Summary
The bleeding patterns of severe von Willebrand’s disease (VWD) adversely affect quality of life, and may be life threatening. There is a presumed role for prophylaxis with VWF-containing concentrates, but data are scarce. The von Willebrand Disease Prophylaxis Network (VWD PN) was formed to investigate the role of prophylaxis in clinically severe VWD that is not responsive to other treatment(s). Using a retrospective design, the effect of prophylaxis was studied. Availability of records to document, or reliably assess, the type and frequency of bleeding episodes prior to, and after, the initiation of prophylaxis was required. Annualized bleeding rates were calculated for the period prior to prophylaxis, during prophylaxis and by primary bleeding indication defined as the site accounting for more than half of all bleeding symptoms. The Wilcoxon signed-rank test of differences in the medians was used. Sixty-one subjects from 20 centres in 10 countries were enrolled. Data for 59 were used in the analysis. The median age at onset of prophylaxis was 22.4 years. Type 3 VWD accounted for the largest number (N = 34, 57.6%). Differences in bleeding rates within individuals during compared with before prophylaxis were significant for the total group (P < 0.0001), and for those with primary bleeding indications of epistaxis (P = 0.0005), joint bleeding (P = 0.002) and GI bleeding (P = 0.001). The effect of prophylaxis was similar among those age < 18 years and those ≥18. One person developed an inhibitor during treatment. We conclude that prophylactic treatment of VWD is efficacious.
Keywords: bleeding rate, epistaxis, gastrointestinal bleeding, joint bleeding, prophylaxis, severe VWD
Introduction
von Willebrand’s disease (VWD) is the most common bleeding disorder [1], and is caused by quantitative (types 1 and 3) or qualitative (types 2A, 2B, 2M, 2N) defects of von Willebrand factor (VWF) [2]. Type 1 is the most prevalent form, affecting approximately 55–70% of those with symptomatic disease [3]. Type 3, the most severe form of VWD, is rare, estimated to affect from 0.1 to 5.3 per million of the population [4,5]. The bleeding patterns of severe VWD adversely affect short- and long-term quality of life [6,7], and may be life threatening. The index case of VWD, described by Erik von Willebrand in 1926, was a girl who had a history of serious bleeds involving mucous membranes and ankle joints [8]. She subsequently died during her fourth menstrual period.
Clinically, the leading symptom in VWD is bleeding, chiefly of mucosal origin, e.g. epistaxis, gingival or GI bleeding and heavy menstrual bleeding. In the most serious forms of VWD, characterized by reduced levels of VWF activity measured as ristocetin cofactor (VWF:RCo <10 U dL−1) and of FVIII:C (<20 U dL−1), joint and muscle bleeding resembling that seen in mild or moderate haemophilia A may also be observed. Strategies for treatment vary by type and severity, and include DDAVP (desmopressin acetate), use of antifibrinolytics and therapy with VWF-containing concentrates to replace the VWF protein that is missing and/or abnormal [9].
It is logical to translate the success of prophylaxis in haemophilia to severe VWD. Prophylaxis can be implemented early in life in a home setting, and prevention of bleeding and its consequences is possible [10,11]. The documented experience with long-term prophylaxis in VWD, however, is limited. In a Swedish multicentre study of subjects with VWF:RCo <8% and FVIII:C <10%, 37 were on long-term prophylaxis and 13 were treated on demand [12]. The study showed that those beginning prophylaxis at a young age (less than 5 years) had few or no bleeding episodes, and none had clinical signs of arthropathy or reported joint bleeding. Subjects beginning prophylaxis at >15 years of age usually reported a substantial reduction in joint bleeding, but had clinical and radiological signs of joint disease. Prophylaxis led to reductions in other types of bleeding, including epistaxis. The investigators concluded that long-term prophylactic treatment in VWD is warranted in the majority of cases with type 3, and in some cases, depending on the clinical phenotype, for those with other types of VWD. Similar conclusions were reached in another retrospective study performed in a small cohort of Italian patients [13]. Halimeh and colleagues have also reported on the use of secondary prophylaxis, finding a significant decrease in bleeding frequency [14].
