Abstract
Although alcohol abuse is associated with a spectrum of pancreatic diseases from acute self-limiting episodes of pancreatitis to recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer, the majority of those who drink excessive amounts of alcohol do not develop pancreatic disease. One overarching hypothesis is that alcohol abuse requires additional risk factors, either environmental or genetic, for disease to occur. However, another reason why alcohol abuse leads to pancreatic disease in so few individuals could also be a result of alcohol-induced activation of adaptive systems that protect the pancreas from the toxic effects of alcohol. We have turned to investigating the potential role of the unfolded protein response (UPR) of the endoplasmic reticulum (ER) to identify potential pathways that can lead to protection of the pancreas from pancreatic diseases with alcohol abuse. We discuss the pathways involved in protection as well as those involved in development of pancreatic pathology. The remarkable ability of the pancreas to adapt its machinery to alcohol abuse using UPR systems and continue functioning is the likely reason that pancreatitis from alcohol abuse does not occur in the majority of heavy drinkers. These findings additionally indicate that methods to enhance the protective responses of the UPR can provide opportunities for treatment of pancreatic diseases.
Introduction
Alcohol abuse is associated with a spectrum of pancreatic diseases from acute self-limiting episodes of pancreatitis to recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer.1–3 However, clinically detected pancreatic disease occurs in only a small minority of heavy drinkers.1 The reasons why most heavy drinkers do not develop clinically manifest pancreatic diseases are not known. One overarching hypothesis is that alcohol abuse requires additional risk factors, either environmental or genetic, for disease to occur.4–6 Of note, in animal models we and others also have found that alcohol by itself does not cause pancreatitis but that alcohol feeding sensitizes that pancreas to pancreatitis caused by other pancreatic stressors.7, 8
Another reason why alcohol abuse leads to pancreatic disease in so few individuals could also be a result of alcohol-induced activation of adaptive systems that protect the pancreas from the toxic effects of alcohol. In other words, alcohol could activate both damaging effects and protective effects; and that disease occurs when the damaging effects outweigh the protective effects or when the adaptive mechanisms are impaired. Such a combination could also explain the combined actions of alcohol and another risk factor resulting in pancreatic disease.4–6, 9 That is, with an additional risk factor such as smoking or gene mutations, the alcohol-mediated protective responses are overwhelmed by the combination of its toxic effects and those of the second “hit.”
In order to pursue the hypothesis that alcohol intake induces both toxic and protective mechanisms in the pancreas we have turned to investigating the potential role of the unfolded protein response (UPR) of the endoplasmic reticulum (ER) in these dual actions of alcohol using animal models. In our research we have focus mainly on the acinar cell of exocrine pancreas. However, the mechanisms discussed here may also be relevant to the ductal and/or endocrine cells of the pancreas.
Pancreatic Acinar Cell Endoplasmic Reticulum (ER)
The acinar cell requires an extensive endoplasmic reticulum network and protein secretory system to sustain its high rate of digestive enzyme production. ER biogenesis, function and turnover are regulated according to the demands of the secretory pathway. The ER recruits translating ribosomes, translocates newly synthesized polypeptides into its lumin, and accommodates post-translational modifications including glycosylation and disulfide bond formation, and chaperone-facilitated protein folding. Correctly folded proteins are tagged, sorted into specific vesicular compartments, and transported to the Golgi, where they are further processed, sorted and stored in mature zymogen granules and other organelles. Upon neurohormonal stimulation, zymogen granules undergo exocytosis at the apical pole of the cell, secreting their contents into the acinar lumin and ductal system of the exocrine pancreas. Correct ER processing and sorting are especially critical to prevent inappropriate intracellular activation of digestive proenzymes in the acinar cell.10
In eukaryotic cells, protein folding is governed by an efficient team of ER molecular chaperones and folding enzymes that include disulfide isomerases, oxidoreductases and enzymes related to glycosylation of newly synthesized proteins. This process is monitored by quality control machinery to ensure that only properly folding proteins progress into the secretory pathway. Aberrant proteins are retro-translocated into the cytosol for proteosomal degradation by a process known as ER-associated degradation (ERAD). Autophagy is also an important mechanism for degradation of superfluous damaged or misfolded proteins and obsolescent organelles. Accumulated evidence underscores the importance of both ERAD and autophagy in preventing the accumulation of toxic proteins within the ER.11, 12
Protein-folding and chaperone functions within the ER are dependent on the presence of sufficient levels of intralimenal Ca2+ and ATP, and an oxidizing environment that favors disulfide bond formation in folding polypeptides. Thus, major perturbations in ER luminal and cellular Ca2+ fluxes, ATP levels and redox that can occur in the pancreas induce ER “stress” by hindering chaperone and folding activities.13–18 Ca2+, ATP and a controlled oxidative environment are key factors to maintain ER homeostasis for protein synthesis, processing and transport.
