Abstract
Although diabetic cardiomyopathy is widely recognised, there are no specific treatments available. Altered myocardial substrate selection has emerged as a candidate mechanism behind the development of cardiac dysfunction in diabetes. As pyruvate dehydrogenase (PDH) activity appears central to the balance of substrate utilisation, we aimed to investigate the relationship between PDH flux and myocardial function in a rodent model of type-II diabetes and to explore whether or not increasing PDH flux, with dichloroacetate, would restore the balance of substrate utilisation and improve cardiac function.
All animals underwent in vivo hyperpolarized [1-13C]pyruvate magnetic resonance spectroscopy and echocardiography to assess cardiac PDH flux and function respectively. Diabetic animals showed significantly higher blood glucose (10.8±0.7mM vs 8.4±0.5mM), lower PDH flux (0.005±0.001s−1 vs 0.017±0.002s−1) and significantly impaired diastolic function (E/E’ 12.2±0.8 vs 20±2) in keeping with early diabetic cardiomyopathy. Twenty-eight days treatment with dichloroacetate restored PDH flux to normal levels (0.018±0.002s−1), reversed diastolic dysfunction (E/E’ 14±1) and normalized blood glucose (7.5±0.7mM).
Treatment of diabetes with dichloroacetate therefore restored the balance of myocardial substrate selection, reversed diastolic dysfunction and normalised blood glucose levels. This suggests that PDH modulation could be a novel therapy for the treatment and/or prevention of diabetic cardiomyopathy.
Keywords: Diastolic Dysfunction, Echocardiography, Fuel Selection, Magnetic Resonance Spectroscopy, Metabolism, MRI, Pyruvate Dehydrogenase, Pyruvate Dehydrogenase Kinase, Type 2 Diabetes
Introduction
It is now firmly established that type-II diabetes contributes to an increased risk for the development of heart failure1. Although some of this risk can be attributed to increased coronary artery disease and hypertension, it is becoming clear that patients with type-II diabetes are also at risk of developing “diabetic cardiomyopathy”2-5, which manifests across a spectrum from subclinical left ventricular diastolic dysfunction to overt systolic failure6. As the incidence of type-II diabetes is rapidly increasing, understanding the pathophysiology behind diabetic cardiomyopathy and developing new treatment strategies is of increasing clinical importance.
Cardiac metabolism and altered substrate utilisation are now emerging as candidate mechanisms underpinning diabetic cardiomyopathy, and as such, are a target for novel treatments7, 8. The cardiac metabolic changes in type-II diabetes are linked to an increase in circulating fatty acids that results from insulin insensitivity and a failure to suppress adipose tissue hormone-sensitive lipase9. This increase in fatty acid availability, and consequently increased cardiac usage, is thought to result in a loss of efficiency between substrate utilization and ATP production in the diabetic heart10. Changes in cardiac substrate utilisation precede functional changes in the diabetic heart11. As a result, metabolic therapies aimed at restoring the balance of substrate utilization are an attractive target to improve, or even prevent, diabetic cardiomyopathy12. Specifically, diastolic dysfunction could be a good initial indicator of the effect of metabolic therapy, given dysfunction is seen in up to 60% of diabetic patients13, precedes systolic dysfunction14 and is more sensitive to changes in myocardial energetics than systolic function15.
One of the potential targets for metabolic therapy is pyruvate dehydrogenase (PDH), a key enzyme that regulates the balance between carbohydrate and fat metabolism in the heart. In diabetes, PDH activity is decreased and pyruvate oxidation impaired16, 17, resulting in a lack of metabolic flexibility in substrate selection, contributing to the over-utilization of fatty acids. Therefore, it follows that restoration of PDH activity may re-establish a normal fuel balance, thereby restoring cardiac function.
Pyruvate dehydrogenase kinase (PDK) is responsible for the phosphorylation, and consequent deactivation, of PDH. Dichloroacetate (DCA) is a pyruvate mimetic that inhibits all isoforms of PDK, leading to an increase in PDH activity and glucose oxidation18. The mechanism of action and isoform specific inhibition kinetics of DCA have been extensively studied and reported19, 20. Previous studies have shown that increasing glucose utilization via DCA, or via carnitine palmitoyltransferase-1 inhibition, improved left ventricular function in the isolated perfused diabetic rat heart21, 22. As yet there have not been any in vivo studies investigating whether DCA improves cardiac metabolism or function as a potential therapy for diabetic cardiomyopathy.
Until recently we have been limited in our ability to measure metabolism in vivo. However advances in hyperpolarized 13C magnetic resonance (MR) spectroscopy23 have been made that now enable us to obtain a real-time, in vivo assessment of carbohydrate metabolism24. In combination with an echocardiographic assessment of cardiac function, hyperpolarized 13C MR provides an ideal tool to investigate the effects of systemic treatments on diabetic myocardial metabolism and function in vivo.
In this study we aimed 1) to use hyperpolarized [1-13C]pyruvate MR spectroscopy to assess cardiac PDH flux, and echocardiography to assess cardiac function, in a rodent model of type-II diabetes25 and 2) to investigate the effects of increasing PDH flux using DCA on cardiac substrate selection and function in the diabetic rodent myocardium.
Methods
Twenty-four male Wistar rats (initial body weight ~200g, Harlan, UK) were housed on a 12:12h light/dark cycle in animal facilities at the University of Oxford. All metabolic studies were performed between 7am and 1pm with animals in the fed state. All procedures conformed to the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act of 1986 and to University of Oxford institutional guidelines. To generate a model of type-II diabetes, two groups of eight rats were fed a high fat diet for three weeks (60% fat, 35% protein, 5% carbohydrate; Special Diet Services, UK). After twelve days, these high-fat diet-fed rats were fasted overnight before receiving an intraperitoneal injection of 25mg/kg streptozotocin (STZ, freshly prepared in citrate buffer, 0.655g citric acid, 0.552g sodium citrate in 100ml ddH2O, pH4)25. These animals then continued on the high fat diet for the remainder of the study. This model aimed to generate a moderate type-II diabetic phenotype by using the high-fat feeding to induce peripheral insulin resistance, followed by a low dose of the pancreatic β-cell toxin STZ. STZ is traditionally used at high doses to induce type-I diabetes, as it results in impaired insulin secretion from the β-cell. Reed et al proposed that if a low dose of STZ was used after high-fat feeding, the function of the β-cell mass would be modestly impaired without completely compromising insulin secretion, resulting in a moderate impairment in glucose tolerance (i.e. hyperglycaemia in the absence of hypoinsulinaemia)26. The final group of animals (n=8) were fed a standard chow diet and received no injection of STZ to act as controls.