The von Willebrand Disease Prophylaxis Network (VWD PN) was formed to investigate the role of prophylaxis in clinically severe VWD requiring use of VWF-containing concentrates due to lack of response to DDAVP or other treatments. In a network-sponsored survey of 74 treatment centres conducted in 2005–2006, investigators reported that approximately 70% of their patients with type 3 VWD had been treated with VWF-containing plasma-derived products in the previous 12 months, and 22% were on prophylaxis. Use of prophylaxis for patients with type 1 and type 2 VWD was rare; the most commonly cited reasons for initiating prophylaxis were joint bleeding (40%), epistaxis/oral bleeding (23%), gastrointestinal (GI) bleeding (14%) and menorrhagia (5%) [15].
The VWD International Prophylaxis (VIP) study, which contains both retrospective and prospective study components, is an initiative of the VWD PN. The current report highlights results from a retrospective study of the effect of prophylaxis on bleeding frequency.
Materials and methods
Population
To be eligible, subjects must have been on a prophylactic regimen for VWD that was initiated at least 6 months prior to enrolment, or have a history of prophylaxis use for a period of at least 6 months that was subsequently discontinued because it was no longer required. Availability of records to document, or reliably assess, the type and frequency of bleeding episodes prior to, and after, the initiation of prophylaxis was required. Subjects were excluded if, in the judgment of the investigator, the subject had a history of non-compliance with his or her treatment regimen. Data were collected between 2008 and 2011. The human-subjects committees of collaborating institutions approved the VIP study in compliance with the guidelines of the Declaration of Helsinki. The VIP study is registered at www.ClinicalTrials.gov.
Clinical data collection
Patients were diagnosed locally at their centres. Variables collected included subject demographics, VWD type, site and frequency of bleeding episodes prior to, and after, the initiation of prophylaxis, and whether an inhibitor to VWF had ever been detected. Bleeding history was derived from centre records or registries, diaries and logs. Records were available for every bleeding episode during the period of study for nine (15%) participants. For all others, the investigator made an assessment of available documentation to determine the average number of bleeding episodes that occurred each month, and the distribution of the sites of bleeding. The primary indication for prophylaxis was defined as the bleeding symptom accounting for one half or more of a subject’s bleeding episodes. For four subjects the percentages were unknown, so a primary indication could not be identified. Other variables collected included the usual dose (U VWF:RCo per kg) per infusion during prophylaxis, and number of infusions administered per week or per menstrual cycle.
Statistical methods
Annualized bleeding rates were calculated for the periods prior to prophylaxis and during prophylaxis. For those participants with complete bleeding records, this was done by dividing the number of bleeding episodes by the duration of the period(s) of interest (prior and during) in years. For those whose records did not capture every bleed, either prior to or during prophylaxis, the reported number of bleeding episodes per month was multiplied by 12. Annualized bleeding rates were calculated for the primary indication by multiplying the total annual number of bleeds by the proportion that occurred at the primary indication site. Medians and interquartile ranges (IQR) are used to describe bleeding rates. In addition, a ‘paired’ approach was used to calculate the percent change in number of bleeding episodes within individuals by subtracting the number of bleeds that occurred before prophylaxis from the number of bleeds after prophylaxis, then dividing by the number of bleeds that occurred before prophylaxis. A paired Wilcoxon signed-rank test of the differences in the medians was used to compare the bleed rate overall and by primary indication.
