Abstract
Activating and inactivating mutations in numerous human G protein-coupled receptors (GPCRs) are associated with a wide range of disease phenotypes. Here we use several class A GPCRs with a particularly large set of identified disease-associated mutations, many of which were biochemically characterized, along with known GPCR structures and current models of GPCR activation, to understand the molecular mechanisms yielding pathological phenotypes. Based on this mechanistic understanding we also propose different therapeutic approaches, both conventional, using small molecule ligands, and novel, involving gene therapy.
Keywords: Activation, Agonist, Mutation, Gene therapy, Genetic disorder, GPCR
Introduction
Seven transmembrane domain architecture of G protein-coupled receptors (GPCRs) appeared very early in evolution. Striking structural similarity between a group of photopigments from bacteria and archaebacteria on the one hand, and rhodopsins and related GPCRs from eukaryotes on the other, is often cited as an example of convergent evolution. However, recent demonstration that the order of helices can be scrambled in sequence and the resulting protein still forms functional photopigment1 suggests that all prokaryotic and eukaryotic rhodopsins evolved from a common ancestor, even though this happened so long ago that sequence homology cannot be traced. Animals have more GPCR subtypes than other groups of living organisms. The genomes of primates (including humans) and bats have at least 800 GPCRs,2 whereas other mammals have a lot more, with elephants expressing >3,200 GPCR subtypes currently holding the record (sevens.cbrc.jp/). Thus, it is pretty clear that GPCR design was a huge evolutionary success.3 Here using several well-studied class A (rhodopsin-like) human GPCRs we analyze why this particular protein architecture happened to be so suitable for transmembrane signaling, what are the functional consequences of various mutations in these receptors, how molecular errors translate into disease phenotypes, and what can be done to treat or cure genetic disorders associated with GPCR mutations.
The key feature that makes GPCRs particularly good signal transducers is their flexibility.4 Every protein molecule is a lot more flexible than crystal structures imply, exploring numerous conformations in physiological conditions. Special feature of GPCRs appears to be that they have several energy minima that are not dramatically different, and relatively low energy barriers between these conformational states.4 The affinity of many endogenous agonists is relatively low, with KDs varying from 0.1 to 10 mM. There is a simple relationship between the interaction energy and affinity: ΔG0 = −RTlnKA, where ΔG0 is free energy of association, R is a gas constant, T is temperature Kelvin, and KA is association constant; it is the inverse of equilibrium dissociation constant, KA = 1/KD. The calculation shows that interaction energy of these compounds at physiological temperature is ∼7–10 kcal/mol. This is certainly insufficient to push the receptor from one state to another, suggesting that ligands essentially act by conformational selection. In simple terms, flexible GPCRs exist in equilibrium of many conformations. By preferentially binding to “active” ones agonists shift the equilibrium towards activation, whereas inverse agonists preferentially bind “inactive” states, pushing the equilibrium in the opposite direction. The only obvious exception that cannot work by conformational selection is light receptor rhodopsin.5 In the dark it has covalently linked 11-cis-retinal, which acts as an inverse agonist. The photon of light isomerizes 11-cis to all-trans-retinal, which acts as an agonist, while remaining covalently bound. Structural similarity of rhodopsin to other receptors, first discovered after sequencing of β2-adrenergic receptor,6 which lead to the concept of GPCRs, suggests that the mechanisms of activation are likely similar in this super-family. Interestingly, the energy of a photon with wavelength of 500 nm (the peak of rhodopsin absorption) is much greater, ∼57 kcal/mol, which might be sufficient to “push” rhodopsin into the active state. Even though every protein explores pretty wide conformational space and exists in a multitude of conformations, for the purposes of this review we will refer to all states that are unfavorable for G protein binding as inactive (R), and all states that can couple to G proteins as “active” (R*). We would like the reader to keep in mind that in reality this long-established tradition, however convenient, is a gross over-simplification.
Classification of GPCR mutations
Mutations altering GPCR function are usually classified according to the net change in signaling ability. A general decrease in relevant signaling is termed loss of function (LOF), a general increase as gain of function (GOF). Considering the complexity of the GPCR signaling process, the simple discrimination between LOF and GOF does not fully reflect the variety of disease-causing mutations. More detailed mechanism-based classification helps to understand receptor malfunctions and devise appropriate therapy.
Mutations affecting GPCR basal activity
As GPCRs are very flexible, there is a definite probability for the receptor to adopt an active R* conformation even without agonist binding. This probability and the level of so called constitutive or basal activity varies highly among wild type (WT) GPCRs (for extensive list see7) and has physiological importance in many cases. The melanocortin-4-receptor (MC4R) exhibits a fairly high basal activity, which appears to be essential for the maintenance of normal energy homeostasis.8, 9 Loss of MC4R constitutive activity is associated with the risk of obesity.9 Both decreases and increases in constitutive activity can lead to disease phenotypes.
It is believed that the level of basal activity is determined by intra-molecular constrains,10, 11 which limit the GPCR flexibility, and the ability of the receptor to adopt a conformation in which it can activate G-protein even without agonist binding. In many GPCRs Asp(6.30) and Arg(3.50) form the so called “ionic lock”, a salt bridge, which has been associated with modulation of basal activity.12 Mutation of Asp(6.30) into a variety of different amino acids breaks this salt bridge, relieving the constraint, thereby increasing constitutive activity in several GPCRs.13, 14, 15, 16 In glycoprotein hormone receptors the conserved residue Asp(6.44) was shown to play an essential role in dampening basal activity.17 Substitutions of Asp(6.44) lead to increased basal activity in the thyroid stimulating hormone receptor (TSHR)18 and the luteinizing hormone/chorionic gonadotropin receptor (LHCGR).19 The active conformation made more probable by those mutations does not always have to be identical to the one stabilized by agonists. In fact, there are mutations, which increase basal activity, but impair agonist stimulation. In the TSHR those mutations are scattered all over the receptor structure, and their effects range from total loss to slight decrease in TSH response, along with increased constitutive activity. A decrease in constitutive activity would be expected to reflect limited conformational flexibility of the receptor, e.g., when the mutation introduces additional intra-molecular constraints. In reality most mutations decreasing basal activity also cause other defects, such as impaired G protein coupling, impaired agonist binding, or general decrease in response to agonist simulation.
Mutations affecting ligand binding
At the level of ligand binding, a mutation can change the response by altering: (1) agonist affinity, (2) efficacy, or (3) receptor selectivity. 1. Although altering binding affinity does not change maximum response, it influences the EC50 value. The concentration at which a response is achieved is either decreased (in GOF mutants) or increased (in LOF mutants). Residues essential for agonist binding, directly and indirectly, can be found within the extracellular receptor elements and in the trans-membrane domains, and are expected to be involved in affinity modulation.10, 20 2. A mutation increasing the efficacy of a ligand can do so by facilitating the formation of active receptor conformation, which, when stabilized by agonist binding, provides a more favorable interface for G-protein activation. In this case an agonist would induce a stronger response while retaining the same affinity. 3. As far as the specificity is concerned, the glycoprotein hormone receptors provide a perfect example. The substantial sequence homology of the three receptors (TSHR, follicle-stimulating hormone receptor (FSHR), LHCGR) and their cognate agonists (TSH, FSH, LH and hCG) requires an exact specificity barrier.10 Mutations broadening receptor specificity have been found both in the large N-terminal ectodomain and within the serpentine trans-membrane (TM) domain. The mechanisms that alter specificity appear to be different in these two cases. Site-directed mutagenesis suggests that substitutions within the N-terminal ectodomain of the glycoprotein hormone receptors alter the recognition specificity and the accessibility of the receptor.20, 21 Mutations within the TM domain are expected to change the energy barrier for activation by an alternative ligand, thereby altering functional selectivity of the receptor. In case of the FSHR both types of mutations have been associated with a defect known as spontaneous ovarian hyper-stimulation syndrome (sOHSS). sOHSS is the result of FSHR stimulation by elevated serum hCG levels during pregnancy, while the receptor retains normal ability to respond to FSH. This promiscuous response leads to overstimulation of the ovaries despite normal levels of FSH.10
Mutations affecting GPCR-G protein interaction
Upon GPCR activation, the cytoplasmic ends of the trans-membrane helices V and VI move considerably to form an interface for G-protein binding and activation.22,23 G-protein binding residues are mainly located within helices III, V and VI.20 Mutations can interfere with the process of coupling to downstream effectors by altering the exposure or the structure of the interaction interface. Experimentally this would also increase the affinity of the agonist–receptor interaction. It should be mentioned that many GPCRs have the ability to interact with several different downstream effectors, such as different G-proteins or arrestins. Consequently there is the possibility of a mutation influencing the signaling outcome by a changed bias. In most cases, functional characterization of disease-causing mutations has been limited to determining cAMP and IP3 levels, both of which are mediated by G-proteins. Possible effects of those mutations on arrestin-mediated signaling are only beginning to be unraveled. Recent structure of the GPCR complex with arrestin24 is the first step to understanding of the structural basis of mutation-induced bias.
Mutations affecting cell surface expression
The discussion above was based on the assumption that mutations do not significantly affect receptor biosynthesis and trafficking. In reality, impaired receptor expression is the most common defect.25 While receptor biosynthesis does not seem to be affected in most cases, the critical point in trafficking of mutant receptors appears to be the ER, where the first quality control mechanism ensures that misfolded receptors are not allowed to move to the Golgi, but are instead trafficked to lysosomes and degraded.26,27 Several molecular defects can lead to misfolding, ranging from the inability to bind necessary chaperones due to missing interaction sequences to general receptor instability. Any disruption of the disulfide bridge between TM3 (C3.25) and the extracellular loop by a mutation has been reported to lead to receptor instability and malfunction.20 Sometimes misfolding can be prevented by the application of pharmacological chaperones, as has been shown experimentally for several different diseases caused by GPCR mutations.26,27 Another cause of faulty trafficking is the disruption or deletion of signaling motifs. A motif within the C-terminal tail of glycoprotein hormone receptor has been suggested to be essential for plasma membrane targeting. Mutation of several residues in this motif leads to intracellular retention.28 Many of the receptor defects discussed above, both GOF and LOF, are often combined with a general decrease in cell surface expression. In fact, partial intracellular retention appears to be a general characteristic of GOF mutations. Considering the increased flexibility leading to the enhanced signaling in the first place, it is conceivable that excessive flexibility increases the chance of unfolding and/or misfolding.
Frequent combination of several defects makes a detailed functional characterization and the identification of the ultimate cause of receptor malfunction difficult. Nevertheless, extensive studies of the glycoprotein hormone receptors, the melanocortin-4 receptor and the vasopressin V2 receptor provide insights about structural basis of disease-causing effects of many mutations.
GPCR mutations in diseases
GPCR malfunctions due to mutations have been associated with many diseases, including immunological, metabolic and reproductive disorders, cancer and neurodegenerative diseases,29 but only a fraction of disease-associated GPCR mutations have been characterized functionally (Table 1a, Table 1b, Table 2, Table 3; Supplemental Table S1). For a detailed analysis of structure-function relationships of disease-causing mutations here we chose five most intensively studied GPCRs: three glycoprotein hormone receptors (GPHR), TSHR (thyroid stimulating hormone receptor), LHCGR (luteinizing hormone/choriogonadotropin receptor) and FSHR (follicle stimulating hormone receptor), the melanocortin receptor MC4R and the V2R (arginine vasopressin type 2 receptor).
Table 1a.
Basal Activity (GOF).
| Receptor | BW | Mutation | Size | Charge | Hydrophicity | Ref. |
|---|---|---|---|---|---|---|
| TSHR | 1.49 | G → S | Increased | More hydrophilic | 19, 152 | |
| MC4R | 1.49 | E → K | − → + | 153 | ||
| TSHR LHCGR | 2.43 | M → T | Decreased | More hydrophilic | 19, 59, 154 | |
| 19, 60, 155, 156 | ||||||
| TSHR | 3.43 | L → Q | Increased | More hydrophilic | 25, 157 | |
| TSHR LHCGR | 3.43 | L → R | Increased | More hydrophilic | 19, 63 | |
| 19, 64, 74 | ||||||
| TSHR | 5.54 | V → F | Increased | 158 | ||
| TSHR | 5.54 | V → L | Increased | 159 | ||
| LHCGR | 5.54 | I → L | 14, 19 | |||
| TSHR, FSHR | 6.30 | D → G | Decreased | − → neutral | 13, 19, 160, 161 | |
| 15, 19 | ||||||
| LHCGR, FSHR | 6.30 | D → N | − → neutral | 14, 19 | ||
| 19, 162, 163, 164 | ||||||
| TSHR | 6.34 | A → I | Increased | 13, 19, 160 | ||
| TSHR, LHCGR | 6.34 | A → V | Increased | 19, 160, 161, 165, 166 | ||
| 19, 167 | ||||||
| TSHR | 6.34 | A → F | Increased | 62 | ||
| TSHR, LHCGR | 6.37 | M → I | Decreased | 19, 168 | ||
| 19, 169 | ||||||
| TSHR, LHCGR | 6.38 | A → V | Increased | 170 | ||
| 19, 171 | ||||||
| TSHR | 6.40 | L → F | Increased | 18, 19, 61 | ||
| TSHR, MC4R | 6.40 | L → Q | Increased | More hydrophilic | 18, 19, 61 | |
| 19, 46, 48, 172, 173 | ||||||
| TSHR | 6.41 | I → M | Increased | 174 | ||
| TSHR, LHCGR | 6.41 | I → L | 19, 175 | |||
| 19, 176 | ||||||
| TSHR, LHCGR | 6.43 | T → I | Increased | More hydrophobic | 19, 161, 177, 178, 179 | |
| 19, 169 | ||||||
| TSHR | 6.44 | D → A | Decreased | − → neutral | 18, 19 | |
| TSHR, LHCGR | 6.44 | D → E | Increased | 18, 19, 178, 179 | ||
| 19, 180 | ||||||
| TSHR, LHCGR | 6.44 | D → Y | Increased | − → neutral | 18, 19, 161, 178, 179, 181 | |
| 14, 19, 36 | ||||||
| LHCGR | 6.44 | D → G | Decreased | − → neutral | 19, 182, 183 | |
| LHCGR | 6.44 | D → H | − → + | 19, 36 |
Table 1b.
Basal activity (LOF).
| Receptor | BW | Mutation | Ref. |
|---|---|---|---|
| MC4R | 1.57 | I → T | 48, 153 |
| MC4R | 2.62 | I → S,T | 19,25,173,184,185 |
| 16,19,25,48,186 | |||
| MC4R | 3.24 | I → T | 45 |
| MC4R | 3.53 | T → I | 48 |
| MC4R | 4.41 | R → W | 25, 45, 48, 172, 173, 184 |
| MC4R | 4.50 | W → C | 95 |
| MC4R | 6.34 | A → E | 45, 184 |
| MC4R | 6.42 | G → S | 45, 48, 185 |
| MC4R | 6.61 | C → R | 25, 173, 187 |
| MC4R | 7.52 | I → T | 48, 172 |
Table 2.
Transduction ability.
| Receptor | BW | Mutation | Ref. | |
|---|---|---|---|---|
| G protein binding pocket | V2R | 3.50 | R → H | 73 |
| MC4R | 3.53 | T → I | 48 | |
| LHCGR | 6.34 | A → V | 167 | |
| FSHR | 6.36 | R → C | 188 | |
| LHCGR | 7.55 | I → K | 25, 74, 189 | |
| Central cluster | MC4R | 2.50 | D → N | 190 |
| V2R | 2.50 | D → N | 191, 192, 193 | |
| MC4R | 3.40 | I → T | 194 | |
| LHCGR | 3.43 | L → R | 64 | |
| MC4R | 6.43 | V → I | 172, 184, 195 | |
| LHCGR | 6.45 | C → R | 14 | |
| TSHR | 7.45 | N → S | 196 | |
| V2R | 7.50 | P → S | 75 | |
| Cluster helices I and II | V2R | 1.39 | L → F | 197 |
| LHCGR | 1.41 | L → P | 198 | |
| MC4R | 1.43 | G → D | 153 | |
| MC4R | 1.43 | G → V | 153 | |
| FSHR | 2.61 | A → T | 199 | |
| MC4R | 2.62 | I → S | 184 | |
| Outliners | MC4R | 4.51 | A → T | 49, 195 |
| LHCGR | 6.59 | A → P | 76 |
Table 3.
