Key Points
Donor treatment with agonistic DR3 antibody induces selective expansion of Tregs and reduced activation of conventional T cells.
T cells from DR3 antibody–treated donors result in reduced acute GVHD and preserved GVT effects.
Abstract
The paucity of regulatory T cells (Tregs) limits clinical translation to control aberrant immune reactions including graft-versus-host disease (GVHD). Recent studies showed that the agonistic antibody to DR3 (αDR3) expanded CD4+FoxP3+ Tregs in vivo. We investigated whether treating donor mice with a single dose of αDR3 could alleviate acute GVHD in a MHC-mismatched bone marrow transplantation model. αDR3 induced selective proliferation of functional Tregs. CD4+ T cells isolated from αDR3-treated mice contained higher numbers of Tregs and were less proliferative to allogeneic stimuli. In vivo GVHD studies confirmed that Tregs from αDR3-treated donors expanded robustly and higher frequencies of Tregs within donor CD4+ T cells were maintained, resulting in improved survival. Conventional T cells derived from αDR3-treated donors showed reduced activation and proliferation. Serum levels of proinflammatory cytokines (IFNγ, IL-1β, and TNFα) and infiltration of donor T cells into GVHD target tissues (gastrointestinal tract and liver) were decreased. T cells from αDR3-treated donors retained graft-vs-tumor (GVT) effects. In conclusion, a single dose of αDR3 alleviates acute GVHD while preserving GVT effects by selectively expanding and maintaining donor Tregs. This novel strategy will facilitate the clinical application of Treg-based therapies.
Introduction
Regulatory T cells (Tregs) defined by the cell surface phenotype of CD4+CD25+ express the transcription factor FoxP3 and regulate not only autoimmune but also alloimmune responses.1 Several groups have shown in different murine models that donor Tregs prevent the development of acute graft-versus-host disease (GVHD).2-5 These findings have been extended to the clinic with encouraging results.6-9 The timed addition of Tregs and CD4+ and CD8+ T cells preserve graft-vs-tumor (GVT) effects.2,5 However, the adoptive transfer of Tregs remains challenging because of their rare frequency and the paucity of surface markers that unequivocally distinguish Tregs from conventional T cells. A number of strategies have been reported including the isolation of natural Tregs with immunomagnetic beads and cell sorting as well as ex vivo expansion of Tregs.10 Ex vivo expansion of Tregs has been accomplished by treating purified cells with IL-2 combined with antibodies directed against CD3/CD28.11,12 Overgrowth of effector T cells has been frequently observed after ex vivo expansion, and rapamycin has been used to reduce it.13 Isolation and expansion of Tregs requires specialized facilities and can be challenging to obtain sufficient numbers of cells for clinical applications.10
Alternative approaches have been used to expand induced Tregs from human naïve T cells by the addition of transforming growth factor-β and rapamycin.14 Epigenetic modulations through the addition of agents such as azacitidine, decitabine, and vorinostat have also been shown to induce FoxP3 expression in CD4+CD25– T cells and suppress acute GVHD in murine models.15,16 We previously reported that the combined administration of rapamycin and IL-2 expanded donor Tregs and reduced acute GVHD in a murine model.17 However, these agents could work nonspecifically and might adversely affect other cell populations. Therefore, alternative strategies for the activation and expansion of Tregs are needed.
Death receptor 3 (DR3, TNFRSF25) belongs to the tumor necrosis factor (TNF) receptor superfamily and is primarily expressed on Tregs, lymphoid tissue inducer cells, and natural killer T cells. The natural ligand of DR3, TL1a, is expressed on endothelial cells and antigen-presenting cells.18 Early studies reported that the activation of TL1a-DR3 signaling induced pathogenic inflammation in some disease models.19 It was recently shown that an agonistic monoclonal antibody (mAb) to DR3 (αDR3) significantly expanded Tregs in vivo. DR3-induced Treg expansion in vivo required T-cell receptor (TCR), IL-2, and MHC II signaling. Although the mechanism is not fully understood, αDR3 treatment prevented the development of lung inflammation by increasing the proportion of local Tregs.20 αDR3 also promoted the survival of cardiac allografts by increasing the proportion of Tregs within peripheral CD4+ T cells.21 In our study, we treated donors with αDR3 and performed bone marrow transplantation (BMT) in an MHC I/II–mismatched murine model.17,22 We found that donor Tregs markedly expanded, resulting in significant reduction of acute GVHD without impairment of GVT effects.
Materials and methods
Mice and cell line
C57BL/6 (H-2kb CD45.2+) and BALB/c (H-2kd CD45.2+) mice were purchased from the Jackson Laboratory (Sacramento, CA). Luc+ C57BL/6 (H-2kb Thy-1.1+ CD45.1+) and Thy-1.1+ CD45.1+ gfp+ C57BL/6 mice were bred in our animal facility at Stanford University.23 C57BL/6-FoxP3.Luci.DTR-4 mice were provided from the University of Würzburg, Germany.24 Mice between the ages of 8 to 16 weeks were used and sex-matched combinations were used for transplant experiments. A20 and luc+/gfp+-BCL1, B-cell lymphoma cell lines (derived from Balb/c mice) were used for GVT experiments.25,26 All animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Antibodies and reagents
The FcR-blocking reagent, magnetic microbeads, LS columns were purchased from Miltenyi Biotec (Auburn, CA). Agonistic anti-DR3 mAb (clone: 4C12) and Hamster IgG isotype control mAb (clone: HTK888) were purchased from Biolegend (San Diego, CA). Antibodies to the following molecules were used for flow cytometric analysis: H-2Kb (AF6-88.5), CD45.1 (A20), CD3ε (145-2C11), CD4 (GK1.5), CD8 (53-6.7), FoxP3 (FJK-16s), CD25 (3C7), T-bet (4B10), granzyme B (GB11), IFNγ (XMG1.2), TNFα (MP6-XT22), and BrdU (BU20A) from Biolegend and eBioscience (San Diego, CA). Fixable Viability Dye eFluor 506 (eBioscience) was used to exclude dead cells. FoxP3 Fixation/Permeabilization buffer set was purchased from eBioscience. BrdU and DNase I were obtained from Sigma (St. Louis, MO) and [3H] thymidine from PerkinElmer (Shelton, CT).
