Abstract
Since the early days of gene therapy, muscle has been one the most studied tissue targets for the correction of enzyme deficiencies and myopathies. Several preclinical and clinical studies have been conducted using adeno-associated virus (AAV) vectors. Exciting progress has been made in the gene delivery technologies, from the identification of novel AAV serotypes to the development of novel vector delivery techniques. In parallel, significant knowledge has been generated on the host immune system and its interaction with both the vector and the transgene at the muscle level. In particular, the role of underlying muscle inflammation, characteristic of several diseases affecting the muscle, has been defined in terms of its potential detrimental impact on gene transfer with AAV vectors. At the same time, feedback immunomodulatory mechanisms peculiar of skeletal muscle involving resident regulatory T cells have been identified, which seem to play an important role in maintaining, at least to some extent, muscle homeostasis during inflammation and regenerative processes. Devising strategies to tip this balance towards unresponsiveness may represent an avenue to improve the safety and efficacy of muscle gene transfer with AAV vectors.
Keywords: AAV vectors, muscle, clinical trials, immune responses, tolerance
INTRODUCTION
Recombinant adeno-associated virus (AAV) vectors (1) have gained broad interest as tools to achieve therapeutic gene transfer in vivo in a variety of models of disorders affecting muscle, brain, eye, and liver due to their excellent safety profile and their ability to transduce a wide variety of post-mitotic tissues, providing efficient and stable transgene expression (2). Recently, the first gene therapy drug based on an AAV vector injected intramuscularly, Glybera, has been approved by the European Medicine Agency for the treatment of lipoprotein lipase deficiency (3).
Since the early days of gene therapy, skeletal muscle was considered as a potential target for in vivo genetic engineering to create a site for the production of secreted proteins following AAV vector-mediated gene transfer (4-9). However, muscle tissue is also the hotbed of immune reactions, and intramuscular injection is commonly used for vaccination purposes. As a consequence, local immune reactions need to be cautiously addressed upon gene delivery to muscle, as they may represent an obstacle to the success of therapies aiming at restoring normal protein expression in enzyme deficiencies (6-9) and hereditary muscular disorders (6-8, 10-17). In addition, the vast heterogeneity the disease state of muscle in neuromuscular disorders provides an additional layer complexity to the understanding of immunity in muscle gene transfer, since tissue remodeling and/or disease-related inflammation may impact the context in which either the vector or the encoded transgene will be presented to the immune system (18).
Finally, recombinant AAV vectors are derived from their wild-type counterpart, to which humans are exposed early in life (19-21). This results in development of both humoral (22, 23) and cellular (24) immunity to the vector capsid, which may prevent or reduce therapeutic efficacy following gene transfer.
In this review, we will focus on AAV-based gene transfer to skeletal muscle and highlight the limitations that could be encountered due to the immune response against the vector and/or the transgene.
IMMUNE RESPONSES DIRECTED AGAINST THE AAV CAPSID
Wild-type AAV is a replication-defective parvovirus initially isolated from preparations of viruses infecting humans through the airways (25). While no known pathology is associated with AAV infection, it is known that this small non-enveloped single-stranded DNA virus triggers both innate (26) and adaptive immunity (27, 28), resulting in long-term humoral and cellular immune responses against the structural proteins of the capsid. When it comes to gene transfer with AAV vectors, these immune responses can abolish transgene expression, either by neutralizing the vector before it reaches the desired target tissue (29) or by clearing the transduced cells (29-32).
Anti-AAV Neutralizing Antibodies
Following the exposure to the wild-type virus, a significant proportion of humans develop humoral immunity against the capsid early in life, starting around 2 years of age (19-21). Additionally, shortly after birth, maternal anti-AAV antibodies can be found in newborns (19), resulting in a narrow time window, if any, in which the majority of humans is naive to anti-AAV antibodies.
Because of the high degree of conservation in the amino acid sequence across AAVs (33), anti-AAV antibodies show cross-reactivity with a wide range of serotypes (22). In healthy donors, anti-AAV1 and -AAV2 antibodies appear to be the most prevalent (more than 60% of the population is seropositive to AAV2) and display the highest neutralizing titers (19, 21-23, 34). Conversely, about one third of healthy humans are seropositive for AAV8 and AAV9, and generally titers are low (22).
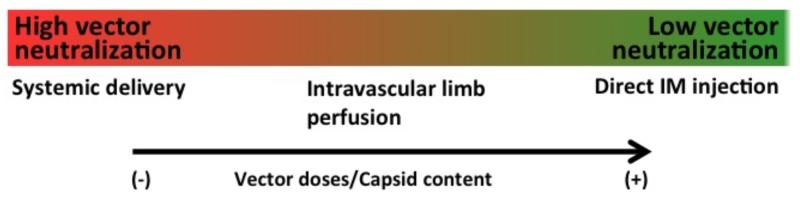
Anti-AAV antibodies can have a profound impact on the efficiency of tissue transduction with AAV vectors, as even low-titer antibodies can neutralize the virus following systemic delivery (29, 35-37), a route of vector administration used to target several tissues, including liver (29, 31, 32), muscle (13, 35), and central nervous system (38). Thus, pre-screening of subjects for pre-existing immunity to AAV prior to enrollment in clinical trials is a necessary step to guarantee therapeutic efficacy following AAV vector systemic administration. Conversely, neutralizing antibodies to AAV are not an issue when the vector is delivered to certain immunoprivileged body sites (e.g. to the subretinal space (39-41)) or intraparenchymally (Table 1). Importantly, while recombinant AAV1 vectors have been delivered via local injection into the skeletal muscle (Table 1), several gene therapy approaches in which AAV vectors are delivered intravascularly or systemically have been recently proposed to target skeletal muscle (13, 35, 42, 43), or myocardium (44), which makes the issue of pre-existing humoral immunity to AAV particularly relevant (Fig. 1). Notably, neutralizing antibodies to AAV constitute also a major obstacle to vector readministration, as they are elicited at high titers following gene transfer and these titers persist for several years. This is a particularly important issue when it comes to systemic disorders, like muscle disorders, in which whole body transduction is necessary and may require repeated vector administrations. Furthermore, the fact that AAV vectors do not integrate efficiently into the host genome and do not transduce satellite cells (the muscle stem cells) (45), together with the fact that transgene expression may not persist long-term in a diseased muscle (46), make safe and effective AAV vector readministration an important goal for the future.
Table 1. T cell responses to AAV in intramuscular gene transfer trials.
| Serotype | Transgene | Route | Dose (vg) | Capsid T cell reactivity |
Transgene expression in muscle biopsies |
Reference (ClinicalTrials.gov ID#) |
|---|---|---|---|---|---|---|
| AAV1 | Gamma sarcoglycan | IM | 3×109 | 0/3 | 0/3 | Herson et al., Brain 2012 (NCT01344798) |
| 1.5×1010 | 0/3 | 0/3 | ||||
| 4.5×1010 | 1/3 | 3/3 | ||||
| AAV1 | Alpha sarcoglycan | IM | 3.25×1011 | 1/3 | 3/3 | Mendell et al., Ann Neurol 2009 (NCT00494195) |
| 3.25×1011 | 2/3 | 2/3 | Mendell et al., Ann Neurol 2010 (NCT00494195) | |||
| AAV1 | Acid-alpha glucosi-dase | IM (diaphragm) | 1×1012 | 0/3 ([H3] prolif.) | N/A | Smith et al., Hum Gene Ther 2013 (NCT00976352) |
| 5×1012 | 0/2 ([H3] prolif.) | N/A | ||||
| AAV2.5 | Minidystrophin | IM | 6×1011 | 1/2 (IS) | 0/2 | Bowles et al., Mol Ther 2012 Mendell et al., NEJM 2009 (NCT00428935) |
| 3×1012 | 0/2 | 1/2 | ||||
| 3×1012 6.6×1012 capsid particles | 0/2 (immunosup-pression, IS) | 1/2 | ||||
| AAV1 | Follistatin | IM | 2.1×1012 | 1/3 | 2/2 | Mendell et al., Mol Ther 2015 (NCT01519349) |
| 4.2×1012 | 3/3 | 2/2 | ||||
| AAV1 | Lipoprotein lipase | IM | 7.0×1012 | 2/4 | 2/3 | Mingozzi et al., Blood 2009 |
| 2.1×1013 | 3/6 | 3/6 | ||||
| 2.1×1013 | 1/4 (IS) | 1/2 | Ferreira et al. , Front immunol 2014 (NCT01109498; NCT00891306) | |||
| 7.0×1013 | 4/13 (IS) | 6/8 | ||||
| AAV1 | Alpha-1 Antitrypsin | IM | 6.9×1012 | N/A | 0/3* | Brantly et al., PNAS 2009 (NCT00430768) |
| 2.2×1013 | 2/2 | Transient* | ||||
| 6.0×1013 | 3/3 | 3/3* | ||||
| 4.2×1013 | 3/3 | 1/3* | Flotte et al., Hum Gene Ther 2012 Mueller et al. , J Clin Invest 2013 (NCT01054339) | |||
| 1.3×1014 | 3/3 | 2/3* | ||||
| 4.2×1014 | 3/3 | 3/3* |
Fig. (1). Route of vector administration and impact of neutralizing antibodies on transduction efficiency.
