Abstract
Mesenchymal stem cells (MSCs), adherent fibroblastoid cells, present in bone marrow and many other tissues can be easily isolated and expanded in vitro. They are capable of differentiating into different cell types such as osteoblasts, chondrocytes, adipocytes, cardiomyocytes, hepatocytes, endothelial cells and neuronal cells. Such immense plasticity coupled with their ability to modulate the activity of immune cells makes them attractive for stem cell-based therapy aimed at treating previously incurable disorders. Preclinical studies have reported successful use of MSCs for delivering therapeutic proteins and repairing defects in a variety of disease models. These studies highlighted the in vivo potential of MSCs and their ability to home to injury sites and modify the microenvironment by secreting paracrine factors to augment tissue repair. Their therapeutic applicability has been widened by genetic modification to enhance differentiation and tissue targeting, and use in tissue engineering. Clinical trials for diseases such as osteogenesis imperfecta, graft-versus-host disease and myocardial infarction have shown some promise, demonstrating the safe use of both allogeneic and autologous cells. However, lack of knowledge of MSC behaviour and responses in vitro and in vivo force the need for basic and animal studies before heading to the clinic. Contrasting reports on immunomodulatory functions and tumorigenicity along with issues such as mode of cell delivery, lack of specific marker, low survival and engraftment require urgent attention to harness the potential of MSC-based therapy in the near future.
Keywords: mesenchymal stem cells, stem cell therapy, genetic modification, protein therapy, tissue engineering
Introduction
Mesenchymal stem cells and its characteristics
- Experimental/preclinical MSC-based studies
- MSC transplantation
- Genetically modified MSC-based therapy
- MSC-based protein therapy
- Tissue engineering using MSCs
Clinical studies
Challenges and future prospects
Introduction
Bone marrow harbours cells of haematopoietic and non-haematopoietic lineages and their precursors, known as stem/progenitor cells. The non-haematopoietic stem/progenitor cell compartment contains mesenchymal stem cells (MSCs), which are involved in remodelling of the mesenchymal tissues throughout adult life. These multi-potent cells are easily isolated from bone marrow and are capable of expansion and differentiation into mesodermal lineage cells including osteoblasts, chondrocytes and adipocytes, under appropriate conditions, in culture [1, 2]. This led to the evaluation of their potential for treating diseases and the birth of MSC-based therapy.
Recent clinical trials with MSCs for treating debilitating disorders like osteogenesis imperfecta, myocardial infarction (MI), stroke and graft-versus-host disease (GVHD) have shown some promise [3–6]. Numerous preclinical studies have established the therapeutic potential of MSCs in tissue engineering and as cellular protein factory for delivery of cytokines and anticancer agents [7–9]. Genetically modified MSCs have also been successfully evaluated in animal models for diabetes, skeletal defects and MI [10–14]. Co-transplantation of MSCs with haematopoietic stem cells (HSCs) has been documented to improve HSC engraftment in mice [15, 16]. Although there has been a surge in preclinical and clinical trials using MSCs, caution must be taken in planning such studies because MSC biology is only beginning to be understood. However, the question arises: what makes MSCs unique and preferable for cell-based therapies?
In this review, we focus on the suitability of MSCs in the field of regenerative medicine. We provide an overview of the current status of research on MSC-based therapies in experimental animals and human beings. Different therapeutic designs along with preclinical cases that also address the mechanisms of MSC action are discussed. Clinical trials with MSCs are critically evaluated, followed by a discussion on the controversies surrounding the use of MSCs and the challenges that need to be overcome for translation of the therapy from the bench to the clinic.
Mesenchymal stem cells and its characteristics
MSCs were first identified about 30 years ago by Friedenstein and colleagues as an adherent fibroblast-like population in the bone marrow capable of differentiating into bone [17]. Since then MSCs have been isolated from human bone marrow based on their ability to adhere to tissue culture plastic [1]. Although occurring at a very low frequency of 1 in 10,000 to 100,000 bone marrow mononuclear cells, these cells are capable of proliferating in vitro without significant loss of differentiation potential during early passages [1, 2, 18].
Originally isolated from the bone marrow, similar populations have also been isolated from peripheral blood [19], periosteum [20], umbilical cord blood [21], synovial membrane [22], trabecular bone [23], adipose tissue [24], limbal stroma [25], amniotic fluid [26], lung [27], dermis and muscle [28]. These populations have been functionally characterized on the basis of their ability to differentiate into osteoblasts, chondrocytes and adipocytes in culture upon induction due to lack of specific markers for MSCs [29]. However, phenotypically they are defined as positive for CD105, CD73 and CD90, and negative for haematopoietic markers (CD34, CD45, CD11b and CD19) and HLA-DR [30]. Because these surface markers are used for characterizing cultured MSCs, immense efforts are underway to identify markers for their direct isolation from tissues. Positive selection approaches using antibodies against low-affinity nerve growth factor receptor [31], stage-specific embryonic antigen (SSEA)-1 [32] and SSEA-4 [33] have been used for isolation of primitive MSCs. Transplantation of a single cell-derived population of SSEA-1+ mesenchymal cells in mice is the first in vivo study demonstrating their capability of differentiating into different mesenchymal cell types, thus showing their true stem cell properties. However, these cell populations were similar phenotypically but heterogeneous in their functionality because all clones did not demonstrate same differentiation potential, suggesting only enrichment of MSCs using these markers. Antibodies have also been raised against MSCs for their prospective isolation such as STRO-1, SH-2, SH-3 and SH-4, but none of them recognize an epitope exclusively present on MSCs [34]. Although the use of non-homogenous MSCs in preclinical and clinical studies has proved safe and effective (as discussed ahead), the search for surface markers exclusive to MSCs for their isolation and characterization is extremely important.
Playing a role in the homeostasis of mesenchymal lineages, these cells differentiate into osteoblasts, adipocytes, chondrocytes, tenocytes, myoblasts and stromal fibroblasts [1, 35, 36]. Recent identification of various MSC populations such as mesodermal progenitor cells [35], marrow-isolated adult multi-lineage inducible cells [37], very small embryonic-like stem cells [38] and SSEA-1+ mesenchymal cells [32] has demonstrated their differentiation into mesodermal, endodermal and neuroectodermal lineages, such as cardiomyocytes, hepatocytes, neural cells and endothelial cells [32, 35, 39–41]. Haematopoietic differentiation has also been observed upon transplantation of SSEA-1+ cells in mice, signifying their primitiveness compared to all other populations [32]. However, the transdifferentiation potential of MSCs has been questioned due to differences in the MSC populations, culture conditions, experimental models and evaluation methods [42]. Many of the observed morphological changes could be a culture artefact or a result of fusion with somatic cell [43, 44]. Therefore, a thorough evaluation of the plasticity of MSCs in vivo is essential because in vitro conditions might not represent the true in vivo milieu.
Another distinguishing feature of MSCs is their ability to expand in vitro under normal culture conditions [2]. We have observed 88- to 560-fold expansion in a single passage (15–20 days) upon culturing early passage MSCs at a density of 50–500 cells/cm2 (our unpublished data). Colter et al. have reported extensive expansion of a subpopulation of MSCs, designated recycling stem cells, to the order of 109-fold in 6 weeks by culturing cells at low density of 1.5 or 3 cells/cm2[45]. Clinical feasibility of culture-expanded MSCs has been validated by a number of studies [4, 46–49]. Thus, a small amount of bone marrow aspirate is sufficient for generation of large number of cells needed for transplantation following in vitro expansion.
Immunological characterization of human MSCs revealed intermediate expression levels of human leucocyte antigen major histocompatibility complex (MHC) class I, and no expression of MHC class II antigen and co-stimulatory molecules CD40, CD80 and CD86 [50–52]. The expression of MHC class I prevents them from the action of natural killer cells, whereas absence of co-stimulatory molecules leaves T cells anergic (reviewed in [53, 54]). In addition, MSCs have been demonstrated to suppress T-lymphocyte proliferation and activation [50, 51]. As a consequence, MSCs are able to modulate the immune response, making them immune privileged and suitable for allogeneic transplantation, as has been reported in numerous clinical studies [3, 55, 56]. Further, MSCs have been reported to home to sites of injury and disease following intravenous infusion and contribute to the repair process [5, 48, 57]. The expression of chemokine receptors on MSCs might be responsible for their ability to sense and respond to signals such as chemokines expressed by injured tissues [58], causing them to extravasate from the blood vessels, such as immune cells [59], via a co-ordinated rolling and adhesion behaviour on endothelial cells in a P-selectin- and VCAM1-dependent manner [60]. Their contribution to tissue repair is also mediated by secretion of paracrine factors having angiogenic and anti-apoptotic properties [61–63]. These paracrine factors not only attract endothelial cells and macrophages but are also likely to stimulate the resident stem/progenitor cells to aid in the process of tissue repair [64].