After the initial three-week period to establish the diabetic model, echocardiography was carried out on both control and diabetic groups. Following this, one group of diabetic animals began treatment with dichloroacetate for a period of 28 days. Dichloroacetic acid (Sigma-Aldrich, UK) was dissolved in the animals’ drinking water (to a final concentration of 1mM) and neutralized to pH7.2 with NaOH. At the end of the 28-day treatment period, all animals underwent metabolic and functional analysis before sacrifice and plasma and tissue samples were collected. For all in vivo studies, animals were anaesthetised using 2% isoflurane with 2L/min oxygen.
Echocardiography
Echocardiographic indices were obtained according to the recommendations of the British Society of Echocardiography. Transthoracic echocardiography was performed in control and diabetic animals, by blinded observers, with the use of a commercially available Vivid I echocardiography system (GE Healthcare) using an 11.5MHz phased array 10S-RS paediatric echo probe. Wall thickness and left ventricular (LV) dimensions were obtained from a short-axis view at the level of the papillary muscles. LV fractional shortening was calculated as (LVd-LVs)/LVd×100, where LVs is LV end-systolic diameter and LVd is the LV end-diastolic diameter. 2D-guided pulsed-wave Doppler recordings of LV inflow were obtained from the apical 4-chamber view to measure maximal early diastolic peak velocity (E) and late peak velocity (A). The E parameter provides a measure of the peak early diastolic mitral inflow velocity, and is affected by left atrial pressure, LV relaxation, and LV systolic pressure. This results in it being preload sensitive. Tissue Doppler Imaging was therefore also recorded from the medial mitral valve annulus to record early (E’) and active (A’) left ventricular diastolic myocardial velocities. E’ provides a measure of the velocity of the mitral medial annulus and is considered a non-invasive surrogate for LV relaxation. It is significantly less preload dependent and the ratio of E and E’ is therefore assumed to overcome the influence of ventricular relaxation on E and provide a preload independent reflection of left ventricular filling pressure.
MR Spectroscopy
Rats were positioned in a 7T horizontal bore MR scanner interfaced to a Direct Drive console (Varian Medical Systems, UK), and a home-built 1H/13C butterfly coil (loop diameter, 2cm) was placed over the chest, localizing signal from the heart27. Correct positioning was confirmed by the acquisition of an axial proton FLASH image (TE/TR, 1.17/2.33ms; matrix size, 64×64; FOV, 60×60mm; slice thickness, 2.5mm; excitation flip angle, 15°). An ECG-gated shim was used to reduce the proton linewidth to ~120Hz. Hyperpolarized [1-13C]pyruvate (Sigma-Aldrich, UK) was prepared by 40 minutes of hyperpolarization at ~1K as described by Ardenkjaer-Larsen et al.23, before being rapidly dissolved in a pressurised and heated alkaline solution. This produced a solution of 80mM hyperpolarized sodium [1-13C]pyruvate at physiological temperature and pH, with a polarization of ~30%. One millilitre of this solution was injected over ten seconds via a tail vein cannula (dose of ~0.32mmol/kg). Sixty individual ECG-gated 13C MR slice selective, pulse-acquire cardiac spectra were acquired over 60s after injection (TR, 1s; excitation flip angle, 5°; slice thickness 10mm, sweep width 13,593Hz; acquired points 2,048; frequency centred on the C1 pyruvate resonance)28. The combination of surface coil localisation with slice selection ensures that the signals measured are accurately localised to the cardiac lumen (pyruvate) and the front wall of the myocardium (bicarbonate, lactate, alanine). Minimal contribution is provided from the skeletal muscle due to the large differential in metabolic rate between the contracting myocardium and the resting skeletal muscle.
Tissue Collection
Animals were sacrificed in the fasted state with an overdose of isoflurane (5% isoflurane with 2L/min oxygen). The heart was removed, washed briefly in phosphate buffered saline, and frozen in liquid nitrogen. Epididymal fat pads were removed and weighed, with weights reported relative to body weight. Blood samples were taken and placed in heparinised tubes before centrifugation at 13,000rpm for 10 minutes at 4°C. The plasma was then frozen in liquid nitrogen for later biochemical analyses.
Biochemical Analyses
Western Blotting
Frozen tissue was crushed and lysis buffer added before tissue was homogenised; a protein assay established the protein concentration of each lysate. The same concentration of protein from each sample was loaded on to 12.5% SDS-PAGE gels and separated by electrophoresis29. Primary antibodies for PDK1 and 2 were purchased from New England Biolabs and Abgent, respectively; an antibody for PDK4 was kindly donated by Prof. Mary Sugden (Queen Mary’s, University of London, UK); primary antibodies for uncoupling protein 3 (UCP3) and medium chain acyl-CoA dehydrogenase (MCAD) were purchased from Abcam. Even protein loading and transfer were confirmed by Ponceau staining (0.1% w/v in 5% v/v acetic acid, Sigma-Aldrich), and internal standards were used to ensure homogeneity between samples and gels. Bands were quantified using UN-SCAN-IT gel software (Silk Scientific, USA) and all samples were run in duplicate on separate gels to confirm results.
Blood Analyses
Fasting blood glucose levels were assessed using an Optium blood glucose monitor. Fasting insulin levels were assessed on post mortem plasma using an insulin ELISA kit (Mercodia, Sweden). Plasma metabolites (Non-Esterified Fatty Acids (NEFA), low density lipoproteins (LDL), high density lipoproteins (HDL), triglycerides (TAG) and cholesterol) were assessed using an ABX Pentra 400 (Horiba ABX Diagnostics).