Cell isolation and sorting
αDR3 or isotype control mAb was administered by intraperitoneal injection at a dose of 0.5 mg kg−1 4 days before BMT, and animals were sacrificed on day 0. Lymph nodes (LNs) plus spleen were harvested, and donor T cells were isolated by magnetic-activated cell sorting using anti-CD4, anti-CD8 microbeads. For CD25+ Treg isolation, cells were stained with anti-CD25 APC, incubated with anti-APC microbeads, and positively selected with an LS column. CD4+CD25+ cells were isolated using the ARIA cell sorter (BD Biosciences, San Jose, CA). The purity of CD4+CD25+FoxP3+ was >95%.27
Murine models: MHC-mismatch GVHD and tumor models
GVHD was induced as described previously.17,22 Briefly, BALB/c recipient mice were irradiated with 850 cGy in 2 split doses on day 0. T cell–depleted bone marrow (TCD-BM) cells were isolated from wild-type nontreated C57BL/6 mice, whereas donor T cells were obtained from mAb-treated (αDR3 or isotype-mAb) C57BL/6 donor mice. 5 × 106 TCD-BM cells and 1 × 106 T cells were intravenously injected to recipients. For some experiments, 5 × 105 T cells and 5 × 106 TCD-BM cells were injected. Clinical evidence of GVHD was scored as previously described.28 To evaluate GVT effects, 2 × 104 luc+/gfp+-A20 or BCL1 cells were injected IV into the Balb/c mice via a tail vein.2
Mixed-lymphocyte reaction (MLR)
T cells (2 × 105/well) were isolated from αDR3 or isotype-treated C57BL/6 mice by magnetic-activated cell sorting and cocultured with γ-irradiated (3000 cGy) Balb/c splenocytes (2 × 105/well) as stimulator cells. After 96 hours, cells were pulsed with 0.037 MBq per well (1 μCi/well) [3H] thymidine for 16 hours. [3H] Thymidine incorporation was measured with a Wallac Betaplate counter (PerkinElmer). For some MLR experiments, nontreated C57BL/6 T cells (2 × 105/well), γ-irradiated Balb/c splenocytes (4 × 105/well) and CD25+ Tregs sorted from mAb-treated donors were cultured.
Flow cytometric analysis: FoxP3, cytokines, and BrdU
Cells were harvested from MLR plates after culture for 96 hours or were reisolated from BMT recipients on days 3, 7, 14, and 28.17 For intracellular cytokine staining, cells were stimulated with Cell Stimulation Cocktail (eBioscience) for 5 hours, fixed with FoxP3 Fixation/Permeabilization buffer (eBioscience), and stained for intracellular molecules. For BrdU staining, the recipient mice were injected intraperitoneally with 1 mg of BrdU at 1 and 2 days before analysis. All assays were performed with an LSR II cytometer (BD Biosciences). The data were analyzed using FlowJo software (TreeStar, Ashland, OR) and Cytobank (www.cytobank.org).29,30
Bioluminescence imaging
In vivo bioluminescence imaging (BLI) was performed as described previously.2 Briefly, mice were injected intraperitoneally with luciferin (10 μg ⋅ g−1 of body weight). The mice were anesthetized and imaged using an IVIS Spectrum charge-coupled device imaging system (Caliper-Xenogen, Alameda, CA) for up to 5 minutes. Imaging data were analyzed with Living Image Software (Caliper Life Sciences, Hopkinton, MA).
Multiplex cytokine assays
Sera were collected from the recipient mice on days 3, 7, 14, and 28. Twenty different cytokines were analyzed in a multiplex assay system (Cytokine Mouse 20-plex Panel for the Luminex platform, LMC0006; Invitrogen, Carlsbad, CA) and quantitated using the Luminex 200 system (Luminex, Austin, TX).
Fluorescence microscopy
Gastrointestinal tract and liver were harvested from the recipient mice on days 7 and 14. Five-micrometer (5 μm)-thickness cryosections were prepared. Slides were fixed with 4% paraformaldehyde, stained with 4,6 diamidino-2-phenylindole, and observed on a fluorescence microscope (Olympus BX41; Olympus, Center Valley, PA). Images were analyzed with ImageJ (version 1.47, National Institutes of Health).
Statistical analysis
Survival curves were plotted with the Kaplan-Meier method and compared by a log-rank test. Data from repeated experiments were combined and plotted in the box-and-whiskers (Tukey) style.31 Data were displayed with mean and standard error of the mean on a bar or scatter dot plot. The multiple Student t tests with Holm-Sidak correction were performed to analyze serially collected data to deal with multiple comparisons across several time points. Otherwise, data were analyzed using the 2-tailed Student t test. Prism 6 (GraphPad Software, La Jolla, CA) was used for all statistical analyses.
Results
αDR3 treatment selectively expands functional Tregs in vivo
Donor mice were treated with a single dose of αDR3, and T cells were isolated to investigate whether this mAb could expand Tregs in LNs and spleen. The proportion of Tregs was significantly increased in CD4+ T cells obtained from αDR3-treated donor mice (Figure 1A, left; P < .0001). Absolute numbers of Tregs per donor were also increased with αDR3 treatment (Figure 1A, right; P < .0001).
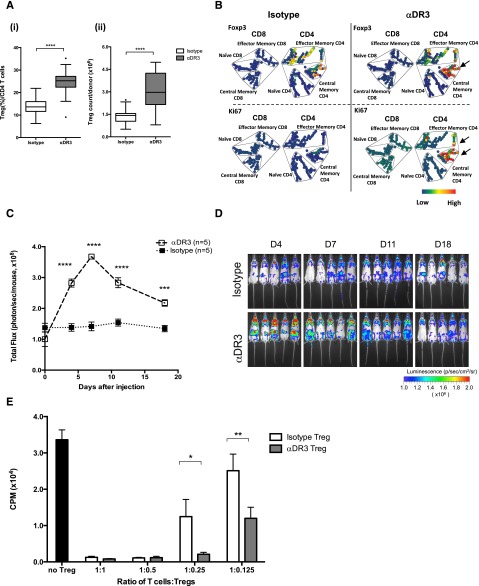
Figure 1.
αDR3 induces the selective expansion of functional Tregs. (A) T cells were isolated from mAb-treated C57BL/6 (αDR3 vs isotype) mice and stained for FoxP3. Frequencies of FoxP3+ Tregs in CD4+ T cells (left) and total Tregs numbers per C57BL/6 donor mice (right) treated with αDR3 vs isotype control mAb. Tregs number/donor = (the percentage of FoxP3+ of live cells) × (live cell counts) / (n of donor mice). The pooled 24-T cells isolations were analyzed (****P < .0001). (B) T cells from αDR3 or isotype mAb-treated C57BL/6 mice were stained for CD4, CD8, CD44, CD62L, FoxP3, and Ki67. SPADE analysis was performed and the representative results are shown. Node colors were scaled to FoxP3 (upper) and Ki67 (lower) expression levels. Naïve (CD62L+CD44–), central memory (CD62L+CD44+), and effector memory (CD62L–CD44+) phenotypes were defined by the expression of CD44 and CD62L. Note that proliferating Ki67+FoxP3+CD4+ Tregs were observed in T cells from αDR3-treated mice (arrow). (C-D) C57BL/6-FoxP3.Luc.DTR-4 mice were treated with αDR3 or isotype mAb (n = 5/group). (C) BLI signals from Tregs were quantitated and compared on days 4, 7, 11, and 18 by multiple Student t tests with Holm-Sidak correction (***P < .001, ****P < .0001). (D) Images at indicated days after treatment. Representative results of more than 2 experiments are displayed. (E) CD4+CD25+ Tregs were isolated from αDR3 or isotype mAb–treated C57BL/6 mice and plated at various ratios with freshly isolated T cells (2 × 105/well) from nontreated C57BL/6 mice and γ-irradiated splenocytes from Balb/c mice (4 × 105/well). [3H] Thymidine incorporation was measured and compared by multiple Student t tests using Holm-Sidak correction (*P < .05, **P < .01). The representative data of 2 independent experiments are shown.