MSCs can be easily isolated from readily accessible blood and bone marrow compared to other stem cells from tissues such as brain, heart and liver [65, 66]. Additionally, ex vivo expansion potential enables generation of a sufficient number of cells for transplantation [45]. Immunomodulatory functions, homing ability to injured sites and capability to modify the microenvironment by paracrine factors make intravenous delivery feasible in comparison to site-specific delivery of neural [67], cardiac [68] and muscle stem cells [69], thus, making MSCs a promising candidate for stem cell-based therapy (Fig. 1).
Figure 1.
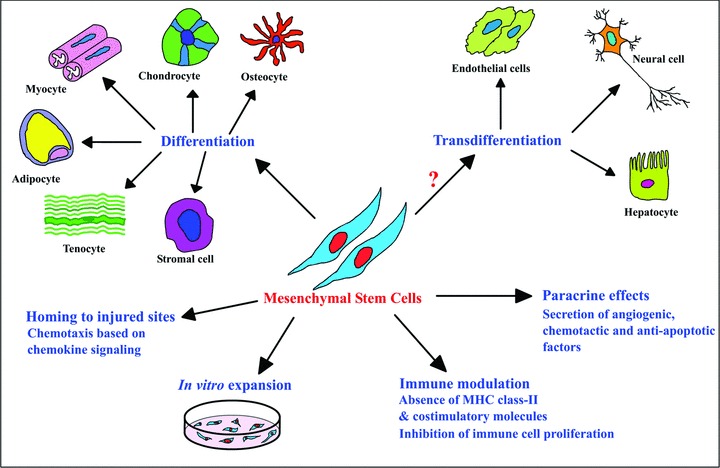
Therapeutically significant properties of MSCs. MSCs are capable of in vitro expansion and differentiation, though their transdifferentiation ability is questionable. They do not express MHC class II and co-stimulatory molecules (CD40, CD80 and CD86) preventing immune response upon transplant and inhibit immune cell (B cells, T cells, natural killer cells and dendritic cells) proliferation and activation. Their ability to respond to damage signals such as chemokines aids in homing to the injured sites, and enhance tissue repair by facilitating recruitment of endothelial cells and macrophages by secretion of angiogenic and chemotactic factors.
Experimental/preclinical MSC-based studies
Capitalizing the extraordinary properties of MSCs, several studies have been undertaken to evaluate their potential for tissue repair in animal models. Depending on the type of disease/injury, different strategies involving site-specific delivery, genetic modification and use of scaffolds have been designed. Basic studies to identify the mode of action of MSCs and their responses to damages have also been addressed, highlighting the therapeutic potential as well as safety and efficacy of using MSCs. However, certain issues remain to be resolved before translation of MSC-based therapy to the clinic (Fig. 2).
Figure 2.
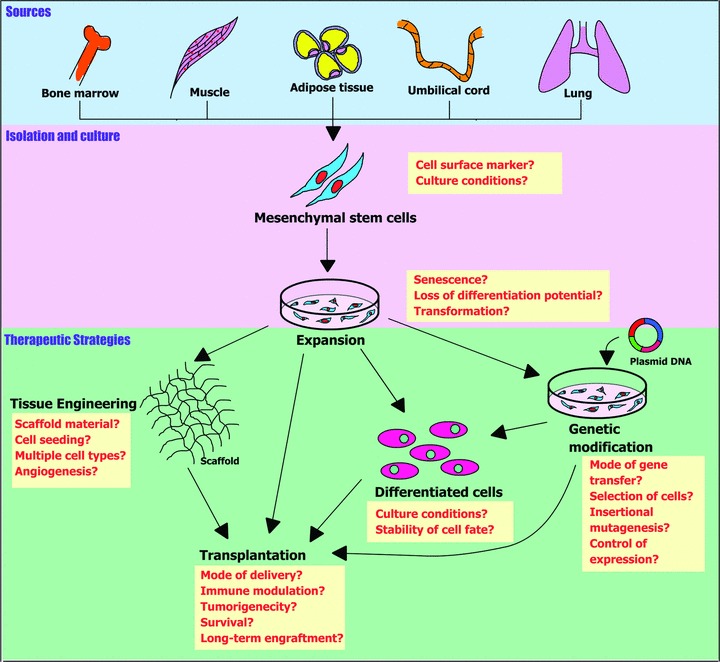
MSC-based approaches and challenges. MSCs isolated from various tissues can be expanded in culture and then used directly or after genetic modification or combining them with scaffolds for treating disorders. Important issues to be resolved to increase MSC utilization in clinics are highlighted in boxes along the steps involved in MSC-based therapies
MSC transplantation
To begin with, numerous studies using systemic administration of MSCs have been performed at preclinical level to assess their in vivo behaviour and suitability for the treatment of a number of injuries and diseases (Table 1). Ortiz and colleagues evaluated the ability of intravenously infused MSCs in bleomycin-exposed mice, which represents a lung injury model, to engraft in the lung tissue [70]. Bleomycin treatment resulted in a 23-fold increase in engraftment levels of MSCs compared to mice not exposed to bleomycin. Further, the engrafted cells adopted an epithelium-like morphology and reduced bleomycin-induced inflammation and collagen deposition in the lung [70, 71]. Whether MSCs actually underwent transdifferentiation into alveolar epithelial type II cells or fused with epithelial cells was not evaluated. However, transplanted mice exhibited increased level of G-CSF and GM-CSF, which might have mobilized endogenous stem cells aiding in repair [71]. The anti-inflammatory action of MSCs was mediated by secretion of IL-1 receptor antagonist (IL1RN), which suppressed expression of pro-inflammatory cytokines TNF-α and IL-1α[72]. In vitro migration assays demonstrated the release of, as yet unknown, chemotactic factors from damaged lung cells that attracted MSCs to the injury site [71].
Table 1.
Experimental mesenchymal stem cell-based therapies
| Disease/injury | Study organism | Cells | Mode of delivery | Outcome | References |
|---|---|---|---|---|---|
| None | Pre-immune foetal sheep | Human MSCs | Intrahepatic | Generation of hepatocytes | [172] |
| Abdominal irradiation | NOD/SCID mouse | Human MSCs | Intravenous | Recovery of small intestine structure with increase in villus height | [173] |
| Renal failure | Mouse | Murine GFP-MSCs | Intravenous | Kidney engraftment, tubular epithelial differentiation, recovery from renal failure | [174] |
| Spinal cord injury | Rhesus monkey | Autologous MSCs differentiated into neural cells in vitro | Injected into damaged site | De novo neurogenesis and functional recovery of senses | [175] |
| Retinitis pigmentosa | Rhodopsin knockout mouse | Murine GFP-MSCs | Injected to the retina | Rescue photoreceptor cells via differentiation | [176] |
| Chronic wound | Mouse | Murine GFP-MSCs | Intradermally around the wound | Accelerated wound closure with increased re-epithelialization, cellularity and angiogenesis | [74] |
| MI | Pig | Allogeneic MSCs | Intramyocardial | Long-term engraftment, reduction in scar formation and no rejection | [145] |
| Diabetes | NOD/SCID mouse | Human MSCs | Intracardiac | Lower blood glucose levels and increased insulin levels | [78] |
| Acute lung injury | Mouse | Murine MSCs | Intrapulmonary | Down-regulation of pro-inflammatory responses to endotoxin | [177] |
| Chemically burned cornea | Rat | Human MSCs | Injected into the cornea | Reconstruction of corneal surface associated with inhibition of inflammation and angiogenesis | [178] |
Shimizu’s group standardized transdifferentiation of MSCs into keratinocytes in culture and investigated whether MSCs could migrate and engraft into wounded skin in murine model. They found that intravenously injected MSCs transdifferentiated into keratinocytes, endothelial cells and pericytes at the wound site, thereby accelerating the repair process [73]. Evaluating the migratory mechanism using in vitro and in vivo migration assays, they identified chemokine receptor CCR7 to play a major role because its ligand SLC/CCL21 induced MSC migration [73]. Expression of keratin by transplanted MSCs and formation of glandular structures were reported by Wu and colleagues upon injection of MSCs around wound in an excisional wound splinting model in diabetic mice [74]. They observed reduction in the number of donor-derived cells in the wound during the 4-week follow-up, suggesting that MSC effects are transient and do not provide long-term self-renewal stem cells for keratinocytes. Because MSCs have also been observed to return to the bone marrow after wound healing [75], the local concentration of the chemokine signals or the expression of a particular chemokine in response to injury at the site might have a significant role in retaining MSCs [76], which needs further evaluation. Apart from undergoing transdifferentiation, MSCs are also likely to contribute to the repair process by secreting paracrine factors including VEGF-α, EGF, keratinocyte growth factor, SDF-1, IGF-1 and angiopoietin-1 (Ang-1), which facilitate the recruitment of macrophages, keratinocytes and endothelial cells to the wound site and enhance angiogenesis and wound healing [63, 74]. Thus, reduction in inflammatory responses and accelerated angiogenesis contribute to the ongoing reparative process, but functionality of MSC-generated tissues such as sebaceous and sweat glands, if any, is not known. Therefore, complete regeneration of the tissue is debatable [77].