Spectroscopic Data Analysis
All cardiac 13C spectra were analysed using the AMARES algorithm in the jMRUI software package30. Spectra were DC offset-corrected based on the last half of acquired points. The peak areas of [1-13C]pyruvate, [1-13C]lactate, [1-13C]alanine and [13C]bicarbonate at each time point were quantified and used as input data for a kinetic model based on that developed by Zierhut et al.31 and Atherton et al.24. PDH flux was quantified as the rate of 13C label transfer from pyruvate to bicarbonate32. The rate of 13C label transfer from pyruvate to lactate and alanine was used as a marker of lactate dehydrogenase activity and alanine aminotransferase activity respectively.
Statistical Analyses
Values are reported as the mean±SEM. Differences between the three groups were assessed using analysis of variance with a Holm-Sidak post-hoc correction. A Pearson correlation was used to assess the correlation between cardiac PDH flux and diastolic dysfunction (E/E’). Statistical significance was considered if p≤0.05.
Results
Blood Metabolites and Protein Expression
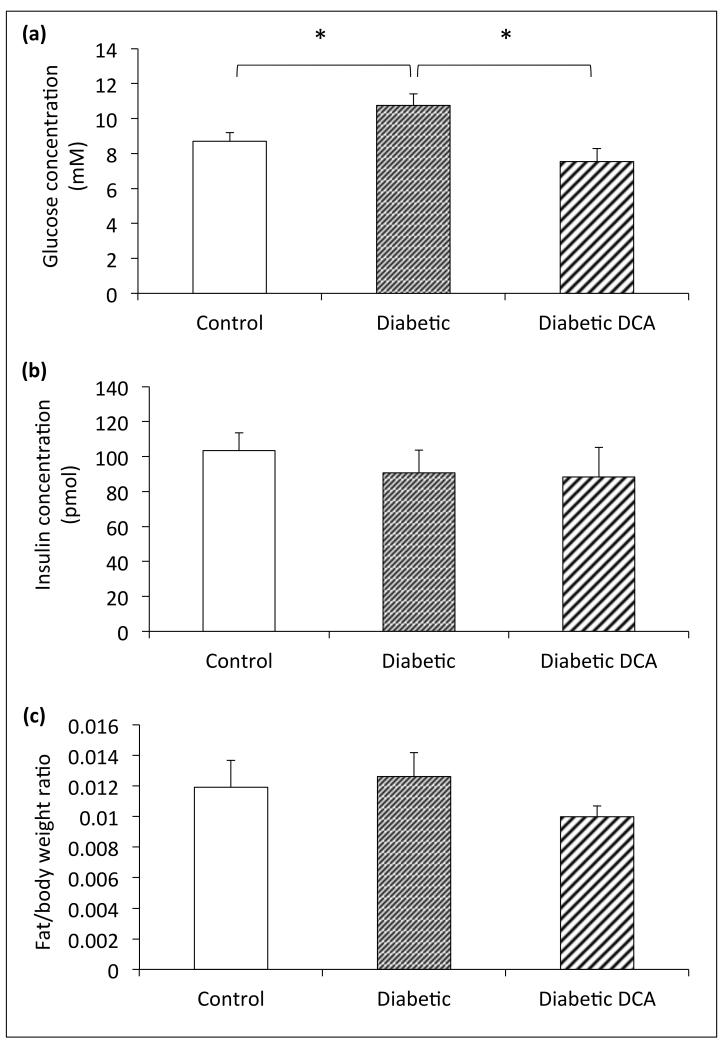
At the end of the 7-week study period, the diabetic group had significantly elevated fasting blood glucose levels compared to control animals (Figure 1a). Fasting plasma insulin levels, epididymal fat pad weight, and body weights were not significantly different between control and diabetic groups (Figure 1b/c). Diabetic animals treated with DCA for 28-days demonstrated fasting plasma glucose concentrations significantly lower than untreated diabetic animals (Figure 1a). Fasting plasma insulin levels were not different in treated diabetic animals compared to untreated (p=0.30) and control groups (p=0.13) (Figure 1b). Epididymal fat pads were similarly unaffected in comparison to both untreated diabetic (p=0.39) and control (p=0.20) groups (Figure 1c).
Figure 1.
Biochemical characterization of the type II diabetic model - (a) fasting blood glucose concentration (mM), (b) fasting plasma insulin concentration (pmol) and (c) epididymal fat pad weight normalised to body weight. *p≤0.05
The plasma metabolites (Table 1) showed a significant reduction in the plasma TAG levels in the untreated diabetic and DCA treated diabetic groups relative to controls. This finding potentially supports a greater rate of fatty acid oxidation in the diabetic animals that was unaffected by DCA treatment. The only other difference in the plasma lipid profile was a small decrease in the LDL levels measured in the DCA treated diabetic animals relative to controls. Taken together, these results provide little evidence for a significant effect of the DCA treatment on fatty acid metabolism in the diabetic animals.
Table 1. Plasma Lipid Metabolites.
| Plasma Metabolite | Control | Diabetic | Diabetic DCA |
|---|---|---|---|
| NEFA/mM | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.30 ± 0.03 |
| LDL/mM | 0.44 ± 0.04 | 0.38 ± 0.05 | 0.29 ± 0.02* |
| HDL/mM | 0.65 ± 0.04 | 0.62 ± 0.04 | 0.61 ± 0.05 |
| Triglycerides/mM | 0.98 ± 0.10 | 0.51 ± 0.06** | 0.53 ± 0.09** |
| Cholesterol/mM | 1.9 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 |
p<0.05 vs Control
p<0.01 vs Control
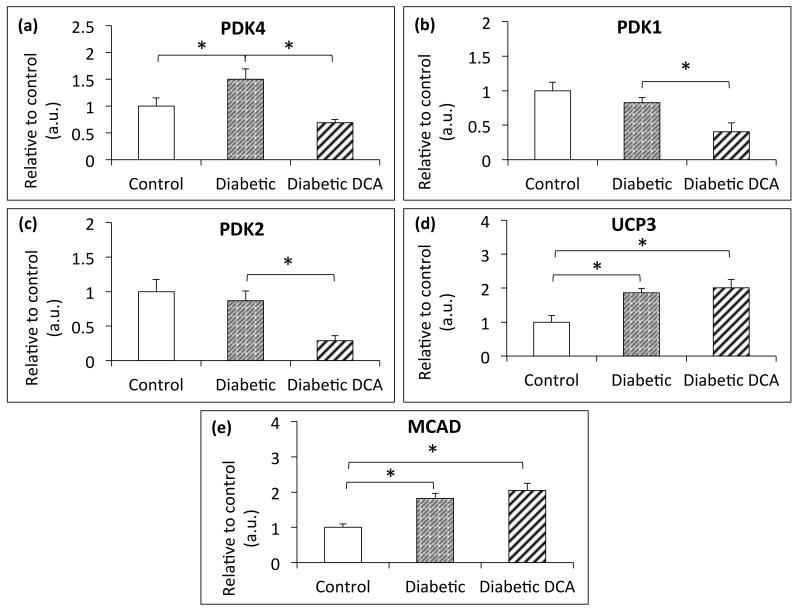
Cardiac PDK4 expression was elevated (Figure 2a) in diabetic compared with control animals, but PDK1 and 2 expression was unaffected by diabetes (Figure 2b/c). Cardiac PDK1, 2, and 4 were significantly decreased in DCA-treated diabetic animals compared to untreated diabetic animals (p=0.023, 0.009 and 0.002 respectively). PDK4 returned to a level not significantly different to control (p=0.2) (Figure 2a). Protein expression of the PPARα target genes, UCP3 and MCAD, was elevated (Figure 2d/e) in the untreated and DCA treated diabetic animals relative to controls indicating and increased rate of fatty acid oxidation in the diabetic animals that was unaffected by the DCA treatment.