Spanning-tree progression analysis of density-normalized events (SPADE) analysis was reported as a tool to analyze high-dimensional flow cytometry data in an objective manner.29 The resulting tree structure can be colored to display how surface/intracellular molecules behave across the entire heterogeneous cell population. CD4+ and CD8+ T cells contain several heterogeneous subsets including CD4+FoxP3+Tregs. To investigate the effects of αDR3 in the spectrum of T cells comprehensively, we performed SPADE analysis using flow cytometric data of T cells obtained from αDR3-treated animals (Figure 1B and supplemental Figure 1A, available on the Blood Web site). Tree structure was constructed according to the expression level of CD4, CD8, CD44, and CD62L (supplemental Figure 1B). FoxP3 expression was limited primarily to central memory (CD44+CD62L+) CD4+ T cells (Figure 1B). αDR3 treatment selectively expanded Tregs as demonstrated by upregulation of the proliferation marker Ki67 primarily in the FoxP3+ CD4+ central memory T-cells subset (Figure 1B and supplemental Figure 1A-B).
We visualized the time course of Treg expansion in vivo using C57BL/6-FoxP3.Luci.DTR-4 mice that express the luc/gfp protein under control of the FoxP3 promoter.24 Mice were treated with αDR3 or isotype mAb and the BLI signal emitted by expanding Tregs was significantly higher in αDR3-treated mice (Figure 1C-D; P < .0001 on days 4, 7, and 11, and P < .001 on day 18). The expanded Tregs persisted for 2 weeks and gradually contracted to the normal level. These data suggested that the systemic Treg expansion mediated by αDR3 treatment is transient.
Next, to determine the functional capability of Tregs expanded by αDR3 treatment, we performed MLR experiments. C57BL/6 mice were treated with αDR3 or isotype control mAb on day −4, and CD4+CD25+ Tregs were purified on day 0. Subsequently, we cocultured Tregs with freshly isolated T cells from nontreated C57BL/6 mice and irradiated splenocytes from Balb/c mice as stimulators at various ratios. Both Tregs isolated from αDR3 and isotype-treated mice were functional as demonstrated by the ability of these cells to suppress the proliferation of fresh T cells in the MLR at T cell:Treg ratios of 1:1 and 1:0.5. However, even lower Treg frequencies from αDR3-treated donors retained suppressive function (Figure 1E; P < .05). Taken together, these data demonstrate that αDR3 treatment not only resulted in an increase in the number of Tregs but also enhanced their function.
T cells from αDR3 mice are less proliferative and maintain higher Treg frequency
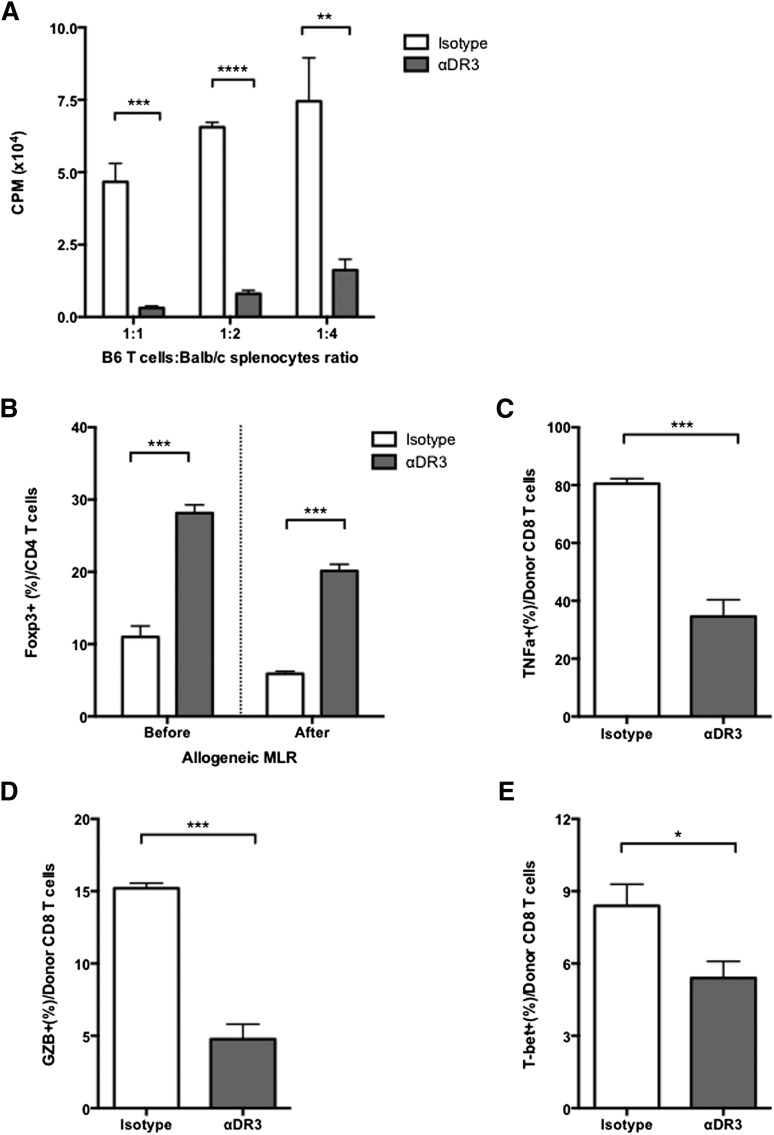
Next, we performed MLR experiments to investigate how αDR3-treated T cells responded to allogeneic stimuli. T cells were isolated from αDR3-treated donors and cocultured with γ-irradiated Balb/c splenocytes for 96 hours. T cells from αDR3-treated animals showed significantly reduced proliferation (Figure 2A; P < .001 for 1:1, P < .0001 for 1:2, P < .01 for 1:4 ratio) and maintained a higher frequency of Tregs compared with T cells from isotype-treated donors (Figure 2B and supplemental Figure 2A; P < .001). We also evaluated the activation of CD8+ T cells in response to allogeneic stimuli because they are the major effector cells of acute GVHD.32,33 CD8+ T cells from αDR3-treated donors produced significantly less TNFα (Figure 2C and supplemental Figure 2B; P < .001) and granzyme B (Figure 2D and supplemental Figure 2B; P < .001) than did those from isotype-treated donors. T-bet expression was also significantly decreased (Figure 2E and supplemental Figure 2B; P < .05).
Figure 2.
