Abstract
Background
The K-complex (KC) is a brain potential characteristic of non-rapid eye movement (NREM) sleep resulting from the synchronous activity of a large population of neurons and hypothesized to reflect brain integrity. KC amplitude is lower in individuals with Alcohol Use Disorder (AUD) compared with age-matched controls but its recovery with short-term abstinence has not been studied. Therefore, we investigated whether the KC shows significant recovery over the first four months of abstinence in individuals with AUD.
Methods
16 recently abstinent AUD (46.6 ± 9.3 years) and 13 gender and age-matched healthy controls (41.6 ± 8.3 years) were studied on three occasions: the Initial session was within 1 month of the AUD last drink, then 1 month and 3 months later. Overnight electroencephalogram (EEG) was recorded while participants were presented with tones during stage 2 NREM sleep to elicit KCs.
Results
At the Initial session, AUD showed significantly lower KC amplitude and incidence compared with controls. In the AUD individuals, KC amplitude increased significantly from the Initial to the 1 month session. KC incidence showed a marginally significant increase. Neither KC amplitude nor incidence changed from the 1 month to the 3 month session. No changes in KC amplitude or incidence across sessions were observed in the control group.
Conclusions
Our results demonstrate partial KC recovery during the first two months of abstinence. This recovery is consistent with the time course of structural brain recovery in abstinent AUD demonstrated by recent neuroimaging results.
Keywords: K-complex, EEG, sleep, abstinence, Alcohol Use Disorder
Introduction
Alcohol Use Disorder (AUD) is not only a significant public health problem but also debilitating to AUD themselves. Besides the well-known physical and psychological problems AUD face (Rehm 2011), they also suffer from persistent sleep difficulties – both during the period of dependence and during abstinence (Brower et al. 2001; Arnedt et al. 2007). Even long into abstinence, AUD have difficulty falling asleep, increased sleep fragmentation, reduced slow wave sleep and increased rapid eye movement (REM) sleep compared with healthy, age-matched controls. It has been hypothesized that one major factor in relapse is self-medication with alcohol to alleviate sleep problems (for comprehensive reviews of alcohol’s effects on sleep, see (Brower 2003) and (Colrain et al. 2014)).
One of the most consistent findings in the sleep electroencephalogram (EEG) of abstinent AUD is a lower slow wave delta power (0.3–4Hz range) compared with healthy individuals, which has also been shown to predict relapse (Brower 2003). Abstinent AUD also show a lower incidence and amplitude of K-complexes (KC) (Colrain et al. 2009 ) – large, slow potentials characteristic of stage 2 non-rapid eye movement (NREM) sleep, thought to be single instances of a delta frequency wave (Colrain 2005). While KCs occur spontaneously, they can also be evoked using external stimuli. In experimental settings, this is typically done by playing a short auditory tone, although other stimuli can also be used (e.g. airway occlusions) (Gora et al. 1999). The KC waveform consists of three easily identifiable components – the P200, the N550 and the P900. The N550 (a negative going deflection peaking approximately 550ms following stimulus presentation) is the most prominent component whose amplitude can reach over 100μV and is largest over frontal electrode sites. The exact functional role of the KC is still unknown. It is thought to be a forerunner of slow waves (De Gennaro et al. 2000) and there is evidence to suggest that it reflects a process that protects sleep from disruption (Nicholas et al. 2002; Czisch et al. 2009). More recent evidence suggests that KCs limit normal cortical processing of external stimuli by inducing a widespread “down-state” (i.e. a period of reduced firing) of cortical neurons (Cash et al. 2009; Laurino et al. 2014; Mak-McCully et al. 2014). While much less is known about the P200 component than the N550 (Crowley et al. 2004), recent research suggests it may be related to KC generation (Laurino et al. 2014).
Aging and AUD appear to have parallel effects on both the KC and brain structural integrity, leading to the hypothesis that N550 amplitude is related to quality of brain integrity (Colrain et al. 2010). Both KC incidence and N550 amplitude are reduced with age (Crowley et al. 2002; Colrain et al. 2010) and in abstinent AUD (Nicholas et al. 2002; Colrain et al. 2009). Evidence from a regression analysis of 42 abstinent AUD and 42 healthy age-matched controls over a wide age range suggests the effects of long-term AUD on N550 amplitude are, on average, equivalent to an extra 23 years of aging (Colrain et al. 2009). Brain structure is also compromised in both aging (Resnick et al. 2003; Sullivan et al. 2007) and AUD populations (Pfefferbaum et al. 1988; Chanraud et al. 2007) with the frontal cortex being particularly susceptible (Abernathy et al. 2010). Consistent with this hypothesis, in a cross-sectional study of 40 AUD and 40 healthy controls (Colrain et al. 2011) we previously demonstrated that cortical grey matter volume significantly predicted N550 amplitude at all seven electrode recording sites. AUD diagnosis improved N550 amplitude prediction, but only at frontal electrode sites, consistent with alcohol’s predominant effects at frontal brain regions.
