Abstract
Osteoarthritis is a heterogeneous disorder. The goals of this review are (1) To stimulate use of standardized nomenclature for osteoarthritis (OA) that could serve as building blocks for describing OA and defining OA phenotypes, in short to provide unifying disease concepts for a heterogeneous disorder; and (2) To stimulate establishment of ROAD (Risk of Osteoarthritis Development) and ROAP (Risk of Osteoarthritis Progression) tools analogous to the FRAX™ instrument for predicting risk of fracture in osteoporosis; and (3) To stimulate formulation of tools for identifying disease in its early preradiographic and/or molecular stages -- REDI (Reliable Early Disease Identification). Consensus around more sensitive and specific diagnostic criteria for OA could spur development of disease modifying therapies for this entity that has proved so recalcitrant to date. We fully acknowledge that as we move forward, we expect to develop more sophisticated definitions, terminology and tools.
Purpose
New specific and sensitive disease endpoints are critically needed to alleviate roadblocks to development of disease modifying therapeutics and strategies for secondary prevention of osteoarthritis (OA). A key step in this process is the development of standardized definitions of OA. Standardization of OA definitions would aid communication across the field and help advance drug development for OA and research by achieving consensus on globally recognized definitions of disease and globally recognized standards for classifying the disease. We anticipate that these definitions could facilitate communication about the disease among industry and non-industry researchers, regulatory agencies, funding agencies, third party payers, and patients. We further anticipate that these definitions would be maintained by the Osteoarthritis Research Society International (OARSI) and subjected to regular refinement as new scientific advances demand. Definitions proposed are not intended to distinguish an OA patient uniquely from patients with other forms of arthritis; rather, the draft definitions can be viewed as the building blocks for defining OA phenotypes. We fully acknowledge that these building blocks are likely most applicable to knee and hip OA, possibly helpful for hand OA, but will require modification for spine OA. In this review we therefore propose broad OA definitions with the intent to refine them through “crowd-sourcing” from the OARSI membership via a WIKI on the OARSI website.
Outdated Disease Classification System
According to the United States (US) Food and Drug Administration (FDA) [1], “Currently used disease classification systems define diseases primarily on the basis of their signs and symptoms. These systems do not easily accommodate emerging information about disease mechanisms, particularly when it is at odds with traditional physical descriptions. As a result, many disease subtypes with distinct molecular causes are still classified as one disease, while multiple, different diseases that share a common molecular cause are not properly linked. The failure of our outdated disease classification systems to incorporate optimally new biological insights serves as a fundamental barrier to progress in personalized medicine. The US National Academy of Sciences has called for the creation of a ‘New Taxonomy’ of disease that is designed to advance our understanding of disease pathogenesis and improve health and that defines and describes diseases on the basis of their intrinsic biology in addition to traditional signs and symptoms [2].”
Several different strategies have been proposed for describing OA phenotypes, including phenotyping based on modern imaging [3] or pathophysiological mechanisms [4]. Based on phenotypes, OA is considered highly heterogeneous. Nevertheless, it is often considered a common pathological process triggered by a variety of inciting events and agents. These entities that share a common molecular cause would benefit from a ‘new taxonomy’ that would enable us to communicate about the disease on the basis of intrinsic characteristics. The purpose here is to begin to develop and reach consensus on a shared nomenclature. This would be designed to facilitate our understanding of the pathogenesis of OA and would be updated and refined as new disease insights are gained. The US National Research Council Committee suggested a framework for creating an information system called a Knowledge Network of disease that integrates the rapidly expanding range of information on the causes of disease and allows researchers, health-care providers, and the public to share and update this information [2]. Such a long-term goal is indeed tantalizing for OA, and could be envisioned as an ongoing project by the OARSI membership.
Disease versus Illness
“Disease” refers to abnormalities of the structure and function of body organs and systems that can be specifically identified and described by reference to certain biological, chemical or other evidence [5]. A disease has specific properties and a recurring identity in whichever setting it appears. Because a particular disease is assumed to be universal in its form, progress and content [5], we seek here to define OA as disease, not by patient phenotype but rather by its universal form.
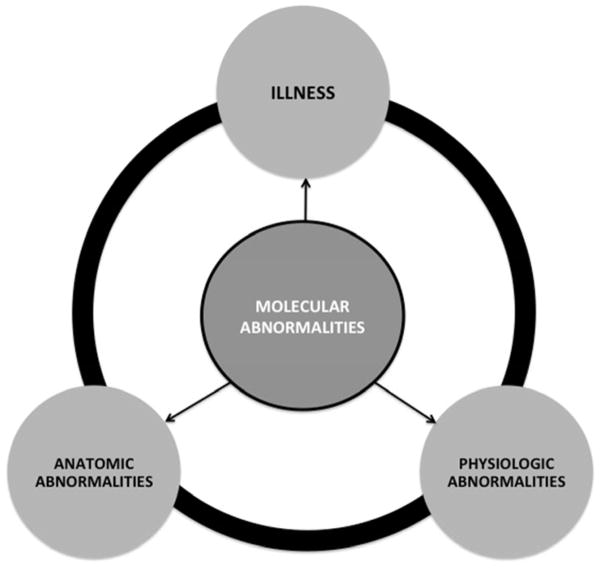
An “illness” refers to the human response to disease [5]. Cassell in 1978 described illness to mean “what the patient feels when he goes to the doctor”, and disease to mean “what he has on the way home from the doctor’s office”; in short, disease is something an organ has and illness is something a person has (summarized by Helman [5]). The specific manifestations of illness are likely to differ according to OA phenotype. Although they may coexist, and often do, it is possible for disease to occur in the absence of illness. Like osteoporosis, OA may be manifest by a prolonged period of musculoskeletal tissue abnormalities at a molecular but clinically silent level that can precede the anatomic organ system disease and illness manifestations by years or even decades (Figure 1). Thus, very early OA would be characterized by an asymptomatic disease state. It is common in OA that disease does not coincide with illness; definite radiographic features of OA are often found in joints of persons without symptoms. In this sense, the disease and its radiographic features could be considered risk factors for the illness. A precedent exists in spondyloarthropathies for which preradiographic diagnostic disease criteria were developed [6] that subsequently stimulated treatment trials [7]. By analogy, we hope that a greater understanding and consensus regarding the disease versus illness aspects of OA would similarly stimulate treatment trials for early OA.
