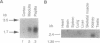Abstract
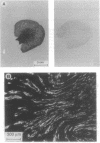
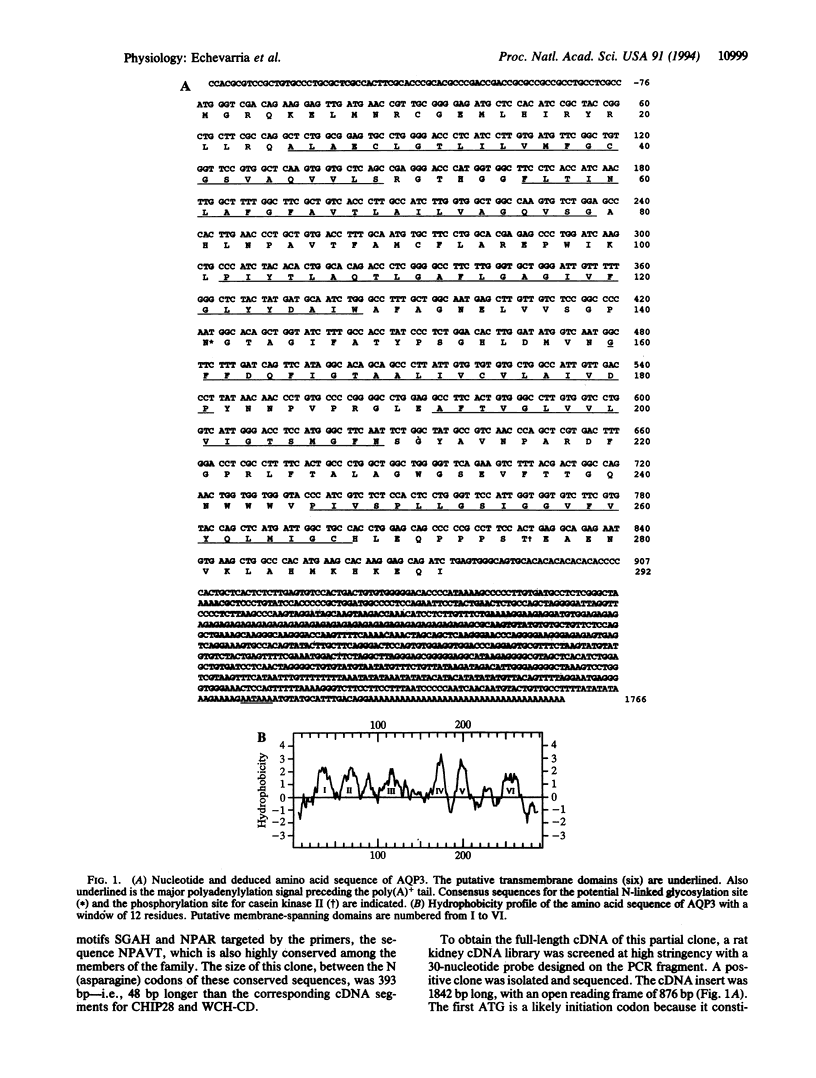
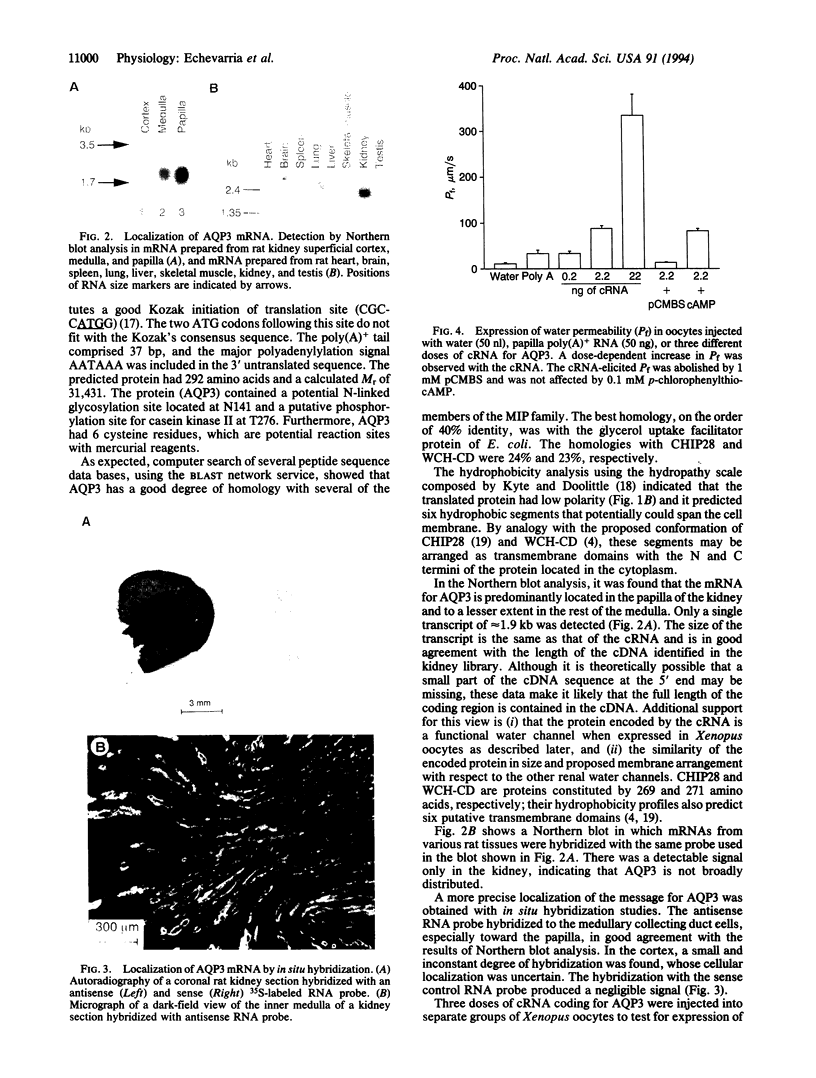
The terminal part of the inner medullary collecting duct exhibits a high degree of water permeability that is independent of increased intracellular cAMP and not accounted for by the activity of the known renal epithelial water channels CHIP28 (28-kDa channel-forming integral protein) and WCH-CD (collecting duct water channel protein). Starting with rat kidney papilla mRNA, reverse transcription PCR was performed with degenerate primers assuming that the putative channel would be a member of the major intrinsic protein (MIP) family of proteins. A cDNA fragment was identified and used to screen a rat kidney cDNA library. A 1.9-kb cDNA clone was isolated. The open reading frame of 876 bp coded for a protein of 292 amino acids (M(r), 31,431). Aquaporin 3 (AQP3; 31.4-kDa water channel protein) is a newly discovered member of the MIP family. Northern blot analysis showed a single transcript for AQP3 of approximately 1.9 kb present in the renal medulla, predominantly in the inner medulla. With in situ hybridization, abundant message was found in the cells of the medullary collecting ducts. Injection of the complementary RNA of AQP3 into Xenopus oocytes markedly increased the osmotic water permeability. This permeability had an energy of activation of 3.0 kcal/mol (1 cal = 4.184 J), it was fully blocked by 1 mM p-chloromercuriphenylsulfonate, and this inhibition was reversed by 5 mM dithiothreitol. cAMP did not increase this water permeability. AQP3 did not permit passage of monovalent ions (Na, K, Cl); however, it is slightly permeable to urea. The present study demonstrates the existence of an additional water channel, AQP3, in epithelial cells of the medullary collecting duct.