Results
Sixty-one subjects from 20 treatment centres in 10 countries located in Europe (67%) and North America (33%) were enrolled. One patient was excluded because there were no records to reliably evaluate the type and frequency of bleeding episodes prior to the onset of prophylaxis. Among those with type 3 VWD, one patient had a history of an inhibitor diagnosed during childhood, a number of years prior to the onset of prophylaxis, and had been on prophylaxis for a period of just over 1 year. Testing conducted 2 months prior to enrolment in the current study showed an inhibitor concentration of 1 Bethesda Unit (BU). This patient was excluded from the analysis. A second subject was diagnosed with an inhibitor during prophylaxis and the regimen was subsequently discontinued. Data for this subject were used for the period prior to the detection of the inhibitor. Thus, the current analysis was completed with data for 59 subjects.
The median age (range) of subjects at start of prophylaxis was 22.4 (2.3–77.2). Age at start varied considerably by the indication for prophylaxis. For example, for those whose bleeding was primarily epistaxis, the median age at start was 6.9 years, whereas for those with GI bleeding it was 55.8. The median period of time on prophylaxis was 2.2 years. Duration was somewhat longer, but not significantly so, among subjects from centres in Europe, median of 3.4 years, compared with centres in North America, median of 2.1 years. Other demographic and VWD-related characteristics of the study group are shown in Table 1 Male and female subjects were represented almost equally. The vast majority of subjects were of European descent, with smaller proportions of participants of Hispanic, Asian, African descent and other races. Type 3 VWD accounted for the largest number: 34 (57.6%).
Table 1.
Demographic and von Willebrand’s disease (VWD)-related characteristics (N = 59).
| N (%) | |
|---|---|
| Gender | |
| Female | 28 (47.5) |
| Male | 31 (52.5) |
| Race/ethnicity | |
| White | 43 (72.9) |
| Hispanic | 9 (15.3) |
| Asian | 5 (8.5) |
| African descent | 1 (1.7) |
| Other | 1 (1.7) |
| VWD type | |
| 1 | 5 (8.5) |
| 2A | 10 (17.0) |
| 2B | 8 (13.6) |
| 2M | 2 (3.4) |
| 2N | 0 (0.0) |
| 3 | 34 (57.6) |
Table 2 summarizes, by bleeding indication, characteristics for the study group including frequency of bleeding before and during prophylaxis; usual dose in U VWF:RCo/kg and the median number of infusions during prophylaxis. Overall, the median (IQR) rate of bleeding episodes in the year prior to prophylaxis was 12 (6–24), compared with a median (IQR) rate of 3.6 (0.96–9.4) during prophylaxis. In the case of occurrences of heavy menstrual bleeding, the changes represent a reduction in the number of days or intensity of bleeding with each cycle. In the year prior to prophylaxis, the median number of cycles in which heavy menstrual bleeding was reported was 12, compared with four per year during prophylaxis.
Table 2.
Frequency distribution of primary indication for prophylaxis, characteristics of treatment during prophylaxis and outcome (N = 55).
| Bleeding site | N (%) | Usual dose U VWF: RCo/kg Median (IQR) |
Number of infusions/week Median (IQR) |
Annualized number of episodes prior to prophylaxis Median (IQR) |
Annualized number of episodes during prophylaxis Median (IQR) |
|---|---|---|---|---|---|
| Epistaxis | 13 (23.6) | 48 (40–60) | 3 (2–3) | 24 (12–48) | 6 (2.9–12) |
| GI bleeding | 13 (23.6) | 60 (47–60) | 3 (2–3) | 8.4 (6–12) | 6 (3–6) |
| Joint Bleeding | 12 (21.8) | 40 (30–50) | 2 (2–3) | 15.6 (5–21.5) | 1.3 (0.3–3.2) |
| Other* | 8 (14.6) | 59 (17–70) | 2.5 (1.5–3) | 3 (1–10.5) | 0.5 (0.2–2.8) |
| Mixed† | 5 (9.1) | 42 (33–49) | 2.5 (2–3) | 12 (6–18) | 6 (1.2–12) |
| Abnormally heavy bleeding at menstruation | 4 (7.3) | 39 (38–40) | 3 (2.5–3)‡ | 12 (12–12) | 4 (1–9) |
Other: the primary indication occurred with low frequency. This category includes intracranial haemorrhage, haematomas in soft tissue, oral, dental extraction, scraped knees, ovarian cysts or bleeding.