Figure 2.
Protein expression of the three cardiac isoforms of pyruvate dehydrogenase kinase (PDK), along with uncoupling protein 3 (UCP3) and medium chain acyl-CoA dehydrogenase (MCAD) – (a) cardiac PDK4 expression, (b) cardiac PDK1 expression, (c) cardiac PDK2 expression, (d) cardiac UCP3 expression and (e) cardiac MCAD expression. *p≤0.05
In Vivo Cardiac Carbohydrate Metabolism
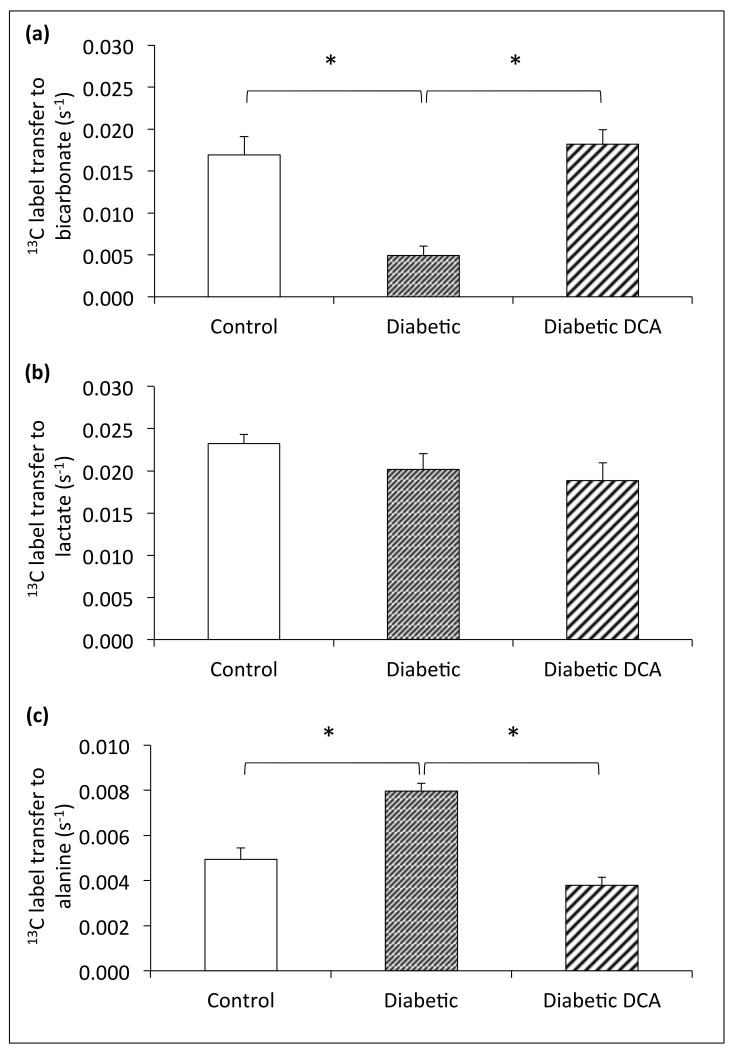
The rate of 13C-label transfer from pyruvate to bicarbonate in vivo was used as a real-time assessment of PDH flux (Figure 3). Control animals showed a PDH flux of 0.017±0.002s−1, compared with a significantly decreased cardiac PDH flux in diabetic animals of 0.005±0.001s−1 (p=0.0002, Figure 4a). 13C label transfer to lactate in diabetic animals was not significantly different to controls, however 13C label transfer to alanine was significantly increased (0.0080±0.0003s−1 in diabetics compared with controls at 0.0049±0.0005s−1, p=0.0001, Figure 4b/c). Cardiac PDH flux was significantly increased by DCA treatment (0.018±0.002s−1, p<0.0001, Figure 4a), compared to untreated diabetic animals, to the extent that the rate of flux was not different to that in control animals (p=0.6). The rate of 13C label transfer to lactate was unchanged, but 13C label transfer to alanine was decreased in treated diabetic animal hearts compared to untreated diabetics (0.0038±0.0004s−1 compared to 0.0080±0.0003s−1, p<0.0001), to a level not significantly different to controls (p=0.06, Figures 4b/c).
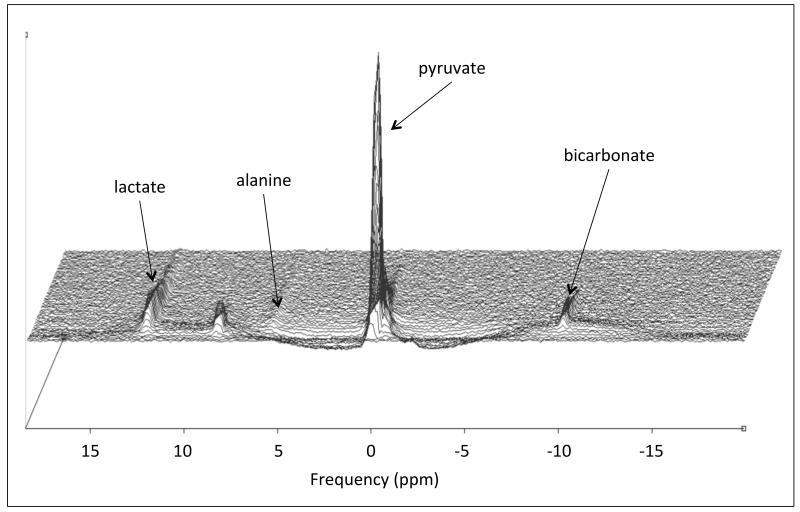
Figure 3.