Similarly, transplantation of human MSCs in hyperglycaemic NOD/SCID mice resulted in homing to islets associated with an increase in pancreatic islets and mouse insulin production [78]. No human insulin was detected in blood, and the reduction in blood glucose levels was mainly a result of stimulation of islets and β-cells [78–80], similar to that observed for neural stem cells in mice [81], as well as inhibition of T-cell responses against the new β-cells [82]. These studies bring to light the potential of MSCs to migrate to injury site and modify the microenvironment, thereby modulating the immune response and facilitating tissue repair by stimulating endogenous stem/progenitor cells. It is, therefore, necessary that studies suggesting transdifferentiation clearly define the experimental conditions and thoroughly evaluate the true nature of the differentiated cells by expression profiling and functional assays. Genetic marking approach may be useful in assessing the differentiation potential of putative MSCs upon transplantation in animal model systems [83]. Further, these animal models represent excellent systems to elucidate the mechanism of action of MSCs in mediating various therapeutic effects, in order to improve the present treatment regimens and facilitate the development of new approaches.
Recently, MSCs have also been shown to improve haematopoietic transplantation [15, 16, 47, 84, 85]. Transplantation of HSCs is used for the treatment of oncohaematological disorders, but marrow-ablative therapy (involving high-dose chemotherapy and radiotherapy) destroys not only haematopoietic cells but also damages the stroma [86, 87]. This is likely to cause reduction in the engraftment of HSCs in the hostile environment as has been demonstrated in mice [88], thereby, decreasing the success of transplant. Koc et al. reported rapid haematopoietic recovery in 28 breast cancer patients undergone high-dose chemotherapy following co-infusion of HSCs and MSCs [47]. Enhanced haematopoietic engraftment was also reported upon infusion of limiting number of umbilical cord blood stem cells with unrelated MSCs in mice [84]. Co-transplanting MSCs with HSCs (CD34+ cells) has been shown to improve engraftment in the bone marrow in mice, though the underlying mechanism needs to be elucidated [15, 16]. This will not only help in improving the present regimens to enhance HSC engraftment, but represents a useful strategy that can be employed to enhance success of transplantation of other adult stem cells, as documented by increased survival of MHC-mismatched skin grafts in immunocompetent baboons [89].
Migration of MSCs to the sites of injury and disease has also been well documented in animal models for MI and cerebral ischaemia [90, 91]. Also, culture-expanded human MSCs have been shown to home to radiation-injured tissues in NOD/SCID mouse model [92]. This portrays their ability to sense and respond to damage signals, thereby avoiding the need for targeted delivery (such as intramyocardial and intrahepatic) to damaged tissues. However, intravenous infusion would cause distribution of cells throughout the body, reducing the fraction of cells homing to the damaged site [93, 94]. Another issue is entrapment of a large fraction of cells in the lung [90] resulting in very low engraftment levels of the order of 0.1–2.7% in the tissues [3, 93]. In vitro expansion of MSCs is also likely to result in low homing as demonstrated in murine study [95], but whether human MSCs also exhibit similar effect remains to be determined. Another contributing factor is low cell survival rate after transplantation [96, 97]. Thus, preconditioning of MSCs prior to transplant by culturing in presence of SDF-1 [98] or under hypoxic conditions [99, 100] is a useful strategy, which enhances cell survival in the hostile environment in vivo. Such preconditioning leads to the activation of Akt survival pathway as well as increased expression of pro-survival and pro-angiogenic factors such as hypoxia-inducible factor 1, VEGF, erythropoietin (EPO), Ang-1 and Bcl-2. Also, increased expression of c-met leads to higher migration rates to ischaemic tissue in response to secreted hepatocyte growth factor as demonstrated in rat hind limb ischaemia model [100].
Further studies using disease models need to be carried out to elucidate the molecular mechanism involved in MSC homing for the improvement of current therapies. For instance, studies have revealed the involvement of integrin β1 in MSC migration and engraftment in ischaemic myocardium in mice [101], whereas CD44 has been implicated in migration and localization of MSCs to kidneys in mouse model of acute renal failure [102]. Cytokine-mediated up-regulation of CXCR4 expression in Flk1+ MSCs improved their engraftment in bone marrow of sublethally irradiated NOD/SCID mice [103], whereas ectopic expression of α4 integrin on mouse MSCs resulted in significant increase in bone-specific retention of transplanted MSCs in mouse [104]. These studies offer molecular targets for genetic engineering of MSCs to enhance their homing and engraftment to injury sites and accelerate recovery. Alternatively, cytokine treatment of MSCs to enhance expression of tissue-specific adhesion molecule or tissue-specific administration of chemotactic factors such as SDF-1α[105], CCL12 [73] and MCP-3 [76] is likely to facilitate targeting to a particular tissue.
Genetically modified MSC-based therapy
Integrating the strengths of genetic engineering and stem cell biology holds tremendous potential for designing treatments for critical injuries and diseases by inducing differentiation into a specific lineage and improving adhesion potential. Following transplantation, the fate of MSCs would be determined stochastically in vivo depending on the niches they home, and therefore, not all transplanted cells might contribute to the repair of the damage. As recently demonstrated in mice, transplanted MSCs differentiated into osteoblasts in the heart [106]. Thus, site-specific transplantation of functional, differentiated cells would be advantageous under certain conditions. Although differentiated cells can be generated by chemical stimulants or differentiation factors in vitro, the differentiation state might not be stable upon transplantation. Such reversal of differentiation (i.e. dedifferentiation) has been shown in vitro for MSCs upon the withdrawal of stimulants [107]. Therefore, genetically modifying stem cells by a key differentiation factor would help to achieve directed and complete differentiation into the desired lineage.
Studies on the therapeutic applicability of genetically modified MSCs (GM-MSCs) have been carried out in animal models (Table 2). MSCs transduced with BMP2 and BMP4 have been shown to successfully repair a variety of musculoskeletal defects in animal models as BMPs are potent inducers of osteogenic differentiation [11, 12, 108]. The cells not only themselves undergo differentiation but also stimulate the neighbouring cells to participate in the repair process. It has also been reported that short-term expression (for 6 days) of BMP-2 in MSCs was sufficient to irreversibly induce osteochondral bone formation upon implantation into tibialis anterior muscle or joints of SCID mice [109].
Table 2.