Mixed: bleeding pattern was mixed with no primary indication.
Number of infusions per menstrual cycle.
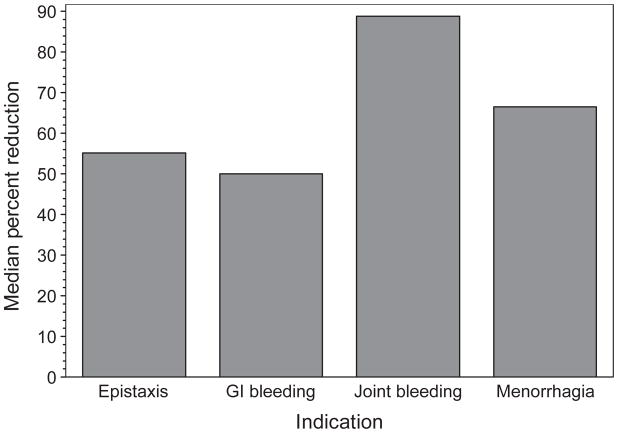
While Table 2 presents the median numbers of bleeding episodes before and after prophylaxis for the group overall, perhaps more meaningful are the percent reductions within individuals that occurred during the period of evaluation (Fig. 1). Differences in annualized bleeding rates within individuals (during prophylaxis – before prophylaxis) were significant for the total group (P < 0.0001), and for those with primary indications of epistaxis (P = 0.0005), joint bleeding (P = 0.002) and GI bleeding (P = 0.001), and of borderline significance (P = 0.055), for those in the category of ‘other’ indications. The within-individual difference in the group whose primary indication for treatment was abnormally heavy bleeding at menstruation (n = 4) was not significant (P = 0.25).
Fig. 1.
Outcomes measured as percent reduction in bleeding within individuals during prophylaxis, according to primary indication for treatment.
When we examined the effect of prophylaxis by age for subjects <18 (n = 26), and those ≥18 (n = 33), we found that it was similar in both groups. The median within-individual number of bleeds per year after prophylaxis compared with before was significantly lower, P < 0.0001 in both groups. A primary indication of joint bleeding occurred somewhat more frequently among those <18; however, GI bleeding and menorrhagia were not reported as the primary bleeding indication for prophylaxis for any subjects in that age group. Epistaxis was almost twice as likely to be the primary indication for prophylaxis among those <18 compared with those aged ≥18 years (32.0% vs. 16.7%).
While the specifics of individual bleeding episodes were not available for all bleeds in the year prior to and following onset of prophylaxis, a total of 604 bleeds were reported. Of these, 529 (87.6%) were treated with a VWF-containing concentrate. The most commonly used products were Humate P, 77.1% (CSL Behring GmbH); Fandhi, 16.5% (Grifols); and Alphanate, 4.5% (Grifols).
A review of reasons for inpatient and outpatient hospitalizations, and supplemental comments on study data collection forms revealed no reports of thrombotic events among those in the study group.
Discussion and conclusions
The data presented provide support for the use of long-term prophylaxis with VWF-containing concentrates in cases of VWD that are not responsive to other treatments. The benefits of prophylaxis in haemophilia have been demonstrated repeatedly. Prevention of bleeding and arthropathy [10,11], better quality of life [16,17], fewer school absences and higher academic achievement in young school-age children have been documented [18]. Importantly, children with VWD in a Swedish cohort who started prophylaxis early never developed joint disease [12].