Figure 1. Relationships of disease and illness components.
We posit that the disease may be manifest by a prolonged period of isolated musculoskeletal tissue abnormalities at a molecular and clinically silent level (molecular). Further, the molecular abnormalities could precede the anatomic and physiologic level organ system disease and illness manifestations by years or even decades. In addition, abnormalities of two domains or all in combination could be imagined (depicted by arrows and the ring connecting the components).
There is much we do not know about the chronology of the OA trajectory from molecular disease to anatomic disease to illness. As noted in Figure 1, we posit that molecular abnormalities may coexist with anatomic abnormalities in the absence of illness; we observe this as radiographic or MRI abnormalities in the absence of symptoms. We also posit that molecular abnormalities may coexist with illness in the absence of measurable anatomic abnormalities; this may be due, for instance, to cartilage degradation products activating innate immunity, subclinical synovitis and pain in the absence of discernible anatomic derangements by the current imaging tools. Illness might also occur in the absence of discernible disease; if truly OA related, then this could be due to a lack of sensitive enough biochemical and imaging biomarker tools to detect disease. In OA, it will be challenging to confidently rule out disease at early stages until we have sensitive tools for identifying the early molecular derangements of the joint organ. Of course, any aspect of disease may coexist with illness [8].
In addition to illness and molecular and anatomic aspects of disease, there can be physiologic aspects of disease (Figure 2A). It is said that anatomy studies the form, while physiology looks at the function - anatomy looks at what it is, while physiology looks at what it does [3]. A holistic description of the pathology requires an understanding and description of all these aspects. Consider the real life illustration of these concepts for the example of heart failure. The anatomic severity of heart failure can be quantified by degree of left ventricular dilatation. The physiologic severity of heart failure can be quantified by percent ejection fraction. There are even molecular biomarkers, such B-type natriuretic protein and N-terminal pro-B-type natriuretic peptide, produced by the ventricles in the heart in response to excessive stretching of heart muscle cells, that correlate with severity of heart failure, and whose use clinically may be superior to symptom-guided therapy [9, 10]. Multiple symptoms of illness can arise in heart failure, among them most notably, shortness of breath. Now consider a possible example for OA (Figure 2B). The anatomic severity of knee OA can be quantified by degree of cartilage degradation (manifested by joint space narrowing) or osteophyte formation. The physiologic severity can be quantified by cartilage indentation to measure cartilage stiffness that sensitively reflects alterations in both the proteoglycan concentration and superficial collagen layer of articular cartilage [11]. The collagen II marker, urinary C-telopeptide of type II collagen (uCTXII) could be considered the molecular biomarker with the greatest amount of data supporting its association with OA [12]. The hallmark of illness in OA is of course joint pain. These examples illustrate the utility and clarity provided by attending to all these aspects of pathology. This taxonomy can be applied to define the stages of OA (Figure 3). Below follows a more detailed discussion of this taxonomy and these components of pathology.
Figure 2. Taxonomy of Osteoarthritis (OA).
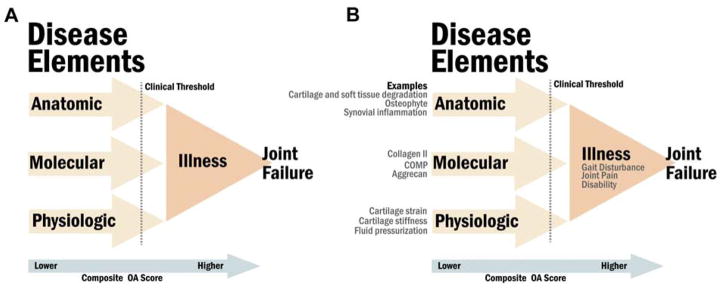
We propose a new ‘taxonomy of OA’ based on the standardized nomenclature of disease (made up of molecular, anatomic and physiologic components, domains or disease elements) and illness (panel A). As illustrated here, a clinical threshold would be anticipated that would result in the transition from disease to illness. This taxonomy anticipates the development of composite indices of OA (arrow) that by analogy to the Disease Activity Score (DAS) in rheumatoid arthritis, would encompass all three-disease domains (molecular, anatomic and physiologic) and illness that could be useful for clinical evaluation and trials. Varying weights might be ascribed to the different elements in the composite score (illustrated by the terms lower and higher within the arrow). Osteoarthritis specific examples for each domain are included in panel B.
Figure 3. Stages of Osteoarthritis incorporating the new taxonomy.
