Abstract
Background
Public health and clinical interventions for obesity in free-living adults may be diminished by individual compensation for the intervention. Approaches to predict weight outcomes do not account for all mechanisms of compensation, so they are not well suited to predict outcomes in free-living adults. Our objective was to quantify the range of compensation in energy intake or expenditure observed in human randomized controlled trials (RCTs).
Methods
We searched multiple databases (PubMed, CINAHL, SCOPUS, Cochrane, ProQuest, PsycInfo) up to August 1, 2012 for RCTs evaluating the effect dietary and/or physical activity interventions on body weight/composition. Inclusion Criteria: subjects per treatment arm ≥ 5; ≥1 week intervention; a reported outcome of body weight /body composition; the intervention was either a prescribed amount of over- or underfeeding and/or supervised or monitored physical activity was prescribed; ≥ 80% compliance; an objective method was used to verify compliance with the intervention (e.g., observation, electronic monitoring). Data were independently extracted and analyzed by multiple reviewers with consensus reached by discussion. We compared observed weight change to predicted weight change using two models that predict weight change accounting only for metabolic compensation.
Findings
Twenty-eight studies met inclusion criteria. Overfeeding studies indicate 96% less weight gain than expected if no compensation occurred. Dietary restriction and exercise studies may result in up to 12–44% and 55–64% less weight loss than expected, respectively, under an assumption of no behavioral compensation.
Interpretation
Compensation is substantial even in high-compliance conditions, resulting in far less weight change than would be expected. The simple algorithm we report allows for more realistic predictions of intervention effects in free-living populations by accounting for the significant compensation that occurs.
Keywords: obesity, weight loss, weight gain, meta-analysis, energy balance
Introduction
Obesity is a serious and prevalent public health concern (1). New public health and clinical interventions to reduce obesity are frequently advocated or implemented based on hypothetical estimates of an outcome that may have little empirical support (e.g., the 3500 kcal rule). For example, imagine an initiative from a large company that replaces its 250 kcal candy bars in its vending machines with 50 kcal protein bars in order to reduce energy intake (EI) from snacking among its employees. This initiative can be expected to produce (in those who consume at least 250 kcal per day from such snacks), on average, 5.7 kg of weight loss after one year (e.g., for a 35 year old man who is 183 cm tall and weighs 100 kg at baseline, body mass index = 30). This estimate is based on one of the mathematically validated prediction models (2) sometimes used to justify such interventions.(3) But is this estimate realistic?
Based on the evidence, this estimate is likely optimistic because current models for predicting weight change are not well-suited for use in free-living subjects. A common rule of thumb used for decades to predict weight change outcomes is that losing or gaining one pound of fat requires a deficit of 3,500 kcals of energy (4). This rule does not consider that human energy balance is a dynamic and adaptable system, or that lean and fat mass is lost during negative energy balance, and this leads to an underestimation of the change in EI or energy expenditure (EE) needed to produce weight change.(5–8) Recently, more sophisticated models have been developed to predict weight changes which consider the metabolic adaptations that occur during weight change.(9–12) To accurately predict weight change in free-living individuals, however, both 1) metabolic, and 2) behavioral compensatory mechanisms must be accounted for.
Specifically, we define the modes of possible compensation as follows:
Metabolic compensation: Compensation for an energy balance intervention through physiological changes in metabolism. For example, current mathematical models account for changes in resting metabolic rate, fluid balance, the thermic effect of food, and spontaneous physical activity resulting from an energy balance intervention.(11–13)
Behavioral compensation: Compensation for an energy balance intervention through behavior changes. For example, when a dietary or physical activity intervention attempts to create negative energy balance, an individual may respond by reducing voluntary EE and/or increasing EI if these avenues are not strictly controlled. Similarly, during an energy balance intervention of added energy, voluntary EE may increase and/or EI may decrease from other sources.
Others have shown that behavioral compensation occurs for physical activity interventions (14). Behavioral compensation may also occur for interventions that reduce caloric intake or add calorie-containing foods to the diet. (15, 16) Current prediction models are intended for use where interventions are implemented with high fidelity (i.e., intended intervention exposure was achieved) in isolation, and when metabolic compensation is the only route of compensation for the intervention possible. During interventions in free-living subjects, however, compensation can occur through metabolic compensation and through behavioral compensation. Behavioral compensation may diminish the effects of an intervention, making it important to quantify and account for it when predicting outcomes in free-living populations. It is imperative that more realistic models be used for predicting outcomes, for the reasons stated recently:
“…to establish a less controversial legacy for this important field, we should avoid past traps and be explicit about reasonable expectations. Implausible results that are “too good to be true” still threaten nutritional research on many fronts, including survey measurements, observational associations, treatment effects in randomized trials, and estimates of the impact on populations.”(17)
We therefore set out to build an empirically-based model to predict weight change outcomes in free-living subjects, and to quantify the extent to which observed weight change in free-living subjects differs from that predicted under the assumption of no behavioral compensation. The approach we took was to use systematic review techniques to collect study data and conduct meta-regression on studies meeting a priori inclusion criteria. These criteria guided identification of high fidelity interventions implemented in free-living adults. The subjects had some ability to behaviorally compensate for the intervention, yet the reported information about the intervention and compliance verification allowed for a high degree of confidence in treatment fidelity. For our main analysis, we compared the predictions from models which assume no active compensation (2, 18) to observed outcomes as an estimate of the effects of behavioral compensation.
Methods
Systematic Review of the Literature and Study Selection
Articles, abstracts and doctoral dissertations were retrieved using searches performed on the following electronic databases: PubMed, Cochrane Library, SCOPUS, PsycInfo, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Dissertation Abstracts. We searched PubMed without MeSH headings to identify publications for inclusion, using the following limits: date August 1, 2012 back to earliest records of human studies. Detailed search methods are provided on the PROSPERO registry website (Registry #CRD42013002912). No ethics committee approval was required since the data used are published summary statistics.