αDR3-treated T cells are less proliferative and maintain higher Treg frequency. T cells from αDR3 or isotype mAb–treated C57BL/6 mice were plated at 1:1 ratio to γ-irradiated Balb/c splenocytes in an MLR. (A) [3H] Thymidine incorporation of allogeneic responder cells (**P < .01, ***P < .001, ****P < .0001). (B) T cells were stained for FoxP3 before and 96 hours after MLR to assess Tregs (%) of CD4+ T cells (***P < .001). (C-E) Frequencies of TNFα (C), granzyme B (D), and T-bet (E) in CD8+ T cells are shown (*P < .05, ***P < .001). The results of more than 2 independent experiments are shown (N = 4, in total).
T cells from αDR3-treated donors reduce GVHD mortality and morbidity
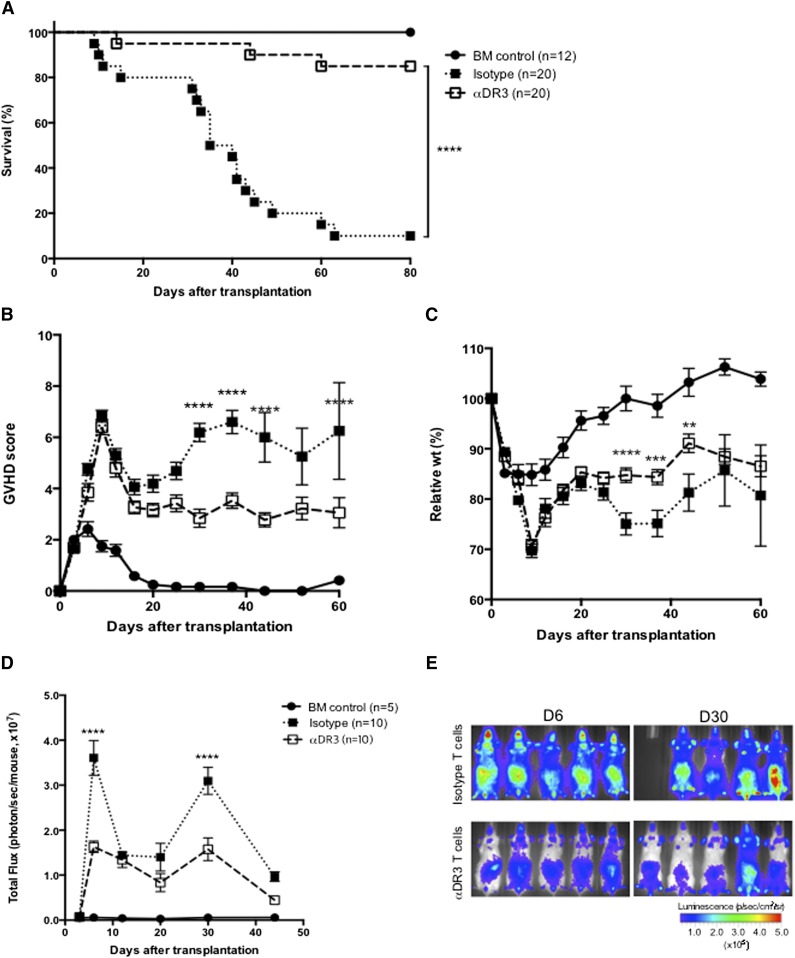
To investigate whether a single αDR3 injection to donors could reduce acute GVHD, we used a well-established MHC major mismatch GVHD model (Figure 3 and supplemental Figure 3).17,22 Survival improved significantly in recipients of T cells from αDR3-treated donors compared with mice receiving T cells from isotype-treated donors (Figure 3A; P < .0001). All recipients displayed high GVHD scores for the first 2 weeks (Figure 3B) when the cytokine storm induced by irradiation reached its peak. Thereafter, GVHD worsened in T-cell recipients from isotype-treated donors and most of these animals died between days 30 and 60. In contrast, T-cell recipients from αDR3-treated donors experienced only mild weight loss (Figure 3C; P < .0001 on day 30, P < .001 on day 37, P < .01 on day 44) and showed consistently lower GVHD scores at multiple time points (Figure 3B; P < .0001). To track the in vivo proliferation of donor T cells with BLI, T cells were transplanted from luciferase-expressing (luc+) C57BL/6 donors.34 Although T cells from isotype-treated donors showed higher proliferation and strong target organ infiltration in recipient mice (Figure 3E), T cells from αDR3-treated donors expanded significantly less and demonstrated a weaker organ infiltration in recipient mice (Figure 3D-E; P < .0001 on days 6 and 30).
Figure 3.
Donor treatment with αDR3 reduces GVHD mortality and alloreactive T-cell expansion after BMT. T cells were isolated from luc+ CD45.1+ C57BL/6 donors that were treated with αDR3 vs isotype mAb. Wild-type Balb/c recipients were lethally irradiated and transplanted with 1 × 106 T cells and 5 × 106 TCD-BM cells. Survival (A), GVHD score (B), and the relative weights (C) of recipient mice were assessed. Results are combined from 3 independent experiments. Survival (A) curves were compared by the log-rank test (****P < .0001). GVHD scores (B) and the relative weights (C) were compared by multiple Student t tests with Holm-Sidak correction (**P < .01, ***P < .001, ****P < .0001). (D) The bioluminescence signal from donor T cells on days 3, 6, 12, 20, 30, and 44 after BMT are analyzed. The total photon flux from either group (αDR3 vs isotype mAb–treated T cells) are compared by multiple Student t tests with Holm-Sidak correction (****P < .0001). The data were derived from 3 independent experiments. (E) Representative images of recipient mice on days 6 and 30 are shown.