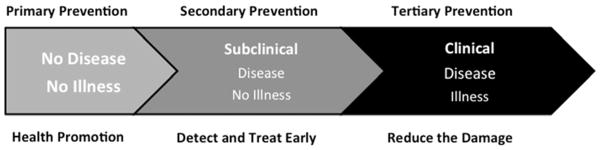
Three stages can be imagined -- a no disease/no illness stage, a subclinical stage (with disease manifestations only) and a clinical stage (with illness manifestations). The goal at the predisease stage is to promote health through education on healthy lifestyle choices and specific prevention against the inception of disease by modifying risk factors in a favorable direction. The goal at the subclinical stage is to be able to make a presymptomatic diagnosis to prevent its progression to symptomatic disease and thereby prevent illness and associated disability. The goal at the clinical stage is to provide treatment in an effort to prevent its progression to disability; this includes maximizing the remaining capabilities and functions via pain management, symptom control, stress relief, disease management, rehabilitation and risk reduction. (Levels of prevention adapted from Katz et al. [79]).
The Disease
Molecular indicators of joint health and disease
This level definition of OA is founded on the molecular abnormalities of the joint organ as detected by omics technologies such as genomics, proteomics, transcriptomics, metabolomics etc. In theory, a molecular level of interrogation is the only one able to detect the very initial and very early phases of the disease process before changes in structure are detected with for instance, radiography, magnetic resonance imaging (MRI), positron emission tomography (PET) or ultrasound. It is anticipated that one or more biomarkers will in future be qualified for identifying molecular joint disease in its early stages. It is also plausible that the molecular features that characterize the abnormal joint will change within a given individual over the course of their disease. Currently, there are only candidate biomarkers for this early stage of disease that would be identified solely by molecular abnormalities [13–15].
Improved understanding of joint physiology and the molecular pathogenesis of OA can potentially provide tools for defining and identifying molecular OA. To date, our understanding is that under physiologic loading, chondrocytes maintain the balance between degradation and synthesis of matrix macromolecules [16]. Under injury or loading that exceeds the capacity of the tissue, degradation exceeds synthesis, causing joint tissue degeneration and eventually OA [16]. Markers of in vivo tissue turnover (such as deamidated and racemized protein epitopes) suggest that cartilage is capable of some spontaneous repair and that this response is upregulated in OA cartilage of the knee but not the hip [17–19]. The mechanisms underlying these different repair responses by joint site are currently unknown, however migration of cells with chondrogenic capacity from synovium and bone marrow to damaged cartilage has been suggested by several authors [20, 21]. Mechanosensors mediate the homeostatic joint response to load [16, 22]. Mechanoresponses of chondrocytes play an important role in the development of OA and cartilage overloading elicits metabolic stress reactions and enzymes that mediate tissue degradation in vivo in a mechanosensitive manner [16, 23, 24]. Thus, an abnormal physiology in the joint is driven by mechanical ‘wear’ that actively drives the enzymes that produce the ‘tear’ [23].
Proteomics methods have identified many proteins that may relate to pathological mechanisms of OA (for review see [25]). For instance, the OA synovial fluid proteome implicates proteins related to formation and remodeling of the extracellular matrix [26], and the acute-phase response signaling pathway, the complement pathway, and the coagulation pathway [27]. Indeed, proteomic analyses have defined sets of serum proteins that distinguish patients with radiographic knee OA from controls; proteins observed only in patients with severe radiographic knee OA suggested that molecular markers may become useful for staging disease [28]. Some other molecular leads or ‘fingerprints’ that could be helpful might include molecular entities associated with cell stress, extracellular matrix degradation, wound healing, pro-inflammatory pathways of innate immunity, analytes associated with hypertrophic and senescent chondrocytes, as well as hypocellularity and autophagy of cartilage [29–33]. It will be important to investigate these pathways for their ability to detect the molecular phase of the disease process as determined by their ability to predict incident OA defined by established imaging criteria.
To date there is only scant evidence to support the ability of these pathways to distinguish OA uniquely from other arthritides, such as rheumatoid arthritis [34]. This important knowledge gap needs to be a focus of future research. However, there are data to suggest that cartilage from different joint sites differ in their biochemical constituents [35] and that therefore, it may ultimately be possible to identify molecular OA according to specific joint sites.
We considered the term metabolic to describe the molecular phase of the OA process. Although the term metabolic is attractive for its ability to encompass the concept of joint tissue metabolism or turnover, this term could create confusion with the metabolic phenotype of OA (the association of OA with obesity, hypertension, and diabetes mellitus, etc.). We therefore propose the term molecular OA as a better option to avoid confusion with the metabolic phenotype of OA or metabolic syndrome often associated with OA.
Anatomic indicators of joint health and disease
Anatomy deals with the branch of science concerned with the bodily structure of humans, animals, and other living organisms. In contrast to molecular disease, defined on the basis of omics technologies, the structural abnormalities comprising anatomic disease are mainly revealed by imaging methodologies (radiography, MRI, PET or ultrasound) or arthroscopy. By histology, the abnormalities of OA include cartilage fibrillation, fissuring and denudation to bone, loss of proteoglycan, chondrocyte death or proliferation and osteophyte formation [36]. By radiography, the primary anatomic abnormalities of OA are loss of articular and meniscal cartilage (reflected in joint space narrowing), osteophyte formation, bone sclerosis and bone cysts, pathological bone contour alterations and joint malalignment. By MRI more subtle anatomic abnormalities are discernible [37–39]. These structural changes may be present in the absence of the illness characterized by the experience of pain or other symptoms [38]; therefore, an anatomic description of the disease can be independent of illness (as described below). For example, early disease may be characterized by increased cartilage thickness and high T1rho signal (due to cartilage swelling), abnormal intrameniscal signal representing meniscal degeneration, and alterations in bone shape or subchondral trabecular bone [40, 41]. These structural changes may be used in different combinations to optimize specificity or sensitivity for an OA diagnosis, as has been proposed for MRI findings [42]. By ultrasound, additional pathological anatomic abnormalities, such synovitis and angiogenesis, can be appreciated in the clinical setting [43–45]. In some cases, erosions are also a manifestation of disease, in particular in a subset of hand OA, which is likely to reflect a specific phenotype [46]. The prevalence of erosions increases with the sensitivity of imaging modality; a new MRI-based scoring system has been developed to better identify and classify features of hand OA [47].
