Abstract
Viral vector vaccines designed to elicit CD8+ T cells in non-human primates exert potent control of immunodeficiency virus infections; however, similar approaches have been unsuccessful in humans. Adenoviral vectors elicit potent T cell responses but also induce production of immunosuppressive interleukin-10 (IL-10), which can limit the expansion of T cell responses. We investigated whether inhibiting IL-10 signaling prior to immunization with a candidate adenovirus vectored-HIV-1 vaccine, ChAdV63.HIVconsv, could modulate innate and adaptive immune responses in BALB/c mice. Transient IL-10 receptor blockade led to a modest but significant increase in the total magnitude CD8+ T cell response to HIVconsv, but did not affect T cell responses to immunodominant epitopes. Anti-IL-10R-treated animals also exhibited greater expression of CD86 on CD11c+ dendritic cells. Our data support further investigation and optimization of IL-10 blocking strategies to improve the immunogenicity of vaccines based on replication-defective adenoviruses.
Keywords: interleukin-10, HIV-1, mouse, T cells
Introduction
Development of a universal vaccine for HIV-1 remains the best hope for ending the AIDS pandemic. 1 Replication-deficient adenoviruses are attractive vectors for HIV-1 immunogen delivery due to their excellent safety profile and capacity to induce potent immune responses. 2 However, development of human adenovirus 5 (HAdV5) vectored HIV-1 vaccines has ceased due to poor immunogenicity and disappointing clinical trial results,3,4 as well as concerns regarding an association between pre-existing humoral immunity to HAdV5 and increased HIV-1 acquisition. 3 The field has now turned to rare human adenoviruses (HAdVs 26 and 35) and simian adenovirus ChAdV63 for which seroprevalence in humans is low.5 However, such vectors may be less potent than HAdV5,6 though capable of eliciting strong immune responses when included in heterologous prime-boost regimens.5
A potential approach to increasing the immunogenicity of adenovirus vectors is to modulate inhibitory signals, such as interleukin-10 (IL-10), that are induced alongside pro-inflammatory cytokines and chemokines as a compensatory anti-inflammatory response.7 IL-10 inhibits the activation and migration of naïve T cells by downregulating MHC class II and CD86 on antigen-presenting cells8,9 and inhibiting the production of cytokines and chemokines such as IL-1α, MCP-1, IP-10 and TNF-α.10,11 Antibody blockade of the IL-10R restored proliferation and cytokine production by HIV-1-specific CD4+ and CD8+ T cells in vitro.12,13
We investigated whether inhibition of IL-10 signaling at the time of immunization could enhance CD8+ T cell responses to an adenovirus-vectored HIV-1 vaccine candidate, ChAdV63.HIVconsv, which was recently shown to be immunogenic in a phase I clinical trial. 5 We report that IL-10R blockade significantly enhanced both the frequency and function of CD8+ T cell responses to ChAdV63.HIVconsv.
Materials and Methods
Immunogen design and vector production
HIVconsv has been described in detail elsewhere.14 ChAdV63.HIVconsv was produced at the Clinical Biomanufacturing Facility (CBF), University of Oxford, UK, as described previously.5
Experimental animals and immunizations
Six-week-old female BALB/c mice were purchased from Harlan. All procedures were conducted in accordance with the UK Animals (Scientific Procedures) Act under Project License PPL 30/2833 and Personal License PIL 40/1027 and approved by the University of Oxford Animal Care and Ethical Review Committee. Animals were injected intraperitoneally with either 200 μg low endotoxin azide free (LEAF) αIL-10R antibody (Biolegend; clone 1B1.3a) or 200 μg LEAF rat IgG1κ isotype control antibody (Biolegend) 24 hours pre-immunization. Mice were immunized intramuscularly with 5 x 108 viral particles of ChAdV63.HIVconsv. In selected experiments, blood was collected by venipuncture of the lateral tail vein 24 hours post-immunization (Fig. S1).