All studies were evaluated according to the following inclusion criteria: 1) the data were from adult human RCTs in free-living subjects, 2) the intervention was either a prescribed amount of over- or underfeeding given and reported (or could be converted) in kcal and/or supervised or monitored physical activity was prescribed and verified, 3) an objective verification method was used to verify the intervention at ≥ 80% (e.g., observation, electronic monitoring, provision of food with returned unused portions), 4) the study had a total sample size of at least 5 participants at enrollment, 5) the study protocol included an intervention period of at least 7 days, 6) the publication was available in the English language, and 7) the study was published and listed in the above databases on or before August 1, 2012.
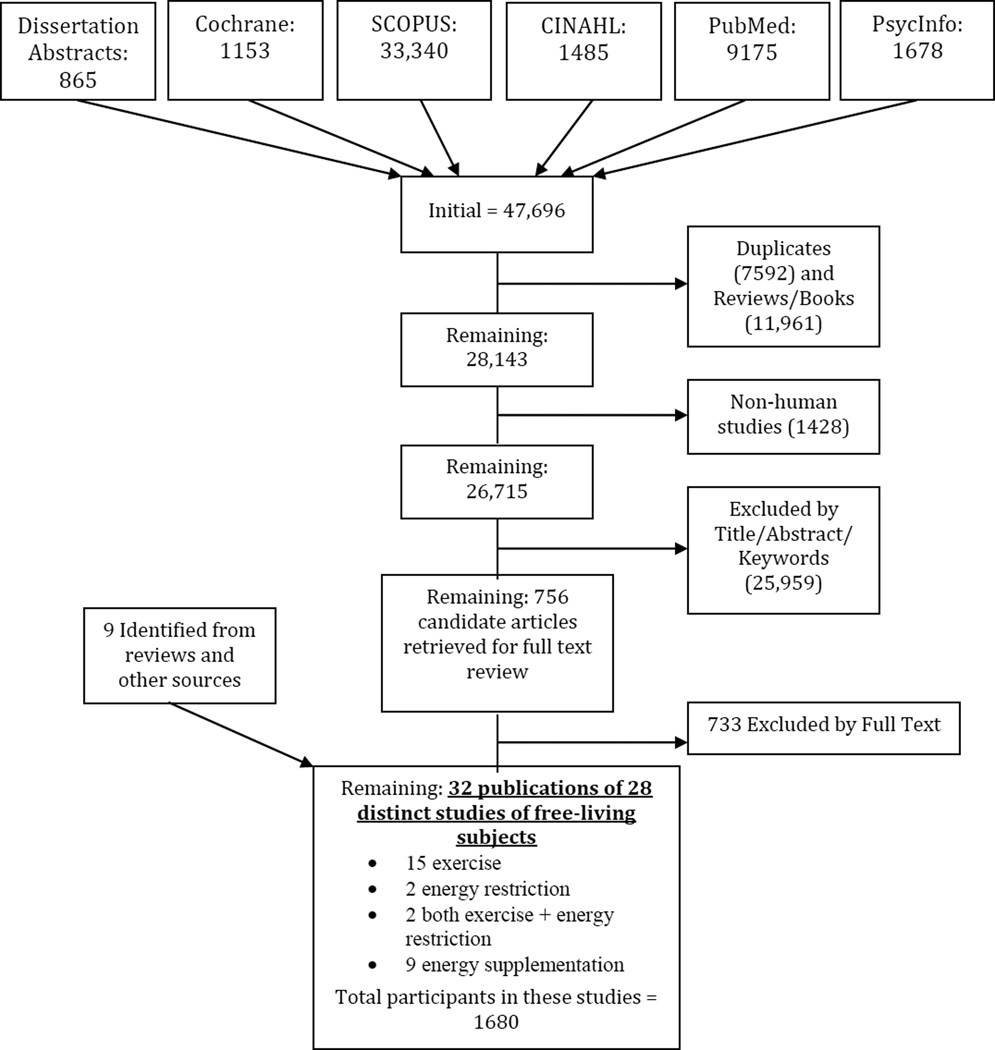
Our exclusion criteria are detailed in the online supplementary material. Briefly, we excluded studies on samples that were completely or predominantly made up of individuals younger than 18 years old or older than 60 years, or having any health conditions that may affect weight. The filtering process of the initial search results is detailed in Figure 1 and also described in more detail in the online supplement.
Figure 1.
PRISMA Diagram - Literature search and study selection process
Statistical Analysis
Quantifying the effect of behavioral compensation– comparison to metabolic compensation models
We entered sample demographic and intervention data into each of the metabolic compensation model calculators to most closely represent each intervention as described in the published papers to estimate weight changes that would occur if only metabolic compensation occurred. Since we included data that had samples of both men and women where separate baseline data and results were not reported (only combined summaries), we entered the data for both genders and mathematically adjusted the outputs for the relative proportions of men and women. For the NIDDK simulator (2), we assumed a baseline value (when not otherwise reported) of sedentary activity level (1.4 METs). The difference between the observed weight change for each study and the weight change predicted by these models is indicative of the degree of behavioral compensation that is observed for the interventions in free-living adults included in our review and meta-analysis.
All model data were analyzed with R routines (19) and descriptive summaries were generated with Microsoft® Excel version 2010. Further details of statistical approaches used for the predictive model building are on the online supplement. Risk of bias was assessed by two authors (EJD and KAK) independently and discrepancies were discussed until consensus was reached.
Role of Funding Source
The funding agency (International Life Science Institute – North America) had no role in the design, conduct, analysis, manuscript preparation or decision to publish the results of this study.