αDR3-stimulated Tregs expand robustly and suppress donor T cells
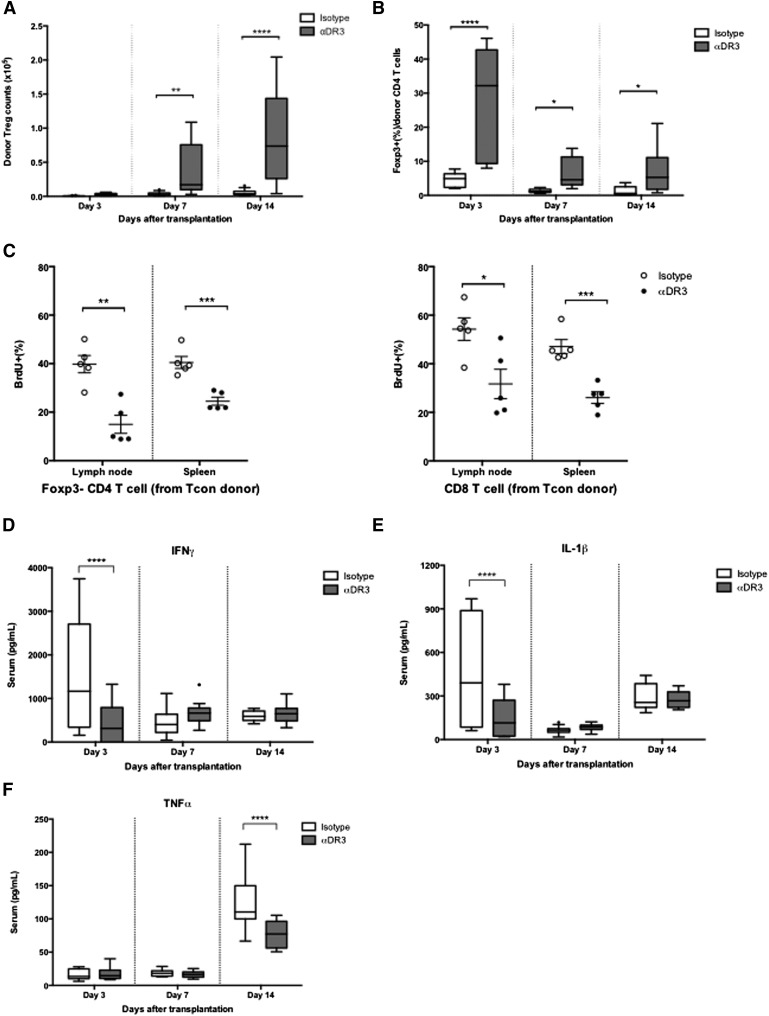
Because Treg frequency is strongly associated with GVHD risk,35-37 we assessed the absolute donor Treg number and frequency of Tregs within the donor CD4+ T-cell population. On days 3, 7, and 14 after BMT, we measured CD45.1+ donor T cells from the LNs and spleens of recipient animals. In contrast to the very low Treg numbers from the control group, Treg numbers increased significantly over time in the recipients of αDR3-treated donors in the spleen (Figure 4A; P < .01 on day 7, P < .0001 on day 14) and LNs (supplemental Figure 4A; P < .0001 on day 14). The Treg frequency rapidly decreased early after BMT in the recipient mice of T cells from isotype-treated donors. However, recipient mice of T cells from αDR3-treated donors maintained higher Treg proportions in the spleen (Figure 4B; P < .0001 on day 3, P < .05 on day 7, P < .05 on day 14) and LNs (supplemental Figure 4B; P < .001 on day 3, P < .0001 on day 7, P < .05 on day 14).
Figure 4.
αDR3-stimulated Tregs expand over time to reduce donor T-cell activation and inflammatory cytokines after BMT. T cells were isolated from mAb-treated (αDR3 vs isotype) CD45.1+ C57BL/6 mice and transplanted to lethally irradiated Balb/c recipients. (A-B) Spleens were harvested from recipient mice on days 3, 7, and 14. Absolute number of donor Tregs (A) and FoxP3+(%) of donor CD4+ T cells (B) are shown (*P < .05, **P < .01, ****P < .0001). These are the combined results from 3 independent experiments (n = 15 [day 3], 18 [day 7], 14 [day 14] for isotype and n = 15 [day 3], 19 [day 7], 16 [day 14] for the αDR3 group). (C) LNs and spleen were harvested on day 7 after in vivo BrdU labeling. BrdU uptake in CD45.1+ FoxP3– CD4+ T cells (left) and CD45.1+ CD8+ T cells (right) are shown. Representative data of 3 independent experiments are shown (n = 5 for each group; *P < .05, **P < .01, ***P < .001). (D-F) Serum levels of IFNγ (D), IL-1β (E), and TNFα (F) in recipient mice were measured on days 3, 7, and 14 by multiplex assay. Data of either group were compared by multiple Student t tests with Holm-Sidak correction (****P < .0001). These are the combined results of 7 independent experiments (n = 10 [day 3], 15 [day 7], 13 [day 14] for isotype and n = 10 [day 3], 16 [day 7], 13 [day 14] for αDR3 group; ****P < .0001).
We treated recipient mice with BrdU to measure the proliferating fraction of donor T cells. Significantly fewer donor conventional T cells proliferated on day 7 when derived from αDR3-treated donors than those from isotype-treated donors (Figure 4C: left, donor FoxP3– CD4+ T cells, P < .01 in LN, P < .001 in spleen; right, donor CD8+ T cells, P < .05 in LN, P < .001 in spleen). Donor conventional T cells derived from αDR3-treated donors also downregulated CD25, a common activation marker of T cells (supplemental Figure 5: left, donor FoxP3– CD4+ T cells, not significant in LN, P < .01 in spleen; right, donor CD8+ T cells, P < .01 in LN, P < .001 in spleen).38 These results suggest that activation of donor conventional T cells was suppressed in T-cell recipients from αDR3-treated donors.
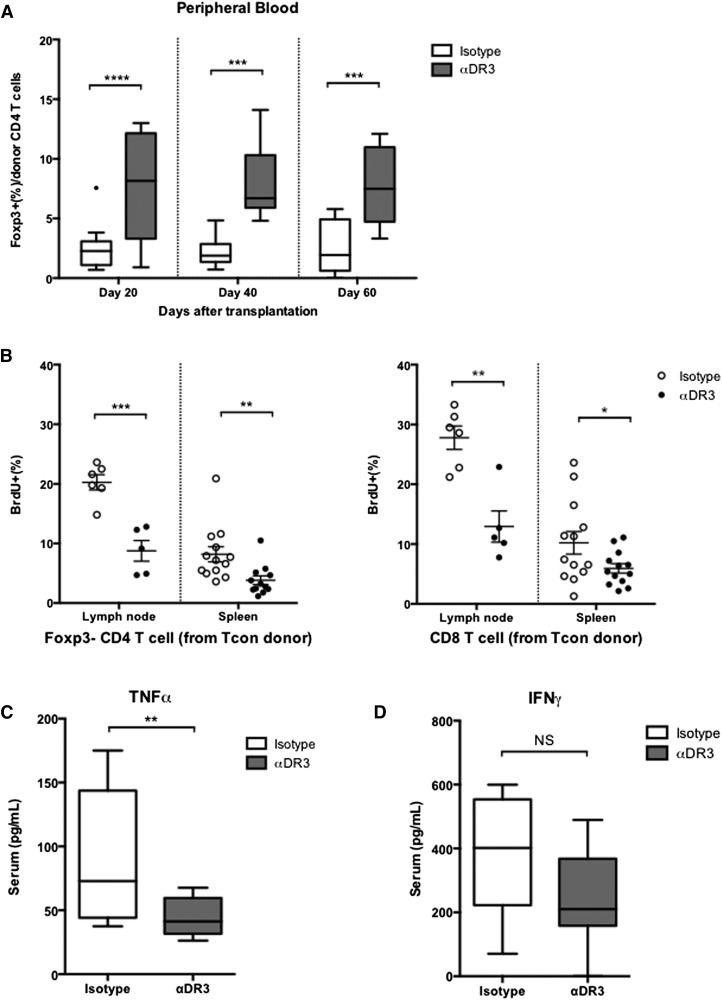
Proinflammatory cytokines such as IFNγ, IL-1β, and TNFα play an important role in acute GVHD pathogenesis39 and their serum levels were measured on days 3, 7, and 14 after BMT. IFNγ (Figure 4D) and IL-1β (Figure 4E) levels were immediately elevated after BMT and then gradually decreased in control recipients. However, recipients of T cells from αDR3-treated donors showed significantly lower levels of these cytokines (Figure 4D-E; P < .0001 on day 3). TNFα levels were stable and equivalent between the 2 groups until day 7; yet the recipients of T cells from isotype-treated donors had significantly higher levels of TNFα on day 14 compared with the T-cell recipients from αDR3-treated donors (Figure 4F; P < .0001 on day 14).