Genetically modified mesenchymal stem cell-based therapies
| Disease/injury | Study organism | Cells | Mode of delivery | Outcome | References |
|---|---|---|---|---|---|
| Diabetes | Mouse | Human insulin gene-transfected murine GFP-MSCs | Intrahepatic | Diabetes relieved for 6 weeks | [179] |
| Diabetes | Mouse | PDX-1 gene-modified human MSCs | Transplanted under renal capsule | MSCs differentiate into functional insulin-producing cells and restore back normal glucose levels | [115] |
| MI | Rat | Human angiopoietin-1-modified rat MSCs | Intramyocardial | Improved heart function, enhanced angiogenesis and reduced cardiac remodelling | [180] |
| MI | Rat | Calreticulin-modified autologous MSCs | Injected into injured site | Enhanced cell adhesiveness, migration and survival post-transplant | [181] |
| None | Athymic nude mouse | BMP-9-transduced human MSCs | Paraspinal injection | Spinal fusions (i.e. ectopic bone formation at injected sites) | [182] |
| Myeloma bone disease | NOD/SCID mouse | Human OPG-transduced MSCs | Intravenous | Reduced osteoclast activation and trabecular bone loss | [183] |
| GVHD | Mouse | IL-10 transduced MSCs | Intravenous | Reduced inflammatory response and enhanced survival | [184] |
Differentiation being a process co-ordinately regulated by number of factors, expression of combination of genes has proved more fruitful for orthopaedic gene therapy. BMP-2/7 and BMP-4/7 heterodimers exhibit higher activity than homodimers; therefore, simultaneous transduction with BMP-2 or BMP-4 and BMP-7 in mesenchymal cells resulted in 2- to 3-fold more bone formation in mice [110, 111]. However, BMPs are secreted factors and their constitutive overexpression is likely to cause abnormal bone formation in vivo. Therefore, regulated overexpression of osteogenic transcription factor Runx2 (using tetracycline-regulated Tet-Off expression system) has been demonstrated to offer control over osteoblast differentiation of engineered MSCs in mice [112]. These engineered cells provide a novel approach for treatment of osteochondral disorders and use of regulatable expression systems to prevent undesirable effects, but studies aimed at mapping the fate of GM-MSCs following repair of the defect are required before leaping at the prospect of using them for human clinical trials.
Use of GM-MSCs has been investigated in culture as a choice for the treatment of genetic disorders. Genetic modification of MSCs with dominant-negative collagen type I protein successfully repaired bones derived from osteogenesis imperfecta patients [113], whereas dystrophin-transfected MSCs participated in myogenesis through cellular fusion and complemented the genetic defect of muscular dystrophy myotubes in vitro[114].
Generation of cells of different tissues for the purpose of transplantation can also be achieved by genetic modification. Pancreatic transplantation is the only cure for type 1 diabetic patients. However, shortage of pancreas donors calls for the development of alternative cell-replacement therapy. Transdifferentiation of human bone marrow MSCs into insulin-producing cells by overexpression of pancreatic duodenal factor 1 (PDX1) has been achieved in vitro[10, 115]. Only 50% of the cells expressed insulin and secreted it in response to glucose in culture, whereas other islet hormones were expressed by all cells. Because these cells did not differentiate completely in vitro, as determined by microarray, transplantation under the renal capsule in streptozotocin-diabetic immunodeficient mice induced further differentiation and resulted in the reduction of hyperglycaemia and stabilization of blood glucose levels during the 5-week follow-up [10]. None of the transplanted cells were observed to migrate to the pancreas, signifying the advantage of site-specific transplantation and avoidance of unwanted effects due to homing to undamaged organs following systemic infusion. However, to assess the maintenance of differentiated state, the cells can be transfected with vector containing GFP or YFP cloned under the control of cell-type-specific transcription factor prior to transplantation to evaluate their fate in vivo, specifically when they are transplanted in another tissue/organ because the microenvironment can alter their fate.
The cells must also be labelled properly (dyes such as PHK26 or genetically such as GFP) to track them following transplantation in animal models. For instance, using GFP-labelled Akt-overexpressing murine MSCs, Noiseux et al. tracked MSC fate following intramyocardial injection in mouse model of MI [116]. They observed transient engraftment of MSCs in the infarct zone and fusion of MSCs with recipient cardiomyocytes as early as 3 days after injection, raising concern regarding safety and long-term outcome of the fusion events. Although a very small fraction of cells likely differentiated into cardiomyocytes, the reduction in infarct size and improvement in cardiac function were possibly mediated by secreted paracrine factors [117]. Enhanced expression (100-fold) of secreted frizzled-related protein 2 (SFRP2) by Akt-overexpressing MSCs was determined to exert a pro-survival effect on myocardium by increasing nuclear β-catenin, which activated anti-apoptotic gene transcription in ischaemic cardiomyocytes [118].
Cell replacement is also an attractive opportunity for treating a number of neurological disorders. Kim et al. demonstrated that Neurogenin1 (Ngn1) overexpression was capable of inducing neuronal differentiation of MSCs in vitro[119]. The differentiated cells expressed voltage-gated L-type Ca2+ channels and TTX-sensitive voltage-gated Na+ channels, which are critical for initiation and propagation of action potential in neurons [120]. These cells on intracranial transplantation in rat stroke model engrafted in the ischaemic brain, formed connections with host neurons and improved motor functions compared to control transplanted with normal MSCs. MSCs modified with Ngn1 were detected even after 8 weeks following transplant compared to normal MSCs, which disappear within 4 weeks. Both animal groups receiving normal and GM-MSCs documented proliferation of neural progenitors and protected delayed cell death, as shown in earlier studies, as a result of paracrine effects of MSCs [121, 122]. Taken together, these studies clearly demonstrate the significance of GM-MSCs exhibiting enhanced functional capabilities as a suitable system for the generation of transplantable cells in vitro as well as their efficacy in vivo.
Apart from modifying the differentiation potential of MSCs, they can also be engineered for targeting to specific tissues. For instance, MSCs transduced with CXCR4 exhibited enhanced homing to infarcted myocardium in rats following intravenous delivery [123, 124]. CXCR4 overexpression in MSCs facilitated their mobilization and engraftment in the collagenous tissue of the infarcted area, perhaps via up-regulation of matrix metalloproteinases, and led to significant neoangiogenesis compared to normal MSCs [125]. Such strategies will help in the development of non-invasive cell therapy. Route of delivery of GM-MSCs and tissue targeting is also important in order to avoid formation of heterotopic tissue, especially in case of cells modified to favour differentiation into a particular lineage. Low cell survival following transplantation is a hurdle in MSC-based therapy as mentioned earlier. Genetic modification of MSCs with hypoxia-regulated heme oxygenase-1 [125], Bcl-2 [126] and Akt1 [127] resulted in enhanced cell survival upon transplant in animal models by inhibition of apoptosis and represents a potential opportunity. Another important issue is the mode of gene transfer. The use of viral vectors because of their high transduction efficiency is likely to be associated with activation of immune responses and problem of insertional mutagenesis despite the development of different generations of viral vectors [128]. Thus, the use of non-viral approaches is an alternative, which has been documented to repair critical size bone defect in mice even though their transfection efficiencies are very low [129].
MSC-based protein therapy
MSCs can also serve as ‘protein factory/production unit’ for the treatment of disorders caused as a result of attenuated production of cytokine/growth factor or synthesis of a mutated inactive protein (Table 3). They are genetically modified to synthesize the desired factor and then transplanted either intravenously or at the required site depending on the situation. This therapeutic approach has the advantage of continuous supply of the protein (or can be controlled by use of inducible expression systems), delivery of potentially more physiological levels compared to conventional protein therapy and comfortable for the patient. It might be possible to design treatments for blood disorders such as haemophilia and anaemia, autoimmune disease and tumours, using engineered MSCs in the near future. Transplantation of EPO-transduced MSCs in baboons showed the presence of EPO in serum for up to 137 days and displayed increase in haematocrit [130]. Further improvements are required because such short-term expression can only be useful in conditions such as MI and is not suitable for treating genetic disorders. However, the feasibility of the system for allogeneic transplantation is sceptical with recent observation that allogeneic murine EPO expressing MSCs resulted in the development of severe anaemia in mice because of induction of neutralizing anti-EPO antibodies [131]. Intravenous injection of INFβ-transfected MSCs into SCID mice with established tumours resulted in incorporation of MSCs in tumour architecture and inhibition of tumour growth [8]. Mice injected with INFβ-overexpressing MSCs survived for longer time compared to those receiving INFβ injection only, suggesting involvement of other secreted factors as well. With their ability to home to damaged sites, MSCs can be used as vehicles for targeted delivery of therapeutic proteins, eliminating effects on other tissues. This strategy can also be applied under certain situations to stimulate the resident stem cell population via paracrine action of cytokines, thereby inducing natural repair systems or accelerating the ongoing regeneration process. The problems associated with genetic modification are already mentioned earlier. Another important concern is the level of transgene expression and sustenance of expression in vivo. Use of inducible expression system is likely to prevent undesirable effects because of high level of expression as well as offer control on timing of expression of the transgene [112].
Table 3.