Results
Results of Publication Search
We retrieved citations dated back to 1935, but more than two thirds of the initial publications retrieved were published after 2001. The final dataset for building the predictive model consisted of 28 studies published between 1987 and 2012 including 15 exercise studies, 9 studies with added energy, 3 dietary restriction studies, and 2 studies that included both dietary restriction and exercise in the intervention (see Table 1 for a complete listing of included studies with select summary data and intervention descriptions). The primary reasons for exclusion after full text review were studies not being truly randomized or not having a control group, followed by reliance only on self-report for EI or physical activity without any objective verification of compliance. Studies were all published journal articles except for two dissertations.(20, 21) Eleven studies had samples that were either 100% men or 100% women. Three other studies reported results by gender separately if both males and females were included in the sample. Only six studies (21%) reported the racial makeup of the samples; therefore, this factor was excluded from further analysis. Mean ages of the samples ranged from 20.6 years to 60 years. Mean baseline body mass index (BMI) of the samples ranged from 22.6 to 35.1 kg/m2.
Table 1.
Master list and summary of included studies grouped by treatment type and sorted in ascending year of publication
| Reference(s) | Intervention | Sample Studied (mean age - yrs, pct female, baseline BMI kg/m2) |
Adjusted Daily Dose(s) (kcal: treatment - control) |
Study Duration (weeks) |
Intervention Notes | N Randomized, Completed, Analyzed |
Method of Missing Data Handling |
Overall Mean Compliance |
|---|---|---|---|---|---|---|---|---|
| Johnstone, AM, et al. 2008 (22) | Diet | 38, 0%, 35.1 | −167.2 | 4 | High protein, ketogenic diet | 20, 17, 17 | completers | 100 |
| Das SK, et al., 2009 (23) | Diet | 35, 76.3%, 27.6 | −285.6 | 26 | Caloric restriction | 46, 39, 39 | completers | 100 |
| Zachwieja JJ, et al., 2001 (24) | Diet and exercise | 24, 45.8%, 24.1 | −675 | 2 | Caloric restriction and daily treadmill exercise | 24, 24, 24 | no drops | 90 |
| Moreira EA, et al., 2011 (25) | Diet and exercise (separate treatments) | 49, 68, 30 | −556.0, −753.3 | 11 | 25% caloric restriction (controlled feeding) vs. aerobic exercise (individualized and supervised sessions 3×/week) | 36, 35, 36 | ITT | 99 |
| Leon AS, et al., 1996 (26) | Exercise | 32.6, 0%, 26 | −245.6 | 12 | Walking and stair climbing | 22, 16, 16 | completers | 86 |
| Van Etten LMLA, et al., 1997 (27) | Exercise | 33.7, 0%, 23.7 | −31.6 | 18 | Weight training | 26, 26, 26 | completers | 95 |
| Murphy MH, et al., 1998 (28) | Exercise | 44.4, 100%, 25.76 | −81.6, −84.5 | 10 | Long versus short bouts of walking | 47, 34, 34 | completers | 86.5 |
| Crandall KJ, 1999 (21) | Exercise | 51.75, 44, 30.8 | −76.7 | 12 | Recumbent cycle ergometer | 13, 13, 13 | no drops | 100 |
| Shaw I & Shaw BS, 2006 (29) | Exercise | 41, 92%, 32.6 | −13.7 | 8 | Resistance training | 28, 28, 28 | completers | 91.1 |
| Kirk EP, et al., 2007 (30) | Exercise | 20.6, 0%, 28.2 | −104.7 | 24 | High-intensity resistance training | 25, 19, 19 | completers | 96 |
| Whybrow S, et al., 2008 (31) | Exercise | 27.2, 50%, 23.6 | −455.6, −513.6, −907.1 | 2 | Progressive exercise on cycle ergometer or treadmill | 12, 12, 12 | no drops | 100 |
| Guadalupe-Grau A, et al., 2009 (32) | Exercise | 23.7, 65.2%, 23.03 | −51.7 | 9 | Strength training and plyometric jumps | 88, 72, 66 | completers | 85 |
| Alves JG, et al., 2009 (33) | Exercise | 38.2, 100%, 30 | −106.1 | 26 | Group exercises | 156, 146, 156 | ITT, BOCF | 96 |
| Turner JE, et al., 2010 (34) | Exercise | 54,0%, 28 | −187.3 | 24 | Structured exercise | 54, 41, 29 | completers | 94 |
| Bell GJ, et al., 2010 (35) | Exercise | 49, 100%, 34.7 | −399.0, −395.1 | 24 | Pedometer based walking program | 211, 128, 128 | completers | 84.77 |