An example in vivo spectral time-course taken from a control Wistar rat heart showing the injection and subsequent decay of the hyperpolarized [1-13C]pyruvate due to the recovery back to thermal equilibrium and exchange with [1-13C]lactate, [1-13C]alanine and 13C bicarbonate.
Figure 4.
Assessment of in vivo cardiac carbohydrate metabolism using hyperpolarized [1-13C]pyruvate magnetic resonance spectroscopy – (a) cardiac PDH flux, (b) cardiac 13C-label transfer to lactate and (c) cardiac 13C-label transfer to alanine. *p≤0.05.
Cardiac Function
Cardiac function, both systolic and diastolic, was assessed by echocardiography to investigate the differences between control and diabetic animals at both 3-weeks and 7-weeks (3-week data not shown, 7-week data - Figure 5). At both time points, there was no difference in ejection fraction between groups, nor in early:active ventricular filling rates. A further measure of diastolic function, E/E’, was shown to be significantly increased in the diabetic animals at both 3-weeks and 7-weeks indicating diastolic dysfunction (Figure 5c). At 7-weeks, ejection fraction and E/A ventricular filling rates were not different in DCA-treated diabetic animals compared to either control or untreated diabetic animals (Figures 5a/b). Treatment with DCA did however result in a decreased E/E’ ratio compared to diabetic animals (14±1 compared to 20±2, p=0.019, Figure 5c), restoring diastolic function back to that in control rats (p=0.4). Thus, cardiac PDH flux and diastolic dysfunction (as assessed by E/E’ ratio) were significantly negatively correlated (p=0.02).
Figure 5.
Assessment of cardiac systolic and diastolic function using echocardiography – (a) left ventricular ejection fraction, (b) maximal early diastolic peak velocity : late peak velocity (E/A) and (c) pre-load independent E/E’. *p≤0.05.
Summary
Overall this suggests that, similar to that seen in humans, this diabetic model is characterised by hyperglycaemia, reduced PDH flux, elevated PDK4 expression, and diastolic but not systolic dysfunction. Treatment with DCA reversed hyperglycaemia, decreased PDK4 expression, improved PDH flux and reversed diastolic dysfunction. This suggests that modulating substrate selection may be a potential therapeutic target for the treatment and/or prevention of diabetic cardiomyopathy.
Discussion
Type-II diabetes is a growing global health concern. It is now well established that type-II diabetes markedly elevates the risk of developing of heart failure1. Given the fact that the incidence of type-II diabetes is rapidly increasing, it is likely that the rates of diabetic cardiomyopathy and heart failure will also continue to rise. As a result, further understanding of the aetiology of diabetic cardiomyopathy and the development of novel therapeutic strategies aimed at preventing or treating diabetic cardiomyopathy is of great clinical importance.
Using the combination of hyperpolarized [1-13C]pyruvate spectroscopy and echocardiography, we have shown for the first time that 1) the diastolic dysfunction associated with diabetes is linked with a reduction in PDH flux, and 2) short term treatment with the PDK inhibitor DCA can restore PDH flux, reverse diastolic dysfunction and improve whole body glycaemic control in a rodent model of type-II diabetes.
Linking Pyruvate Dehydrogenase Activity and Diastolic Function in Diabetes
Substrate selection is a fundamental step in myocardial metabolism. In the normal resting heart, most (60-90%) of the acetyl CoA that enters the Krebs cycle comes from the β-oxidation of free fatty acids with 10–40% of acetyl CoA coming from the oxidation of pyruvate, which itself is derived from either glycolysis or lactate oxidation8, 33. However, the heart displays great flexibility in this choice of substrate, depending on the prevailing metabolic conditions34. In the diabetic myocardium, because of the combined effects of insulin resistance, high circulating free fatty acids and PDH inhibition, fatty acids are used almost exclusively to support ATP synthesis22. This shift towards fatty acid metabolism and reduced carbohydrate oxidation appears to be a combination of several factors, including reduced insulin-induced GLUT4-mediated glucose uptake, suppressed glycolysis, and reduced mitochondrial pyruvate dehydrogenase activity involving PDK435, 36. Further, the inhibition of glucose oxidation by fatty acids at the level of the PDH complex is universally reported37-39.
The crucial importance of this increase in fatty acid metabolism lies in the fact that the mitochondrial redox state, and as a result, also the free energy of hydrolysis of ATP, is negatively affected by a change in the balance of substrate utilization40. In agreement with this, a decreased efficiency of substrate utilization to create ATP has been demonstrated in diabetes and reduced myocardial energetics and diastolic dysfunction have been shown in multiple studies41, 42. This is in line with the concept that an impairment in high-energy phosphate metabolism initially affects the ability of the sarcoplasmic reticular Ca2+ ATPase (SERCA), the most energetically demanding of all enzymes involved in contractile function43, to lower cytosolic Ca2+, impairing diastolic function.
This is the first study to show a relationship between cardiac substrate selection and diastolic function in vivo. We have showed that in the heart, the presence of diabetes is associated with reduced PDH flux (as a result of elevated PDK4 levels) and, importantly, that this reduction in PDH flux is related to impaired diastolic function. This establishes PDH as a potential therapeutic target for improving diastolic function in diabetes.
Pyruvate Dehydrogenase as a Potential Therapeutic Target
DCA inhibits pyruvate dehydrogenase kinase, which results in an increase in the proportion of active pyruvate dehydrogenase18. Although the PDK isoforms PDK1, PDK2 and PDK4 are present in the heart19, it has been widely shown in diabetes that the inhibition of the pyruvate dehydrogenase complex and an inability to metabolise glucose is mediated by the up-regulation of PDK444. In agreement with this, we have shown that diabetes was associated with increased PDK4 expression and with reduced PDH flux (Figures 2a and 4a).