αDR3-stimulated Tregs maintain higher frequency and function during the effector phase of GVHD
To evaluate the effector phase of acute GVHD and minimize early deaths of recipient mice, we modified the acute GVHD model by using a lower donor T-cell dose (5 × 105).2 Peripheral blood was collected and analyzed every 20 days (Figure 5A). Interestingly, recipients from αDR3-treated donors showed significantly higher frequencies of Tregs among donor-derived CD4+ T cells for up to 60 days after BMT (Figure 5A; P < .0001 on day 20, P < .001 on days 40 and 60). Next, we treated recipient mice with BrdU to analyze proliferating cells on day 28. Significantly fewer conventional T cells derived from αDR3-treated donors proliferated (Figure 5B: left, donor FoxP3– CD4+ T cells, P < .001 in LN, P < .01 in spleen; right, donor CD8+ T cells, P < .01 in LN, P < .05 in spleen). On day 28, T-cell recipients from αDR3-treated donors also had lower serum TNFα levels (Figure 5C; P < .01) and lower serum levels of IFNγ, although this did not reach statistical significance (Figure 5D). These data suggest that the effects of αDR3 on donor T cells were sustainable throughout the effector phase of acute GVHD.
Figure 5.
Higher Tregs frequency and reduced donor T-cell proliferation in αDR3 group during the effector phase. 5 × 105 donor T cells from αDR3 or isotype mAb–treated CD45.1+ C57BL/6 mice and 5 × 106 TCD-BM cells from C57BL/6 mice were transplanted into lethally irradiated Balb/c mice. (A) The frequencies of FoxP3+ cells in donor CD4+ T cells in peripheral blood were analyzed on days 20, 40, and 60 after BMT. FoxP3+(%) of CD45.1+ CD4+ T cells of either group were compared by multiple Student t tests with Holm-Sidak correction. These are the combined results of 2 experiments (n=11 [day 20], 9 [day 40], 8 [day 60] for isotype and n = 9 [day 20], 9 [day 40], 8 [day 60] for αDR3 group; ***P < .001, ****P < .0001). (B) LNs and spleen were harvested on day 28 after in vivo BrdU labeling. BrdU uptakes of CD45.1+ FoxP3– CD4+ (left) and CD45.1+ CD8+ T cells (right) are shown (*P < .05, **P < .01, ***P < .001). These are the combined results of 2 experiments (n= 6 [LN], 13 [spleen] for isotype and n = 5 [LN], 13 [spleen] for αDR3 group). (C-D) Serum levels of TNFα (C) and IFNγ (D) were measured in recipient mice on day 28 by multiplex assay. The combined results of 2 independent experiments are shown (n = 13 in each group; **P < .01; NS, not significant).
T cells from αDR3-treated donors display lower infiltration of GVHD target tissues
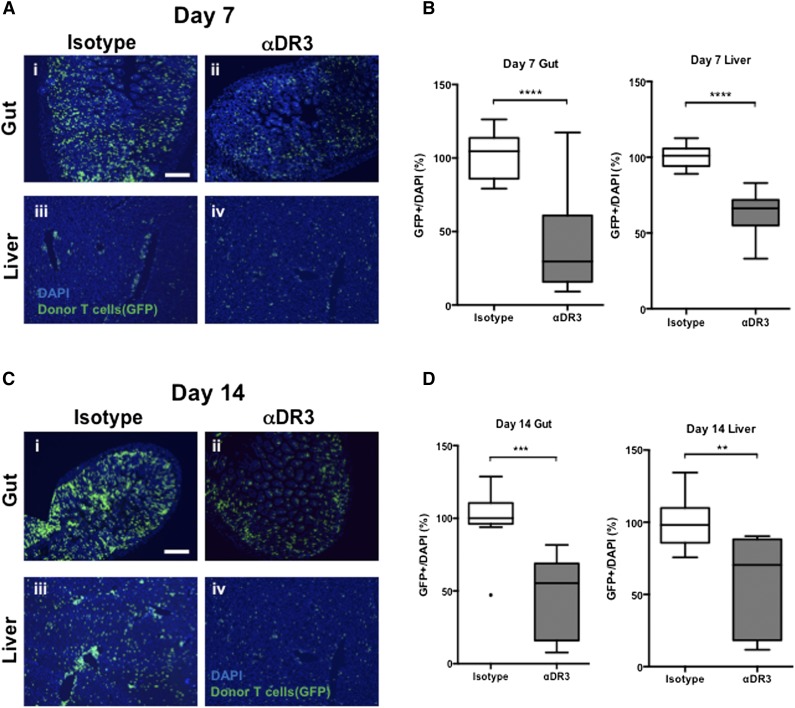
To investigate T-cell infiltration into GVHD target tissues, we isolated T cells from gfp+ C57BL/6 donors. Liver and small bowel (distal ileum) were harvested from recipient mice on days 7 and 14 after BMT and analyzed for the frequency of gfp+ tissue–infiltrating donor T cells. T cells infiltrated preferentially in the crypts of the small intestine (distal ileum) and periportal areas of the liver by 7 days after BMT (Figure 6A). T cells from isotype-treated donors formed focal collections, whereas T cells from αDR3-treated donors infiltrated in a more diffuse fashion. Lower T cell numbers infiltrated target tissues in the T-cell recipients from αDR3-treated donors (Figure 6B, left; P < .0001 in the small bowel; right, P < .0001 in the liver). Small bowel and liver harvested on day 14 showed similar findings (Figure 6C) with lower infiltrating T cell numbers in the αDR3-group on day 14 as well (Figure 6D, left; P < .001 in the small bowel; right, P < .01 in the liver). Because alloreactive T cells have been reported to start infiltrating intestinal tissues as soon as days 4 to 6 after BMT,40 we measured serum chemokine levels on day 3. T-cell recipients from αDR3-treated donors had significantly lower serum levels for CCL2 (supplemental Figure 6A; P < .001) and CXCL10 (supplemental Figure 6B; P < .01) and a similar trend for CXCL9 (supplemental Figure 6C) and CXCL1 (supplemental Figure 6D) that did not reach statistical significance.
Figure 6.
