Abstract
One of the aims of evolutionary developmental biology is to discover the developmental origins of morphological variation. The discipline has mainly focused on qualitative morphological differences (e.g., presence or absence of a structure) between species. Studies addressing subtle, quantitative variation are less common. The Drosophila wing is a model for the study of development and evolution, making it suitable to investigate the developmental mechanisms underlying the subtle quantitative morphological variation observed in nature. Previous reviews have focused on the processes involved in wing differentiation, patterning and growth. Here, we investigate what is known about how the wing achieves its final shape, and what variation in development is capable of generating the variation in wing shape observed in nature. Three major developmental stages need to be considered: larval development, pupariation, and pupal development. The major cellular processes involved in the determination of tissue size and shape are cell proliferation, cell death, oriented cell division and oriented cell intercalation. We review how variation in temporal and spatial distribution of growth and transcription factors affects these cellular mechanisms, which in turn affects wing shape. We then discuss which aspects of the wing morphological variation are predictable on the basis of these mechanisms.
Keywords: Evo-Devo, Developmental systematics, Quantitative development, Evolutionary morphology, Drosophila wing morphogenesis
Introduction
A major goal in evolutionary developmental biology (Evo-Devo) is to discover the developmental origins of morphological variation. To date, most such studies have considered only gross qualitative variation of well-defined traits, such as the gain or loss of a morphological feature. The question of how subtle changes in development give rise to subtle, quantitative variation observed in populations or between closely related species has not often been addressed (Nunes et al., 2013; Parsons & Albertson, 2013), although exceptions exist (e.g., Salazar-Ciudad & Jernvall, 2010; Mallarino et al., 2012; Arif et al., 2013). This is an important class of variation since natural selection acts on this variation at the population level, and magnifies it over evolutionary time leading to differences between species.
The wing of the fruit fly Drosophila is an ideal model to study the developmental origins of quantitative morphological variation because it is one of the most studied systems in developmental biology, and it has also been under the interest of quantitative geneticists. Early studies focused on the genetic pathways and developmental processes involved in the determination of wing identity (e.g., Kim et al., 1996) and, later, on the presence or absence of some morphological characters (Crozatier et al., 2004; Gompel et al., 2005). But what about the subtle variation in shape that is actually observed among and within species? The wing is a morphological structure that exhibits abundant quantitative multivariate variation at both the intra-specific and inter-specific levels that, in most cases, needs to be precisely measured in order to be detected (Houle et al., 2003; Mezey & Houle, 2005). Another important but unexplained property of the wing shape variation is its integration: some parts of the wing have strong patterns of covariation (Klingenberg & Zaklan, 2000), while others are relatively independent (Weber, 1992). Mutations with strong effects on one part also tend to affect the remainder as well. This has important evolutionary implications because it implies that natural selection acting on any morphological aspect of the wing would lead to indirect changes in the whole organ. Therefore, if we want to predict the response of wing shape to natural selection, it is necessary to understand the mechanisms that generate the (co)variation and so the genotype-phenotype (GP) map of the fly wing.
Variation in wing shape depends on many genetic factors. In wing tissues, approximately 80% of the fly genes have detectable expression, and 50% of the transcriptome exhibits changes in expression during a time course of wing development (O'Keefe et al., 2012). Quantitative Trait Locus (QTL) studies have repeatedly detected multiple loci affecting aspects of wing shape (Weber et al., 1999, 2001; Zimmerman et al., 2000; Mezey et al., 2005). When 191 lines of D. melanogaster homozygous for a single P-element insertion were tested, 63 % of them were found to be associated with variation in wing shape (Carreira et al., 2011). The large number of genes having an effect on wing shape variation, together with environmental variables which also impact wing shape (Bitner-Mathé & Klaczko, 1999), suggests that even in a relatively simple system such as the wing, the GP map is complex. It is, however, possible that understanding variation may be simpler at another level of organization such as development.
We will try to address this complexity by focusing at the development level. Much attention has been focused on the processes that lead to determination of new structures during development, but for an organ such as the Drosophila wing where most variation is subtle, the major determinants of size and shape are more likely to involve just four major morphogenetic processes. These processes are i) spatial regulation of mitotic density, ii) orientation of cell division, iii) biased rearrangements and intercalation of cells, and iv) differential cell death (Lecuit & Le Goff, 2007). Such processes are also well known in other systems. For example, heterogeneities in mitotic density across a tissue account for organ shape distortions during development in wings of two Lepidopteran species (Nijhout et al., 2014) and in mammalian teeth (Salazar-Ciudad & Jernvall, 2002). Orientation of division plays a key role in determining organ shape (Gillies & Cabernard, 2011). In many tissues, cells can change relative positions by remodeling their contacts with neighbor cells. Biased orientation of the rearrangements results in tissue elongation, as is observed during the elongation of the Drosophila embryo (Bertet et al., 2004) and also in vertebrate tissues (Wallingford et al., 2002). Finally, differential cell death can result in a dramatic remodeling of tissue shape. For example, spacing between vertebrate digits is the consequence of inter-digital cell death (Montero & Hurlé, 2010). Variation in the shape of the wing is likely to be the result of variation in a combination of these four processes. An explanation of how variation in wing shape is generated requires a prior understanding of how these processes are regulated during development.
Signals regulating morphogenesis can be placed in two categories. On the one hand, morphogenetic cell behaviors are governed by extracellular signals secreted by cells, or by membrane-bound signals. For example, Bmp and Wnt-like proteins are generally involved in the control of cell proliferation and establish tissue fates, thus also playing a crucial role in cell differentiation (Lander, 2011). Moreover, because of their capacity to move from cell to cell, these proteins can establish concentration gradients pointing towards the source. Cells can sense the direction of such global gradients and translate these signals to establish their planar polarity (i.e, the polarity in the plane of the tissue) (Heisenberg et al., 2000; Myers et al., 2002; von der Hardt et al., 2007; Gao et al., 2011; Sagner et al., 2012; Wu et al., 2013). This is important because in many cases polarity defines the orientation of cell intercalations and divisions (Gong et al., 2004; Segalen & Bellaïche, 2009; Gray et al., 2011). In addition, the magnitude of that polarity has been proposed to regulate growth in some cases (Rogulja et al., 2008).
The second category of signals are mechanical forces, such as stretching or compression of a tissue, and the local growth environment, such as availability of nutrients. Mechanotransduction is the sensitivity of cells to mechanical signals. For example, the mammalian YAP/TAZ pathway, involved in growth regulation, is modulated by mechanical properties of the extracellular matrix (Dupont et al., 2011). Forces extrinsic to epithelial cells can reorganize orientation patterns of cell rearrangements and divisions as well as cell fate, differentiation, and shape (Heisenberg & Bellaïche, 2013). Cell shape plays a special role in morphogenesis because it can directly modify tissue shape, and also regulates the morphogenetic processes as for example orientation of cell division (Minc & Piel, 2012) and growth (Chen et al., 1997). Finally, there is growing evidence that cell death can be triggered by mechanical forces, as well as chemical signals (Marinari et al., 2012; Vincent et al., 2013).
Clearly, integration of developmental approaches with a comparative framework has allowed us to fully address some cases of qualitative variation (e.g., Gompel et al., 2005; Chan et al., 2010). We raise the question: Is our understanding of morphogenesis and of its regulation mature enough to explain multivariate and quantitative morphological variation of the kind observed for the wing shape? Here, we review current knowledge of wing development, and we propose hypotheses for how the spatial and the temporal patterns of gene expression affect morphogenetic processes, and how variation in these processes can change wing shape. These hypotheses remain to be tested, but provide a roadmap for future experiments that should ultimately answer our overriding question.
Natural variation of wing shape
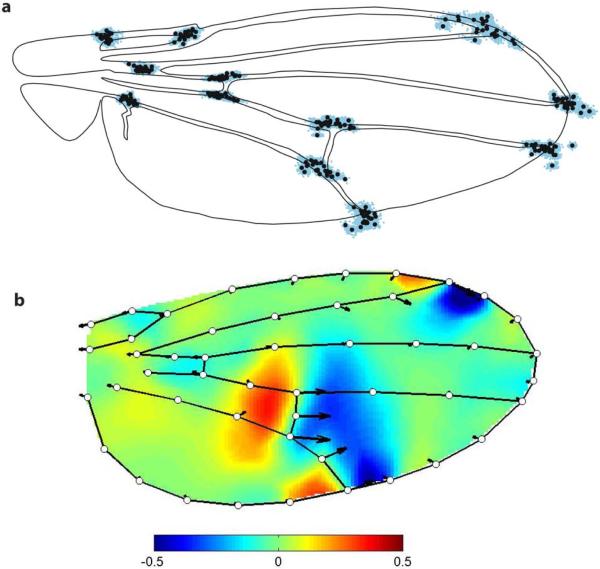
The wing shape of Drosophila is a model to study evolution of traits with multidimensional and quantitative variation. “Multidimensional” implies that variation affects many aspects of the organ simultaneously, such that it is not adequately described by any single measurement. In the case of the wing, shape is usually measured using vein intersections because they are homologous positions which can easily be compared between individuals. In addition, vein intersections are widely spaced on the wing, giving a reasonable sampling of its overall shape (Fig. 1a). Wing shape variation is captured by the pattern of changes in relative positions of landmarks at veins intersections. Such variation falls into a mathematical space (called “phenotype space”, or “morphospace”) with axes defined by each measured variable (in this case, the x and y position of each measured landmark). Mezey and Houle (2005) analyzed the number of axes or dimensions with significant variation in a natural D. melanogaster population and found variation in 20 out of 20 possible dimensions in phenotype space in female flies and 18 dimensions in males. Mutation accumulation lines in a lab population have detectable variation in at least 15 dimensions (Houle & Fierst, 2013). Our unpublished analyses of variation among inbred lines in the Drosophila Genome Reference Panel (Fig. 1b; Mackay et al., 2012) shows variation in 39 of 39 possible dimensions. Thus, the natural variation of the wing shape is multidimensional in nature.
Figure 1. Intra and inter-specific variation of Drosophila wing shape.
a. Variation in the position of 12 vein intersections in Drosophila wings. Black circles show species means of vein intersections positions, for 25 species within the genus Drosophila. Blue dots are the landmarks positions in each of the 2406 specimens analyzed by Houle et al. (2003). b Heat map representing relative changes along the first principal component of genetic variation in the Drosophila Genome Reference Panel (unpublished). Warm colours are local expansions, cool colours contractions. Scale is log2 area change relative to the reference. Changes shown correspond to three genetic standard deviations. Note that for both analyses, the landmarks positions are adjusted for differences and size and thus only shape differences are visible. Fig. 1a is from Houle et al. (2003).