Intracellular cytokine staining
Cytokine production was assessed as described previously. 15 T cell responses to HIVconsv were measured by stimulating splenocytes, in the presence of CD107a-FITC (eBioscience), with pools of peptides (2 μg/ml each) spanning the HIVconsv immunogen (15-mers overlapping by 11 amino acids; Sigma Genosys), or with individual peptides corresponding to a single epitope (H, P and G1). All peptides were suspended in R10 medium (RPMI 1640 supplemented with 10% fetal calf serum, 2mM L-glutamine and penicillin-streptomycin antibiotics). R10 medium containing 0.05% DMSO (the same concentration as in the peptide pools) was used as a negative control, and PMA (50 ng/ml) and ionomycin (2 μg/ml) as a positive control. The mean response to the negative control was 0.07% of CD8+ T cells IFN-γ+, with a standard deviation of 0.07 (data not shown). Following stimulation, splenocytes were stained with anti-CD16/32, CD4-Pacific Blue, IFN-γ- APC, IL-2-PE (all eBioscience), LIVE/DEAD® fixable aqua dead cell stain (Invitrogen), and CD8α-PE-Texas red (Abcam). Cytokine secretion by CD11c+ cells was assessed after a 6-and-a-half-hour culture period in the absence of peptide. Splenocytes were stained with anti-CD16/32, CD11c-PE (eBioscience), LIVE/DEAD® fixable aqua dead cell stain (Invitrogen), CD8α-PE-Texas Red (Abcam), IL-12-APC, and CD86-FITC (Biolegend). Samples were acquired on an LSRII flow cytometer (BD Biosciences), and data analyzed using FlowJo version 9.5.2 (Tree Star).
Analysis of serum cytokines and chemokines
Serum obtained 24 hours post-immunization was stored at −80°C prior to use. The Milliplex MAP luminex assay (Millipore) was performed according to the manufacturer's instructions. Briefly, serum samples were incubated with magnetic beads conjugated to anti-IP-10, MCP-1 and TNF-α antibodies, followed by biotinylated detection antibodies and then Streptavidin-PE. Samples were analyzed using the Bio-Plex 100 system (Bio-Rad). Serum IL-10 concentration was measured in BABL/c mice 24 hours after intradermal immunization with 5 x 109 viral particles of ChAdV9.ME.TRAP, a vector that belongs to the same serogroup as ChAdV63.6 IL-10 was quantified using a BD Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences) according to the manufacturer's instructions.
Enzyme-linked immunosorbent spot assay (ELISPOT)
Responses to the entire HIVconsv immunogen were assessed using 15-mer peptides overlapping by 11 amino acids combined into 80 pools to produce a 4-dimensional matrix (i.e. each peptide was present in 4 different pools). ELISPOTS were performed on splenocytes harvested 21 d post-immunization as previously.14 Spots were quantified using an AID ELISPOT Reader and version 5.0 software (AID GmbH), and results expressed as spot-forming units (SFU) per 106 cells. Pools that elicited a response of above 100 spot-forming units (SFU)/106 splenocytes were entered into the analysis software Deconvolute This! (Mario Roederer, Vaccine Research Center, NIAID, NIH) to identify peptides to be retested individually in a second IFN-γ ELISPOT assay. Only peptides that were present in 4 pools that elicited a response in the first-round assay were selected for retesting. In all ELISPOT assays, R10 medium containing 0.05% DMSO (the same concentration as in the peptide pools) was used as a negative control, and PMA (50 ng/ml) and ionomycin (2 μg/ml) as a positive control. The mean response in the negative control wells was 15 SFU/106 splenocytes, with a standard deviation of 15 (data not shown).
In vivo killing assay
The in vivo killing assay was performed as previously16 using HIVconsv peptides H (RGPGRAFVTI), P (IFQSSMTKI), G1 (AMQMLKDTI) and unpulsed splenocytes labeled with 400 nM, 132 nM, 80nM and 16 nM carboxyfluorescein diacetate, succinimidyl ester (CFSE) respectively. In separate experiments splenocytes pulsed with HIVconsv peptide 42 (KAIGTVLVGPTPVNI) and unpulsed splenocytes were labeled with 400 nM and 16 nM CFSE respectively. In total, 107 splenocytes (containing equal numbers of cells stimulated with each peptide or control) were injected into the lateral tail vein of each mouse. After 16 hours, all splenocytes were harvested and acquired using an LSRII flow cytometer. Data were analyzed using FlowJo version 9.5.2. For each peptide-pulsed population, percentage lysis was calculated using the following formula: % lysis = (no. unpulsed splenocytes – no. pulsed splenocytes)/ no. unpulsed splenocytes x 100%.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5. Comparisons were performed using an unpaired t test for parametric data and the Mann-Whitney test for non-parametric data. The normality of the data was determined using the Kolmogorov–Smirnov one-sample test. p values <0.05 were considered statistically significant.