In addition, we have shown that treatment with DCA resulted in a restoration of PDH flux to a level seen in normal, non-diabetic animals, along with down regulation of all isoforms of PDK in the heart (Figures 2a and 4a). It is likely that the restoration of PDH flux was a result of both the down regulation of PDK expression and the direct effect of DCA on PDK activity, although PDK isoform activity was not directly assessed in this work19. Furthermore, in association with this increase in PDH flux we have shown not only a reversal of diastolic dysfunction but also, crucially, that PDH flux remains related to diastolic function after DCA treatment. This suggests that restoration of the balance of cardiac substrate selection in diabetes by increasing flux through PDH, and increasing glucose oxidation, may be a central mechanism behind the observed restoration of diastolic function. This provides a therapeutic target in the form of cardiac substrate selection, and more specifically, the PDK/PDH interaction for the treatment, or potentially prevention, of diabetic cardiomyopathy.
Several other investigators have explored the physiological relevance and therapeutic potential of the PDK/PDH interaction using a PDK4 deficient mouse model in addition to pharmacological modulation with DCA. Jeoung et al showed that starvation lowered blood glucose more in mice lacking PDK4 than in wild-type mice45. They further showed that the activity state of pyruvate dehydrogenase was greater in kidney, gastrocnemius muscle, diaphragm and heart but not in the liver of starved PDK4−/− mice, indicating that the up-regulation of PDK4 in tissues other than the liver was clearly important during starvation for regulation of glucose homoeostasis. Jeoung et al went on to show that fasting blood glucose levels were lower, glucose tolerance was slightly improved, and insulin sensitivity was slightly greater in PDK4−/− mice compared with wild-type mice subjected to diet induced obesity46. Work by Ussher et al. demonstrated that direct stimulation of PDH in mice with DCA significantly decreased infarct size following temporary ligation of the left anterior descending coronary artery47. These results were then recapitulated in PDK4 deficient mice, which had enhanced myocardial PDH activity. Finally, Mori et al. showed that deletion of PDK4 prevented diastolic dysfunction and normalised blood glucose levels in a rodent model of cardiac hypertrophy48.
Whilst fatty acid oxidation rates were not specifically measured in this study, the protein expression of the PPARα target genes UCP3 and MCAD were shown to be elevated in the diabetic animals irrespective of treatment with DCA. This would suggest an increase in fatty acid oxidation rates in diabetes that was unaffected by treatment with DCA. This finding is supported by the work of Ussher et al who have shown that palmitate oxidation rates are unaffected by DCA treatment in isolated perfused mouse hearts during ischemia/reperfusion experiments47.
Beneficial Effects Outside the Heart
In addition to the cardiac glucose oxidation increase seen in this study as a result of DCA treatment, we observed a more systemic effect, in the reduction in circulating glucose levels. This is likely to be related to the direct effects of DCA on the liver49. We have also shown a significant increase in cardiac 13C label transfer to alanine in diabetic animals (Figure 4c), which is reduced to normal levels after DCA treatment, potentially demonstrating a reduction in the supply of this gluconeogenic precursor to the liver, facilitated by the glucose-alanine cycle. It is likely that this is at least partially involved in the restoration of normal blood glucose levels seen with DCA treatment. This also suggests that targeting the PDK family in diabetes is likely to result in positive effects in blood glucose management.
Whilst our data supports very strongly a direct effect of DCA on the heart (i.e. metabolic changes that correlate with the change in function), we cannot say that the whole-body effects of DCA are not having a contributory effect on the changes in cardiac function that we have observed. In support of the hypothesis that the direct cardiac effects of DCA are causing the changes in cardiac function is the study by Nicholl et al where they provided DCA to an isolated working heart preparation of an STZ induced diabetic rat21. In this study they demonstrated that isolated diabetic hearts provided with DCA revealed a marked improvement in function (both in terms of heart rate and rate pressure product). This work provides evidence of a direct effect of enhanced glucose oxidation on cardiac function but cannot exclude the potential effect of changes in whole-body metabolism from having made a contribution to the results observed in our study.
Clinical Relevance
The incidence of diabetes is continually increasing, with over 600-million people worldwide expected to have type-II diabetes by 203050. Further, subclinical diastolic dysfunction has been linked to an increased risk of heart failure51. Therefore a therapeutic target that improves both generalised glycaemic control and reverses cardiac dysfunction is likely to be of great clinical importance. DCA has previously been the subject of clinical trials as a diabetic treatment52. However, despite the benefits in glycaemic control, it has not been widely used as a diabetic treatment due to side effects that include peripheral neuropathy53. Although other agents are now available to increase PDH activity, such as phenylbutyrate54, they have, in general, been limited to the treatment of rare conditions of inborn errors in metabolism, including congenital PDH deficiency55. The findings in our study support further work into the development of pyruvate dehydrogenase kinase and pyruvate dehydrogenase modulators as targets for the treatment of type-II diabetes. Finally, with clinical hyperpolarization studies imminent, studies that can identify reversible changes in cardiac substrate selection that, if targeted, could improve cardiac dysfunction in diabetes, are likely to become a reality in the near future56.
Study Limitations
As with any study, the limitations of the techniques used need to be taken into consideration when interpreting the results. One limitation of the current study was that the use of hyperpolarized pyruvate only probes one side of the metabolic process, namely carbohydrate metabolism, and is unable to report directly on fatty acid oxidation due to the low solubility and short hyperpolarized lifetimes of the physiologically relevant long-chain fatty acids.
Another limitation was that due to the echocardiography equipment used, we were only able to assess the diastolic parameters E/A and E/E’. Use of a higher frequency echo probe operating at >12MHz would have allowed a more in depth study of the diastolic dysfunction observed in our diabetic animals, e.g. IVRT, myocardial performance index, propagation of mitral inflow, Vp etc. However, we feel that the use of the load independent parameter, E/E’ offered a sensitive and valuable assessment of diastolic dysfunction in our model and its normalization under DCA treatment.
