Abstract
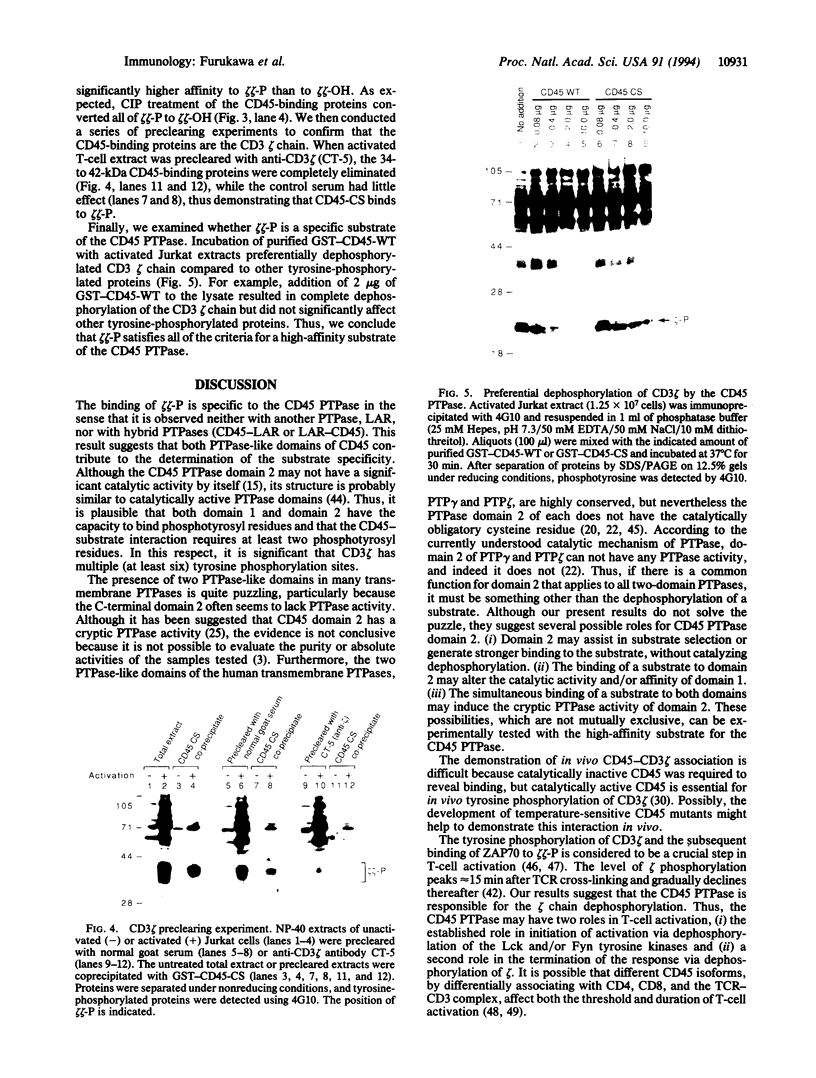
The CD45 transmembrane protein-tyrosine phosphatase (PTPase, EC 3.1.3.48) plays an essential role in T-cell activation by activating the Lck and/or Fyn protein-tyrosine kinases. However, numerous experiments have indicated that CD45 may have both stimulatory and inhibitory roles in T-cell activation. Thus, it is unlikely that the two kinases are the sole substrates of the CD45 PTPase. Furthermore, the complex regulation of the alternative splicing of the extracellular domain in various leukocyte lineages also suggests additional roles for the CD45 PTPase. To identify such functions, it is necessary to identify physiologically relevant substrates of the CD45 PTPase other than the two protein-tyrosine kinases. To this end, we searched for high-affinity substrates of the CD45 PTPase among the tyrosine-phosphorylated T-cell proteins by using purified glutathione S-transferase-CD45 fusion molecules. The enzymatically inactive CD45 C828S mutant protein, in which the cysteine residue at the catalytic center was changed to a serine residue, bound tightly to the phosphorylated CD3 zeta chain. This binding was specific to CD45 PTPase, as neither the leukocyte common antigen-related molecule (LAR) PTPase nor the CD45-LAR hybrid PTPases bound the phosphorylated CD3 zeta chain. Furthermore, phosphorylated CD3 zeta chain was preferentially dephosphorylated by the wild-type CD45 PTPase under conditions that did not significantly dephosphorylate other cellular proteins. Thus, the phosphorylated CD3 zeta chain is a specific and high-affinity substrate of the CD45 PTPase. These results suggest that CD45 is involved in the termination of the T-cell response via dephosphorylation of CD3 zeta chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autero M., Saharinen J., Pessa-Morikawa T., Soula-Rothhut M., Oetken C., Gassmann M., Bergman M., Alitalo K., Burn P., Gahmberg C. G. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994 Feb;14(2):1308–1321. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D., Flint A. J., Tonks N. K. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994 Mar 11;263(5152):1397–1404. [PubMed] [Google Scholar]
- Barnea G., Silvennoinen O., Shaanan B., Honegger A. M., Canoll P. D., D'Eustachio P., Morse B., Levy J. B., Laforgia S., Huebner K. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP gamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993 Mar;13(3):1497–1506. doi: 10.1128/mcb.13.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir McFarland E. D., Hurley T. R., Pingel J. T., Sefton B. M., Shaw A., Thomas M. L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Cho H., Ramer S. E., Itoh M., Kitas E., Bannwarth W., Burn P., Saito H., Walsh C. T. Catalytic domains of the LAR and CD45 protein tyrosine phosphatases from Escherichia coli expression systems: purification and characterization for specificity and mechanism. Biochemistry. 1992 Jan 14;31(1):133–138. doi: 10.1021/bi00116a019. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Redoglia V., Malavasi F., Bragardo M., Pileri A., Janeway C. A., Jr, Bottomly K. Isoform-specific associations of CD45 with accessory molecules in human T lymphocytes. Eur J Immunol. 1992 Feb;22(2):365–371. doi: 10.1002/eji.1830220212. [DOI] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Hall C. G., Sancho J., Terhorst C. Reconstitution of T cell receptor zeta-mediated calcium mobilization in nonlymphoid cells. Science. 1993 Aug 13;261(5123):915–918. doi: 10.1126/science.8346442. [DOI] [PubMed] [Google Scholar]
- Hall L. R., Streuli M., Schlossman S. F., Saito H. Complete exon-intron organization of the human leukocyte common antigen (CD45) gene. J Immunol. 1988 Oct 15;141(8):2781–2787. [PubMed] [Google Scholar]
- Hurley T. R., Hyman R., Sefton B. M. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993 Mar;13(3):1651–1656. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Streuli M., Krueger N. X., Saito H. Purification and characterization of the catalytic domains of the human receptor-linked protein tyrosine phosphatases HPTP beta, leukocyte common antigen (LCA), and leukocyte common antigen-related molecule (LAR). J Biol Chem. 1992 Jun 15;267(17):12356–12363. [PubMed] [Google Scholar]
