Abstract
Cessation from chronic alcohol abuse often produces a dysphoric state that can persist into protracted withdrawal. This dysphoric state is theorized to function as a negative reinforcer that maintains excessive alcohol consumption and/or precipitates relapse in those struggling to abstain from alcohol. However, we know relatively little regarding the impact of cessation from binge drinking on behavioral measures of negative affect and related neurobiology. Male C57BL/6J mice were given access to unsweetened 20% alcohol for 6 weeks under modified Drinking-in-the-Dark procedures, followed by behavioral testing beginning either 1 or 21 days into withdrawal. Mice were administered a behavioral test battery consisting of: the elevated plus maze, light/dark box, novel object test, marble burying test, Porsolt forced swim test and sucrose preference test to assess anxiogenic and depressive signs. Egr1 immunostaining was used to quantify cellular activity within the central nucleus of the amygdala (CEA), basolateral amygdala (BLA), bed nucleus of the stria terminalis (BNST), and the nucleus accumbens (Acb) shell (AcbSh) and core (AcbC). Compared to water controls, alcohol-drinking mice exhibited higher indices of emotionality in the majority of behavioral assays. The hyper-emotionality exhibited by binge drinking mice was apparent at both withdrawal time-points and correlated with higher Egr1+ cell counts in the CEA and BNST, compared to controls. These data show that affective symptoms emerge very early after cessation of binge drinking and persist into protracted withdrawal. A history of binge drinking is capable of producing enduring neuroadaptations within brain circuits mediating emotional arousal.
Keywords: binge drinking, anxiety, amygdala, BNST, nucleus accumbens
1. Introduction
Binge drinking is the most common pattern of excessive alcohol consumption, with over 38 million Americans admitting to binge drinking an average of four times per month [1]. Binge drinking is defined as a pattern of alcohol intake sufficient to produce a blood alcohol concentration (BAC) of ≥80mg% in a 2-hr period [2]. Repeated bouts of binge drinking can result in physical dependence, leading to symptoms of withdrawal during periods of abstinence. Withdrawal symptoms often include insomnia, confusion, anxiety, depression, irritability, tachycardia, and in severe cases, delirium tremens and seizures [3]. Frequent binge drinking is also a significant risk factor for the development of alcoholism [4]. As such, characterizing the neurobiological impact of binge drinking is crucial for understanding the consequences of this pattern of excessive alcohol consumption, which will aid in the development of pharmacotherapies for this prevalent form of alcohol-use disorder.
The elevated anxiety and depression during alcohol withdrawal is an aversive state frequently reported in the human population [5] that is theorized to be a compelling source of negative reinforcement fueling compulsive drug-seeking behavior and relapse [6]. However, confounding subject factors render it difficult to discern whether or not binge-drinking history is a sufficient antecedent to a negative affect state in humans. Moreover, it is impossible to study the cellular and molecular mechanisms underpinning emotional disturbances during alcohol withdrawal in a systematic, controlled, manner through studies of humans. As such, we have gained the majority of our insight into the psychobiological impact of alcohol from studies of animal models of alcohol use disorders. This being said, there exists limited literature describing emotional anomalies during withdrawal in animal models of alcohol abuse; these studies have typically relied on either non-contingent alcohol delivery paradigms (e.g., vapor inhalation, injection, or gavage) or contingent models based prolonged access to adulterated alcohol solutions (e.g., liquid diet) [7]. While effective at producing physical dependence and robust behavioral indices of negative affect, such models arguably lack face validity and often involve higher alcohol doses than animals would consume voluntarily [8-11]. Given the gaps in our extant knowledge concerning the emotional consequences of binge drinking, herein, we characterized the effects of voluntary alcohol intake on behavioral measures of anxiety and depression within the context of a modified version of the well-established “Drinking in the Dark” (DID) murine model of binge drinking [e.g. 12].
Emotional dysregulation during alcohol withdrawal is largely attributed to changes within the extended amygdala [e.g., 7]. The extended amygdala is a basal forebrain macrosystem that acts as a subcortical relay station between the brainstem, thalamus, and cortical areas and includes the bed nucleus of the stria terminalis (BNST), the shell of the nucleus accumbens (AcbSh), and the central nucleus of the amygdala (CEA) [13]. These areas are heavily implicated in the processing of anxiety, fear, depression, reward, and reinforcement and alcohol-induced changes within this macrosystem are implicated in the emotional disturbances that can occur during alcohol abstinence [14]. As for withdrawal-induced negative affect (see above), the vast majority of our understanding of how alcohol withdrawal impacts extended amygdala function of relevance to emotional dysregulation has been derived from studies employing non-contingent alcohol administration or the use of adulterated alcohol diets [7]. However, consistent with this prior work, a dysregulation of extended amygdala function is reported during alcohol withdrawal in animal models of binge drinking. For instance, increased indices of glutamate transmission within extended amygdala structures are observed at 24 hrs following a month-long history of binge drinking [15, 16], while changes in the expression of genes related to synaptic transmission and neuronal plasticity occur within the AcbSh and CeA of binging rodents during early withdrawal (i.e., 1-6 hrs post-binge) [17, 18]. Thus, binge alcohol drinking appears to induce neuroadaptations within the extended amygdala similar to those reported in traditional models of alcohol dependence [19]. However, to the best of our knowledge, no study to date has related binge drinking-induced changes in extended amygdala function to the manifestation of negative affect during withdrawal from binge drinking. Given the gaps in the extant literature, we conducted an immunohistochemical analysis of the expression of the transcription factor Egr-1 within the extended amygdala and adjacent structures to correlate cellular activity [20] with the manifestation of anxiety and depressive behavior during withdrawal from binge drinking. We hypothesized that a history of binge drinking would augment behavioral indices of negative affect and that withdrawal-induced negative affect would be associated with increased cellular activity within the extended amygdala.
2. Methods
2.1 Subjects
This study used 60 adult C57BL/6J (B6) male mice that were 8 weeks of age at onset of drinking and weighed 25-30g (Jackson Laboratories, Sacramento, CA). Animals were randomly divided into an alcohol-drinking group (n=30; hereafter referred to as Alcohol mice) and a water-drinking group (n=30; hereafter referred to as Water mice) and then individually housed in standard, Plexiglas cages, under a 12-hour-reverse light/dark cycle (lights off at 10am), in a temperature-controlled vivarium (23°C). Food and water were available ad libitum, with the exception of the 2-hr alcohol drinking period, during which time the home cage water bottle was removed. All experiments were conducted in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 2010) and approved by the IACUC of the University of California, Santa Barbara.
2.2 Drinking-in-the Dark (DID) Procedures
To elicit consistently high alcohol consumption, we employed a modified version of the DID model, which results in alcohol intakes between 3.5-5.0 g/kg alcohol in a 2-hr period and yields blood alcohol concentrations (BACs) in excess of 80mg% [e.g. 12]. Three hours after lights out, home cage water bottles were replaced with sipper tubes containing a 20% (v/v) unsweetened alcohol solution in filtered tap water and mice were allowed to drink for 2 hrs, at which point the alcohol bottles were removed and the home cage water bottles were replaced. Control animals received an identical sipper tube of filtered tap water in lieu of alcohol. For practical reasons, mice were subjected to these drinking conditions 5 days per week (M-F) over the course of 6 weeks (total drinking days = 30). Each day, the amount of alcohol consumed was calculated by bottle weight immediately before and after the drinking period. Unfortunately, technical difficulties with our Analox Analyzer precluded our ability to determine BACs in this study. Thus, BACs were estimated from observed intakes and based on the results of published correlational analyses in B6 mice [12, 21, 22], as conducted previously [23].
2.3 Behavioral testing
We administered a 2-day test battery to assay for alcohol withdrawal-induced changes in behavior in both the short-term and long-term (respectively 1-2 vs. 21-22 days following last alcohol presentation; n=15/group/withdrawal time-point). At both time-points, testing for affect began with an overnight test for sucrose preference and the remaining tests were conducted across the following 2 days. On the first test day after sucrose preference testing, mice were assayed first in a light/dark shuttle box or novel object encounter (order randomized across cohorts), followed by a 15-min swim test. The 15-min swim test occurred at the end of the first day to allow mice time to recuperate, as per our IACUC's request. On the second test day, mice were tested first on the elevated plus maze or for marble burying (order randomized across cohorts), followed by a 5-min swim retest and animals were sacrificed and brain tissue was harvested immediately upon completion of the swim retest. All tests were conducted under standard ambient lighting and the details of the procedures employed for each of these paradigms are provided below.
2.3.1. Sucrose preference
Anhedonia, an absence of pleasure from previously enjoyable activities, is a characteristic symptom of depression in humans. Low sucrose preference is a well-established index of anhedonia in animal models [24] and thus, we examined for the effects of early versus late withdrawal from binge drinking on sucrose preference in our mice. For this, animals were given overnight access to 2 identical sipper tubes, one contained 5% sucrose and the other contained tap water. The bottles were weighed prior to being placed on the home cage at 17:00 h. Sixteen hours later (09:00 h the next day), the bottles were removed from the home cage and weighed to determine the total volume consumed, as well as the relative preference for sucrose.