Low-level neutralizing antibodies (NAb) can block target tissue transduction following systemic AAV vector administration. The effect of NAb may be less important when vector is delivered via isolated limb perfusion, which somewhat limits exposure to the systemic circulation. Efficiency of muscle transduction following direct intramuscular (IM) administration of AAV is, conversely, not affected by the presence of NAb. Higher capsid doses have a beneficial effect on transduction efficiency in the presence of NAb to AAV.
To date, the only strategy adopted in clinical trials to prevent vector neutralization by anti-AAV antibodies has been to exclude seropositive subjects from enrollment. This approach is far from being optimal, as it prevents a large proportion of subjects from being enrolled in potentially beneficial clinical trials; furthermore, exclusion of seropositive subjects requires the use of reliable and sensitive antibody assays (Table 2).
Table 2. Assays used to measure anti-AAV neutralizing antibodies.
| Assay | Advantages | Limitations |
|---|---|---|
| Binding antibody assay Capsid particles or pep-tides are coated onto test wells and binding antibodies measured with a secondary anti-Ig antibody |
|
|
| In vitro cell-based assay The neutralizing antibody titer (NAb) is determined by measuring the residual activity of a reporter vector after incubation with a test sample |
|
|
| In vivo neutralization assay Residual expression of the vector is measured in mice passively immunized with the test sample |
|
|
To allow efficient vector delivery and transgene expression in seropositive patients, several strategies have been tested to lower preexisting humoral responses to AAV (28). These include pharmacological, physical, and capsid engineering methods, each carrying advantages and potential drawbacks. While examples of prevention of anti-AAV antibody following vector administration using immunosuppression are reported in the literature, for example by using a non-depleting anti-CD4 antibody (47, 48), achieving this goal in large animal models of gene transfer has been challenging even when intensive regimens were used (49). Similarly, eradication of anti-AAV antibodies via immunosuppression is likely to be difficult (34) and to require very aggressive drug regimens in order to target both B and T cells and in particular plasma cells; this raises concerns over possible toxicities related to immunosuppression, such as the increased risk of developing malignancies and infections, or over possible interactions of immunosuppressive drugs with gene transfer (50, 51). Plasmapheresis has recently gained attention as a clinically approved, safe method that can be used to lower pre-existing anti-AAV antibodies. In humans, Monteilhet and colleagues showed that up to five cycles of plasmapheresis resulted in a dramatic drop in anti-AAV neutralizing antibodies (52), a result that was also confirmed in other studies (53, 54). While results obtained are promising, the technology likely requires some improvement, as it is non-specific, resulting in the depletion of all immunoglobulins, thus requiring supplementation with intravenous immunoglobulin (IVIg), and it is not very efficient, as it requires multiple cycles of immunoabsorption over time to remove circulating and extravascular IgG (52). Furthermore, the efficacy of plasmapheresis is limited when high-titer anti-AAV antibodies are present, such as in the context of vector readministration. The use of capsid decoys to shield AAV vectors from antibody-mediated neutralization has also been recently proposed (55), however this approach needs to be weighted against the potential issue of T cell immunity directed against the AAV capsid (30).
T Cell Responses Directed against the AAV Capsid
In addition to neutralizing antibodies, natural infection with wild-type AAV also triggers cell-mediated immune responses against the capsid, which results in a reservoir of memory CD8+ T cells that can be reactivated upon vector administration. This can cause the destruction of transduced cells presenting AAV capsid antigen in the context of MHC class I (56, 57), as it has been observed in subjects enrolled in AAV vector mediated liver gene transfer trials (29-32). While several studies looked at humoral immunity to AAV, only few reports on T cell reactivity to AAV in healthy humans are available in the literature. Veron and colleagues used a lentiviral vector expressing the capsid protein VP1 to restimulate peripheral blood mononuclear cells (PBMCs) and evaluate T cell reactivity to AAV1 in healthy donors. The survey, performed both by IFN-gamma ELISpot and polyfunctional flow cytometry assay, showed a prevalence of capsid T cell reactivity of about 30% in humans (24). In another study, T cell reactivity to AAV2 was detected at higher frequency, >70%, in splenocytes collected from human subjects restimulated with peptides derived from the AAV VP1 protein (30, 58). These T cells showed a memory phenotype, and appeared to recognize specifically MHC class I epitopes against which reactivity was also found in subjects dosed with AAV vectors (30). Importantly, several of the identified AAV capsid MHC class I epitopes are highly conserved and cross recognized by T cells (30), making the relatively simple maneuver of switching to “non-human” serotypes of AAV vectors (33) to avoid memory T cell responses to the capsid unlikely to succeed. While the fact that AAV vector administration elicits T cell-mediated immunity is not completely surprising, as AAV vectors are essentially perfect copies of wild-type AAV to which humans are exposed, several open questions remain on the role of these capsid-specific CD8+ T cells in the outcome of gene transfer, as detection of T cell reactivity to the capsid in PBMCs has not always been associated with detrimental effects on gene transfer in liver (31, 32) and muscle trials (Table 1).
Reemergence of memory anti-capsid CD8+ T cell responses (or de novo stimulation of a cytotoxic response to the AAV capsid) following AAV administration has been extensively characterized in humans undergoing AAV vector gene transfer to the liver, in which an increase in liver enzymes and loss of transgene expression was documented (29-32). In a first clinical trial in which an AAV2 vector was introduced into the liver of severe hemophilia B subjects (29), two subjects developed transient elevation of liver enzymes and loss of oagulation factor IX transgene expression around week 4 post vector delivery due to the immune rejection of transduced hepatocytes mediated by capsid-specific CD8+ T cells (30). A similar set of observations was made in the context of another hemophilia B clinical trial in which an AAV8 vector was infused systemically (31, 32). These studies showed that, like for AAV2, AAV8 vector administration in humans results in activation of capsid-specific T cells, and in some cases this is associated with the loss of transgene expression and an increase in liver enzymes. In the AAV8 hemophilia trial, this phenomenon has been documented in 4 out 6 subjects dosed with 2×1012 vector genomes per kg (32) and has been controlled with a short course of steroids administered at the time of liver enzyme elevation and loss of transgene expression. Notably, timely administration of immunosuppression at the time of liver enzyme elevation resulted in better rescue of transgene expression, underscoring the need for careful monitoring of adverse immune responses in gene transfer (32).
Preclinical studies in small and large animal models of gene transfer failed to predict the outcome of gene transfer observed in human trials. In mice, induction of anti-capsid cytotoxic T lymphocytes (CTLs) responses directed against AAV2 vectors did not result in clearance of AAV2-transduced cells neither in the liver nor in the muscle (59-61), with the exception of a recent study in which CD8+ T cells primed against AAV capsid epitopes were adoptively transferred into immunodeficient animals transduced with AAV vectors (62). Cellular immunoreactivity to the capsid has nevertheless been detected in a study in dogs following intramuscular administration of AAV2 and AAV6 vectors (63). In this study, immunosuppression was required to allow for long-term expression of the transduced myocytes (63).