- Jiang Y. P., Wang H., D'Eustachio P., Musacchio J. M., Schlessinger J., Sap J. Cloning and characterization of R-PTP-kappa, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993 May;13(5):2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Greenbaum L., Bottomly K., Trowbridge I. S. Identification of the alternatively spliced exons of murine CD45 (T200) required for reactivity with B220 and other T200-restricted antibodies. J Exp Med. 1989 Mar 1;169(3):1179–1184. doi: 10.1084/jem.169.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Ostergaard H. L., Wasden C., Trowbridge I. S. Mutational analysis of CD45. A leukocyte-specific protein tyrosine phosphatase. J Biol Chem. 1992 Apr 25;267(12):8035–8041. [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Schultz T., Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman M., Johnson P., Trowbridge I., Bottomly K. Differential expression of the alternatively spliced exons of murine CD45 in Th1 and Th2 cell clones. Eur J Immunol. 1991 Jan;21(1):17–22. doi: 10.1002/eji.1830210104. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mustelin T., Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45). Oncogene. 1990 Jun;5(6):809–813. [PubMed] [Google Scholar]
- Mustelin T., Pessa-Morikawa T., Autero M., Gassmann M., Andersson L. C., Gahmberg C. G., Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992 May;22(5):1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Farber D., Leitenberg D., Hong S. C., Johnson P., Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994 May;1(2):109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Levin S. D., Appleby M. W., Anderson S. J., Alberola-Ila J. Regulation of lymphocyte function by protein phosphorylation. Annu Rev Immunol. 1993;11:451–499. doi: 10.1146/annurev.iy.11.040193.002315. [DOI] [PubMed] [Google Scholar]
- Pingel J. T., Thomas M. L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989 Sep 22;58(6):1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. Active site labeling of a receptor-like protein tyrosine phosphatase. J Biol Chem. 1992 Jan 5;267(1):140–143. [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Morimoto C., Wong L. L., Schlossman S. F. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987 Dec 1;166(6):1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Alternative use of 5' exons in the specification of Ly-5 isoforms distinguishing hematopoietic cell lineages. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5364–5368. doi: 10.1073/pnas.84.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Pancoast T. C., Boyse E. A. Organization of the Ly-5 gene. Mol Cell Biol. 1988 Nov;8(11):4889–4895. doi: 10.1128/mcb.8.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Morimoto C., Schrieber M., Schlossman S. F., Saito H. Characterization of CD45 and CD45R monoclonal antibodies using transfected mouse cell lines that express individual human leukocyte common antigens. J Immunol. 1988 Dec 1;141(11):3910–3914. [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tan X., Stover D. R., Walsh K. A. Demonstration of protein tyrosine phosphatase activity in the second of two homologous domains of CD45. J Biol Chem. 1993 Apr 5;268(10):6835–6838. [PubMed] [Google Scholar]
- Thomas M. L., Barclay A. N., Gagnon J., Williams A. F. Evidence from cDNA clones that the rat leukocyte-common antigen (T200) spans the lipid bilayer and contains a cytoplasmic domain of 80,000 Mr. Cell. 1985 May;41(1):83–93. doi: 10.1016/0092-8674(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Thomas M. L. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Diltz C. D., Fischer E. H. CD45, an integral membrane protein tyrosine phosphatase. Characterization of enzyme activity. J Biol Chem. 1990 Jun 25;265(18):10674–10680. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991 Dec 15;266(35):23517–23520. [PubMed] [Google Scholar]
- Trowbridge I. S., Thomas M. L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- Wange R. L., Malek S. N., Desiderio S., Samelson L. E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993 Sep 15;268(26):19797–19801. [PubMed] [Google Scholar]