The Unfolded Protein Response (UPR)
In order to adjust to changing demands encountered by the ER protein synthesis and processing machinery, eukaryotic cells have developed the Unfolded Protein Response (UPR) signaling system. The UPR is activated by accumulation of unfolded proteins in the ER lumin, a condition termed “ER stress”.19 ER stress has several sources, including a physiologic increase in the demand for protein folding, decreased chaperone function, accumulation of permanently misfolded mutant proteins, restricted ER-Golgi protein trafficking, decreases in cellular ATP levels or in [Ca2+]ER, and perturbed ER redox status. In particular, repeated cycles of folding-refolding of misfolded proteins via thioredoxin-fold protein disulfide isomerase (PDI) family and ER oxidase-1 (Ero1) activities consumes cellular energy reserves and generates high levels of reactive oxygen species (ROS) and redox imbalance. Thus, protein misfolding shifts the redox status of the lumin to more oxidizing, possibly favoring aberrant disulfide formation.
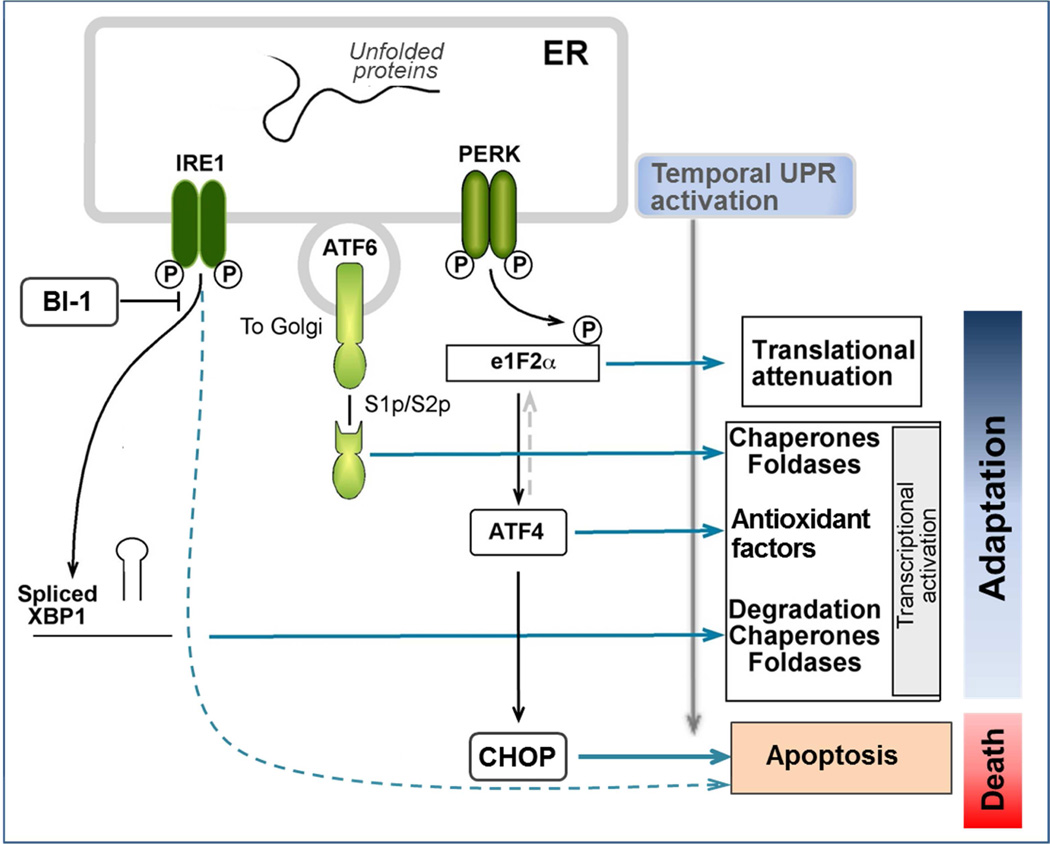
The UPR has three major outputs that coordinate to maintain ER homeostasis: 1) global reduction in mRNA translation decreases the demand for processing newly synthesized proteins; 2) increased transcription of numerous chaperones and foldases, and phospholipid synthesis to augment the ER folding and export capacity and to expand the ER network, 3) activation of ERAD and autophagic systems to eliminate accumulated unfolded and misfolded proteins, and 4) degradation of ER associated mRNAs to reduce protein folding load.12, 19–22 Three trans-membrane ER stress sensor-transducers are responsible for these UPR outputs.19, 20 Inositol-requiring protein-1 α (IRE1α), activating transcription factor-6 (ATF6), and RNA-activated protein kinase (PKR)-like ER kinase (PERK). Each sensor transmits information from the folding status of proteins in the ER lumin to the nucleus by distinct mechanistic pathways (Figure 1).