After long-term abstinence (at least eight months), several studies have shown recovery in brain structure and metabolic function (Johnson-Greene et al. 1997; Cardenas et al. 2007; Alhassoon et al. 2012). However, a number of studies have also demonstrated that recovery starts within the first weeks of abstinence. (Pfefferbaum et al. 1995) demonstrated both an increase in anterior grey matter volume and a decrease in posterior sulcal and lateral ventricle volume (indicating an increase in brain volume) after only 21 days of abstinence. (Gazdzinski et al. 2005) showed similar results of increased tissue volume and decreased sulcal and ventricle size after one month of abstinence; in addition, they also noted that while abstinent AUD continued to gain brain volume over the next 6–12 months, the rate of gain was much slower than that observed in the first month. (Bartsch et al. 2007) also reported both increased tissue volume and an increase in frontomedial N-acetylaspartate (NAA) following 6–7 weeks of abstinence. NAA is an amino acid present at high concentrations in the CNS and while its functions remain something of a mystery, it is thought to be involved in both neuronal energy production and axonal myelination by oligodendrocytes. Both these functions are consistent with the hypothesis that brain volume increase with abstinence is at least partly due to dendritic re-arborization and glial recovery (Harper et al. 1990).. A more recent study by (van Eijk et al. 2013) demonstrated decreased cerebro-spinal fluid (CSF) volume and increased grey matter volume (but no change in white matter volume) following just two weeks of abstinence.
We have previously investigated evoked KC recovery during sleep in 15 abstinent AUD over the course of a year (Colrain et al. 2012). The results demonstrated recovery of N550 and P900 amplitudes at anterior scalp sites, although there was no evidence of change in the P200 component. However, the initial measurements were taken after several months (2–14) of abstinence. Given the evidence of early recovery in brain structure, we designed the current study to investigate short term KC recovery in AUD during the first few months of abstinence. We predicted that KC incidence and frontal N550 amplitude would be smaller in abstinent AUD than control participants and that AUD would show significant recovery between the Initial and 1 month sessions with further recovery from the 1 month to 3 month session.
Materials and Methods
Participants
Seventeen recently abstinent and detoxified AUD and thirteen healthy controls, matched for age and sex, participated in this study. AUD were recruited from local residential treatment centers and controls from the local community. Exclusion criteria were: current use of psychoactive medication, severe current or past medical or psychiatric disorder, head injury with associated loss of consciousness for more than 30 minutes, alcohol withdrawal symptoms (for the AUD individuals) and clinically significant sleep apnea. All participants completed a structured alcohol history (Pfefferbaum et al. 1988) and the structured clinical interview for DSM-IV-TR for Axis I DSM IV disorders(First et al. 1994). All AUD met the DSM-IV-TR criteria for alcohol dependence for at least three years. Eleven had no other drug dependency apart from nicotine (n=5). Of the remaining six AUD, three met criteria for amphetamine dependence, one for both cannabis and amphetamine dependence, one for both cannabis and opioid dependence and one for both cannabis and cocaine dependence. Control participants did not meet the criteria for any Axis I psychopathology. Three AUD relapsed between the Initial and 1 month follow-up session and a further five relapsed between the 1 month and 3 month follow-up sessions. Two control participants missed their 1 month follow-up session and one participant withdrew from the study following the 1 month follow-up session. Two night’s data were lost due to technical problems – the Initial session for one AUD patient and the 1 month follow-up session for one control participant. Participant numbers, demographic details and alcohol consumption history are shown in Table 1.
Table 1.
Participant numbers, demographic details and alcohol consumption history for AUD and control participants. Where shown, means and standard deviations (in parentheses) reflect the values for participants at the Initial session. P-values are derived from independent samples t-tests of Initial session values.
| Controls | AUD | p-value | |
|---|---|---|---|
| Number | Initial: 13 1 month: 10 3 month: 12 |
Initial: 17 1 month: 14 3 month: 9 |
- |
| Sex | F: 6 M: 7 |
F: 7 M: 10 |
- |
| Age (years) | 46.62 (9.29) | 41.59 (8.25) | p = 0.13 |
| Education (years) | 17.14 (2.14) | 12.41 (1.80) | p < 0.001 |
| Ethnicity | Caucasian: 9 African-American: 1 Hispanic: 1 Asian: 2 |
Caucasian: 12 African-American: 2 Hispanic: 3 Asian: 0 |
- |
| Body Mass Index (kg/m2) | 24.45 (3.30) | 26.16 (3.33) | p = 0.16 |
| Time since last drink (days) | - | 17.12 (6.75) | - |
| Length of alcohol dependence (years) | - | 15.41 (8.73) | - |
| Estimated Lifetime Alcohol Consumption (kg) | 49.57 (112.55) | 1436.12 (1268.14) | p < 0.001 |
The study was approved by the Institutional Review Board at SRI International. All participants gave informed consent prior to the study and were compensated for their participation.