In muscle gene transfer clinical trials, the emergence of CTLs against the AAV capsid after intramuscular administration of AAV vectors (Table 1) appears to be, at least to some extent, dose-dependent and is accompanied by the detection of T cell infiltrates in the injected muscle; in some instances, T cell reactivity to the AAV capsid has been associated with the apparent lack of transgene expression (64, 65).
Despite the detection of T cell reactivity against AAV in PBMCs and the identification of infiltrates in the muscle of AAV-treated patients, the transgene was still expressed in subjects who received an AAV1 vector encoding for alpha-1 antitrypsin (66-68). Interestingly, CD4+CD25+FoxP3+ regulatory T cells (Tregs) were also found within the infiltrating cells in vector-injected muscle, which may account for the control of capsid cytotoxic T lymphocyte (CTL) responses locally (68). In this study, the persistence of AAV antigen locally in muscle and the expression of PD-1/PDL-1 by T cells may explain the expansion and maintenance of Tregs in muscle. Interestingly, it has been shown that alpha-1-antitrypsin has tolerogenic properties as, for example, it promotes expansion of regulatory T cells in vitro [69].
Treg infiltrates were also found in a study of AAV1 muscle gene transfer for lipoprotein lipase deficiency (Table 1), however the administration of an intensive immunosuppressive regimen in this study may also have affected the outcome of gene transfer (70, 71). Nevertheless, data emerging from preclinical (72) and clinical (64) studies of direct intramuscular injection of AAV vectors indicate that the muscle has unique features when it comes to immune response to AAV gene transfer, which may result in apoptosis of reactive T cells and, therefore, allow for long-term transgene expression.
One caveat in the interpretation of results from several of the AAV trials in which muscle was targeted is that the measurement of endpoints of safety (e.g. muscle enzymes) and efficacy (transgene expression) has not always been straightforward. The presence of underlying muscle inflammation (73), the fact that several transgenes are not secreted or their effect not easily measurable (Table 1), and the fact that in some cases immunosuppression was used together with gene transfer (70, 71), further complicates the interpretation of results.
No conclusive data, no conclusive data are available on the impact of contaminants in AAV vector preparations on the immune response detected following gene transfer. However, improvements in vector design (74) and production processes (for example aimed at removing empty capsids) (56) may represent important steps towards the enhancement of the safety of gene transfer in humans.
Future studies will have to better decipher the factors contributing to vector immunogenicity in muscle gene transfer, and devise strategies to reduce the therapeutic dose of vector (75) to improve the efficacy of the approach while reducing potential immune-mediated toxicities.
IMMUNE RESPONSES DIRECTED AGAINST THE TRANSGENE PRODUCT: WHAT WE HAVE LEARNED FROM INTRAMUSCULAR VECTOR ADMINISTRATION
Direct injection of AAV vectors has been extensively tested as a strategy to engineer the muscle to produce lacking enzymes (67, 76-78), to provide expression of proteins vital for muscle physiology (10, 64, 79, 80), or to correct metabolic defects (81). This work initially focused on AAV serotype 2 (77, 82), however today most gene therapy approaches rely on AAV serotypes such as 1, 6, 8, 9 (33, 83-85), and some derivative of these serotypes (10, 86-88), which target the muscle with higher specificity and efficiency than AAV2. From an immunology perspective, pre-clinical and clinical work on intramuscular muscle gene transfer has been extremely important to understand the role of transgene immunity in gene transfer, and to understand the roles played by the context of gene transfer, such as the genetic background of the host and the muscle environment itself.
THE EXPERIENCE WITH INTRAMUSCULAR GENE TRANSFER TO RESTORE METABOLIC DEFECTS AND ENZYME DEFICIENCIES
In gene-replacement therapy, the therapeutic transgene product encoded by the AAV vector is typically a neo antigen to which the host may lack central tolerance. This, and other concurring factors (Table 3), may result in transgene-specific immune responses and activate effector mechanisms mediated by either neutralizing antibodies (89) or T cells (7).
Table 3. Factors contributing to transgene immunogenicity in AAV gene transfer to muscle.
| Factor | Increased immunogenicity | Decreased immunogenicity | References |
|---|---|---|---|
| Muscle environment | Inflamed | Normal | (18) |
| Route of vector administration | Intramuscular | Intravascular | (35, 161, 164) |
| Vector dose/site (intramuscular) | High dose/site of injection | Low dose/site of injection | (111) |
| Genetic background | Lack of endogenous protein | Presence of non-functional endogenous protein antigen | (108) |
| Expression cassette | Constitutive expression cassette | Muscle-specific cassette/detargeting of APCs | (100, 102) |
| Vector genome conformation | Self-complementary | Single-stranded | (108, 112) |
| Transgene | Strong T and B cell epitopes | (101) |
Initial gene transfer studies in mice were very encouraging as they showed that AAV2 vectors were able to allow for efficient and long-term expression of an immunogenic transgene in normal skeletal muscle following intramuscular injection. This outcome of gene transfer was strikingly different from what was observed in animals injected with adenoviral (Ad) vectors (4, 5, 90), in which a strong humoral and cellular immune response to the transgene was observed. Differently, little to no responses were observed following AAV vector intramuscular injection (91), and the difference in immunogenicity of Ad vs. AAV vectors was attributed to differences in the ability to transduce antigen-presenting cells (APC), particularly dendritic cells (DC), in vivo (92). These results were confirmed in studies in which stable expression was reported for transgenes like erythropoietin (4), alpha-1-antitrypsin (90), and coagulation factor IX (F.IX) (9) following intramuscular AAV vector administration. Further evidence that AAV vectors are less immunogenic than Ad vectors (89, 90) also comes from the observation that, when used as vaccines, AAV vector elicit weak, poorly protective CD8+ T cell responses (93). Furthermore, when highly immunogenic proteins such as influenza hemagglutinin HA are expressed in the muscle with AAV vectors, strong cell-mediated immune responses are triggered, which results in the elimination of transduced fibers within four weeks from vector administration (94). However, in this setting the kinetics of induction of antigen-specific CD4+ T cells appears delayed when the transgene is delivered with AAV as compared to Ad vectors, suggesting differences between the two vectors in either the signals delivered to the immune cells or the way the antigen is presented. Since no efficient transduction of DC has been observed with AAV vectors, uptake of antigen by APCs appears to be the prevalent mechanism of antigen presentation in the context of AAV gene transfer (94). Indeed, CD8+ T cell activation against the GFP transgene was documented in AAV vector intramuscular gene transfer via cross-presentation of the antigen by dendritic cells (95). Nevertheless, some degree of transduction of DC in vitro has been evidenced also in dogs and humans (96, 97), and in vivo in mice (98). Further indirect evidence that the transduction of APC in vivo is a key determinant of the transgene immune responses in AAV gene transfer comes from studies in which detargeting of transgene expression in APC with mir142-regulated (99) AAV vectors abolished CD4+ and CD8+ T cell responses against an immunogenic transgene in mice (100). This strategy was effective in reducing immunogenicity of different transgenes (100-102), however it failed to prevent immune responses to the transgene in inflamed muscle, where cross-presentation of antigen at the DC level is presumably more efficient (103). Finally, recent work from Carpentier and colleagues suggests that CD8+ T cell responses directed against transgenes in AAV gene transfer to the muscle are determined by the presence of MHC class I epitopes together with strong MHC class II epitopes and also B cell epitopes (101). Conversely, it has been shown that poor APC transduction associated with other factors such as T cell exhaustion and low upregulation of MHC class I molecules on target cells can result in induction of tolerance (104), suggesting that the combination of multiple parameters drives the balance between tolerance, ignorance, and immunity in muscle gene transfer.
Although transgene-specific CD8+ T cells (105) and muscle infiltrates have been detected in several AAV muscle trials, the impact of cell mediated immunity on AAV-transduced myofibers has been questioned (72). Indeed, it has been shown that T cells infiltrating the muscle display an exhausted phenotype, lose their cytotoxic potential, and eventually undergo apoptosis (64, 72). While this phenomenon does not seem to be associated with the induction of active tolerance to the transgene, as it is has been described for liver gene transfer with AAV vectors (106), more complex and complementary mechanisms may concur to shaping transgene immunogenicity in the muscle.