Figure 1. Activation and outputs of the Unfolded Protein Response (UPR).
The scheme summarizes the signaling pathways and outputs of the three branches of the mammalian UPR: IRE1α, ATF-6 and PERK. Upon disturbances of ER function, unfolded proteins accumulate within the ER lumin and the UPR sensors respond by activating adaptive signaling pathways. Cell growth arrest and cell death signaling prevail when ER stress is persistent or too severe.
Although these sensors can be simultaneously activated by ER stressors, the UPR signaling outputs vary depending on the nature and duration of the ER stress, and the cell type.23 For example, the IRE1 branch is critical for adaptation to long term ER stress.24 Upon its activation, endonuclease activity within the IRE1α polypeptide splices X-box binding protein-1 (XBP1) mRNA resulting in the translation of a multifunctional transcription factor, called sXBP1. sXBP1 regulates a broad spectrum of UPR genes involved in protein folding, including chaperones and oxidoreductases of the PDI family, protein degradation (ERAD), vesicular trafficking and redox metabolism, as well as lipid biosynthesis/metabolism and ER/Golgi biogenesis, in a cell-specific manner.22, 25 The effects of sXBP1 generally adapt to ER stressors and enhance normal function in many cells, but play an especially important supportive role in secretory cells (described in the next section).
Activation of the PERK pathway by ER stressors results in inhibition of general protein translation by phosphorylation and inhibition of eukaryotic Initiation factor 2-α (eIF2α), a factor necessary for most protein translation. In the short term, this inhibition of protein synthesis relieves the cell of the demands of protein processing and can be beneficial. However, in the face of general translational inhibition, eIF2α favors translation of the transcription factor ATF4. ATF4 expression upregulates the proapoptotic transcription factor, C/EBP homologous protein (CHOP), as well as many target genes involved in translation, amino acid import and redox metabolism. The ER sensor ATF6 regulates the expression of XBP1 and several ER chaperones in response to short term ER stress, providing protective adaptation.
Whereas short term perturbations of ER function are normally resolved by the UPR, severe ER stress or defective UPR can lead to inflammation and cell death (10).27 Several intermediate responses have been identified. Sustained severe ER stress can trigger cell death downstream of PERK/CHOP and IRE1/JNK activation, and as a result of promiscuous IRE1α-dependent decay (RIDD) of ER associated mRNAs.26,27
ER stress can favor translocation of proapoptotic Bax/Bak to the ER membrane causing Ca2+ release and mitochondrial dysfunction. This process can be positively regulated by Bim-only proteins such as Puma and Noxa that may be transcriptionally induced by ER stress.28 Notably, it was reported that Bax or Bak exogenous expression positively modulates the activity of IRE1α, possibly via the formation of protein-protein complexes.29 Further, an ER membrane protein termed Bax inhibitor-1 (BI-1) exerts an opposite effect on IRE1α, such that in cells lacking BI-1, IRE1 activity is prolonged (ibid).30 Thus, IRE1 activity is regulated by distinct ER proteins. The UPR can also promote NF-κB activation by limiting translation of inhibitor of κB proteins. As our knowledge of the regulation of ER function has evolved, more human disorders have been associated with ER stress and/or dysregulation of the UPR.