“Quantitative” refers to variation which falls along a continuous range of possible values, rather than into discrete categories. The comparison of aligned landmarks positions for 25 species in the sub-family Drosophilinae (Fig. 1a) shows that among species variation in wing shape is continuous, despite millions years of divergence time between these species (Obbard et al., 2012). Wing shapes at the species level are nevertheless morphologically distinct, although the differences are often not apparent without detailed analysis (Houle et al., 2003; Fig. 1a). This relative conservatism of wing shape is not due to a lack of genetic or developmental variation, as new wing shapes (often beyond the range of variation in the genus Drosophila) can be obtained in the lab by performing artificial selection on wing shape (Carter & Houle, 2011; Houle et al., 2003; Le Rouzic et al., 2011; Pélabon et al., 2006, 2010; Weber, 1990, 1992).
Subtle, continuous variation of wing shape is also observed at the population level in D. melanogaster (Fig. 1b). Interestingly, there are strong patterns of covariation among different parts of the wing, as is apparent from the shifts in veins II, IV and the distal crossvein shown in Fig. 1b. This pattern could be due to developmental changes which result in widespread morphological variation, but the origins of such covariation patterns remains unknown. The range of morphological variation observed at the specific level is not qualitatively different from the one observed at the inter-specific level. A possible advantage of the similarity of the pattern of variation at these different phylogenetic levels is that the insights obtained from developmental variation within the model species D. melanogaster might be extrapolated at the inter-specific level, i.e., the same developmental changes might account for the variation at the populational and inter-specific levels. Here follows a description of the developmental processes in D. melanogaster that we think are relevant in order to understand natural variation in wing shape and size
Overview of wing development
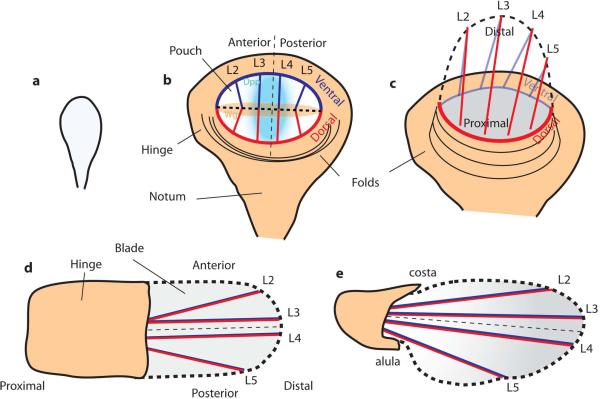
The wing is derived from a precursor group of ~ 30 cells that invaginates from the embryonic ectoderm. This occurs in the anterior/ mid-part of the embryo, at the boundary between the second and third parasegments (the parasegments are sections of the ectoderm established along the whole anterior-posterior axis of the embryo) (Bate & Martinez-Arias, 1991). These cells form the wing imaginal disc, a mono-layered sac of epithelial cells that will undergo growth and patterning during the larval stages (Figs. 2, 4). The two halves of the sac-shaped wing imaginal disc differentiate into a layer of columnar cells that will develop into the adult structures of the wing and the dorsal thorax (notum), and a peripodial membrane consisting of squamous cells, which does not give rise to adult tissue (Fig. 4a). The wing disc proper consists of a roughly circular patch of cells, termed the wing pouch, in the columnar cell layer, which is surrounded on all sides by the cells that will become the hinge that connects the wing to the thorax of the adult (Fig. 2b). The apical surface of the columnar cells is on the interior of the imaginal disc.
Figure 2. Overview of Drosophila wing development.
a- 2nd instar larval disc. b- 3rd instar larval disc with compartments defined by the dorso/ventral (D/V) and anterior/posterior (A/P) boundaries, and morphogen gradients of Dpp, (produced by cells at the A/P boundary) and Wg, (produced by cells at the D/V boundary). . c – Evagination of the disc. The wing pouch folds along its D/V boundary (thick dashed line), apposing dorsal and ventral compartments, and the blade extends and become elongated along the proximal-distal axis. The part of the hinge behind the blade folds back and elongates as the blade does. d – Early pupal wing after evagination and expansion. e – Late-pupal wing. The hinge contraction creates tension that drives the elongation of the wing blade. The posterior/proximal margin becomes curved and an indentation becomes visible at the intersection between L5 and the wing margin. The costa and alula are visible. At this stage the shape of the wing blade is similar to adult shape.
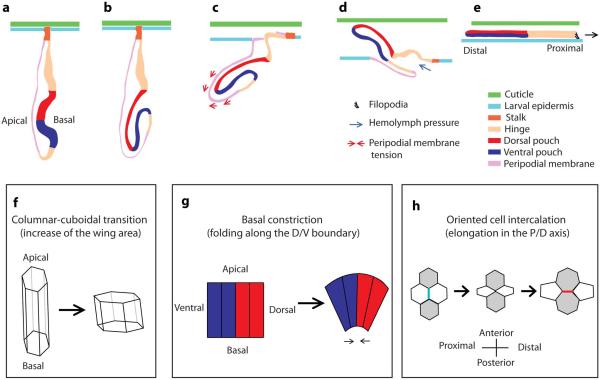
Figure 4. Eversion of the wing imaginal disc (a-e, redrawn from Fristrom and Fristrom (1993) and from Pastor-Pareja et al. [2004]) and some processes involved in subsequent changes in tissue size and shape (f-h).
a - Lateral view of the late 3rd instar disc. b - Folding along the D/V boundary apposing the ventral and dorsal compartments on each other. c - The larval cuticle is degraded and replaced by the pupal cuticle (apolysis). Spacing appears between the epidermis and the cuticle. The peripodial membrane invades the larval epidermis (the cells of the epidermis are replaced by those of the peripodial membrane) and delaminates, thus creating a lumen through which the appendage exits into the space between the epidermis and the cuticle. Contractions of the peripodial membrane could contribute to the movement of the wing outside of the epidermis. d - Exit of the appendage through the lumen created by the peripodial membrane. Hemolymph injected into the epithelial sac could contribute to the exit of the wing. e - The wing is completely everted. The blade extends its area and the whole tissue crawls over the epidermis towards the dorsal midline, where the fusion with the other imaginal wing disc takes place. f - 3rd instar disc have columnar cells whereas early pupal wings have cuboidal cells. The transition from one shape to the other contributes to the expansion of the wing blade area. g - Basal constriction of the cells along the D/V boundary could contribute to the folding of the disc. h - Oriented cell intercalation contributes to the elongation of the wing blade in the P/D direction (only the apical area of the cells is illustrated). Cell rearrangement is driven by shrinkage of one junction (blue) and expansion of a newly formed junction (red).
In the first larval instar (24 to 49 h after fertilization (AF)), the wing disc grows moderately and without cell division (Madhavan & Schneiderman, 1977). Growth becomes exponential during the second (49-72 h AF) and early third instar (72-150 h AF), then achieving ~ 10-11 rounds of cell divisions, each of which takes ~ 8.5 hours at 25 °C (Garcia-Bellido & Merriam, 1971). Concurrently with growth, disc patterning results in the establishment of the three body axes (anterior-posterior (A/P); dorsal-ventral (D/V) and proximal-distal (P/D)) and of five longitudinal proveins (L1 – L5) (Fig. 2b). In the larval phase, all the compartments (dorsal, ventral, anterior, posterior, proximal, distal) are roughly situated in the same plane. Wing disc shape changes during the growth phase, from a nearly circular shape to an ellipsoid shape (Fig. 2b). This change in wing disc shape is the result of anisotropic growth, as growth is weakest in the axis defined by the A/P boundary, and it is strongest in the direction of the axis defined by the D/V boundary (Bittig et al., 2009). Thus, by the end of the 3rd larval instar, the wing disc is an organized structure consisting of about 30.000 – 50.000 morphologically and molecularly differentiated cells.
At the beginning of metamorphosis, the wing imaginal disc folds to form a two-layered epithelium with the ventral and dorsal compartments apposed to each other (Fig. 2c). The tissue turns inside out so that the apical surface of the epithelial cells faces outward (Fig. 4b). At the same time, the tissue elongates along the proximal-distal axis. The folding and extension processes take place from 6 h before pupariation to 6 h after pupariation (AP) (Fristrom & Fristrom, 1993). This process is termed evagination, and it involves cell rearrangements, changes in cell shape, and cell division (Taylor & Adler, 2008; Kanca et al., 2014).
Other key events of wing development take place during metamorphosis. At ~ 4 h AP, the larval epidermis is perforated by the peripodial membrane, and the wing epithelium protrudes in a space between the cuticle and the larval epidermis (Figs. 4c and 4d). Once the wing disc epithelium penetrates the larval epidermis, the proximal region of the wing imaginal disc, the notum, spreads by crawling over the larval epidermis and fuses with the other wing imaginal disc at the dorsal midline (Fig. 4e). Such disc movement is achieved through the extension of filopodia at the leading edge of the disc, which exert mechanical forces and sense positional cues in order to achieve proper meeting between the discs at the dorsal midline. JNK signaling is required to achieve proper disc eversion, spreading over the larval epidermis and fusion with the other imaginal disc (Martin-Blanco et al., 2000; Usui & Simpson, 2000; Pastor-Pareja et al., 2004). At 6 h AP, the anterior-cross vein can be visualized by patterns of protein expression (Matakatsu & Blair, 2004).
From ~ 6 h AP to ~ 18 h AP, proliferation is arrested (Milán et al., 1996), but the blade is considerably elongated, possibly as a result of differential increase of cell area along the P/D axis (Fristrom & Fristrom, 1993). Very little is known, however, about the developmental processes taking place during this period because the rate of change is large, and the tissue is fragile and difficult to isolate.
From ~ 18 to 35 h AP, the shape of the wing is profoundly altered to become quite similar to the adult wing (Figs. 2d and 2e). Crucial to this morphogenetic process is the synthesis at ~ 14 h AP of a pupal cuticle, which embeds the wing epithelium. Thereafter, the proximal part of the wing, the hinge, contracts. This contraction, together with attachments of the distal parts of the wing to the external and rigid pupal cuticle, exerts a force that extends the blade along the P/D axis, and contracts it on the A/P axis. This force modifies wing shape by orienting cell intercalations and divisions (Aigouy et al., 2010; Sugimura & Ishihara, 2013). There is approximately one round of cell division during that period but the area of the wing blade remains unchanged (Aigouy et al., 2010), probably because of a reduction in cell area. This remodeling gives the wing its A/P asymmetry. Vein cells become morphologically differentiated during that period (O'Keefe et al., 2012).
