which has independently been associated with worse outcomes20,21 and shown to be decreased by reduction of filling pressures with intravenous vasodilation.22 Of the signs and symptoms of congestion assessed in the outpatient setting at one month after discharge, orthopnea was the strongest single predictor of adverse outcomes, associated with 62% rate of death or urgent transplant, compared to 23% in patients free of orthopnea at one month.9
Weight Change and Decongestion
In the current analysis, weight loss did not consistently correlate with congestion status as measured by orthodema. Patients enrolled with high-grade orthodema at baseline that resolved completely by discharge lost an average of 14.6 pounds. Comparatively, of the patients with baseline high-grade orthodema, 24% were discharged with persistent high-grade orthodema, despite a similar weight loss of 14.2 pounds. These findings are consistent with the results of a previous analysis of the DOSE-AHF trial, which showed that increased weight loss at 72 hours was not associated with decongestion as assessed by dyspnea relief.23 Weight change relative to an initial and optimized weight assessment may help determine how weight loss is associated to congestion relief during hospitalization for ADHF. This lack of correlation reflects marked inter-individual variation in the amount of fluid retention that precipitates symptoms and the severity of symptoms that lead patients to seek hospitalization.
Congestion Status at Discharge and Predicting Outcomes
A previous consensus document proposed an approach to grading congestion at the end of HF hospitalization by assigning point values to individual physical examination findings, patient symptoms, exercise testing, and laboratory values.8 More recently a congestion score from the EVEREST trial 24,25 in patients with reduced EF was calculated by summing scores for orthopnea, JVD, and peripheral edema for maximum score of 9. By discharge, only 10% of patients had a score of 3 or more, and experienced a HF rehospitalization rate of 35% versus 26% after a mean follow-up of 10 months. Only in the smallest subset of patients with the highest discharge congestion score was there an independent association between congestion status and worse clinical outcomes.
In the present analysis, patients with evidence of orthodema at discharge had significantly higher morbidity and mortality compared to those without congestion, but the difference was modest. The 60-day adverse event rates were high irrespective of congestion status at discharge: 50% for patients free from orthodema, 52% for those with low-grade orthodema and 68% with high-grade orthodema.
Recurrence of Congestion after Discharge
This study demonstrates the burden of recurrent congestion even after initial relief of congestion during acute hospitalization. Rates of recurrent congestion at one month were 51% and 27% for moderate and severe congestion, respectively, in the ESCAPE trial.9 JVP, peripheral edema, and weight were also increased at 3 months in the ESCAPE trial, with slightly more recurrence in the arm that had been randomized to therapy guided by pulmonary artery catheters. 26 The rates of recurrent orthodema in the current study are comparable, with 27% and 35% of patients going on to develop low and high-grade recurrent congestion by 2 months.
Focusing on the first month after discharge for HF patients with reduced EF, Lucas et. al.9 developed a 0–5 point score based on presence of orthopnea, edema, JVD > 8 cm H20, weight gain and need to increase daily diuretic dose. They found that patients free of these markers at one month after discharge had a two-year survival of 87%, compared to 67% in patients with 1–2 components of recurrent congestion, and 41% in patients with 3–5 components (p=0.00001). The most significant single predictor at one month was orthopnea.
This 5-point assessment of congestion at one month after discharge was validated by Rogers et. al.27 in the more contemporary ESCAPE trial. Patients with no evidence of recurrent congestion at one month had a 9% risk of death and 31% risk of hospitalization at 6 months compared to patients with at least 3 components of recurrent congestion, who had 28% risk of death and 68% risk of re-hospitalization. Furthermore, the one-month congestion influenced quality of life and ambulation. Minnesota Living with Heart Failure questionnaire scores remained improved at 6 months after discharge in 50% of patients free of congestion at one month, compared to 37% of patients with at least 3 components of recurrent congestion. At 6 months, the 6 minute walk distance was over 900 feet in 73% of patients free of congestion for the first month, compared to 46% of patients with 1–2 components of congestion and only 26% of patients with 3–5 components of recurrent congestion at 1 month.
The 2 NHLBI-sponsored trials in this study, as well as the ESCAPE28 study, were performed at centers with recognized expertise in care of acute HF, and offered patients the benefit of additional surveillance through designated research staff, which has long been recognized to improve outcomes for patients in clinical trials whether receiving new or standard therapies. This is in addition to the established benefits of patient education and access to mid-level providers that are routine for patients cared for at the HF centers included in the NHLBI Heart Failure Network. Recurrent congestion remains a fundamental challenge that we have not been able to address with our current approach to management of patients after hospital discharge. Until we can better understand and address this challenge of “re-congestion” after hospital discharge, our ability to discern benefits of selected additional therapies during HF hospitalization may be limited.
Limitations
This study is a post hoc retrospective analysis of two trials testing different therapies, but both required clinical evidence of congestion at enrollment and measured the impact of therapies to reduce circulating volume during hospitalization. The entry criteria and physical assessment forms were developed to collect data about the presence and changes in the components of congestion. Increasingly a focus of attention, the definition and quantification of congestion remain challenging and controversial. Any congestion score is somewhat arbitrary, and multiple thresholds and combinations could be constructed. In addition, individual clinical assessment of the presence and severity of JVD and edema is prone to variation, and patient assessment of orthopnea is not only variable between patients, but possibly between time points for a given patient, who may be anxious to describe resolution of orthopnea perhaps to accelerate discharge.