The presence of misfolded proteins and a pathological UPR response continue to emerge as common denominators in diabetes, although lipotoxicity and glucotoxicity also play roles in pancreas disease.31 Proteins implicated in diabetes and/or exocrine pancreas disease whose pathogenic mechanisms relate to mutation-induced misfolding include carboxyl ester lipase,32 colipase,33 proinsulin,34 and the K+ and other ion channel subunits.35 Mutant trypsins are established as monogenic causes of hereditary pancreatitis, a number of which induce ER stress in experimental models.36 Thus, the list of proteins misfolded within the ER that may contribute specifically to diabetes and pancreatitis appears to be growing longer. Other mechanisms of ER stress may also play a part. Specifically, regulation of protein chain elongation occurs at the level of EF2 kinase,37 a key protein whose regulation may contribute to pathology and represent a novel target of therapy. De novo biosynthesized elements of translation, glycosylation and protein trafficking machinery within the pancreatic acinar cell are susceptible to global changes in protein folding capacity. ER protein misfolding and associated UPR are mitigated by the actions of HSP70 chaperones, oxidative folding enzymes and chemical chaperones, and pharmacologic strategies to approach this issue are currently a focus of drug development.
sXBP1 as a Mediator of Pancreatic Protection and its Secretory Phenotype
The most evolutionarily conserved arm of UPR signaling is that governed by IRE1α. Upon ER stress IRE1α homodimerizes and trans-auto phosphorylates, thereby enhancing its cytosolic RNAse activity to induce splicing of XBP1 and generate sXBP1.38 sXBP1 is a basic-region leucine zipper (bZIP) transcription factor of the CREB-ATF family with higher stability than the unspliced protein (uXBP1) that lacks transcriptional activity. As described above, IRE1α/sXBP1 signaling primarily mediates adaptive responses to reestablish cellular homeostasis. Adaptive responses needed in the acinar cell include sXBP1-induced upregulation of many ER chaperones, PDI family oxidoreductases, lipid synthases to expand the ER network, ERAD system components, and Mist1, a bHLH-family transcription factor required for normal organelle localization and Ca2+ signaling in the acinar cell.11, 39 Collectively, these gene products act to lessen ER stress and to reinforce critical aspects of ER protein folding and export to support the secretory pathway, as well as protein degradation systems (ERAD and autophagy). The importance of ER chaperone function for pancreatitis was underscored by the enhanced experimental pancreatitis severity we found in GRP78+/− mice.40
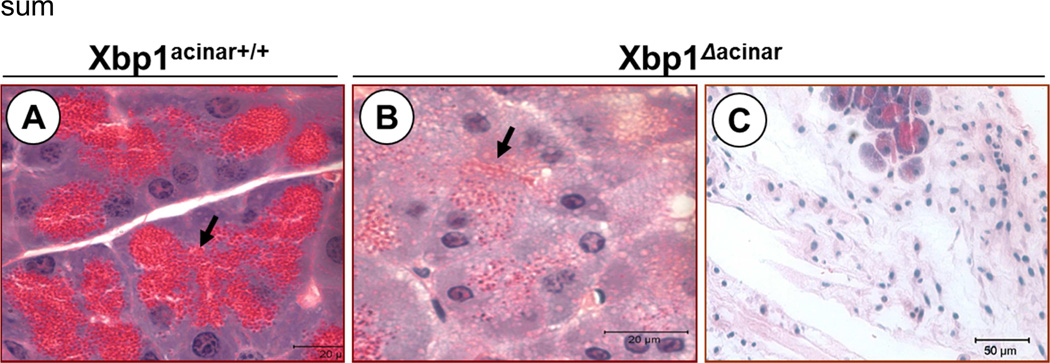
Genetic ablation of Xbp1 in mice results in embryonic lethality due to liver hypoplasia. Xbp1 null mice expressing an Xbp1 transgene in liver to rescue embryonic lethality died shortly after birth due to poor ER development and digestive enzyme synthesis in the acinar cells and severe exocrine insufficiency.25 The critical importance of sXBP1 for the function of the pancreatic acinar cell is also supported by studies using Xbp1+/− mice10, 40 and acinar cell specific XBP1 null mice25, 41 (and Figure 2). XBP1 deficiency results in extensive acinar cell loss and inflammation (Figure 2) as well as severe pathology in the remaining acinar cells, as evidenced by reduced levels of ER chaperones, a poorly developed ER network and secretory system, marked reduction in zymogen granules and digestive enzymes, and accumulation of autophagic vacuoles.10, 40
Figure 2. Specific deletion of Xbp1 in the pancreatic acinar cell leads to a decrease in secretory capacity and acinar cell pathology.