Results and Discussion
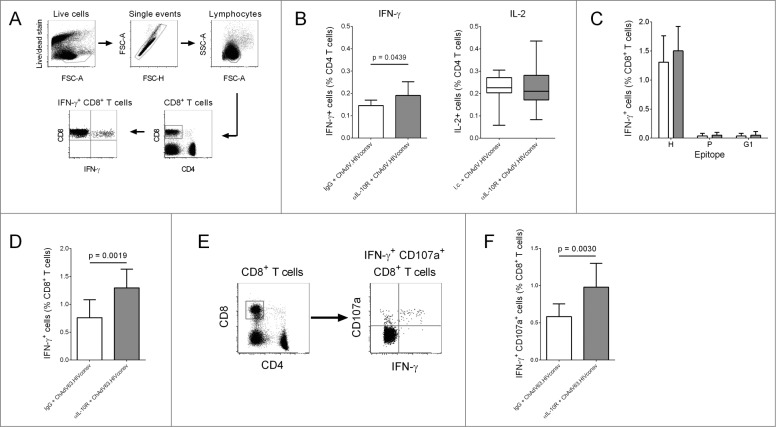
In a previous clinical trial using an adenovirus-vectored HIV vaccine, lack of efficacy was accompanied by relatively low-magnitude T cell responses in vaccinees.3 To assess whether IL-10R blockade could enhance vaccine immunogenicity, CD4+ and CD8+ T cell IFN-γ responses to ChAdV63.HIVconsv were compared in mice receiving αIL-10R or isotype control antibody 24 hours prior to immunization (Fig. S1). Responses were assessed at 14 and 21 d post-immunization, using overlapping peptides spanning the HIVconsv immunogen. ChAdV63.HIVconsv induced robust CD8+ T cell responses on day 14, but with no statistically significant difference between the 2 groups (Fig. S2). By contrast, CD4+ T cell IFN-γ responses to HIVconsv (defined by production of IFN-γ or IL-2) were lower but, by day 21, HIVconsv-specific IFN-γ production by CD4+ T cells was significantly enhanced in αIL-10R-treated mice (Fig. 1B; Fig. S2). At 21 d post-immunization, HIVconsv-specific CD8+ T cell responses (defined by production of IFN-γ) were dominated by a previously-described epitope, H (Fig. 1C). 16 Responses to H, and to 2 previously-defined subdominant epitopes, P and G1, were not enhanced by IL-10R blockade (Fig. 1C). However, the total magnitude CD8+ T response to HIVconsv was significantly increased in mice that had received αIL-10R (Fig. 1D), as was the frequency of HIVconsv-specific CD8+ T cells co-expressing IFN-γ and the degranulation marker CD107a (Fig. 1E, F). In all mice, CD8+ T cell IFN-γ responses to HIVconsv exceeded the mean background plus 2 standard deviations (Fig. 1D). These observations suggested that IL-10R blockade increased responses to previously-undefined epitopes in HIVconsv.
Figure 1.
IL-10R blockade enhanced CD8+ T cell responses to ChAdV63.HIVconsv in mice. (A) Gating strategy for the identification of IFN-γ+ CD8+ T cells. (B) IFN-γ and IL-2 production by HIVconsv-specific CD4+ T cells 21 d post-immunization. Mice were immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bars; n = 10) or isotype control antibody (white bars; n = 10). Statistical significance calculated using an unpaired t test (IFN-γ responses) or Mann-Whitney test (IL-2 responses). (C) Frequency of H-, P- and G1-specific IFN-γ+ CD8+ T cells 21 d post-immunization in mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bars; n = 10) or isotype control antibody (white bars; n = 10). (D) Total frequency of antigen-specific IFN-γ+ CD8+ T cells 21 d post-immunization in mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bar; n = 10) or isotype control antibody (white bar; n = 10). Data are representative of 2 independent experiments; statistical significance calculated using an unpaired t test. (E) Gating strategy for the identification of IFN-γ+ CD107a+ CD8+ T cells. (F) Total frequency of antigen-specific IFN-γ+ CD107a+ CD8+ T cells in mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bar; n = 10) or isotype control antibody (white bar; n = 10). Statistical significance calculated using an unpaired t test.