Procedure
The experiment was conducted at the Human Sleep Laboratory at SRI International. Prior to the Initial experimental session, participants underwent a clinical polysomnography (PSG) night to screen for major sleep disorders and to acclimatize participants to the sleep lab environment. For AUD individuals, the Initial session was conducted within one month of their last drink (17.12±6.75 days). Follow-up sessions were conducted 1 month and 3 months subsequently, using identical procedures. Inter-session intervals for control participants were the same duration as for AUD individuals. The mean duration between the Initial session and 1 month follow-up for AUD was 30.31±7.54 days, and 35.55±14.50 days for controls; between the 1 month follow-up and 3 month follow-up it was 54.75±6.18 days for AUD and 62.70±13.00 days for controls. A breath alcohol concentration test (S75 Pro, BACtrack Breathalyzers, San Francisco, CA, USA) confirmed none of the participants had consumed alcohol (Breath Alcohol Concentration = 0.0%) prior to testing. Sleep on the nights prior to study was not controlled or monitored.
When participants had completed 30 minutes of stable NREM sleep, tones were presented binaurally using Compumedics NeuroScan Stim software (Compumedics Ltd, Abbotsford, Victoria, Australia) though E-A-RTONE 3A insert earphones (3M Auditory Systems, Indianapolis, IN, USA). The tones were of 1000Hz frequency, presented at 80dB for 50ms (2ms rise and fall time) with a random inter-stimulus interval of 15–30s as per our standard laboratory practice (Colrain et al. 1999; Colrain et al. 2012). All participants knew they were going to be presented with tones during the night, reported normal hearing and confirmed they could hear the tones before they went to sleep. Presentation of the tones was controlled automatically, monitored by a sleep technician throughout the night. If the participants showed signs of arousal or transitioned to REM sleep, the sleep technician paused the presentation of the tones until at least 10 minutes of stable NREM sleep had occurred.
PSG/EEG recording
The polysomnogram (PSG) recording consisted of nine electroencephalogram (EEG) electrodes (Fp1, FP2, Fz, FCz, Cz, CPz, Pz, O1 and O2), two electrooculogram (EOG) electrodes (placed one centimeter outside and below the outer canthus of each eye) and two submental chin electromyogram (EMG) electrodes. Data were recorded at 1000Hz using Compumedics NeuroScan Synamps amplifiers. Thirty second epochs were classified according to standard criteria into wake, N1, N2, N3 and REM stages (Iber et al. 2007).
EEG analysis
The EEG was analyzed offline using the EEGLAB (Delorme et al. 2004) and ERPLAB (Lopez-Calderon et al. 2014) toolboxes for MATLAB (MathWorks, Natick, MA, USA). EEG was re-referenced to the average mastoid and filtered at 0.3–30Hz using a 4th order Butterworth filter. Epochs 500ms before to 2000ms after tone presentation were extracted and baseline corrected to the 500ms pre-stimulus period. Epochs of N2 sleep containing a KC elicited by the tone were identified by visual inspection of the Fz and Cz channels according to our standard laboratory procedure (Crowley et al. 2002; Colrain et al. 2009) and blind to group membership. For a KC to be considered stimulus-related, its negative peak had to occur 400–900ms following tone presentation; because differences in KC amplitude were predicted, no amplitude criterion was used in identifying KCs. Epochs showing significant artefactual activity were removed from any further analysis. KC incidence was calculated as the proportion of artefact-free epochs containing a tone-related KC. Trials on which a KC was present (KC+ trials) were averaged to create a mean KC waveform for each participant. N550 amplitude was calculated as the mean of the 11ms around the maximum negative peak 400–900ms following tone presentation (i.e. the average of the maximum plus five data points either side). P200 amplitude was calculated using the same technique around the maximum positive peak 100–400ms following tone presentation.
Statistical analysis
In order to test differences between AUD and control groups, independent sample t-tests were conducted on data collected at the Initial session. Due to the different subject numbers at each session, paired t-tests were used to assess any changes within participant groups between the Initial and 1 month follow-up sessions, and between the 1 month and the 3 month follow-up sessions. Because we made a priori predictions of increased KC incidence and N550 amplitude across sessions in AUD individuals, a one-tailed test was used. The Benjamini-Hochberg procedure was applied to control for false discovery rate and corrected p-values are reported. To explore possible differences in KC amplitude recovery across electrode sites within the AUD group, we used separate repeated measures ANOVAs with electrode and session as within-subject factors to test for differences between the Initial and 1 month sessions, and between the 1 month and 3 month sessions. To ensure anterior-posterior scalp coverage, electrodes Fp1, FCz, CPz and O1 were used in the repeated measures ANOVAs. Results were similar when the analysis included just electrodes Fz, Cz and Pz, or all nine electrodes. The Greenhouse-Geisser correction was applied to correct for violations of sphericity. Finally, exploratory analyses were conducted to compare differences between groups in N550 amplitude and KC incidence at each time point, using independent t-tests.