Ligand binding affinity.
| GOF: Decreased KD |
LOF: Increased KD |
||||||
|---|---|---|---|---|---|---|---|
| Receptor | Mutation | BW | Ref. | Receptor | Mutation | BW | Ref. |
| LHCGR | M → T | 2.43 | 19, 60, 155, 156 | MC4R | E → K | 1.49 | 153 |
| TSHR | M → V | 2.53 | 19, 200 | MC4R | N → S | 1.50 | 25, 49, 173, 184, 201 |
| TSHR | S → R | 3.36 | 19, 160, 196 | MC4R | N → D | 2.57 | 25, 49, 173, 195 |
| TSHR | V → A | 3.40 | 19, 160, 202, 203 | MC4R | L → P | 2.66 | 25, 173, 201 |
| TSHR | Y → N | 5.58 | 19, 204 | V2R | R → W | 3.26 | 192, 205, 206, 207, 208 |
| TSHR | D → G | 6.30 | 13, 19, 160 | MC4R | I → K | 3.28 | 25, 49, 173, 185, 195 |
| TSHR | A → I,V | 6.34 | 13, 19, 160, 165, 166 | MC4R | I → T | 3.40 | 25, 172, 173, 194 |
| MC4R | L → Q | 6.40 | 19, 46, 48, 172, 173 | V2R | Y → C | 5.39 | 191, 208, 209, 210 |
| TSHR | L → F | 6.40 | 18, 19, 61 | V2R | P → H | 7.50 | 75 |
| TSHR | I → M | 6.41 | 174 | ||||
| TSHR | F → L | 6.42 | 19, 211 | ||||
| TSHR | T → I | 6.43 | 19, 178, 179 | ||||
| TSHR | C → W | 6.47 | 212 | ||||
| MC4R | F → S | 6.51 | 95 | ||||
| MC4R | I → T | 7.52 | 48, 172 | ||||
The glycoprotein hormone receptors
All three GPHRs function in the endocrine system. Their cognate ligands are produced by the anterior pituitary gland, secreted into the bloodstream, and transported to their target organ. Upon binding to their receptors, the glycoprotein hormones initiate their response mainly via GS protein, although at high ligand concentrations the GPHRs were shown to activate the Gq/11 proteins as well. Mutations in GPHRs are responsible for a variety of diseases: activating mutations generally cause ligand-independent activity of the target tissue, giving rise to classical hyper-phenotypes and in some cases initiate tumor development. Inactivating mutations result in tissue resistance to the agonist and classical hypo-phenotypes. While GOF mutations are generally dominant (Table 1a), LOF mutations follow mainly an autosomal or X-linked recessive transmission pattern (Table 1b). The presence of dominant-negative effects is unclear in many cases. It appears that those effects are more likely linked to receptor biosynthesis and trafficking to the cell surface than receptor function.
Activation of TSHR by its cognate agonist thyroid-stimulating hormone (TSH) is essential for thyroid cell proliferation and differentiation and stimulates the synthesis and secretion of thyroid hormones.25 The thyroid hormones, T3 and T4, exert stimulatory effects on metabolism. Both GOF and LOF of the TSHR have been associated with various thyroid diseases. The physiological and pathological aspects of TSHR signaling have been reviewed comprehensively.30, 31, 32 Constitutive activation of the TSHR by GOF mutations generally results in genetic non-autoimmune hyper-thyroidism, defined by excessive release of thyroid hormones. Somatic GOF mutations, originally affecting only a single thyrocyte cell, result in the formation of a benign, well defined and encapsulated adenoma, demonstrating both unregulated growth and autonomous hyperfunction.33 The tumor progressively takes over the function of the thyroid tissue ultimately leading to thyrotoxicosis, characterized by elevated plasma T3 and/or T4 levels.30 Although a similar effect can be achieved by defects within other components of the cAMP cascade, activating TSHR mutations are responsible for 70%–80% of all toxic adenomas.32 Germline GOF mutations make the whole thyroid tissue autonomous (insensitive to regulation), ultimately resulting in hereditary toxic thyroid hyperplasia (HTTH, also known as familial non-autoimmune hyperthyroidism), which is an autosomal dominant disorder.30 In the case of spontaneous germline GOF mutations the condition is known as sporadic congenital non-autoimmune hyperthyroidism; patients generally tend to display a more severe phenotype than patients suffering from HTTH.32 For an extensive summary of clinical aspects for all three conditions see Hébrant et al (2011).32 While somatic LOF mutations remain asymptomatic, germline LOF mutations cause resistance to TSH, resulting either in euthyroid hyperthyrotropinemia or hypothyroidism, depending on the severity of the mutation. Euthyroid hyperthyrotropinemia remains mainly asymptomatic. Only TSH plasma levels are chronically elevated to compensate for the loss in TSHR sensitivity and to maintain T3/T4 levels within the physiological range.34 This condition is therefore also termed compensated hypothyroidism. More severe LOF mutations result in congenital hypothyroidism with hypoplasia of the thyroid glands, but TSHR mutations appear to be causative only in a small proportion of patients.30
Both LH and FSH are released from the anterior pituitary gland upon stimulation by the gonadotropin-releasing hormone and in turn regulate gonadal development and function via their cognate receptors, LHCGR and FSHR, respectively. Defects of both receptors have therefore been associated with various reproductive diseases. Importantly, the impact of mutated receptor varies between genders.
Being expressed on the Leydig cells, LHCGR stimulates testosterone production, which is required for male sex differentiation. In females, LHCGR is expressed on theca and granulosa cells, where it influences the ovarian cycle. In males, constitutive activation of LHCGR results in a precocious development of sexual characteristics due to LH-independent production of testosterone at an early age, a condition termed familial male-limited precocious puberty (FMPP). Asp578Gly represents the most frequent mutation, causing 76% of all FMPP cases.35 Interestingly, patients suffering from FMPP display normal reproductive function as adults.19 GOF mutations were also suspected to be responsible for Leydig cell tumor development, but to our knowledge only one mutation has been reported to induce tumor development.36 In females, the constitutive activation of LHCGR remains asymptomatic due to the necessity of both LH and FSH for female gonadal development. LHCGR LOF mutations were reported to cause Leydig cell hypoplasia in males. Depending on the residual activity of the mutant receptor, patients display phenotypes ranging from micropenis (in case of certain residual activity) to complete pseudohermaphroditism (in case of total loss of function), accompanied by an ambiguous phenotype.37 In females, LOF mutations result in hypergonadotrophic hypogonadism and primary amenorrhea, but do not affect follicular development. Symptoms are generally mild and present with a late onset.35
FSHR is expressed on the granulosa cells of growing follicles in females. Stimulation by FSH is absolutely required for normal female gonadal development, maturation and function. Subsequently, LOF mutations lead to various degrees of gonadal malfunction, ranging from ovarian dysgenesis (ODG), to primary and secondary amenorrhea. Again, the residual activity of the FSHR correlates with the severity of the phenotype, a knockout of the FSHR gene was shown to result in complete infertility in female mice.38 The first and most prominent LOF mutation was identified in a Finnish female patient with ODG.39 Since GOF mutations in TSHR and LHCGR are associated with tumor development, FSHR, which also mediates cell proliferation, was expected to be responsible for granulose cell tumor. Analysis never confirmed this hypothesis. Instead, all FSHR GOF mutations identified so far are associated with ovarian hyperstimulation syndrome (OHSS), which, as mentioned earlier, results from a relaxation of specificity borders. In males, FSHR is expressed on testicular Sertoli cells. Although spermatogenesis can be initiated without FSH, FSH action appears to be important for viability and mobility of sperm.38 LOF mutations in males therefore lead to small testes with various degrees of impaired spermatogenesis but do not result in complete azoospermia.40 Only two GOF mutation were reported in male patients. The first mutation, Asp567Gly, was discovered in a hypophysectomized and hypogonadotrophic male, who remained fertile with only testosterone treatment. The second mutation remained asymptomatic.41
The melanocortin-4 receptor
The melanocortin-4 receptor (MC4R) belongs to a subfamily consisting of five receptors, which respond to several agonists (α-MSH, β-MSH, γ-MSH and ACTH) and two endogenous inverse agonists (Agouti and Agouti-related protein (AgRP)). The MC4R is mainly expressed in neurons in brain regions associated with feeding behavior and food intake. As part of the melanocortin circuit, MC4R is involved in the regulation of energy homeostasis. Leptin, secreted into the blood proportionally to the amount of body fat, stimulates the production of proopiomelanocortin (POMC), a precursor of several active neuropeptides. POMC is cleaved to α-MSH, which exerts an anorexigenic effect via stimulation of MC4R. At the same time, leptin inhibits the biosynthesis of AgRP, which normally exerts an orexigenic effect by functioning as an inverse agonist to the MC4R.42 Animal studies provided further evidence about the effects of these ligands: α-MSH stimulation decreases,43 while AgRP increases weight gain.44 Any disruption of this circuit can be expected to cause a metabolic disease, termed obesity. Generally obesity is defined as the chronic imbalance between food intake and energy expenditure, resulting in excessive lipid accumulation and an increased body mass index (>30). Although obesity is believed to be a multifactorial disease, with both environmental and genetic factors contributing to its severity, MC4R mutations have been suggested to cause a monogenic form of obesity. MC4R mutations have variable prevalence, with between 0.5% and 6% of severe obese adults reported as carriers of MC4R mutations.42 Indeed, more than 100 mutations, both LOF and GOF have been reported, covering 32% of MC4R residues. While LOF mutations are associated with excessive weight gain (obesity), GOF mutations were expected to cause an especially lean or anorexic phenotype. Interestingly, most of the GOF mutations reported so far were discovered in obese subjects.45, 46, 47, 48 Their modes of action have not been elucidated yet. Most LOF mutants are retained intracellularly,25 others have been reported to display decreased basal activity. Since MC4R basal activity is believed to provide a constant tonic signal of satiety, a decrease results in increased weight gain.9 A correlation between residual activity and severity of the phenotype can be observed.49 In contrast to many other GPCRs, MC4R LOF mutations display an autosomal dominant transmission pattern. Both haplo-insufficiency and dominant negative effects due to dimerization have been proposed as pathogenic mechanism.42 Physiology and pathology of MC4R have been reviewed comprehensively recently.42, 50 The MC3R has also been associated with energy homeostasis, but, in contrast to MC4R, its contribution to obesity is controversial.
The arginine vasopressin type 2 receptor
The arginine vasopressin type 2 receptor (V2R) belongs to a group of three receptors, all responding to the neurohypophyseal nonapeptide arginine-vasopressin (AVP), but differing in expression patterns, downstream signaling pathways and ultimate functions. V2R is expressed in several tissues, ranging from the kidney to the inner ear, with its function remaining elusive in many cases. In the kidney, V2R action has been studied extensively. There V2R is expressed on the basolateral membrane of the collecting ducts cells, mediating diuresis. Upon activation by AVP, V2R exerts its effect via the stimulatory G protein pathway, resulting in increased cytoplasmic cAMP levels and subsequent activation of protein kinase A (PKA). PKA in turn mediates the insertion of the water channel aquaporin-2 (AQP2) into the luminal plasma membrane, increasing water permeability. In addition, PKA elevates urea permeability and stimulates sodium retention.27, 51 Over 190 V2R mutations, both LOF and GOF, have been reported over the last years. LOF generally leads to the inability to concentrate urine despite normal levels of AVP, known as nephrogenic diabetes insipidus (NDI). Clinically NDI is characterized by polyuria, polydipsia, hyposthenuria. Since the V2R gene is located on the X-chromosome, this form of NDI is considered as X-linked NDI (XNDI). XNDI is generally a rare disease with about 90% of XNDI patients being males.27 GOF mutations have been reported in patients with a condition known as nephrogenic syndrome of inappropriate antidiuresis (NSIAD), the inability to excrete excessive water, resulting in hyponatremia, hypo-osmolality and natriuresis. Only a few GOF have been reported to date.52
Mutations affecting GPCR signaling
For analysis of disease-causing mutations, we focused on the extended trans-membrane region, including the elements responsible for ligand binding, signal transduction and G-protein coupling. Although different receptors within the class A GPCR sub-family show low sequence identity, the trans-membrane part shows the highest homology in their sequence. The Ballesteros–Weinstein numbering scheme53 allows comparison of equivalent residues in different receptors, which otherwise show very little sequence homology.
The intracellular and extracellular loops are regions of high variance, both in sequence and in secondary structure, complicating direct comparison of different receptors. While the extracellular structures regulate ligand specificity, ligand pocket accessibility and, in the case of the GPHR, even ligand binding, the intracellular parts have been mainly associated with G-protein binding, receptor desensitization and internalization. Despite the undeniable functional importance of both regions, we will primarily focus on mutations located within the trans-membrane part (Table 1a, Table 1b, Table 2, Table 3).
To analyze the location and the structural influence of mutations, we visualized all mutations on the backbone of inactive and active structure of β2AR (PDB ID 2RH1 and 3SN6). We ask the reader to keep in mind that the analyzed receptors may differ significantly both in sequence and structure from the β2AR. Therefore, proposed ideas do not necessarily apply to all GPCRs.
Characterization of mutations according to the net change in signaling ability
We mapped disease-causing mutations on the inactive structure of β2AR, according to their net change in signaling ability. LOF mutations appear to be scattered all over the receptor structure without a clear pattern. If we focus on mutations observed in at least two different receptors, this decreases the number of LOF mutations drastically and reveals their predominant localization in two main clusters: a) at the interfaces of helices I, II and VII, and b) at the interface between helices III and VI (Fig. 1A). GOF are also mainly limited to the helical interfaces, further restrictions only slightly change this picture. Interestingly, the localization of GOF is generally similar to that of LOF mutations, but GOF mutations appear more concentrated in the cluster around helix VI (Fig. 1B).
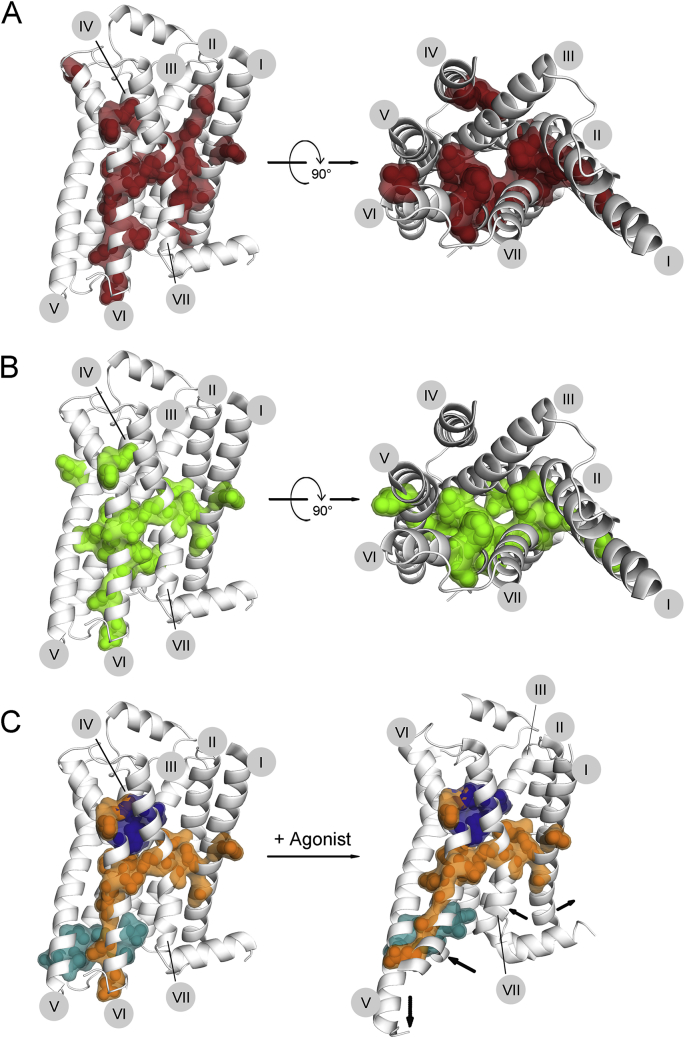
Figure 1.
Characterization of mutations according to net change in signaling ability. Disease-causing mutations, reported in any of the five chosen receptors (TSHR, LHCGR, FSHR, MC4R, V2R), were characterized according to the net change in signaling ability. For direct comparison, the mutations were converted according to the Ballesteros–Weinstein (BW) numbering scheme53: each residue is given an identifier, consisting two numbers. The first identifies the helix, the second corresponds to the position of the residue relative to the most conserved residue within this helix; the most conserved residue is assigned the number 50. To visualize the mutations, we chose the crystal structure of the β2-adrenoreceptor (β2AR), which was numbered according to an advanced numbering scheme, taking into account helical irregularities (can be accessed at http://tools.gpcr.org/docs/numbering). Loss of function (A) and gain of function (B) mutations, reported in more than one receptor, are mapped separately on the β2AR structure and depicted both in side view (left panels) and top view (right panels; as seen from the extracellular side; the ECL2 helix was deleted for better visualization). C. Positions where mutations were reported to cause LOF or GOF, depending on the substituting amino acid, are shown in orange in both the inactive (left) and active (right) β2AR crystal structure. The area containing most of the mutations partially bridges the common ligand binding pocket (dark blue) and the common G protein interface (turquois), both defined by Venkatakrishnan et al (2013).20 Black arrows within the active structure indicate which helical regions undergo major movements during activation. The numbers of trans-membrane helices I–VII are indicated in gray circles.