Donor treatment of αDR3 decreases T-cell infiltration into GVHD target tissues. αDR3 or isotype mAb-treated gfp+ CD45.1+ C57BL/6 mice were used as T cell donors and BMT was performed. Gut (distal ileum) and liver were harvested from recipients on days 7 (A-B) and 14 (C-D). (A,C) Representative fluorescence microscopic images of gut (upper) and liver (lower) are shown. The scale bar represents 200 μm. The frequencies of donor gfp+ T cells into gut (B, left; D, left) and liver (B, right; D, right) were calculated (αDR3 vs isotype mAb; **P < .01, ***P < .001, ****P < .0001). These are the combined results of 3 experiments (Gut, n = 15 [day 7], 9 [day 14] for isotype and n = 14 [day 7], 9 [day 14] for αDR3 group; Liver, n = 13 [day 7], 9 [day 14] for isotype and n = 13 [day 7], 8 [day 14] for αDR3 group).
Allogeneic T cells from αDR3-treated donors retain GVT effects
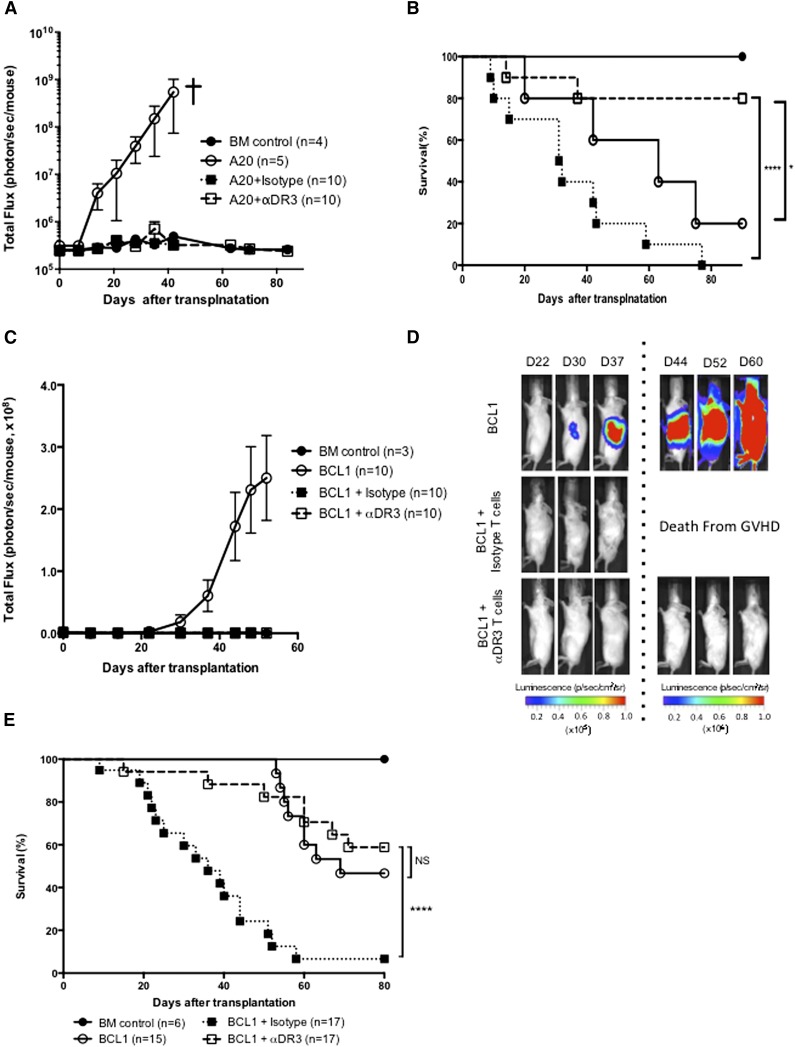
To evaluate the impact of αDR3 treatment on T-cell function, we evaluated GVT effects using 2 different lymphoma models (A20 and BCL1 lymphoma cell line; Figure 7).2 BLI revealed that engraftment and early growth of both A20 and BCL1 cells occurred rapidly in animals that received TCD-BM cells alone (Figure 7A,C). The recipients of T cells isolated from αDR3 or isotype-treated donors showed no signs of tumor in both models (Figure 7A,C). Mice that received A20 and BCL1 together with T cells from isotype-treated donors died with GVHD (10/10 mice in the A20 model, 16/17 mice in the BCL1 model; Figure 7B,E). In the A20 model, the recipients who had received T cells from αDR3-treated donors showed significantly improved survival (Figure 7B; P < .0001 vs isotype T cells, or P < .05 vs animals that received only TCD-BM). In the BCL1 model, although the mice that received BCL1 and TCD-BM cells showed statistically similar overall survival as the recipients of T cells from αDR3-treated donors, most of the animals that received BCL1 and TCD-BM cells were distressed by tumor growth (Figure 7D), whereas the recipients of T cells from αDR3-treated donors showed mild symptoms of GVHD and were in a stable condition without evidence of tumor. Because lymphomas were eradicated in both tumor models, these results demonstrate that T cells from αDR3-treated donors retained function as assessed by GVT activity.
Figure 7.
T cells from αDR3-treated donors retained function. (A-B) Lethally irradiated Balb/c recipients were intravenously injected with 2 × 104 luc+/gfp+-A20 mouse lymphoma cells together with TCD-BM (5 × 106) and isotype/αDR3-treated T cells (1 × 106) on day 0. Tumor burden (A) was monitored by BLI at indicated time points and survival (B) was monitored (*P < .05, ****P < .0001). Representative results of 2 independent experiments are shown. (C-E) Balb/c recipients were injected IV with 2 × 104 luc+/gfp+-BCL1 cells on day −8 and tumor engraftment was confirmed on day −1. TCD-BM (5 × 106) and isotype/αDR3-treated T cells (1 × 106) were injected into lethally irradiated recipient mice with tumor. (C-D) Tumor burden was monitored by BLI at indicated time points. Results are representative of 2 independent experiments. (E) Survival data of 2 independent experiments were combined. Survival curves were compared by the log-rank test (****P < .0001; NS, not significant).
Discussion
Tregs have enormous promise for the treatment and prevention of several immunologic disorders and for the promotion of organ transplantation tolerance.41 In allogeneic BMT, Tregs prevent GVHD in several different models with retention of GVT effects.2-5 Results of clinical studies also show an apparent reduction in GVHD risk and, interestingly, a low risk of disease relapse.6,7,42 A major concern is that the paucity of Tregs makes clinical translation challenging. Tregs can be expanded ex vivo; however, this approach is still technically difficult and expensive.