Results
K-complex Incidence
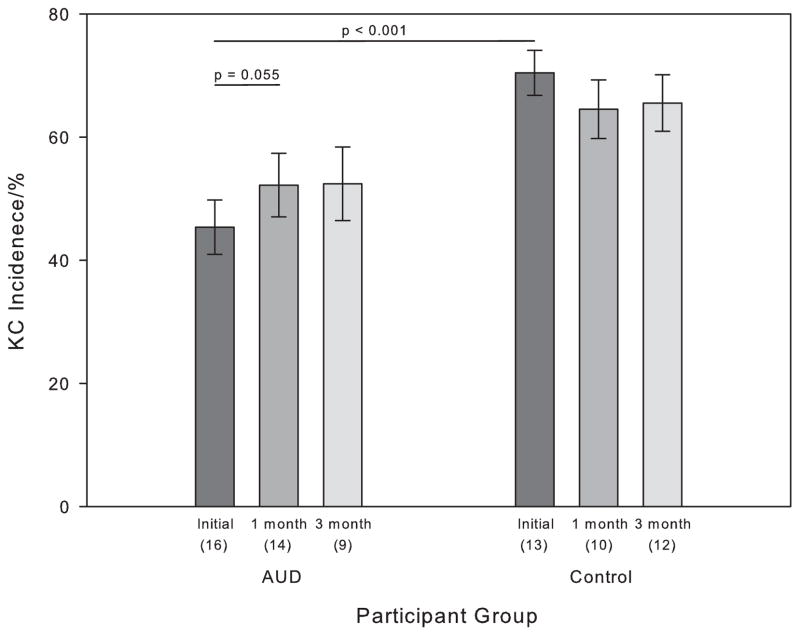
The numbers of tones presented in N2 sleep did not differ between groups or nights (Alcoholics: Baseline 219±71; 1 month 211±80; 3 month 181±83; Controls: baseline 200±30; 1 month 236±70; 3 months 192±76). Figure 1 shows KC incidence for AUD and control participants at each session (for all participants available at each session). Within the group of 13 AUD who completed both Initial and 1-month follow-up sessions, there was a trend for increased KC incidence (Initial session: 41.92±17.31%; 1 month session: 50.41±18.87%; t(12) = −2.13, p = 0.055). There was no difference in KC incidence between the 1 month (53.62±21.35%) and 3 month (52.41±17.92%) follow-up sessions (N = 9, p = 0.80). For the control participants, there were no differences in KC incidence between the Initial and 1 month sessions (N = 10, p = 0.41) or between the 1 month and 3 month sessions (N = 9, p = 0.31). KC incidence at the Initial session was significantly lower in AUD (45.36±17.69%) compared with controls (70.45±13.15%; t(27) = 4.24, p < 0.001). There was a trend for the difference between AUD (52.19±19.31%) and controls (64.54±15.02%) to persist at 1 month (t(22) = 1.69, p = 0.053). At 3 months, KC incidence was again significantly lower in AUD (52.41±17.91%) compared with controls (65.54±15.91%; t(19) = 1.78, p = 0.046).
Figure 1.
KC incidence in AUD and Control participants over the three sessions. All available subjects for each session are plotted - the number of subjects contributing to the overall mean is shown in parentheses below the bar. Error bars represent the standard error of the mean.
N550 Amplitude
Because the N550 is typically largest over frontal brain regions, we tested amplitude differences at electrode Fz.
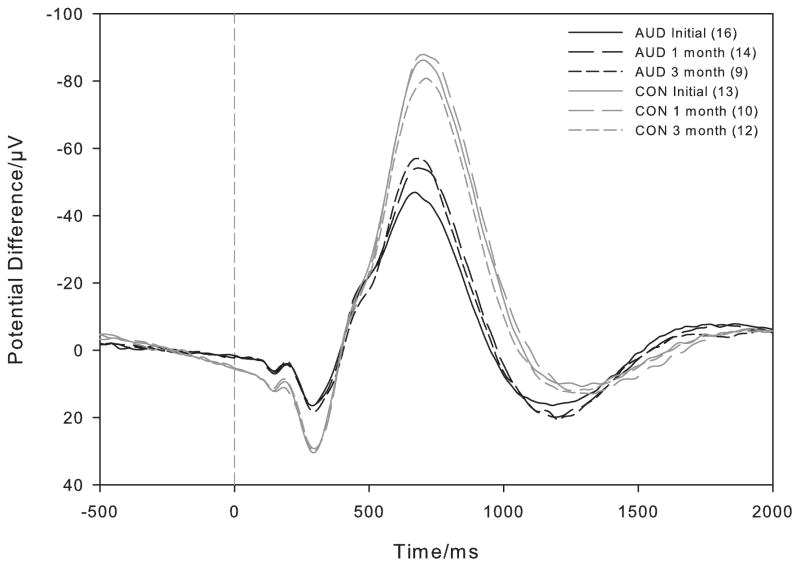
Within the group of AUD who returned for a 1 month follow-up session, N550 amplitude at Fz increased from the Initial (−47.03±15.67μV) to the 1 month follow-up session (−56.88±22.04μV; t(12) = 2.91, p = 0.013). There was no significant difference in N550 amplitude between the 1 month (−56.06±20.00μV) and 3 month (−60.26±22.49μV; t(8) = 1.38, p = 0.21) follow-up sessions in AUD (Figure 2, dark lines). There were no changes in N550 amplitude in the control group between the Initial and 1 month sessions (n = 10, p = 0.36) or between the 1 month and 3 month sessions (n = 9, p = 0.90; Figure 2, light lines).