A few additional investigations have been reported using different VWF-containing concentrates [19,20], [21,22]. Common among these was the finding that prophylaxis appears to be effective at decreasing or eliminating bleeding, and that side effects are mild. No cases of thromboembolism have been reported. In the Swedish cohort, one patient developed an inhibitor. In a recent publication from Germany, a retrospective study of 32 patients was reported. Following a 12-month period, the monthly bleeding frequency was significantly reduced compared with the preprophylaxis values (3 vs. 0.07), and an inhibitor developed in one patient.
Allo-antibodies against VWF are a rare complication of treatment with plasma-derived concentrates containing VWF [23]. They usually occur in type 3 VWD characterized by large deletions of the VWF gene; however, there are currently no data regarding the clinical and molecular markers of these complications. In particular, there is no evidence that prophylaxis with VWF concentrates triggers their appearance as in almost all cases reported, the antibodies developed during on-demand treatment.
In this study, the effect of prophylaxis appeared to be most pronounced in the case of joint bleeding, as has been observed in other investigations [12]. Joint haemorrhage occurs when FVIII levels are low. Haemarthroses are prevented primarily by the increase in FVIII levels during prophylaxis and not impacted by the VWF levels, per se. Mucosal bleeding, i.e. epistaxis, GI bleeding and menorrhagia, was reduced but not to the same degree, perhaps because these haemorrhages are not only dependent on normal circulating levels of active VWF, but also on the presence of discrete concentrations of normal VWF inside the platelets and within endothelial matrices. These considerations of the biology and physiopathology of VWF should be kept in mind when therapeutic approaches are chosen to stop or prevent mucosal bleeding, especially in patients with VWD types 3 or 2A, which are characterized by absent or abnormal VWF in platelet and endothelial sites.
In ex vivo experiments, the lack of normal platelet VWF was reported to be the major factor for impaired platelet adhesion to subendothelium in patients with VWD types 3 and 2A [24]. More importantly, when patients with VWD type 3 were given large doses of cryoprecipitate containing all the VWF multimers, all could correct VWF:RCo whereas 60% still showed prolonged bleeding time (BT), the surrogate marker of the cellular defect of VWF at the vascular sites. A complete correction of BT could be obtained only when normal platelet concentrates were given after cryoprecipitate [25].
The fact that BT cannot always be normalized by exogenous VWF, despite the complete correction of circulating VWF:RCo and/or the intact multimeric structure of VWF in the concentrates, was clearly confirmed in a crossover randomized trial in type 3 VWD patients using four concentrates with different VWF and FVIII content [26]. Our results of a more modest resolution of mucosal bleeding during prophylaxis with VWF-containing concentrates, therefore, may not be surprising. Further investigation of response to prophylaxis by bleeding indication and VWD type requires a larger sample size, and is a planned topic of future exploration for the VIP study.
Epistaxis is frequent in haemostatically normal subjects, especially children, indicating that mechanisms in addition to the haemostatic system are involved. Gastrointestinal bleeding is known to be severe and difficult to treat in VWD, especially among patients with type 2 disease [12]. The results in this study corroborate the findings of the few other published reports in the field, with higher doses used for that indication. Frequency of infusions and dosages reported were quite similar in our study compared to other studies. Determination of dose for prophylactic treatment of VWD has relied on prophylaxis in haemophilia as a template. Whether or not this is optimal has not been investigated. In the prospective component of the VIP study, participants undergo an escalation of treatment from one to three doses of VWF concentrate per week [15] with the objective of establishing optimal treatment regimens for joint bleeding, GI bleeding, epistaxis and menorrhagia.
No occurrence of thromboembolism was reported as a reason for inpatient or outpatient hospitalization. In fact, thromboembolism is rare in VWD [27,28] and the higher levels of FVIII/VWF obtained during prophylaxis are of short duration, probably explaining why these patients do not appear to be at high risk even though FVIII and VWF are both considered to be risk factors for venous thromboembolism.