Key residues affecting GPCR functions are clustered at the helical interfaces
The residues where mutations can cause either LOF or GOF appear to function as switches, which when mutated determine the fate of the receptor. The area where these key residues are located partially bridges a common ligand binding pocket and common G-protein interaction site (identified on the basis of the comparison of active and inactive crystal structures of several different receptors20), without affecting these key areas themselves. Comparison of active and inactive structures reveals that these residues are mainly located in areas, which undergo major activation-induced movements (Fig. 1C). Especially interesting are the residues along helix VI, which experiences the most dramatic structural change. The clusters between helix III and VI and between helix VI and VII are located around the kink area of helix VI, suggesting their involvement in movement regulation. It is expected that many of these residues are also involved in modulation of basal activity or general signaling ability.
Both clusters of key residues mainly have hydrophobic side chains in the WT sequence, which presumably stabilize the helical organization via hydrophobic interactions. Tight packing in this area is necessary for a functional receptor, so that any substitutions here can be expected to lead to instability and functional changes.
Mutational alteration of GPCR basal activity
GPCR signal transduction
The mechanism of GPCR activation has been the subject of intensive investigation, and the crystal structures elucidated over the past 10 years are finally beginning to shed some light on the complex process of receptor activation. The comparison of receptor structures in active and inactive conformation suggests that the signal is transduced from a common ligand-binding pocket to a common G-protein docking interface via a conserved mechanism.20 The variety of GPCR-induced responses was expected to be reflected by the complexity of activation mechanism. Since little is known about the specific features of this mechanism in most receptors, here we focus on elucidated conserved structures and the mechanisms proposed so far.
GPCRs bind a variety of different ligands, ranging from ions and nucleotides to small molecule hormones, peptides, and proteins. Despite this variety, Venkatakrishnan et al suggested the existence of a common ligand-binding pocket, deep within the trans-membrane bundle.20 This ligand-binding pocket contains certain trigger residues, sensing the presence of a ligand. The conserved CWxP motif, located near the extracellular end of helix VI, is one of these triggers. Upon ligand binding, Trp6.48 experiences a slight shift, thereby inducing a conformational change in helix VI, which is amplified by a proline-induced kink. Water cluster within the ligand-binding pocket was proposed to stabilize a non-proline kink of helix III by interacting with Ile3.28 and Val3.32. Upon activation helix III straightens, probably due to rearrangement of water cluster triggered by ligand binding.54 Both ligand-activated triggers lead to large-scale rearrangements of the trans-membrane structure, culminating in the opening of a cytoplasmic cavity between helices III, V and VI. These large-scale changes are accompanied by rotameric changes within conserved micro-switches, which stabilize the active conformation. The D/ERY motif in helix III is one of the most conserved structural motifs in class A GPCRs, with 96% conservation for the central Arg3.50. Both Asp3.49 and Arg3.50 stabilize the inactive conformation by forming a number of possible interactions: Asp3.49 interacts with a conserved Tyr in IL2, tethering the loop to the helical bundle. Arg3.50 forms a salt bridge with Asp/Glu6.30, connecting the cytoplasmic ends of helices III and VI, thereby closing the cavity. This so called ionic lock is not conserved in all GPCRs. In some receptors Arg3.50 appears to participate in hydrogen bonding with polar residues in helix VI.55 In addition, Arg3.50 and Asp3.49 were also reported to interact electrostatically.56 Upon activation, Arg3.50 experiences a rotameric conformational change and interacts with the C-terminal helix of Gα.23 The comparison of crystal structures of GPCRs in different active conformations suggests that the rotameric change of Arg3.50 requires the presence of Gα.55 A similarly conserved motif, NPxxY, is located near the cytoplasmic end of helix VII. Tyr7.53 functions as one of the main activation switches: upon activation, Tyr7.53 changes its orientation towards the middle axis of the helical bundle, forming new interactions, for example with Tyr5.58 in rhodopsin and the β2AR. In GPHR this activation switch is controlled by signature motif within helix VI, the FTD motif. In the inactive conformation, Asp6.44 of the FTD motif likely interacts with the Asn7.49 of the NPxxY motif, thereby sequestering the Arg7.49 from other interactions. Upon activation, helix VI experiences both a rotation and a translocation, breaking this interaction. Asn7.49 is now free to establish new interactions, thereby stabilizing the active conformation. Mutagenesis experiments show that mutation of any of these two residues leads to constitutive activity.17
Over the past years another aspect of GPCR activation has developed: the idea of fine-tuning GPCR function by allosteric modulators. A high-resolution crystal structure of A2A-adrenoreceptor revealed internal water molecules, forming a continuous water channel within a central cavity, which binds a sodium ion as allosteric modulator. The water channel was suggested to be involved in receptor activation: while the inactive structure shows the continuous water channel, the active structure reveals two hydrophobic layers, disrupting the channel and decreasing the size of the cavity from 200 A3 to 70 A3, thus releasing the sodium ion.54 Molecular dynamic simulations have suggested an opposite mechanism.57 In any case, residues lining the channel, especially within the cavity, can be expected to affect receptor activation if mutated. Because of this contradiction, we will not consider the concept of allosteric modulation further.
Potential mechanisms altering basal activity of GPCRs
An increase in basal activity requires a mutation that leads to a ligand-independent opening of the G-protein-binding cavity. This conformational change can be induced mutationally in multiple ways. Keeping in mind the mechanism of receptor activation by the ligand binding, it is conceivable that a mutation partially mimics this process by affecting key positions, such as ligand-dependent trigger residues, micro-switches, or residues directly involved in G-protein interaction. Such mutations could be identified by simple comparison to reported common receptor elements involved in activation. It is also generally believed that the receptor is maintained in an inactive conformation by restraining interactions. Releasing those constraints would consequently lead to increased ligand-independent activation. Another possibility is mutations in positions maintaining general receptor stability. Increased flexibility can obviate the necessity for ligand binding to open the G protein-binding cavity. Such mutations can be localized everywhere within the helical bundle, where they loosen up inter-helical interactions and therefore increase the conformational flexibility of the receptor.
Mutations that enhance basal activity by increasing the accessibility of the G-protein interaction site
Mapping the residues, mutations of which were reported to increase basal activity, reveals significant overlap with positions of mutations causing general GOF, suggesting that an increase in basal activity is an important disease-causing mechanism. These mutations are mainly concentrated on the cytoplasmic half of the interfaces of helices III, V and VI (Fig. 2B). A central cluster of residues from all three helices is located close to the proline-induced kink in helix VI. Additional mutations are located all along the cytoplasmic half of helix VI. In contrast to general GOF mutations, the second cluster detected between helices I, II and VII is absent in this group of mutations.
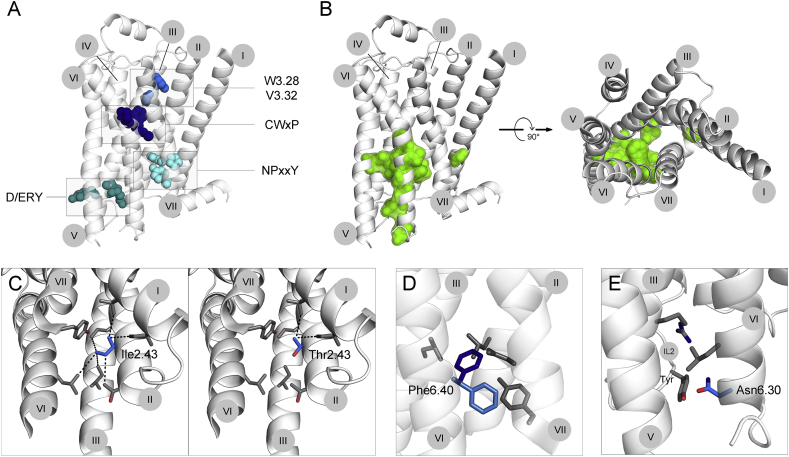
Figure 2.
Mutational alteration of GPCR basal activity. A. GPCR activation is mediated by conserved structural elements. The conserved CWxY motif and the residues 3.28 and 3.32, within the ligand binding pocket function as triggers, inducing conformational changes after ligand binding. These changes include rotameric rearrangements in the D/ERY motif in helix III and the NPxxY motif in helix VII, which stabilize the active conformation. Mutations affecting any of these essential elements are believed to alter GPCR activation. B. Mutations increasing basal activity. The residues mutated in at least two receptors are shown in green on the inactive structure of β2AR (left, side view; right, top view, as seen from the extracellular side; ECL2 helix was deleted for better visualization). C,D,E. Depending on the change in chemical properties introduced by the substituting amino acid, we propose three mechanisms, by which the mutation increases basal activity. C. Mutation of Ile2.43 to Thr2.43 decreases hydrophobicity, thereby weakening the tight helical packing. D. Introduction of Phe at position 6.40 results in physical clashes with surrounding residues and therefore probably leads to conformational changes within the helical bundle. E. Mutation of Asp6.30 to Asn changes the charge. In other receptors this Asp6.30 was reported to engage in an electrostatic interaction, which is broken by the introduction of Asn. In the β2AR this interaction rather results in repulsion with a Tyr residue in ICL2. The numbers of trans-membrane helices I-VII are indicated in gray circles.
Analysis of chemical changes introduced by particular mutations allows us to hypothesize how these mutations increase basal activity. Considering mutation-induced changes in size, charge and hydrophobicity of the side chain (Table 1a, Table 1b), we propose three possible mechanisms.
1. One powerful driving force of general protein folding is the “hydrophobic collapse”, the assembly of hydrophobic side chains within the core of the protein to minimize water contacts.58 This applies to GPCRs: the structural and functional integrity of the trans-membrane domain largely depends on the stabilizing effect of hydrophobic contacts on the helical interfaces. By increasing the hydrophilicity of the side chains within the helical bundle, a mutation can destabilize the receptor. The introduced side chain would not fit into hydrophobic tightly packed helical bundle, thereby not only loosening the hydrophobic core, but also introducing major structural changes within the seven trans-membrane domain. Ultimately this change can lead to the opening of the cytoplasmic cavity. Mutation of a conserved hydrophobic Met2.43 into polar Thr was shown to increase basal activity in both TSHR59 and LHCGR.60 As part of helix II, Met2.43 points inward and likely establishes extensive hydrophobic contacts with residues in close proximity (<5A). Introduced Thr would disrupt this packing (Fig. 2C). However, it is important to keep in mind that the exact orientation and therefore the definite interactions might vary between the β2AR, which carries an Ile in this position, and the GPHR. Fig. 2C only shows a likely scenario of what Met2.43Thr mutation causes within the GPHR.
2. In a tightly packed helical core, the size of the side chains matters. Increasing the size may result in clashes with surrounding side chains, which would require small conformational changes in the immediate surrounding structures to accommodate the new side chain. This is particularly important around the kink area of helix VI, where a small conformational change is sufficient to turn the helix and open up the cavity. For example, Leu6.40Phe is an activating mutation in TSHR.18, 61 Leu6.40 is located near the cytoplasmic end of helix VI. Its substitution by Phe, which is a generally conservative change, is accompanied by an increase in size. Modeling shows that the newly introduced Phe clashes with surrounding side chains in any possible rotameric conformation (two representative conformations are shown in Fig. 2D), suggesting that Phe physically pushed the helices III, V, VI and VII apart from each other. Ultimately this would lead to an opening of the G protein binding cavity. The conservative substitution of Ala6.34 with several other hydrophobic amino acids, such as Ile, Val or Phe, provides another example. In GPHR Ala6.34, located at the cytoplasmic tip of helix VI (right where the action happens), forms a hydrophobic interaction with Ile5.61, contributing to the tight packing within the helical bundle. Increasing the size of Ala6.34 probably disrupts the tight packing, resulting in the opening of the helical bundle.62
3. Several mutations increasing basal activity introduce an alteration in charge, either a change from negative to positive or from negative to neutral. Generally, charged side chains within the helical bundle are likely involved in electrostatic interactions. Therefore, mutating the side chain breaks these interactions, releasing a constraint, which possibly keeps the receptor inactive. A charge reversal would even result in repulsion between the two intended interaction partners, creating the force that pushes helices apart. For example, Asp6.30, located at the very tip of helix VI, was reported to form an electrostatic interaction with Arg3.50, thereby constraining the receptor in the inactive conformation. By neutralizing the negative charge (Asp to Asn mutation), this constraint is released, facilitating the transition from inactive to active conformation. In the crystal structure of β2AR Asp6.30 and Arg3.50 do not interact, but the introduction of Asn leads to repulsion due to Tyr residues within intracellular loop 2, suggesting an opening of the cavity (Fig. 2E).
Please note that the charge is not always the most important factor. The actual chemical environment has to be taken into account. The best example is the residue D6.44, which is mutated into a variety of different amino acids in different receptors. Due to its negative charge and its orientation towards the middle of the bundle, D6.44 was suggested to be involved in an electrostatic interaction. In the Drosophila GPHR homolog D6.44 was reported to interact with N7.49 of the conserved NPxxY motif. This interaction restrains the receptor in the inactive state. Thus, breaking this interaction by either decreasing the size of the side chain or by altering the charge would be expected to cause an increase in the basal activity.10 Interestingly, mutation into Asn, although neutralizing the charge, does not lead to constitutive activity, suggesting that hydrogen bonding is the crucial function of D6.44. In any case, D6.44 mutations invariably lead to constitutive activity.
Several mutations involve more than one of the changes mentioned above, opening the possibility of combining several effects. For example, the substitution of Leu3.43 by Arg within the TSHR63 and the LHCGR64 changes both the size and the polarity of the residue. Any rotameric conformation of the introduced Arg requires conformational adjustments. The introduction of the positive charge further destabilizes the helical arrangement.
All three scenarios listed above have one thing in common: they lead to a general destabilization of the receptor, thereby increasing its flexibility. Interestingly, no mutations were discovered in positions essential to the common receptor activation process, such as micro-switches or G-protein interaction sites. The exception is the GPHR-specific FTD motif, where mutations of two out of three residues were found, further emphasizing the importance of this motif for GPHR activation. The majority of mutations seem to exert their positive effect on the basal activity, mostly through general destabilization of the structure, rather than through mimicking the effects of ligand binding.
Mutations of very few residues were reported to decrease or abolish basal activity; they are scattered all over the receptor structure without a clear pattern, so it is impossible to suggest a unifying hypothesis regarding the mechanism(s) of their action.
Mutations affecting GPCRs ability to transduce a signal from the extra- to the intracellular side
The GPCR-G protein interaction
The ability of a GPCR to respond to the presence of a ligand by signaling of the appropriate strength is essential to any physiological process. The receptor adopts an active conformation, which is characterized by the opening of the cavity at the cytoplasmic side. Cross-linking experiments of helices III and VI showed this cavity to be essential for G protein binding and activation.65 Most of the main interaction sites between GPCR and G protein are therefore expected to be located in and around this cavity.
For a long time most of the evidence about the exact location and composition of this G-protein interaction surface came from competition, mutagenesis and cross-linking experiments. Early studies with the 5HT1A receptor provided evidence for the importance of the intracellular loops, especially ICL2 for G protein coupling; when expressed as a separated peptide, ICL2 competes with the receptor for the Gi protein, inhibiting AC activity.66 Further studies identified several residues of importance within or in close proximity to ICL2, among them the conserved R3.50 (DRY motif),67 a conserved hydrophobic Leu68 and a stretch of residues on the junction of ICL2 and helix IV.69 A number of mutational studies of all three GPHRs also implicated ICL3, a hydrophobic motif within ICL3 in particular, in Gs coupling; it remained unclear whether ICL3 was involved in G protein coupling directly or indirectly by influencing the packing of helices V and VI.67 Cross-linking studies using MC3R interacting with Gq protein confirmed the importance of ICL2 and of a hydrophobic residue within ICL2, and identified additional residues within helix VI and the cytoplasmic helix VIII.70 Collectively, biochemical studies point to the cytoplasmic ends of helices III, V and VI, the intracellular loops 2 and 3 and the intracellular helix VIII as elements important for G-protein activation. Despite extensive progress in identifying the GPCR-G protein interactions by using those biochemical methods (for an extensive list of residues associated with G-protein coupling see71), actual crystal structures of GPCRs in complex with G-proteins were needed to confirm the evidence.