Figure 2.
Overall KC+ waveforms at electrode Fz for AUD (dark) and Control participants (light) over the Initial (solid line), 1 month (long dashed line) and 3 month (short dashed line) follow-up sessions. All available subjects for each session are plotted - the number of subjects contributing to the overall mean is shown in parentheses in the legend. The tone was presented at 0ms and negative values are plotted up.
At the Initial session, the amplitude of the N550 generated by AUD at Fz (−50.68±22.19μV) was significantly smaller than that generated by controls (−87.76±25.65μV; t(27) = −4.17, p < 0.001). Differences persisted at 1 month (AUD: −59.62±23.52μV; control: −94.32±32.08μV; t(22) = −3.06, p = 0.003) and at three months (AUD: −60.26±22.49μV; control: −83.66±28.33μV; t(19) = −2.04, p = 0.028).
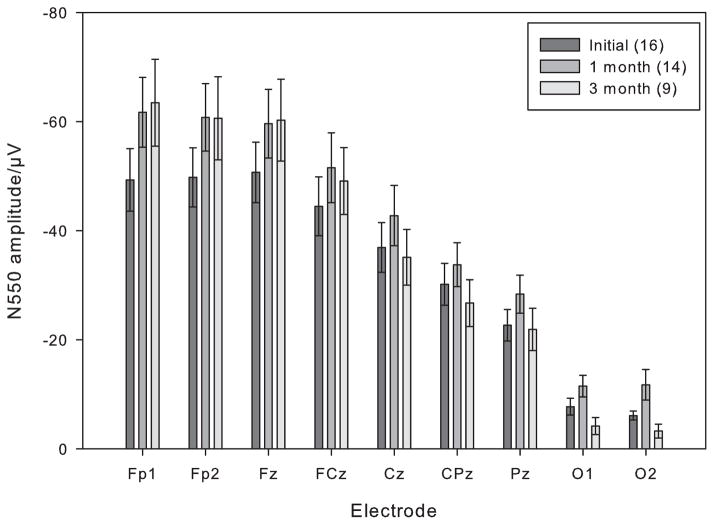
Figure 3 shows N550 amplitude for AUD across all nine electrode sites at each session (for all participants available at each session). These results show the typical pattern of reduction in N550 amplitude from anterior to posterior electrode sites. The repeated measures ANOVA comparing recovery in N550 amplitude in AUD at electrodes Fp1, FCz, CPz and O1 from the Initial to 1-month follow up session revealed significant main effects for Electrode (F(3.36) = 60.40, p < 0.001), Session (F(1,12) = 8.06, p = 0.015) but no Electrode x Session interaction (p = 0.068). This suggests there was a similar increase in amplitude from the Initial to the 1 month follow-up session across all electrode sites. The comparison of N550 amplitude between the 1 month and 3 month follow-up sessions again showed a main effect for Electrode (F(3,24) = 39.96, p < 0.001), but no effect for Session (p = 0.47). However, there was a Session x Electrode interaction (F(3.24) = 5.98, p = 0.019), reflecting a decrease in N550 amplitude at posterior electrode sites. Post hoc paired sample t-tests revealed that there was a significant decrease in N550 amplitude at O1 in the AUD group between the 1 month and 3 month follow-up sessions (t (8) = −2.46, p = 0.039, two-tailed) but not at the other electrode locations (all p values > 0.15).
Figure 3.
N550 amplitude for AUD across all the electrode sites for the Initial (dark), 1 month (medium) and 3 month (light) follow-up sessions. All available subjects for each session are plotted - the number of subjects contributing to the overall mean is shown in parentheses below the bar. Error bars represent the standard error of the mean.
P200 Amplitude
We tested P200 amplitude differences at electrode Cz, because its amplitude is largest at central sites. As with the N550 amplitude, P200 amplitude was significantly greater in controls than AUD at the Initial session (Control: 35.78±13.12μV; AUD: 21.25±9.74μV; t(27) = 3.42, p = 0.002). However, there were no significant changes in P200 amplitude across sessions in either the AUD or the control group (all p values > 0.5).