Several studies analyzed immune responses directed against the transgene in AAV gene transfer for coagulation factor IX (F.IX) to muscle in preclinical models of hemophilia B. This work allowed identifying the genetic background of the host as one of the crucial determinants of transgene immunogenicity (107-110), with the magnitude of the immune responses directed against the F.IX transgene directly correlated to the degree of disruption of the endogenous F.IX gene; particularly with null mutations resulting in the highest immunogenicity (107). Data in hemophilia B dogs are in agreement with these findings, and show that in intramuscular gene transfer with AAV vectors higher vector doses injected locally result in enhanced transgene immunogenicity (111). In addition to genetic background and local vector dose, recent studies in mice showed that self-complementary AAV1 vectors encoding for F.IX injected intramuscularly can lead to a stronger CD8+ T cell response to the transgene compared to single-stranded vectors (108), a result in agreement with previous findings in the context of AAV gene transfer to the liver (112). These results are in agreement with the reported higher levels of transduction in DC with self-complementary vectors as compared to single-strand vectors after intramuscular injection in mice (98) and in in vitro in human DC (96)
The availability of animal models to test transgene immunogenicity in different gene therapy scenarios has been crucial for the design of clinical gene transfer trials (77, 78), in which only subjects with residual protein levels were enrolled and received multiple intramuscular injections of AAV vectors to maintain the local vector dose below a critical threshold for immunogenicity (111).
Finally, preexisting immunity to the transgene may be found in patients suffering from lysosomal storage diseases affecting the muscle, such as Pompe disease, in which immunity triggered against the therapeutic protein is relatively common (113). In hemophilia, eradication of pre-existing immunity to coagulation factor VIII and F.IX has been achieved with liver gene transfer with AAV vectors (114, 115); in intramuscular gene transfer, high doses of AAV vectors expressing F.IX resulted in induction of anti-transgene antibodies in mice, which were then cleared over time (116). Whether clearance of pre-existing humoral immunity to a protein via muscle directed gene transfer is a result consistently achievable remains to be tested.
One alternative strategy to reduce or avoid the induction of immunity to the transgene product, or to overcome preexisting immunity that would prevent gene transfer, is to overexpress a specific endogenous protein whose function can correct the disease phenotype. For example, activated coagulation factor VII (F.VIIa) has been used to achieve correction of the bleeding phenotype in dog models of hemophilia by expressing the transgene in the liver via AAV8-mediated gene transfer (117). While the approach may be feasible in muscle, it should be noted that supraphysiological levels of expression of a transgene in muscle might break tolerance to the transgene itself. For example, an autoimmune response to erythropoietin (Epo) has been reported following intramuscular injection of AAV-Epo vectors in macaques (118).
The Experience with Neuromuscular Disorders
Considering the broad heterogeneity of muscular dystrophies, it is worth mentioning that it appears that gene expression profiles of hindlimb muscle is markedly different between severely and mildly affected animal models (119). Similarities in the expression profile of dystrophin- and sarcoglycan-deficient mice, including inflammatory and remodeling processes, were also found (119). A chronic inflammatory response characterized by local cellular infiltrates, including macrophages, is a shared feature in several dystrophic mouse models, and chemokines such as CCL2 and CCL6 correlate with macrophages and T cell recruitment in inflamed muscle (120). Therefore, the dystrophic muscle already contains all the signals and the components of a productive immune response. In dystrophic patients and in murine models of muscular dystrophies, anti-inflammatory and/or immunosuppressant drugs have a beneficial effect, further highlighting the role of the immune system in the disease pathophysiology (121-123). Studies in mice support the involvement of B and/or T lymphocytes in the course of Duchenne muscular dystrophy (DMD) and limb girdle muscular dystrophy (LGMD) type D (124, 125). Additionally, in the absence of immunomodulatory treatment, activated T cells were found in the muscle of the mdx mice, a model of DMD, and depletion of either CD4+ or CD8+ T cells resulted in an attenuation of the disease (126). These results correlate with findings in humans, as muscle biopsies from DMD patients show the presence of clonal populations of T cells with conserved TCR sequences (127), and T cell reactivity directed against dystrophin was recently documented in a large proportion of DMD patients, with over 50% of the subjects showing a positive signal in PBMC restimulated with peptides derived for dystrophin and tested in an IFN-γ ELISpot assay (73). T cell reactivity in these subjects appeared to increase with age and was decreased in subjects treated with corticosteroids (73).
In dog models of muscular dystrophy, systemic delivery of an AAV9 vector constitutively expressing minidystrophin to neonate animals resulted in widespread muscle transduction (as predicted in prior studies conducted in neonate dogs with a reporter transgene (128)) and detection of transgene-positive myofibers. The procedure, however, was also associated with marked inflammation and consequent muscle atrophy and contractures (83). This prompted the use of immunosuppressive regimens (129, 130) to prevent or lower immune reactivity following intramuscular gene transfer (for a review, see (131)). Optimization of the microdystrophin sequence, together with the use of a muscle-restricted promoter and serotype 8 AAV vectors has been shown to reduce the immunogenicity of the approach (132), as shown also in a recent study of AAV8 vector mediated exon skipping in Golden Retriever Muscular Dystrophy dogs (13).
In clinical gene transfer trials, dystrophin immunity in DMD was documented in a clinical trial of intramuscular gene transfer with an AAV2.5 vector (a hybrid vector between AAV1 and AAV2 (10)) encoding for mini-dystrophin in DMD subjects (Table 1, (105)). In this study, apparent loss of transgene expression following gene transfer was associated with detection of T cell reactivity against CD4+ and CD8+ T cell epitopes in the mini-dystrophin transgene that were not present in the endogenous dystrophin gene due to the underlying disease causing mutation. Notably, baseline reactivity to dystrophin was also detected, indicating the presence of immune reactivity directed against revertant fibers. Revertant fibers are found DMD patients and are thought to arise form somatic mutations in the revertant nucleai, which restore frameshift mutations originating from small deletions in the dystrophin gene (133). Unlike in mdx mice (134), in humans, revertant fibers do not seem to increase over time, possibly reflecting immune mediated clearance of newly-formed fibers, and their detection is not associated with a clinically relevant improvement in the disease course (135). Together, findings on revertant fibers and the basic pathophysiology of DMD should be taken into careful considerations in the design of future gene transfer trials, in which inflamed muscle is being targeted and immunomodulation may be necessary to modulate preexisting transgene-directed immune responses.
As in the case of metabolic diseases (117), also for muscle diseases it might be possible to overexpress endogenous muscle proteins to achieve correction of the disease phenotype. This approach was recently tested in humans in a phase I/II clinical trial of AAV1 vector-mediated gene transfer for follistatin in Becker muscular dystrophy patients (136), which showed amelioration of the disease phenotype. Similarly, overexpression of utrophin, a dystrophin-related protein, has been proposed as an alternative for the treatment of DMD (for a review see (137)). In mdx transgenic mice over-expressing utrophin, a significant amelioration of the disease phenotype was observed (138); similarly AAV vectors have also been used to deliver to the muscle a functional truncated form of the utrophin (139) or upstream factors involved in the upregulation of the utrophin expression, such a PGC-1α and Jazz (140, 141). These strategies could allow for avoidance of immune responses to dystrophin, as it has been observed in the context of Adenovirus-mediated gene transfer (142). However, it should be kept in mind that the structural organization of dystrophin vs. utrophin differs significantly in muscle (143), arguing for the potential limitation in full correction of muscle function with this approach.