Physiologic indicators of joint health and disease
Physiology is the study of the function of body parts and the body as a whole. Physiology is often complex and involves interactions between multiple organs and tissues. OA can lead to functional limitation and impairment at the level of the cell, tissue, organ or person and thereby lead to abnormal functioning at these levels [24, 48]. Much of OA disease progression is mediated by aberrant physiological interaction of the components of the musculoskeletal system, such as aberrant biomechanical forces or a pathologic response to these forces [49]. Many interventions, such as exercise and walking aids, are designed to correct abnormal physiology [50]. The physiologic aspect of disease is therefore an integral and important descriptor of OA. Physiologic measures that might be used to characterized and grade OA include evaluation of cartilage degeneration using indentation [11, 51], dynamic MRI [52] including site-specific variations in cartilage strain with activity [53] constituting a non-invasive in vivo cartilage “stress test”, and gait biomechanics [54]. Traditional OA risk factors, such as strength, joint stability (functional or structural), obesity, and age are all likely to impact joint physiology but could also impact molecular and anatomic indicators of disease. Moreover, different domains of disease can and will interact such as malalignment (anatomic indicator) which will impact gait mechanics joint load and tissue strain (physiologic indicator).
The Illness
Illness refers to the human response to disease, in other words “what the patient feels when he goes to the doctor”. The latter description would entail inclusion of patient symptoms, such as pain aching or stiffness, and disability (a physical or mental impairment that substantially limits one or more major life activities of such individual) as defining the illness of OA. There are multiple potential causes of joint symptoms; symptoms in the absence of anatomic structural changes of OA cannot currently be definitively diagnosed as attributable to an OA disease process [55]. Recommendations have already been proposed for making a diagnosis of concurrent radiographic OA without the need for imaging, based on clinical signs (crepitus, restricted movement and bony enlargement) and symptoms (knee pain, short-lived morning stiffness and functional limitation) [56]. Work is in progress to develop classification criteria for early OA (through the OARSI endorsed International Early Knee Osteoarthritis working group). The validation of such criteria will be their ability to predict with high likelihood, the subsequent development of anatomic OA. In future, the concurrence of OA-related molecular abnormities with symptoms might also allow for a diagnosis of OA despite the absence of anatomic abnormalities. [56]
Classically, two types of control groups have been used in OA studies, those lacking symptoms (illness) or those lacking radiographic OA (anatomic disease). Since disease may not necessarily coincide with illness, the optimal control group for predicting risk of early OA will lack illness and disease (at both molecular and anatomic levels).
Clinical Thresholds
To better understand the concepts of disease and illness in OA, it is instructive to consider the interface of disease and illness for other organ systems. The thresholds for clinical manifestations of illness differ by organ system. For instance, 50% of men and 64% of women who die suddenly of coronary heart disease have no previous clinical symptoms of the disease [57] (Figure 4). Other ‘high functioning’ organs, liver and kidney, have 90% excess functional capacity from birth. For these organ systems, the threshold for transition from disease to illness is high.
Figure 4. Disease versus illness.
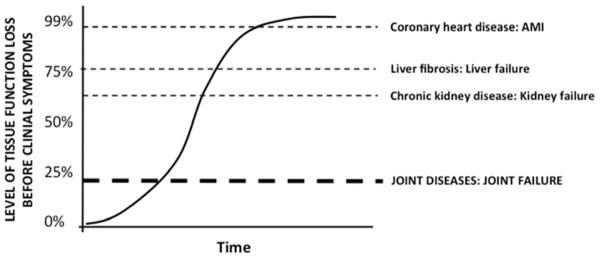
The tissue functional threshold for establishment of a clinical symptomatic disease differs by organ system. The horizontal dashed lines depict the transition from disease to illness for different diseases. The threshold is relatively high in heart, liver and kidney disease but anticipated to be relatively low for the transition of joint disease to illness (symptoms, disability and joint failure). It is possible that the threshold will vary according to type of joint disease. Both the kidney and liver have a large “functional reserve”. This contributes to their being silent killer diseases [80], in which asymptomatic late stage disease suddenly becomes clinically apparent with a possibly fatal outcome for some patients [63]. AMI=acute myocardial infarction due to coronary heart disease.
Due to a large “renal reserve”, traditional markers of renal injury lack the sensitivity and/or specificity to adequately detect nephrotoxicity prior to significant loss of renal function [58, 59]. This has, in part, been responsible for efforts stimulated by the FDA to develop a kidney damage panel. Such a safety pharmacology panel may be pertinent to other disease indications [60]. The function of the kidney is highly age dependent and GFR declines dramatically with age. Thus, age alone can account for more than 75% decrease in kidney function, without any associated illness. This illustrates that the kidney is an organ with a large overcapacity, and that a large functional decline is possible before clinical manifestations of illness may be observed [61, 62].
The liver is another organ with a large overcapacity. Liver function can decline as much as 70% before diagnosis and symptoms occur such as the presence of full blown cirrhosis (end stage fibrosis) with portal hypertension [63]. The liver is the only human internal organ capable of natural regeneration of lost tissue. In the absence of an associated illness, as little as 25% of a liver can regenerate into a whole liver [64–67]. The liver fibrosis field shares many similar needs with the OA field, i.e. a need for anti-fibrotic treatments and a large medical need for early diagnostics and prognostics [63].