A–C. Control and Ela1-Cre-ERT2;Xbp1F/F mice received tamoxifen for 5 days to delete the Xbp1 gene specifically in acinar cells (Xbp1Δacinar). Then mice were sacrificed at 7 (a and b) or 17 days (c) after tamoxifen administration. Panels show pancreas histology in tamoxifen-treated control mice (panel a) and Xbp1Δacinar mice (panels b and c). As illustrated, acinar cells lacking XBP1 exhibit severe loss of secretory granules (closed arrow) and vacuolation (especially shortly after tamoxifen) followed by death and loss of acinar cells.
Whereas IRE1α/XBP1 signaling primarily mediates adaptive responses to reestablish ER function, this protective signal can be prematurely attenuated during severe or prolonged ER stress resulting in upregulation of proapoptotic CHOP and cell death.23 Similarly, genetic ablation of Xbp1 in pancreas and other tissues is unequivocally associated with potent upregulation of CHOP and cell death.25, 40 Interestingly, Lin et al. demonstrated that forced and sustained IRE1α/XBP1 activity enhances cell survival in conditions of severe stress,23 further supporting a protective role for sXBP1 signaling.
The PERK/CHOP Pathway
The PERK UPR branch rapidly adjusts the cell to ER stress causing a general attenuation of protein synthesis.19 Activated PERK becomes autophosphorylated at Thr980 and rapidly phosphorylates the eukaryotic translation initiation factor, eIF2α at Ser51.42, 43 This modification generally impedes eIF2α-mediated translation initiation. Much evidence indicates that PERK/eIF2α activation is protective, preventing depletion of ER resources during the ER stress response.14, 44, 45 In support of a protective role for PERK in the exocrine pancreas, acinar cell-specific PERK ablation in mice increased acinar cell death but did not affect enzyme secretion.46 Sustained phosphorylation of eIF2α (by PERK or other kinases) leads to upregulation of the transcription factors ATF4, that targets genes involved in antioxidant activities including glutathione synthesis47, and CHOP that promotes ER stress-related cell death responses.48 Emerging data indicate that CHOP leads to cell death by upregulation of ER factors that govern protein folding resulting in excessive protein synthesis and ER ATP depletion.14 CHOP also promotes inflammation by regulating cytokine production and promoting the survival of inflammatory cells.49, 50 Importantly, we and others found that depriving the acinar cell from using the IRE1-sXBP1 to adapt to ER stress is associated with sustained activation of the PERK-CHOP pathway and development of pancreatic pathology.40 Indeed, Chop−/− mice exhibited less pancreatic inflammation and histological damage than wild type when challenged with cerulein and LPS.51 In sum, although the PERK branch can play a transient protective role, unresolved ER stresses (e.g., due to sXBP1 deficiency) upregulate CHOP and promote inflammation leading to pancreas pathology.