The Muscle Environment and Transgene Immunogenicity
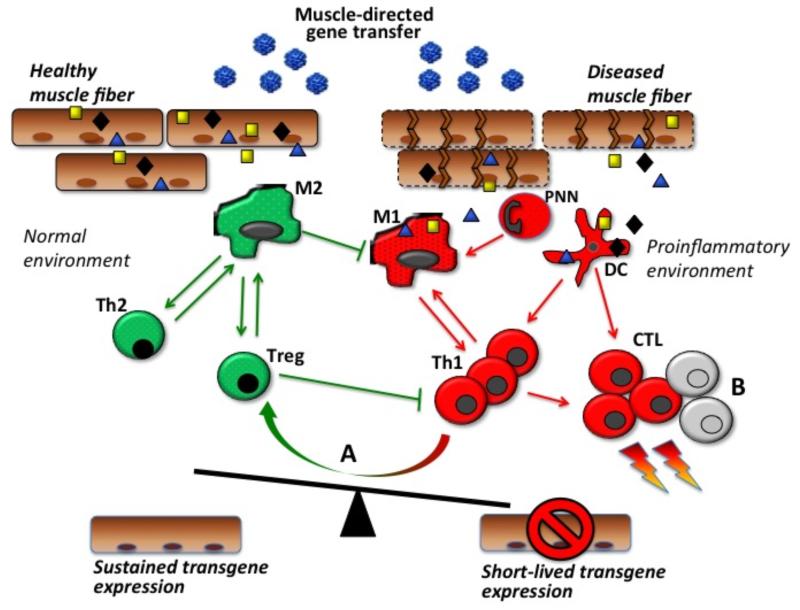
Many different immune cells involved in innate immunity contribute to the pathogenesis of dystrophies by intervening either in the degenerative or regenerative process of the muscle characteristic of this family of diseases, these cells include neutrophils, eosinophils, macrophages, mast cells (for review, (144, 145)). Early depletion of myeloid cells in mdx mice have been reported to decrease necrotic fibers by 70-80% (146), probably by containing the effects of proinflammatory M1 macrophages, which can worsen the disease by promoting muscle damage. Conversely, anti-inflammatory M2 macrophages seem to play an essential role in muscle regeneration: M2a macrophages reduce the lytic activity of M1 macrophages, while M2c macrophages promote muscle regeneration (145). These populations of macrophages are highly versatile (147) and can be influenced by the cytokine milieu. The cross talk between T cells and macrophages in fact plays a role in muscle pathology of DMD (144) and may also play an important role in AAV vector-mediated gene transfer (Fig. 2). Both DC and macrophages are able to present transgene antigen to CD4+ T helper cells in draining lymph nodes of dystrophic and healthy muscle; however in dystrophic muscle antigen presentation is enhanced (103). As a result, an increase in transgene immunogenicity may be observed in dystrophic vs. normal muscle (Fig. 2). Indeed, increased immunogenicity of the reporter transgene β-galactosidase expressed with an AAV2 vector was observed in dystrophic mice compared with wild-type animals (148).
Fig. (2). Transgene expression in muscle and the balance between tolerance and immunity.
The release of immunogenic antigens by leaky, damaged muscle fibers upon rAAV intramuscular administration and their uptake by dendritic cells (DC) contribute to the activation of immune responses and destruction of transduced muscle fibers. The proinflammatory conditions provided by the diseased muscle environment drive the activation of effector cells including type 1 macrophages (M1), granulocytes (PNN), type 1 CD4+ T helper cells (Th1), and CD8+ cytotoxic T lymphocytes (CTL). In a normal muscle environment, type 2 macrophages (M2), type 2 CD4+ T helper cells (Th2), and regulatory T cells (Treg) may dampen the effector immune response and contribute to sustained transgene expression. (A) in inflamed muscle, resident Tregs may be induced and contribute to modulate immune responses at the muscle level; similarly (B) effector T cell exhaustion and/or apoptosis may contribute to limit muscle inflammation.
Concomitant to enhanced antigen presentation, a high concentration of macrophages reactive against myofibers (50,000 macrophages/mm3) is found in muscle of mdx mice (146). These macrophages may influence the induction of T cell-mediated responses in gene transfer. Indeed, we found that macrophages were at least partially involved in the immune-mediated loss of transgene expression following AAV vector-mediated muscle gene transfer in a murine model of LGMD type D (103). CD8+ T cells also contributed to the elimination of transduced muscle cells in dystrophic mice but not in normal mice (103).
Changes in the immune environment during muscle injury also include anti-inflammatory responses. For example, the anti-inflammatory M2c macrophage subpopulation that emerges during muscle regeneration after injury responds to IL-10 (149). Interestingly, a population of muscle-specific regulatory T cells (150) have been identified in dystrophic muscle of mdx mice and also detected in muscle infiltrates from DMD patients (151). These IL-10 secreting Tregs improve the dystrophic phenotype by decreasing inflammation associated with the disease, and their depletion results in worsening of the disease phenotype, while induction of Tregs with IL-2 has a beneficial effect (151). Thus, treatments soliciting the emergence of a stable Treg population in muscle at the time of gene transfer may reduce muscle inflammation and favor maintenance of transgene expression. Indeed, administration of exogenous transgene-specific Tregs concomitantly to AAV vector gene transfer was shown to lower anti-transgene immune reactivity and to allow stable transgene expression (152) in normal muscle. Moving forward it will be important to evaluate the efficacy of the approach in a dystrophic context, since inflammatory conditions can challenge the stability of Tregs and give rise to proinflammatory Th17 cells (153, 154). This challenge might be resolved by acting directly on muscle Tregs (150, 151), which seem to retain their immunomodulatory potential despite the inflammatory environment, for example by administering low doses of IL-2 (151, 155).
THE ROUTE OF ADMINISTRATION: INTRAMUSCULAR, INTRAVASCULAR, AND SYSTEMIC DELIVERY OF AAV VECTORS TO TARGET THE MUSCLE
Proof-of-concept studies on intramuscular gene transfer with AAV vectors were initially conducted in mice. These studies revealed that the approach has the potential to result in immunity to the transgene encoded by the vector, as evidenced in several studies with vectors encoding for human F.IX (9) and other transgenes (156-158). In some cases, the substitution of strong constitutive promoters with muscle-specific promoters resulted in lower transgene immunogenicity, particularly in the context of muscular dystrophies (157, 158). Scaling up to large animal models of gene transfer confirmed results in mice, showing that intramuscular administration of AAV vectors has the potential of triggering immune responses to the transgene, particularly when large doses of vector are injected in one site (63, 109, 111, 116, 118, 129).
While immunosuppression has proven efficacious in preventing immunity to the transgene following intramuscular vector delivery (63, 129, 130, 148), the approach has several potential risks and limitations. Additional limitations of the intramuscular vector delivery approach, for example the subtherapeutic levels of transgene expression (67, 77) and the limited vector spread across the muscle, prompted investigators to devise novel vector delivery technologies to transduce large portions to the muscle via the intravascular route (159). The safety and efficacy of this technology has been tested in large animal clotting deficiencies (35), muscle disease (13, 43), and in non-human primates (160-162). Recently the intravascular delivery technology has also been tested in muscular dystrophy patients, demonstrating that the delivery technique is well tolerated (163). From the perspective of immune responses to capsid or transgene, intravascular delivery of AAV to target the muscle exposes the vector to neutralization by pre-existing antibodies (35) and results in systemic exposure to the vector itself, with all possible consequences in terms of activation of T cell responses directed against the capsid (30); furthermore, the delivery technique requires specific skills. Notably, one important advantage of intravascular delivery over intramuscular injection of the vector relies on the fact that it results in dampened transgene immunogenicity (160, 164), which has been associated with induction of protolerogenic T cells in the case of F.IX gene transfer in hemophilia B dogs (164).
In several neuromuscular diseases, the need to target skeletal muscle body-wide and the identification of AAV serotypes able to target muscle following systemic delivery (33, 43, 83, 128), generated broad interest on this route of vector delivery as a one-time treatment solution for diseases affecting skeletal muscle.
Systemic vector delivery, however, results in broad vector biodistribution and targeting of various organs, including the liver, the heart, and, in some cases, the central nervous system. This forces to carefully consider potential toxicities related to immune responses to vector or transgene, and devise intervention strategies to modulate them (31, 32). The use of muscle-specific promoters may help restricting the expression of the transgene to the muscle, thus preventing ectopic expression of the therapeutic protein and potentially reducing its immunogenicity (132). Engineering of chimeric vectors or novel AAV serotypes may also help detargeting tissues in which vector transduction is not desired for therapeutic efficacy (86, 165).