The final steps of wing morphogenesis (35 h AP to eclosion) are less understood than the earlier ones because during this period the wing epithelium is embedded in the adult cuticle, which is synthesized from ~ 36 h to ~70 h AP by the apical surface of the wing epithelial cells (Fristrom & Fristrom, 1993). Cell division is arrested, and the tissue area expands further due to an increase in cell size, accompanied by folding of the wing within the puparium (Waddington, 1940). In the final stages, following eclosion of the adult, the epithelial cells undergo an epithelial-mesenchymal transition: epithelial cells delaminate (they lose contact with each other), and the cells migrate from the wing into the thorax (Kiger et al., 2007), leaving only a few living nerve cells along veins L1 and L3, and the folded adult cuticle. Cell death has also been reported at this stage (Kimura et al., 2004). Thereafter, the cuticle is expanded and flattened by increased pressure from the hemolymph, transmitted along the veins. Final tanning of the expanded cuticle gives it its relatively rigid adult form (Honegger et al., 2008).
Morphological variation generated during development
a- Larval stages
Larval development of the wing imaginal disc is studied by many labs and it is thus the best known aspect of wing development. Of the four processes outlined above, differential cell proliferation, oriented cell division and cell death play an important role, while cell intercalation does not.
Orientation of cell division
Many studies have shown that the orientation of cell division is not random during wing disc growth. Moreover, disruption of the pattern of oriented cell divisions affects the shape of the adult wing (González-Gaitán et al., 1994; Baena-López et al., 2005; Mao et al., 2011, 2013; Le Goff et al., 2013; Repiso et al., 2013; Heemskerk et al., 2014). The orientation of cell division during larval development is thus important with respect to the adult wing morphology. All these studies have deduced patterns of orientation of cell divisions by looking at the shape of cell clones and, for some studies, by looking also at the orientation of the mitotic spindle. The approaches gave consistent results indicating that the shape of the clones is a good readout of growth direction, and is not strongly affected by other processes that could also shape the clones, such as cell intercalation.
The emerging picture is that during the late third instar, cell division in the wing pouch is oriented radially (i.e., from the center towards the periphery ), except in the peripheral areas (Fig. 3c). Thus, in the area forming the DV boundary itself, growth is oriented in the direction of the DV boundary, and the same rule applies for the cells located in the AP boundary, where growth is oriented in the direction of the AP boundary (Baena-López et al., 2005). In the central regions that do not belong to any of these boundaries, growth is also oriented towards the periphery of the pouch, perpendicularly to the pouch outline. In all the peripheral areas, however, growth is oriented tangentially with respect to the pouch outline, and not perpendicularly (Le Goff et al., 2013; Mao et al., 2013).
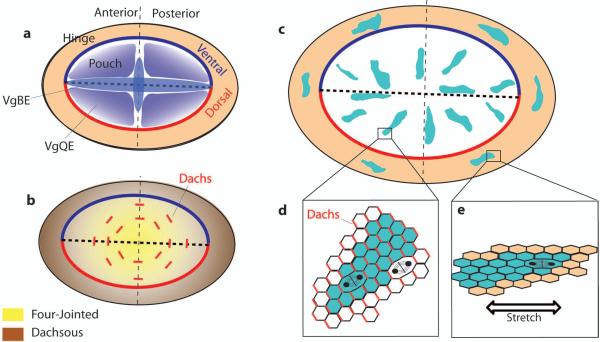
Figure 3. Gene expression and pattern of oriented cell division in 3rd instar wing disc.
a - Expression of the transcription factor Vestigial depends on two enhancers (VgBE and VgQE) regulated by dpp, wingless and notch signalling, driving expression of vg in throughout the wing pouch. b - Vestigial represses the expression of Dachsous (brown) and induces the expression of Four-jointed (yellow). Fj and Ds are expressed as opposing gradients oriented radially with respect to the pouch. These two gradients are necessary to localise the atypical myosin Dachs (red lines in b) at cell junctions perpendicular to the direction of Fj-Ds gradients. c - Patterns of growth during late 3rd instar, shown by the orientation of cell clones (coloured blue). In the pouch, growth is radial and this pattern depends on the control of the orientation of cell divisions by the localisation of Dachs (red lines in d). In the hinge, growth is oriented tangentially to the hinge / pouch boundary and this pattern depends on the orientation of cell division controlled by cell stretching arising from differential growth rate between the pouch and the hinge (e).
Both mechanical and molecular signals govern orientation of cell division in the larval stages. A major determinant is the Fat (Ft) Dachsous (Ds) Four-jointed (Fj) system. ds and fj genes are expressed as opposed gradients in the disc: ds expression is high in the hinge region and it gradually decreases to be low in the central region of the blade, whereas fj has the opposite pattern (Ambegaonkar et al., 2012) (Fig. 3b). These two gradients are oriented radially, in the same orientation as the direction of growth, suggesting a relationship between the direction of these gradients and mitotic orientation. This relationship has been proven in an experiment whereby a reorientation of the direction of the Ds gradient resulted in the reorientation of the divisions in a predictable way, affecting the shape of the adult wing (Mao et al., 2011). In addition, the characteristic radial orientation of mitosis is lost in flies carrying mutant alleles for ft or ds, with clear effects on the shape of the disc and of the adult wing (Baena-López et al., 2005; Mao et al., 2011) (Fig. 6d). The regulation of PCP by the ft-ds-fj system has been more thoroughly reviewed elsewhere (Thomas & Strutt, 2012). Briefly, the gradients of Ds and Fj polarize the atypical myosin Dachs (Figs. 3b, d), which is localized in the distal, apical membrane of the cells (Rogulja et al., 2008; Ambegaonkar et al., 2012; Bosveld et al., 2012; Brittle et al., 2012). Dachs is thought to orient cell divisions, but whether it does so by regulating cell shape or not is unclear (Mao et al., 2011; Le Goff et al., 2013). Noteworthy, elimination of the Ds expression gradient does not result in a complete randomization of the orientation of cell divisions, but in a pattern where growth is oriented parallel to the DV boundary (García-Bellido, 2009). This suggests the existence of other signals polarizing growth even in the absence of the ft-ds PCP system that remain to be discovered.
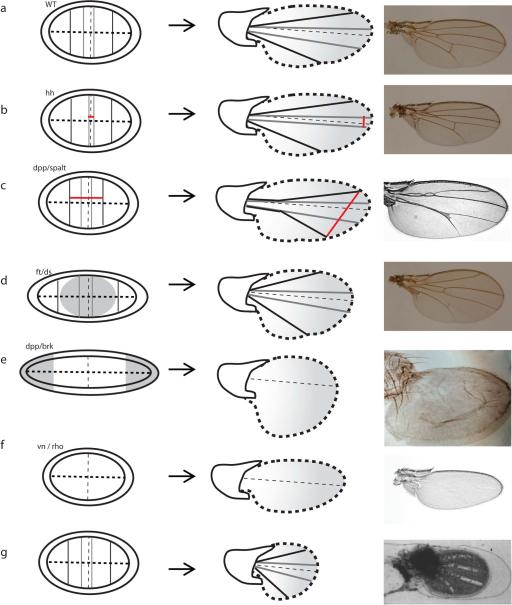
Figure 6. Examples of shape variation in the adult wing obtained from manipulation of known developmental processes.
a - Wild-type disc and adult wing. b - Manipulation of hedgehog signalling affects the spacing between L3 and L4, due to shifted positions of the corresponding proveins domains in the wing disc. c - Manipulation of the expression of dpp/spalt affects the spacing between L2 and L5. As in b, change in vein position result from change in proveins domains in the wing disc (picture: Bier [2005]). d - Manipulation of the fat/dachsous system during larval growth results in a disc more elongated along the D/V boundary, further to the disruption of the pattern of oriented cell division and of cell proliferation in the central part of the disc (shaded grey). The adult wing has increased roundness as compared with the wild-type (Baena-López et al., 2005). e - Manipulation of the dpp/brk system during larval growth results in discs very elongated along the D/V boundary, further to important overgrowth in the lateral areas of the disc (shaded grey). The adult wing lacks venation, and presents an increased posterior compartment (picture: Martín et al. [2004]). f - vn and rho mutants lack provein domains in the wing disc and veins in the adult wing. In addition, the posterior margin does not have any indentation at the presumpted intersection with L5, and the wing is smaller (picture: Roch et al. [2002]). g - During pupal development, disruption of the anchorage of the wing blade to the cuticle result in the retraction of the wing affecting its shape (picture: Turner & Adler [1995]). Pictures in a, b and d (courtesy of O. Shimmi and Y. Huang) are in the same scale.
Since changes in the orientation of growth by the fat-ds-fj system have effects on the adult wing shape, it is important to understand how these gradients are established. A key element is the transcription factor Vestigial (Vg). Vestigial is expressed in a graded fashion in a pattern which is very similar to Fj expression and complementary to Ds expression (Baena-López & Garcia-Bellido, 2006) (Fig. 3a). This suggests a network in which vg activates fj expression and represses ds. Such interactions have been validated experimentally (Cho & Irvine, 2004; Zecca & Struhl, 2010). Moreover, there is evidence for a relationship between the graded expression of Vg in the blade and the orientation of cell division. Flattening the gradient of Vg results in a misorientation of cell divisions in the wing disc and in changes in adult wing shape very similar to those observed in flies carrying a mutant allele of Ds (Baena-López & Garcia-Bellido, 2006). In this context, mitosis seems to be randomly oriented though some mitosis oriented in the direction of the D/V boundary remain. According to this hypothesis, the regulation of ds and fj by vg is crucial for the control of growth direction, raising the question of how the gradient of Vg is established.
The regulation of vg gene expression is complex. It is mediated by Wingless - Notch- Dpp signaling, but also by Vestigial itself, Fat, Dachsous, Yorkie and Hippo (Kim et al., 1996; Klein & Arias, 1999; Zecca & Struhl, 2010). vestigial expression is controlled at least by two different enhancers: the quadrant enhancer (vgQE) and the boundary enhancer (vgBE) (Kim et al., 1996). vgBE is active in the DV boundary and, later, in the AP boundary. Later in developement, vgQE is active in four quadrants that fill the prospective wing blade, and it is not active at the DV and AP boundaries (Fig. 3a). The domain of activity of the two enhancers is thus complementary in time and space. The activation of the vgBE is made by Wingless and Notch signaling. Thereafter, Vg resulting from the activity of the vgBE acts synergistically with Wg and Dpp to activate the vgQE in the blade. More precisely, the vgQE is activated in a cell when the cell or one of her neighbors produces Vg, and when it receives some Dpp or Wg. As a result, “the activity of the vgQE is initiated at the intersection of wg and dpp expression and radiates from this focus” (Klein & Arias, 1999). Moreover, recruitment of new cells producing Vg requires a feed-forward signal in which Fat, Dachsous, Hippo and Yorkie are involved (Zecca & Struhl, 2010).