No direct assessment of either glucose tolerance or cardiac insulin resistance was undertaken in this work and so we cannot make any comment about the contribution that improved cardiac insulin sensitivity may have played in the restoration of PDH flux and the improvement in diastolic function we observed. However, previous work by Lloyd et al has demonstrated distinct effects of insulin and DCA in the isolated perfused rat heart57, with DCA specifically enhancing pyruvate oxidation, whilst insulin was shown to increase glucose uptake and glycolytic flux. It would therefore seem likely that the results observed in our study are via the direct effect of DCA on PDH flux and pyruvate oxidation rather than via a secondary effect on cardiac insulin resistance.
Conclusion
Diabetes is a significant global health burden and is strongly linked to the development of widespread cardiovascular problems including heart failure. We have shown here, that by specifically targeting the PDK/PDH control of substrate selection in diabetes, benefits in both diastolic function and general glycaemic control can be achieved.
Acknowledgements
D.J.T is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. L.M.L.P. undertook the experiments, analyzed the data & wrote the manuscript, O.J.R. undertook the experiments, analyzed the data and reviewed/edited the manuscript, A.J.L. undertook the experiments and reviewed/edited the manuscript, V.B. undertook experiments and reviewed/edited the manuscript, K.C. reviewed/edited the manuscript, E.J. reviewed/edited the manuscript, C.A.C. undertook the experiments and reviewed/edited the manuscript, L.C.H. undertook the experiments and reviewed/edited the manuscript, D.J.T. conceived the study, analyzed the data, wrote the manuscript and reviewed/edited the manuscript. The authors would like to thank Miss Lucia Giles, Miss Lucy Ambrose, Mr Latt Mansor and Mr Jack Miller for their technical assistance, and Professor Mary Sugden for the kind donation of a primary antibody for PDK4. This study was funded by the British Heart Foundation (FS/10/002/28078 & FS/14/17/30634), Diabetes UK (11/0004175) and equipment support was provided by GE Healthcare.
Disclosures
L.M.L.P. was supported in the form of a partial contribution to her DPhil studies by AstraZeneca PLC, London, UK, E.J. is an employee of Astra Zeneca PLC, London, UK, D.J.T. has previously received grant support from GE Healthcare, O.J.R., A.J.L., V.B., K.C., C.A.C. & L.C.H have no financial disclosures relevant to the material described in this manuscript.
Footnotes
Publisher's Disclaimer: This is an author-created, uncopyedited electronic version of an article accepted for publication in Diabetes. The American Diabetes Association (ADA), publisher of Diabetes, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties.
The definitive publisher-authenticated version will be available in a future issue of Diabetes in print and is currently online at http://diabetes.diabetesjournals.org/content/early/2015/03/18/db14-1560.abstract?sid=eb562e76-bbad-42ae-abff-8adcd41fbae5.
References
- 1.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 2.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. The American journal of medicine. 2008;121:748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Fein FS. Diabetic cardiomyopathy. Diabetes care. 1990;13:1169–79. doi: 10.2337/diacare.13.11.1169. [DOI] [PubMed] [Google Scholar]
- 4.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. The Journal of clinical investigation. 1977;60:884–99. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosyns B, Droogmans S, Weytjens C, Lahoutte T, Van Camp G, Schoors D, Franken PR. Effect of streptozotocin-induced diabetes on left ventricular function in adult rats: an in vivo Pinhole Gated SPECT study. Cardiovascular diabetology. 2007;6:30. doi: 10.1186/1475-2840-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajan SK, Gokhale SM. Cardiovascular function in patients with insulin-dependent diabetes mellitus: a study using noninvasive methods. Annals of the New York Academy of Sciences. 2002;958:425–30. doi: 10.1111/j.1749-6632.2002.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 7.Isfort M, Stevens SC, Schaffer S, Jong CJ, Wold LE. Metabolic dysfunction in diabetic cardiomyopathy. Heart failure reviews. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovascular research. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 9.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–4. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 10.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 11.Chatham JC, Forder JR. Relationship between cardiac function and substrate oxidation in hearts of diabetic rats. The American journal of physiology. 1997;273:H52–8. doi: 10.1152/ajpheart.1997.273.1.H52. [DOI] [PubMed] [Google Scholar]
- 12.Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. Journal of molecular and cellular cardiology. 2011;50:598–605. doi: 10.1016/j.yjmcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Bell DS. Diabetic cardiomyopathy. Diabetes care. 2003;26:2949–51. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 14.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart failure clinics. 2012;8:619–31. doi: 10.1016/j.hfc.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neubauer S. The failing heart--an engine out of fuel. The New England journal of medicine. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 16.Hall JL, Stanley WC, Lopaschuk GD, Wisneski JA, Pizzurro RD, Hamilton CD, McCormack JG. Impaired pyruvate oxidation but normal glucose uptake in diabetic pig heart during dobutamine-induced work. The American journal of physiology. 1996;271:H2320–9. doi: 10.1152/ajpheart.1996.271.6.H2320. [DOI] [PubMed] [Google Scholar]
- 17.Seymour AM, Chatham JC. The effects of hypertrophy and diabetes on cardiac pyruvate dehydrogenase activity. Journal of molecular and cellular cardiology. 1997;29:2771–8. doi: 10.1006/jmcc.1997.0512. [DOI] [PubMed] [Google Scholar]
- 18.Stacpoole PW, Greene YJ. Dichloroacetate. Diabetes care. 1992;15:785–91. doi: 10.2337/diacare.15.6.785. [DOI] [PubMed] [Google Scholar]
- 19.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. The Biochemical journal. 1998;329(Pt 1):191–6. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato M, Li J, Chuang JL, Chuang DT. Distinct structural mechanisms for inhibition of pyruvate dehydrogenase kinase isoforms by AZD7545, dichloroacetate, and radicicol. Structure. 2007;15:992–1004. doi: 10.1016/j.str.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholl TA, Lopaschuk GD, McNeill JH. Effects of free fatty acids and dichloroacetate on isolated working diabetic rat heart. The American journal of physiology. 1991;261:H1053–9. doi: 10.1152/ajpheart.1991.261.4.H1053. [DOI] [PubMed] [Google Scholar]