Generalizability of assessments conducted in a clinical research setting requires validation in routine clinical practice. These trials were designed to achieve relief of congestion in rigorous clinical research environments that likely represent a level of vigilant attention above that feasible in routine practice. However, the components have been simplified to orthopnea and edema, which are classic clinical features universally recognized as descriptors of decompensated HF and typical targets for relief during hospitalization and noted during clinical evaluation.
Clinical Implications
The findings of the present analysis have distinct implications. First it provides a simple symptom-based tool that may be used as a target for therapy in the hospital and outpatient setting. Second, it points out challenges in achieving decongestion despite rigorous clinical trial settings designed to evaluate decongestive therapies. Third, it highlights that even in those patients who are relieved of congestion, adverse event rates are high, underscoring the poor prognosis marked by hospitalization for AHF. Finally rates of recongestion are high in follow up, suggesting a need to better understand the factors leading to recurrent congestion once patients return home.
Conclusions
Orthodema provides a clear target for therapy during hospitalization for ADHF and continuing care after discharge. Higher orthodema scores were associated with more post-discharge events, which supports continued emphasis on decongestion during hospitalization. However, patients with relief of orthodema by discharge still experienced high event rates after hospitalization for ADHF, in part due to frequent recurrent congestion after discharge, which should be a major focus of efforts to prevent readmission with heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
DOSE-AHF and CARRESS-HF were funded by the National Heart, Lung, and Blood Institute. Training Grant: U10HL110337
CHF Solutions/Gambro provided research grant support and some equipment to Duke to conduct the CARRESS-HF trial. S.R.G. reports honoraria from CHF solutions. Dr. Stevenson has received travel support for consultation with St. Jude Medical.
Footnotes
Disclosures
The other authors report no relevant conflicts of interest.
References
- 1.Braunwald E. Heart Failure. JACC Heart failure. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. American College of Cardiology F and American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Network NHFCR Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E, Heart Failure Clinical Research N. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. The New England journal of medicine. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker GM, O’Connor CM, Braunwald E. Heart Failure Clinical Research Network I.Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circulation Heart failure. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bart BA, Goldsmith SR, Lee KL, Redfield MM, Felker GM, O’Connor CM, Chen HH, Rouleau JL, Givertz MM, Semigran MJ, Mann D, Deswal A, Bull DA, Lewinter MM, Braunwald E. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research Network. Journal of cardiac failure. 2012;18:176–182. doi: 10.1016/j.cardfail.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. The New England journal of medicine. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. European Society of C European Society of Intensive Care M Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. European journal of heart failure. 2010;12:423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 9.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA, Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. American heart journal. 2000;140:840–847. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 10.van’t Laar A. Why is the measurement of jugular venous pressure discredited? The Netherlands journal of medicine. 2003;61:268–272. [PubMed] [Google Scholar]
- 11.Nohria A, Stevenson LW. Observation is never obsolete. JACC Heart failure. 2014;2:32–34. doi: 10.1016/j.jchf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–506. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. The American journal of medicine. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiade M, Shin DD, Thomas TO, Brandimarte F, Fonarow GC, Abraham WT. Congestion is an important diagnostic and therapeutic target in heart failure. Reviews in cardiovascular medicine. 2006;7(Suppl 1):S12–S24. [PubMed] [Google Scholar]
- 15.Konstam MA. Natriuretic peptides and cardiovascular events: more than a stretch. JAMA : the journal of the American Medical Association. 2007;297:212–214. doi: 10.1001/jama.297.2.212. [DOI] [PubMed] [Google Scholar]
- 16.Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. The American journal of cardiology. 1989;63:709–713. doi: 10.1016/0002-9149(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, Young JB, Califf RM, Nohria A. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circulation Heart failure. 2008;1:170–177. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley D, Brozena SC. Assessing risk by hemodynamic profile in patients awaiting cardiac transplantation. The American journal of cardiology. 1994;73:379–383. doi: 10.1016/0002-9149(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Stevenson LW, Palardy M, Campbell PM, May CW, Lakdawala NK, Stewart G, Nohria A, Rogers JG, Heywood JT, Gheorghiade M, Lewis EF, Mi X, Setoguchi S. The worst symptom as defined by patients during heart failure hospitalization: implications for response to therapy. Journal of cardiac failure. 2012;18:524–533. doi: 10.1016/j.cardfail.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khayat R, Small R, Rathman L, Krueger S, Gocke B, Clark L, Yamokoski L, Abraham WT. Sleep-disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. Journal of cardiac failure. 2013;19:431–444. doi: 10.1016/j.cardfail.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. Journal of cardiac failure. 2009;15:739–746. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson TP, Frantz RP, Snyder EM, O’Malley KA, Beck KC, Johnson BD. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. American heart journal. 2007;153:104, e1–e7. doi: 10.1016/j.ahj.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, Braunwald E, O’Connor CM, Felker GM. Committee NHFNS and Investigators. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circulation Heart failure. 2013;6:240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M, Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. European heart journal. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA : the journal of the American Medical Association. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 26.Palardy M, Stevenson LW, Tasissa G, Hamilton MA, Bourge RC, Disalvo TG, Elkayam U, Hill JA, Reimold SC, Investigators E. Reduction in mitral regurgitation during therapy guided by measured filling pressures in the ESCAPE trial. Circulation Heart failure. 2009;2:181–188. doi: 10.1161/CIRCHEARTFAILURE.108.822999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers JG. TLow Congestion Score One Month Following Heart Failure Hospitalization Predicts Better Function and Survival. 2007 [Google Scholar]
- 28.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, Investigators E, Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA : the journal of the American Medical Association. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.