Alcohol, ER Stress and Pancreatitis
Ethanol feeding in rodents induces structural changes in the acinar cell consistent with ER stress and impaired protein trafficking, such as extensive ER dilation and disorganization of the cellular location of zymogen granules.40, 52, 53 However, despite these morphologic changes, chronic ethanol-fed animals, as humans, do not develop pancreatitis unless challenged with other toxic factors.7, 54,55 We found that pancreatic levels of sXBP1 were markedly increased in mice and rats fed ethanol-containing diets.40 In order to determine whether the upregulation of sXBP1 by alcohol feeding is necessary to maintain homeostasis and prevent pancreatitis, we used Xbp1 heterozygous mice. Compared to ethanol-fed wild-type mice (XBP1+/+), histological analysis of pancreatic tissue in ethanol-fed XBP1+/− mice revealed morphologic features of ER dysfunction in acinar cells: disorganized and dilated ER, accumulation of dense material within the ER, and a reduced number of mature zymogen granules. These features were accompanied by accumulation of autophagic vacuoles, and activation of apoptotic signals including upregulation of CHOP within patchy areas of inflammatory pancreatitis.10, 40 In a separate study, Alahari et al found that mice deficient in MIST1, a sXBP1 gene target, are more susceptible to ethanol toxicity and displayed deficient UPR activity, supporting a critical role for sXBP1 in regulating ER function and UPR during chronic alcohol abuse.56
Conclusions
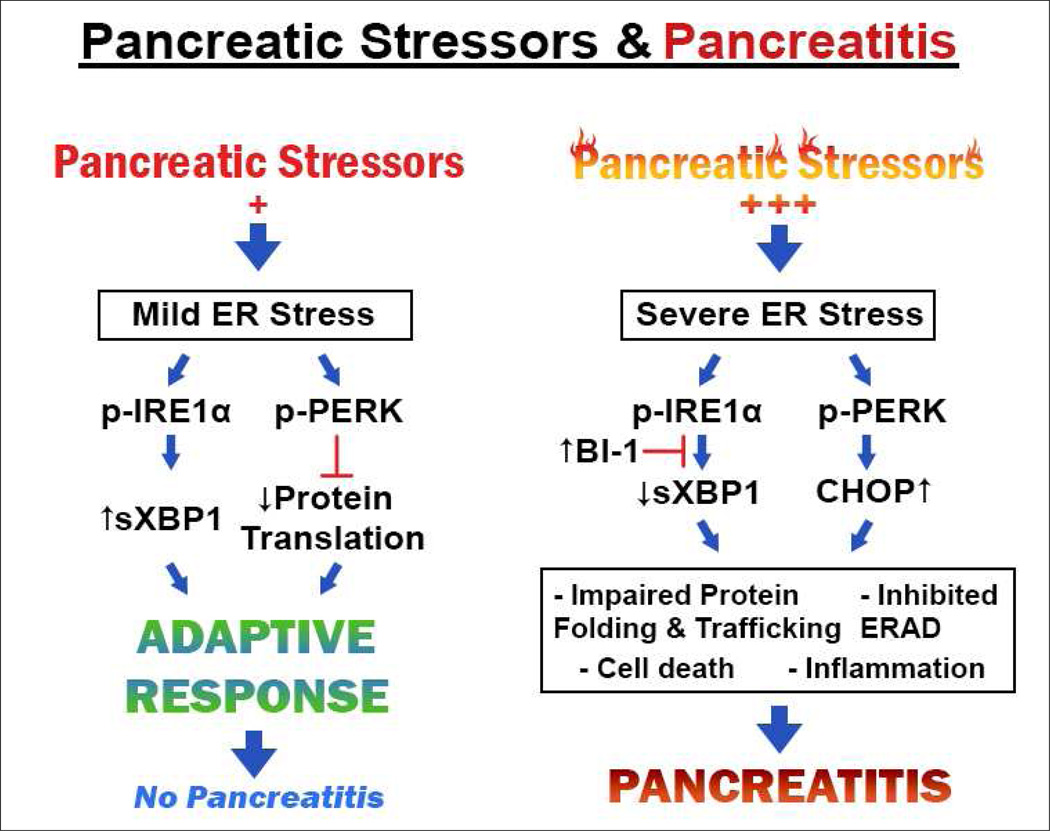
In conclusion, our work identifies sXBP1 as a key protective factor against ethanol induced toxicity in pancreas. Based on our data, we envision a model in which chronic alcohol abuse alters mitochondria and increases ROS levels producing changes in redox state of ER components and ER oxidative stress. This alters the redox structure and function of ER oxidoreductases as well as their client proteins, thereby compromising protein folding and trafficking through the secretory pathway. Acinar cells respond by activating IRE1/XBP1 that acts to restore levels of ER chaperones and oxidoreductases needed for protein folding, trafficking, and degradation pathways. This sequence of events is adaptive and limits deleterious PERK/CHOP outputs that lead to cell death and inflammation. However, in the presence of a second ER stressor such as a genetic mutation in one of the secretory proteins or an environmental factor such as smoking there may be an inability of the IRE1/XBP1 pathway to adapt sufficiently to the stressors resulting in pathology. The remarkable ability of the pancreas to adapt its machinery to alcohol abuse and continue functioning is the likely reason that pancreatitis from alcohol abuse does not occur in the majority of heavy drinkers. These findings additionally indicate that methods to enhance the protective responses of the UPR can provide opportunities for treatment of pancreatic diseases. See Figure 3 summarizing our hypothesis below.