| Vispute SS, et al., 2011 (36) | Exercise | 23.66, 41.7%, 24.6 | −41.9 | 6 | Abdominal exercises | 24, 24, 24 | no drops | 95.71 |
| Hornbuckle LM, et al., 2012 (37) | Exercise | 28.5, 0%, 25.42 | −57.7 | 12 | Resistance training | 44, 32, 44 | ITT | 96 |
| Heydari M, et al., 2012 (38) | Exercise | 37.7, 56.3%, 27.8 | −186.4 | 12 | High-intensity intermittent exercise | 46, 38, 38 | completers | 100 |
| Thompson AM, et al., 2008 (39) Church, T. S., et al., 2010 (40) | Exercise | 49.7, 72.8%, 31.8 | −174.8 | 16 | Supervised aerobic exercise | 162, 137, 162 | ITT | 91 |
| Addington EA, 1998 (20) | Feeding | 38.74, 63.8%, 32.09 | 2.9 (aspartame group), 142.9 (SSB group) | 4 | Artificially sweetened beverage (aspartame) versus Sugar Sweetened Beverage (SSB) | 150, 111, 111 | completers | 100 |
| Lammert, O., et al., 2000 (41) | Feeding | 22.4,0%, 22.61 | 191 | 3 | Overfeeding carbohydrate or fat | 20, 20, 20 | no drops | 100 |
| Martin A, et al., 2000 (42) | Feeding | 37.7, 56.3%, 27.8 | 597.1 | 2 | Low versus high calorie breakfast | 10, 10, 10 | no drops | 100 |
| SabateJ, et al., 2005 (43) | Feeding | 42.6, 45.2%, 23.7 | 219 | 26 | Walnuts | 90, 90, 90 | no drops | 95 |
| Whybrow S, et al., 2006 (44) | Feeding | 60, 26.7%, 27.7 | 122.8, 227.5 | 8 | Added fruits and vegetables | 90, 62, 62 | completers | 92.6 |
| Whybrow S, et al., 2007 (45) | Feeding | 35.05, 50%, 25.35 | 343.9, 687.9 | 2 | Added snacks | 100, 87, 72 | completers | 96 |
| Sheridan MJ, et al., 2007 (46) | Feeding | 24.9, 0%, 28.7 | 314.8 | 4 | Pistachio nuts | 15, 15, 15 | no drops | 99 |
| Casas-Agustench P, et al., 2011 (47) | Feeding | 54.4, 56.3%, 26.5 | 176.9 | 12 | Mixed nuts | 52, 50, 50 | completers | 94 |
| Maersk M, et al., 2012 (48) | Feeding | 28, 0%, 22.2 | 3.1, 365.2, 385.5 | 26 | 1 liter per day of diet soda, SSB or milk versus water | 60, 47, 47 | completers | 85 |
ITT = Intention to Treat analysis reported
BOCF = Baseline Observation Carried Forward
Building a Predictive Model
We expected to find enough studies to build a robust regression model, incorporating mean participant characteristics and evaluating any significant interactions. However, the relatively low number and sparsely distributed data prevented reliable estimates from our final model. Details of the model and its estimations can be found in the online supplement, Figure S1 and Table S2.
Comparison to Metabolic Compensation Models – Estimating Behavioral Compensation
To address our main research question (What is the effect of behavioral compensation that occurs in free-living subjects who receive an energy balance intervention on weight outcomes?),we generated output for each study using the NIDDK and Pennington weight change prediction calculators (2, 18) to estimate weight changes that would occur if only metabolic compensation occurred. The difference between the observed weight loss for each study and the weight change predicted by these models is indicative of behavioral compensation occurring during the intervention. The NIDDK and Pennington models are highly correlated (Pearson’s r = 0.98, p <.0001) in predicted weight change (Figure S2). In general, the Pennington calculator is slightly more conservative than the predictions made by the NIDDK calculator.
The overall degree of behavioral compensation estimated by the gap between the observed and metabolic compensation-only predicted values is illustrated in Figure S3, panels A & B. Both slopes being less than 1 (i.e., 0.344 and 0.399 for the NIDDK and Pennington Models, respectively) indicates that the observed weight change is less than predicted after accounting for metabolic compensation. This quantifies the degree of behavioral compensation that is occurring (i.e., the compensation that is in addition to the metabolic compensation, resulting in less weight change than expected).
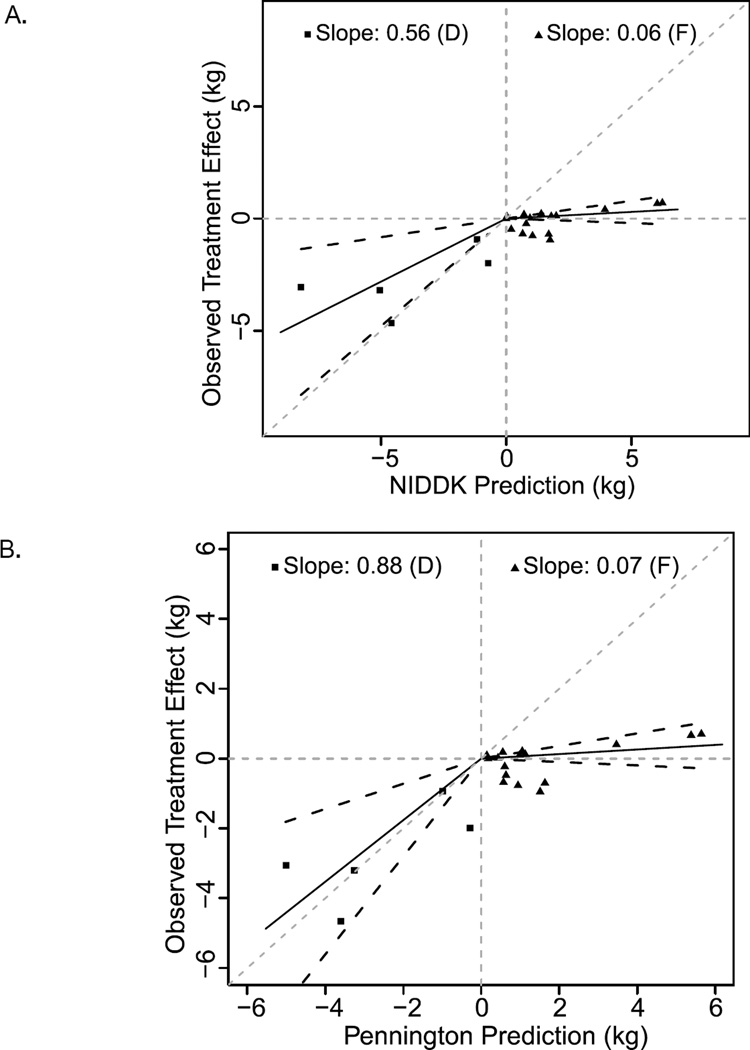
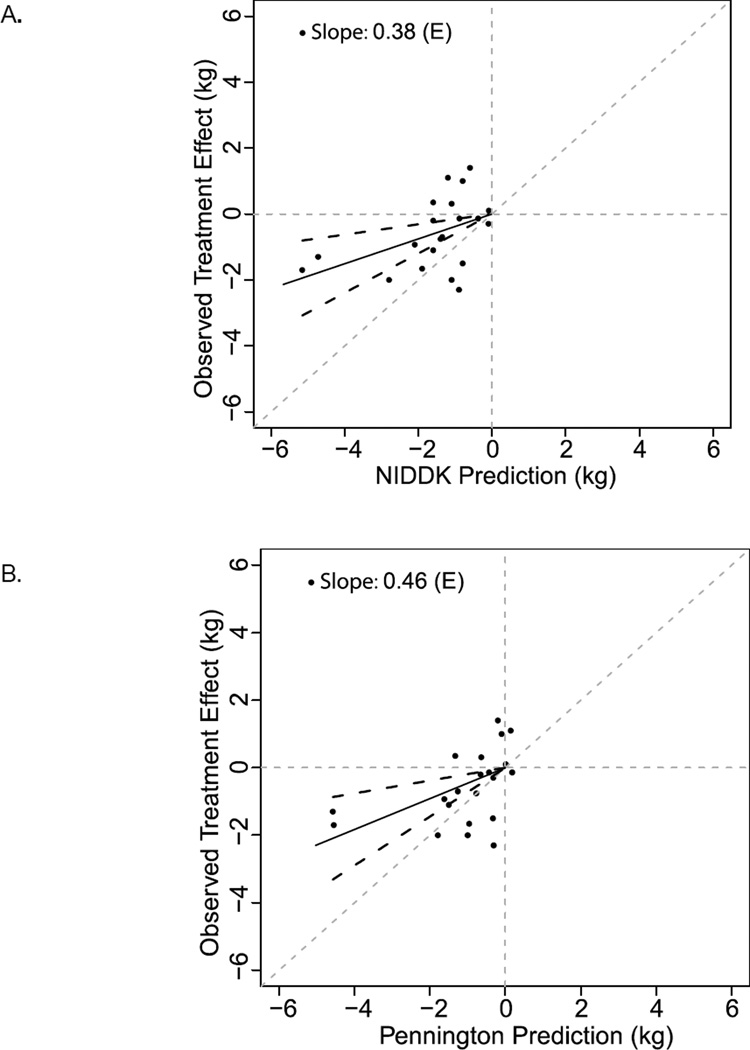