Discussion
The data demonstrated partial KC recovery within the first two months of sobriety in AUD – there was a substantial and statistically significant increase in N550 amplitude from the Initial to the 1 month follow-up session (approximately 10μV or 20%) and a marginally significant increase in KC incidence over the same period. Consistent with previous studies (Nicholas et al. 2002; Colrain et al. 2009; Colrain et al. 2012), our results provide evidence that KC incidence and N550 amplitude is reduced in abstinent AUD compared with matched controls.
Using a similar experimental protocol, (Colrain et al. 2012) reported an increase in amplitude of approximately 12μV (approximately 42%) after 12 months of abstinence following initial assessment. While these results are similar to the ones reported here, there are several reasons why it is difficult to directly compare the two data sets. There are differences in participants’ age, years of alcohol dependence, total volume of alcohol consumed, and length of sobriety at the first visit. The AUD in the present study are, on average, around 10 years younger and have 14 fewer years of alcohol dependence than those reported in (Colrain et al. 2012). Despite these differences, the present participants reported a higher lifetime volume of alcohol consumed (1436 ± 1268kg) than those in (Colrain et al. 2012) (1283 ± 814kg), which can be explained by the exclusive recruitment from inpatient rehabilitation centers in the present study. This nonetheless highlights an important interaction between age and alcoholism. Despite the higher volume of alcohol consumed, the present AUD had substantially larger N550 components than the older AUD reported in (Colrain et al. 2012) and (Colrain et al. 2009), consistent with the strong effect of age on N550 amplitude (Colrain et al. 2010) and the lack of an effect of volume of alcohol consumed on N550 amplitude in (Colrain et al. 2009). Finally, while the present experiment was designed to study AUD at the Initial session within the first month of abstinence, the AUD in (Colrain et al. 2012) had been sober for a variable duration, ranging between 2–14 months, at the initial visit. Therefore, much of the recovery observed in the present study at the 1 month follow-up may already have already occurred before the baseline assessment in (Colrain et al. 2012). Nevertheless, the implication of these two studies is that there is rapid KC recovery within the first two months of abstinence followed by an extended period of slower recovery. The results of the exploratory t-tests comparing the two groups at each time point need to be interpreted with caution given the small numbers of subjects and the variable numbers in each group at 1 month and 3 months relative to baseline. Nonetheless, they highlight that despite some beneficial effects of abstinence, AUD do not fully recover, within the period studied, to levels seen in age-matched controls.
The time course of KC recovery demonstrated here appears similar to that of brain structural recovery reported by others (Pfefferbaum et al. 1995; Gazdzinski et al. 2005; Bartsch et al. 2007; van Eijk et al. 2013). One particularly striking parallel is that between the results of this study and those of (Gazdzinski et al. 2005), who demonstrated rapid increase in brain tissue volume in the first month of abstinence, followed by continued, but slower recovery over the following 6–12 months. Given the dependence of KC amplitude on the synchronous activity of a large population of healthy neurons, it is likely that brain structural recovery underlies, at least in part, the increase in KC amplitude that we observed.
One feature of brain recovery studies in humans is they primarily investigate changes in gross brain volume with abstinence, which are thought to reflect neural re-arborization and glial recovery (Harper et al. 1990). However, in addition to these morphological changes, alcohol also has many effects at the synaptic and neurotransmitter levels. GABA is an inhibitory neurotransmitter thought to be involved in EEG delta generation (Steriade et al. 1993) and in animal models of AUD the GABAA receptor subtype shows both pre-synaptic and post-synaptic down-regulation (Lovinger et al. 2013). Alcohol also inhibits the excitatory glutamatergic neurotransmitter system, with sustained alcohol use leading to up-regulation of glutamatergic activity (Lovinger et al. 2013) including metabotropic glutamate receptors, which have also been implicated in delta generation (Hughes et al. 2002). Prolonged exposure also results in down-regulation of adenosine receptors (Sharma et al. 2010) which alter the neuromodulatory effects of adenosine on multiple neurotransmitter systems (Dohrman et al. 1997 ; Nam et al. 2012; Sharma et al. 2014). Abstinence from alcohol in AUD individuals, therefore, leads to an immediate imbalance in these neurotransmitter systems which is, in part, responsible for many of the acute withdrawal symptoms. The increase in KC amplitude seen between the Initial and 1 month sessions may therefore reflect recovery in neurochemical systems in addition to structural recovery. Unfortunately, animal model data showing the time course of recovery of neurotransmitter systems following cessation of alcohol administration are currently lacking. Given that the risk of seizure activity passes within days (Foy et al. 1997), it is possible that GABAergic and glutamatergic systems recover very quickly. However, at least in the case of adenosine, other manipulations (such as sleep deprivation) have been shown to have effects, including increased sensitivity to acute alcohol, that last for several weeks (Clasadonte et al. 2014).