Mesenchymal stem cell-based protein therapies
| Disease/injury | Study organism | Cells | Mode of delivery | Outcome | References |
|---|---|---|---|---|---|
| Anaemia | Mouse | Epo-gene modified MSCs | Subcutaneous implantation | Anaemia corrected | [9] |
| Stroke | Rat | BDNF-modified telomerized human MSCs | Intracerebral | BDNF production improved functional recovery with fewer number of cells undergoing apoptosis in ischaemic boundary zone | [185] |
| Glioma | Rat | Human IL-2-modified MSCs | Intratumoral | Inhibited tumour growth and prolonged survival of tumour-bearing rats | [186] |
| Lung metastasis | Mouse | NK4-transduced MSCs | Intravenous | Inhibited development of lung metastasis; prolonged survival by inhibiting tumour-associated angiogenesis and lymphangiogenesis and apoptosis of tumour cells | [187] |
Tissue engineering using MSCs
Another out-branch of stem cell therapy involves the generation of graftable tissues in vitro combining cells (normal or engineered) or parts thereof and scaffolds to generate three-dimensional implants. It involves trying to recapitulate the in vivo environment to favour the development of the desired tissue for transplantation. Various approaches such as protein-impregnated scaffolds [132], gene vector-incorporated matrices [133] and combinations of cells and scaffold have been designed (Table 4). Scaffolds alone have been useful in repairing certain kinds of damages by incorporating into them differentiation signals such as BMP2, which stimulates the endogenous cells at the defect site [132]. However, seeding scaffolds with MSCs has greater regeneration ability because it augments the in situ repair process by supplying progenitors as well as stimulatory factors. To further enhance the therapeutic potential of tissue-engineered implants, GM-MSCs can be seeded onto scaffolds. It offers the advantage of directed and irreversible differentiation and greater responsiveness to extracellular signals [111].
Table 4.
Tissue engineering therapies using MSCs
| Disease/injury | Study organism | Cells and scaffold | Mode of delivery | Outcome | References |
|---|---|---|---|---|---|
| Osteochondral defect | Rabbit | Autologous MSCs in an injectable synthetic ECM | In situ | Cartilage filled the full-thickness defect | [188] |
| Spinal cord injury | Rat | Autologous MSCs seeded on hydrogels | In situ | Enhanced ingrowth of axons in the lesion and improvement in function | [189] |
| None | In vitro | eNOS-modified rat MSCs seeded onto tubular poly (propylene carbonate) scaffold | – | Generation of engineered blood vessels | [190] |
| Critical size bone defect | Mouse | OSX-modified murine MSCs seeded in type I collagen sponge | In situ | Enhanced bone formation | [191] |
| Tendon defect | Rat | C3H10T1/2 cells stably transfected with BMP-2 and active Smad8 variant seeded onto collagen scaffold | In situ | Tendon regeneration | [192] |
| Articular cartilage defect | Rabbit | Autologous MSCs modified with TGF-β1 seeded onto chitosan scaffold | In situ | Enhanced repair; defect filled with hyaline cartilage | [193] |
The choice of biomaterial used for making the scaffold is important because its physical and chemical properties affect MSC differentiation. For instance, the elasticity of the polyacrylamide matrix seeded with MSCs determines their differentiation into neuronal, muscle or bone lineages depending on the cross-linking density [134]. Presence of carboxyl or hydroxyl groups on scaffold surface favour chondrogenic differentiation, whereas amino and sulfhydryl groups promote osteogenic differentiation of MSCs [135]. MSCs have been exploited in bone and cartilage tissue engineering using a variety of polymer materials such as hydroxyapatite and tricalcium phosphate ceramics, alumina and titanium metal alloys, synthetic polymers made of polyglycolic and polylactic acids and natural polymers such as collagen-I, cellulose, agarose and demineralised bone composites (reviewed in [136]). Arinzeh and colleagues transplanted allogeneic MSCs loaded onto a hollow ceramic cylinder made of hydroxyapatite-tricalcium phosphate, into critical-sized bone defect in the femoral diaphysis in dogs without the use of immunosuppressive therapy [137]. A critical size bone defect cannot be healed by the body’s own regenerative potential. The ‘test’ group receiving the implant exhibited no adverse host response as documented by absence of lymphocyte infiltration and antibodies against allogeneic cells. Radiological and histological evaluation post-implantation demonstrated new bone formation after 16 weeks throughout the implant with significantly greater amount of bone within the pore space of implants loaded with MSCs than cell-free implants [137]. This study highlights the immunomodulatory functions of MSCs, which prevented any immune rejection against transplanted cells as well as ability of MSCs to differentiate into osteoblasts and repair the bone defect.
Generation of complex three-dimensional tissue grafts is confronted by problem of supply of nutrients to the cells deep inside the graft. Vascularization of the graft is essential for the survival of cells and sustenance of the implant. Although host blood vessels invade the implant in response to signals secreted by implanted cells because of oxygen deficiency, it occurs at very slow pace and would require weeks to vasculate an implant of few millimetres [138], leading to death of cells inside the implant. Endothelial precursor cells (EPCs) and pro-angiogenic factors such as VEGF have been used for the generation of vascularized grafts [139]. They can be used either by mixing EPCs and MSCs or by transfecting MSCs with VEGF gene to promote angiogenesis in vivo upon transplant [140]. Human MSCs coupled with human umbilical vein endothelial cells were used to generate vascularized bone in vitro, but no perfusion was observed upon implantation [141]. No vascularization strategy is available at present that can support large constructs after implantation. Current approaches such as in vivo prevascularization, in vitro prevascularization, use of scaffold and angiogenic factor delivery (reviewed in [142]) are only likely to increase the chances of vascularization of the implant, because each has certain limitations. In vivo evaluation of proper integration of the implant at the injury site and its long-term persistence using imaging techniques are required to ensure safety and facilitate further improvements because neovascularization mediated by VEGF alone may produce non-functional vessel with defective cellular differentiation [143].
Clinical studies
Encouraging results of tissue repair and immunomodulation in animal studies have led to limited clinical studies with MSCs for some debilitating disorders (Table 5). Metachromatic leucodystrophy (MLD) and Hurler syndrome are autosomal recessive disorders due to deficiency of enzymes arylsulfatase A and α-L-iduronidase, respectively. These patients develop neurological and musculoskeletal defects that limit their survival [56]. HSC transplantation significantly improves survival of patients but abnormalities still persist. Koc and colleagues postulated that infusion of MSCs might correct the defects because they are capable of differentiating into mesenchymal and neuronal cells [56]. Patients undergone HSC transplant were infused with MSCs from the same donor and demonstrated no infusion-related toxicity. Donor derived-MSCs constituted only 0.4 and 2% of MSCs from 2 of 11 patients enrolled in the study. Although MLD patients showed significant improvement in nerve conduction velocity, no change in overall health of the patients was apparent. The study demonstrated the safety of allogeneic MSC transplantation and highlights the low engraftment efficiency of culture-expanded MSCs, which could be either because of poor survival following transplant, proliferative defect or low homing ability of cultured MSCs [56]. However more studies are required to investigate any role of MSC in the treatment of MLD and Hurler syndrome.
Table 5.
Clinical mesenchymal stem cell-based therapies
| Disease/injury | Cells | Mode of delivery | Outcome | References |
|---|---|---|---|---|
| Stroke | Autologous MSCs | Intravenous | Improved functional recovery | [5] |
| Osteogenesis imperfecta | Allogeneic MSCs | Intravenous | Increased growth velocity and no clinically significant toxicity | [48] |
| Radiation burns | Autologous MSCs | Injected at burn site | Promoted tissue regeneration, inhibited recurrence of inflammation | [49] |
| Multiple sclerosis | Autologous MSCs | Intrathecal | Some degree of improvement in sensory, pyramidal and cerebellar functions | [194] |
| MI | Autologous MSCs | Intracoronary | Improved left ventricular function | [146] |
| Crohn’s fistula | Autologous MSCs | Injected into wall of track or rectal mucosa | Six of eight fistulas were covered with epithelium; two showed incomplete closure | [195] |
| GVHD | Autologous and allogeneic MSCs | Intravenous | GVHD disappeared in six of eight patients | [148] |
| Spinal cord injury | HLA-matched MSCs | Injected into subarachnoid space | Improved sensory perception and movement in hips and thighs | [196] |
MI caused by an imbalance between the oxygen supply and the demand of the myocardium results in the development of myocardial necrosis. Thus, the restoration of functional cardiomyocytes in the infarcted myocardium is the only solution. Because MSCs have been demonstrated to differentiate into cardiomyocytes in vitro as well as in animal model of MI [144, 145], Chen and colleagues planned a randomized study to investigate the effectiveness of intracoronary injection of autologous culture-expanded MSCs in patients with MI [4]. During the 6-month follow-up study, the percentage of hypokinetic, akinetic and dyskinetic segments decreased whereas wall movement velocity and left ventricular ejection fraction increased significantly in transplant recipients compared with control group. Most of the improvement was observed after 3 months of transplant, without much improvement thereafter, implicating only short-term benefit [4]. Thus, it is not justifiable to judge the clinical potential for MI based on few small-scale studies [4, 146]. Moreover, low efficiency of engraftment, transient effects and insufficient evidence supporting the presence of MSC-derived cells at the infarct site as documented in animal studies emphasize the need to determine the optimal cell dose [147], number of infusions, route of delivery and timing of transplant.