To summarize, the orientation of cell divisions in the central areas of the blade and in the AP and DV boundaries seems to originate from cell signaling by Notch and by the morphogens Dpp and Wg. These signals then generate a gradient of Vestigial expression along the P/D axis, which patterns the expression of Dachsous, Four-jointed and Dachs. This results in a pattern where growth of the central wing disc blade is oriented radially. By contrast, the tangential orientation of cell division in the peripheral areas of the blade does not depend on these signals. Two studies (Le Goff et al., 2013; Mao et al., 2013) have shown that the growth rate in the peripheral areas of the wing blade is, at least in early stages of development, slightly lower than in the central areas. A consequence of the differential growth between central and peripheral areas is that cells in the peripheral disc tissue stretch parallel to the wing blade boundary. Following Hertwig's rule, a general property in animals according to which cells cleavage plan is made perpendicularly to cell apical area longest's axis (Minc & Piel, 2012), the direction of divisions at the periphery of the disc occurs tangentially to the blade outline (Fig. 3e).
How does variation in the mechanisms orienting growth in the larval wing generate variation in the adult shape? Clearly, failure to orient growth radially in the blade affects the roundness of the adult wing: mutants for ds have wings less elongated along the PD axis but broader along the AP axis than wild-type flies (Baena-López et al., 2005; Mao et al., 2011) (Fig. 6d). It could be that such morphological variation is encountered in nature. If so, such variation could be obtained in several ways. For example, changes in the parameters of the morphogen production, degradation and diffusion could affect the direction of the Fj and Ds gradients and growth direction. Another possibility would be cells failing to correctly transduce the morphogen signals into planar cell polarity. This would involve changes in the interactions between the morphogens, Vg and the components of the Ft-Fj-Ds pathway and Dachs. Regarding the peripheral tangential growth, nothing is known about wing shape in individuals in which it doesn't occur, although it is plausible that changes in this would affect mechanical forces and then the orientation of divisions.
Regulation of mitotic density
Mitotic density in the developing larval wing was originally described in D. melanogaster as being spatially homogeneous (Milán et al., 1996). More recently, it has been shown that during early stages proliferation is slightly higher in central areas of the blade than in the peripheral areas (Le Goff et al., 2013; Mao et al., 2013). Besides this slight difference in growth between central and peripheral areas, there are no important spatial heterogeneities of mitotic density in the larval wing that could account for adult wing shape, contrary to what is known for example in Lepidopteran wings (Nijhout et al., 2014). However, several labs have created flies with wing discs having spatially heterogeneous growth rates (Martín et al., 2004; Rogulja et al., 2008; Schwank et al., 2011). Interestingly, these flies have very unusual adult wings shapes and it could be that more subtle manipulations of spatial control of growth rate could be a source of natural variation of wing shape.
Growth control in the wing disc is not fully understood despite being the target of much research (for reviews see Wartlick et al., 2011; Hamaratoglu et al., 2014; Restrepo et al., 2014). It is clear however that spatial heterogeneities in growth are possible because growth in the central parts of the disc is under the control of different mechanisms than in the lateral ones (Rogulja et al., 2008; Schwank et al., 2011). Manipulation of the fat pathway results in overgrowth of the central regions of the dics through a mechanism dependent on Dachs (which is a regulator of the Hippo pathway), whereas manipulation of the dpp/brk system induces overgrowth only in the lateral parts of the disc, through a mechanism independent of Dachs (Rogulja et al., 2008; Schwank et al., 2011). Manipulation of the dpp/brk or fat signals results in a spatial heterogeneity in growth rate which has clear effects in wing size and shape and it could be that during evolution differential regulation of growth in central and lateral areas has been used to generate variation in wing shape (Fig. 6d and 6e).
The pathway regulating orientation of cell divisions, described in the preceding section, is also involved in the spatial regulation of growth rate. Indeed, the intracellular localization of Dachs, as well as the spatially graded expression of vg, ds-fj, dpp, and also the temporal variation in Dpp levels, are all known to promote growth (Baena-López & Garcia-Bellido, 2006; Rogulja et al., 2008; Willecke et al., 2008; O Wartlick et al., 2011) . This shows that many genes have pleiotropic effects on size and shape, and that identifying the contributions of growth and of oriented cell division to shape variation through manipulation of gene expression will not be straightforward.
Growth control, together with cell size, plays an essential role in determining wing size and it has an important role in explaining inter-specific differences in wing size. The mechanisms determining the final size of the wing are subject of much research, showing that the Hippo pathway is an essential determinant of wing growth control (Irvine, 2012). It consists of at least 35 proteins, and its upstream regulation is complex, as it integrates inputs from the above described planar polarity ft-ds system, from the cell apico-basal polarity system, and also from mechanical cues such as cell-cell contacts, and cell membrane tension (Harvey et al., 2013; Gaspar & Tapon, 2014). There are many targets here for generating morphological variation.
Vein patterning
Vein positions are generally used to characterize the shape of the wing in a quantitative way using morphometric methods (Fig. 1). Once they are determined, veins act as signaling centers (e.g., Matsuda et al., 2013) and they might have mechanical effects on tissue shape (see below). For these reasons, veins are important determinants of wing shape. Veins morphologically differentiate late in pupal development but provein domains, i.e., groups of cells expressing vein-specific proteins, appear during the larval phase. Longitudinal veins L1 to L5 are visible in 3rd instar wing discs by staining Delta or Rho proteins, and flies with defects in the proveins have altered vein pattern in the adult wing as well (Biehs et al., 1998; Bangi & Wharton, 2006).
In order to understand how variation in provein determination generates variation in the adult wing, the developmental processes involved in vein positioning can be distinguished from those involved in vein differentiation. The former will define vein localizations whereas the latter will define presence or absence of veins. Thus, manipulation of the processes involved in the differentiation will result in wings with missing veins or altered vein morphology (e.g., Sturtevant & Bier, 1995; de Celis, 1997), whereas manipulation of the processes involved in positioning will result in wings with changed veins position (e.g., Gorfinkiel et al., 2005).
Mechanisms regulating vein differentiation and positioning have been thoroughly reviewed elsewhere (De Celis, 2003; Blair, 2007). The regulation of vein positioning is of special interest for us because it is an important source of quantitative variation in Drosophila wing shape (Fig. 1). Briefly, positioning of longitudinal veins L2, L3, L4 and L5 seems to result from morphogen gradients in a dose-dependent manner: close to the signal source, at high morphogen concentration, a specific set of genes is activated while at larger distances a different one gets activated. Cells having a specific set of genes activated will differentiate into proveins. Accordingly, the position of L2 and L5 is given by the dpp gradient whereas the positions of L3 and L4 are dependent on hh diffusion. Experimental manipulation of hh or dpp signalling results in changes in the spacing between veins (Blair, 2007) (Figs. 6b and 6c). Regarding the anterior and posterior cross-veins, the causes of their differentiation are coming to the light (Shimmi et al., 2005; Matsuda et al., 2013) but further research is needed to understand what defines their relative positions in the wing.
Apoptosis
There is apoptosis in normal larval wing discs of D. melanogaster but cell death is both homogeneous across the disc and rare (Milán et al., 1997). Thus, apoptosis does not seem to account for shape properties in the D. melanogaster wild-type wing. Experimentally-induced cell death in the larva can have important effects on adult wing shape. Such wings can, for example, lack entire patches of tissue with dramatic effect on the overall shape (e.g., Bejarano et al., 2010). As in the case of mitotic density, it could be that mutations creating subtle patterns of cell death and resulting in morphological variation shaped differences between species. Apoptosis plays, however, an important role in limiting variability of wing disc size. Although blocking completely apoptosis during larval development does not lead to disc overgrowth, it does result in increased variability in disc size (De La Cova et al., 2004).
Cell intercalation
Cell intercalation is rare in the developing wing imaginal disc. This is supported by live imaging (Le Goff et al., 2013), and by the observation that cell clones normally stay in coherent groups and do not intermix (Knox & Brown, 2002). This suggests that oriented cell intercalation during larval development does not play a direct role in the definition of the wing disc morphology.
b- Larval to pupal transition
During the larval to pupal transition the wing disc undergoes evagination (Figs. 4a-e), which consists of folding and an expansion of the wing epithelium. In the larva, the longest axis of the tissue is perpendicular to the A/P boundary, and the dorsal and ventral compartments are in the same plane (Fig. 2b). By the end of the evagination process dramatic changes in tissue shape reverse the relative lengths of the pouch, D/V and A/P boundaries. The D/V boundary expands to become the edge of the wing, while the boundary between the blade and hinge contracts greatly. The A/P boundary expands considerably in the direction of the P/D axis (Figs. 2c and 2d). In addition, the ventral and dorsal tissues become apposed on each other, thus ending in different planes, and the area of the wing pouch increases considerably (Figs. 4a-e). Such tissue size and shape reconfigurations are mediated by changes in cell shape, oriented cell intercalation, and cell division (Figs. 4f-h). Folds in the peripheral areas of blade that appear by the late 3rd instar unfold during evagination and contribute to the extension of the wing (Fig. 2c).
Cell size and shape
Fristrom and Fristrom (1993) report that changes in cell size and shape participate in the two phases of wing evagination: folding and elongation. According to these authors, the folding of the wing pouch along the dorsal/ventral boundary is initiated by the cells located on either side of the wing margin, which become wedge-shaped as the basal area of the cells shrinks (Fig. 4g). Such cell shape changes are a very common way to initiate tissue folding (Sawyer et al., 2010). Thereafter, the cells undergo a columnar to cuboidal change in cell shape, which reduces cell height and increases cell apico-basal area, thus expanding the wing (Fig. 4f). By the end of the 3rd instar, cell apical area is relatively small in the central areas of the wing blade, and gradually increases in the more peripheral areas (Aegerter-Wilmsen et al., 2012) but by 4 h AP, all the cells of the wing blade have approximately the same apical area (Fristrom & Fristrom, 1993). As the initial differences in cell area disappear, it can be deduced that the columnar to cuboidal transition results in a differential expansion of the wing blade, because it implies that the more central/distal cells expand their apical area more than the peripheral/proximal ones. Such differential expansion could contribute to the elongation of the blade along the P/D axis (Fristrom & Fristrom, 1993).
Changes in cell shape during pupariation are regulated by integrins distributed in the basolateral membranes of the cells, which control cell shape by attaching cells membrane to the basal extracellular matrix. Domínguez-Giménez et al. (2007) show that the disruption of integrin function results in cells adopting a cuboidal shape. In addition, interfering with integrins in early stages of the evagination process causes a failure to achieve proper contact between the dorsal and the ventral epithelium during their apposition, suggesting that the columnar shape of cells facilitates this process. Wings in which apposition fails have fluid-filled blisters on the adult wings. The interactions between the integrins and the extra-cellular matrix are mediated by a Raf-dependent transduction pathway (Domínguez-Giménez et al. 2007).
Oriented cell intercalation
Oriented cell intercalation contributes to the expansion along the P/D axis and contraction along the A/P axis of the wing during early pupal development (Fig. 4h). Two different studies (Taylor & Adler, 2008; Kanca et al., 2014) have marked cell clones during the late third instar and have followed the evolution of the shape of these clones during the larval to pupal transition. In both cases, they found that the clones became narrower along the A/P axis and more elongated along the P/D axis during the early pupal stages, as compared to the larval phase. In addition, Taylor & Adler (2008) tracked individual cells and found that they intercalate. They did not find any evidence for oriented cell divisions. Changes in the shape of the clones are thus due to cell intercalations.