2.3.2. Light/dark shuttle box
The light/dark shuttle box test was used to assess exploratory and anxiety-like behaviors [25, 26]. Animals were placed into a polycarbonate box measuring 46 cm long×24 cm high×22 cm wide containing 2 distinct environments for a 15-minute trial. Half of the box was white and uncovered, the other half black and covered, and these 2 environments were separated by a central divider with an opening. The animals were first placed on the dark side and the latency to enter the light side, number of light-side entries, and total time spent in the light-side of the shuttle box were recorded using Any-maze™ tracking software (Stoelting co, Wood Dale, IL). Increased reluctance to venture into the light, uncovered, side was interpreted as an index of anxiety.
2.3.3. Novel object encounter
To test reactivity to a novel object as an index of neophobia-related anxiety [27, 28], animals were placed in an activity arena measuring 46 cm long×42 cm wide×40 cm high. In the center of the arena was placed a novel, inedible, object (candlestick holder; measuring approximately 6cm in diameter×12cm high). The animals’ interaction with the novel object was observed during a 2-minute trial. The number of contacts, total time spent in contact with the novel object, and fecal count were recorded by a trained observer who was blind to the drinking condition of the animals.
2.3.4. Porsolt forced swim test
Floating behavior during the Porsolt forced swim test serves as an index of behavioral despair in laboratory animals [29] and is a model with high predictive validity for the clinical efficacy of anti-depressant drugs [29]. On day 1 of behavioral testing, each animal was placed into a 26-cm diameter pool of room-temperature water, deep enough so animals were unable to touch the bottom. Behavior was monitored every 30 seconds by a trained observer for 15 min. On day 2 of behavioral testing (approximately 24 hrs following the first swim test), the animals were re-exposed to the pool for a 5-min retest session, in which behavior was monitored every 30 seconds. On both test days, the animal's behavior at each observation was classified as floating (all 4 limbs completely immobile), treading (minimal limb motion with no forward movement), or swimming (active paddling with forward movement). Only 1 behavior was recorded per observation. The latency to first float was also recorded using a stopwatch.
2.3.5. Elevated plus maze
The elevated plus maze is a well-established paradigm in which to measure anxiety in laboratory animals, with high predictive validity for anxiolytic drugs [30, 31]. Animals were placed on the center intersection of a 4-arm radial plus maze with 2 white open arms and 2 black walled arms 24cm high. Each arm measured 123cm long × 5cm wide. Latency to first open-arm entry, number of open-arm entries, and total time spent in an open arm were monitored for the 4-minute trial by a trained observer who was blind to the drinking history of the mice. Differences in the amount of time spent in an open versus enclosed arm were also used to assess anxiety.
2.3.6. Marble burying
The marble burying test was used to measure anxiety-induced defensive burying [32]. In our paradigm, 12 square glass pieces (2.5cm2×1.25cm tall) were placed in the animals’ home cage, 6 at each end. Latency to start burying the marbles was determined by a blind observer using a stopwatch and the total number of marbles buried following a 20-minute trial was recorded.
2.4 Brain tissue collection
At the conclusion of behavioral testing on day 2 (i.e., immediately following the 5-min swim re-test), mice were euthanized with an overdose of Euthasol® (Virbac Animal Health, Fort Worth, TX) and perfused transcardially with 10 ml of phosphate-buffered saline (PBS) followed by 10 ml of a 4% paraformaldehyde solution. Brains were extracted and post-fixed for 24hrs in 2% paraformaldehyde in PBS, then stored in PBS containing 30% (w/v) sucrose until cryosectioning. A Leica CM1800 cryostat (Leica Microsystems Inc., Buffalo Grove, IL) was used to collect 20 μm slices of brain tissue along the coronal plane, which were then mounted onto Superfrost Plus microscope slides (Fisher Scientific, Chino, CA). Using the Paxinos & Franklin [33] mouse brain atlas as a guide, the following areas were sampled (co-ordinates relative to Bregma): the AcbSh and AcbC (+1.98-0.86 mm), the dorsal BNST (+0.74-0.82 mm) and the CeA and BLA (-0.94-2.06 mm). The AcbC and the BLA are functionally and anatomically associated with the extended amygdala, but are not considered part of this macrosystem. Thus, these subregions were included in our analysis to determine whether or not any observed changes in cellular activity were exclusive to the extended amygdala. Additional water-drinking and alcohol-drinking groups were included in this study to control for the effects of behavioral testing upon basal Egr1 levels. These controls were subjected to identical drinking and withdrawal schedules as water/alcohol mice but did not undergo behavioral testing prior to brain collection.
2.5 Immunohistochemistry
Egr1 (also known as Zif268, krox-24, and NGFI-A) is a transcription factor encoded by an immediate early gene and is commonly used as a marker of localized brain activation in laboratory animals [20, 34]. Egr1 is uniquely advantageous over other common immediate early genes (e.g., c-Fos) due to its constitutive expression, making it sensitive to either increases or decreases in activation. To assess Egr1 levels throughout the extended amygdala, brain tissue slides were prepared using the ABC method [35]. Slides were washed twice with Tris-buffered saline (0.05 M, pH 7.6 at room temperature) between each of the different treatments. Sections were treated with 0.25% Triton X-100 (Sigma #X-100, St. Louis, MO, USA) and 5% dimethyl sulfoxide (Sigma D-5879), and then incubated for 1 hr in 20% normal horse serum (NHS; Sigma G6767) + 1% bovine serum albumin (BSA-Fract V; Fisher Scientific, Los Angeles, CA, USA, BP1605-100) to block non-specific binding. Slides were then incubated for 24 hrs in a rabbit Egr1 primary antibody 1:1000 (c-19 anti-Egr1; Santa Cruz Biotechnology, Santa Cruz, CA, USA) + 0.5% Triton X-100 + 1% NHS. Next, sections were incubated for 1 hr in the secondary anti-rabbit IgG antibody (Vector Laboratories BA110, Burlingame, CA, USA), and for 30 min in the avidin–biotin horseradish peroxidase complex (Elite Vectastain Universal ABC Kit, Vector Laboratories PK6200, Burlingame, CA, USA). Staining was visualized using the chromogen 3,3’-diaminobenzidine (DAB) (Vector Laboratories Peroxidase Substrate Kit SK-4100). Following staining, sections were dehydrated and cover-slipped. Egr1-positive (Egr1+) cell counts were recorded by visual inspection at 40× magnification, restricted to an area defined by a 0.25 mm2 grid by experimenters blind to the treatment of the animals. Cell counts for each region were averaged across 3 different adjacent sections per animal.
2.6 Statistical analyses
For each week of alcohol consumption, the average alcohol intake (expressed as g/kg body weight) was determined and data were analyzed using a within-subjects ANOVA with repeated measures on the Week factor (6 levels), with Fisher LSD post-hoc pairwise comparisons. Statistical analyses of all behavioral testing data were conducted using between-subjects two-way analyses of variance (ANOVAs). Cell count data were analyzed with a 2*2*2 ANOVA to compare group differences based on treatment, behavioral testing, and withdrawal duration. ANOVAs were followed by post-hoc t-test comparisons, when appropriate. α=0.05 for all ANOVAs and t-tests. Correlational analyses were conducted between Egr1+ cell counts in discrete regions and individual behavioral measures to determine if cellular activation of a particular region was related to behavioral signs of anxiety or depression. Correlational analyses were also conducted between brain regions to assess regional co-activation using cell counts from both behaviorally tested and untested alcohol/water-drinking animals in order enhance statistical power. Correlations were conducted using Bonferroni adjusted alpha levels for each set of comparisons. Statistical outliers were identified using the ±1.5*IQR rule and excluded from analyses. Any animals identified as outliers during behavioral analysis were excluded from the cell count analysis. All calculations were performed using SPSS v.21 statistical software (IBM, 2012).
3. Results
3.1.1 Animal attrition
Over the course of the study, 6 animals were lost from the initial 30. One Alcohol and one water mouse were found dead during drinking procedures from no apparent cause. Two Water mice had to be euthanized due to congenital malocclusion and two Water mice died shortly after arrival of apparent dehydration. This resulted in the following group sizes by the end of the behavioral component of this study: Alcohol-short-term withdrawal= 14, Alcohol-long-term withdrawal= 15, Water-short-term withdrawal= 12; Water-long-term withdrawal= 15. During tissue preparation, we encountered technical difficulties with cryostat slicing, which resulted in a significant reduction in the sample sizes for the BNST tissue. Issues related either to a malfunctioning cryostat or weak immunostaining contributed to lowering the sample sizes employed in our analysis of other brain regions.
3.1.2 Alcohol intake
The B6 mice in this study consumed on average 4.0 ± 0.06 g/kgl/2 h over the entire 6-week alcohol drinking period with very low variability. Although blood alcohol levels were not assayed in this study, this level of intake is predicted to result in BACs ≥80 mg%, based on published results [12, 21]. Importantly, there was no difference in the average amount of alcohol consumed between animals tested for behavior at 1-day (average= 4.06 ± 0.08 g/kg/2hr) versus 21-days withdrawal (average= 4.07 ± 0.08 g/kg/2hr) [t(28)=0.304, p>0.05].