The crystal structure of rhodopsin in complex with the key interacting peptide of transducin, the C-terminus of the α-subunit, identified positions within helices III, V, VI and VIII as potential G-protein interaction sites. The complex structure further illustrated the importance of the DRY motif as a central activation motif.72 In 2011 Rasmussen et al23 solved the crystal structure of β2AR in complex with Gs protein, providing further definite evidence of receptor-G protein interactions. According to this structure the interaction sites are mainly located at the cytoplasmic tips of helices V and VI and within the second intracellular loop (ICL2) of the receptor, forming a 1,276 A2 interface. Interestingly, the receptor directly interacts only with Gαs, while Gβ appears to function in positioning and stabilizing the Gαs N-terminal α helix. Some of the extensive intermolecular interactions between β2AR and Gαs can be identified. As predicted by biochemical studies, ICL2 appears to interact with Gαs via hydrophobic contacts. F139 is buried within a hydrophobic pocket formed by Gαs. A similar interaction was earlier reported in studies using the Hm1R68 and the MC3R.70 The exact position and orientation of ICL2 is stabilized by an intramolecular interaction with the Asp3.49 of the DRY motif within helix III. The DRY motif is further involved in a direct interaction with Gαs; the complex structure shows that Arg3.50 packs against Tyr 391 of Gαs. Both ICL2 and the DRY motif appear to be crucial for Gαs activation.
Venkatakrishnan et al20 compared the residues identified in both structures and defined a common G protein interface, consisting of residues at the cytoplasmic tips of helices III, V and VI and within ICL2. We will refer to this common interface when comparing mutations to essential G protein interaction residues.
We would like to mention that very little is known about G-protein selectivity. The attempts to define specific signature sequences within the putative G protein-binding site within the receptor were impeded by the fact that many GPCRs can interact with more than one type of G-protein. Current thinking is that the spatial arrangement (secondary and tertiary), i.e., the active conformation adopted by the receptor in general and binding interface in particular, rather than the exact residues in the contact site, determines the selectivity for G-proteins.23 Most disease-causing mutations were characterized by determining the cAMP response, providing only evidence about the interaction with Gs protein. A full profile of receptor-G protein interaction defects requires the determination of other downstream responses as well.
Incomplete information impedes a reliable judgment of the signaling ability of the receptor
When analyzing disease-causing mutants for their ability to respond to ligand, there are several parameters to be taken into account: 1. The maximum response can generally reflect both the cell surface expression and the ability to respond to the presence of the ligand. We therefore consider a decrease in maximum response despite unchanged cell surface expression as an indication of defective transduction ability. 2. The EC50 value by itself does not necessarily provide a clear answer about the receptor ability to respond, since both binding issues and signaling issues are reflected in the EC50 value. 3. The efficacy eliminates this dual-dependency, by combining the EC50 and the binding affinity; mathematically the efficacy can be presented as the ratio of binding affinity (KD) to EC50. While the KD value is independent of the receptor number, the EC50 decreases with increased cell surface expression. Both parameters, therefore, need to be determined in the same experimental setup. For a reliable analysis of a mutant's ability to respond to a signal, the cell surface expression, the maximum response, the EC50 and the KD value need to be determined. This extensive characterization has only been done for a few receptor mutants. Therefore, we will focus on the mutations that show a decreased or increased maximum response despite normal cell surface expression.
Interestingly, most disease-associated mutations decrease, rather than increase the ability to respond to a ligand (Table 2). To our knowledge A6.34V, found in the TSHR is the only mutation reported to increase maximum response. Since this mutation also shows increased binding affinity, it is unclear to which extend the increase in signaling ability leads to the phenotype of general GOF.
The mutations causing a decrease in maximum response, visualized in the active conformation of β2AR, (Fig. 3B) are scattered all over the receptor structure. We want to stress, that there is only one mutation, which occurred in multiple receptors. Depending on the location of the mutation we propose two hypotheses how those mutations influence receptor responsiveness:
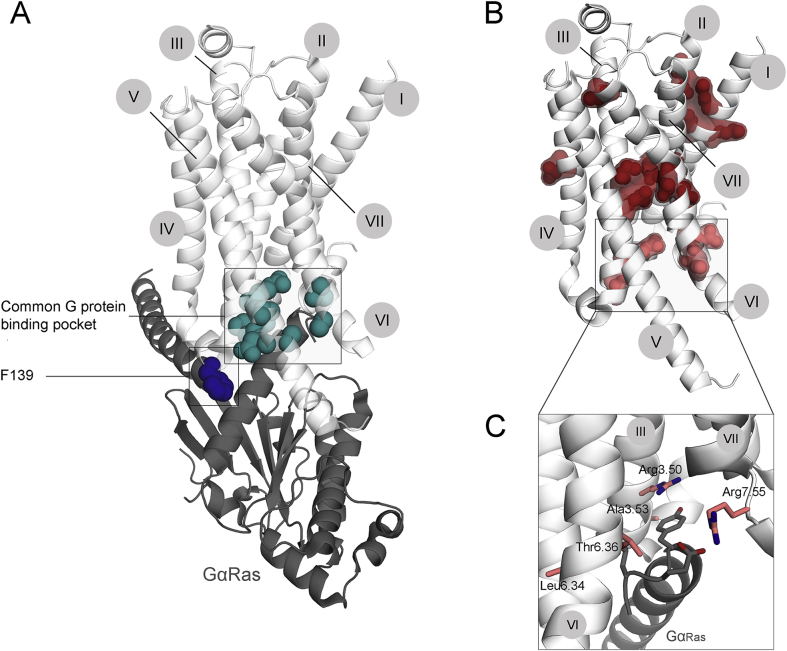
Figure 3.
Mutations alter the ability of the receptor to transduce the signal. A. Receptor residues essential for GPCR-G protein interaction are shown on the structure of active β2AR in complex with the Gs protein (Ras-like domain of Gα-subunit is shown in dark gray). They include several residues within the cytoplasmic cavity (turquois), and the Phe139, which is engaged in a hydrophobic interaction with a number of residues of Gα Ras-like domain. Mutations of any of these residues can be expected to alter GPCR-G protein coupling and thus transduction ability. B. Mutations, altering maximum response along with normal cell surface receptor expression, are shown on the active structure of β2AR. Depending on the localization of the mutation, we propose two different mechanisms of its action. Residues located within the center or far from the cytoplasmic site of the receptor (dark red) are expected to alter overall receptor conformational equilibrium; residues located at or within close proximity to the G protein-binding interface (light red) are expected to directly alter the GPCR-G protein interaction. For a detailed view of those residues (C) the perspective was changed slightly for better visualization. The numbers of trans-membrane helices I-VII are indicated in gray circles.
1. By affecting the residues in and around the G protein-binding interface, a mutation can directly influence the affinity of the receptor for the G protein. Indeed, mutations of five residues within close proximity of the C-terminal Gαs helix have been reported (Fig. 3C). Of those five residues, positions 3.50, 3.53 and 6.36 belong to the common G protein-binding interface. Arg3.50 as the key residue of G protein activation is directly interacting with the C-terminal Gαs helix. Mutation to His in the V2R was found to decrease activation of adenylyl cyclase due to impaired G protein coupling.73 Interestingly, this residue was also found to be substituted by Cys and Leu in the V2R, but a comprehensive analysis of these mutants is not available so far. Both residues Thr3.53 and Arg6.36 point towards the cavity; any change in size or charge at these positions might lead to clashes with Tyr391 and Leu393 of the C terminal helix of Gαs, respectively. Position 7.55 was not reported to be directly involved in G protein activation. Characterization of this LHCGR mutant revealed a decrease in maximum response that could not be explained by the decrease in cell surface expression.74 We therefore hypothesize that its orientation towards the cavity might lead to a physical clash between the newly introduced residue and the C-terminal helix of Gαs. Again we want the reader to keep in mind that the orientation of this residue within the LHCGR could be completely different and therefore cause its defect via a different mechanism.
2. To interact with a G protein, the receptor has to be able to adopt an active conformation, characterized by the cytoplasmic opening of the G protein-binding cavity. A mutation can alter receptor structure and thereby interfere with the opening of the cavity, while leaving binding affinity for the ligand unchanged. We suspect that most of the mutations, located in the central cluster or in the cluster on the interface of helices I and II work via this mechanism (Fig. 3C). A very clear example is Pro7.50, which is responsible for the kink towards the cytoplasmic side of helix VII. In the V2R this residue is mutated to a Ser,75 altering the structure of helix VII. Although helix VII has not been considered essential for G protein activation, this structural change could have an effect on the formation of the G protein-binding cavity and thereby decrease G protein activation. Interestingly, A6.59, located close to the binding pocket, was found mutated to Pro in the LHCGR, leading to a decrease in cAMP response, likely due to a defect in coupling. At the same time the mutant demonstrates wild type binding affinity.76 The newly introduced Pro is expected to induce structural changes, which despite its general localization close to the binding pocket more likely influences the general organization of the trans-membrane domain, than ligand binding.
It appears that the majority of GPCR mutations exert their effects by causing general structural rearrangements in receptors, rather than by affecting key residues responsible for ligand or G protein interactions. In addition to the residues discussed above, mutations of a number of residues within the second and third intracellular loop were found to decrease transduction ability, in agreement with the structural and biochemical experimental results.
Mutational alterations of GPCR-ligand interactions
The ligand binding pocket
In a physiological context ligand recognition by a cognate receptor is a crucial event, both in terms of specificity and affinity. Both parameters are determined by the interface between ligand and receptor, with a specific structure and composition, and can therefore be influenced by mutations.
Although different class A GPCRs bind ligands with various structures, there appears to be a consensus ligand-binding pocket, consisting of residues essential to general receptor-ligand contact. Interestingly, this ligand-binding pocket is buried deeply within the trans-membrane domain (Fig. 4B), leaving the extracellular loops with the important role of modulating accessibility. The consensus ligand binding pocket, as defined by comparison of different crystal structures, is lined by residues in helices III, VI and VII.20 It can be expected that additional residues, specific for different GPCRs, further shape the ligand-binding pocket (an example is shown in Fig. 4A for the β2AR). Collectively, common and specific residues determine the size, shape and electrostatic properties of the ligand-binding pocket and thereby create specificity.
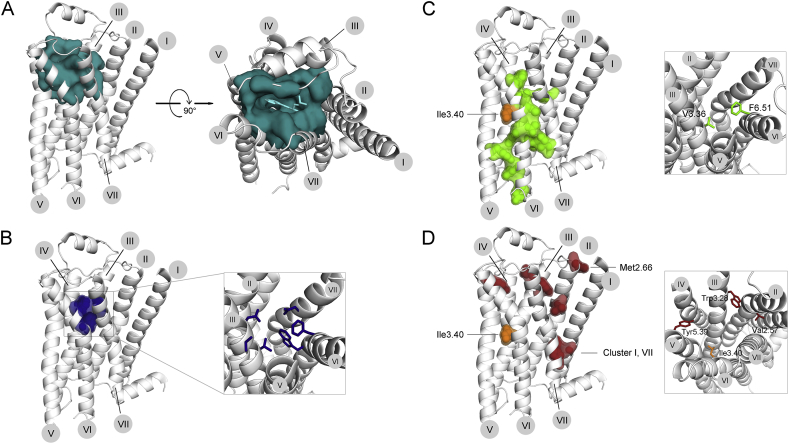
Figure 4.
The effect of GPCR mutations on agonist binding affinity. Most class A GPCRs have a cavity on the extracellular side, which in most cases functions as a ligand-binding pocket. A. To visualize this cavity, we highlighted all residues within a 5A distance from the agonist (left panel, side view; right panel, top view from the extracellular side). B. A common ligand-binding pocket (as defined by Venkatakrishnan et al (2013)20), consisting of residues involved in ligand binding in several GPCR subtypes, is located at the bottom of this cavity. Mutations of any of the residues lining this pocket can be expected to change ligand binding affinity. C, D. Mutations associated with increased or decreased agonist affinity are shown on the inactive structure of β2AR. C. Mutations increasing agonist affinity of any receptor are mostly located on the interfaces of helices III, V, VI, VII. Two residues belong to the common ligand-binding pocket (right panel, detailed view from the extracellular side). D. Mutations decreasing agonist affinity of any receptor are mostly located towards the extracellular side (right panel, detailed view from the extracellular side) or in a cluster on the interface of helices I and VII near the cytoplasmic site. The only position (Ile3.43) where mutations were reported to increase or decrease agonist affinity, depending on the substituting amino acid, is shown in orange on the active structure of β2AR. The numbers of trans-membrane helices I-VII are indicated in gray circles.
The GPHRs represent a special case in terms of ligand binding. Their large extracellular domain (ECD) is responsible for specific ligand binding with high affinity. In all three GPHRs the ECD consists of a horseshoe-shaped leucine-rich repeat (LRR) domain and a Cys rich hinge domain, connecting the ECD to the trans-membrane domain. The crystal structure of the FSHR ECD in complex with FSH not only provided evidence for the mode of hormone binding77 but also suggested the involvement of this domain in receptor activation.78
Although both regions appear to contact the hormone directly, the LRR domain constitutes the primary, high-affinity binding site. Mutagenesis experiments suggest a number of non-conserved residues within the inner concave surface of the LRR domain to be important for recognition specificity.10 This initial binding leads to conformational changes in the hormone, creating new binding sites for interactions with the hinge region. Among those newly generated binding sites is a pocket for a sulfated Tyr (sTyr), located within the hinge region.78 In case of GPHRs, this sTyr has been shown to be essential for hormone binding and receptor activation.79 The hinge region has a distinct structure, stabilized by a number of disulfide bonds. Part of this structure is a hairpin loop, which is normally positioned near the extracellular loops, and functions as a tethered inverse agonist, inhibiting receptor basal activity.80 By drawing the sulfated Tyr into its binding pocket, the hormone is believed to lift the hairpin loop up, thereby releasing this inhibitory effect.81 This lifting motion is converted into a structural change within helix I, which is expected to lead to similar overall changes within the trans-membrane region as seen for other GPCRs. This proposed mechanism also implies that the hormone itself never contacts a ligand-binding pocket within the trans-membrane domain, in contrast to what is generally believed for other class A GPCRs. Little is known about how the hinge region exerts its inhibitory effect on the receptor. One possible explanation is that it contacts residues within the ligand-binding pocket, thereby stabilizing the receptor in inactive state. Contacts with extracellular loops rather suggest an allosteric mode of inhibition.
Potential mechanisms of mutation-induced changes in ligand affinity
We propose two mechanisms by which a mutation can affect the affinity for the ligand: 1. Mutations in or in close proximity to the ligand-binding pocket can either directly affect ligand–receptor interactions or alter the structure of the ligand-binding pocket to change the binding affinity. Mutations exerting their effect in this way are expected in different areas for the GPHR and for the prototypical class A GPCRs (e.g., MC4R and V2R). For the classical GPCRs we would expect those mutations within the common ligand-binding pocket, for GPHRs we would expect mutations within the extracellular loops and the ECD itself. 2. A second, and more general way to influence ligand-binding affinity is to alter the conformational equilibrium of the receptor. It was shown that the active receptor binds agonists with a much higher affinity than the inactive receptor.82 By rendering the receptor more active, or in other words by increasing basal activity, a mutation can at the same time increase agonist affinity.
Mutations altering the affinity for ligands are located in two separate areas and exert their effects via two different mechanisms
Mutations that were reported to increase or decrease the KD value of the receptor for the corresponding agonist and were mapped the inactive structure of β2AR. GOF and LOF mutations overlap only in one residue (3.40). With this exception, the phenotypes appear to be restricted to separate areas: GOF mutations, reported for TSHR, LHCGR and MC4R, are mostly located towards the central and cytoplasmic side, especially along helix VI. Some additional mutations are located in helices II, III, V and VII, all pointing inward (Fig. 4C). Due to their central location, most of these mutations can be expected to exert their effect on ligand binding affinity by influencing general receptor flexibility. Indeed, all but one (I7.52T) mutation associated with increased agonist affinity also show an elevated basal activity. Interestingly, two of the reported mutations belong to the common ligand-binding pocket. The first mutation S3.36R was discovered in the TSHR. Since the TSH does not contact the common ligand binding pocket of the TSHR, the mutation S3.36R is expected to exert its effect via general structural changes increasing receptor flexibility as most of the other mutations. The second mutation, F6.51S, was reported in the MC4R. The changes, both in size and polarity, introduced by this mutation appear to be significant enough to influence the interaction between MC4R and the cognate agonist MSH. Overall, direct action of the mutation via changes in ligand-binding pocket is rare. It appears that most mutations increasing binding affinity exert their effect in the most effective and general way, by changing conformational equilibrium. From an evolutionary perspective, this finding is comprehensible: natural selection probably perfected the binding pocket, leaving little room to further enhance binding affinity by manipulating those residues directly.