Joints may be more sensitive than internal organs with respect to threshold for manifestation of illness in the form of clinical symptoms. In rheumatoid arthritis and OA, clinical manifestations are incident decades before organ failure – defined as the necessity for joint replacement. We therefore speculate that the threshold for clinical manifestations may be 20% loss of joint organ function. Several studies seem to suggest a low illness threshold related to the OA disease in some individuals [68, 69]. The association between illness and radiographic anatomic alteration is very modest--some patients experience pain very early, and others far later or never. The presence of multiple joints in the musculoskeletal system complicates the interaction of disease and illness. Disease and/or illness may affect “just” one joint or multiple joints simultaneously. Whereas, a 30% loss of function of the liver or kidney would be hardly noticeable, a 30% loss of function of even one joint could be debilitating for an OA patient and have a negative impact on other joints. Just one abnormal joint can lead to pain while many abnormal joints can further and alter the pain experience; the worse the pain the lower the pain threshold [70], i.e. illness threshold. In addition, small losses in cartilage volume are correlated with pain worsening, suggesting a much lower threshold to illness in joint disease compared to liver and kidney disease [71]. Among other factors, the illness threshold could also be impacted by central sensitization of pain [70] and genetic polymorphisms of pain sensitivity [72]. The estimate of a 20% threshold is arbitrary. Further research is needed, including the input of patients, to determine the exact threshold that defines the transition to illness in different individuals and settings.
Draft Definitions of Osteoarthritis
Draft OARSI
“Osteoarthritis is a disorder involving movable joints characterized by cell stress and extracellular matrix degradation initiated by micro- and macro-injury that activates maladaptive repair responses including pro-inflammatory pathways of innate immunity. The disease manifests first as a molecular derangement (abnormal joint tissue metabolism) followed by anatomic, and/or physiologic derangements (characterized by cartilage degradation, bone remodeling, osteophyte formation, joint inflammation and loss of normal joint function), that can culminate in illness.”
NICE guideline (http://www.nice.org.uk/guidance/index.jsp?action=folder&o=64496)
“Osteoarthritis is characterized pathologically by localized loss of cartilage, remodeling of adjacent bone and associated inflammation. A variety of traumas may trigger the need for a joint to repair itself. Osteoarthritis includes a slow but efficient repair process that often compensates for the initial trauma, resulting in a structurally altered but symptom-free joint. In some people, because of either overwhelming trauma or compromised repair, the process cannot compensate, resulting in eventual presentation with symptomatic osteoarthritis; this might be thought of as ‘joint failure’. This explains the extreme variability in clinical presentation and outcome that can be observed between people, and also at different joints in the same person.”
Global Burden of Osteoarthritis in the Year 2000 by Deborah Symmons, Colin Mathers, and Bruce Pfleger) (www.who.int/healthinfo/.../bod_osteoarthritis.pdf)
“Osteoarthritis is a complex disease entity that is difficult to diagnose and define. The Subcommittee on Osteoarthritis of the American College of Rheumatology Diagnostic and Therapeutic Criteria Committee defined osteoarthritis as “A heterogeneous group of conditions that lead to joint symptoms and signs which are associated with defective integrity of articular cartilage, in addition to related changes in the underlying bone at the joint margins” [73]. Clinically, the condition is characterized by joint pain, tenderness, limitation of movement, crepitus, occasional effusion, and variable degrees of local inflammation.”
Centers for Disease Control (http://www.cdc.gov/arthritis/basics/osteoarthritis.htm)
“Osteoarthritis is a disease characterized by degeneration of cartilage and its underlying bone within a joint as well as bony overgrowth. The breakdown of these tissues eventually leads to pain and joint stiffness. The joints most commonly affected are the knees, hips, and those in the hands and spine. The specific causes of osteoarthritis are unknown, but are believed to be a result of both mechanical and molecular events in the affected joint. Disease onset is gradual and usually begins after the age of 40.”
Taxonomy of OA -- Building Blocks to Phenotypes
In describing and classifying OA, we anticipate that it would be possible to use a new ‘taxonomy of OA’ (Figure 2), as building blocks to systematically describe and classify OA phenotypes or subtypes. We can gain insights into such a nomenclature by contemplating clinical descriptions of rheumatoid arthritis and the 2010 classification criteria for rheumatoid arthritis of the American College of Rheumatology (ACR) and EULAR (http://www.rheumatology.org/practice/clinical/classification/ra/ra_2010.asp) (7, 8). As illustrated by the following example, rheumatoid arthritis is typically described clinically with a string of qualifiers that encompass all three disease domains (molecular, anatomic and physiologic) and illness: sero-positive/negative (molecular domain), erosive/non-erosive (anatomic domain), deforming/non-deforming (anatomic domain), pattern of joint involvement (anatomic domain), joint range of motion (physiologic domain), and acute/chronic duration of symptoms and disability (Illness domain). The ACR/EULAR system scores the pattern of joint involvement (anatomic domain), serology and acute phase reactants (molecular domain), and duration of symptoms (illness domain). These means of describing rheumatoid arthritis have served the field well. In this regard, we believe the disease and illness domains, suggested above for OA, would represent an advance for the OA research field.
Needs of the Field
Risk Prediction Tools
The FRAX® tool (http://www.shef.ac.uk/FRAX/) [74] was developed under the aegis of the World Health Organization, and designed to predict the 10-year probabilities of sustaining major osteoporotic fractures (clinical spine, forearm, hip or shoulder fracture) [75]. FRAX is an example of a composite risk score that integrates the risks associated with clinical risk factors (such as country, age, sex, weight, height, family history, patient history (e.g. previous fractures)), as well as bone mineral density (BMD) at the femoral neck [75]. The osteoporosis field may have been particularly “lucky” as bone mineral density (BMD) is both diagnostic of osteoporosis and prognostic for fracture; the OA field has yet to define such an opportune marker. Although the 10 year risk of fracture can be estimated quite well in the absence of BMD, BMD data narrow the confidence interval of the estimate.