3.2 Sucrose preference
No group differences in sucrose intake or preference were observed following the overnight sucrose preference test (2-way ANOVAs, p's>0.05).
3.3. Novel object
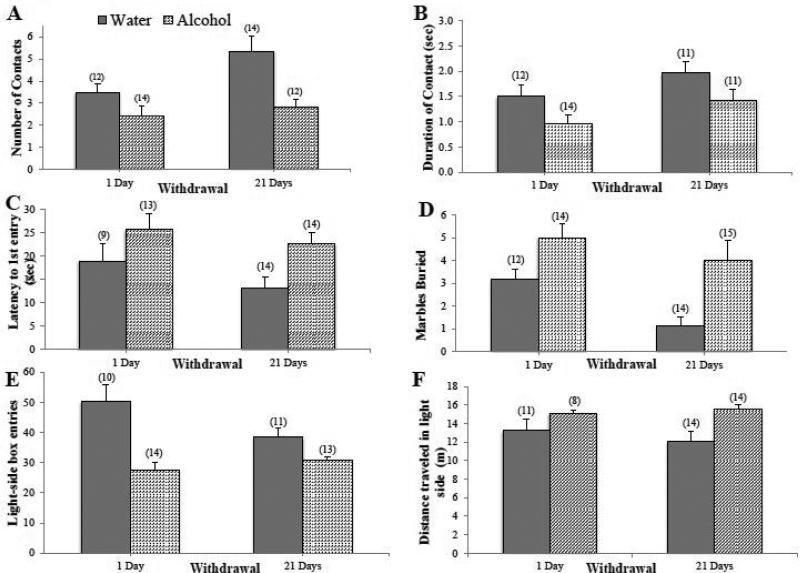
Alcohol mice exhibited less exploration of a novel object during withdrawal compared to Water controls. Alcohol mice also made fewer contacts with the object (Fig. 1A) [Treatment effect: F(1,48)=12.74, p=0.001] and spent less time interacting with the object (Fig. 1B) [Treatment effect: F(1,44)=6.31, p=0.016] during the 2-minute trial. Importantly, the main treatment effect was independent of withdrawal duration (for both variables, no withdrawal effects or interactions, p's>0.05), indicating that the hyper-anxious state persisted into protracted withdrawal. However, there were no significant group differences in the latency to first contact the novel object or in fecal count (data not shown; 2-way ANOVAs, all p's>0.05).
Figure 1. Withdrawal from binge drinking increases behavioral signs of anxiety across a variety of paradigms.
Compared to water-drinking controls (Water), mice with a 30-day history of binge drinking (Alcohol): (A) made fewer contacts with the object and (B) spent less time interacting with the novel object in a 2-min novel object test. Alcohol mice also (C) exhibited a longer latency (in sec) to first enter into the open-arm in a 5-min elevated plus-maze test, (D) buried more marbles in a 20-min marble-burying test, (E) made fewer light-side box entries in a 5-min light-dark shuttle box test and (F) travelled a greater distance in the light side of the shuttle box. The heightened behavioral signs of anxiety were observed in Alcohol mice during both short- (1-day) and long-term (21 days) withdrawal. Data represent mean ± SEM of the number of animals indicated in parentheses. Each graph depicts a main effect of alcohol,p < 0.05 vs. water control.
3.4 Elevated plus maze
Alcohol mice showed a significantly longer latency to first open-arm entry compared to Water controls, independent of withdrawal duration (Fig. 1C) [Treatment effect: F(1,46)=7.60, p=0.008; other p's>0.05]. However, there were no group differences in the total number of open-arm entries, total entries, or total time spent in an open arm (data not shown; 2-way ANOVAs, p's>0.05).
3.5 Marble burying
Alcohol mice exhibited an increase in marble burying compared to Water controls, independent of withdrawal duration (Fig. 1D) [Treatment effect: F(1,51)=13.24, p=0.001; other p's>0.05]. However, there were no significant group differences in the latency to begin burying (data not shown; 2-way ANOVA, p's>0.05).
3.6 Light/dark shuttle box
Binge drinking influenced the number of entries into the light-side of a light/dark shuttle box, but the magnitude of this effect varied with withdrawal (Fig. 1E) [Treatment effect: F(1,44)=22.27, p<0.001; Treatment X Withdrawal: F(1,44)=5.10, p=0.029]. Deconstruction of the significant interaction for light-side entries along the Withdrawal factor indicated that the Alcohol mice made fewer light-side entries than Water controls at both withdrawal time- points [Short-term: t(19)=3.742, p=0.001; Long-term: t(25)=2.496, p=0.023], with a more prominent group difference during short-term withdrawal, as is apparent in Fig. 1E. While there were no time-dependent differences within the Alcohol mice [t(22)=1.13, p>0.05], Water mice showed a trend for a withdrawal effect [t(22)=1.953, p=0.064). This latter result was likely the source of the interaction revealed by the 2-way ANOVA. The cause of this trend is uncertain, but for the purposes of this study, it is most relevant that Alcohol mice made fewer light-side entries at both time points and their behavior was consistent between the time-points. Overall, Alcohol mice also traveled a greater distance in the light side compared to water controls (Fig. 1F) [Treatment effect: F(1,43)=6.9, p=0.012; interaction: p>0.05]. However, there were no group differences in the latency to first light-side entry or the total time spent in the light-side (data not shown; 2-way ANOVAs, p's>0.05).
3.7 Porsolt forced swim test
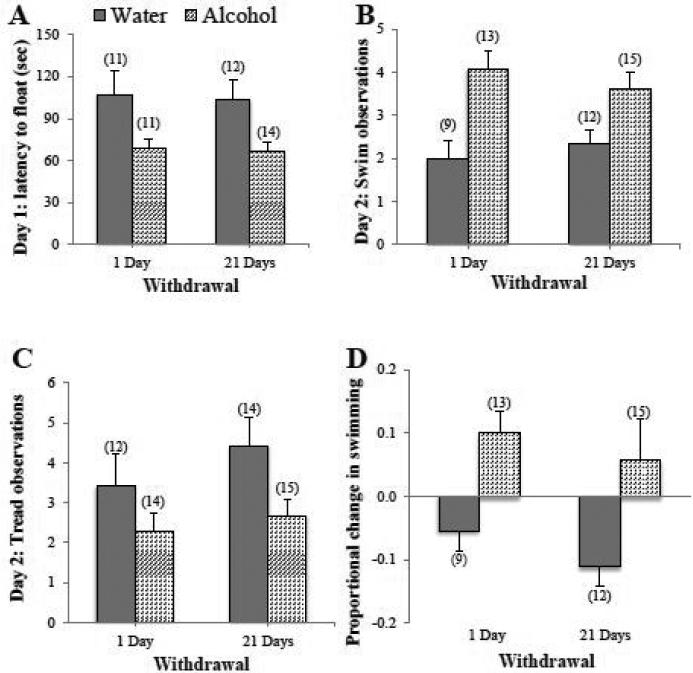
Alcohol mice exhibited a shorter latency to first float than Water mice during the 15-minute trial on test day 1 (Fig. 2A) [Treatment effect: F(1,44)=10.38, p=0.002]. However, there were no group differences in swimming, treading, or floating observed during this initial test (data not shown; 2-way ANOVAs, p's>0.05). During the 5-minute re-exposure test on day 2, Alcohol mice showed significantly more swimming (Fig. 2B) [Treatment effect: F(1,45)=16.32, p<0.001] and less treading than Water controls (Fig. 2C) [Treatment effect: F(1,51)=5.77, p=0.02]. Again, the treatment differences did not vary as a function of withdrawal duration (no main effect or interaction, p's>0.05). There were no group differences in latency to first float or total number of floats on this re-exposure test (data not shown; 2-way ANOVAs, p's>0.05).
Figure 2. Withdrawal from binge drinking produces mixed effects on measures of behavioral despair.
(A) During the first session of the Porsolt swim test, mice with a 30-day history of binge drinking (Alcohol) displayed a shorter latency to first float during the 15 min trial, compared to water-drinking controls (Water) and this effect was observed during both short- (1-day) and long-term (21 days) withdrawal. When assayed 24 hours later in a 5-min re-exposure test, mice with a history of binge drinking exhibited (B) more swimming behavior and (C) less treading behavior than water controls. (D) Alcohol mice also showed a significant increase in the proportion of swim observations between the 2 swim tests, while water-drinking mice displayed a test-dependent reduction in the proportion of swim observations. Data represent mean ± SEM of the number of animals indicated in parentheses. Each graph depicts a main effect of alcohol, p < 0.05 vs. water control.