Osteogenesis imperfecta, a genetic disorder of mesenchymal cells caused due to mutation in collagen type I gene, results in osteopenia, multiple fractures, bone deformities and short stature. Allogeneic bone marrow transplantation (BMT) in children with osteogenesis imperfecta demonstrated 1.5–2.0% donor-derived osteoblasts with an increase in total bone mineral content as well as improvement in body growth and reduced fracture incidence in all children. This study highlights the ability of MSCs and their progenitors to engraftment in the bone, and subsequently differentiate into functional osteoblasts [3]. Follow-up over 18–36 months showed increase in total bone mineral content with decreasing growth rates. Hence, it was hypothesized that additional MSC transplantation without marrow-ablative treatment would safely boost responses in these patients undergone BMT. After two rounds of infusions, five of six children showed engraftment of MSCs and their differentiation into osteoblast as well as skin fibroblast [48]. Thus, a small fraction of allogeneic MSCs engrafted in the bone and underwent osteogenic differentiation without causing any immune problems, signifying the feasibility of allogeneic MSC transplantation in human beings. However, the benefit from a single transplant was short-lived and subsequent transplants were performed, highlighting the need to modify transplant strategies to improve MSC homing and engraftment in vivo for potential long-term gains.
Use of MSCs for the treatment of steroid-resistant, severe, acute GVHD has also been initiated following demonstration of the safety of allogeneic MSC infusion and immune suppression by MSCs (mentioned earlier). In an earlier study on 8 patients with steroid refractory grades III–IV acute GVHD, MSC infusion resulted in disappearance of GVHD in 6 of 8 patients [148]. Henceforth, the study was extended to phase II trial involving 55 patients. Out of 55 patients treated during the 5-year study, 39 patients responded with 30 showing complete response. The response was independent of the HLA match and resulted in higher overall survival 2 years after HSC transplantation, 53% among complete responders compared to 16% among partial or non-responders [149]. No side effects were observed after HLA-identical or mismatched MSC infusions [148], and the response rate was not related to donor HLA match [149]. On the other hand, in a multi-centric study by Lazarus et al., MSC co-infusion with HLA-identical HSCs in patients undergoing allogeneic transplant for GVHD did not produce any effect such as prevention of graft rejection or accelerated haematopoietic recovery [150]. Co-transplantation of MSCs and MHC-identical allogeneic HSCs in patients suffering from haematopoietic malignancies was reported to have lower rate of GVHD but higher relapse rate than patients receiving HSC transplant alone [151]. Hence, evaluation of their mechanism of action is extremely essential before using them in clinical settings.
Limited not only to simple transplantation, MSCs and scaffold have been combined and used in clinic. In a classical study of bone tissue engineering, Quarto and colleagues used culture-expanded autologous MSCs to treat large bone defects in three patients [152]. The patients had loss of 4–7 cm bone segments, and bone grafting is the only approach for treating such large defects. Each patient was implanted at the lesion site with expanded MSCs seeded on hydroxyapatite scaffolds of appropriate size and shape. None of the patients demonstrated any complications over more than 15-month follow-up and all of them recovered limb function [152], but as no biopsies were taken, it remains unclear whether the callus was induced by implanted MSCs or by endogenous bone-forming cells. Non-cultured, enriched bone marrow-derived MSCs combined with porous β-tricalcium phosphate (β-TCP) have been used for posterior spinal fusion [153]. The enriched MSCs were mixed with β-TCP granules and incubated for 2 hrs for cells to adhere, and thereafter implanted in patients with spondylolysis or thoracolumbar fracture. None of the patients had neurological deterioration after operation and there was no deep vein thrombosis or pulmonary embolism. After about 3 years, 95% cases had good spinal fusion signifying the potential of the approach over conventional bone grafting, which is associated with problems such as donor-site morbidity [153]. The use of MSC enrichment technique would likely be of great advantage because it diminishes the effects of culture conditions on MSC behaviour and might result in higher level of engraftment, which must be evaluated in subsequent studies. Thus, all these studies are suggestive of the clinical potential of MSCs and document their safe use in human beings. Hence, the likelihood of establishing MSC banks, which expand and cryopreserve an individual’s MSCs, can be of great therapeutic significance. However, because these early studies have been done on small set of patients without complete knowledge of MSC biology, it emphasizes the need to examine certain critical issues to harness complete potential of MSCs.
Challenges and future prospects
Numerous animal model studies have documented the therapeutic potential of MSCs and their safety and efficacy in vivo. But the regenerative capacity of MSCs in human beings is controversial because of limited human studies performed on very small set of patients. Large-scale multi-centric clinical trials designed with great caution need to be performed for complete validation of MSC-based therapy [154]. However, before planning and initiating such trials, certain issues related to MSC biology need to be addressed at basic and preclinical levels (Fig. 2).
Because the true identity of MSCs in vivo remains elusive, current approaches used for their isolation have resulted in heterogeneous subpopulations exhibiting MSC-like characteristics. Therefore, identification of MSC-specific marker for isolation of a homogenous population of cells directly from tissue is necessary. Such homogenous population would help in determining the true potential of MSCs as well as deciphering their exact anatomical location. Because they are believed to play role in regulation of haematopoiesis, their true identification will aid in delineating the underlying signalling events and possible cell–cell interactions with HSCs. In addition, it will accelerate the pace of research on MSCs as comparison of results among laboratories would then be feasible. Hence, concerted efforts employing high-end techniques such as two-dimensional gel electrophoresis and mass spectrometry (MS and combination with chromatography LC-MS) are required for identification of a novel surface molecule expressed exclusively on the putative MSC.
A potential block in the applicability of these therapies is the requirement of large number of cells for direct transplant or for the generation of an implant. For example, BMT requires on average 5 × 106 cells/kg body-weight. Although MSCs are easy to isolate and undergo in vitro proliferation, extended expansion was observed to alter their properties [18, 155–157]. Although stem cells must exhibit indefinite self-renewal as per definition, human MSCs have been shown to undergo senescence and exhibit reduced differentiation potential 6th passage onwards [18], which is in agreement with other studies showing that about 13–25 population doublings result in complete senescence [157]. Senescence associated changes in cellular morphology, expression of surface markers and global gene profile have been observed with increasing number of passages beginning from the first passage itself [157]. Increase in expression of cell-cycle inhibitor p16INK4A[158] and decrease in telomere length during culture contribute to the process of senescence [18]. However, variation in culture conditions such as passing at low density [45], use of cytokines such as FGF2 [159, 160] and overexpression of hTERT [161] are likely to delay the progress of senescence, thereby helping in achieving greater number of doublings.
Continuous passaging has also been observed to lead to the transformation of murine bone marrow-derived MSCs, which formed fibrosarcoma upon transplantation in mice [162]. These cells lost their osteogenic capacity after 13 passages and became malignant after 29 passages [162]. Human bone marrow-derived MSCs appear to be resistant to transformation compared to murine MSCs as revealed by genetic characterization using comparative genomic hybridization, karyotyping and subtelomeric fluorescent in situ hybridization analysis at different passages during long-term culture [163]. However, Rubio and colleagues demonstrated that long-term culture (4–5 months) of adipose tissue-derived human MSCs led to spontaneous transformation. The transformed cells exhibited chromosomal abnormalities, increased c-myc levels and telomerase activity, and formed tumours on transplantation [164]. Therefore, great caution needs to be taken in clinical studies because transplantation of MSCs is likely to be associated with potential risk of tumour generation and ability to enhance the growth of previously undetected tumour (discussed ahead). Genetic characterization and expression profiling of expanded MSCs might be a way to screen for changes in the cells before using them for transplant.