The FRAX® is highly instructive for possible future advances in OA. The OA field is in need of analogous tools for predicting Risk of OA Development (ROAD), Risk of OA Progression (ROAP), and Reliable Early Disease Identification (REDI). It will take time to accumulate good enough predictor data from more than one population to develop these tools. These endeavors are optimally conducted as an international collaborative project and represent one of the grand challenges in OA research. We can also anticipate tools for predicting risk of altered physiology and illness. Preliminary work is ongoing to develop a tool for predicting risk of radiographic OA development (i.e. anatomic OA based on our proposed taxonomy) [76]. To date, models predict 34% of the variance in radiographic OA incidence (defined as knee Kellgren Lawrence grade <2 at baseline and grade ≥2 at follow up of a mean of 4–10 years). The addition of either clinical/questionnaire-based variables, a genetic risk score or concentration of a biochemical marker, urinary CTX-II, to age, gender and BMI added little to no predictive value to the model. However, the addition of symptoms and baseline radiographic knee OA (Kellgren Lawrence score 1) improved predictive capabilities of the model. The Kellgren Lawrence score of 1 was the best predictor of future knee OA and stronger than age, gender and BMI alone. As aptly stated by Sharma et al, and in agreement with our taxonomy of OA, incident radiographic OA is likely capturing early progression of disease rather than disease development. A robust risk predictor of anatomic OA development will likely require a sensitive and objective molecular indicator of early disease.
Composite indices of disease and illness
For clinical trials in rheumatoid arthritis, the Disease Activity Score (DAS) together with ACR20, 50 and 70 response rates are becoming the gold standard outcomes. At the patient level, the DAS score is receiving much deserved attention for its use in intention to “treat until remission”, that is until lowering of DAS to below 2.6 [77]. The DAS score is a composite index combining objective (disease) and subjective (illness) measures including number of tender joints, number of swollen joints, a serological inflammation measure, such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) and a general patient health assessment on a visual analog scale (VAS). The DAS score has been useful for correlating disease activity with molecular biomarkers in rheumatoid arthritis [78]. For OA, it will undoubtedly be useful to develop one or more composite indices (Figure 2) combining disease and illness parameters that could be used for diagnosis, prognosis and monitoring of a treatment response.
Knowledge Network of OA
A Knowledge Network of disease would integrate the rapidly expanding range of information on the causes of OA and allow OARSI researchers to share and update this information. We hope that the exercise of posting this draft taxonomy of OA to the web and engaging the membership in its refinement will be a start to an expanding range of information on the disease and illness of OA that can facilitate and invigorate the research enterprise.
Acknowledgments
Declaration of Funding and Role of Funding Sources
This work was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P01 AR050245) and the National Institute of Aging (P30 AG028716) at the National Institutes of Health (VBK), and the Instituto de Salud Carlos III- FIS PI 12/0329 (FJB).
Footnotes
Author Contributions: All authors contributed to the writing and revision of the manuscript and approved the final version.
Conflicts of Interest
Virginia Byers Kraus -- Has received salary support through NIH grants PO1 AR050245 and AG028716; lecture/consultancy fees from Merrimack Pharmaceuticals, Flexion Therapeutics, Bioiberica and Abbott. She is an Associate Editor of Osteoarthritis & Cartilage.
Francisco J Blanco -- has received Grants (Clinical Trials, conferences, advisor and publications) from: Abbvie, Amgen, Bioiberica, Bristol Mayer, Celgene, Celltrion, Cellerix, Grunenthal, Gebro Pharma, Lilly, MSD, Merck Serono, Pfizer, Pierre-Fabra, Roche, Sanofi, Servier and UCB.
Martin Englund – has received honorarium for lectures in a course in clinical epidemiology from Pfizer and for a lecture in knee OA from Össur.
Morten Karsdal - is a full time employee of Nordic Bioscience, a company engaged in biomarker and medicinal product research and development.