Previous studies of prophylaxis in VWD have been limited to examination of data from a small number of haemophilia treatment centres. A major strength of this investigation is that it is multicentre with standardized data collection conducted in 20 centres across 10 countries in Europe and North America. The number of patients with type 3 VWD (n = 34) was substantially higher than in other reports of prophylaxis in VWD, and the number of patients with type 2 VWD (n = 20) was greater as well. Results were generally consistent across bleeding indications and even age groups. The weakness of many retrospective studies, including this investigation, is that data collection depends on the ability to reliably assess or document, from varying sources, the occurrence of bleeding. As a requirement for participation in the VIP study, subjects must have been on prophylaxis for at least 6 months, and have demonstrated compliance with their regimen. While this may bias the study group by removing from observation those for whom prophylaxis was abandoned because of lack of success or acceptability, it nonetheless encourages continued evaluation, both retrospective and prospective.
We conclude from this international, multicentre cohort study that prophylactic treatment of VWD is efficacious. This appears to be most evident in FVIII-dependent haemorrhages. A network-initiated prospective study is underway to confirm these findings, and address issues of cost-effectiveness and quality of life.
Acknowledgments
The von Willebrand Disease Prophylaxis Network is funded through an investigator-initiated grant from CSL Behring. We are grateful to the participants who volunteered to participate in this study. The following are the members of the Steering Committee of the von Willebrand Disease Prophylaxis Study (VWD PN) or contributors to the initiatives of the network: E. Berntorp, Malmö, Sweden (principal investigator) and T. Abshire, Milwaukee, Wisconsin, US (principal investigator); M. Alvárez, Madrid, Spain; J. Astermark, Malmö, Sweden; J. Blatny, Brno, Czech Republic; P. Bolton-Maggs, Manchester, UK; L. Bowles, London, UK; M. Carcao, Toronto, Ontario, Canada; S. Crary, Dallas, Texas, US; A. B. Federici, Milan, Italy; A. Geddis, San Diego, California, US; P. Giardina, New York, New York, US; J. Cox Gill, Milwaukee, Wisconsin, US; K. Kavakli, İzmir, Turkey; C. Kempton, Atlanta, Georgia, US; B. Kerlin, Columbus, Ohio, US; N. Key, Chapel Hill, North Carolina, US; R. Klamroth, Berlin, Germany; E. Kraut, Columbus, Ohio, US; P. Kouides, Rochester, New York, US; K. Kurnik, Munich, Germany; A. Landorph, Copenhagen, Denmark; F. Leebeek, Rotterdam, The Netherlands; S. Lethagen, Copenhagen, Denmark; M. Makris, Sheffield, UK; P. M. Mannucci, Milan, Italy; P. Mathew, Albequerque, New Mexico, US; D. Nugent, Orange County, California, US; S. Pavord, Leicester, UK; A. Shapiro, Indianapolis, Indiana, US; J. Wilde, Birmingham, UK; L. Valentino, Chicago, Illinois, US; R. Winikoff, Montreal, Quebec, Canada; T. Yee, London, UK.
Footnotes
Author Contributions
E. Berntorp designed research, performed research, interpreted data, wrote the manuscript, gave final approval of the version to be published; T. Abshire designed research, performed research, interpreted data, wrote the manuscript, gave final approval of the version to be published; A. Federici designed research, performed research, interpreted data, wrote the manuscript, gave final approval of the version to be published; M. Alvárez performed research; J. Bowen collected data, analysed data, wrote the manuscript; M. Carcao designed research, performed research, gave critical review of the content; J. Gill designed research, performed research; N. Key performed research, gave critical review of the content; P. Kouides designed research, performed research; K. Kurnik designed research, gave critical review of the content; A. Lail analysed the data, wrote the manuscript; F. Leebeek designed research, performed research, gave critical review of the content; M. Makris designed research, performed research, gave critical review of the content; P. Mannucci designed research, performed research, gave critical review of the content; R. Winikoff designed research, performed research.