The degree of behavioral compensation appears to differ depending on intervention type. As shown in Figure S3, Panels A & B, all types of interventions demonstrated less weight change than either the Pennington or NIDDK calculators predicted. The plot of overfeeding trials has a slope (95% confidence interval) of 0.06 (−0.04, 0.16) and 0.07 (−0.05, 0.18), plotted against the NIDDK and Pennington calculators, respectively (Figure 2, Panels A & B). A slope of 1 would indicate that, on average, the interventions produced exactly as much weight change as expected from the mathematical models, which assume no behavioral compensation. As such, this suggests that behavioral compensation may result in as much as 96% less weight gain than predicted by metabolic calculators when adding energy to the diet. The slopes of the plots for dietary restriction and exercise studies are more similar to each other. Specifically, slopes (95% confidence interval) of 0.56 (0.17, 0.96) and 0.88 (0.36, 1.40) were plotted against the NIDDK and Pennington calculators, respectively, for dietary restriction studies (Figure 2). For exercise intervention studies, slopes (confidence interval) of 0.38 (0.16, 0.60) and 0.46 (0.19, 0.72) were plotted against the NIDDK and Pennington calculators, respectively (Figure 3). Thus, behavioral compensation may result in up to 12–44% less weight loss than predicted for dietary restriction studies and 55–64% less weight loss than predicted for exercise intervention studies.
Figure 2.
NIDDK and Pennington calculator predictions for caloric restriction (D, squares) and overfeeding (F, triangles) interventions. NIDDK (A) and Pennington (B) model predictions (x-axis) versus actual observed weight changes for all studies (y-axis) Each individual point represents a control vs. treatment comparison; the solid lines are lines of best fit for slope and black dashed lines are 95% confidence intervals. Gray dashed lines are axes and lines of identity. Overall, predictions are an overestimate of observed weight change.
Figure 3.
NIDDK and Pennington calculator predictions for exercise interventions (E). NIDDK (A) and Pennington (B) model predictions (x-axis) versus actual observed weight changes for all studies (y-axis). Each individual point represents a treatment vs. control comparison; the solid lines are lines of best fit for slope and black dashed lines are 95% confidence intervals. Gray dashed lines are axes and lines of identity. Overall, predictions are an overestimate of observed weight change.
Risk of Bias Assessment for Included Studies
See online supplement for risk of bias summary and detailed ratings figure (Figure S4) for each included study. The greatest proportions of study aspects with high risk of bias were judged to be lack of analysis for incomplete data (attrition bias – e.g., use of intention to treat analysis, ITT) and lack of attention placebo for control groups. Four studies reported results using ITT.
Discussion
We generated simple adjustment factors to predict weight change resulting from energy balance interventions in free-living adult populations, with the ability to compensate both behaviorally and metabolically, using 73 treatment versus control arm comparisons from 28 studies. One of the notable findings was the small number of studies meeting our inclusion criteria (i.e., where compliance was objectively measured) making it difficult to study the role of behavioral compensation in a free-living context beyond a very basic level. Although our estimates are the only ones for this purpose to-date based on the currently available literature, this highlights a gap in the literature of studies designed to determine the impact of energy balance perturbations in humans in the context of a full range of compensation that prevents a more precise estimate. Since these studies are crucial to understanding the effect of public health interventions, their limited quantity underscores a need for future research in this area.