LOF mutations (Fig. 4D) are mostly localized near the extracellular side of the receptor. Three additional residues are located close to the cytoplasmic opening on the interface of helices VII and I (7.50, 1.49, 1.50). Depending on the location within the receptors LOF mutations appear to exert their effect via one of the mechanisms proposed above. 1. Mutations at positions 2.57, 3.28, 3.40 and 5.39 are in the large cavity, where most ligands bind. All of those mutations introduce major changes in polarity, size and charge, thereby altering the properties of the ligand-binding pocket, likely directly decreasing the ligand affinity. 2. Mutations, which are not directly associated with the common ligand-binding pocket, appear to exert their effect via structural modifications. Mutations within the cluster at the interface of helices I and VII (1.49, 1.50 and 7.50) possibly decrease the ability of the receptor to adopt an active conformation, thereby decreasing agonist affinity. A mutation introducing a proline at position 2.66 at the junction of helix II and the extracellular loop 1 can also be expected to introduce structural changes. Its position close to the extracellular loops suggest that it rather alters the structure of the extracellular regions, thereby altering ligand recognition and/or ligand access and ultimately decreasing ligand binding affinity.
Mutations in only one position (Fig. 4C and D, labeled in orange) were reported to both reduce and increase binding affinity, depending on the receptor and the nature of the replacing amino acid. Residue 3.40 appears to be located at a key position within the receptor, having the access to the ligand-binding pocket and at the same time controlling GPCR structural changes. To judge the exact effect of each of these mutations, crystal structures are required.
In addition to affecting conformational equilibrium, a mutation can also change the specificity of the receptor-ligand interaction. This phenomenon has been studied for the FSHR, where hypersensitivity towards hCG leads to spontaneous ovarian hyper-stimulation syndrome (sOHSS). The mechanism of broadening receptor specificity varies depending on the location of the mutation within the receptor structure. FSHR receptors with mutations within the transmembrane region in most cases show a dose-dependent response towards both hCG and TSH and at the same time display increased basal activity. Vassart et al10 suggested that these mutations likely lower the intra-molecular energy barrier to activation, rather than affect binding affinity as such. In other words, the mutation alters receptor conformation, thereby increasing the efficacy of hCG and TSH without altering binding affinity. Even low-affinity ligands can now initiate a significant response. The only mutation found within the ECD of the FSHR was responsive towards hCG but not TSH. Both the location and the very specific defect of the mutation suggest, that it directly affects the binding affinity of the receptor towards hCG, turning a low-affinity ligand into a high-affinity ligand. Interestingly, a mutation with a similar phenotype was found in TSHR ECD.83
Alterations in GPCR cell surface expression as a major mutation-induced defect
GPCR biogenesis and maturation
In the cell the level of protein is determined by the rates of biosynthesis and degradation. In the case of GPCRs this implies the balance between the process of trafficking of the receptor to the cell surface and the processes of internalization and degradation. Both factors have to be taken into account when interpreting alterations in cell surface expression of disease-causing mutants. In fact, decreased expression is one of the most common defects, accounting for almost 70% of V2R mutants.25, 27 Importantly, due to spare receptors the level of cell surface expression has to be decreased drastically in order to achieve a significant change in signaling. Interestingly, only few mutations have been discovered that result in an increased number of receptors. Since those mutants also have other defects, such as increased basal activity, the extent to which increased cell surface expression contributes to the phenotype is unclear. We will therefore focus on mutants decreasing cell surface expression. Of the two factors, faulty trafficking to the cell surface has been studied more intensively than the effect mutations have on the internalization rate. Our main focus will therefore be turned towards the GPCR targeting to the cell surface.
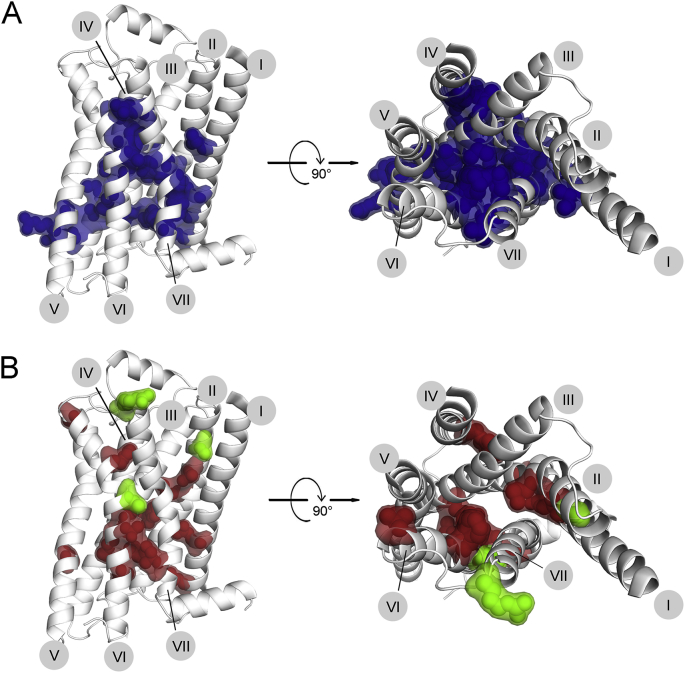
GPCRs, like other transmembrane proteins, are synthesized and folded in the endoplasmatic reticulum (ER), from where they are exported via the ER-Golgi intermediate complex (ERGIC), the Golgi apparatus and the trans-Golgi network (TGN) to the plasma membrane. The process of protein transport is highly regulated. Much effort has been invested into investigation of the sequence determinants controlling the fate of a receptor. Several motifs, mostly consisting of a certain arrangements of hydrophobic residues, have been identified both in the C- and the N-termini. While the role of the C-terminal tail as a major regulatory region for ER export is widely accepted, the role of the N-terminus in the trafficking process is less clear; several motifs in this element have been associated with GPCR export from the Golgi apparatus.84 In addition, the ER provides an extensive quality control system, sorting out misfolded proteins and targeting them for proteasomal degradation. Mutations are thought to interfere with this biogenesis pathway at two steps. 1. Direct deletion or mutation of ER/Golgi export signals have been shown to decrease or abolish cell surface expression.84 It can be expected that mutations within the C-terminal tail target those motifs, thereby leading to the retention of the GPCR in the ER. To our knowledge, there have been no trafficking motifs identified within the trans-membrane portion of the receptor. We therefore exclude this mode of action as a possible mechanism exerted by mutations within the trans-membrane domain. 2. The ER quality control system disposes of any proteins incompletely folded or misfolded. Mutations can therefore exert their effect by destabilizing the receptor and thereby interfering with correct folding. We would expect these mutations to be located on the helical interfaces, involved in interactions stabilizing the overall GPCR structure. Venkatakrishnan et al defined a consensus scaffold, consisting of 24 residues which form an extensive network of non-covalent interactions (Fig. 5A).20 Remaining unaffected by receptor activation, this consensus network probably preserves the structural integrity of a GPCR. Although there appears to be a certain tolerance for variability within this network, mutations can be expected to lead to receptor instability and thus retention in the ER.
Figure 5.
Changes in cell surface expression induced by mutations. A. The structural integrity of the GPCR fold is believed to be maintained by a network of non-covalent inter-helical contacts (described in20), visualized here on the β2AR structure. Disruption of this network can be expected to result in increased receptor instability. B. Mutations, increasing (green) or decreasing (red) receptor cell surface expression in at least two different receptors, are shown on the inactive structure of β2AR. A, B. Left panel, side view; right panel, top view from the extra-cellular side; for the latter the ECL2 helix was removed for better visualization. The numbers of trans-membrane helices I–VII are indicated in gray circles.
Cell surface expression is generally decreased by mutational destabilization and ER retention of the receptor
Mutations decreasing cell surface expression are numerous and appear to affect most parts of the transmembrane domain. If we focus on mutations observed in at least two receptors, this decreases the number of mutations and largely limits them to a cluster between helices VI, VII and III, and to the interface of helices I and II. Two additional residues are located within helices IV and V. (Fig. 5B) The location on the helical interfaces suggests that these mutations would cause helical rearrangements, possibly leading to receptor instability. Interestingly, 50% of those residues are also considered part of the consensus scaffold, further supporting this hypothesis.
Many signaling defects are accompanied by a reduction in cell surface expression. For mutants displaying an increased basal activity this reduction appears to be almost a general characteristic. Defects in signaling and binding affinity are accompanied by a decreased cell surface expression in 45% and over 80% of the cases, respectively. All of those defects are in part associated with increased receptor instability, making the receptor flexible enough to show ligand-independent activity. At the same time this flexibility increases the chance of misfolding, leading to retention in the ER. We want to emphasize that a reduction in cell surface expression affects the interpretation of other defects in terms of comparison between mutants. Normalization to cell surface expression is a prerequisite for reliable comparison of the mutants.
Therapeutic strategies for correcting genetic errors in GPCRs
Genetic disorders represent medical problem that is arguably the hardest to address. Here we will discuss several approaches used to counteract signaling imbalances caused by the molecular errors in GPCRs. As a rule, mutations in receptors create complex multi-faceted problems, whereas existing therapies address only some aspects in each case. There are quite a few human disorders associated with mutations in different GPCRs (discussed in the previous section; see also85).
Pharmacological chaperones to increase receptor cell surface expression
Many mutants reported to show decreased cell surface expression are indeed functional.86 Therapeutically speaking, this means that a simple increase in cell surface expression could re-establish a close to physiological state and relieve disease symptoms. Several approaches have been used to increase cell surface expression, many of them are especially used in the attempt of deorphanizing GPCR receptors, where sufficient receptor expression in heterologous cells poses the main challenge.87 One of these approaches, the applications of so-called pharmacological chaperones, has shown promise as a therapeutic strategy in diseases associated with protein misfolding (reviewed in86,88, 89, 90, 91, 92). The concept of pharmacological chaperones (also known as pharmacochaperones or pharmacopherones) has been studied in vitro, in vivo and clinically for several different diseases, not limited to GPCRs. Pharmacological chaperones function by directly assisting in protein biogenesis and/or by correcting misfolding of a specific protein, having the advantage of avoiding disruption of general proteostasis. To exert their effect, they have to cross the membrane; the potential chaperone therefore has to be not only target-specific, but also small in size and hydrophobic enough to freely diffuse into the cell.
Mechanistically, several modes of action have been proposed90: pharmacological chaperones can stabilize the native conformation, compensating for the destabilizing effect of mutations. Protein stability is generally increased by intra-molecular interactions, such as hydrogen bonds, disulfide bonds, and electrostatic interactions. Mutational disruption of any of these interactions can be rescued by pharmacological chaperones mimicking them. The gonadotropin-releasing hormone receptor (GnRHR) mutant Asp90Lys can be rescued by the application of a pharmacological chaperone, which recreates the salt bridge between Asp98 ad Lys121 to compensate for the change in charge and resulting disruption of a salt bridge.93 This mode of action applies only to a very limited number of receptors. A more general approach is the application of receptor ligands, both agonists and antagonists, since ligand binding limits receptor conformational freedom and stabilizes a native conformation. The application of receptor ligands requires the careful consideration of pharmacological parameters. The chaperone should increase folding efficiency significantly at non-toxic concentrations (low EC50) and be easily replaceable by physiological concentrations of endogenous receptor agonist (low IC50) to achieve an increase in functionality in addition to the increase in cell surface expression. In the case of NBP, a compound specifically rescuing MC4R, its high binding affinity towards misfolded MC4R increases the receptor recovery rate, but inhibits receptor functionality because the endogenous agonist has to compete with NBP.94 Pharmacological chaperones have also been used to affect protein oligomerization either by facilitating the formation or by stabilizing already formed oligomers.
This approach has been applied to several GPCRs, among them two of the receptors we described in this review, the MC4R and the V2R. Mutant MC4R as the cause of monogenic obesity is the perfect target for pharmacological chaperones. Several compounds have been identified in vitro that affect both cell surface expression and receptor functionality to different extent. Most of these demonstrated a limited rescue potential and/or a narrow rescue profile, not qualifying as a general therapeutic.26, 94, 95 Recently, new MC4R antagonists, Ipsen 5i and Ipsen 17, were identified, which rescue a broader spectrum of MC4R mutants with a high efficiency at concentrations as low as 10−9 and 10−8 M, respectively.96,97 Functionality was restored in most of the studied cases. As expected, these chaperones were unable to functionally rescue mutants with additional defects, such as impaired ligand binding ability. The action of pharmacological chaperones is limited to the cell surface expression. Several MC4R mutants (e.g., P299H) appear to be resistant to the stabilizing effect of all pharmacological chaperones studied to date. The authors suggested that the conformational change introduced by this mutation was too strong to be complemented.94 So, despite the general success of the pharmacological chaperones, their rescuing ability does not apply to every MC4R mutant, killing the idea of a single compound acting as a universal pharmacological chaperone. Thus, these compounds can only be used in personalized therapeutics. In vivo experiments are required to demonstrate specificity and pharmacological potential of these compounds in the treatment of obesity caused by MC4R mutations.
In contrast to the MC4R chaperones, V2R chaperones have been studied in vitro and used in clinical trials to treat XNDI. Two compounds are especially interesting. The non-peptide V2R specific antagonist SR121463, available as different salts,98, 99 was shown to partially rescue a V2R mutation responsible for XNDI. In vitro studies revealed a dramatic increase in cell surface expression and a significant increase in receptor function, although full functionality could not be restored.100 Comparable data have been obtained in two additional in vitro studies.101,102 The action of the second chaperone, the non-peptide V1aR antagonist SR49059, has been demonstrated both in vitro and in vivo.103 Within several hours after application, SR49095 was able to decrease the urine volume and the water intake of NDI patients, demonstrating successful symptom relief. In vitro data provide further evidence that SR49095 exerts its therapeutic effect by increasing cell surface expression of otherwise retained V2R mutant. However, interference with cytochrome P450 metabolic pathway precluded the clinical application of SR49095.103 Again, several mutants did not respond to the stabilizing effect of this pharmacological chaperone due to severe distortion of receptor structure.
Compensation by re-engineered proteins
LOF mutations are usually recessive, i.e., the product of the “good” allele is sufficient to do the job. In contrast, GOF mutations are dominant and always result in excessive signaling, causing a problem.7 Inverse agonists, which shift the equilibrium towards inactive receptor conformations, can suppress this signaling,104 sometimes even to the point of restoring the balance. Despite its simplicity, this concept is hardly implemented yet in GPCR therapeutics.19 A TSHR specific inverse agonist has been shown in vitro to exert an inhibitory effect on the basal activity of both WT and four constitutively active TSHR mutants. Basal activity was lowered down to 36%–78% of WT.105 In vivo studies and clinical trials to treat hyperthyroidism, Graves' Disease and metastatic thyroid cancer are on their way. Equivalent compounds for LHCGR and FSHR, both associated with classical hyper-phenotypes have not been identified yet. Clinical studies in combination with in vitro experiments using H2R revealed a possible down-side of inverse agonism in therapeutics: upregulation of receptor number appears to compensate for the inhibitory effect, thereby leading to tolerance observed after chronic treatment.106 In vitro studies with β2AR and α1B-adrenoreceptor have provided further evidence that this could be a general problem of the long-term application of inverse agonists.107, 108
G-protein-mediated signaling by most GPCRs is terminated by a conserved two-step mechanism109: active receptors are phosphorylated by G protein-coupled receptor kinases (GRKs),110 whereupon the receptor acquires high affinity for a cognate arrestin.111 The formation of the arrestin-receptor complex precludes further coupling to G-proteins,109 and initiates the second, G-protein-independent wave of signaling.112, 113 This mechanism stops working when mutations eliminate GRK phosphorylation sites, so that resulting receptor is perfectly normal in every way, but its signaling cannot be stopped via GRK phosphorylation and subsequent arrestin binding.114, 115 This type of GOF mutation is also dominant: the presence of even a small amount of inactivation-deficient receptor results in excessive signaling.116 WT arrestins preferentially bind active phosphorylated receptors.117 Extensive mutagenesis studies118, 119, 120, 121, 122, 123 supported by X-ray crystallography of all four vertebrate arrestin subtypes124, 125, 126, 127 revealed the mechanism of function of arrestin sensor that detects receptor-attached phosphates and yielded a number of “enhanced” phosphorylation-independent mutants that bind with high affinity even unphosphorylated active receptors and quench their signaling via G proteins.122, 123, 128 The ability of enhanced mutant of visual arrestin-1 to compensate for defects of rhodopsin phosphorylation in rod photoreceptors in vivo in genetically modified mice was recently tested.129 The good news was that this compensational approach to gene therapy of GOF mutations works: the expression of enhanced arrestin-1 in photoreceptors where rhodopsin was not phosphorylated due to the absence of rhodopsin kinase improved retinal morphology, prolonged photoreceptor survival, and improved their functional performance.129 However, the rate of signaling shutoff in “compensated” rods was slower than in WT animals, suggesting that more potent phosphorylation-independent versions of arrestin-1 are needed.129 New arrestin-1 mutants specifically designed to target unphosphorylated rhodopsin were constructed,130 and their compensational ability needs to be tested in vivo.