How the amount and the orientation of cell intercalation is modulated during wing evagination has not been studied. Cell intercalation can be modulated in an active way by adhesive and tensile molecules located at the cell-cell junctions, the balance of which determines the “viscosity” of the tissue (Lecuit & Lenne, 2007). Since these molecules can respond to planar polarized cues, they can lead to oriented cell intercalations. Cell intercalation can also be driven as a response of an external tension applied on the tissue (Aigouy et al., 2010). In this case, the orientation of the intercalations dissipates the stress produced by the external tension (Sugimura & Ishihara, 2013). Both processes are known to happen in Drosophila tissues (e.g., Bertet et al., 2004; Aigouy et al., 2010; Bosveld et al., 2012; Sugimura & Ishihara, 2013) and can in principle account for the patterns observed during wing evagination.
It is known that the gradients of Dachsous and Four-Jointed established during the larval stages are maintained in early pupal stages (Matakatsu & Blair, 2004). Since the graded distribution of these two proteins polarizes the myosin Dachs in the P/D axis (see above), it could be that Dachs modulates the pattern of cell intercalation along the P/D axis by causing shrinkage of the junctions oriented perpendicularly to the P/D axis, as it does in the development of the pupal thorax (Bosveld et al., 2012). Another possibility is that the pathway leading to oriented cell intercalations in the fly embryo, through the Zipper, Sqh and RhoA proteins (Bertet et al., 2004), would also be involved in polarized junction shrinkage and cell intercalation during wing evagination.
On the other hand, it is known that tension mediated by myosin II originates from the peripodial membrane. Such tension is necessary to achieve proper evagination and eversion (Aldaz et al., 2013) and it could be that it also orients cell intercalation. Another external force that could be acting on cell behaviours during evagination is hydraulic pressure from the hemolymph. The wing discs are open to the body at their proximal end. It could be that hemolymph pumped into the epithelial sac contributes to evagination and eversion by imposing a mechanical force which could orient cell intercalations (Taylor & Adler, 2008) (Fig. 4d).
Cell division and cell death
Cell division was thought to be arrested during the larval to pupal transition (Milán et al., 1996) but two studies have tracked cell clones during this developmental period and reported an increase in the number of cells per clone, suggesting that cell division occurs (Taylor & Adler, 2008; Kanca et al., 2014). More precisely, Taylor & Adler (2008) propose that ~ 40% of the wing blade cells divide during evagination, and that mitotic activity occurs homogeneously throughout the wing blade. Neither of these studies imaged the spindle orientation in the dividing cells in the wing so the orientation of these divisions is not known. There is no evidence for patterned cell death during wing disc evagination (Milán et al., 1997; Taylor & Adler, 2008).
While there is as yet no direct evidence that modulation of the processes occurring during the transitions from larva to early pupa (folding, expansion and exit of the wing through the larval epidermis) generates quantitative variation in wing shape, they could in principle do so. Regulation of cell size and shape is known to be driven by integrins under the control of the Ras-signaling cascade for changes in cell shape (Domínguez-Giménez et al., 2007), whereas patterns of oriented cell intercalation could be regulated by the Ft-Ds-Fj system as it is the case in the thorax (Bosveld et al., 2012) or by the Zipper, Sqh and RhoA proteins, as is the case in the embryo (Bertet et al., 2004). Intercalation could also be driven by external forces coming from myosin II activity in the peripodal membrane or from the hydrolic pressure of pumped hemolymph (Taylor & Adler, 2008; Aldaz et al., 2013). Note that the peripodial epithelium is also necessary to perforate the larval epidermis and it presumably contracts in order to allow the exit of the notum and of the wing blade through the larval epidermis (Fristrom & Fristrom, 1993; Pastor-Pareja et al., 2004) (Figs. 4c and 4d).
c- Late pupal development
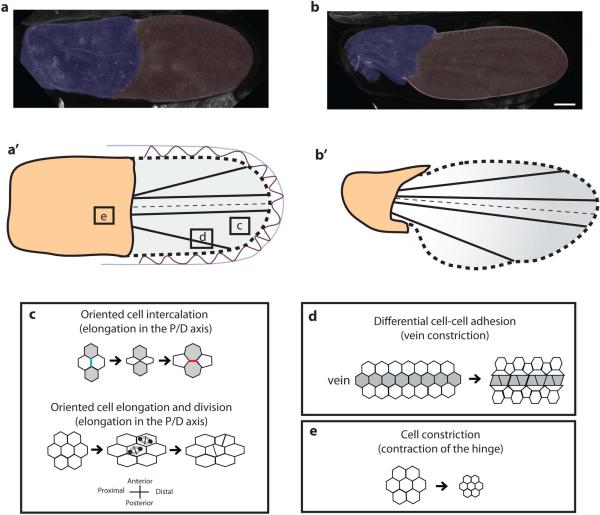
The morphology of the wing is further modified during late pupal development, and the processes involved in these changes are well known, in great part thanks to the study of Aigouy et al., (2010), which is the basis for the following description, unless stated otherwise. At ~ 15 h AP, the length of the wing is about three times bigger than its width, and its shape is symmetrical relative to the A/P boundary. The wing is evenly divided into the proximal hinge and the distal blade, derived from the wing pouch (Figs. 5a and 5a’). At ~ 32 h AP, the hinge has contracted to about half its initial area, and its anterior and posterior margins have indented to form the alula and the costa. The blade maintains its area but changes its shape. It becomes more elongated in the P/D direction, and narrower on the A/P axis. Its posterior/proximal margin changes its curvature, thus giving rise to an asymmetry along the A/P axis (Figs. 5b and 5b’).
Figure 5. Elongation of the wing driven by hinge contraction.
a,b - Pictures and diagrams showing the pupal wing at 15 h AP (a, a’) and 33 h AP (b, b’). The process is driven by contraction of the hinge, together with attachments (red in a’) of the cells outlining the wing blade to the pupal cuticle (bluein a’). The contraction of the hinge, together with tension mediated by the fat-ds-fj system, controls a pattern of oriented cell intercalation, cell elongation and oriented cell division that elongates the wing in the P/D axis (c). At the late pupal stage, vein cells become morphologically differentiated from the non-vein cells. Change in cell shape arises from differential adhesion force at vein-vein cell junctions, with respect to vein/non-vein junction. Such force creates a tension oriented in the direction of the veins, which could modify tissue shape, as illustrated by the indentation at the intersection between L5 and the wing margin (d, redrawn from O'Keefe et al. [2012]). e - Apical cell area in the hinge shrinks thus reducing hinge area. Pictures in a and b are from Aigouy et al. (2010).
These changes in relative proportions and in shape are driven by two processes. On the one hand, the hinge cells constrict their apical area (B. Aigouy personal communication), thus reducing the area of the proximal half of the wing (Fig. 5e). This takes place from 15 to 32 h AP. On the other hand, cells at the margin of the blade are attached to the surrounding pupal cuticle by extracellular connections described in in vitro wings by Turner & Adler (1995) (Fig. 5a’). This results in the elongation of the blade in the proximo- distal direction, by orienting both cell intercalations and cell divisions, although these two cell processes are affected in an asynchronous pattern. From 15 to 24 h AP, the elongation of the blade is mainly driven by oriented cell division, whereas from 24 to 32 h AP, it is mostly by oriented cell intercalation.
Oriented cell division
There is about one round of cell division during the late pupal stage, occurring from 15 to 24 h AP. The direction of the divisions is biased such that the cleavage plane is oriented perpendicularly to the P/D axis and thus favors the elongation of the blade in this axis (Fig. 5c). It is likely that tension generated by the hinge contraction pulling on the blade orients the divisions via changes in cell shape. It is known that cell shape influences the orientation of the mitotic spindle and positions it in the direction of the longest axis of the cell (Gibson et al., 2011; Mao et al., 2011; reviewed in Minc & Piel, 2012).
Two lines of evidence described by Aigouy et al. (2010) support the hypothesis that tension orients cell division in the P/D direction. First, when the hinge is severed from the blade, the pattern of oriented cell division is strongly reduced, and cell shape does not elongate in the P/D direction. This shows that the tension exerted by hinge contraction on the blade regulates the orientation of mitosis, and that this regulation is likely to occur in part via changes in cell shape. Second, in dachsous mutant flies the patterns of oriented cell division and elongated cell shape are impaired, but not completely lost. Graded Dachsous distribution in the pupal wing polarizes the myosin Dachs, which increases line-tension at cell junctions (see above). It could be that this gives the tissue enough rigidity to transmit the effects of the tension. We hypothesize that the loss of Dachs in cell junctions makes the tissue behaves more like a fluid, restricting the propagation of the tension driving the patterns of oriented cell divisions.
Oriented cell intercalation
Contrary to the larval stage, where relatively few events of cell intercalation are thought to occur, there is a lot of cell rearrangement during late pupal wing development. Most intercalation takes place from 14 to 32 h AP, after the last round of divisions has occurred, but still within the period of hinge contraction. As for cell divisions, the orientation of cell intercalation is biased in favor of elongation in the direction of the P/D axis; cell junctions which are perpendicular to the P/D axis tend to vanish whereas new junctions form parallel to it (Fig. 5c). As in the case of oriented cell division, intercalation is also governed by both the tension coming from the contraction of the hinge and dachsous, as the pattern of cell intercalation is disrupted in both dachsous mutants and severed wings (Aigouy et al., 2010; Sugimura & Ishihara, 2013).
Despite the importance of tension, other processes govern the patterns of cell intercalation during late pupal development. Cell intercalation is a two-step process during which one cell junction vanishes and a new one is created (Fig. 5c). These events rely on regulation of Myosin II (MyoII), in addition to external forces exerted on the tissue. Bardet et al. (2013) show that in pupal wing epithelium, accumulation of MyoII at the junction is necessary for its shortening during the first step of the intercalation event (as already observed in other Drosophila tissues). They also show that the release of MyoII at the newly formed junction is necessary to allow its lengthening. Such release of MyoII is regulated by the tumor suppressor gene pten. Loss of function of this gene disregulates cell junction length, resulting in aberrant cell packing. The characteristic honeycomb-like hexagonal cell packing of Drosophila epithelia becomes cobblestone-like. Such wings present subtle changes in their overall shape (Bardet et al., 2013).