Perhaps the most robust finding from our study most relevant to public health is that currently available predictions consistently overestimate weight change, which is evidence of significantly diminished weight change resulting from behavioral compensation. This is in spite of some instances where explicit instructions were given to make no other changes in routine habits, a form of compliance that is less commonly tracked or verified. In particular, the treatment effect of added calories was only, on average, ~5% of the weight gain predicted from models assuming no behavioral compensation. Several included studies reported a mean weight loss effect from added energy. This indicates that even if a new food is introduced to the diet, for example adding a daily snack or beverage, EI and/or EE can be adjusted reasonably well, resulting in very little weight gain relative to how much would be expected if this behavioral compensation did not occur. Behavioral compensation for negative energy balance interventions such as exercise or dietary restriction is also evident from our analysis, and results in 37–45%, and 56–88% of the weight loss predicted from metabolic-only compensation models, respectively. In our initial example of reducing EI via snacks by 200 kcals per day for the hypothetical man, the adjusted estimate of weight change after one year would be closer to 3.2 kg. This is lower than the 5.7 kg estimate given by the body weight simulator that predicts metabolic compensation only.
Therefore, our results suggest that current public health interventions or clinical interventions that alter one aspect of energy balance, without holding other aspects constant, may result in more modest weight changes than predicted or desired. A similar approach has been reported in pediatric studies (3), but it did not attempt to account for both behavioral and metabolic compensation components. It is important to take all modes of compensation into consideration when planning an intervention with targeted amounts of weight change, and when anticipating its outcomes. It is likely that increased doses of energy perturbations are required. Increased control over compliance and compensation are necessary to achieve target outcomes. Estimates of what is required to achieve a specific weight change may be made more accurate for the purposes of public health recommendations if the present estimations are considered.
Our results suggest that there might be a differential effect of treatment type on the degree of behavioral compensation. However, an aspect of our dataset needs to be considered in interpreting this result. Dietary restriction interventions are associated with greater treatment effects, and less behavioral compensation, than either exercise or overfeeding interventions. However, this finding may be because the dietary restriction interventions included in our analysis only allowed for behavioral compensation through EE changes, whereas all exercise and overfeeding interventions allowed for behavioral compensation through both dietary intake and EE changes.
Our approach has strengths and limitations. First, our inclusion criteria were rigorous. All included studies have at least 80% compliance with the prescribed intervention, with compliance verified objectively (no reliance solely on self-report). In addition, the dose was corrected in our calculations for the level of compliance reported in the study. Further, included studies were RCTs, and our outcome for generating the predictive model and for comparing to metabolic compensation models was the control group adjusted weight change. Therefore, our models are built to assess true treatment effect, and are corrected for any weight change due to factors such as regression to the mean, maturation, historical factors, behaviors that result from simply participating in a study, rather than from the treatment itself.
Several limitations should also be considered when interpreting our analysis. Weight was not always the primary outcome in studies that met our inclusion criteria. This is particularly true for those with added EI in the form of nuts. Differences in stated outcomes of interest, time with researchers and other factors may affect weight outcomes for individual studies. In addition, body composition may be an important outcome that we were not able to adequately analyze because of the limited number of studies including body composition measurements such as changes in fat mass and fat-free mass. Because of our rigorous inclusion criteria, our dataset is small (28 studies). The types of studies we selected are necessary for making definitive conclusions about the impact of perturbations in one aspect of energy balance on body weight. Studies also tended to be shorter in duration, thus it is difficult to make conclusions about long-term effects. This is a large gap in the literature, and a more systematic approach to large, well-controlled studies to answer these questions is warranted. Additionally, 16 of the 28 studies reported data only for those participants who completed the intervention period, and across all studies there was a 17.8% dropout rate (Table 1), which may have biased our estimates of weight change towards overestimation. We used the intention to treat data when reported (four studies). Eight studies reported no dropouts.
Future research is needed to understand potential differences in compensation between dietary interventions (added or reduced energy), different food forms, macronutrient compositions. Also, certain factors should be considered as potential confounders when quantifying the compensatory response to a specific intervention. For example, bioavailability of energy in food, efficiencies in physical activity and food utilization, seasonal effects, and durations of interventions may all influence both the metabolic and behavioral compensatory response to an intervention. It is also unclear if compensation would remain constant over time. Moreover, evaluating the influence of participant characteristics related to eating behavior (cognitive restraint, dis-inhibition and hunger) and compensation during interventions is needed as this may hold promise for optimizing treatment effectiveness.
To conclude, we have presented the first empirically-based, quantitative estimation for the range of behavioral compensation that may be observed for energy balance interventions. This information may assist in the estimation of weight outcomes of clinical health interventions. It may also inform public health projections for obesity interventions or public health initiatives.
Supplementary Material
Acknowledgments
Acknowledgements/Disclosures:
This project was sponsored by the International Life Sciences Institute – North America (Dhurandhar & Kaiser, co-PIs). The authors wish to thank the following experts for their helpful comments on earlier versions of this manuscript: Steve Blair, Steve Heymsfield, Rick Mattes, Robert Matthews, Diana Thomas and Kevin Fontaine.
D. B. Allison has received consulting fees and his university has received gifts, grants, and donations from multiple non-profit and for-profit organizations with interests in obesity including publishers, litigators, and food and pharmaceutical companies. K.A. Kaiser has received a speaker honorarium from Coca-Cola Iberia.
Funding Source: International Life Science Institute - North America
Footnotes
Conflict of Interest: No other authors have any information to disclose pertinent to conflict of interest.
Authors' contributions: EJD, KAK and DBA conceived the study and developed the design and selection criteria. KAK performed the literature searches. KAK and EJD reviewed the literature, selected studies, extracted data, evaluated risk of bias and wrote significant portions of the manuscript. ASA assisted with literature selection, data extraction and summary calculations. JAD and KDK performed the statistical analysis and wrote some portions of the manuscript. DBA directed the statistical analysis and wrote some portions of the manuscript.