Figure 3. Proposed mechanisms of sXBP1-related responses during pancreatitis.
Acknowledgements
NIH grants: R01 AA019954 (to A.L.), P50 AA11999 (S.P.), P01 CA163200 (S.P.), P01 DK098108 (S.P.); and Department of Veterans Affairs (S.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yadav D, Eigenbrodt ML, Briggs MJ, et al. Pancreatitis: prevalence and risk factors among male veterans in a detoxification program. Pancreas. 2007;34:390–398. doi: 10.1097/mpa.0b013e318040b332. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sand J, Lankisch PG, Nordback I. Alcohol consumption in patients with acute or chronic pancreatitis. Pancreatology. 2007;7:147–156. doi: 10.1159/000104251. [DOI] [PubMed] [Google Scholar]
- 4.Whitcomb DC. Genetics and alcohol: a lethal combination in pancreatic disease? Alcohol Clin Exp Res. 2011;35:838–842. doi: 10.1111/j.1530-0277.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson MA, Zolotarevsky E, Cooper KL, et al. Alcohol and tobacco lower the age of presentation in sporadic pancreatic cancer in a dose-dependent manner: a multicenter study. Am J Gastroenterol. 2012;107:1730–1739. doi: 10.1038/ajg.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44:1349–1354. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandol SJ, Periskic S, Gukovsky I, et al. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706–716. doi: 10.1016/s0016-5085(99)70465-8. [DOI] [PubMed] [Google Scholar]
- 8.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–794. doi: 10.1016/s0016-5085(00)70148-x. [DOI] [PubMed] [Google Scholar]
- 9.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol. 2010;7:131–145. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 10.Lugea A, Waldron RT, French SW, et al. Drinking and driving pancreatitis: links between endoplasmic reticulum stress and autophagy. Autophagy. 2011;7:783–785. doi: 10.4161/auto.7.7.15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Direnzo D, Hess DA, Damsz B, et al. Induced MIST1 Expression Promotes Remodeling Of Mouse Pancreatic Acinar Cells. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 13.Mandl J, Mészáros T, Bánhegyi G, et al. Minireview: endoplasmic reticulum stress: control in protein, lipid, and signal homeostasis. Mol Endocrinol. 2013;27:384–393. doi: 10.1210/me.2012-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton R, Petersen OH, Pandol SJ. Pancreatitis and calcium signaling: Report of an international workshop. 2008 doi: 10.1097/MPA.0b013e3181675010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee R, Criddle DN, Gukovskaya A, et al. Mitochondrial injury in pancreatitis. Cell Calcium. 2008;44:14–23. doi: 10.1016/j.ceca.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 18.Kim MS, Lee KP, Yang D, et al. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. 2115 e1–2115 e4. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 20.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Marciniak SJ, Garcia-Bonilla L, Hu J, et al. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J Cell Biol. 2006;172:201–209. doi: 10.1083/jcb.200508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JH, Li H, Yasumura D, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers JE, Marciniak SJ. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease 2. Protein misfolding and ER stress. Am J Physiol Cell Physiol. 2014;307:C657–C570. doi: 10.1152/ajpcell.00183.2014. [DOI] [PubMed] [Google Scholar]
- 25.Lee AH, Chu GC, Iwakoshi NN, et al. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh R, Wang L, Wang ES, et al. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetz C, Martinon F, Rodriguez D, et al. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 29.Hetz C, Bernasconi P, Fisher J, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 30.Lisbona F, Rojas-Rivera D, Thielen P, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yalcin A, Hotamisligil GS. Impact of ER protein homeostasis on metabolism. Diabetes. 2013;62:691–693. doi: 10.2337/db12-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson BB, Torsvik J, Bjorkhaug L, et al. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem. 2011;286:34593–34605. doi: 10.1074/jbc.M111.