CONCLUSIONS
Muscle has been one of the first tissues targeted with gene transfer with AAV vectors. Over the years efforts have been concentrated on achieving widespread muscle transduction using novel serotypes and delivery techniques. However for muscle diseases requiring whole-body transduction the goal of phenotype correction has not been achieved yet in human trials. Similarly, in several early clinical studies of intramuscular administration of AAV vectors to correct enzyme deficiencies, levels obtained were subtherapeutic (67, 77). Yet, muscle remains a key target for gene therapy, for the treatment of the numerous diseases affecting this organ, and for the fact that it is a valuable target tissues for gene replacement therapy when, for example, it is not possible to target the liver. Importantly, encouraging safety data are emerging from muscle gene transfer trials, and evidence of multi-year expression of vector genomes injected into human muscle has been obtained (166).
As the field moves towards systemic approaches of delivery and higher vector doses, it will be essential to keep into careful consideration the interactions between vector and host immune system. In particular anti-AAV antibodies can prevent efficient targeting of the muscle via intravascular and systemic vector administration routes. Novel strategies are being tested to administer the vector in seropositive subjects, and promising results are being obtained, particularly using physical methods to remove antibodies or to block their neutralizing action (52, 53).
Systemic administration of AAV vectors will also likely result in significant spreading of the virus to organs other than the muscle, thus requiring careful monitoring of activation of T cell mediated immunity directed against the capsid or the transgene and potential related toxicities. While data emerging from hemophilia B trials indicate that it is feasible to simply use immunosuppression on demand if liver enzyme elevation is detected (32), this strategy is going to be more difficult to implement in the context of some diseases such as DMD, due to the ongoing disease-related muscle inflammation (167). To this end, a question that remains open, and requires careful risk/benefit evaluation, is whether upfront immunosuppression is warranted, particularly when gene transfer is performed in background of inflamed muscle.
Importantly, from an immunological point of view the muscle is a peculiar environment, where proinflammatory and feedback protolerogenic signals overlap (150, 151). Moving forward towards successful gene transfer in humans it will be key to understand the mechanisms regulating the balance between these two arms of immunity. Devising strategies to skew this balance towards a tolerance state may hold the potential to ameliorating muscle diseases associated with inflammation, allowing for safe and effective gene transfer with AAV vectors.
ACKNOWLEDGEMENTS
This work was supported by Genethon and by the European Union FP7-PE0PLE-2012-CIG Grant 333628 and European Research Council ERC - 2013 - CoG grant 617432.
Biography

F. Mingozzi
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- [1].Muzyczka N, Berns KI. In: Parvoviridae: The viruses and their replication. 4th ed. Knipe DM, Howley PM, editors. Lippincott, Williams and Wilkins; Philadelphia: 2001. [Google Scholar]
- [2].Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature reviews Genetics. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- [3].Buning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO molecular medicine. 2013;5(1):1–3. doi: 10.1002/emmm.201202291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996;93(24):14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70(11):8098–108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fraites TJ, Jr., Schleissing MR, Shanely RA, Walter GA, Cloutier DA, Zolotukhin I, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther. 2002;5(5 Pt 1):571–8. doi: 10.1006/mthe.2002.0580. [DOI] [PubMed] [Google Scholar]
- [7].Ross CJ, Twisk J, Bakker AC, Miao F, Verbart D, Rip J, et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum Gene Ther. 2006;17(5):487–99. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- [8].Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1998;95(24):14384–8. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94(11):5804–9. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20(2):443–55. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foster H, Sharp PS, Athanasopoulos T, Trollet C, Graham IR, Foster K, et al. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther. 2008;16(11):1825–32. doi: 10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
- [12].Lai Y, Yue Y, Liu M, Ghosh A, Engelhardt JF, Chamberlain JS, et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nature biotechnology. 2005;23(11):1435–9. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Le Guiner C, Montus M, Servais L, Cherel Y, Francois V, Thibaud JL, et al. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther. 2014;22(11):1923–35. doi: 10.1038/mt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lostal W, Bartoli M, Bourg N, Roudaut C, Bentaib A, Miyake K, et al. Efficient recovery of dysferlin deficiency by dual adeno-associated vector-mediated gene transfer. Human molecular genetics. 2010;19(10):1897–907. doi: 10.1093/hmg/ddq065. [DOI] [PubMed] [Google Scholar]
- [15].Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. The journal of gene medicine. 2005;7(4):442–51. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- [16].Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97(25):13714–9. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12(7):787–9. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- [19].Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18(9):1586–8. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J Med Virol. 1999;59(3):406–11. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [21].Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene therapy. 2011 doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- [22].Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- [23].Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P, et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. Journal of immunology. 2012;188(12):6418–24. doi: 10.4049/jimmunol.1200620. [DOI] [PubMed] [Google Scholar]
- [25].Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966;55(6):1467–74. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW. Innate Immune Responses to AAV Vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Basner-Tschakarjan E, Mingozzi F. Cell-Mediated Immunity to AAV Vectors, Evolving Concepts and Potential Solutions. Frontiers in immunology. 2014;5:350. doi: 10.3389/fimmu.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discovery medicine. 2013;15(85):379–89. [PubMed] [Google Scholar]
- [29].Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- [30].Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–22. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- [31].Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. The New England journal of medicine. 2011;365(25):2357–65. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. The New England journal of medicine. 2014;371(21):1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mingozzi F, Chen Y, Edmonson SC, Zhou S, Thurlings RM, Tak PP, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene therapy. 2013;20(4):417–24. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arruda VR, Stedman HH, Haurigot V, Buchlis G, Baila S, Favaro P, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115(23):4678–88. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–8. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810–7. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- [38].Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nature biotechnology. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. The New England journal of medicine. 2008;358(21):2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- [40].Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. The New England journal of medicine. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shin JH, Pan X, Hakim CH, Yang HT, Yue Y, Zhang K, et al. Microdystrophin ameliorates muscular dystrophy in the canine model of duchenne muscular dystrophy. Mol Ther. 2013;21(4):750–7. doi: 10.1038/mt.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Childers MK, Joubert R, Poulard K, Moal C, Grange RW, Doering JA, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med. 2014;6(220):220ra10. doi: 10.1126/scitranslmed.3007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15(3):171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Arnett AL, Konieczny P, Ramos JN, Hall J, Odom G, Yablonka-Reuveni Z, et al. Adeno-associated viral (AAV) vectors do not efficiently target muscle satellite cells. Mol Ther Methods Clin Dev. 2014:1. doi: 10.1038/mtm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dupont JB, Tournaire B, Georger C, Marolleau B, Jeanson-Leh L, Ledevin M, et al. Short-lived recombinant adeno-associated virus transgene expression in dystrophic muscle is associated with oxidative damage to transgene mRNA. Mol Ther Methods Clin Dev. 2015;2:15010. doi: 10.1038/mtm.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McIntosh JH, Cochrane M, Cobbold S, Waldmann H, Nathwani SA, Davidoff AM, et al. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene therapy. 2012;19(1):78–85. doi: 10.1038/gt.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Han SO, Li S, Brooks ED, Masat E, Leborgne C, Banugaria S, et al. Enhanced Efficacy from Gene Therapy in Pompe Disease Using Co-receptor Blockade. Hum Gene Ther. 2014 doi: 10.1089/hum.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Unzu C, Hervas-Stubbs S, Sampedro A, Mauleon I, Mancheno U, Alfaro C, et al. Transient and intensive pharmacological immunosuppression fails to improve AAV-based liver gene transfer in non-human primates. Journal of translational medicine. 2012;10:122. doi: 10.1186/1479-5876-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110(7):2334–41. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Montenegro-Miranda PS, ten Bloemendaal L, Kunne C, de Waart DR, Bosma PJ. Mycophenolate mofetil impairs transduction of single-stranded adeno-associated viral vectors. Hum Gene Ther. 2011;22(5):605–12. doi: 10.1089/hum.2010.222. [DOI] [PubMed] [Google Scholar]