- 22.Wall SR, Lopaschuk GD. Glucose oxidation rates in fatty acid-perfused isolated working hearts from diabetic rats. Biochimica et biophysica acta. 1989;1006:97–103. doi: 10.1016/0005-2760(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 23.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherton HJ, Schroeder MA, Dodd MS, Heather LC, Carter EE, Cochlin LE, Nagel S, Sibson NR, Radda GK, Clarke K, Tyler DJ. Validation of the in vivo assessment of pyruvate dehydrogenase activity using hyperpolarised 13C MRS. NMR in biomedicine. 2011;24:201–8. doi: 10.1002/nbm.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansor LS, Gonzalez ER, Cole MA, Tyler DJ, Beeson JH, Clarke K, Carr CA, Heather LC. Cardiac metabolism in a new rat model of type 2 diabetes using high-fat diet with low dose streptozotocin. Cardiovascular diabetology. 2013;12:136. doi: 10.1186/1475-2840-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism: clinical and experimental. 2000;49:1390–4. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 27.Tyler DJ, Schroeder MA, Cochlin LE, Clarke K, Radda GK. Application of hyperpolarized magnetic resonance in the study of cardiac metabolism. Appl Magn Reson. 2008;34:523–531. [Google Scholar]
- 28.Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12051–6. doi: 10.1073/pnas.0805953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. American journal of physiology Heart and circulatory physiology. 2001;280:H977–83. doi: 10.1152/ajpheart.2001.280.3.H977. [DOI] [PubMed] [Google Scholar]
- 30.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12:141–52. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 31.Zierhut ML, Yen YF, Chen AP, Bok R, Albers MJ, Zhang V, Tropp J, Park I, Vigneron DB, Kurhanewicz J, Hurd RE, Nelson SJ. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. Journal of magnetic resonance. 2010;202:85–92. doi: 10.1016/j.jmr.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 2011;123:2552–61. doi: 10.1161/CIRCULATIONAHA.110.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological reviews. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 34.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Annals of the New York Academy of Sciences. 2004;1015:202–13. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 35.Kolter T, Uphues I, Eckel J. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. The American journal of physiology. 1997;273:E59–67. doi: 10.1152/ajpendo.1997.273.1.E59. [DOI] [PubMed] [Google Scholar]
- 36.Randle PJ, Kerbey AL, Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes/metabolism reviews. 1988;4:623–38. doi: 10.1002/dmr.5610040702. [DOI] [PubMed] [Google Scholar]
- 37.Bryson JM, Cooney GJ, Wensley VR, Phuyal JL, Caterson ID. The effects of the inhibition of fatty acid oxidation on pyruvate dehydrogenase complex activity in tissues of lean and obese mice. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1996;20:738–44. [PubMed] [Google Scholar]
- 38.Priestman DA, Orfali KA, Sugden MC. Pyruvate inhibition of pyruvate dehydrogenase kinase. Effects of progressive starvation and hyperthyroidism in vivo, and of dibutyryl cyclic AMP and fatty acids in cultured cardiac myocytes. FEBS letters. 1996;393:174–8. doi: 10.1016/0014-5793(96)00877-0. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsumi E, Takenaka F. Inhibition of pyruvate kinase by free fatty acids in rat heart muscle. Biochimica et biophysica acta. 1969;171:355–7. doi: 10.1016/0005-2744(69)90169-7. [DOI] [PubMed] [Google Scholar]
- 40.Rider OJ, Cox P, Tyler D, Clarke K, Neubauer S. Myocardial substrate metabolism in obesity. International journal of obesity. 2013;37:972–9. doi: 10.1038/ijo.2012.170. [DOI] [PubMed] [Google Scholar]
- 41.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. Journal of the American College of Cardiology. 2003;42:328–35. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 42.Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99:2261–7. doi: 10.1161/01.cir.99.17.2261. [DOI] [PubMed] [Google Scholar]
- 43.Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca(2)(+) pumps accounts for 40-50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PloS one. 2013;8:e68924. doi: 10.1371/journal.pone.0068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. The Biochemical journal. 1998;329(Pt 1):197–201. doi: 10.1042/bj3290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeoung NH, Wu P, Joshi MA, Jaskiewicz J, Bock CB, Depaoli-Roach AA, Harris RA. Role of pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) in glucose homoeostasis during starvation. The Biochemical journal. 2006;397:417–25. doi: 10.1042/BJ20060125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeoung NH, Harris RA. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. American journal of physiology Endocrinology and metabolism. 2008;295:E46–54. doi: 10.1152/ajpendo.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovascular research. 2012;94:359–69. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- 48.Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. American journal of physiology Heart and circulatory physiology. 2013;304:H1103–13. doi: 10.1152/ajpheart.00636.2012. [DOI] [PubMed] [Google Scholar]
- 49.Blackshear PJ, Holloway PA, Alberti KG. The metabolic effects of sodium dichloroacetate in the starved rat. The Biochemical journal. 1974;142:279–86. doi: 10.1042/bj1420279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 51.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 52.Stacpoole PW, Moore GW, Kornhauser DM. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. The New England journal of medicine. 1978;298:526–30. doi: 10.1056/NEJM197803092981002. [DOI] [PubMed] [Google Scholar]
- 53.Calcutt NA, Lopez VL, Bautista AD, Mizisin LM, Torres BR, Shroads AL, Mizisin AP, Stacpoole PW. Peripheral neuropathy in rats exposed to dichloroacetate. Journal of neuropathology and experimental neurology. 2009;68:985–93. doi: 10.1097/NEN.0b013e3181b40217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferriero R, Manco G, Lamantea E, Nusco E, Ferrante MI, Sordino P, Stacpoole PW, Lee B, Zeviani M, Brunetti-Pierri N. Phenylbutyrate therapy for pyruvate dehydrogenase complex deficiency and lactic acidosis. Science translational medicine. 2013;5:175ra31. doi: 10.1126/scitranslmed.3004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonard JV, Morris AA. Diagnosis and early management of inborn errors of metabolism presenting around the time of birth. Acta paediatrica. 2006;95:6–14. doi: 10.1080/08035250500349413. [DOI] [PubMed] [Google Scholar]
- 56.Rider OJ, Tyler DJ. Clinical implications of cardiac hyperpolarized magnetic resonance imaging. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15:93. doi: 10.1186/1532-429X-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lloyd S, Brocks C, Chatham JC. Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart. American journal of physiology Heart and circulatory physiology. 2003;285:H163–72. doi: 10.1152/ajpheart.01117.2002. [DOI] [PubMed] [Google Scholar]