While this study investigated an event-related potential (ERP) during sleep, others have investigated recovery of task-related ERPs during wakefulness in abstinent AUD individuals. Results have generally been inconsistent; for example, (Fein et al. 2004) reported no difference in the mismatch negativity in long-term abstinent AUD individuals, whereas (Ji et al. 1999) demonstrated electrophysiological differences in a category matching task in short-term abstinent AUD individuals. However, results of experiments investigating the P3 component sound a cautionary note in interpreting findings from studies of recovering AUD individuals. Early research showed decreased P3 amplitude in AUD (Porjesz et al. 1981) which later studies showed was preserved in abstinence (Emmerson et al. 1987; Porjesz et al. 1987). However, further research revealed that the P3 is reduced in non-alcoholic relatives of AUD individuals, suggesting a common genetic trait that may result in a reduction in P3 amplitude in AUD prior to drinking (Begleiter et al. 1984) These studies highlight the need for both careful interpretation of experimental results and for well-designed prospective longitudinal studies to investigate genetic predispositions that can manifest as ERP differences. Similarly, in our experiment, we cannot rule out the possibility that KC incidence and N550 amplitude are lower in AUD than controls prior to drinking. However, our finding of partial recovery with abstinence suggests heavy alcohol consumption or alcohol dependence has a damaging effect, even if some premorbid deficits are present.
An unanticipated finding in this study was the pattern of recovery across electrode sites. The increase in N550 amplitude found by (Colrain et al. 2012) was observed only at frontal and fronto-central electrode sites. While a similar pattern of results was seen in this experiment at the 3 month follow-up session, at the 1 month session, N550 amplitude appeared larger at all electrode sites. This finding suggests that there may be a non-specific acute recovery across all brain regions early in abstinence that then develops into recovery of more functionally specific networks. This explanation is highly speculative and further research needs to be conducted to investigate the exact topography of KC recovery.
In addition to the results concerning the N550, we also showed a smaller P200 amplitude in the AUD group than the controls that showed no change with recovery. (Crowley et al. 2004)Laurino et al. (2014) have recently suggested that the P200 may be related to sensory stimulus processing that activates a frontally-mediated KC generation process(Laurino et al. 2014). While (Mak-McCully et al. 2014) suggest that the KC need not be generated in the frontal regions, they do propose a cortico-thalamic mechanism involving frontal pyramidal cortical cells that project widely to reticular nucleus neurons which results in widespread cortical inactivity. While we might tentatively interpret our results as a reflection of diminished sensory processing and KC generation in the AUD group, much more research is needed to investigate the P200 component and its involvement in KC initiation.
Our results should be interpreted within the context of the study limitations. A major problem in any longitudinal study involving AUD is participant recruitment and retention, as a significant proportion of recovering AUD relapse (Brower 2003). While the AUD in this study showed a typical relapse rate, recruitment of a larger number would have been beneficial. Because of low participant numbers and high relapse rate, we did not have a sufficient sample size to conduct further analyses, such as whether KC recovery predicted relapse. Another difficult issue surrounding alcoholism is that AUD frequently suffer from a variety of comorbidities. While the recruitment procedures screened out participants with major psychological or neurological dysfunction, a number of our participants were comorbid for other substance. As yet, nothing is known about the effects of addiction to other substances on the KC; further studies are needed to investigate this. However, in spite of these limitations, we were able to show clear recovery in N550 amplitude within the first two months of abstinence.
In conclusion, our results are consistent with previous research showing impairments in KC generation in AUD which improves with abstinence; and extends it by clearly showing substantial and significant KC recovery early in abstinence.
Acknowledgments
This research was funded by grant AA02565 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Portions of this research were presented at the annual conference of the American Academy of Sleep Medicine and the Sleep Research Society, June 2014, Minneapolis MN, and at the joint meeting of the Research Society on Alcoholism and the International Society of Brain Research on Alcoholism, June 2014, Bellevue, WA.
Conflict of Interest
None of the authors report any biomedical financial interests or potential conflicts of interest.