Disclosures
T. C. Abshire has served on the advisory board of CSL Behring, is a reviewer for the CSL Behring Heimburger award. A. B. Federici has served on medical advisory boards and data monitoring committees, and received honoraria for attending educational events from Baxter, CSL Behring, Grifols, Kedrion, LFB and Octapharma. J. Bowen has received funding from CSL Behring for research carried out on this study. J. Cox Gill has served as a consultant to CSL Behring, Baxter and Octapharma. N. S. Key has served as a consultant to Baxter, Inspiration, and Novo Nordisk. P. A. Kouides has served on the advisory board for CSL Behring. K. Kurnik has received research grants, and reimbursement for attending meetings and lecturing from Baxter, Bayer, Biotest, CSL Behring and Novo Nordisk. A. E. Lail has received funding from CSL Behring for research carried out on this study. F. W. G. Leebeek has served on advisory boards and received research funding from Baxter and CSL Behring. M. Makris has served as a consultant and received honoraria for lecturing from CSL Behring. P. M. Mannucci receives speaker fees from Grifols and serves on the scientific board of Baxter. R. Winikoff has received reimbursement from CSL Behring for attending symposiums. E. Berntorp has received research grants from CSL Behring.
References
- 1.Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood. 1987;69:454–9. [PubMed] [Google Scholar]
- 2.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–14. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 3.Federici AB, Mannucci PM. Management of inherited von Willebrand disease in 2007. Ann Med. 2007;39:346–58. doi: 10.1080/07853890701513738. [DOI] [PubMed] [Google Scholar]
- 4.Baronciani L, Federici AB, Eikenboom JCJ. Clinical, laboratory, and molecular markers of type 3 von Willebrand disease. In: Federici CALAB, Berntorp EE, Lillicrap D, Montgomery RR, editors. Von Willebrand Disease: Basic and Clinical Aspects. Oxford, UK: Wiley-Blackwell; 2011. pp. 207–13. [Google Scholar]
- 5.Rodeghiero F, Castaman G. Textbook of Hemophilia. Oxford, UK: Blackwell Publishing; 2005. Von Willebrand disease: epidemiology; pp. 265–71. [Google Scholar]
- 6.De Wee EM, Mauser Bunschoten EP, vander Bom JG, et al. Health related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8:1492–9. doi: 10.1111/j.1538-7836.2010.03864.x. [DOI] [PubMed] [Google Scholar]
- 7.De Wee EM, Fijnvandraat K, de Goede Bolder A, et al. Impact of von Willebrand disease on health related quality of life in a pediatric population. J Thromb Haemost. 2011;9:502–9. doi: 10.1111/j.1538-7836.2010.04175.x. [DOI] [PubMed] [Google Scholar]
- 8.Von Willebrand EA. Hereditär pseudo hemofili. Fin Läkaresällsk Handl. 1926;68:87–112. [Google Scholar]
- 9.Nichols W, Hutlin M, James A, Manco-Johnson M, Montgomery R, Ortel T. NIH Publication No. 08–5832. US Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute; 2007. The Diagnosis, Evaluation, and Management of von Willebrand Disease; pp. 1–112. [Google Scholar]
- 10.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 12.Berntorp E, Petrini P. Long-term prophylaxis in von Willebrand disease. Blood Coagul Fibrinolysis. 2005;16:S23. doi: 10.1097/01.mbc.0000167659.23262.18. [DOI] [PubMed] [Google Scholar]
- 13.Federici AB. Highly purified VWF/FVIII concentrates in the treatment and prophylaxis of von Willebrand disease: the PRO. WILL Study. Haemophilia. 2007;13:15–24. doi: 10.1111/j.1365-2516.2007.01573.x. [DOI] [PubMed] [Google Scholar]