Visual signaling is characterized by much faster kinetics and is generally significantly more demanding than any other GPCR-driven system. So, if enhanced mutants partially compensate for defects of rhodopsin phosphorylation, they are likely to work much better in suppressing excessive signaling by non-visual GPCRs. Due to conservation of the overall arrestin fold,124, 125, 126, 127 mutations homologous to those that make visual arrestin-1 phosphorylation-independent have the same effect on non-visual arrestins.122, 123, 128, 131 Thus, enhanced versions of non-visual arrestins can be expected to be effective suppressors of excessive signaling by GPCRs with GOF mutations. However, there is a problem: both arrestin-2 and -3 are fairly promiscuous, interacting with many GPCRs.132, 133 As most cells express numerous GPCR subtypes, the introduction of an enhanced non-visual arrestin would suppress signaling not only by GOF mutant that needs to be targeted, but also by all the other GPCRs in the same cell, likely producing unwanted side effects. Arrestin-1 is naturally selective for rhodopsin,134 demonstrating the feasibility of constructing receptor-specific arrestins. Subsequent studies established that relatively few exposed residues on the extensive receptor-binding surface determine receptor specificity of arrestins.135 Indeed, substitutions of these “receptor-discriminator” residues in bovine arrestin-3 with those found in homologous positions in arrestins from various species112 yielded mutants of this most promiscuous non-visual subtype with greatly increased receptor specificity, with up to 60-fold preference for some receptors over others.136, 137 These results suggest that non-visual arrestins selectively targeting individual GPCRs with GOF can be constructed and used for compensational therapy.138
Genome editing
Recently developed methods of targeting selected genomic sequences in vivo made it possible to correct the errors in genes, thereby eliminating the very cause of disorders associated with receptor mutations. Three types of tools can be used to this end: Zinc finger nucleases (ZFNs), TALENs, and CRISPR/Cas system (recently reviewed in detail in139,140). The use of each of these tools, just like the use of compensational approach described above, requires the equivalent of gene therapy: the delivery of DNA encoding appropriate tools to particular cells in the body. Recent success of three clinical trials of Leber's congenital amaurosis, a blinding disorder that is caused by LOF mutations in retinal pigment epithelium-specific 65 kDa protein (RPE65), which lead to a deficit of the 11-cis-retinal necessary for photopigment regeneration, demonstrated the feasibility of this approach.141, 142, 143, 144 Obviously, LOF, not GOF mutation was corrected in this case, and gene delivery to a fraction of RPE cells was sufficient to restore the supply of 11-cis-retinal to photoreceptors. If correction in all or even the majority of cells expressing “offending” mutant is necessary, it cannot be achieved using current viral or non-viral delivery vehicles. Luckily, it appears that this is not going to be required in most cases. One recent study used lentiviral delivery of GRK6 gene to strengthen homologous desensitization machinery in the striatum to suppress dyskinesia, a devastating side effect of L-DOPA therapy, which is the most effective in Parkinson's disease.145 It turned out that in both rats and monkeys increased expression of GRK6 in a fraction of striatal neurons had a clear beneficial effect.145 Although a few existing successes may not tell the whole story, it appears likely that in most disorders correction of the signaling in a fraction of affected cells might be sufficient for therapeutic outcome.
Every method of in vivo manipulation of genome and protein expression has its drawbacks. Replacement of LOF mutant with normal allele via gene delivery can only be successful when the expression in a fraction of affected cells is sufficient, as there are no methods that guarantee gene delivery to the majority of targeted cells. The same is true for compensational approach: the signaling can be rebalanced only in cells that received cDNAs encoding re-engineered proteins, so many cells will remain uncorrected. Another danger of re-engineered signaling proteins is that in many cases more than one function might be affected, even though the mutant was designed to change just the desired one. We do not know enough about most proteins to be sure that only the targeted function is changed. A good example is arrestin-3 where the residues responsible for GPCR binding were replaced with alanines. As expected, it lost the ability to bind GPCRs.135 Unexpectedly it was found that, although this mutant binds all kinases in the ASK1-MKK4-JNK3 pathway, like parental arrestin-3 or even better, in contrast to WT protein it does not facilitate JNK3 activation.146 Thus, even though JNK3 and upstream kinases interact with the other side of the arrestin-3 molecule than the receptor,147 mutations on the receptor-binding side affect their binding. This example illustrates the point that creating a mutant protein where only one function is changed is not an easy task.
Targeted regulation of gene expression
Importantly, the tools that enable gene targeting and repair can also be used to selectively increase or suppress the expression of a normal protein. CRISPR can enhance the transcription of endogenous genes when inactive mutant Cas9 and guide RNA for targeting transcription activation elements to specific promoters is used.148, 149, 150, 151 Similarly, catalytically inactive Cas9 targeted to promoters can repress transcription.148, 149, 150, 151
Conclusions and future prospects
Safety is an important consideration for any therapeutic approach. Gene delivery is necessary for gene therapy to correct LOF (replacement) and GOF (compensational) mutations, as well as to repair mutations using ZFNs, TALENs, or CRISPR-Cas. Thus, any side effects associated with viral or non-viral methods of targeted gene delivery are the same in all these cases. From the prospective of safety, modified catalytically inactive Cas targeted to particular sites in the genome to increase or suppress transcription, as well as replacement gene therapy, are probably the least likely to cause unwanted side effects: the proteins expressed as the result of gene activation are WT, “approved” by evolution, and therefore harmless in most cells. The expression of modified proteins, e.g., necessary for compensational gene therapy, is less safe: in many cases harm can be done by simultaneous changes in functions that were not expected to be modified. The least safe is probably genome editing involving the expression of active nucleases of any kind in vivo: in many cases these constructs were found to be cytotoxic, most likely because they hit unintended places in the genome, despite careful targeting. In this sense CRISPR-Cas, that targets only one 23-base sequence, is more prone to off-target activity than ZFNs and TALENs, which use two sequences of similar size located at a partivcuilar distance from each other.139
Therapeutic use of any approach in all cases would require careful estimate of potential dangers and benefits.140 It is highly unlikely that “one size fits all” approach to correcting genetic errors in GPCRs or any other protein class will be ever developed. The better we understand the mechanisms underlying disease phenotypes, the more informed decisions we can make regarding advisability of each therapeutic strategy.
Conflicts of interest
All authors have none to declare.
Acknowledgments
This work was supported by NIH grants GM077561, EY011500, and GM109955 (VVG) and DAAD RISE worldwide summer scholarship and Eberhard Karls Universität Tübingen (Germany) Master's program (HS).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.gendis.2015.02.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Mackin K.A., Roy R.A., Theobald D.L. An empirical test of convergent evolution in rhodopsins. Mol Biol Evol. 2014;31:85–95. doi: 10.1093/molbev/mst171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fredriksson R., Lagerstrom M.C., Lundin L.G., Schioth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 3.Bockaert J., Pin J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manglik A., Kobilka B. The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin. Curr Opin Cell Biol. 2014;27:136–143. doi: 10.1016/j.ceb.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugh E.N., Jr., Lamb T.D. Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga D.G., DeGrip W.J., Pugh E.N. Jr., editors. Handbook of Biological Physics. Molecular Mechanisms in Visual Transduction. Elsevier; Amsterdam: 2000. pp. 183–255. [Google Scholar]
- 6.Dixon R.A., Kobilka B.K., Strader D.J. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- 7.Seifert R., Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg's Archives Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 8.Tao Y.X. Constitutive activity in melanocortin-4 receptor: biased signaling of inverse agonists. Adv Pharmacol. 2014;70:135–154. doi: 10.1016/B978-0-12-417197-8.00005-5. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S., Lubrano-Berthelier C., Govaerts C. Constitutive activity of the melanocortin-4 receptor is maintained by its N-terminal domain and plays a role in energy homeostasis in humans. J Clin Invest. 2004;114:1158–1164. doi: 10.1172/JCI21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassart G., Pardo L., Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Parnot C., Miserey-Lenkei S., Bardin S., Corvol P., Clauser E. Lessons from constitutively active mutants of G protein-coupled receptors. Trends Endocrinol Metab. 2002;13:336–343. doi: 10.1016/s1043-2760(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 12.Lebon G., Warne T., Tate C.G. Agonist-bound structures of G protein-coupled receptors. Curr Opin Struct Biol. 2012;22:482–490. doi: 10.1016/j.sbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Parma J., Duprez L., Van Sande J. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- 14.Laue L., Chan W.Y., Hsueh A.J. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci U S A. 1995;92:1906–1910. doi: 10.1073/pnas.92.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gromoll J., Simoni M., Nordhoff V., Behre H.M., De Geyter C., Nieschlag E. Functional and clinical consequences of mutations in the FSH receptor. Mol Cell Endocrinol. 1996;125:177–182. doi: 10.1016/s0303-7207(96)03949-4. [DOI] [PubMed] [Google Scholar]
- 16.Tao Y.X., Segaloff D.L. Functional analyses of melanocortin-4 receptor mutations identified from patients with binge eating disorder and nonobese or obese subjects. J Clin Endocrinol Metabolism. 2005;90:5632–5638. doi: 10.1210/jc.2005-0519. [DOI] [PubMed] [Google Scholar]
- 17.Govaerts C., Lefort A., Costagliola S. A conserved Asn in transmembrane helix 7 is an on/off switch in the activation of the thyrotropin receptor. J Biological Chem. 2001;276:22991–22999. doi: 10.1074/jbc.M102244200. [DOI] [PubMed] [Google Scholar]
- 18.Parma J., Duprez L., Van Sande J. Diversity and prevalence of somatic mutations in the thyrotropin receptor and Gs alpha genes as a cause of toxic thyroid adenomas. J Clin Endocrinol Metabolism. 1997;82:2695–2701. doi: 10.1210/jcem.82.8.4144. [DOI] [PubMed] [Google Scholar]
- 19.Tao Y.X. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol Ther. 2008;120:129–148. doi: 10.1016/j.pharmthera.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatakrishnan A.J., Deupi X., Lebon G., Tate C.G., Schertler G.F., Babu M.M. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 21.Smits G., Campillo M., Govaerts C. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22:2692–2703. doi: 10.1093/emboj/cdg260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen S.G., Choi H.J., Fung J.J. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen S.G., DeVree B.T., Zou Y. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Y., Zhou X.E., Gao X. Crystal structure of rhodopsin bound to arrestin determined by femtosecond X-ray laser. Nature. 2014 doi: 10.1038/nature14656. [revised and resubmitted] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao Y.X. Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther. 2006;111:949–973. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Ward N.A., Hirst S., Williams J., Findlay J.B. Pharmacological chaperones increase the cell-surface expression of intracellularly retained mutants of the melanocortin 4 receptor with unique rescuing efficacy profiles. Biochem Soc Trans. 2012;40:717–720. doi: 10.1042/BST20110764. [DOI] [PubMed] [Google Scholar]
- 27.Bichet D.G. V2R mutations and nephrogenic diabetes insipidus. Prog Mol Biol Transl Sci. 2009;89:15–29. doi: 10.1016/S1877-1173(09)89002-9. [DOI] [PubMed] [Google Scholar]
- 28.Ulloa-Aguirre A., Dias J.A., Bousfield G., Huhtaniemi I., Reiter E. Trafficking of the follitropin receptor. Meth Enzymol. 2013;521:17–45. doi: 10.1016/B978-0-12-391862-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heng B.C., Aubel D., Fussenegger M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol Adv. 2013;31:1676–1694. doi: 10.1016/j.biotechadv.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Corvilain B., Van Sande J., Dumont J.E., Vassart G. Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol. 2001;55:143–158. [PubMed] [Google Scholar]
- 31.Kleinau G., Neumann S., Gruters A., Krude H., Biebermann H. Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev. 2013;34:691–724. doi: 10.1210/er.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hebrant A., van Staveren W.C., Maenhaut C., Dumont J.E., Leclere J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol/Eur Fed Endocr Soc. 2011;164:1–9. doi: 10.1530/EJE-10-0775. [DOI] [PubMed] [Google Scholar]
- 33.Hamburger J.I. The autonomously functioning thyroid nodule: Goetsch's disease. Endocr Rev. 1987;8:439–447. doi: 10.1210/edrv-8-4-439. [DOI] [PubMed] [Google Scholar]
- 34.Sunthornthepvarakui T., Gottschalk M.E., Hayashi Y., Refetoff S. Brief report: resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med. 1995;332:155–160. doi: 10.1056/NEJM199501193320305. [DOI] [PubMed] [Google Scholar]
- 35.Chan W.Y. Molecular genetic, biochemical, and clinical implications of gonadotropin receptor mutations. Mol Genet Metab. 1998;63:75–84. doi: 10.1006/mgme.1997.2650. [DOI] [PubMed] [Google Scholar]
- 36.Liu G., Duranteau L., Carel J.C., Monroe J., Doyle D.A., Shenker A. Leydig-cell tumors caused by an activating mutation of the gene encoding the luteinizing hormone receptor. N Engl J Med. 1999;341:1731–1736. doi: 10.1056/NEJM199912023412304. [DOI] [PubMed] [Google Scholar]
- 37.Stavrou S.S., Zhu Y.S., Cai L.Q. A novel mutation of the human luteinizing hormone receptor in 46XY and 46XX sisters. J Clin Endocrinol Metabolism. 1998;83:2091–2098. doi: 10.1210/jcem.83.6.4855. [DOI] [PubMed] [Google Scholar]
- 38.Dierich A., Sairam M.R., Monaco L. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aittomaki K., Lucena J.L., Pakarinen P. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 40.Tao Y.X., Segaloff D.L. Follicle stimulating hormone receptor mutations and reproductive disorders. Prog Mol Biol Transl Sci. 2009;89:115–131. doi: 10.1016/S1877-1173(09)89005-4. [DOI] [PubMed] [Google Scholar]
- 41.Desai S.S., Roy B.S., Mahale S.D. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction. 2013;146:R235–R248. doi: 10.1530/REP-13-0351. [DOI] [PubMed] [Google Scholar]
- 42.Santini F., Maffei M., Pelosini C., Salvetti G., Scartabelli G., Pinchera A. Melanocortin-4 receptor mutations in obesity. Adv Clin Chem. 2009;48:95–109. [PubMed] [Google Scholar]
- 43.Soos S., Petervari E., Szekely M., Jech-Mihalffy A., Balasko M. Complex catabolic effects of central alpha-MSH infusion in rats of altered nutritional states: differences from leptin. J Mol Neurosci. 2011;43:209–216. doi: 10.1007/s12031-010-9462-6. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y., Elmquist J.K., Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinney A., Hohmann S., Geller F. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J Clin Endocrinol Metabolism. 2003;88:4258–4267. doi: 10.1210/jc.2003-030233. [DOI] [PubMed] [Google Scholar]
- 46.Vaisse C., Clement K., Durand E., Hercberg S., Guy-Grand B., Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinney A., Bettecken T., Tarnow P. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metabolism. 2006;91:1761–1769. doi: 10.1210/jc.2005-2056. [DOI] [PubMed] [Google Scholar]
- 48.Lubrano-Berthelier C., Dubern B., Lacorte J.M. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metabolism. 2006;91:1811–1818. doi: 10.1210/jc.2005-1411. [DOI] [PubMed] [Google Scholar]
- 49.Farooqi I.S., Keogh J.M., Yeo G.S., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 50.Tao Y.X. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juul K.V., Bichet D.G., Nielsen S., Norgaard J.P. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiology Ren Physiology. 2014;306:F931–F940. doi: 10.1152/ajprenal.00604.2013. [DOI] [PubMed] [Google Scholar]