3.8 Correlational analyses between binge drinking and behavioral measures
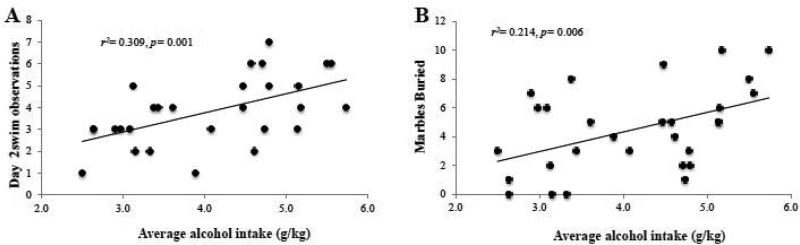
To determine whether or not the binge alcohol intake of the mice during the 30-day drinking period predicted the behavioral outcomes in our test battery, we conducted correlational analyses between the average alcohol intake (in g/kg) and each of our behavioral measures using a Bonferroni adjusted alpha level of 0.005 per test (0.05/10). Despite alcohol-exposed animals exhibiting greater indices of anxiety than water controls across a variety of measures (Figs. 1-2), the average amount of alcohol intake across the 30 days of drinking correlated with only a few behavioral outcomes (Fig. 3), likely due to the very low variability in intake. Nevertheless, higher alcohol intake was significantly correlated with the amount of swimming behavior during the re-exposure swim test (Fig. 3A) [r(28)=0.56, p=0.006] and there was a notable trend associating alcohol intake with the total number of marbles buried in the marble burying test (Fig. 3B) [r(29)=0.46, p= 0.006]. No other behavioral outcomes were correlated in a statistically significant manner with alcohol intake (p's>0.05; data not shown).
Figure 3. Binge alcohol intake correlates with the intensity of behavioral signs of negative affect in withdrawal.
Using a Bonferroni adjusted alpha of .005, the correlational analyses between the average total amount of alcohol intake exhibited by the B6 mice across the 30-day drinking period and our behavioral measures of anxiety and behavioral despair revealed a significant correlation with (A) the swimming behavior on day 2 of the forced swim test. (B) There was also a notable trend associating alcohol intake with the total number of marbles buried. For sufficient statistical power, the data were collapsed across both withdrawal time-points for this analysis.
3.9 Immunohistochemistry
3.9.1.Amygdala
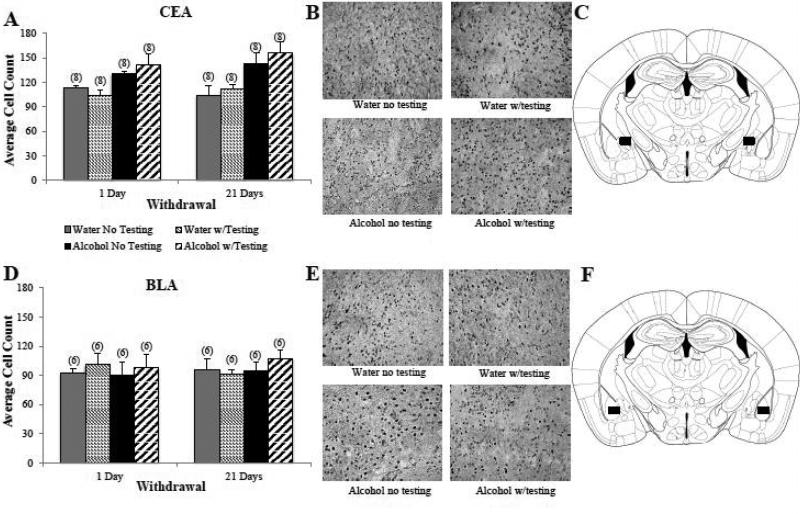
Overall, Alcohol mice showed significantly higher Egr1+ cell counts in the CEA than water controls (Fig. 4A,B) [main treatment effect: F(1,56)= 24.49, p<0.001; n=8/group]. This alcohol effect was independent of behavioral testing as indicated by no main Testing effect or interactions with the Testing factor (p's>0.05). As behavioral testing did not influence the number of Egr1+ cell counts, the elevated cellular activity within the CEA of Alcohol mice was likely due to their history of binge drinking and not a drinking-induced vulnerability to the cellular effects of testing stress. The alcohol-induced increase in Egr1 staining was present in the CEA as early as 24 hrs into withdrawal and persisted unchanged for at least 22 days into withdrawal, as indicated by no main Withdrawal effect or interactions with the Withdrawal factor (Fig. 6A; p's>0.05). In contrast to the CEA, there were no significant effects of binge drinking, behavioral testing, or withdrawal duration on Egr1+ cell counts in the BLA (Fig. 4D,E; 3-way ANOVA, p's>0.05).
Figure 4. Subregional differences in the effects of withdrawal from binge drinking upon Egr1 expression in the amygdala.
(A) Summary of the average Erg+ cell counts within the CeA exhibited by water-drinking controls (Water) and B6 mice with a 30-day history of binge drinking (Alcohol) during short-term (1-day) and long-term (21 days) withdrawal. Compared to Water mice, Alcohol mice exhibited higher Egr1+ cell counts in the CEA, irrespective of the withdrawal time-point or whether or not the mice were subjected to our behavioral test battery (Testing vs. No Testing) prior to tissue collection. (B) Representative micrographs of Egr1 immunostaining within the CeA of the four treatment groups, indicating greater Egr1 immunostaning in Alcohol versus Water mice. (C) Schematic illustrating a coronal section through the amygdala, highlighting (black square) the size and location of the sampling region used to assay the number of Egr1+ cells within the CeA. (D) In contrast to the CeA, Egr1 expression within the BLA was unaffected by either alcohol treatment or behavioral testing. (E) Representative micrographs of Egr1 immunostaining within the BLA indicating comparable immunostaining within the four treatment groups. (F) Schematic illustrating a coronal section through the amygdala, highlighting (black square) the size and location of the sampling region used to assay the number of Egr1+ cells within the BLA. The data in panels A and D represent mean ± SEM of the number of animals indicated in parentheses.
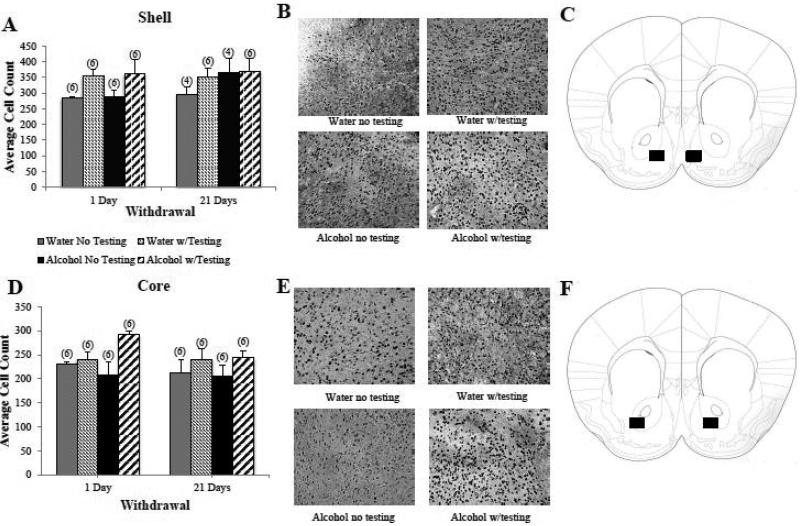
Figure 6. Egr1 expression within the Acb is regulated by behavioral testing.
(A) Both water-drinking controls (Water) and mice with a 30-day history of binge drinking (Alcohol) exhibited higher Egr1+ cell counts within the AcbSh if they underwent behavioral testing procedures (Testing) and this main effect of the behavioral testing was apparent in animals during both short- (2-day) and long-term (22 days) withdrawal. (B) Representative micrographs of Egr1 immunostaining within the AcbSh of the four treatment groups, indicating higher immunostaining in behaviorally tested mice. (C) Schematic illustrating a coronal section through the striatum, highlighting (black square) the size and location of the sampling region used to assay the number of Egr1+ cells within the AcbSh. (D) A similar pattern of group differences was observed within the AcbC. (E) Representative micrographs of Egr1 immunostaining within the AcbC of the four treatment groups, indicating higher immunostaining in behaviorally tested mice. (F) Schematic illustrating a coronal section through the striatum, highlighting (black square) the size and location of the sampling region used to assay the number of Egr1+ cells within the AcbC. The data in panels A and D represent mean ± SEM of the number of animals indicated in parentheses.