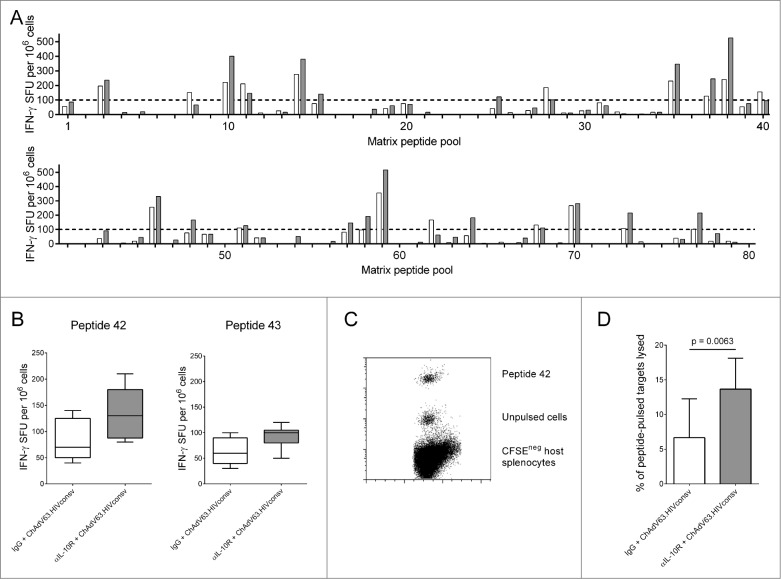
To identify new epitopes, we mapped responses to HIVconsv by ELISPOT using peptide pools spanning the entire immunogen (Methods and Fig. 2A). We identified 2 candidate overlapping peptides, 42 and 43, which were retested individually. Peptide 42, comprising Pol residues 126–140, contains a previously-defined mouse 14,17 and human CD8+ T cell epitope. IL-10R blockade enhanced IFN-γ production and in vivo cytotoxicity in response to peptide 42 at 21 days, although only the latter was statistically significant (Fig. 2B–D). Blockade did not enhance in vivo cytotoxicity in response to peptides H, P or G1 (Fig. S3).
Figure 2.
IL-10R blockade enhanced lysis of targets pulsed with a subdominant CTL epitope. (A) IFN-γ ELISPOT responses 21 d post immunization to 80 pools containing peptides spanning the HIVconsv immunogen in mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bars; n = 2) or isotype control antibody (white bars; n = 2). (B) IFN-γ ELISPOT responses to peptide 42 (left panel) and peptide 43 (right panel) in mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bars; n = 5) or isotype control antibody (white bars; n = 5). (C) Representative plot showing peptide 42-pulsed and unpulsed target cells isolated 16 hours after injection into a mouse immunized 21 d previously with ChAdV63.HIVconsv. (D) Proportion of peptide 42-pulsed targets killed in vivo in mice immunized 21 d previously with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bar; n = 10) or isotype control antibody (white bar; n = 10). Statistical significance calculated using an unpaired t test.