Immunomodulatory effects of MSCs also require due consideration because studies have demonstrated the ability of MSCs to home to sites of tumour and suppress or stimulate tumour growth in animal models [165]. For instance, inhibition of primary tumour growth was observed upon co-injection of MSCs with tumour cells in models of Lewis lung carcinoma and B16 melanoma [166], whereas co-culture of MSCs with breast cancer cells enhanced tumour cell proliferation [167]. Molecular studies beginning to elucidate the underlying mechanism suggest the role of IL-6 secreted by MSCs in promoting multiple myeloma proliferation [168]. Karnoub et al. recently also demonstrated integration of MSCs into breast cancer stroma and enhancement of cancer cell metastasis by MSC-secreted chemokine CCL5-dependent signalling [169]. Thus, understanding the interrelationship between MSCs and cancer is essential for clinical utilization of MSCs and the development of suitable anticancer therapies. Further, the interaction between the immune cells and MSCs needs to be studied in vivo because MSC transplant proved beneficial in animal models for autoimmune diseases such as type 1 diabetes [82], experimental autoimmune encephalomyelitis [170], but had no effect on collagen-induced arthritis in murine model of rheumatoid arthritis [171].
Thus, the future research on MSCs needs to focus on (i) identification of exclusive marker on MSCs; (ii) assessment of differentiation potential; (iii) standardization of culture conditions; (iv) state of cells to be transplanted MSCs, progenitors or differentiated cells; (v) survival and long-term engraftment on transplant; (vi) in vivo behaviour of MSCs; (vii) immunomodulatory functions and (viii) paracrine effects. Addressing these issues would require time and patience as well as thorough studies at basic, preclinical and clinical levels. Hence, with the continued efforts of hundreds of scientists and clinicians around the world, and step-by-step progress in the field and related areas, all kinds of diseases and damages would be repairable in the near future.
Acknowledgments
We are thankful to Dr R. P. Tripathi, Institute of Nuclear Medicine and Allied Sciences, DRDO, Lucknow Road, Delhi 110054, for providing us necessary facilities and support. Mr Neeraj Kumar Satija in particular thanks CSIR (India) for the award of Senior Research Fellowship.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryipreservation. J Cell Biochem. 1997;64:278–94. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz ED, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 6.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplant. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 8.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 9.Eliopoulos N, Gagnon RF, Francois M, et al. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol. 2006;17:1576–84. doi: 10.1681/ASN.2005101035. [DOI] [PubMed] [Google Scholar]
- 10.Karnieli O, Izhar-Prato Y, Bulvik S, et al. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic modification. Stem Cells. 2007;25:2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 11.Gugala Z, Olmsted-Davis EA, Gannon FH, et al. Osteoinduction by ex vivo adenovirus-mediated BMP2 delivery is independent of cell type. Gene Ther. 2003;10:1289–96. doi: 10.1038/sj.gt.3302006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XS, Linkhart TA, Chen ST, et al. Local ex vivo gene therapy with bone marrow stromal cells expressing human BMP4 promotes endosteal bone formation in mice. J Gene Med. 2004;6:4–15. doi: 10.1002/jgm.477. [DOI] [PubMed] [Google Scholar]
- 13.Gersbach CA, Le Doux JM, Guldberg RE, et al. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13:873–82. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–84. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Noort WA, Kruisselbrink AB, In’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 16.Park SK, Won JH, Kim HJ, et al. Co-transplantation of human mesenchymal stem cells promotes human cd34+ cells engraftment in a dose-dependent fashion in NOD/SCID mice. J Korean Med Sci. 2007;22:412–9. doi: 10.3346/jkms.2007.22.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 18.Mohyeddin-Bonab M, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cells in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 22.De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Tuli R, Tuli S, Nandi S, et al. Characterisation of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells. 2003;21:681–93. doi: 10.1634/stemcells.21-6-681. [DOI] [PubMed] [Google Scholar]
- 24.Boquest AC, Shahdadfar A, Fronsdal K, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alternation of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–41. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polisetty N, Fatima A, Madhira SL, et al. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–42. [PMC free article] [PubMed] [Google Scholar]
- 26.In’t Anker PS, Scherion SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 27.Martin J, Helm K, Ruegg P, et al. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–51. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- 28.Young HE, Steele TA, Bray RA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 29.Da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 30.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 31.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 32.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 33.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 34.Satija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 35.Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 36.Pittenger MF, Marshak DR. Mesenchymal stem cells of human adult bone marrow. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem cell biology. Cold Spring Harbor Laboratory Press; 2001. pp. 349–73. In:, editors.. New York: [Google Scholar]
- 37.D’lppolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–81. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 38.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4+ SSEA-1+ Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 39.Kadivar M, Khatami S, Mortazavi Y, et al. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–47. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 40.Kang XQ, Zang WJ, Bao LJ, et al. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461–5. doi: 10.3748/wjg.v11.i47.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung SC, Chen H, Pan CY, et al. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522–9. doi: 10.1634/stemcells.20-6-522. [DOI] [PubMed] [Google Scholar]
- 42.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multi-potent stromal cells (MSCs). The state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 43.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 44.Spees JL, Olson SD, Ylostalo J, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–64. [PubMed] [Google Scholar]
- 47.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz EW, Gordon PL, Koo WKK, et al. Isolated allogeneic bone marrow-derived mesenchymal stem cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lataillade JJ, Doucet C, Bey E, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–94. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 51.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 52.Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–41. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 53.Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stem cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 55.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 56.Koc ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 57.Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41. [PubMed] [Google Scholar]
- 58.Chamberlain G, Fox J, Ashton B, et al. Concise review. Mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 59.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–44. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 61.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 62.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakanishi C, Yamagishi M, Yamahara K, et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–6. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 65.Oettgen P. Cardiac stem cell therapy. Need for optimization of efficiency and safety monitoring. Circulation. 2006;114:353–8. doi: 10.1161/CIRCULATIONAHA.106.639385. [DOI] [PubMed] [Google Scholar]
- 66.Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14:53–60. doi: 10.1089/teb.2007.0216. [DOI] [PubMed] [Google Scholar]
- 67.Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 69.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in the repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortiz LA, DuTreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 74.Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 75.Li HH, Fu XB, Ouyang YS, et al. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;14:325–35. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 76.Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–51. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 77.Fu XB, Fang LJ, Li XK, et al. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Rep Reg. 2006;14:325–35. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 78.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/SCID mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao X, Song L, Shen K, et al. Transplantation of bone marrow derived cells promotes pancreatic islet repair in diabetic mice. Biochem Biophys Res Commun. 2008;371:132–7. doi: 10.1016/j.bbrc.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 80.Boumaza I, Srinivasan S, Witt WT, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009;32:33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Munoz JR, Stoutenger BR, Robinson AP, et al. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–6. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Urbán VS, Kiss J, Kovács J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 83.Shi PA, Hematti P, Von Kalle C, et al. Genetic marking as an approach to study in vivo hematopoiesis: progress in the non-human primate model. Oncogene. 2002;21:3274–83. doi: 10.1038/sj.onc.1205320. [DOI] [PubMed] [Google Scholar]
- 84.Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 85.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–34. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Fliedner TM, Nothdirft W, Calvo W. The development of radiation late effects to the bone marrow after single and chronic exposure. Int J Radiat Biol Stud Phys Chem Med. 1986;49:35–46. doi: 10.1080/09553008514552211. [DOI] [PubMed] [Google Scholar]
- 87.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27:1460–66. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 88.Madhusudhan T, Majumdar SS, Mukhopadhyay A. Degeneration of stroma reduces retention of homed cells in bone marrow of lethally irradiated mice. Stem Cell Dev. 2004;13:173–82. doi: 10.1089/154732804323046774. [DOI] [PubMed] [Google Scholar]
- 89.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 90.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 91.Mahmood A, Lu D, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 92.Mouiseddine M, François S, Semont A, et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80:S49–55. doi: 10.1259/bjr/25927054. Spec No 1: [DOI] [PubMed] [Google Scholar]