Stefan Lohmander -- Relevant financial activities outside the submitted work include consultancy for Abbvie, Flexion, Galapagos, Medivir, MerckSerono, Teijin, Össur. Employment as Editor-in-Chief of Osteoarthritis and Cartilage.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simoncell T, Barclay L, Bouri K, Burns K, Cook K, Filice R, et al. Paving the Way for Personalized Medicine: FDA’s Role in a New Era of Medical Product Development. Silver Spring, MD: Oct, 2013. pp. 1–61. [Google Scholar]
- 2.Desmond-Hellmann S, Sawyers C, Cox D, Fraser-Liggett C, Galli S, Goldstein D, et al. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington DC: National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 3.Nordqvist C. Medical News Today: Medical News Today. 2014. What is physiology. [Google Scholar]
- 4.Castaneda S, Roman-Blas JA, Largo R, Herrero-Beaumont G. Osteoarthritis: a progressive disease with changing phenotypes. Rheumatology (Oxford) 2014;53:1–3. doi: 10.1093/rheumatology/ket247. [DOI] [PubMed] [Google Scholar]
- 5.Helman CG. Disease versus illness in general practice. J R Coll Gen Pract. 1981;31:548–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Treatment trials for nonradiologic axSpA (nr-axSpA) followed the development of diagnostic criteria by ASAS in 2009. Rheumatology News. 2014 [Google Scholar]
- 8.Cibere J, Zhang H, Garnero P, Poole AR, Lobanok T, Saxne T, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60:1372–1380. doi: 10.1002/art.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vecchis R, Esposito C, Di Biase G, Ariano C, Giasi A, Cioppa C. B-type natriuretic peptide-guided versus symptom-guided therapy in outpatients with chronic heart failure: a systematic review with meta-analysis. J Cardiovasc Med (Hagerstown) 2014;15:122–134. doi: 10.2459/JCM.0b013e328364bde1. [DOI] [PubMed] [Google Scholar]
- 10.Savarese G, Trimarco B, Dellegrottaglie S, Prastaro M, Gambardella F, Rengo G, et al. Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2,686 patients in 12 randomized trials. PLoS One. 2013;8:e58287. doi: 10.1371/journal.pone.0058287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasara AI, Jurvelin JS, Peterson L, Kiviranta I. Arthroscopic cartilage indentation and cartilage lesions of anterior cruciate ligament-deficient knees. Am J Sports Med. 2005;33:408–414. doi: 10.1177/0363546504268040. [DOI] [PubMed] [Google Scholar]
- 12.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17:43–48. doi: 10.1016/j.joca.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golightly Y, Marshall1 S, Kraus V, Renner J, Villaveces A, Casteel1 C, et al. Serum cartilage oligomeric matrix protein, hyalutonan, high-sensitivity C-reactive protein and keratan sulfate as predictors of incident radiographic knee osteoarthritis: differences by chronic knee symptoms. Osteoarthritis & Cartilage. 2010;18:S62–63. [Google Scholar]
- 15.Ruiz-Romero C, Blanco FJ. Proteomics role in the search for improved diagnosis, prognosis and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18:500–509. doi: 10.1016/j.joca.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Tambe DT, Deng L, Yang L. Biomechanical properties and mechanobiology of the articular chondrocyte. Am J Physiol Cell Physiol. 2013;305:C1202–1208. doi: 10.1152/ajpcell.00242.2013. [DOI] [PubMed] [Google Scholar]
- 17.Catterall JB, Hsueh MF, Stabler TV, McCudden CR, Bolognesi M, Zura R, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catterall J, Zura R, Bolognesi M, Kraus V. Aspartic acid racemization reveals a high state of repair in knee compared with hip osteoarthritic cartilage. 2015 doi: 10.1016/j.joca.2015.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stabler TV, Byers SS, Zura RD, Kraus VB. Amino acid racemization reveals differential protein turnover in osteoarthritic articular and meniscal cartilages. Arthritis Res Ther. 2009;11:R34. doi: 10.1186/ar2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco FJ, Ruiz-Romero C. New targets for disease modifying osteoarthritis drugs: chondrogenesis and Runx1. Ann Rheum Dis. 2013;72:631–634. doi: 10.1136/annrheumdis-2012-202652. [DOI] [PubMed] [Google Scholar]
- 21.Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 22.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13:449–454. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Milner P, Wilkins R, Gibson J. Cellular physiology of articular cartilage in health and disease. In: Rothschild B, editor. Principles of Osteoarthritis- Its Definition, Character, Derivation and Modality-Related Recognition. InTech; 2012. [Google Scholar]
- 25.Hsueh MF, Onnerfjord P, Kraus VB. Biomarkers and proteomic analysis of osteoarthritis. Matrix Biol. 2014;39C:56–66. doi: 10.1016/j.matbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Mateos J, Lourido L, Fernandez-Puente P, Calamia V, Fernandez-Lopez C, Oreiro N, et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC-MALDI TOF/TOF. J Proteomics. 2012;75:2869–2878. doi: 10.1016/j.jprot.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 27.Ritter SY, Subbaiah R, Bebek G, Crish J, Scanzello CR, Krastins B, et al. Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum. 2013;65:981–992. doi: 10.1002/art.37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Puente P, Mateos J, Fernandez-Costa C, Oreiro N, Fernandez-Lopez C, Ruiz-Romero C, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res. 2011;10:5095–5101. doi: 10.1021/pr200695p. [DOI] [PubMed] [Google Scholar]
- 29.Rigoglou S, Papavassiliou AG. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Kraus VB. Osteoarthritis: The zinc link. Nature. 2014;507:441–442. doi: 10.1038/507441a. [DOI] [PubMed] [Google Scholar]
- 31.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 32.Takayama K, Kawakami Y, Lee S, Greco N, Lavasani M, Mifune Y, et al. Involvement of ERCC1 in the pathogenesis of osteoarthritis through the modulation of apoptosis and cellular senescence. J Orthop Res. 2014;32:1326–1332. doi: 10.1002/jor.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mobasheri A, Matta C, Zakany R, Musumeci G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80:237–244. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Heard BJ, Martin L, Rattner JB, Frank CB, Hart DA, Krawetz R. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet Disord. 2012;13:126. doi: 10.1186/1471-2474-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegard D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem. 2012 doi: 10.1074/jbc.M111.298968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) Bmj. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JA, Gold GE. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am. 2011;19:249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter D, Lohmander L, Makovey J, Tamez-Pena J, Totterman S, Schreyer E, et al. The effect of anterior cruciate ligament injury on bone curvature: The KANON Trial. Osteoarthritis & Cartilage. 2014 doi: 10.1016/j.joca.2014.05.014. in press. [DOI] [PubMed] [Google Scholar]