3.9.2. BNST
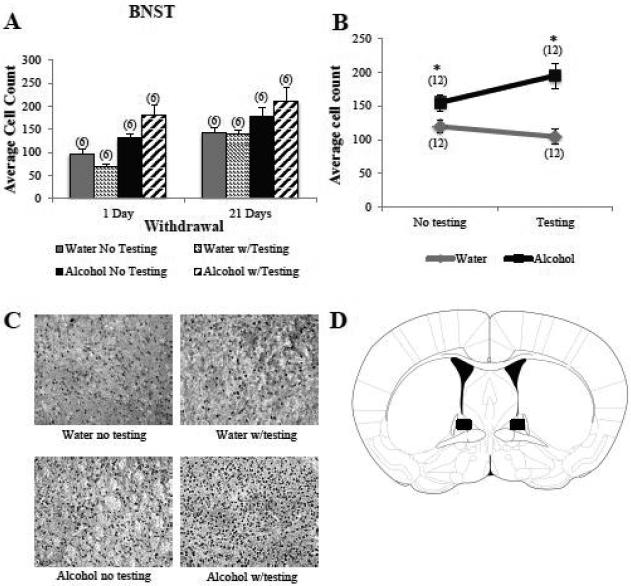
As observed in the CEA (Fig. 6)], overall, there were significantly more Egr1+ cells in the dorsal BNST of binge-drinking versus water-drinking animals (Fig. 5A,C) [main treatment effect: F(1,40)=28.95,p<0.001; n=6/group]. However, in contrast to the CEA, we observed a significant interaction between treatment and behavioral testing with respect to Egr1+ cell counts in the BNST [treatment by testing interaction: F(1, 40)=5.53, p=0.02], indicating that the binge alcohol-water differences in cellular activity within this region depended upon behavioral testing. Post-hoc t-test comparisons of tested versus untested animals separately for each withdrawal time-point confirmed that a history of binge drinking increased cellular activity in the BNST, regardless of behavioral testing, although the magnitude of the treatment difference was greater in the behaviorally tested animals (Fig. 5B) [no testing: t(22)=2.29, p=0.03; testing: t(22)=4.87, p<0.001]. While inspection of Fig. 5B suggested opposite influences of behavioral testing procedures upon the number of Egr1+ cells within the BNST of alcohol-exposed (increase) and water-exposed (decrease) mice, t-test comparisons of tested versus untested animals for each treatment group separately indicated that the effects of behavioral testing were not statistically reliable [1 day: t(10)=1.90, p= 0.11; 21 days: t(10)=0.93, p= 0.38]. Also in contrast to the CEA (Fig. 4A), Egr1+ cell counts within the BNST were higher overall at 21 days versus 1 day withdrawal (Fig. 5A) [main time effect: F(1,40)=16.78, p<0.001]. This time-dependent difference in cell activity was independent of both treatment group and behavioral testing (no interactions with either factor, p's>0.05) and, thus, may reflect some age-related change in BNST activity.
Figure 5. Egr1 expression in the BNST depends upon treatment and testing.
(A) Compared to water controls (Water), B6 mice with a 30-day history of binge drinking (Alcohol) exhibited higher Egr1+ cell counts in the BNST. Overall, animals had higher Egr1 expression during long-term withdrawal (21 days), compared to short-term withdrawal (1 day). (B) Graphical depiction of the significant interaction effect between treatment group and behavioral testing collapsed across both withdrawal time points, illustrating that behavioral testing exerted a greater stimulatory effect on Egr1 expression in Alcohol mice compared to Water controls. The data in panels A and B represent mean ± SEM of the number of animals indicated in parentheses. *Denotes treatment effect, p<0.05. (C) Representative micrographs of Egr1 immunostaining within the BNST of the four treatment groups, indicating the combinatorial effect of binge drinking history and behavioral testing upon Egr1 immunostaining within the BNST. (D) Schematic illustrating a coronal section through the BNST, highlighting (black square) the size and location of the sampling region used to assay the number of Egr1+ cells.
3.9.3. Acb
Unlike the CEA and BNST, we failed to detect any influence of prior alcohol experience upon Egr1 expression within either the AcbC or AcbSh (Fig. 6; no main effects of, or interactions with, the treatment factor, p's>0.05]. Moreover, we failed to detect any withdrawal-dependent change in Egr1+ cell counts within either subregion (no main effects of, or interactions with, the time factor, p's>0.05). However, we did detect significant main effects of behavioral testing upon Egr1 levels in both the AcbSh (Fig. 6A,B) [F(1,36)=5.28, p=0.03; n=6/group] and the AcbC (Fig. 6D,E) [F(1,40)=8.01, p=0.007; n=6/group] ], with behaviorally tested animals exhibiting higher Egr1+ cell counts than untested controls, regardless of drinking history or withdrawal duration (no interactions with the testing factor, p's>0.05).
3.94 Correlational Analyses of Egr1 Expression Between Brain Regions of Interest
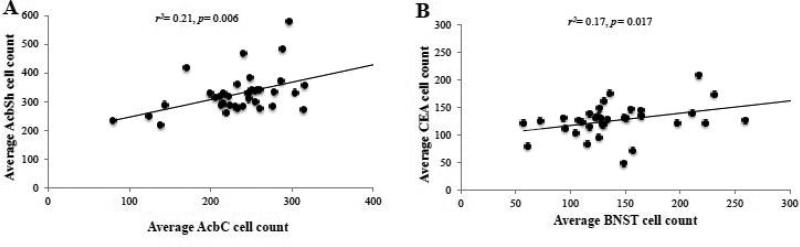
Within-subjects correlational analyses were conducted using a Bonferroni adjusted alpha of 0.008 (0.05/6) to compare Egr1 activation in the CEA, BNST, AcSh, and AcbC to examine for inter-region relations in Egr1 levels. This analysis was conducted across all animals for which we obtained tissue sections from all five regions of interest, irrespective of drinking history or behavioral testing in order to obtain sufficient statistical power. We observed a significant correlation for Egr1+ cell counts between the AcbC and AcbSh (Fig. 7A) [r(34)=0.455, p= 0.006] and a positive trend between cell counts within the CEA and those within the BNST (Fig. 7B) [r(33)= 0.412, p= 0.017]. However, Egr1+ cell counts within the CEA and BNST were not correlated with those observed in either the AcbC or AcbSh (p's>0.05).
Figure 7. Egr1 activation is correlated across multiple extended amygdala structures.
Correlational analyses were conducted between the average number of Egr1+ cell counts observed within our brain regions of interest to examine for concordant activation within these structures. (A) Egr1 activation within the CEA was positively correlated with activation in the BNST. (B) Concurrent activation was also observed between the AcbC and AcbSh. The statistical results of the analyses are represented in each individual panel.
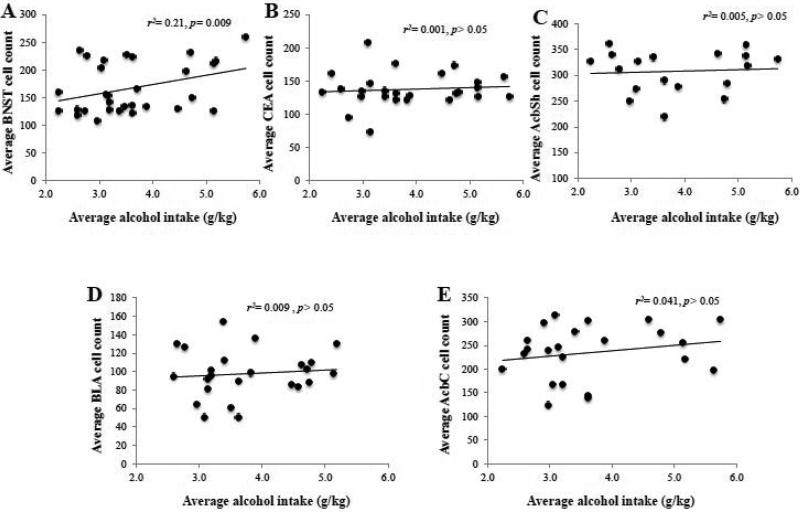
3.10. Correlational analyses between binge alcohol intake and Egr1 expression
To determine whether or not a relation existed between alcohol intake during the 30-day drinking period and the activational state of our brain regions of interest and drinking behavior, we also conducted within-subjects correlational analyses between the average alcohol intake and Egr1+ cell counts within the CEA, BLA, BNST, AcbSh and AcbC. Using a Bonferroni adjusted alpha of 0.01 (0.05/5), we found that alcohol intake was positively correlated with Egr1+ cell counts only within the BNST (Fig. 10A) [r(31)=0.462, p=0.009]. Egr1+ cell counts within other regions were not significantly correlated with alcohol intake [p's> 0.05].
3.11. Correlational Analyses between Egr1 expression and behavioral measures
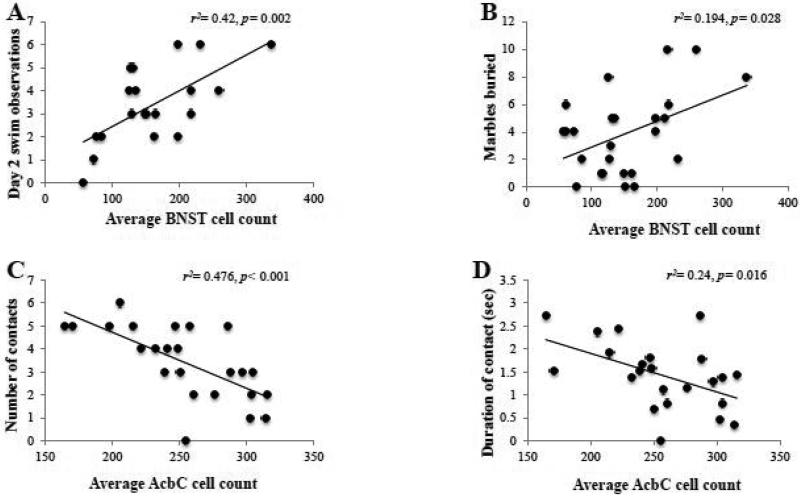
To determine whether or not a relation existed between the activational state of our brain regions of interest and negative affect, we also conducted within-subjects correlational analyses between the average Egr1+ cell counts within the CEA, BLA, BNST, AcbSh and AcbC and our different behavioral outcomes from the test battery using a Bonferroni adjusted alpha of 0.005 (0.05/10) for each region. Analyses indicated a strong positive relation between Egr1+ cell counts within the BNST and the number of swim observations during the re-exposure test (Fig. 9A) [r(20)=0.65, p=0.002] and a positive trend between BNST Egr1+ cell count and the number of marbles buried (Fig. 9C) [r(25)=0.44, p=0.028]. In contrast to the BNST, there were no predictive relations between Egr1 expression within the CEA or BLA and any of our behavioral measures (data not shown). In the novel object test, analyses indicated a negative relationship between cellular activation within the AcbC and the number of object contacts (Fig. 9E) [r(24)=-0.69, p<0.001] and a negative trend with total contact duration (Fig. 9F) [r(23)=−0.49, p=0.016]. There were no significant relations observed between Egr1+ cell counts within the AcbSh and any of our behavioral measures (data not shown).