- 52.Feldman B.J., Rosenthal S.M., Vargas G.A. Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med. 2005;352:1884–1890. doi: 10.1056/NEJMoa042743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballesteros J.A., Weinstein H. Integrated methods for the construction of three- dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 54.Liu W., Chun E., Thompson A.A. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katritch V., Cherezov V., Stevens R.C. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogel R., Mahalingam M., Ludeke S., Huber T., Siebert F., Sakmar T.P. Functional role of the “ionic lock”–an interhelical hydrogen-bond network in family A heptahelical receptors. J Mol Biol. 2008;380:648–655. doi: 10.1016/j.jmb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 57.Yuan S., Filipek S., Palczewski K., Vogel H. Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway. Nat Commun. 2014;5:4733. doi: 10.1038/ncomms5733. [DOI] [PubMed] [Google Scholar]
- 58.Dill K.A. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 59.de Roux N., Polak M., Couet J. A neomutation of the thyroid-stimulating hormone receptor in a severe neonatal hyperthyroidism. J Clin Endocrinol Metabolism. 1996;81:2023–2026. doi: 10.1210/jcem.81.6.8964822. [DOI] [PubMed] [Google Scholar]
- 60.Yano K., Kohn L.D., Saji M., Kataoka N., Okuno A., Cutler G.B., Jr. A case of male-limited precocious puberty caused by a point mutation in the second transmembrane domain of the luteinizing hormone choriogonadotropin receptor gene. Biochem Biophys Res Commun. 1996;220:1036–1042. doi: 10.1006/bbrc.1996.0528. [DOI] [PubMed] [Google Scholar]
- 61.Wonerow P., Chey S., Fuhrer D., Holzapfel H.P., Paschke R. Functional characterization of five constitutively activating thyrotrophin receptor mutations. Clin Endocrinol. 2000;53:461–468. doi: 10.1046/j.1365-2265.2000.01119.x. [DOI] [PubMed] [Google Scholar]
- 62.Castro I., Lima L., Seoane R., Lado-Abeal J. Identification and functional characterization of two novel activating thyrotropin receptor mutants in toxic thyroid follicular adenomas. Thyroid. 2009;19:645–649. doi: 10.1089/thy.2009.0002. [DOI] [PubMed] [Google Scholar]
- 63.Kosugi S., Hai N., Okamoto H., Sugawa H., Mori T. A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur J Endocrinol. 2000;143:471–477. doi: 10.1530/eje.0.1430471. [DOI] [PubMed] [Google Scholar]
- 64.Latronico A.C., Abell A.N., Arnhold I.J. A unique constitutively activating mutation in third transmembrane helix of luteinizing hormone receptor causes sporadic male gonadotropin-independent precocious puberty. J Clin Endocrinol Metabolism. 1998;83:2435–2440. doi: 10.1210/jcem.83.7.4968. [DOI] [PubMed] [Google Scholar]
- 65.Sheikh S.P., Vilardarga J.P., Baranski T.J. Similar structures and shared switch mechanisms of the beta2-adrenoceptor and the parathyroid hormone receptor. Zn(II) bridges between helices III and VI block activation. J Biological Chem. 1999;274:17033–17041. doi: 10.1074/jbc.274.24.17033. [DOI] [PubMed] [Google Scholar]
- 66.Varrault A., Le Nguyen D., McClue S., Harris B., Jouin P., Bockaert J. 5-Hydroxytryptamine1A receptor synthetic peptides. Mechanisms of adenylyl cyclase inhibition. J Biological Chem. 1994;269:16720–16725. [PubMed] [Google Scholar]
- 67.Ulloa-Aguirre A., Uribe A., Zarinan T., Bustos-Jaimes I., Perez-Solis M.A., Dias J.A. Role of the intracellular domains of the human FSH receptor in G(alphaS) protein coupling and receptor expression. Mol Cell Endocrinol. 2007;260–262:153–162. doi: 10.1016/j.mce.2005.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moro O., Lameh J., Hogger P., Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biological Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- 69.McClue S.J., Baron B.M., Harris B.A. Activation of Gi protein by peptide structures of the muscarinic M2 receptor second intracellular loop. Eur J Pharmacol. 1994;267:185–193. doi: 10.1016/0922-4106(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 70.Hu J., Wang Y., Zhang X. Structural basis of G protein-coupled receptor-G protein interactions. Nat Chem Biol. 2010;6:541–548. doi: 10.1038/nchembio.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreira I.S. Structural features of the G-protein/GPCR interactions. Biochim Biophys Acta. 2014;1840:16–33. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 72.Scheerer P., Park J.H., Hildebrand P.W. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 73.Rosenthal W., Antaramian A., Gilbert S., Birnbaumer M. Nephrogenic diabetes insipidus. A V2 vasopressin receptor unable to stimulate adenylyl cyclase. J Biological Chem. 1993;268:13030–13033. [PubMed] [Google Scholar]
- 74.Min L., Ascoli M. Effect of activating and inactivating mutations on the phosphorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol Endocrinol. 2000;14:1797–1810. doi: 10.1210/mend.14.11.0555. [DOI] [PubMed] [Google Scholar]
- 75.Ala Y., Morin D., Mouillac B. Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus: molecular basis of a mild clinical phenotype. J Am Soc Nephrol. 1998;9:1861–1872. doi: 10.1681/ASN.V9101861. [DOI] [PubMed] [Google Scholar]
- 76.Kremer H., Kraaij R., Toledo S.P. Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9:160–164. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- 77.Jiang X., Liu H., Chen X. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci U. S. A. 2012;109:12491–12496. doi: 10.1073/pnas.1206643109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang X., Dias J.A., He X. Structural biology of glycoprotein hormones and their receptors: insights to signaling. Mol Cell Endocrinol. 2014;382:424–451. doi: 10.1016/j.mce.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 79.Costagliola S., Panneels V., Bonomi M. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002;21:504–513. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agrawal G., Dighe R.R. Critical involvement of the hinge region of the follicle-stimulating hormone receptor in the activation of the receptor. J Biol Chem. 2009;284:2636–2647. doi: 10.1074/jbc.M808199200. [DOI] [PubMed] [Google Scholar]
- 81.Majumdar R., Dighe R.R. The hinge region of human thyroid-stimulating hormone (TSH) receptor operates as a tunable switch between hormone binding and receptor activation. PloS One. 2012;7:e40291. doi: 10.1371/journal.pone.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samama P., Cotecchia S., Costa T., Lefkowitz R.J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biological Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 83.Rodien P., Bremont C., Sanson M.L. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823–1826. doi: 10.1056/NEJM199812173392505. [DOI] [PubMed] [Google Scholar]
- 84.Dong C., Filipeanu C.M., Duvernay M.T., Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoneberg T., Schulz A., Biebermann H., Hermsdorf T., Rompler H., Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Conn P.M., Ulloa-Aguirre A., Ito J., Janovick J.A. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 87.Dunham J.H., Hall R.A. Enhancement of the surface expression of G protein-coupled receptors. Trends Biotechnol. 2009;27:541–545. doi: 10.1016/j.tibtech.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen F.E., Kelly J.W. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 89.Conn P.M., Ulloa-Aguirre A. Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs. Trends Endocrinol Metab. 2010;21:190–197. doi: 10.1016/j.tem.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leidenheimer N.J., Ryder K.G. Pharmacological chaperoning: a primer on mechanism and pharmacology. Pharm Res. 2014;83:10–19. doi: 10.1016/j.phrs.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maya-Nunez G., Ulloa-Aguirre A., Janovick J.A., Conn P.M. Pharmacological chaperones correct misfolded GPCRs and rescue function: protein trafficking as a therapeutic target. Sub-cellular Biochem. 2012;63:263–289. doi: 10.1007/978-94-007-4765-4_14. [DOI] [PubMed] [Google Scholar]
- 92.Muntau A.C., Leandro J., Staudigl M., Mayer F., Gersting S.W. Innovative strategies to treat protein misfolding in inborn errors of metabolism: pharmacological chaperones and proteostasis regulators. J Inherit Metab Dis. 2014;37:505–523. doi: 10.1007/s10545-014-9701-z. [DOI] [PubMed] [Google Scholar]
- 93.Conn P.M., Janovick J.A. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol. 2009;299:137–145. doi: 10.1016/j.mce.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rene P., Le Gouill C., Pogozheva I.D. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. J Pharmacol Exp Ther. 2010;335:520–532. doi: 10.1124/jpet.110.172098. [DOI] [PubMed] [Google Scholar]
- 95.Fan Z.C., Tao Y.X. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J Cell Mol Med. 2009;13:3268–3282. doi: 10.1111/j.1582-4934.2009.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tao Y.X., Huang H. Ipsen 5i is a novel potent pharmacoperone for intracellularly retained Melanocortin-4 receptor mutants. Front Endocrinol. 2014;5:131. doi: 10.3389/fendo.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X.H., Wang H.M., Zhao B.L., Yu P., Fan Z.C. Rescue of defective MC4R cell-surface expression and signaling by a novel pharmacoperone Ipsen 17. J Mol Endocrinol. 2014;53:17–29. doi: 10.1530/JME-14-0005. [DOI] [PubMed] [Google Scholar]
- 98.Serradeil-Le Gal C., Lacour C., Valette G. Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest. 1996;98:2729–2738. doi: 10.1172/JCI119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Serradeil-Le Gal C. An overview of SR121463, a selective non-peptide vasopressin V(2) receptor antagonist. Cardiovasc Drug Rev. 2001;19:201–214. doi: 10.1111/j.1527-3466.2001.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 100.Ranadive S.A., Ersoy B., Favre H. Identification, characterization and rescue of a novel vasopressin-2 receptor mutation causing nephrogenic diabetes insipidus. Clin Endocrinol. 2009;71:388–393. doi: 10.1111/j.1365-2265.2008.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morello J.P., Salahpour A., Laperriere A. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wuller S., Wiesner B., Loffler A. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biological Chem. 2004;279:47254–47263. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 103.Bernier V., Morello J.P., Zarruk A. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 104.Bond R.A., Ijzerman A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Huang W., Englund E., Titus S. Identification of thyroid stimulating hormone receptor inverse agonists. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US) 2011 Mar 31 [updated 2013 Feb 28] [PubMed] [Google Scholar]
- 106.Smit M.J., Leurs R., Alewijnse A.E. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U. S. A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacEwan D.J., Milligan G. Inverse agonist-induced up-regulation of the human beta2-adrenoceptor in transfected neuroblastoma X glioma hybrid cells. Mol Pharmacol. 1996;50:1479–1486. [PubMed] [Google Scholar]
- 108.Lee T.W., Cotecchia S., Milligan G. Up-regulation of the levels of expression and function of a constitutively active mutant of the hamster alpha1B-adrenoceptor by ligands that act as inverse agonists. Biochem J. 1997;325:733–739. doi: 10.1042/bj3250733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carman C.V., Benovic J.L. G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol. 1998;8:335–344. doi: 10.1016/s0959-4388(98)80058-5. [DOI] [PubMed] [Google Scholar]
- 110.Gurevich E.V., Tesmer J.J., Mushegian A., Gurevich V.V. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gurevich V.V., Gurevich E.V. The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gurevich E.V., Gurevich V.V. Arrestins are ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 114.Kim R.Y., al-Maghtheh M., Fitzke F.W. Dominant retinitis pigmentosa associated with two rhodopsin gene mutations. Leu-40-Arg and an insertion disrupting the 5'-splice junction of exon 5. Arch Ophthalmol. 1993;111:1518–1524. doi: 10.1001/archopht.1993.01090110084030. [DOI] [PubMed] [Google Scholar]
- 115.Restagno G., Maghtheh M., Bhattacharya S. A large deletion at the 3' end of the rhodopsin gene in an Italian family with a diffuse form of autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1993;2:207–208. doi: 10.1093/hmg/2.2.207. [DOI] [PubMed] [Google Scholar]
- 116.Chen J., Makino C.L., Peachey N.S., Baylor D.A., Simon M.I. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 1995;267:374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- 117.Gurevich V.V., Gurevich E.V. The molecular acrobatics of arrestin activation. Trends Pharmacol Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 118.Gurevich V.V. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J Biological Chem. 1998;273:15501–15506. doi: 10.1074/jbc.273.25.15501. [DOI] [PubMed] [Google Scholar]
- 119.Gurevich V.V., Benovic J.L. Visual arrestin binding to rhodopsin: diverse functional roles of positively charged residues within the phosphorylation-recignition region of arrestin. J Biol Chem. 1995;270:6010–6016. doi: 10.1074/jbc.270.11.6010. [DOI] [PubMed] [Google Scholar]
- 120.Gurevich V.V., Benovic J.L. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol. 1997;51:161–169. doi: 10.1124/mol.51.1.161. [DOI] [PubMed] [Google Scholar]
- 121.Vishnivetskiy S.A., Paz C.L., Schubert C., Hirsch J.A., Sigler P.B., Gurevich V.V. How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem. 1999;274:11451–11454. doi: 10.1074/jbc.274.17.11451. [DOI] [PubMed] [Google Scholar]
- 122.Celver J., Vishnivetskiy S.A., Chavkin C., Gurevich V.V. Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J Biological Chem. 2002;277:9043–9048. doi: 10.1074/jbc.M107400200. [DOI] [PubMed] [Google Scholar]
- 123.Kovoor A., Celver J., Abdryashitov R.I., Chavkin C., Gurevich V.V. Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biological Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- 124.Hirsch J.A., Schubert C., Gurevich V.V., Sigler P.B. The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell. 1999;97:257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 125.Han M., Gurevich V.V., Vishnivetskiy S.A., Sigler P.B., Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 126.Sutton R.B., Vishnivetskiy S.A., Robert J. Crystal structure of cone arrestin at 2.3Å: evolution of receptor specificity. J Mol Biol. 2005;354:1069–1080. doi: 10.1016/j.jmb.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 127.Zhan X., Gimenez L.E., Gurevich V.V., Spiller B.W. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual arrestins. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gray-Keller M.P., Detwiler P.B., Benovic J.L., Gurevich V.V. Arrestin with a single amino acid sustitution quenches light-activated rhodopsin in a phosphorylation-independent fashion. Biochemistry. 1997;36:7058–7063. doi: 10.1021/bi963110k. [DOI] [PubMed] [Google Scholar]
- 129.Song X., Vishnivetskiy S.A., Gross O.P. Enhanced arrestin facilitates recovery and protects rod photoreceptors deficient in rhodopsin phosphorylation. Curr Biol. 2009;19:700–705. doi: 10.1016/j.cub.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vishnivetskiy S.A., Chen Q., Palazzo M.C. Engineering visual arrestin-1 with special functional characteristics. J Biological Chem. 2013;288:11741–11750. doi: 10.1074/jbc.M112.445437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pan L., Gurevich E.V., Gurevich V.V. The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biological Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- 132.Barak L.S., Ferguson S.S., Zhang J., Caron M.G. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biological Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 133.Gurevich V.V., Dion S.B., Onorato J.J. Arrestin interaction with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, b2-adrenergic, and m2 muscarinic cholinergic receptors. J Biological Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- 134.Vishnivetskiy S.A., Hosey M.M., Benovic J.L., Gurevich V.V. Mapping the arrestin-receptor interface: structural elements responsible for receptor specificity of arrestin proteins. J Biol Chem. 2004;279:1262–1268. doi: 10.1074/jbc.M308834200. [DOI] [PubMed] [Google Scholar]