Junction remodeling underlying cell intercalation is thus not solely a passive process, but is actively regulated by the dynamics of the acto-myosin network. Regulation of this network has been reviewed elsewhere (Rauzi et al., 2008). During pupal development, it is worth emphasizing that MyoII is positioned, in part, in response to forces external to the cell. Sugimura & Ishihara (2013) show that there is MyoII accumulation in the junctions oriented in the P/D axis of the wing, and that this accumulation is a response to the anisotropic tension caused by hinge contraction. Thus, the orientation of cell intercalation during pupal development is under the control of both molecular and mechanical signals and these two cues exert feed-backs on each other.
Mitotic density
There is approximately one round of cell division during the period ranging from 15 to 25 h AP. As in the case of the larval growth, there are no strong patterns suggesting differential rates of mitotic density across time and space, which could affect shape. Wings with mutations on rho or vn lacking veins are smaller (e.g., Roch et al., 2002) (Fig. 6f) and it could be that the veins act as signaling centers with respect to growth regulation. Dpp, a major regulator of wing growth is produced by the vein cells during that period. The idea that veins act as signaling centers regulating wing growth is supported by the heterogeneous proliferation dynamics described by Milán et al. (1996): cell cycling starts in veins earlier than in intervein regions.
A second possible regulator of wing growth during pupal development is the tension arising from hinge contraction. When developing wings are severed from the hinge, the number of cell divisions is only about ~ 80 % of that in normal conditions (Aigouy et al., 2010). It is known that in some cases cell proliferation is modulated by mechanical cues such as cell density and contacts (Gaspar & Tapon, 2014). It could be that the tension generated during hinge contraction regulates growth.
Mechanical effect from the veins
Vein differentiation has been well studied (O'Keefe et al., 2012). Veins morphologically differentiate during late pupal development, and they could have a mechanical effect on the shape of the wing. This is most clearly visible at the intersection between L5 and the wing margin, where there is some curvature suggesting that the vein is pulling on the margin. Interestingly, this effect does not happen in wings mutant for rho or vn genes. Such wings lack veins, and they have an overall shape different from wild-type, suggesting a relationship between the presence of the veins and the deformation of the shape of the margin (Figs. 6a and 6f). Vein cells are morphologically different from the other cells. Their apical area does not show the characteristic hexagonal pattern of the other cells of the blade (Fig. 5d). It has been shown that these morphological differences arise from differential adhesion under the control of Ras/Egfr signaling. Junctions located at the interface between two vein cells have higher adhesion/lower line-tension, as compared with junctions at vein-intervein interface. Thus, there is a relatively high tension at the vein-intervein junctions and it could be that this tension reflects a force pulling on the margin.
To summarize, patterns of mitotic density, orientation of cell division and of cell intercalation, which all account for the distortions of wing size and shape during late pupal development, are driven by two classes of processes. On the one hand, the contraction of the hinge and possibly the attachments of the blade margin cells to the cuticle generate a tension on the blade. Hinge contraction has been shown to directly influence the patterns of cell intercalation and of cell division, and it is likely that it affects the amount of growth. Interfering with the contraction of the hinge affects the shape and the size of the wing blade. On the other hand, the regulation of the tensile and adhesive forces at the cell junctions by the acto-myosin network is essential to control the visco-elastic properties of the tissue and its topology. Such forces can arise from the accumulation of MyoII as a response to tissue tension (Sugimura & Ishihara, 2013), or by instructive molecular signals such as the positioning of Dachs by Dachsous and Four-Jointed gradients, the regulation of MyoII release by pten, or vein-intervein differential cell adhesion under the control of Ras/Egfr signalling. In principle, natural variation in wing shape morphology could result from some inter-individual variation in any of these processes.
Perspectives
The Drosophila wing has become an important model system for developmental and evolutionary biology. However, these two fields have not yet succeeded in integrating advances on wing development to explain natural variation of wing size and shape. Our review of wing development shows that several well-known developmental processes are capable of modifying wing size and shape. Variation comparable to what can be observed in nature can be created by manipulating these processes in the laboratory, but it remains to be established whether these processes have been modified during wing evolution, or if natural variation comes from other developmental processes.
There is, to our knowledge, no study reporting the developmental events responsible for natural variation in the wing shape or size in Drosophila. We thus foresee a research program of developmental systematics, consisting in investigating the variation in development within natural populations that causes differences in wing morphology. Such a program must involve comparisons at many different biological scales, including DNA sequencing; comparative analysis of gene expression in time and space; comparison of cellular morphogenetic behaviors; comparison of morphology of developing structures and, of course, comparison of adult structures. While there have been several QTL and association studies of the effects of genomic variation with wing shape, these are insufficient to identify the mechanisms producing variation. An understanding of the natural variation of wing morphology requires attention on the other levels of the genotype-phenotype map in order to causally link genotype variation with phenotypic variation, as exemplified in many vertebrate studies.
This review highlights several possible “hot-spots” to look at, such as the distribution of morphogens in the wing disc; patterns of expression of Vestigial and of the proteins defining planar cell polarity; patterns of growth during larval development; wing expansion during the early pupal phase; and pattern of elongation during the late pupal stage (Fig. 6).
The developmental basis of natural morphological variation has been studied in other Drosophila organs. Notable examples include the study of inter-populational and inter-specific variation in trichome patterns in larval and adult structures (Stern, 1998; Sucena et al., 2003; Arif et al., 2013); and inter-specific variation in head morphology (Posnien et al., 2012; Arif et al., 2013) and in wing pigmentation (Arnoult et al., 2013). Such efforts need to be applied to the study of natural wing variation. This organ presents modest variation that must be measured precisely in order to be detected, even between species separated by long divergence times (Fig. 1). It is thus likely that the detection of the differences in developmental processes leading to differences in wing shape will require the use of a quantitative framework of the same precision as the one used to measure variation in the adult wings. Evo-Devo research has long emphasized the qualitative description of developmental processes, with relatively few efforts to quantify more subtle variation in developmental processes that are likely to cause variation in multidimensional phenotypes (Parsons & Albertson, 2013). The study of the fly wing fits well into this latter category of analysis. The recent advances in our knowledge of wing development reviewed here should enable studies that associate wing variation with specific developmental processes.
In recent years, considerable progress has been made in quantifying developmental processes in the fly wing and in other Drosophila organs thanks to improved imaging techniques, especially live imaging. For example, the distribution of morphogens in the wing disc can be measured with a cell-level precision (Kicheva et al., 2007; Bollenbach et al., 2008); local patterns of cell intercalation and oriented cell division have been quantitatively described during thorax and pupal wing development (Aigouy et al., 2010; Bosveld et al., 2012; Sugimura & Ishihara, 2013); patterns of planar cell polarity have been quantified in larval and pupal wing tissues (Sagner et al., 2012; Merkel et al., 2014); local patterns of shape distortion have been described during wing disc growth (Heemskerk et al., 2014); and there are quantitative predictions about how the distribution of the Fat and Dachsous proteins should affect polarity and growth (Mani et al., 2013). Until now, these techniques have been used to compare control and experimental genotypes in D. melanogaster. We advocate the application of such techniques for comparison of natural variants resulting from inter-sexual, inter-population or inter-specific morphological evolutionary divergence. In the latter case, it is likely that applying these tools outside D. melanogaster will be challenging, but the fact that interspecific differences in development have been unraveled successfully in the examples mentioned above is encouraging.
For example, a good starting point in identifying developmental changes accounting for natural variation could be to identify at which stage development has been modified. This can be done first by comparing morphology of 3rd instar wing discs between individuals differing by their adult wing characteristics, using the tools routinely used to measure the adult morphology (Fig. 1). If the wing discs are different (e.g., Figs. 6 a-f), then this will suggest that developmental changes occurred during the larval phase. In this case, the patterns of the key proteins involved in larval wing development (e.g., growth factors, planar cell polarity proteins, provein determinants), as well as patterns of proliferation and of oriented cell division in the larva will need to be checked in order to find out which differences during larval development account for differences in the adult wing. Alternatively, if no morphological differences are found in 3rd instar wing discs, this will suggest that differences in development between the two variants appeared later. In this case, a comparison of the morphology of the early pupal wing will tell whether it is the larval to pupal transition or the late pupal development that changed. Depending on the result, specific developmental processes (e.g. wing folding and expansion during pupariation; hinge contraction during the late pual phase ) could be screened for differences.
The use of computer simulations provides a useful framework to test our ability to connect quantitative differences in development with changes in adult wing morphology. An explicit, mathematical model of development brings our verbal models into potential conflict with experimental results, allowing rigorous tests of our current understanding. In the case of the fly, many aspects of wing development such as wing disc growth (Hufnagel et al., 2007; Mao et al., 2011, 2013; Aegerter-Wilmsen et al., 2012); signalling (Wartlick et al., 2011); boundary formation (Landsberg et al., 2009; Canela-Xandri et al., 2011; Schilling et al., 2011; Aliee et al., 2012); planar cell polarity (Aigouy et al., 2010; Merkel et al., 2014); cell packing (Farhadifar et al., 2007); mitotic cleavage patterns (Gibson et al., 2011) have been successfully simulated using cell-based models. Computational models of development in many other Drosophila tissues have also been developed (for review see Fletcher et al., 2014). None of these models, however account for variation in wing disc or adult shape. For example, existing simulations of wing disc growth do not account for the ellipsoid shape of the disc and the anisotropies in growth described by Bittig et al. (2009) in wild-type-flies. The application of such models to the study of variation in shape will test our capacity to predict how changes in development affect wing shape. This process has begun in other developmental systems (Salazar-Ciudad & Jernvall, 2010).
In the Introduction, we noted that the genetic basis of variation in wing size and shape is highly polygenic (i.e., influenced by a large number of genes), making it unlikely that a general understanding of variation can be achieved by studying individual genes. This review of development is in part motivated by the possibility that variation in wing shape will be simpler to study at the developmental level than at the genetic level. While this remains to be tested experimentally, the knowledge about wing development summarized here suggests that complexity may remain at this level. There are many events that must occur during wing development. Perhaps more significant is that the same genes and pathways are often involved in the regulation of several developmental processes. For example, changes in dpp expression affect both vein patterning and tissue growth, and the fat/dachsous system is involved in the regulation of proliferation, orientation of division and of cell rearrangements during both larval and pupal development. Moreover, biophysical forces emerging during development actively regulate morphogenesis and gene expression itself. Variation in discrete traits such as presence/absence of an organ or feature can sometimes (but not always [Stern & Orgogozo, 2009]) be explained by precise changes in the regulation of expression of one or a small number of genes (see Carroll (2008) for examples). Future work will need to address whether the diffuse, multivariate differences in Drosophila wing shape rely on such a simple explanation.
Acknowledgments
We thank B. Aigouy, A. Baena-Lopez, F. Bosveld, L. Le Goff, A. Martinez-Arias, A. Repiso, O. Shimmi, and B. Thompson for helpful advice and discussion of specific aspects of wing development, and two reviewers for constructive comments. We thank B. Aigouy, E. Bier, Y. Huang, F. Roch and O. Shimmi for sharing original picture files for figures 5 and 6.