Figure 9. Activation of extended amygdala structures predicts behavioral indices of anxiety.
(A-B) The statistically significant results of correlational analyses between different behavioral results from our test battery and the number of Egr1+ cell counts within BSNT. (C-D) The statistically significant results of correlational analyses between different behavioral result from our test battery and the number of Egr1+ cell counts within the AcbC. The data represent the data from 18-24 mice and the statistical results are presented in each individual panel.
4. Discussion
Many alcoholics report that anxiety reduction is a key motivator for drinking [36]. Therefore, increased anxiety during withdrawal serves as a negative reinforcer that substantially increases the likelihood of relapse during abstinence from chronic alcohol abuse [37]. Individuals who regularly engage in heavy episodic drinking do not necessarily meet the diagnostic criteria for alcoholism [1]; however, frequent binge drinking is a significant risk factor for the development of alcoholism and can produce symptoms during withdrawal similar to those seen with alcohol dependence [38, 39]. Herein, we show that withdrawal from a chronic (30-day) history of binge drinking increases a number of behavioral indices of negative affect, most notably anxiety-related measures, in mice. Moreover, the negative affect observed in our binge-drinking mice manifested as early as 24 hrs into withdrawal and persisted, unchanged, for at least 3 weeks following the last drinking episode. These data demonstrate that repeated bouts of alcohol intake under limited access conditions produces enduring changes in emotionality. Thus, the dysphoria caused by cessation of binge drinking likely promotes re-engaging in the behavior to alleviate these aversive symptoms and perpetuation of this binge-withdrawal cycle likely contributes to the transition from binge drinking to alcoholism.
4.1 Withdrawal from binge drinking elevates indices of anxiety
Binge drinking produced anxiogenic effects during withdrawal, as evidenced by treatment-dependent differences in behavior on a variety of tests. For one, alcohol-drinking animals showed greater reluctance to interact with a novel object compared to water controls, suggesting heightened levels of neophobia-induced anxiety [27, 28]. Consistent with this, Alcohol mice exhibited increased defensive burying in the marble burying test and this result was correlated with average alcohol intake during the 30-day drinking period. These present results are in line with a previous study in rats showing decreased interaction with a novel object during the first 10-30 hrs of withdrawal from a 4-day alcohol liquid diet (7-13 g/kg/day) [40]. However, in that previous study, the hyper-anxious effect dissipated by 70 hrs into withdrawal, while in the present study, the hyper-anxious state of the binge drinking mice persisted for at least 21 days. Although there are a number of different procedural variables between the present study and that prior [40] (including species, adulteration of alcohol, daily duration of alcohol access, number of drinking days), we argue that the relatively brief duration of alcohol exposure in the prior rat study (4 days) was likely insufficient to elicit the enduring behavioral changes observed following more chronic drinking procedures.
Arguably, the longer latency to first open arm entry displayed by Alcohol mice on the EPM is not likely physiologically relevant nor sufficient to suggest a hypersensitivity to the aversive properties of open/brightly-lit areas. However, more meaningful evidence of an increased anxiogenic response to these aversive properties was observed in the light/dark shuttle box test, with alcohol-drinking mice exhibiting fewer light-side entries at both withdrawal time-points. Alcohol-drinking mice traveled a greater distance in the light side, compared to water controls, despite their fewer entries. As there were no group differences in the total time spent in the light side, these data suggest that binging mice exhibited greater hyperactivity during each entry into this aversive environment, compared to water-drinking animals. Although debate exists in the literature pertaining to the interpretation of locomotor activity in aversive environments [41-44]; independent of this interpretational challenge, both hypo- and hyperactive psychomotor dysregulation are symptoms reported in humans during alcohol cessation [45], as well as in those suffering from anxiety disorder [46] and certain depression subtypes [47]. Thus, our data for both neophobia and motor hyperactivity are consistent with, and extend, extant data from humans and laboratory animals to a murine model of binge alcohol drinking and provide further predictive validity for our modified DID procedure as a model of alcohol dependence.
4.2 Withdrawal from binge drinking produces little change in indices of depression
This study found limited evidence of withdrawal-induced depressive symptoms, as indicated by a shorter latency to float in the Porsolt forced swim test for alcohol-drinking mice on the first, but not on the second, day of testing. While a decreased latency to float and increased floating behavior are both traditionally considered indicative of greater behavioral despair in this paradigm [48], no Alcohol-Water differences were noted for the latency to first float during the 5-minute re-exposure to the pool on day 2 of testing and alcohol-drinking mice showed significantly more swimming and less treading than water controls on this re-exposure test. In considering the traditional interpretation of floating behavior in the forced swim test as reflecting behavioral despair, these between-test results seem counterintuitive given the negative affective state manifested by Alcohol mice in the aforementioned anxiety tests. However, one can reconcile this apparent discrepancy in findings by considering the adaptive value of conserving energy by floating when re-exposed to a previously inescapable swim stressor. In this regard, the increase in floating/reduced swimming exhibited by water controls during the re-exposure test may reflect a more adaptive and appropriate response upon recall of the futility of their prior escape efforts. By this rationale, the increase in swimming exhibited by alcohol-drinking mice during the re-exposure test appears to be an anomalous, potentially maladaptive response. This could indicate some sort of memory impairment [49, 50] or perhaps a sensitized behavioral response to a previously stressful situation that might reflect heightened anxiety [51-53]. A memory deficit does not seem as likely, since the Alcohol animals increased their swimming behavior as a function of testing. Given the aforementioned evidence that a history of binge drinking produces a persistent increase in anxiety, Alcohol mice may be more sensitive to inescapable stressor-induced behavioral sensitization, particularly given that the increase in swimming during the swim re-test was positively correlated with the amount of alcohol intake. These interpretations are purely speculative and the impact of binge drinking history upon the subsequent development of repeated stressor-induced behavioral sensitization requires more direct examination.
Although group differences were noted in the Porsolt swim test, we failed to detect alcohol-water differences in the sucrose preference test – a test widely employed for assessing depression-related anhedonia in rodents [24]. However, our data showed only a trend for a subject factor interaction at 1 day withdrawal, but no influence of prior binge history upon this measure in protracted withdrawal. Many studies have shown a relationship between alcohol consumption and increased preference for sweet solutions in both laboratory animals [54, 55] and humans [56, 57], which may be especially prevalent in early withdrawal. Although the elevated sucrose preference in the AD animals at 1-day withdrawal did not quite reach statistical significance, given these previous studies and the other behavioral results described herein, it is argued that the failure to observe significant group differences change in sucrose preference during withdrawal may reflect insensitivity of the test as conducted, rather than a lack of an effect of alcohol withdrawal on anhedonia. Taken together, the results of both the forced swim and sucrose preference tests argue a more robust effect of binge drinking history upon anxiety versus depressive measures.
4.3 Withdrawal from binge drinking increases cellular activity in the CEA, but not the BLA
Using the transcription factor Egr1 to index cellular activity [34], we observed elevated Egr1 in the CEA during withdrawal from binge drinking that was independent of withdrawal duration or behavioral testing. These findings argue that a history of binge drinking is sufficient to produce an enduring increase in the activity of this extended amygdala structure and are consistent with its role in regulating excessive drug consumption [58-61].