We observed that αDR3 treatment resulted in an increased proportion and absolute increase in the number of Tregs 4 days after injection. One of the major concerns about the effect of αDR3 in vivo is the influence of the mAb on other immune cells after antibody treatment. SPADE analysis enables the visualization of the entire spectrum of T cells using multicolor flow cytometry in an objective manner. Colored tree structure analysis demonstrated that this mAb did not induce proliferation of other T-cell populations, and the effects were remarkably limited to the FoxP3-expressing effector memory and central memory population of CD4+ cells (Figure 1B). This expansion of Tregs was transient after αDR3 treatment of the donor mice and they did not show any adverse effects after injection. Expanded Tregs were functionally competent in the MLR and had increased ability to suppress T-cell proliferation even at lower concentrations, a hallmark of Treg activity. Upon adoptive transfer, allogeneic donor T cells from αDR3-treated animals had a significantly reduced capacity for GVHD induction, resulting in markedly enhanced survival of recipients. Tregs contained within the transferred T cells expanded robustly and persisted even after 2 months in the mice that had received αDR3-treated T cells. It is noteworthy that donor Tregs could expand after BMT in the lymphopenic environment.39
It has been well described that Tregs decrease antitumor immunity in some tumor models,43 although we and others have reported that the coinfusion of freshly isolated Tregs did not interfere with GVT effects in allogeneic BMT.2,5 Importantly, GVT effects of αDR3-treated T cells in our model were retained using 2 different tumor models. These results indicated that αDR3 treatment retained functional antitumor capacity of the cytotoxic T cells. Upon translation of these concepts to the clinic using the combined use of Tregs and conventional T cells, very low relapse rates have been observed consistent with the murine results.7,42
The role of TL1a-DR3 signaling of Tregs has not been fully elucidated. Recently, it was reported that murine TL1a-Ig fusion protein expanded preexisting Tregs in vivo.44 On the other hand, it was also reported that the binding of TL1a to DR3 expressed on human T cells caused increased IFNγ production and promoted Th1 differentiation in vitro.45,46 Our data suggest that the major effects of the activation of DR3 signaling in vivo were caused by the expansion and activation of Tregs in donor mice.
In conclusion, our study demonstrates that a single injection of αDR3 to donor animals results in the significant expansion of Tregs with enhanced functional capability of suppressing GVHD while retaining GVT effects. These observations could affect the use of Tregs in the clinic because the paucity of this important cell type challenges translation strategies.
Acknowledgments
The authors thank the Veterinary Service Center at Stanford University and the Stanford Shared FACS Facility.
This research was supported by a grant from the National Heart, Lung and Blood Institute, National Institutes of Health (P01 HL075462); the Daiichi Sankyo Foundation of Life Science (H.N.); the Dr. Mildred Scheel Stiftung (Deutsche Krebshilfe) (D.S.); the Fondazione Italiana per la Ricerca sul Cancro–FIRC (A.P.); and the Interdisciplinary Center for Clinical Research (IZKF), Würzburg, Germany (A.B.).
Footnotes
Presented in abstract form at the 55th annual meeting of the American Society of Hematology, New Orleans, LA, December 8, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.-S.K. designed and performed overall experiments, analyzed data, and wrote the manuscript; H.N. performed experiments, analyzed data, and wrote the manuscript; J.B., A.P., D.S., and Y.P. performed experiments; A.B. and C.-G.P. analyzed and interpreted data and wrote the manuscript; and R.S.N. interpreted data, provided overall research supervision, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Professor of Medicine, Chief, Division of Blood and Marrow Transplantation, Department of Medicine, Stanford University, Center for Clinical Sciences Research Building, Room 2205, 269 Campus Drive, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
- 1.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 2.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 5.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9(4):243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 8.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122(18):3116–3121. doi: 10.1182/blood-2013-08-453126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol. 2011;23(6):462–468. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao T, Soto A, Zhou W, et al. Ex vivo expanded human CD4+CD25+Foxp3+ regulatory T cells prevent lethal xenogenic graft versus host disease (GVHD). Cell Immunol. 2009;258(1):65–71. doi: 10.1016/j.cellimm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178(1):320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 14.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116(1):129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257(1-2):97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118(8):2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244(1):188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber TH, Wolf D, Tsai MS, et al. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120(10):3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf D, Schreiber TH, Tryphonopoulos P, et al. Tregs expanded in vivo by TNFRSF25 agonists promote cardiac allograft survival. Transplantation. 2012;94(6):569–574. doi: 10.1097/TP.0b013e318264d3ef. [DOI] [PubMed] [Google Scholar]
- 22.Leveson-Gower DB, Olson JA, Sega EI, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117(11):3220–3229. doi: 10.1182/blood-2010-08-303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101(1):221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra M, Riedel SS, Biehl M, et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 2013;34(6):1296–1303. doi: 10.1093/carcin/bgt038. [DOI] [PubMed] [Google Scholar]
- 25.Slavin S, Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- 26.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101(2):640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 28.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–3239. [PubMed] [Google Scholar]
- 29.Qiu P, Simonds EF, Bendall SC, et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29(10):886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978;32:12–16. [Google Scholar]
- 32.Rotolo JA, Stancevic B, Lu SX, et al. Cytolytic T cells induce ceramide-rich platforms in target cell membranes to initiate graft-versus-host disease. Blood. 2009;114(17):3693–3706. doi: 10.1182/blood-2008-11-191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda Y, Levy RB, Reddy P, et al. Both perforin and Fas ligand are required for the regulation of alloreactive CD8+ T cells during acute graft-versus-host disease. Blood. 2005;105(5):2023–2027. doi: 10.1182/blood-2004-08-3036. [DOI] [PubMed] [Google Scholar]
- 34.Bäuerlein CA, Riedel SS, Baker J, et al. A diagnostic window for the treatment of acute graft-versus-host disease prior to visible clinical symptoms in a murine model. BMC Med. 2013;11:134. doi: 10.1186/1741-7015-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magenau JM, Qin X, Tawara I, et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant. 2010;16(7):907–914. doi: 10.1016/j.bbmt.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujioka T, Tamaki H, Ikegame K, et al. Frequency of CD4(+)FOXP3(+) regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHD. Bone Marrow Transplant. 2013;48(6):859–864. doi: 10.1038/bmt.2012.232. [DOI] [PubMed] [Google Scholar]
- 38.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, et al. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J Exp Med. 2004;200(2):149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine JE. Implications of TNF-α in the pathogenesis and management of GVHD. Int J Hematol. 2011;93(5):571–577. doi: 10.1007/s12185-011-0803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 42.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 43.Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res. 2010;46(1-3):79–93. doi: 10.1007/s12026-009-8124-7. [DOI] [PubMed] [Google Scholar]
- 44.Khan SQ, Tsai MS, Schreiber TH, Wolf D, Deyev VV, Podack ER. Cloning, expression, and functional characterization of TL1A-Ig. J Immunol. 2013;190(4):1540–1550. doi: 10.4049/jimmunol.1201908. [DOI] [PubMed] [Google Scholar]
- 45.Biener-Ramanujan E, Gonsky R, Ko B, Targan SR. Functional signaling of membrane-bound TL1A induces IFN-gamma expression. FEBS Lett. 2010;584(11):2376–2380. doi: 10.1016/j.febslet.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 46.Papadakis KA, Zhu D, Prehn JL, et al. Dominant role for TL1A/DR3 pathway in IL-12 plus IL-18-induced IFN-gamma production by peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol. 2005;174(8):4985–4990. doi: 10.4049/jimmunol.174.8.4985. [DOI] [PubMed] [Google Scholar]