Registry Information: PROSPERO (http://www.crd.york.ac.uk/prospero/search.asp) CRD42013002912
Supplementary Materials: Supplementary information is available at International Journal of Obesity’s Website.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. Epub 2012/01/19. [DOI] [PubMed] [Google Scholar]
- 2.NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [accessed 09-28-2013]. Body weight simulator. http://bwsimulator.niddk.nih.gov/ Available from: http://bwsimulator.niddk.nih.gov/. [Google Scholar]
- 3.Claire Wang Y, Hsiao A, Tracy Orleans C, Gortmaker SL. The Caloric Calculator: Average Caloric Impact of Childhood Obesity Interventions. American Journal of Preventive Medicine. 2013;45(2):e3–e13. doi: 10.1016/j.amepre.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Wishnofsky M. Caloric equivalents of gained or lost weight. The American Journal of Clinical Nutrition. 1958;6(5):542–546. doi: 10.1093/ajcn/6.5.542. [DOI] [PubMed] [Google Scholar]
- 5.Hall KD. What is the required energy deficit per unit weight loss? Int J Obes (Lond) 2008;32(3):573–576. doi: 10.1038/sj.ijo.0803720. Epub 2007/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall KD, Chow CC. Why is the 3500 kcal per pound weight loss rule wrong? Int J Obes (Lond) 2013;37(12):1614. doi: 10.1038/ijo.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DM, Martin CK, Lettieri S, Bredlau C, Kaiser K, Church T, et al. Response to 'Why is the 3500 kcal per pound weight loss rule wrong?'. Int J Obes (Lond) 2013;37(12):1614–1615. doi: 10.1038/ijo.2013.113. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DM, Martin CK, Lettieri S, Bredlau C, Kaiser K, Church T, et al. Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. Int J Obes (Lond) 2013;37(12):1611–1613. doi: 10.1038/ijo.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KD, Butte BA, Swinburn BA, Chow CC. Dynamics of childhood growth and obesity: development and validation of a quantitative mathematical model. Lancet Diabetes Endocrinol. 2013;1:97–105. doi: 10.1016/s2213-8587(13)70051-2. Epub July 30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. The American journal of clinical nutrition. 2011;94(1):66–74. doi: 10.3945/ajcn.111.014399. Epub 2011/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas DM, Ciesla A, Levine JA, Stevens JG, Martin CK. A mathematical model of weight change with adaptation. Mathematical biosciences and engineering : MBE. 2009;6(4):873–887. doi: 10.3934/mbe.2009.6.873. Epub 2009/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas DM, Schoeller DA, Redman LA, Martin CK, Levine JA, Heymsfield SB. A computational model to determine energy intake during weight loss. The American journal of clinical nutrition. 2010;92(6):1326–1331. doi: 10.3945/ajcn.2010.29687. Epub 2010/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. American journal of physiology Endocrinology and metabolism. 2010;298(3):E449–E466. doi: 10.1152/ajpendo.00559.2009. Epub 2009/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(10):835–847. doi: 10.1111/j.1467-789X.2012.01012.x. Epub 2012/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. Epub 2008/01/17. [DOI] [PubMed] [Google Scholar]
- 16.Alper CM, Mattes RD. Effects of chronic peanut consumption on energy balance and hedonics. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(8):1129–1137. doi: 10.1038/sj.ijo.0802050. Epub 2002/07/18. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JPA. Implausible results in human nutrition research. BMJ. 2013;347 doi: 10.1136/bmj.f6698. [DOI] [PubMed] [Google Scholar]
- 18.Multisubject weight change predictor. http://www.pbrc.edu/research-and-faculty/calculators/mswcp/ Pennington Biomedical Research Center; [accessed 09-28-2013]. Available from: http://www.pbrc.edu/research-and-faculty/calculators/mswcp/. [Google Scholar]
- 19.Team RDC, editor. Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 20.Addington E. Effects on food appetites and mood in young adults (Doctoral dissertation) Manhattan, Kansas: Kansas State University; 1998. Aspartame-or sugar-sweetened beverages. [Google Scholar]
- 21.Crandall KJ. Doctoral dissertation. Colorado: University of Northern Colorado; 1999. The effects of exercise intensity on energy-derived macronutrient intake, caloric intake, body composition, and body weight in the overweight. [Google Scholar]
- 22.Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. The American Journal of Clinical Nutrition. 2008;87(1):44–55. doi: 10.1093/ajcn/87.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Saltzman E, Gilhooly CH, Delany JP, Golden JK, Pittas AG, et al. Low or moderate dietary energy restriction for long-term weight loss: What works best. Obesity. 2009;17(11):2019–2024. doi: 10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachwieja JJ, Ezell DM, Cline AD, Ricketts JC, Vicknair PC, Schorle SM, et al. Short-term dietary energy restriction reduces lean body mass but not performance in physically active men and women. International journal of sports medicine. 2001;(4):310–316. doi: 10.1055/s-2001-13822. [DOI] [PubMed] [Google Scholar]