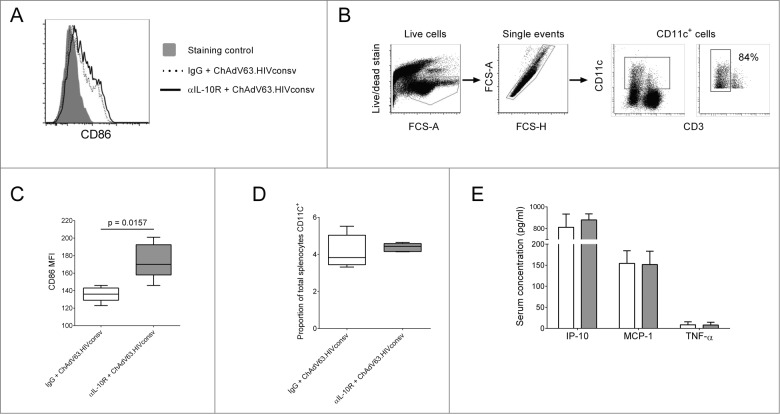
In view of the effects of IL-10R blockade on CD8+ T cell effector responses, we investigated innate responses 24 hours post-immunization (Fig. S1). Blockade significantly increased expression of CD86 on total CD11c+ cells after HIVconsv immunization (Fig. 3A, C). In a separate experiment, we confirmed that the majority of CD11c+ cells were CD3− (Fig. 3B), suggesting that the effect we observed was mediated by dendritic cells (DCs), not activated T cells. Since CD86 is a costimulatory molecule that plays a key role in the priming of naïve T cells,18 this could explain the increased frequency of HIVconsv-specific CD8+ T cells in αIL-10R-treated mice. There was no change in the frequency of splenocytes expressing CD11c, suggesting that inhibition of IL-10 signaling did not affect recruitment of DCs to the spleen (Fig. 3D). Ex vivo production of IL-12 was typically detected in <1% of CD11c+ cells and did not differ significantly between the 2 groups (data not shown). Furthermore, we did not observe a significant difference in the induction of IP-10, MCP-1 or TNF-α (Fig. 3E).
Figure 3.
The effect of IL-10R blockade on innate responses to ChAdV63.HIVconsv. (A) Representative histogram showing CD86 expression on CD11c+ cells 24 hours post-immunization in mice immunized with ChAdV63.HIVconsv in combination with either anti-IL-10R antibody (solid line) or an irrelevant isotype control antibody (dashed line). (B) Gating strategy for the identification of CD11c+ cells and representative plot showing the percentage of CD11c+ cells that are CD3- (far right plot). (C) CD86 expression on CD11c+ cells 24 hours post-immunization in mice immunized with ChAdV63.HIVconsv in combination with either anti-IL-10R antibody (gray bar; n = 5) or isotype control antibody (white bar; n = 5). Statistical significance was determined using the Mann Whitney test. (D) The proportion of splenocytes expressing CD11c 24 hours post-immunization in mice immunized with ChAdV63.HIVconsv after receiving anti-IL-10R (gray box; n = 5) or isotype control antibody (white box; n = 5). (E) Concentration of IP-10, MCP-1 and TNF-α in serum collected 24 hours post-immunization from mice immunized with ChAdV63.HIVconsv in combination with anti-IL-10R (gray bars; n = 10) or isotype control antibody (white bars; n = 10). Data are representative of 2 independent experiments.
Blockade of IL-10 signaling has been used to augment vaccine efficacy in mouse models of chronic LCMV and Leishmania,19,20 and to enhance the efficacy of BCG in mice challenged with Mycobacterium tuberculosis. 21 However, to our knowledge, this is the first demonstration that transient blockade of the IL-10R can boost responses to vaccination in healthy animals, and the first report of such an effect using an adenovirus vaccine. While statistically significant, we note that the effects on the immunogenicity of CD4+ and CD8+ T cells, and lytic capacity of CD8+ T cells, were modest. Immunization did not increase IL-10 levels in serum (Fig. S4), though this does not necessarily preclude its upregulation in the tissues. Notably, Zak et al observed a significant increase in IL-10 expression in the peripheral blood mononuclear cells of volunteers immunized with HAdV5 in a Phase 1B clinical trial,7 suggesting that IL-10R blockade might have a more substantial effect in humans. The use of an immunogen containing an immunodominant murine epitope (peptide H, restricted by murine H-2Dd and H-2Ld) may have obscured responses to other epitopes in HIVconsv that are relevant to control of HIV-1. Whether IL-10R blockade is able to increase responses to subdominant epitopes in the absence of this immunodominant epitope will be the subject of future studies. We did not assess the duration of antibody binding in vivo, but in a previous study of persistent LCMV infection, administration of αIL-10R antibody every 3 d significantly enhanced responses to a DNA vaccine, suggesting that the half-life may be 3 d or longer. 20 While we elected to transiently block IL-10 signaling due to the short-lived increase in IL-10 expression observed after adenoviral immunization in humans,7 repeated administrations of αIL-10R could further increase immunogenicity. Once an IL-10-blocking strategy has been optimized, it will be important to test it in a suitable challenge model, such as a humanized mouse or macaque model. Given our preliminary observations, we believe that this approach merits further investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by a grant from the John Fell OUP Research Fund. GC was funded by a Doctoral Training Award from the Nuffield Department of Medicine, University of Oxford.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Fauci AS, Folkers GK, Dieffenbach CW. HIV-AIDS: much accomplished, much to do. Nat Immunol 2013; 14:1104-7; PMID:24145780; http://dx.doi.org/ 10.1038/ni.2735 [DOI] [PubMed] [Google Scholar]