- [52].Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF, et al. A 10 Patient Case Report on the Impact of Plasmapheresis Upon Neutralizing Factors Against Adeno-associated Virus (AAV) Types 1, 2, 6, and 8. Mol Ther. 2011 doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chicoine LG, Montgomery CL, Bremer WG, Shontz KM, Griffin DA, Heller KN, et al. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther. 2014;22(2):338–47. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hurlbut GD, Ziegler RJ, Nietupski JB, Foley JW, Woodworth LA, Meyers E, et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther. 2010;18(11):1983–94. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mingozzi F, Anguela XM, Pavani G, Chen Y, Davidson RJ, Hui DJ, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688–95. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18(1):135–42. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hui DJ, Basner-Tschakarjan E, Chen Y, Edmonson SC, Maus MV, Podsakoff GM, et al. Characterization of AAV T Cell Epitopes Presented by Splenocytes from Normal Human Donors. Mol Ther. 2012 ASGCT Annual Meeting Abstracts:S557. [Google Scholar]
- [59].Li C, Hirsch M, Asokan A, Zeithaml B, Ma H, Kafri T, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81(14):7540–7. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li H, Murphy SL, Giles-Davis W, Edmonson S, Xiang Z, Li Y, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther. 2007;15(4):792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- [61].Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18(3):185–94. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- [62].Martino AT, Basner-Tschakarjan E, Markusic DM, Finn JD, Hinderer C, Zhou S, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013 doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18(1):18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- [64].Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010a;68(5):629–38. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–86. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106(38):16363–8. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther. 2011;22(10):1239–47. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mueller C, Chulay JD, Trapnell BC, Humphries M, Carey B, Sandhaus RA, et al. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest. 2013;123(12):5310–8. doi: 10.1172/JCI70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ozeri E, Mizrahi M, Shahaf G, Lewis EC. alpha-1 antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. Journal of immunology. 2012;189(1):146–53. doi: 10.4049/jimmunol.1101340. [DOI] [PubMed] [Google Scholar]
- [70].Ferreira V, Petry H, Salmon F. Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Frontiers in immunology. 2014;5:82. doi: 10.3389/fimmu.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J, et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25(3):180–8. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Velazquez VM, Bowen DG, Walker CM. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector-mediated gene therapy. Blood. 2009;113(3):538–45. doi: 10.1182/blood-2008-01-131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Flanigan KM, Campbell K, Viollet L, Wang W, Gomez AM, Walker CM, et al. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther. 2013;24(9):797–806. doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE, et al. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013;123(7):2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Finn JD, Nichols TC, Svoronos N, Merricks EP, Bellenger DA, Zhou S, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120(23):4521–3. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Falk DJ, Mah CS, Soustek MS, Lee KZ, Elmallah MK, Cloutier DA, et al. Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol Ther. 2013;21(9):1661–7. doi: 10.1038/mt.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–72. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- [78].Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28(12):2303–4. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- [79].Herson S, Hentati F, Rigolet A, Behin A, Romero NB, Leturcq F, et al. A phase I trial of adeno-associated virus serotype 1-gamma-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain. 2012;135(Pt 2):483–92. doi: 10.1093/brain/awr342. [DOI] [PubMed] [Google Scholar]
- [80].Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66(3):290–7. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Callejas D, Mann CJ, Ayuso E, Lage R, Grifoll I, Roca C, et al. Treatment of diabetes and long-term survival after insulin and glucokinase gene therapy. Diabetes. 2013;62(5):1718–29. doi: 10.2337/db12-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17(12):1177–86. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- [83].Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, et al. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18(8):1501–8. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nature biotechnology. 2005;23(3):321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- [85].Arnett AL, Beutler LR, Quintana A, Allen J, Finn E, Palmiter RD, et al. Heparin-binding correlates with increased efficiency of AAV1- and AAV6-mediated transduction of striated muscle, but negatively impacts CNS transduction. Gene therapy. 2013;20(5):497–503. doi: 10.1038/gt.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nature biotechnology. 2010;28(1):79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Qiao C, Zhang W, Yuan Z, Shin JH, Li J, Jayandharan GR, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum Gene Ther. 2010;21(10):1343–8. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4(3):201–10. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- [90].Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3(3):306–12. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- [91].Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1(3):225–35. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- [92].Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117(12):3958–70. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O, Jooss K. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J Virol. 2001;75(1):269–77. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Xu D, Walker CM. Continuous CD8(+) T-cell priming by dendritic cell cross-presentation of persistent antigen following adeno-associated virus-mediated gene delivery. J Virol. 2011;85(22):12083–6. doi: 10.1128/JVI.05375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Veron P, Allo V, Riviere C, Bernard J, Douar AM, Masurier C. Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2. J Virol. 2007;81(10):5385–94. doi: 10.1128/JVI.02516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T, et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther. 2009;17(1):73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gernoux G, Guilbaud M, Dubreil L, Larcher T, Babarit C, Ledevin M, et al. Early interaction of adeno-associated virus serotype 8 vector with the host immune system following intramuscular delivery results in weak but detectable lymphocyte and dendritic cell transduction. Hum Gene Ther. 2015;26(1):1–13. doi: 10.1089/hum.2014.070. [DOI] [PubMed] [Google Scholar]
- [99].Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12(5):585–91. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- [100].Boisgerault F, Gross DA, Ferrand M, Poupiot J, Darocha S, Richard I, et al. Prolonged gene expression in muscle is achieved without active immune tolerance using microRNA 142.3p-regulated rAAV gene transfer. Hum Gene Ther. 2013 doi: 10.1089/hum.2012.208. [DOI] [PubMed] [Google Scholar]
- [101].Carpentier M, Lorain S, Chappert P, Lalfer M, Hardet R, Urbain D, et al. Intrinsic transgene immunogenicity gears CD8 T-cell priming after rAAV-mediated muscle gene transfer. Mol Ther. 2014 doi: 10.1038/mt.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Majowicz A, Maczuga P, Kwikkers KL, van der Marel S, van Logtenstein R, Petry H, et al. Mir-142-3p target sequences reduce transgene-directed immunogenicity following intramuscular adeno-associated virus 1 vector-mediated gene delivery. The journal of gene medicine. 2013;15(6-7):219–32. doi: 10.1002/jgm.2712. [DOI] [PubMed] [Google Scholar]
- [103].Ferrand M, Galy A, Boisgerault F. A dystrophic muscle broadens the contribution and activation of immune cells reacting to rAAV gene transfer. Gene therapy. 2014;21(9):828–39. doi: 10.1038/gt.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mays LE, Wang L, Lin J, Bell P, Crawford A, Wherry EJ, et al. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol Ther. 2014;22(1):28–41. doi: 10.1038/mt.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. The New England journal of medicine. 2010b;363(15):1429–37. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–56. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17(10):1733–42. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rogers GL, Martino AT, Zolotukhin I, Ertl HC, Herzog RW. Role of the vector genome and underlying factor IX mutation in immune responses to AAV gene therapy for hemophilia B. Journal of translational medicine. 2014;12:25. doi: 10.1186/1479-5876-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- [110].Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- [111].Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13(11):1281–91. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- [112].Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117(24):6459–68. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Molecular genetics and metabolism. 2010;99(1):26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO molecular medicine. 2013;5(11):1698–709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116(26):5842–8. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103(1):85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Margaritis P, Roy E, Aljamali MN, Downey HD, Giger U, Zhou S, et al. Successful treatment of canine hemophilia by continuous expression of canine FVIIa. Blood. 2009;113(16):3682–9. doi: 10.1182/blood-2008-07-168377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B, et al. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood. 2004;103(9):3300–2. doi: 10.1182/blood-2003-11-3852. [DOI] [PubMed] [Google Scholar]