- 14.Halimeh S, Krümpel A, Rott H, et al. Longterm secondary prophylaxis in children, adolescents and young adults with von Willebrand disease. Thromb Haemost. 2011;104:984–9. doi: 10.1160/TH10-09-0616. [DOI] [PubMed] [Google Scholar]
- 15.Berntorp E, Abshire T. The von Willebrand disease prophylaxis network: exploring a treatment concept. J Thromb Haemost. 2006;4:2511–2. doi: 10.1111/j.1538-7836.2006.02179.x. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Bom J, Mauser Bunschoten E, Roosendaal G, Berg H. Effects of haemophilic arthropathy on health related quality of life and socio economic parameters. Haemophilia. 2005;11:43–8. doi: 10.1111/j.1365-2516.2005.01065.x. [DOI] [PubMed] [Google Scholar]
- 17.Donfield S, Shapiro A, Gomperts E, Lynn H. Haemophilia related morbidity and quality of life. Haemophilia. 2005;11:418. doi: 10.1111/j.1365-2516.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro AD, Donfield SM, Lynn HS, et al. Defining the impact of hemophilia: the Academic Achievement in Children with Hemophilia Study. Pediatrics. 2001;108:e105. doi: 10.1542/peds.108.6.e105. [DOI] [PubMed] [Google Scholar]
- 19.Dunkley S, Baker RI, Pidcock M, et al. Clinical efficacy and safety of the factor VIII/von Willebrand factor concentrate BIOSTATE® in patients with von Willebrand’s disease: a prospective multi centre study. Haemophilia. 2010;16:615–24. doi: 10.1111/j.1365-2516.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- 20.Federici AB, Mannucci PM, Marco P. Von Willebrand factor in high purity factor VIII complex concentrates: chaperone protein or key to therapies? A meeting report. Haemophilia. 2008;14:133–9. doi: 10.1111/j.1365-2516.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 21.Berntorp E, Windyga J. Treatment and prevention of acute bleedings in von Willebrand disease–efficacy and safety of Wilate®, a new generation von Willebrand factor/factor VIII concentrate. Haemophilia. 2009;15:122–30. doi: 10.1111/j.1365-2516.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 22.Borel Derlon A, Federici AB, Roussel-Robert V, et al. Treatment of severe von Willebrand disease with a high purity von Willebrand factor concentrate (Wilfactin®): a prospective study of 50 patients. J Thromb Haemost. 2007;5:1115–24. doi: 10.1111/j.1538-7836.2007.02562.x. [DOI] [PubMed] [Google Scholar]
- 23.Federici AB. Clinical and molecular markers of inherited von Willebrand disease type 3: are deletions of the VWF gene associated with alloantibodies to VWF? J Thromb Haemost. 2008;6:1726–8. doi: 10.1111/j.1538-7836.2008.03146.x. [DOI] [PubMed] [Google Scholar]
- 24.Castillo R, Escolar G, Monteagudo J, et al. Role for platelet von willebrand factor in supporting platelet vessel wall interactions in von willebrand disease. Am J Hematol. 1989;31:153–8. doi: 10.1002/ajh.2830310303. [DOI] [PubMed] [Google Scholar]
- 25.Castillo R, Monteagudo J, Escolar G, Ordinas A, Magallon M, Martin Villar J. Hemostatic effect of normal platelet transfusion in severe von Willebrand disease patients. Blood. 1991;77:1901. [PubMed] [Google Scholar]
- 26.Mannucci PM, Tenconi PM, Castaman G, Rodeghiero F. Comparison of four virus-inactivated plasma concentrates for treatment of severe von Willebrand disease: a cross-over randomized trial. Blood. 1992;79:3130. [PubMed] [Google Scholar]
- 27.Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost. 2002;88:378–9. [PubMed] [Google Scholar]
- 28.Windyga J, von Depka-Prondzinski M. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (Wilate®) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost. 2011;105:1072–9. doi: 10.1160/TH10-10-0631. [DOI] [PubMed] [Google Scholar]