Despite the robust effects of withdrawal from binge drinking upon the cellular activity in the CEA, we failed to detect significant changes in Egr1 levels within the adjacent BLA- a finding consistent with other studies showing a region-specific role of the amygdala in underpinning alcoholism-related behaviors. For example, experimental CEA lesions significantly reduce the anxiogenic effects of restraint stress and also decrease voluntary consumption in rats, while BLA lesions produce no change in these behaviors [Summarized in 62]. Moreover, consistent with the present findings for Egr1, a chronic history of alcohol drinking, under either continuous access [63] or our modified DID binge procedures [16], increases protein indices of glutamatergic signaling in the CEA, but not in the BLA. While we have yet to assay for the long-term glutamatergic consequences of a chronic history of binge drinking upon subregional differences in glutamate protein expression within amygdala, the up-regulation of glutamate-related protein expression within the CEA persists for at least 4 weeks into alcohol withdrawal in rodents consuming alcohol under continuous access conditions [16, 63]. Notwithstanding the possibility that a chronic history of binge drinking changes GABA, glucocorticoid, or other neuropeptide function within the CEA (as reported to occur in rodents withdrawn from high-dose, non-contingent, alcohol vapor exposure [64]), the extant immunoblotting literature [16], coupled with the present results for Egr1 expression provide descriptive evidence that a history of binge drinking produces enduring increases in the activity of the CEA, which might reflect a disruption of the balance between inhibitory and excitatory signaling within this amygdala subregion to elicit withdrawal-induced dysphoria.
4.4 Withdrawal from binge drinking increases cellular activity in the BNST
The BNST exhibits reciprocal interconnections with the CEA [65] and consistent with this neuroanatomical connectivity, we observed a similar alcohol withdrawal-induced increase in Egr1 expression in the BNST, as that observed within the CEA and a predictive relation existed between Egr1 expression between these 2 regions. Additionally, the amount of alcohol intake exhibited by the mice during the 30-day drinking period was positively correlated with the BNST level of Egr1. The BNST exhibits hyper-excitability in response to both acute and repeated stress, as indicated by evoked c-fos induction [66, 67]. Consistent with stressor-induced activation of the BNST, BNST Egr1 levels were positively correlated with behavioral reactivity in the forced swim test. However, in contrast to the CEA, the withdrawal-induced increase in the number of BNST Egr1+ cells was greater in animals subjected to our behavioral test battery than in those that were test-naïve. Thus, factors associated with behavioral testing have a combinatorial effect with alcohol-withdrawal upon the cellular activity of this region and it remains to be determined whether or not the influence of behavioral testing upon BNST Egr1 levels reflects the stressful nature of the behavioral test (and thus, a stressor response) and/or the motor activity associated with exploration/attempts to escape during testing. Nevertheless, it is clear from the present data that, akin to results derived from more conventional models of alcoholism, a chronic history of binge drinking increases the basal activity of BNST neurons and also renders them more sensitive to the effects of stressors on cellular activity within this region. Given prior functional evidence that the BNST regulates alcoholism-related behaviors [68-70], the hyper-reactivity of the BNST observed herein likely contributes heavily to the behavioral manifestation of anxiety during withdrawal from binge drinking and the continued propensity to binge drink when alcohol is next presented.
4.5 Withdrawal from binge drinking does not affect cellular activity within the Acb
The Acb serves as an interface between limbic structures that process emotion and motivation and motor regions governing approach/avoidance behaviors. The AcbSh and AcbC are both anatomically and functionally distinct [71], although both Acb subregions have been implicated in governing different aspects of alcohol reward and alcohol reward-related learning [72] and prior immunoblotting studies indicated changes in excitatory neurochemistry within these subregions in alcohol-experienced animals [15, 23, 63, 73, 74]. Further, dysphoric states during withdrawal are mediated by changes in Acb neurotransmission, with alcohol-induced reductions in monoaminergic function theorized to contribute to the depressive effects of alcohol withdrawal.[23, 74, 75], Moreover, we know that a chronic history of binge drinking elevates indices of glutamatergic signaling within the Acb [15, 73]. Thus, we hypothesized at the outset of this study that a history of binge drinking would augment Acb cellular activity, particularly within the AchSh, and this increased activity would correlate with behavioral indices of negative affect. However, (1) we failed to detect any alcohol-dependent changes in Egr1 expression within either Acb subregion and (2) binge-drinking history did not relate to Egr1 expression, irrespective of subregion. However, when all mice were considered collectively, Egr1 expression within the AcbC, but not the AcbSh, was associated with higher indices of anxiety in the novel object and elevated plus-maze tests and the causative nature of this relation requires further investigation. Interestingly, Egr1 expression within Acb subregions was up-regulated by behavioral testing and there was no subregional distinction in this regard.. Moreover, when all mice were considered collectively, Egr1 expression within the AcbC was associated with higher indices of anxiety in the novel object and elevated plus-maze tests. Thus, cellular hyperactivity was not restricted to the AchSh subregion integrated within the extrahypothalamic stress circuit. Whether or not this observation reflects the motor demand of the swim test conducted just prior to tissue collection, recollection of the prior swim test (or both) cannot be discerned in the present study. However, the fact that Egr1 cell counts in the shell and core subregions were similarly increased in both alcohol-experienced and –naïve mice is consistent with the notion that the Acb integrates motivationally relevant stimuli with motor output [76], but does not provide any support for the regulation of this capacity by prior alcohol experience/withdrawal or for increased cellular activity within Acb subregions as requisite for the manifestation of negative affect during alcohol withdrawal. Across all the animals, the positive correlation between AcbC and ACbSh activation does suggest that these regions are activated concurrently when mice are exposed to an inescapable stressor, which again, is in line with the nature of the behavioral test conducted just prior to tissue collection. While the AcbSh is part of the extended amygdala/extrahypothalamic stress subcircuit and is modulated by BNST's projection to the VTA [77], the discrepancies in the patterns of Egr1 expression observed across the CEA, BSNT and AcbSh argue that the entire extended amygdala does not activate in unison during the behavioral manifestation of alcohol withdrawal-induced negative affect and/or concurrent activation between these structures is not necessary for the manifestation of alcohol withdrawal-induced anxiety.
4.6 Proposed mechanisms underpinning the persistent negative affective state produced by alcohol withdrawal
Immediate early genes (IEGs) such as Egr1 participate in a transcription-level cellular response that is activated transiently and rapidly in response to a wide variety of stimuli. IEGs are a non-specific index of cellular activity and as such, increased levels of Egr1+ cells cannot be used to determine the precise nature of activity. A prudent next step would be to determine the upstream mediators and the downstream consequences of this increase in Egr1 expression. For instance, binge drinking-induced changes in Egr1 expression may be related to an upregulation of stress hormone signaling either by hormone-mediated effects upon Egr1 transcription or by Egr1-dependent transcription of stress hormone-related proteins. There is a preponderance of evidence showing increased corticotropin-releasing hormone (CRH) signaling throughout the extended amygdala in response to alcohol consumption. An upregulation of the CRH system is heavily implicated in alcohol dependence and has been linked to the negative affective states experienced during periods of alcohol abstinence [78-82]. Increased CRH signaling in the CEA and BNST specifically is implicated in withdrawal-induced anxiety. The CEA is a major source of CRH innervation of the BNST and the BNST itself is densely populated with CRH-expressing cell bodies. Antagonism of CRF receptors in the CEA and BNST is sufficient to reduce anxiogenic-like effects of alcohol withdrawal and decrease subsequent consumption [83-87]. Additionally, elevated CRH levels in the BNST during alcohol withdrawal are reduced by subsequent alcohol intake [68], providing a neurophysiological basis for the negative reinforcing properties of withdrawal-induced affective dysregulation.
Anxiety during withdrawal and its enduring role in drug seeking and stress-induced has been demonstrated consistently in both humans and animals [5, 36, 88-91]. However, it is important to note that alcohol withdrawal studies in animals are typically conducted in using models of chronic alcohol dependence. In the human population, binge drinking is the most prevalent form of alcohol abuse [1], yet very little is know about the psychological consequences of this behavior. The present study indicates that this pattern of consumption is capable of producing enduring affective disturbances during abstinence, consistent with those seen in chronic alcoholism. It therefore seems reasonable to speculate that binge drinking causes similar changes to extended amygdala structures, mediated by dysregulation of the HPA axis and CNS hyperexcitability.
4.7 Conclusion
The present study demonstrates that voluntary binge drinking in an animal model is capable of producing emotional dysregulation after as little as 30 days of alcohol exposure. The emotional dysregulation has a rapid onset, persists into protracted withdrawal, and is associated with increased cellular activity in the CEA and BNST, but in the Acb. Further investigation is warranted to better understand the psychophysiological consequences of binge drinking and how this pattern of behavior may contribute to the transition to addiction.
Figure 8. Binge alcohol intake relates to the intensity of Egr1 activation within BNST.