- 2. Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther 2004; 10:616-29; PMID:15451446; http://dx.doi.org/ 10.1016/j.ymthe.2004.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881-93; PMID:19012954; http://dx.doi.org/ 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis 2008; 46:1769-81; PMID:18433307; http://dx.doi.org/ 10.1086/587993 [DOI] [PubMed] [Google Scholar]
- 5. Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, Ebrahimsa U, Hayton EJ, Black A, Bridgeman A, Rosario M, et al. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther 2013; PMID:24166483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med 2012; 4:115ra2; PMID:22218691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, Krishnamurty AT, Chang JT, Adams DJ, Hensley TR, et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8(+) T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci U S A 2012; 109:E3503-12; PMID:23151505; http://dx.doi.org/ 10.1073/pnas.1208972109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991; 174:915-24; PMID:1655948; http://dx.doi.org/ 10.1084/jem.174.4.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitra RS, Judge TA, Nestle FO, Turka LA, Nickoloff BJ. Psoriatic skin-derived dendritic cell function is inhibited by exogenous IL-10. Differential modulation of B7-1 (CD80) and B7-2 (CD86) expression. J Immunol 1995; 154:2668-77; PMID:7533180 [PubMed] [Google Scholar]
- 10. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991; 174:1209-20; PMID:1940799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol 1999; 163:1537-44; PMID:10415057 [PubMed] [Google Scholar]
- 12. Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009; 114:346-56; PMID:19365081; http://dx.doi.org/ 10.1182/blood-2008-12-191296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, Brennan T, Ahmed R, Freeman GJ, Kavanagh DG, et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol 2014; 88:2508-18; PMID:24352453; http://dx.doi.org/ 10.1128/JVI.02034-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS ONE 2007; 2:e984; PMID:17912361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roshorm Y, Cottingham MG, Potash MJ, Volsky DJ, Hanke T. T cells induced by recombinant chimpanzee adenovirus alone and in prime-boost regimens decrease chimeric EcoHIV/NDK challenge virus load. Eur J Immunol 2012; 42:3243-55; PMID:22930183; http://dx.doi.org/ 10.1002/eji.201242624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Im EJ, Hanke T. Short communication: preclinical evaluation of candidate HIV type 1 vaccines in inbred strains and an outbred stock of mice. AIDS Res Hum Retroviruses 2007; 23:857-62; PMID:17678467; http://dx.doi.org/ 10.1089/aid.2007.0009 [DOI] [PubMed] [Google Scholar]
- 17. Hill BJ, Darrah PA, Ende Z, Ambrozak DR, Quinn KM, Darko S, Gostick E, Wooldridge L, van den Berg HA, Venturi V, et al. Epitope specificity delimits the functional capabilities of vaccine-induced CD8 T cell populations. J Immunol 2014. 193:5626-36; PMID:25348625; http://dx.doi.org/ 10.4049/jimmunol.1401017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salek-Ardakani S, Arens R, Flynn R, Sette A, Schoenberger SP, Croft M. Preferential use of B7.2 and not B7.1 in priming of vaccinia virus-specific CD8 T cells. J Immunol 2009; 182:2909-18; PMID:19234186; http://dx.doi.org/ 10.4049/jimmunol.0803545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun 2002; 70:6284-93; PMID:12379707; http://dx.doi.org/ 10.1128/IAI.70.11.6284-6293.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med 2008; 205:533-41; PMID:18332180; http://dx.doi.org/ 10.1084/jem.20071948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitt JM, Stavropoulos E, Redford PS, Beebe AM, Bancroft GJ, Young DB, O'Garra A. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J Immunol 2012; 189:4079-87; PMID:22972927; http://dx.doi.org/ 10.4049/jimmunol.1201061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.