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Echevarria M., Frindt G., Preston G. M., Milovanovic S., Agre P., Fischbarg J., Windhager E. E. Expression of multiple water channel activities in Xenopus oocytes injected with mRNA from rat kidney. J Gen Physiol. 1993 Jun;101(6):827–841. doi: 10.1085/jgp.101.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamion B., Spring K. R. Water permeability of apical and basolateral cell membranes of rat inner medullary collecting duct. Am J Physiol. 1990 Dec;259(6 Pt 2):F986–F999. doi: 10.1152/ajprenal.1990.259.6.F986. [DOI] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Han J. S., Maeda Y., Ecelbarger C., Knepper M. A. Vasopressin-independent regulation of collecting duct water permeability. Am J Physiol. 1994 Jan;266(1 Pt 2):F139–F146. doi: 10.1152/ajprenal.1994.266.1.F139. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Ma T., Skach W., Matthay M. A., Verkman A. S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994 Feb 25;269(8):5497–5500. [PubMed] [Google Scholar]
- Ishibashi K., Sasaki S., Fushimi K., Uchida S., Kuwahara M., Saito H., Furukawa T., Nakajima K., Yamaguchi Y., Gojobori T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lankford S. P., Chou C. L., Terada Y., Wall S. M., Wade J. B., Knepper M. A. Regulation of collecting duct water permeability independent of cAMP-mediated AVP response. Am J Physiol. 1991 Sep;261(3 Pt 2):F554–F566. doi: 10.1152/ajprenal.1991.261.3.F554. [DOI] [PubMed] [Google Scholar]
- Nielsen S., DiGiovanni S. R., Christensen E. I., Knepper M. A., Harris H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Strange K., Spring K. R. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol. 1987;96(1):27–43. doi: 10.1007/BF01869332. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Yan N., Udenfriend S. Expression cloning of a Na(+)-independent neutral amino acid transporter from rat kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):1–5. doi: 10.1073/pnas.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]