- 135.Vishnivetskiy S.A., Gimenez L.E., Francis D.J. Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biological Chem. 2011;286:24288–24299. doi: 10.1074/jbc.M110.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gimenez L.E., Vishnivetskiy S.A., Baameur F., Gurevich V.V. Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J Biological Chem. 2012;287:29495–29505. doi: 10.1074/jbc.M112.366674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gimenez L.E., Babilon S., Wanka L., Beck-Sickinger A.G., Gurevich V.V. Mutations in arrestin-3 differentially affect binding to neuropeptide Y receptor subtypes. Cell Signal. 2014;26:1523–1531. doi: 10.1016/j.cellsig.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gurevich V.V., Gurevich E.V. Synthetic biology with surgical precision: targeted reengineering of signaling proteins. Cell Signal. 2012;24:1899–1908. doi: 10.1016/j.cellsig.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim H., Kim J.S. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 140.Gurevich E.V., Gurevich V.V. Beyond traditional pharmacology: new tools and approaches. Br J Pharmacol. 2015 Jan 9 doi: 10.1111/bph.13066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bainbridge J.W., Smith A.J., Barker S.S. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 142.Cideciyan A.V. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hauswirth W.W., Aleman T.S., Kaushal S. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maguire A.M., Simonelli F., Pierce E.A. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmed M.R., Berthet A., Bychkov E. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000664. 28ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Breitman M., Kook S., Gimenez L.E. Silent scaffolds: inhibition of c-Jun N-terminal kinase 3 activity in the cell by a dominant-negative arrestin-3 mutant. J Biological Chem. 2012;287:19653–19664. doi: 10.1074/jbc.M112.358192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhan X., Perez A., Gimenez L.E., Vishnivetskiy S.A., Gurevich V.V. Arrestin-3 binds the MAP kinase JNK3α2 via multiple sites on both domains. Cell Signal. 2014;26:766–776. doi: 10.1016/j.cellsig.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qi L.S., Larson M.H., Gilbert L.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:343–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maeder M.L., Linder S.J., Cascio V.M., Fu Y., Ho Q.H., Joung J.K. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perez-Pinera P., Ousterout D.G., Brunger J.M. Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat Methods. 2013;10:239–242. doi: 10.1038/nmeth.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Biebermann H., Schoneberg T., Hess C., Germak J., Gudermann T., Gruters A. The first activating TSH receptor mutation in transmembrane domain 1 identified in a family with nonautoimmune hyperthyroidism. J Clin Endocrinol Metab. 2001;86:4429–4433. doi: 10.1210/jcem.86.9.7888. [DOI] [PubMed] [Google Scholar]
- 153.Tan K., Pogozheva I.D., Yeo G.S. Functional characterization and structural modeling of obesity associated mutations in the melanocortin 4 receptor. Endocrinology. 2009;150:114–125. doi: 10.1210/en.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duprez L., Hermans J., Van Sande J., Dumont J.E., Vassart G., Parma J. Two autonomous nodules of a patient with multinodular goiter harbor different activating mutations of the thyrotropin receptor gene. J Clin Endocrinol Metabolism. 1997;82:306–308. doi: 10.1210/jcem.82.1.3691. [DOI] [PubMed] [Google Scholar]
- 155.Evans B.A., Bowen D.J., Smith P.J., Clayton P.E., Gregory J.W. A new point mutation in the luteinising hormone receptor gene in familial and sporadic male limited precocious puberty: genotype does not always correlate with phenotype. J Med Genet. 1996;33:143–147. doi: 10.1136/jmg.33.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kraaij R., Post M., Kremer H. A missense mutation in the second transmembrane segment of the luteinizing hormone receptor causes familial male-limited precocious puberty. J Clin Endocrinol Metabolism. 1995;80:3168–3172. doi: 10.1210/jcem.80.11.7593421. [DOI] [PubMed] [Google Scholar]
- 157.Nishihara E., Fukata S., Hishinuma A. Sporadic congenital hyperthyroidism due to a germline mutation in the thyrotropin receptor gene (Leu 512 Gln) in a Japanese patient. Endocrinol Jpn. 2006;53:735–740. doi: 10.1507/endocrj.k06-090. [DOI] [PubMed] [Google Scholar]
- 158.Alberti L., Proverbio M.C., Costagliola S. A novel germline mutation in the TSH receptor gene causes non-autoimmune autosomal dominant hyperthyroidism. Eur J Endocrinol. 2001;145:249–254. doi: 10.1530/eje.0.1450249. [DOI] [PubMed] [Google Scholar]
- 159.Esapa C.T., Duprez L., Ludgate M. A novel thyrotropin receptor mutation in an infant with severe thyrotoxicosis. Thyroid. 1999;9:1005–1010. doi: 10.1089/thy.1999.9.1005. [DOI] [PubMed] [Google Scholar]
- 160.Van Sande J., Parma J., Tonacchera M., Swillens S., Dumont J., Vassart G. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metabolism. 1995;80:2577–2585. doi: 10.1210/jcem.80.9.7673398. [DOI] [PubMed] [Google Scholar]
- 161.Russo D., Arturi F., Suarez H.G. Thyrotropin receptor gene alterations in thyroid hyperfunctioning adenomas. J Clin Endocrinol Metabolism. 1996;81:1548–1551. doi: 10.1210/jcem.81.4.8636365. [DOI] [PubMed] [Google Scholar]
- 162.Dieterich M., Bolz M., Reimer T., Costagliola S., Gerber B. Two different entities of spontaneous ovarian hyperstimulation in a woman with FSH receptor mutation. Reprod Biomed Online. 2010;20:751–758. doi: 10.1016/j.rbmo.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 163.Smits G., Olatunbosun O., Delbaere A., Pierson R., Vassart G., Costagliola S. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med. 2003;349:760–766. doi: 10.1056/NEJMoa030064. [DOI] [PubMed] [Google Scholar]
- 164.Montanelli L., Van Durme J.J., Smits G. Modulation of ligand selectivity associated with activation of the transmembrane region of the human follitropin receptor. Mol Endocrinol. 2004;18:2061–2073. doi: 10.1210/me.2004-0036. [DOI] [PubMed] [Google Scholar]
- 165.Aycan Z., Agladioglu S.Y., Ceylaner S., Cetinkaya S., Bas V.N., Kendirici H.N. Sporadic nonautoimmune neonatal hyperthyroidism due to A623V germline mutation in the thyrotropin receptor gene. J Clin Res Pediatr Endocrinol. 2010;2:168–172. doi: 10.4274/jcrpe.v2i4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Paschke R., Tonacchera M., Van Sande J., Parma J., Vassart G. Identification and functional characterization of two new somatic mutations causing constitutive activation of the thyrotropin receptor in hyperfunctioning autonomous adenomas of the thyroid. J Clin Endocrinol Metabolism. 1994;79:1785–1789. doi: 10.1210/jcem.79.6.7989485. [DOI] [PubMed] [Google Scholar]
- 167.Latronico A.C., Anasti J., Arnhold I.J. A novel mutation of the luteinizing hormone receptor gene causing male gonadotropin-independent precocious puberty. J Clin Endocrinol Metabolism. 1995;80:2490–2494. doi: 10.1210/jcem.80.8.7629248. [DOI] [PubMed] [Google Scholar]
- 168.Ringkananont U., Van Durme J., Montanelli L. Repulsive separation of the cytoplasmic ends of transmembrane helices 3 and 6 is linked to receptor activation in a novel thyrotropin receptor mutant (M626I) Mol Endocrinol. 2006;20:893–903. doi: 10.1210/me.2005-0339. [DOI] [PubMed] [Google Scholar]
- 169.Kosugi S., Van Dop C., Geffner M.E. Characterization of heterogeneous mutations causing constitutive activation of the luteinizing hormone receptor in familial male precocious puberty. Hum Mol Genet. 1995;4:183–188. doi: 10.1093/hmg/4.2.183. [DOI] [PubMed] [Google Scholar]
- 170.Gozu H.I., Bircan R., Krohn K. Similar prevalence of somatic TSH receptor and Gs alpha mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006;155:535–545. doi: 10.1530/eje.1.02253. [DOI] [PubMed] [Google Scholar]
- 171.Yano K., Saji M., Hidaka A. A new constitutively activating point mutation in the luteinizing hormone/choriogonadotropin receptor gene in cases of male-limited precocious puberty. J Clin Endocrinol Metabolism. 1995;80:1162–1168. doi: 10.1210/jcem.80.4.7714085. [DOI] [PubMed] [Google Scholar]
- 172.Nijenhuis W.A., Garner K.M., van Rozen R.J., Adan R.A. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem. 2003;278:22939–22945. doi: 10.1074/jbc.M211326200. [DOI] [PubMed] [Google Scholar]
- 173.Tao Y.X. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol. 2005;239:1–14. doi: 10.1016/j.mce.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 174.Gozu H., Avsar M., Bircan R. Two novel mutations in the sixth transmembrane segment of the thyrotropin receptor gene causing hyperfunctioning thyroid nodules. Thyroid: Official J Am Thyroid Assoc. 2005;15:389–397. doi: 10.1089/thy.2005.15.389. [DOI] [PubMed] [Google Scholar]
- 175.Tonacchera M., Chiovato L., Pinchera A. Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. J Clin Endocrinol Metab. 1998;83:492–498. doi: 10.1210/jcem.83.2.4559. [DOI] [PubMed] [Google Scholar]
- 176.Laue L., Wu S.M., Kudo M. Heterogeneity of activating mutations of the human luteinizing hormone receptor in male-limited precocious puberty. Biochem Mol Med. 1996;58:192–198. doi: 10.1006/bmme.1996.0048. [DOI] [PubMed] [Google Scholar]
- 177.Kopp P., Muirhead S., Jourdain N., Gu W.X., Jameson J.L., Rodd C. Congenital hyperthyroidism caused by a solitary toxic adenoma harboring a novel somatic mutation (serine281-->isoleucine) in the extracellular domain of the thyrotropin receptor. J Clin Invest. 1997;100:1634–1639. doi: 10.1172/JCI119687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kosugi S., Shenker A., Mori T. Constitutive activation of cyclic AMP but not phosphatidylinositol signaling caused by four mutations in the 6th transmembrane helix of the human thyrotropin receptor. FEBS Lett. 1994;356:291–294. doi: 10.1016/0014-5793(94)01286-5. [DOI] [PubMed] [Google Scholar]
- 179.Porcellini A., Ciullo I., Laviola L., Amabile G., Fenzi G., Avvedimento V.E. Novel mutations of thyrotropin receptor gene in thyroid hyperfunctioning adenomas. Rapid identification by fine needle aspiration biopsy. J Clin Endocrinol Metabolism. 1994;79:657–661. doi: 10.1210/jcem.79.2.8045989. [DOI] [PubMed] [Google Scholar]
- 180.Wu S.M., Leschek E.W., Brain C., Chan W.Y. A novel luteinizing hormone receptor mutation in a patient with familial male-limited precocious puberty: effect of the size of a critical amino acid on receptor activity. Mol Genet Metab. 1999;66:68–73. doi: 10.1006/mgme.1998.2780. [DOI] [PubMed] [Google Scholar]
- 181.Fuhrer D., Tannapfel A., Sabri O., Lamesch P., Paschke R. Two somatic TSH receptor mutations in a patient with toxic metastasising follicular thyroid carcinoma and non-functional lung metastases. Endocrine-related Cancer. 2003;10:591–600. doi: 10.1677/erc.0.0100591. [DOI] [PubMed] [Google Scholar]
- 182.Shenker A., Laue L., Kosugi S., Merendino J.J., Jr., Minegishi T., Cutler G.B., Jr. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature. 1993;365:652–654. doi: 10.1038/365652a0. [DOI] [PubMed] [Google Scholar]
- 183.Yano K., Hidaka A., Saji M. A sporadic case of male-limited precocious puberty has the same constitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J Clin Endocrinol Metabolism. 1994;79:1818–1823. doi: 10.1210/jcem.79.6.7527413. [DOI] [PubMed] [Google Scholar]
- 184.Lubrano-Berthelier C., Durand E., Dubern B. Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet. 2003;12:145–153. doi: 10.1093/hmg/ddg016. [DOI] [PubMed] [Google Scholar]
- 185.MacKenzie R.G. Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides. 2006;27:395–403. doi: 10.1016/j.peptides.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 186.Larsen L.H., Echwald S.M., Sorensen T.I., Andersen T., Wulff B.S., Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metabolism. 2005;90:219–224. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 187.Tarnow P., Schoneberg T., Krude H., Gruters A., Biebermann H. Mutationally induced disulfide bond formation within the third extracellular loop causes melanocortin 4 receptor inactivation in patients with obesity. J Biological Chem. 2003;278:48666–48673. doi: 10.1074/jbc.M309941200. [DOI] [PubMed] [Google Scholar]
- 188.Beau I., Touraine P., Meduri G. A novel phenotype related to partial loss of function mutations of the follicle stimulating hormone receptor. J Clin Invest. 1998;102:1352–1359. doi: 10.1172/JCI3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martens J.W., Verhoef-Post M., Abelin N. A homozygous mutation in the luteinizing hormone receptor causes partial leydig cell hypoplasia: correlation between receptor activity and phenotype. Mol Endocrinol. 1998;12:775–784. doi: 10.1210/mend.12.6.0124. [DOI] [PubMed] [Google Scholar]
- 190.Biebermann H., Krude H., Elsner A., Chubanov V., Gudermann T., Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 191.Birnbaumer M. Vasopressin receptor mutations and nephrogenic diabetes insipidus. Arch Med Res. 1999;30:465–474. doi: 10.1016/s0188-4409(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 192.Pasel K., Schulz A., Timmermann K. Functional characterization of the molecular defects causing nephrogenic diabetes insipidus in eight families. J Clin Endocrinol Metabolism. 2000;85:1703–1710. doi: 10.1210/jcem.85.4.6507. [DOI] [PubMed] [Google Scholar]
- 193.Rocha J.L., Friedman E., Boson W. Molecular analyses of the vasopressin type 2 receptor and aquaporin-2 genes in Brazilian kindreds with nephrogenic diabetes insipidus. Hum Mutat. 1999;14:233–239. doi: 10.1002/(SICI)1098-1004(1999)14:3<233::AID-HUMU6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 194.Gu W., Tu Z., Kleyn P.W. Identification and functional analysis of novel human melanocortin-4 receptor variants. Diabetes. 1999;48:635–639. doi: 10.2337/diabetes.48.3.635. [DOI] [PubMed] [Google Scholar]
- 195.Yeo G.S., Lank E.J., Farooqi I.S., Keogh J., Challis B.G., O'Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet. 2003;12:561–574. doi: 10.1093/hmg/ddg057. [DOI] [PubMed] [Google Scholar]
- 196.Tonacchera M., Van Sande J., Cetani F. Functional characteristics of three new germline mutations of the thyrotropin receptor gene causing autosomal dominant toxic thyroid hyperplasia. J Clin Endocrinol Metabolism. 1996;81:547–554. doi: 10.1210/jcem.81.2.8636266. [DOI] [PubMed] [Google Scholar]
- 197.Oksche A., Schulein R., Rutz C. Vasopressin V2 receptor mutants that cause X-linked nephrogenic diabetes insipidus: analysis of expression, processing, and function. Mol Pharmacol. 1996;50:820–828. [PubMed] [Google Scholar]
- 198.Latronico A.C., Shinozaki H., Guerra G., Jr. Gonadotropin-independent precocious puberty due to luteinizing hormone receptor mutations in Brazilian boys: a novel constitutively activating mutation in the first transmembrane helix. J Clin Endocrinol Metabolism. 2000;85:4799–4805. doi: 10.1210/jcem.85.12.7071. [DOI] [PubMed] [Google Scholar]
- 199.Doherty E., Pakarinen P., Tiitinen A. A novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J Clin Endocrinol Metabolism. 2002;87:1151–1155. doi: 10.1210/jcem.87.3.8319. [DOI] [PubMed] [Google Scholar]
- 200.Fuhrer D., Warner J., Sequeira M., Paschke R., Gregory J., Ludgate M. Novel TSHR germline mutation (Met463Val) masquerading as Graves' disease in a large Welsh kindred with hyperthyroidism. Thyroid. 2000;10:1035–1041. doi: 10.1089/thy.2000.10.1035. [DOI] [PubMed] [Google Scholar]
- 201.Tao Y.X., Segaloff D.L. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology. 2003;144:4544–4551. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- 202.Duprez L., Parma J., Van Sande J. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet. 1994;7:396–401. doi: 10.1038/ng0794-396. [DOI] [PubMed] [Google Scholar]
- 203.Karges B., Krause G., Homoki J., Debatin K.M., de Roux N., Karges W. TSH receptor mutation V509A causes familial hyperthyroidism by release of interhelical constraints between transmembrane helices TMH3 and TMH5. J Endocrinol. 2005;186:377–385. doi: 10.1677/joe.1.06208. [DOI] [PubMed] [Google Scholar]
- 204.Arseven O.K., Wilkes W.P., Jameson J.L., Kopp P. Substitutions of tyrosine 601 in the human thyrotropin receptor result in increase or loss of basal activation of the cyclic adenosine monophosphate pathway and disrupt coupling to Gq/11. Thyroid. 2000;10:3–10. doi: 10.1089/thy.2000.10.3. [DOI] [PubMed] [Google Scholar]
- 205.Albertazzi E., Zanchetta D., Barbier P. Nephrogenic diabetes insipidus: functional analysis of new AVPR2 mutations identified in Italian families. J Am Soc Nephrol. 2000;11:1033–1043. doi: 10.1681/ASN.V1161033. [DOI] [PubMed] [Google Scholar]
- 206.Birnbaumer M., Gilbert S., Rosenthal W. An extracellular congenital nephrogenic diabetes insipidus mutation of the vasopressin receptor reduces cell surface expression, affinity for ligand, and coupling to the Gs/adenylyl cyclase system. Mol Endocrinol. 1994;8:886–894. doi: 10.1210/mend.8.7.7984150. [DOI] [PubMed] [Google Scholar]
- 207.Robben J.H., Knoers N.V., Deen P.M. Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am J Physiol Renal Physiol. 2005;289:F265–F272. doi: 10.1152/ajprenal.00404.2004. [DOI] [PubMed] [Google Scholar]
- 208.Wildin R.S., Cogdell D.E., Valadez V. AVPR2 variants and V2 vasopressin receptor function in nephrogenic diabetes insipidus. Kidney Int. 1998;54:1909–1922. doi: 10.1046/j.1523-1755.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 209.Hermosilla R., Oueslati M., Donalies U. Disease-causing V(2) vasopressin receptors are retained in different compartments of the early secretory pathway. Traffic. 2004;5:993–1005. doi: 10.1111/j.1600-0854.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 210.Yokoyama K., Yamauchi A., Izumi M. A low-affinity vasopressin V2-receptor gene in a kindred with X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 1996;7:410–414. doi: 10.1681/ASN.V73410. [DOI] [PubMed] [Google Scholar]
- 211.Kopp P., van Sande J., Parma J. Brief report: congenital hyperthyroidism caused by a mutation in the thyrotropin-receptor gene. N Engl J Med. 1995;332:150–154. doi: 10.1056/NEJM199501193320304. [DOI] [PubMed] [Google Scholar]
- 212.Winkler F., Kleinau G., Tarnow P. A new phenotype of nongoitrous and nonautoimmune hyperthyroidism caused by a heterozygous thyrotropin receptor mutation in transmembrane helix 6. J Clin Endocrinol Metabolism. 2010;95:3605–3610. doi: 10.1210/jc.2010-0112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.