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao X, Ferguson MR, Magee KE, et al. The Arg92Cys colipase polymorphism impairs function and secretion by increasing protein misfolding. J Lipid Res. 2013;54:514–521. doi: 10.1194/jlr.M034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss MA. Diabetes mellitus due to the toxic misfolding of proinsulin variants. FEBS Lett. 2013;587:1942–1950. doi: 10.1016/j.febslet.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young JC. The role of the cytosolic HSP70 chaperone system in diseases caused by misfolding and aberrant trafficking of ion channels. Dis Model Mech. 2014;7:319–329. doi: 10.1242/dmm.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth BC, Sahin-Toth M. Human cationic trypsinogen (PRSS1) variants and chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G466–G473. doi: 10.1152/ajpgi.00419.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sans MD, Xie Q, Williams JA. Regulation of translation elongation and phosphorylation of eEF2 in rat pancreatic acini. Biochem Biophys Res Commun. 2004;319:144–151. doi: 10.1016/j.bbrc.2004.04.164. [DOI] [PubMed] [Google Scholar]
- 38.Uemura A, Oku M, Mori K, et al. Unconventional splicing of XBP1 mRNA occurs in the cytoplasm during the mammalian unfolded protein response. J Cell Sci. 2009;122:2877–2886. doi: 10.1242/jcs.040584. [DOI] [PubMed] [Google Scholar]
- 39.Luo X, Shin DM, Wang X, et al. Aberrant localization of intracellular organelles, Ca2+ signaling, and exocytosis in Mist1 null mice. J Biol Chem. 2005;280:12668–12675. doi: 10.1074/jbc.M411973200. [DOI] [PubMed] [Google Scholar]
- 40.Lugea A, Tischler D, Nguyen J, et al. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology. 2011;140:987–997. doi: 10.1053/j.gastro.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hess DA, Humphrey SE, Ishibashi J, et al. Extensive pancreas regeneration following acinar-specific disruption of Xbp1 in mice. Gastroenterology. 2011;141:1463–1472. doi: 10.1053/j.gastro.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K, Tirasophon W, Shen X, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blais JD, Chin KT, Zito E, et al. A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J Biol Chem. 2010;285:20993–21003. doi: 10.1074/jbc.M110.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 45.Scheuner D, Song B, McEwen E, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 46.Iida K, Li Y, McGrath BC, et al. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi: 10.1186/1471-2121-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 48.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 49.Goodall JC, Wu C, Zhang Y, et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malhi H, Kropp EM, Clavo VF, et al. C/EBP Homologous Protein-induced Macrophage Apoptosis Protects Mice from Steatohepatitis. J Biol Chem. 2013;288:18624–18642. doi: 10.1074/jbc.M112.442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suyama K, Ohmuraya M, Hirota M, et al. C/EBP homologous protein is crucial for the acceleration of experimental pancreatitis. Biochem Biophys Res Commun. 2008;367:176–182. doi: 10.1016/j.bbrc.2007.12.132. [DOI] [PubMed] [Google Scholar]
- 52.Gukovsky I, Lugea A, Shahsahebi M, et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;294:G68–G79. doi: 10.1152/ajpgi.00006.2007. [DOI] [PubMed] [Google Scholar]
- 53.Lam PP, Cosen Binker LI, Lugea A, et al. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic. 2007;8:605–617. doi: 10.1111/j.1600-0854.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 54.Vonlaufen A, Phillips PA, Xu Z, et al. Withdrawal of alcohol promotes regression while continued alcohol intake promotes persistence of LPS-induced pancreatic injury in alcohol-fed rats. Gut. 2011;60:238–246. doi: 10.1136/gut.2010.211250. [DOI] [PubMed] [Google Scholar]
- 55.Pandol SJ, Gukovsky I, Satoh A, et al. Animal and in vitro models of alcoholic pancreatitis: role of cholecystokinin. Pancreas. 2003;27:297–300. doi: 10.1097/00006676-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Alahari S, Mehmood R, Johnson CL, et al. The absence of MIST1 leads to increased ethanol sensitivity and decreased activity of the unfolded protein response in mouse pancreatic acinar cells. PLoS One. 2011;6:e28863. doi: 10.1371/journal.pone.0028863. [DOI] [PMC free article] [PubMed] [Google Scholar]