Funding: This research was supported by NIH grant R01-GM094424-04 to D. Houle, and by the Finnish Academy grant # WBS 1250271 to I. Salazar-Ciudad.
References
- Aegerter-Wilmsen T, Heimlicher MB, Smith AC, de Reuille PB, Smith RS, Aegerter CM, Basler K. Integrating force-sensing and signaling pathways in a model for the regulation of wing imaginal disc size. Development. 2012;139:3221–3231. doi: 10.1242/dev.082800. [DOI] [PubMed] [Google Scholar]
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J-C, Jülicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Aldaz S, Escudero LM, Freeman M. Dual role of myosin II during Drosophila imaginal disc metamorphosis. Nat Com. 2013;4:1761. doi: 10.1038/ncomms2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliee M, Röper J-C, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr Biol. 2012;22:967–76. doi: 10.1016/j.cub.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-Fat planar cell polarity. Curr Biol. 2012;22:1302–8. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif S, Hilbrant M, Hopfen C, Almudi I, Nunes MDS, Posnien N, Kuncheria L, Tanaka K, Mitteroecker P, Schlötterer C, McGregor AP. Genetic and developmental analysis of differences in eye and face morphology between Drosophila simulans and Drosophila mauritiana. Evol Dev. 2013;15:257–67. doi: 10.1111/ede.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif S, Murat S, Almudi I, Nunes MDS, Bortolamiol-Becet D, McGregor NS, Currie JMS, Hughes H, Ronshaugen M, Sucena É , Lai EC, Schlötterer C, McGregor AP. Evolution of mir-92a underlies natural morphological variation in Drosophila melanogaster. Curr Biol. 2013;23:523–8. doi: 10.1016/j.cub.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult L, Su KFY, Manoel D, Minervino C, Magriña J, Gompel N, Prud'homme B. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science. 2013;339:1423–6. doi: 10.1126/science.1233749. [DOI] [PubMed] [Google Scholar]
- Baena-López LA, Baonza A, García-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–4. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Baena-López LA, Garcia-Bellido A. Control of growth and positional information by the graded vestigial expression pattern in the wing of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2006;103:13734–13739. doi: 10.1073/pnas.0606092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006;295:178–193. doi: 10.1016/j.ydbio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Bardet PL, Guirao B, Paoletti C, Serman F, Léopold V, Bosveld F, Goya Yû, Mirouse V, Graner F, Bellaïche Y. PTEN Controls Junction Lengthening and Stability during Cell Rearrangement in Epithelial Tissue. Dev Cell. 2013;25:534–546. doi: 10.1016/j.devcel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Bate M, Martinez-Arias AM. The embryonic origin of imaginal discs in Drosophila. Development. 1991;112:755–61. doi: 10.1242/dev.112.3.755. [DOI] [PubMed] [Google Scholar]
- Bejarano F, Smibert P, Lai EC. miR-9a prevents apoptosis during wing development by repressing Drosophila LIM-only. Dev Biol. 2010;338:63–73. doi: 10.1016/j.ydbio.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Biehs B, Sturtevant M a, Bier E. Boundaries in the Drosophila wing imaginal disc organize vein-specific genetic programs. Development. 1998;125:4245–57. doi: 10.1242/dev.125.21.4245. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Bitner-Mathé BC, Klaczko LB. Plasticity of Drosophila melanogaster wing morphology: Effects of sex, temperature and density. Genetica. 1999;105:203–210. doi: 10.1023/a:1003765106652. [DOI] [PubMed] [Google Scholar]
- Bittig T, Wartlick O, González-Gaitán M, Jülicher F. Quantification of growth asymmetries in developing epithelia. Eur Phys J E. 2009;30:93–99. doi: 10.1140/epje/i2009-10507-6. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Bollenbach T, Pantazis P, Kicheva A, Bökel C, González-Gaitán M, Jülicher F. Precision of the Dpp gradient. Development. 2008;135:1137–46. doi: 10.1242/dev.012062. [DOI] [PubMed] [Google Scholar]
- Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet P-L, Marcq P, Graner F, Bellaïche Y. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–7. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol. 2012;22:907–14. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela-Xandri O, Sagués F, Casademunt J, Buceta J. Dynamics and mechanical stability of the developing dorsoventral organizer of the wing imaginal disc. PLoS Comput Biol. 2011;7:e1002153. doi: 10.1371/journal.pcbi.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira VP, Soto IM, Mensch J, Fanara JJ. Genetic basis of wing morphogenesis in Drosophila: sexual dimorphism and non-allometric effects of shape variation. BMC Dev Biol. 2011;11:32. doi: 10.1186/1471-213X-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Carter AJR, Houle D. Artificial selection reveals heritable variation for developmental instability. Evolution. 2011;65:3558–3564. doi: 10.1111/j.1558-5646.2011.01393.x. [DOI] [PubMed] [Google Scholar]
- De Celis JF. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997;124:1007–18. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- De Celis JF. Pattern formation in the Drosophila wing: The development of the veins. Bioessays. 2003;25:443–51. doi: 10.1002/bies.10258. [DOI] [PubMed] [Google Scholar]
- Chan YF, Marks ME, Jones FC, Villarreal G, Shapiro MD, Brady SD, Southwick AM, Absher DM, Grimwood J, Schmutz J, Myers RM, Petrov D, Jónsson B, Schluter D, Bell MA, Kingsley DM. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Glise B, Vincent A. Patterns in evolution: veins of the Drosophila wing. Trends Genet. 2004;20:498–505. doi: 10.1016/j.tig.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Domínguez-Giménez P, Brown NH, Martín-Bermudo MD. Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J Cell Sci. 2007;120:1061–71. doi: 10.1242/jcs.03404. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature Publishing Group. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol. 2007;17:2095–104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Fletcher AG, Osterfield M, Baker RE, Shvartsman SY. Vertex models of epithelial morphogenesis. Biophys J. 2014;106:2291–304. doi: 10.1016/j.bpj.2013.11.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrom D, Fristrom J. The metamorphic development of the adult epidermis. In: Bate M, Martinez-Arias AM, editors. Cold Spring Harbour Press; Cold Spring Harbour: 1993. pp. 843–897. [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, Merriam JR. Parameters of the Wing Imaginal Disc Development of Drosophila melanogaster. Dev Biol. 1971;24:61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- García-Bellido A. The cellular and genetic bases of organ size and shape in Drosophila. Int J Dev Biol. 2009;53:1291–303. doi: 10.1387/ijdb.072459ag. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Gibson WT, Veldhuis JH, Rubinstein B, Cartwright HN, Perrimon N, Brodland GW, Nagpal R, Gibson MC. Control of the mitotic cleavage plane by local epithelial topology. Cell. 2011;144:427–38. doi: 10.1016/j.cell.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies TE, Cabernard C. Cell Division Orientation in Animals. Curr Biol. 2011;21:R599–R609. doi: 10.1016/j.cub.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Le Goff L, Rouault H, Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–9. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- Gompel N, Prud'homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature. 2005;433:481–7. doi: 10.1038/nature03235. [DOI] [PubMed] [Google Scholar]
- Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- González-Gaitán M, Capdevila MP, García-Bellido A. Cell proliferation patterns in the wing imaginal disc of Drosophila. Mech Dev. 1994;46:183–200. doi: 10.1016/0925-4773(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Sierra J, Callejo A, Ibañez C, Guerrero I. The Drosophila ortholog of the human Wnt inhibitor factor shifted controls the diffusion of lipid-modified hedgehog. Dev Cell. 2005;8:241–253. doi: 10.1016/j.devcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Affolter M, Pyrowolakis G. Dpp/BMP signaling in flies: from molecules to biology. Semin Cell Dev Biol. 2014;32:128–36. doi: 10.1016/j.semcdb.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg C-P, Hammerschmidt M. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–87. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Heemskerk I, Lecuit T, LeGoff L. Dynamic clonal analysis based on chronic in vivo imaging allows multiscale quantification of growth in the Drosophila wing disc. Development. 2014;141:2339–48. doi: 10.1242/dev.109264. [DOI] [PubMed] [Google Scholar]
- Heisenberg C-P, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–62. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Honegger H-W, Dewey EM, Ewer J. Bursicon, the tanning hormone of insects: recent advances following the discovery of its molecular identity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:989–1005. doi: 10.1007/s00359-008-0386-3. [DOI] [PubMed] [Google Scholar]
- Houle D, Fierst J. Properties of spontaneous mutational variance and covariance for wing size and shape in drosophila melanogaster. Evolution. 2013;67:1116–1130. doi: 10.1111/j.1558-5646.2012.01838.x. [DOI] [PubMed] [Google Scholar]
- Houle D, Mezey J, Galpern P, Carter A. Automated measurement of Drosophila wings. BMC Evol Biol. 2003;3:25. doi: 10.1186/1471-2148-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci U S A. 2007;104:3835–40. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KD. Integration of intercellular signaling through the Hippo pathway. Semin Cell Dev Biol. 2012;23:812–7. doi: 10.1016/j.semcdb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanca O, Caussinus E, Denes AS, Percival-Smith A, Affolter M. Raeppli: a whole-tissue labeling tool for live imaging of Drosophila development. Development. 2014;141:472–80. doi: 10.1242/dev.102913. [DOI] [PubMed] [Google Scholar]
- Kicheva A, Pantazis P, Bollenbach T, Kalaidzidis Y, Bittig T, Jülicher F, González-Gaitán M. Kinetics of morphogen gradient formation. Science. 2007;315:521–5. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- Kiger JA, Natzle JE, Kimbrell DA, Paddy MR, Kleinhesselink K, Green MM. Tissue remodeling during maturation of the Drosophila wing. Dev Biol. 2007;301:178–191. doi: 10.1016/j.ydbio.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kodama A, Hayasaka Y, Ohta T. Activation of the cAMP/PKA signaling pathway is required for post-ecdysial cell death in wing epidermal cells of Drosophila melanogaster. Development. 2004;131:1597–606. doi: 10.1242/dev.01049. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias a M. The vestigial gene product provides a molecular context for the interpretation of signals during the development of the wing in Drosophila. Development. 1999;126:913–25. doi: 10.1242/dev.126.5.913. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Zaklan SD. Morphological intergration between development compartments in the Drosophila wing. Evolution. 2000;54:1273–85. doi: 10.1111/j.0014-3820.2000.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–8. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- De La Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117:107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- Lander AD. Pattern, growth, and control. Cell. 2011;144:955–69. doi: 10.1016/j.cell.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Jülicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–5. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–92. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne P-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RRH, Barrón M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu L-L, Qu C, Ràmia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu Y-Q, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–8. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M, Schneiderman H. Histological Analysis of the Dynamics of Growth of Imaginal Discs and Histoblast Nests During the Larval Development of Drosophila melanogaster. Wilhelm Roux's Arch. 1977;183:269–305. doi: 10.1007/BF00848459. [DOI] [PubMed] [Google Scholar]