- 93.Devine SM, Cobbs C, Jennings M, et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 94.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 95.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–70. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 96.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 97.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cell differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 98.Pasha Z, Wang Y, Sheikh R, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 99.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 100.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–82. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrinβ1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–82. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herrera MB, Bussolati B, Bruno S, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–44. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 103.Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 104.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–27. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 105.Ji JF, He BP, Dheen ST, et al. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–27. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 106.Breitbach M, Bostani T, Roell W, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–9. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 107.Song L, Webb NE, Song Y, et al. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24:1707–18. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- 108.Chang SC, Chuang HL, Chen YR, et al. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther. 2003;10:2013–9. doi: 10.1038/sj.gt.3302106. [DOI] [PubMed] [Google Scholar]
- 109.Noel D, Gazit D, Bouquet C, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22:74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 110.Zhao M, Zhao Z, Koh JT, et al. Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4 and 7. J Cell Biochem. 2005;95:1–16. doi: 10.1002/jcb.20411. [DOI] [PubMed] [Google Scholar]
- 111.Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84:1093–103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- 112.Gersbach CA, Le Doux JM, Guldberg RE, et al. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13:873–82. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 113.Chamberlain JR, Schwarze U, Wang PR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 114.Goncalves MA, De Vries AA, Holkers M, et al. Human mesenchymal stem cells ectopically expressing full-length dystrophin can complement Duchenne muscular dystrophy myotubes by cell fusion. Human Mol Genet. 2005;15:213–21. doi: 10.1093/hmg/ddi438. [DOI] [PubMed] [Google Scholar]
- 115.Li Y, Zhang R, Qiao H, et al. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211:36–44. doi: 10.1002/jcp.20897. [DOI] [PubMed] [Google Scholar]
- 116.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infracted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 117.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 118.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim SS, Yoo SW, Park TS, et al. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–28. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- 120.Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 122.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 123.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 124.Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infracted myocardium. J Mol Cell Cardiol. 2008;44:281–92. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 126.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 127.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 128.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 129.Park J, Ries J, Gelse K, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–98. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 130.Bartholomew A, Patil S, Mackay A, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum. Gene Ther. 2001;12:1527–41. doi: 10.1089/10430340152480258. [DOI] [PubMed] [Google Scholar]
- 131.Campeau PM, Rafei M, Francois M, et al. Mesenchymal stromal cells engineered to express erythropoietin induce anti-erythropoietin antibodies and anemia in allorecipients. Mol Ther. 2009;17:369–72. doi: 10.1038/mt.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 133.Fang J, Zhu YY, Smiley E, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci USA. 1996;93:5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 135.Curran JM, Chen R, Hunt JA. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials. 2006;27:4783–93. doi: 10.1016/j.biomaterials.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 136.Vilquin JT, Rosset P. Mesenchymal stem cells in bone and cartilage repair: current status. Regen Med. 2006;1:589–604. doi: 10.2217/17460751.1.4.589. [DOI] [PubMed] [Google Scholar]
- 137.Arinzeh TL, Peter SJ, Archambault MP, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–35. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 138.Clark ERC. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–301. [Google Scholar]
- 139.Cassell OCS, Hofer SOP, Morrison WA, et al. Vascularisation of tissue-engineered grafts: the regulation of angiogenesis in reconstructive surgery and in disease states. Br J Plastic Surg. 2002;55:603–10. doi: 10.1054/bjps.2002.3950. [DOI] [PubMed] [Google Scholar]
- 140.Yang J, Zhou W, Zheng W, et al. Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology. 2007;107:17–29. doi: 10.1159/000093609. [DOI] [PubMed] [Google Scholar]
- 141.Rouwkema J, De Boer J, Van Blitterswijk CA. Endothelial cells assembly into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 142.Rouwkema J, Rivron NC, Van Blitterswijk CA. Vascularisation in tissue engineering. Trends Biotechnol. 2008;26:434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–64. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 144.Kadivar M, Khatami S, Mortazavi Y, et al. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–47. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 145.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–73. [PubMed] [Google Scholar]
- 147.Tisato V, Naresh K, Girdlestone J, et al. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21:1992–9. doi: 10.1038/sj.leu.2404847. [DOI] [PubMed] [Google Scholar]
- 148.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplant. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 149.Le Blanc K, Frassoni F, Ball L, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 150.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–98. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Ning H, Yand F, Jiang M, et al. The correlation between cotransplantation of MSC and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical trial. Leukemia. 2008;22:593–9. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 152.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Eng J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 153.Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973–82. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 154.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109:3147–51. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 156.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 157.Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shibata KR, Aoyama T, Shima Y, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25:2371–82. doi: 10.1634/stemcells.2007-0225. [DOI] [PubMed] [Google Scholar]
- 159.Bianchi G, Banfi A, Mastrogiacomo M, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 160.Ito T, Sawada R, Fujiwara Y, et al. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-β2. Biochem Biophys Res Commun. 2007;359:108–14. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 161.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–6. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 162.Miura M, Miura Y, Padilla-Nash HM, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 163.Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow-derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 164.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 165.Lazennec G, Jorgensen C. Adult mulipotent stromal cells and cancer: risk or benefit. Stem Cells. 2008;26:1387–94. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maestroni GJ, Hertens E, Galli P. Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55:663–7. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fierro Fa, Sierralta WD, Epuñan MJ, et al. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21:313–9. doi: 10.1023/b:clin.0000046130.79363.33. [DOI] [PubMed] [Google Scholar]
- 168.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of Dkk1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–91. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 169.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumor stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 170.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 171.Djouad F, Fritz V, Apparailly F, et al. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 172.Chamberlain J, Yamagami T, Colletti E, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935–45. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 173.Sémont A, François S, Mouiseddine M, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30. doi: 10.1007/978-0-387-34133-0_2. [DOI] [PubMed] [Google Scholar]
- 174.Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41. [PubMed] [Google Scholar]
- 175.Deng YB, Liu XG, Liu ZG, et al. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy. 2006;8:210–4. doi: 10.1080/14653240600760808. [DOI] [PubMed] [Google Scholar]
- 176.Arnhold S, Absenger Y, Klein H, et al. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007;245:414–22. doi: 10.1007/s00417-006-0382-7. [DOI] [PubMed] [Google Scholar]
- 177.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 178.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–21. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 179.Xu J, Lu Y, Ding F, et al. Reversal of diabetes in mice by intrahepatic injection of bone-derived GFP-murine mesenchymal stem cells infected with the recombinant retrovirus-carrying human insulin gene. World J Surg. 2007;31:1872–82. doi: 10.1007/s00268-007-9168-2. [DOI] [PubMed] [Google Scholar]
- 180.Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–84. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 181.Chang W, Kim JY, Lim S, et al. Mesenchymal stem cells with calreticulin gene modulate cell adhesiveness through an integrin-mediated mechanism. Tissue Eng Regen Med. 2006;3:327–35. [Google Scholar]
- 182.Dumont RJ, Dayoub H, Li JZ, et al. Ex vivo bone morphogenetic protein-9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239–45. doi: 10.1097/00006123-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 183.Rabin N, Kyriakou C, Coulton L, et al. A new xenograft model of myeloma bone disease demonstrating the efficacy of human mesenchymal stem cells expressing osteoprotegerin by lentiviral gene transfer. Leukemia. 2007;21:2181–91. doi: 10.1038/sj.leu.2404814. [DOI] [PubMed] [Google Scholar]
- 184.Min C-K, Kim B-G, Park G, et al. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:637–45. doi: 10.1038/sj.bmt.1705644. [DOI] [PubMed] [Google Scholar]
- 185.Kurozumi K, Nakamura K, Tamiya T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 2004;9:189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 186.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 187.Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14:894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 188.Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3404–16. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 189.Syková E, Jendelová P, Urdzíková L, et al. Bone marrow stem cells and polymer hydrogels – two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–29. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang J, Qi H, Wang H, et al. Engineering of vascular grafts with genetically modified bone marrow mesenchymal stem cells on poly (propylene carbonate) graft. Artif Organs. 2006;30:898–905. doi: 10.1111/j.1525-1594.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 191.Tu Q, Valverde P, Li S, et al. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng. 2007;13:2431–40. doi: 10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940–52. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guo CA, Liu XG, Huo JZ, et al. Novel gene-modified-tissue engineering of cartilage using stable transforming growth factor-β1-transfected mesenchymal stem cells grown on chitosan scaffolds. J Biosci Bioeng. 2007;103:547–56. doi: 10.1263/jbb.103.547. [DOI] [PubMed] [Google Scholar]
- 194.Mohyeddin-Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50–57. [PubMed] [Google Scholar]
- 195.Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 196.Kang KS, Kim SW, Oh YH, et al. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005;7:368–73. doi: 10.1080/14653240500238160. [DOI] [PubMed] [Google Scholar]