The results of correlational analyses between the average total amount of alcohol intake exhibited by the B6 mice across the 30-day drinking period and the number of Egr1+ cell counts within (A) BSNT, (B) CeA, (C) AcbSh, (D) BLA, and (E) AcbC revealed a significant predictive relationship for the BNST only, accounting for approximately 20% of the variation in Egr1 activation. For sufficient statistical power, the data were collapsed across both withdrawal time-points for this analysis. The data represent the data from 22-31 mice and the statistical results are presented in each individual panel.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant AA016650.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion Binge drinking: Nationwide problem, local solutions. CDC Vital Signs. 2012:1–4. [Google Scholar]
- 2.National Institute on Alcohol Abuse and Alcoholism NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter. 2004:3. [Google Scholar]
- 3.Bayard M, et al. Alcohol withdrawal syndrome. Am. Fam. Physician. 2004;69(6):1443–50. [PubMed] [Google Scholar]
- 4.Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol. Clin. Exp. Res. 2005;29(5):902–8. doi: 10.1097/01.alc.0000164544.45746.a7. [DOI] [PubMed] [Google Scholar]
- 5.Driessen M, et al. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36(3):249–55. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24(2):77–84. [PMC free article] [PubMed] [Google Scholar]
- 8.Spanagel R. Recent animal models of alcoholism. Alcohol Res Health. 2000;24(2):124–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict. Biol. 2005;10(4):309–19. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- 10.Becker HC. Animal models of alcohol withdrawal. Alcohol Res Health. 2000;24(2):105–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Freund G. Induction of physical dependence on alcohol in rodents. Adv. Exp. Med. Biol. 1975;56:311–25. doi: 10.1007/978-1-4684-7529-6_16. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes JS, et al. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Alheid GF. Extended amygdala and basal forebrain. Ann. N. Y. Acad. Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 15.Cozzoli DK, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol. Clin. Exp. Res. 2012;36(9):1623–33. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cozzoli DK, et al. Binge alcohol drinking by mice requires intact group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdale. Neuropsychopharmacology. 2014;39(2):435–44. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride WJ, et al. Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44(2):171–83. doi: 10.1016/j.alcohol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman K, et al. Coordinated Dynamic Gene Expression Changes in the Central Nucleus of the Amygdala During Alcohol Withdrawal. Alcohol. Clin. Exp. Res. 2013;37:E88–E100. doi: 10.1111/j.1530-0277.2012.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward RJ, Lallemand F, de Witte P. Biochemical and Neurotransmitter Changes Implicated in Alcohol-Induced Brain Damage in Chronic or ‘Binge Drinking’ Alcohol Abuse. Alcohol Alcohol. 2009;44(2):128–35. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- 20.Kaczmarek L, Robertson HJ. Immediate early genes and inducible transcription factors in mapping of the central nervous system function and dysfunction. Elsevier; Amsterdam Oxford: 2002. p. xix.p. 370. [Google Scholar]
- 21.Crabbe JC, et al. A Line of Mice Selected for High Blood Ethanol Concentrations Shows Drinking in the Dark to Intoxication. Biol. Psychiatry. 2009;65(8):662–70. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes JS, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 23.Cozzoli DK, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J. Neurosci. 2009;29(27):8655–68. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz RJ. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 1982;16(6):965–8. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- 25.Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci. Biobehav. Rev. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 26.Bourin M, Hascoet M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463(1-3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 27.Dulawa SC, et al. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J. Neurosci. 1999;19(21):9550–6. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misslin R, Ropartz P. Responses in Mice to a Novel Object. Behaviour. 1981;78:169–77. [Google Scholar]
- 29.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Therap. 1977;229(2):327–36. [PubMed] [Google Scholar]
- 30.Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003;55(1):69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 31.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2(2):322–28. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Njung'e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991;38(1):63–7. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. Elsevier Academic Press; San Diego, CA: 2004. [Google Scholar]
- 34.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 1998;28(3):370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 35.Su ZI, et al. Prior extended daily access to cocaine elevates the reward threshold in a conditioned place preference test. Addict. Biol. 2013 doi: 10.1111/adb.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am. J. Psychiatry. 1994;151(12):1723–34. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- 37.Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin. Psychol. Rev. 2000;20(2):149–71. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 38.Hasin DS, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 39.Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey on Drug Use and Health (NSDUH) 2012 Available at: http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/DetTabs/NSDUHDetTabsSect5peTabs1to56-2012.htm-Tab5.8A. [PubMed]
- 40.Knapp DJ, Saiers JA, Pohorecky LA. Observations of novel behaviors as indices of ethanol withdrawal-induced anxiety. Alcohol Alcohol. 1993;2:489–93. [PubMed] [Google Scholar]
- 41.Rasmussen DD, et al. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol. Clin. Exp. Res. 2001;25(7):999–1005. [PubMed] [Google Scholar]
- 42.Hilakivi LA, Ota M, Lister RG. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ‘despair’. Pharmacol. Biochem. Behav. 1989;33(2):371–4. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- 43.Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50(6):1129–32. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- 44.Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci. Biobehav. Rev. 2005;28(8):837–50. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Saitz R, O'Malley SS. Pharmacotherapies for alcohol abuse. Withdrawal and treatment. Med. Clin. North Am. 1997;81(4):881–907. doi: 10.1016/s0025-7125(05)70554-x. [DOI] [PubMed] [Google Scholar]
- 46.Tasman A, et al. Psychiatry, 2 Volume Set: Edition 4. John Wiley & Sons; Hoboken, NJ: 2015. pp. 1076–80. [Google Scholar]
- 47.Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am. J. Psychiatry. 1997;154(1):4–17. doi: 10.1176/ajp.154.1.4. [DOI] [PubMed] [Google Scholar]
- 48.Porsolt RD, et al. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2001 doi: 10.1002/0471142301.ns0810as14. Chapter 8: p. Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- 49.Depablo JM, et al. Learned Immobility Explains the Behavior of Rats in the Forced Swimming Test. Physiol. Behav. 1989;46(2):229–37. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- 50.West AP. Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14(6):863–77. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- 51.Polani PE. Attacks of anxiety, panic and frenzy, and their related depression: a hypothesis. Med. Hypotheses. 2004;63(1):124–7. doi: 10.1016/j.mehy.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 52.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol. Bull. 1992;112(2):218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 53.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci. Biobehav. Rev. 2001;25(3):205–18. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 54.Gosnell BA, Krahn DD. The relationship between saccharin and alcohol intake in rats. Alcohol. 1992;9(3):203–6. doi: 10.1016/0741-8329(92)90054-e. [DOI] [PubMed] [Google Scholar]
- 55.Stewart RB, et al. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol. Clin. Exp. Res. 1994;18(2):375–81. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 56.Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am. J. Psychiatry. 1997;154(2):269–70. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- 57.Kranzler HR, Sandstrom KA, Van Kirk J. Sweet taste preference as a risk factor for alcohol dependence. Am. J. Psychiatry. 2001;158(5):813–5. doi: 10.1176/appi.ajp.158.5.813. [DOI] [PubMed] [Google Scholar]
- 58.Lowery-Gionta EG, et al. Corticotropin Releasing Factor Signaling in the Central Amygdala is Recruited during Binge-Like Ethanol Consumption in C57BL/6J Mice. J. Neurosci. 2012;32(10):3405–13. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cozzoli DK, et al. Protein Kinase C Epsilon Activity in the Nucleus Accumbens and Central Nucleus of the Amygdala Mediates Binge Alcohol Consumption. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J, et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4465–70. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci. Biobehav. Rev. 2012;36(2):873–88. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moller C, et al. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760(1-2):94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 63.Obara I, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res. 2009;33(11):1924–34. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberto M, Gilpin NW, Siggins GR. The central amygdala and alcohol: role of gamma-aminobutyric acid, glutamate, and neuropeptides. Cold Spring Harb. Perspect. Med. 2012;2(12):a012195. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Rev. 2001;38(1-2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 66.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138(1):235–43. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 67.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33(4):287–97. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 68.Olive MF, et al. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 2002;72(1-2):213–20. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kash TL. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol. 2012;46(4):303–8. doi: 10.1016/j.alcohol.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito R, Hayen A. Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J. Neurosci. 2011;31(16):6001–7. doi: 10.1523/JNEUROSCI.6588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaudhri N, et al. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–91. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lum EN, et al. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–87. doi: 10.1016/j.neuropharm.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szumlinski KK, et al. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33(6):1365–78. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karkhanis AN, et al. Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cardinal RN, et al. The contribution of the amygdala, nucleus accumbens, and prefrontal cortex to emotion and motivated behaviour. Int Cong Ser. 2003;1250:347–70. [Google Scholar]
- 77.Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J. Neurosci. 2013;33(3):950–60. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behaviour. Addict. Biol. 2004;9(3-4):205–12. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- 79.Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci. 2014;37(4):219–27. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 2001;53(2):209–43. [PubMed] [Google Scholar]
- 81.Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46(4):329–37. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valdez GR, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002;26(10):1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 83.Huang MM, et al. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J. Pharmacol. Exp. Ther. 2010;332(1):298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rassnick S, et al. Microinjection of a Corticotropin-Releasing Factor Antagonist into the Central Nucleus of the Amygdala Reverses Anxiogenic-Like Effects of Ethanol Withdrawal. Brain Res. 1993;605(1):25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 85.Baldwin HA, et al. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl.) 1991;103(2):227–32. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 86.Funk CK, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatry. 2007;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol. Clin. Exp. Res. 2004;28(6):865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 88.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213(1-2):43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silberman Y, et al. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43(7):509–19. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thevos AK, et al. Symptoms of anxiety in inpatient alcoholics with and without DSM-III-R anxiety diagnoses. Alcohol. Clin. Exp. Res. 1991;15(1):102–5. doi: 10.1111/j.1530-0277.1991.tb00525.x. [DOI] [PubMed] [Google Scholar]