- [119].Turk R, Sterrenburg E, van der Wees CG, de Meijer EJ, de Menezes RX, Groh S, et al. Common pathological mechanisms in mouse models for muscular dystrophies. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(1):127–9. doi: 10.1096/fj.05-4678fje. [DOI] [PubMed] [Google Scholar]
- [120].Porter JD, Guo W, Merriam AP, Khanna S, Cheng G, Zhou X, et al. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscular disorders : NMD. 2003;13(3):223–35. doi: 10.1016/s0960-8966(02)00242-0. [DOI] [PubMed] [Google Scholar]
- [121].Connolly AM, Pestronk A, Mehta S, Al-Lozi M. Primary alpha-sarcoglycan deficiency responsive to immunosuppression over three years. Muscle & nerve. 1998;21(11):1549–53. doi: 10.1002/(sici)1097-4598(199811)21:11<1549::aid-mus30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [122].Eghtesad S, Jhunjhunwala S, Little SR, Clemens PR. Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Molecular medicine. 2011;17(9-10):917–24. doi: 10.2119/molmed.2010.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sciorati C, Buono R, Azzoni E, Casati S, Ciuffreda P, D’Angelo G, et al. Co-administration of ibuprofen and nitric oxide is an effective experimental therapy for muscular dystrophy, with immediate applicability to humans. British journal of pharmacology. 2010;160(6):1550–60. doi: 10.1111/j.1476-5381.2010.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Farini A, Meregalli M, Belicchi M, Battistelli M, Parolini D, D’Antona G, et al. T and B lymphocyte depletion has a marked effect on the fibrosis of dystrophic skeletal muscles in the scid/mdx mouse. The Journal of pathology. 2007;213(2):229–38. doi: 10.1002/path.2213. [DOI] [PubMed] [Google Scholar]
- [125].Farini A, Sitzia C, Navarro C, D’Antona G, Belicchi M, Parolini D, et al. Absence of T and B lymphocytes modulates dystrophic features in dysferlin deficient animal model. Experimental cell research. 2012;318(10):1160–74. doi: 10.1016/j.yexcr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- [126].Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clinical immunology. 2001;98(2):235–43. doi: 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- [127].Gussoni E, Pavlath GK, Miller RG, Panzara MA, Powell M, Blau HM, et al. Specific T cell receptor gene rearrangements at the site of muscle degeneration in Duchenne muscular dystrophy. Journal of immunology. 1994;153(10):4798–805. [PubMed] [Google Scholar]
- [128].Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16(12):1944–52. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shin JH, Yue Y, Srivastava A, Smith B, Lai Y, Duan D. A simplified immune suppression scheme leads to persistent micro-dystrophin expression in Duchenne muscular dystrophy dogs. Hum Gene Ther. 2012;23(2):202–9. doi: 10.1089/hum.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15(6):1160–6. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- [131].Wang Z, Tapscott SJ, Chamberlain JS, Storb R. Immunity and AAV-Mediated Gene Therapy for Muscular Dystrophies in Large Animal Models and Human Trials. Front Microbiol. 2011;2:201. doi: 10.3389/fmicb.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Koo T, Okada T, Athanasopoulos T, Foster H, Takeda S, Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. The journal of gene medicine. 2011;13(9):497–506. doi: 10.1002/jgm.1602. [DOI] [PubMed] [Google Scholar]
- [133].Thanh LT, Nguyen TM, Helliwell TR, Morris GE. Characterization of revertant muscle fibers in Duchenne muscular dystrophy, using exon-specific monoclonal antibodies against dystrophin. Am J Hum Genet. 1995;56(3):725–31. [PMC free article] [PubMed] [Google Scholar]
- [134].Pigozzo SR, Da Re L, Romualdi C, Mazzara PG, Galletta E, Fletcher S, et al. Revertant fibers in the mdx murine model of Duchenne muscular dystrophy: an age- and muscle-related reappraisal. PloS one. 2013;8(8):e72147. doi: 10.1371/journal.pone.0072147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Arechavala-Gomeza V, Kinali M, Feng L, Guglieri M, Edge G, Main M, et al. Revertant fibres and dystrophin traces in Duchenne muscular dystrophy: implication for clinical trials. Neuromuscular disorders : NMD. 2010;20(5):295–301. doi: 10.1016/j.nmd.2010.03.007. [DOI] [PubMed] [Google Scholar]
- [136].Mendell JR, Sahenk Z, Malik V, Gomez AM, Flanigan KM, Lowes LP, et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther. 2015;23(1):192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fairclough RJ, Wood MJ, Davies KE. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nature reviews Genetics. 2013;14(6):373–8. doi: 10.1038/nrg3460. [DOI] [PubMed] [Google Scholar]
- [138].Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4(12):1441–4. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- [139].Odom GL, Gregorevic P, Allen JM, Finn E, Chamberlain JS. Microutrophin delivery through rAAV6 increases lifespan and improves muscle function in dystrophic dystrophin/utrophin-deficient mice. Mol Ther. 2008;16(9):1539–45. doi: 10.1038/mt.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Selsby JT, Morine KJ, Pendrak K, Barton ER, Sweeney HL. Rescue of dystrophic skeletal muscle by PGC-1alpha involves a fast to slow fiber type shift in the mdx mouse. PloS one. 2012;7(1):e30063. doi: 10.1371/journal.pone.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Strimpakos G, Corbi N, Pisani C, Di Certo MG, Onori A, Luvisetto S, et al. Novel adeno-associated viral vector delivering the utrophin gene regulator jazz counteracts dystrophic pathology in mdx mice. J Cell Physiol. 2014;229(9):1283–91. doi: 10.1002/jcp.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ebihara S, Guibinga GH, Gilbert R, Nalbantoglu J, Massie B, Karpati G, et al. Differential effects of dystrophin and utrophin gene transfer in immunocompetent muscular dystrophy (mdx) mice. Physiol Genomics. 2000;3(3):133–44. doi: 10.1152/physiolgenomics.2000.3.3.133. [DOI] [PubMed] [Google Scholar]
- [143].Belanto JJ, Mader TL, Eckhoff MD, Strandjord DM, Banks GB, Gardner MK, et al. Microtubule binding distinguishes dystrophin from utrophin. Proc Natl Acad Sci U S A. 2014;111(15):5723–8. doi: 10.1073/pnas.1323842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298(5):R1173–87. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Human molecular genetics. 2009;18(3):482–96. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. The Journal of cell biology. 2001;155(1):123–31. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [148].Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J, et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene therapy. 2002;9(23):1576–88. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- [149].Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Human molecular genetics. 2011;20(4):790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–95. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6(258):258ra142. doi: 10.1126/scitranslmed.3009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Gross DA, Leboeuf M, Gjata B, Danos O, Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102(13):4326–8. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- [153].Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. Journal of neuroimmunology. 2007;191(1-2):51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39(5):949–62. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. The New England journal of medicine. 2011;365(22):2067–77. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- [156].Dressman D, Araishi K, Imamura M, Sasaoka T, Liu LA, Engvall E, et al. Delivery of alpha- and beta-sarcoglycan by recombinant adeno-associated virus: efficient rescue of muscle, but differential toxicity. Hum Gene Ther. 2002;13(13):1631–46. doi: 10.1089/10430340260201725. [DOI] [PubMed] [Google Scholar]
- [157].Fougerousse F, Bartoli M, Poupiot J, Arandel L, Durand M, Guerchet N, et al. Phenotypic correction of alpha-sarcoglycan deficiency by intra-arterial injection of a muscle-specific serotype 1 rAAV vector. Mol Ther. 2007;15(1):53–61. doi: 10.1038/sj.mt.6300022. [DOI] [PubMed] [Google Scholar]
- [158].Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12(2):205–15. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- [159].Su LT, Gopal K, Wang Z, Yin X, Nelson A, Kozyak BW, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112(12):1780–8. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- [160].Toromanoff A, Cherel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME, et al. Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle. Mol Ther. 2008;16(7):1291–9. doi: 10.1038/mt.2008.87. [DOI] [PubMed] [Google Scholar]
- [161].Toromanoff A, Adjali O, Larcher T, Hill M, Guigand L, Chenuaud P, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18(1):151–60. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Rodino-Klapac LR, Montgomery CL, Bremer WG, Shontz KM, Malik V, Davis N, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18(1):109–17. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Fan Z, Kocis K, Valley R, Howard JF, Chopra M, An H, et al. Safety and feasibility of high-pressure transvenous limb perfusion with 0.9% saline in human muscular dystrophy. Mol Ther. 2012;20(2):456–61. doi: 10.1038/mt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Haurigot V, Mingozzi F, Buchlis G, Hui D, Chen Y, Basner-Tschakarjan E, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010 doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bartel M, Schaffer D, Buning H. Enhancing the Clinical Potential of AAV Vectors by Capsid Engineering to Evade Pre-Existing Immunity. Front Microbiol. 2011;2:204. doi: 10.3389/fmicb.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Buchlis G, Podsakoff GM, Radu A, Hawk SM, Flake AW, Mingozzi F, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119(13):3038–41. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].McMillan HJ, Gregas M, Darras BT, Kang PB. Serum transaminase levels in boys with Duchenne and Becker muscular dystrophy. Pediatrics. 2011;127(1):e132–6. doi: 10.1542/peds.2010-0929. [DOI] [PubMed] [Google Scholar]