- 41.Reichenbach S, Guermazi A, Niu J, Neogi T, Hunter DJ, Roemer FW, et al. Prevalence of bone attrition on knee radiographs and MRI in a community-based cohort. Osteoarthritis Cartilage. 2008;16:1005–1010. doi: 10.1016/j.joca.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter DJ, Arden N, Conaghan PG, Eckstein F, Gold G, Grainger A, et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moller I, Bong D, Naredo E, Filippucci E, Carrasco I, Moragues C, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage. 2008;16 (Suppl 3):S4–7. doi: 10.1016/j.joca.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Keen HI, Wakefield RJ, Grainger AJ, Hensor EM, Emery P, Conaghan PG. Can ultrasonography improve on radiographic assessment in osteoarthritis of the hands? A comparison between radiographic and ultrasonographic detected pathology. Ann Rheum Dis. 2008;67:1116–1120. doi: 10.1136/ard.2007.079483. [DOI] [PubMed] [Google Scholar]
- 45.Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20:573–580. doi: 10.1097/BOR.0b013e3283103d12. [DOI] [PubMed] [Google Scholar]
- 46.Kloppenburg M, Kwok WY. Hand osteoarthritis--a heterogeneous disorder. Nat Rev Rheumatol. 2012;8:22–31. doi: 10.1038/nrrheum.2011.170. [DOI] [PubMed] [Google Scholar]
- 47.Haugen IK, Lillegraven S, Slatkowsky-Christensen B, Haavardsholm EA, Sesseng S, Kvien TK, et al. Hand osteoarthritis and MRI: development and first validation step of the proposed Oslo Hand Osteoarthritis MRI score. Ann Rheum Dis. 2011;70:1033–1038. doi: 10.1136/ard.2010.144527. [DOI] [PubMed] [Google Scholar]
- 48.McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. 2010;26:387–399. doi: 10.1016/j.cger.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Curr Rheumatol Rep. 2009;11:15–22. doi: 10.1007/s11926-009-0003-7. [DOI] [PubMed] [Google Scholar]
- 50.Roos EM, Juhl CB. Osteoarthritis 2012 year in review: rehabilitation and outcomes. Osteoarthritis Cartilage. 2012;20:1477–1483. doi: 10.1016/j.joca.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Kiviranta P, Lammentausta E, Toyras J, Kiviranta I, Jurvelin JS. Indentation diagnostics of cartilage degeneration. Osteoarthritis Cartilage. 2008;16:796–804. doi: 10.1016/j.joca.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Shapiro LM, Gold GE. MRI of weight bearing and movement. Osteoarthritis Cartilage. 2012;20:69–78. doi: 10.1016/j.joca.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutter E, Widmyer M, Utturkar G, Spritzer C, Garrett W, DeFrate L. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am J Sports Med. 2014 doi: 10.1177/0363546514559821. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ornetti P, Maillefert JF, Laroche D, Morisset C, Dougados M, Gossec L. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: a systematic review. Joint Bone Spine. 2010;77:421–425. doi: 10.1016/j.jbspin.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Kraus V. Preclinical Osteoarthritis. In: Hochberg M, Silman A, Smolen J, Weinblatt M, Weisman M, editors. Rheumatology. Philadelphia: Mosby Elsevier; 2014. pp. 1498–1547. [Google Scholar]
- 56.Zhang W, Doherty M, Peat G, Bierma-Zeinstra MA, Arden NK, Bresnihan B, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- 57.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henriksen K, Bohren KM, Bay-Jensen AC, Karsdal MA. Should biochemical markers of bone turnover be considered standard practice for safety pharmacology? Biomarkers. 2010;15:195–204. doi: 10.3109/13547500903434519. [DOI] [PubMed] [Google Scholar]
- 61.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63:820–834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7:4. doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karsdal MA, Krarup H, Sand JM, Christensen PB, Gerstoft J, Leeming DJ, et al. Review article: the efficacy of biomarkers in chronic fibroproliferative diseases - early diagnosis and prognosis, with liver fibrosis as an exemplar. Aliment Pharmacol Ther. 2014;40:233–249. doi: 10.1111/apt.12820. [DOI] [PubMed] [Google Scholar]
- 64.Michalopoulos GK. Advances in liver regeneration. Expert Rev Gastroenterol Hepatol. 2014:1–11. doi: 10.1586/17474124.2014.934358. [DOI] [PubMed] [Google Scholar]
- 65.Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 66.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 68.Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25:1–24. [PMC free article] [PubMed] [Google Scholar]
- 69.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 70.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63:264–268. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim DH, Schwartz CE. The genetics of pain: implications for evaluation and treatment of spinal disease. Spine J. 2010;10:827–840. doi: 10.1016/j.spinee.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 74.Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23:2239–2256. doi: 10.1007/s00198-012-1964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fardellone P. Predicting the fracture risk in 2008. Joint Bone Spine. 2008;75:661–664. doi: 10.1016/j.jbspin.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Kerkhof HJ, Bierma-Zeinstra SM, Arden NK, Metrustry S, Castano-Betancourt M, Hart DJ, et al. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203620. [DOI] [PubMed] [Google Scholar]
- 77.Smolen JS, Aletaha D. Forget personalised medicine and focus on abating disease activity. Ann Rheum Dis. 2013;72:3–6. doi: 10.1136/annrheumdis-2012-202361. [DOI] [PubMed] [Google Scholar]
- 78.Misko TP, Radabaugh MR, Highkin M, Abrams M, Friese O, Gallavan R, et al. Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthritis Cartilage. 2013;21:151–156. doi: 10.1016/j.joca.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Katz D, Ather A Preventive Medicine, Integrative Medicine & The Health of The Public. Commissioned for the IOM Summit on Integrative Medicine and the Health of the Public. Institute of Medicine; 2009. pp. 1–45. [Google Scholar]
- 80.El-Zayadi AR. Hepatic steatosis: a benign disease or a silent killer. World J Gastroenterol. 2008;14:4120–4126. doi: 10.3748/wjg.14.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]