- Mallarino R, Campàs O, Fritz JA, Burns KJ, Weeks OG, Brenner MP, Abzhanov A. Closely related bird species demonstrate flexibility between beak morphology and underlying developmental programs. Proc Natl Acad Sci U S A. 2012;109:16222–7. doi: 10.1073/pnas.1206205109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani M, Goyal S, Irvine KD, Shraiman BI. Collective polarization model for gradient sensing via Dachsous-Fat intercellular signaling. Proc Natl Acad Sci U S A. 2013;110:20420–5. doi: 10.1073/pnas.1307459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 2011;25:131–6. doi: 10.1101/gad.610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Hoppe A, Kester L, Thompson BJ, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013;32:2790–803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature Publishing Group. Nature. 2012;484:542–5. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- Martín FA, Pérez-Garijo A, Moreno E, Morata G. The brinker gradient controls wing growth in Drosophila. Development. 2004;131:4921–4930. doi: 10.1242/dev.01385. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E, Pastor-Pareja JC, Garcia-Bellido A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc Natl Acad Sci U S A. 2000;97:7888–7893. doi: 10.1073/pnas.97.14.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–94. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Blanco J, Shimmi O. A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Drosophila Wing. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel M, Sagner A, Gruber FS, Etournay R, Blasse C, Myers E, Eaton S, Jülicher F. The Balance of Prickle/Spiny-Legs Isoforms Controls the Amount of Coupling between Core and Fat PCP Systems. Curr Biol. 2014;24:2111–2123. doi: 10.1016/j.cub.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Mezey JG, Houle D, Nuzhdin S V. Naturally segregating quantitative trait loci affecting wing shape of Drosophila melanogaster. Genetics. 2005;169:2101–2113. doi: 10.1534/genetics.104.036988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey JG, Houle D. The dimensionality of genetic variation for wing shape in Drosophila melanogaster. Evolution. 2005;59:1027–1038. [PubMed] [Google Scholar]
- Milán M, Campuzano S, Barcia-Bellido A. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc Natl Acad Sci U S A. 1996;93:640–645. doi: 10.1073/pnas.93.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milán M, Campuzano S, García-Bellido a. Cell cycling and patterned cell proliferation in the Drosophila wing during metamorphosis. Proc Natl Acad Sci U S A. 1996;93:11687–92. doi: 10.1073/pnas.93.21.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milán M, Campuzano S, García-Bellido a. Developmental parameters of cell death in the wing disc of Drosophila. Proc Natl Acad Sci U S A. 1997;94:5691–6. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc N, Piel M. Predicting division plane position and orientation. Trends Cell Biol. 2012;22:193–200. doi: 10.1016/j.tcb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Montero JA, Hurlé JM. Sculpturing digit shape by cell death. Apoptosis. 2010;15:365–75. doi: 10.1007/s10495-009-0444-5. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Nijhout HF, Cinderella M, Grunert LW. The development of wing shape in Lepidoptera: mitotic density, not orientation, is the primary determinant of shape. Evol Dev. 2014;16:68–77. doi: 10.1111/ede.12065. [DOI] [PubMed] [Google Scholar]
- Nunes MDS, Arif S, Schlötterer C, McGregor AP. A perspective on micro-evo-devo: progress and potential. Genetics. 2013;195:625–34. doi: 10.1534/genetics.113.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe DD, Gonzalez-Niño E, Edgar BA, Curtiss J. Discontinuities in Rap1 activity determine epithelial cell morphology within the developing wing of Drosophila. Dev Biol. 2012;369:223–34. doi: 10.1016/j.ydbio.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe D, Thomas S, Bolin K, Griggs E, Edgar B, Buttitta L. Combinatorial control of temporal gene expression in the Drosophila wing by enhancers and core promoters. BMC Genomics. 2012;13:498. doi: 10.1186/1471-2164-13-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbard DJ, Maclennan J, Kim K-W, Rambaut A, O'Grady PM, Jiggins FM. Estimating divergence dates and substitution rates in the Drosophila phylogeny. Mol Biol Evol. 2012;29:3459–73. doi: 10.1093/molbev/mss150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Albertson RC. Unifying and generalizing the two strands of evo-devo. Trends Ecol Evol. 2013;28:584–91. doi: 10.1016/j.tree.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Grawe F, Martín-Blanco E, García-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Pélabon C, Hansen TF, Carter AJR, Houle D. Response of fluctuating and directional asymmetry to selection on wing shape in Drosophila melanogaster. J Evol Biol. 2006;19:764–776. doi: 10.1111/j.1420-9101.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- Pélabon C, Hansen TF, Carter AJR, Houle D. Evolution of variation and variability under fluctuating, stabilizing, and disruptive selection. Evolution. 2010;64:1912–25. doi: 10.1111/j.1558-5646.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- Posnien N, Hopfen C, Hilbrant M, Ramos-Womack M, Murat S, Schönauer A, Herbert SL, Nunes MDS, Arif S, Breuker CJ, Schlötterer C, Mitteroecker P, McGregor AP. Evolution of eye morphology and rhodopsin expression in the Drosophila melanogaster species subgroup. PLoS One. 2012;7:e37346. doi: 10.1371/journal.pone.0037346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne P-F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–10. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- Repiso A, Bergantiños C, Serras F. Cell fate respecification and cell division orientation drive intercalary regeneration in Drosophila wing discs. Development. 2013;140:3541–51. doi: 10.1242/dev.095760. [DOI] [PubMed] [Google Scholar]
- Restrepo S, Zartman JJ, Basler K. Coordination of patterning and growth by the morphogen DPP. Curr Biol. 2014;24:R245–55. doi: 10.1016/j.cub.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Roch F, Jiménez G, Casanova J. EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development. 2002;129:993–1002. doi: 10.1242/dev.129.4.993. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–21. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rouzic A, Houle D, Hansen TF. A modelling framework for the analysis of artificial-selection time series. Genet Res. 2011;93:155–73. doi: 10.1017/S0016672311000024. [DOI] [PubMed] [Google Scholar]
- Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Jülicher F, Eaton S. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol. 2012;22:1296–301. doi: 10.1016/j.cub.2012.04.066. [DOI] [PubMed] [Google Scholar]
- Salazar-Ciudad I, Jernvall J. A gene network model accounting for development and evolution of mammalian teeth. Proc Natl Acad Sci U S A. 2002;99:8116–20. doi: 10.1073/pnas.132069499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Ciudad I, Jernvall J. A computational model of teeth and the developmental origins of morphological variation. Nature. 2010;464:583–6. doi: 10.1038/nature08838. [DOI] [PubMed] [Google Scholar]
- Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling S, Willecke M, Aegerter-Wilmsen T, Cirpka O a, Basler K, von Mering C. Cell-sorting at the A/P boundary in the Drosophila wing primordium: a computational model to consolidate observed non-local effects of Hh signaling. PLoS Comput Biol. 2011;7:e1002025. doi: 10.1371/journal.pcbi.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Tauriello G, Yagi R, Kranz E, Koumoutsakos P, Basler K. Antagonistic growth regulation by Dpp and Fat drives uniform cell proliferation. Dev Cell. 2011;20:123–30. doi: 10.1016/j.devcel.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Segalen M, Bellaïche Y. Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol. 2009;20:972–7. doi: 10.1016/j.semcdb.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Ralston A, Blair SS, O'Connor MB. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol. 2005;282:70–83. doi: 10.1016/j.ydbio.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Stern DL, Orgogozo V. Is genetic evolution predictable? Science. 2009;323:746–51. doi: 10.1126/science.1158997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–6. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant MA, Bier E. Analysis of the genetic hierarchy guiding wing vein development in Drosophila. Development. 1995;121:785–801. doi: 10.1242/dev.121.3.785. [DOI] [PubMed] [Google Scholar]
- Sucena E, Delon I, Jones I, Payre F, Stern DL. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature. 2003;424:935–8. doi: 10.1038/nature01768. [DOI] [PubMed] [Google Scholar]
- Sugimura K, Ishihara S. The mechanical anisotropy in a tissue promotes ordering in hexagonal cell packing. Development. 2013;140:4091–101. doi: 10.1242/dev.094060. [DOI] [PubMed] [Google Scholar]
- Taylor J, Adler PN. Cell rearrangement and cell division during the tissue level morphogenesis of evaginating Drosophila imaginal discs. Dev Biol. 2008;313:739–51. doi: 10.1016/j.ydbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Dev Dyn. 2012;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Morphogenesis of Drosophila pupal wings in vitro. Mech Dev. 1995;52:247–255. doi: 10.1016/0925-4773(95)00405-p. [DOI] [PubMed] [Google Scholar]
- Usui K, Simpson P. Cellular basis of the dynamic behavior of the imaginal thoracic discs during Drosophila metamorphosis. Dev Biol. 2000;225:13–25. doi: 10.1006/dbio.2000.9766. [DOI] [PubMed] [Google Scholar]
- Vincent J-P, Fletcher AG, Baena-Lopez LA. Mechanisms and mechanics of cell competition in epithelia. Nature Publishing Group. Nat Rev Mol Cell Biol. 2013;14:581–91. doi: 10.1038/nrm3639. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The genetic control of wing development in Drosophila. J Genet. 1940;41:75–139. [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent ExtensionThe Molecular Control of Polarized Cell Movement during Embryonic Development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Jülicher F, Gonzalez-Gaitan M. Understanding morphogenetic growth control - lessons from flies. Nat Rev Mol Cell Biol. 2011;12:594–604. doi: 10.1038/nrm3169. [DOI] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Jülicher F, González-Gaitán M. Dynamics of Dpp signaling and proliferation control. Science. 2011;331:1154–1159. doi: 10.1126/science.1200037. [DOI] [PubMed] [Google Scholar]
- Weber K, Eisman R, Higgins S, Morey L, Patty A, Tausek M, Zeng ZB. An analysis of polygenes affecting wing shape on chromosome 2 in Drosophila melanogaster. Genetics. 2001;159:1045–1057. doi: 10.1093/genetics/159.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Eisman R, Morey L, Patty A, Sparks J, Tausek M, Zeng Z-B. An Analysis of Polygenes Affecting Wing Shape on Chromosome 3 in Drosophila melanogaster. Genetics. 1999;153:773–786. doi: 10.1093/genetics/153.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KE. Selection on wing allometry in Drosophila melanogaster. Genetics. 1990;126:975–989. doi: 10.1093/genetics/126.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KE. How small are the smallest selectable domains of form? Genetics. 1992;130:345–353. doi: 10.1093/genetics/130.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman A-C, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E, Palsson A, Gibson G. Quantitative trait loci affecting components of wing shape in Drosophila melanogaster. Genetics. 2000;155:671–683. doi: 10.1093/genetics/155.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]