References
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, Stephan RA, Schweinsburg BC, Stricker NH, Gongvatana A, Grant I. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36:1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26:41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I. The human K-complex represents an isolated cortical down-state. Science. 2009;324:1084–1087. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci. 2014;34:1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM. The K-complex: A seven-decade history. Sleep. 2005;28:255–273. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Afifi L, Baker FC, Padilla M, Turlington SR, Trinder J. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiology of Aging. 2010;31:874–883. doi: 10.1016/j.neurobiolaging.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Crowley KE, Nicholas CL, Padilla ML, Baker FC. The impact of alcoholism on sleep evoked Delta frequency responses. Biological Psychiatry. 2009;66:177–184. doi: 10.1016/j.biopsych.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain. Handb Clin Neurol. 2014;125:415–431. doi: 10.1016/B978-0-444-62619-6.00024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Padilla ML, Baker FC. Partial recovery of alcohol dependence-related deficits in sleep evoked potentials following twelve months of abstinence. Frontiers in Neurology. 2012 doi: 10.3389/fneur.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Sullivan EV, Rohlfing T, A-LP, Chanraud S, Pfefferbaum A. Relationship between gray matter volume and sleep-specific delta EEG changes in alcoholic men and women. Alcoholism: Clinical and Experimental Research. 2010;34:23A. [Google Scholar]
- Colrain IM, Sullivan EV, Rohlfing T, Baker FC, Nicholas CL, Padilla ML, Chanraud S, Pitel AL, Pfefferbaum A. Independent contributions of cortical gray matter, aging, sex and alcoholism to K-complex amplitude evoked during sleep. Sleep. 2011;34:787–795. doi: 10.5665/SLEEP.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8:273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11:129–140. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Stiegler A, Peters H, Andrade K, Holsboer F, Samann PG. Acoustic oddball during NREM sleep: a combined EEG/fMRI study. PLoS One. 2009;4:e6749. doi: 10.1371/journal.pone.0006749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M. The spontaneous K-complex during stage 2 sleep: is it the ‘forerunner’ of delta waves? Neuroscience Letters. 2000;291:41–43. doi: 10.1016/s0304-3940(00)01366-5. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dohrman DP, Diamond I, Gordon AS. The role of the neuromodulator adenosine in alcohol’s actions. Alcohol Health Res World. 1997;21:136–143. [PMC free article] [PubMed] [Google Scholar]
- Emmerson RY, Dustman RE, Shearer DE, Chamberlin HM. EEG, visually evoked and event related potentials in young abstinent alcoholics. Alcohol. 1987;4:241–248. doi: 10.1016/0741-8329(87)90018-8. [DOI] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Mismatch negativity: no difference between treatment-naive alcoholics and controls. Alcohol Clin Exp Res. 2004;28:1861–1866. doi: 10.1097/01.alc.0000148109.79230.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM IV disorders. New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90:253–261. doi: 10.1093/qjmed/90.4.253. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gora J, Colrain IM, Trinder J. Respiratory-related evoked potentials during the transition from alpha to theta EEG activity in stage 1 NREM sleep. J Sleep Res. 1999;8:123–134. doi: 10.1046/j.1365-2869.1999.00144.x. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of alcoholism. Alcohol and Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifciation. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Ji J, Porjesz B, Begleiter H. Event-related potential index of semantic mnemonic dysfunction in abstinent alcoholics. Biol Psychiatry. 1999;45:494–507. doi: 10.1016/s0006-3223(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, Koeppe RA, Junck L, Kluin KJ, Martorello S, Heumann M. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J Clin Exp Neuropsychol. 1997;19:378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Laurino M, Menicucci D, Piarulli A, Mastorci F, Bedini R, Allegrini P, Gemignani A. Disentangling different functional roles of evoked K-complex components: Mapping the sleeping brain while quenching sensory processing. Neuroimage. 2014;86:433–445. doi: 10.1016/j.neuroimage.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak-McCully RA, Deiss SR, Rosen BQ, Jung KY, Sejnowski TJ, Bastuji H, Rey M, Cash SS, Bazhenov M, Halgren E. Synchronization of isolated downstates (K-complexes) may be caused by cortically-induced disruption of thalamic spindling. PLoS Comput Biol. 2014;10:e1003855. doi: 10.1371/journal.pcbi.1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, McIver SR, Hinton DJ, Thakkar MM, Sari Y, Parkinson FE, Haydon PG, Choi DS. Adenosine and glutamate signaling in neuron-glial interactions: implications in alcoholism and sleep disorders. Alcohol Clin Exp Res. 2012;36:1117–1125. doi: 10.1111/j.1530-0277.2011.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. Journal of Sleep Research. 2002;11:247–253. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- Nicholas CL, Trinder J, Colrain IM. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep. 2002;25:882–887. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human evoked brain potentials and alcohol. Alcohol Clin Exp Res. 1981;5:304–317. doi: 10.1111/j.1530-0277.1981.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987;4:283–287. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135–143. [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Engemann SC, Sahota P, Thakkar MM. Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res. 2010;34:813–818. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Sahota P, Thakkar MM. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep. 2014;37:525–533. doi: 10.5665/sleep.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroradiological characterization of normal adult ageing. British Journal of Radiology. 2007;80(Spec No 2):S99–108. doi: 10.1259/bjr/22893432. [DOI] [PubMed] [Google Scholar]
- van Eijk J, Demirakca T, Frischknecht U, Hermann D, Mann K, Ende G. Rapid partial regeneration of brain volume during the first 14 days of abstinence from alcohol. Alcohol Clin Exp Res. 2013;37:67–74. doi: 10.1111/j.1530-0277.2012.01853.x. [DOI] [PubMed] [Google Scholar]