- 25.Moreira EA, Most M, Howard J, Ravussin E. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2011;26(3):309–315. doi: 10.1177/0884533611405992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon AS, Casal D, Jacobs D., Jr Effects of 2,000 kcal per week of walking and stair climbing on physical fitness and risk factors for coronary heart disease. Journal of Cardiopulmonary Rehabilitation. 1996;16(3):183–192. doi: 10.1097/00008483-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Van Etten LMLA, Westerterp KR, Verstappen FTJ, Boon BJB, Saris WHM. Effect of an 18-wk weight-training program on energy expenditure and physical activity. Journal of Applied Physiology. 1997;82(1):298–304. doi: 10.1152/jappl.1997.82.1.298. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MH, Hardman AE. Training effects of short and long bouts of brisk walking in sedentary women. Medicine and Science in Sports and Exercise. 1998;30(1):152–157. doi: 10.1097/00005768-199801000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Shaw I, Shaw BS. Consequence of resistance training on body composition and coronary artery disease risk. Cardiovascular Journal of South Africa. 2006;17(3):111–116. [PubMed] [Google Scholar]
- 30.Kirk EP, Washburn RA, Bailey BW, LeCheminant JD, Donnelly JE. Six months of supervised high-intensity low-volume resistance training improves strength independent of changes in muscle mass in young overweight men. Journal of strength and conditioning research / National Strength & Conditioning Association. 2007;(1):151–156. doi: 10.1519/00124278-200702000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Whybrow S, Hughes DA, Ritz P, Johnstone AM, Horgan GW, King N, et al. The effect of an incremental increase in exercise on appetite, eating behaviour and energy balance in lean men and women feeding. British Journal of Nutrition. 2008;100(5):1109–1115. doi: 10.1017/S0007114508968240. [DOI] [PubMed] [Google Scholar]
- 32.Guadalupe-Grau A, Perez-Gomez J, Olmedillas H, Chavarren J, Dorado C, Santana A, et al. Strength training combined with plyometric jumps in adults: Sex differences in fat-bone axis adaptations. Journal of Applied Physiology. 2009;106(4):1100–1111. doi: 10.1152/japplphysiol.91469.2008. [DOI] [PubMed] [Google Scholar]
- 33.Alves JG, Gale CR, Mutrie N, Correia JB, Batty GD. A 6-month exercise intervention among inactive and overweight Favela-residing women in Brazil: The caranguejo exercise trial. American Journal of Public Health. 2009;99(1):76–80. doi: 10.2105/AJPH.2007.124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner JE, Markovitch D, Betts JA, Thompson D. Nonprescribed physical activity energy expenditure is maintained with structured exercise and implicates a compensatory increase in energy intake. The American journal of clinical nutrition. 2010;(5):1009–1016. doi: 10.3945/ajcn.2010.29471. [DOI] [PubMed] [Google Scholar]
- 35.Bell GJ, Harber V, Murray T, Courneya KS, Rodgers W. A comparison of fitness training to a pedometer-based walking program matched for total energy cost. Journal of physical activity & health. 2010;7(2):203–213. doi: 10.1123/jpah.7.2.203. [DOI] [PubMed] [Google Scholar]
- 36.Vispute SS, Smith JD, Lecheminant JD, Hurley KS. The effect of abdominal exercise on abdominal fat. Journal of Strength and Conditioning Research. 2011;25(9):2559–2564. doi: 10.1519/JSC.0b013e3181fb4a46. [DOI] [PubMed] [Google Scholar]
- 37.Hornbuckle LM, Liu PY, Ilich JZ, Kim JS, Arjmandi BH, Panton LB. Effects of resistance training and walking on cardiovascular disease risk in African-American Women. Medicine and Science in Sports and Exercise. 2012;44(3):525–533. doi: 10.1249/MSS.0b013e31822e5a12. [DOI] [PubMed] [Google Scholar]
- 38.Heydari M, Freund J, Boutcher SH. The effect of high-intensity intermittent exercise on body composition of overweight young males. Journal of Obesity. 2012;2012:480467. doi: 10.1155/2012/480467. Epub 2012/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson AM, Mikus CR, Rodarte RQ, Distefano B, Priest EL, Sinclair E, et al. Inflammation and exercise (INFLAME): Study rationale, design, and methods. Contemporary Clinical Trials. 2008;29(3):418–427. doi: 10.1016/j.cct.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Church TS, Earnest CP, Thompson AM, Priest EL, Rodarte RQ, Saunders T, et al. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42(4):708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammert O, Grunnet N, Faber P, Bjornsbo KS, Dich J, Larsen LO, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. British Journal of Nutrition. 2000;84(2):233–245. [PubMed] [Google Scholar]
- 42.Martin A, Normand S, Sothier M, Peyrat J, Louche-Pelissier C, Laville M. Is advice for breakfast consumption justified? Results from a short-term dietary and metabolic experiment in young healthy men. Br J Nutr. 2000;84:337–344. doi: 10.1017/s0007114500001616. [DOI] [PubMed] [Google Scholar]
- 43.Sabate J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr. 2005;94(5):859–864. doi: 10.1079/bjn20051567. Epub 2005/11/10. [DOI] [PubMed] [Google Scholar]
- 44.Whybrow S, Harrison CL, Mayer C, James Stubbs R. Effects of added fruits and vegetables on dietary intakes and body weight in Scottish adults. Br J Nutr. 2006;95(3):496–503. doi: 10.1079/bjn20051489. Epub 2006/03/04. [DOI] [PubMed] [Google Scholar]
- 45.Whybrow S, Mayer C, Kirk TR, Mazlan N, Stubbs RJ. Effects of two weeks' mandatory snack consumption on energy intake and energy balance. Obesity. 2007;15(3):673–685. doi: 10.1038/oby.2007.567. [DOI] [PubMed] [Google Scholar]
- 46.Sheridan MJ, Cooper JN, Erario M, Cheifetz CE. Pistachio nut consumption and serum lipid levels. Journal of the American College of Nutrition. 2007;26(2):141–148. doi: 10.1080/07315724.2007.10719595. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 47.Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2011;21(2):126–135. doi: 10.1016/j.numecd.2009.08.005. Epub 2009/12/25. [DOI] [PubMed] [Google Scholar]
- 48.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. The American journal of clinical nutrition. 2012;95(2):283–289. doi: 10.3945/ajcn.111.022533. Epub 2011/12/30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.