Abstract
Background: Little evidence exists on change in diet quality and weight change.
Objectives: We examined the association between change of diet quality indexes and concurrent weight change over 20 y.
Methods: In this analysis we followed 50,603 women in the Nurses’ Health Study (NHS), 22,973 men in the Health Professionals Follow-Up Study (HPFS) between 1986 and 2006, and 72,495 younger women from the Nurses’ Health Study II (NHS II) between 1989 and 2007. Diet was measured every 4 y. We computed the Alternate Mediterranean Diet, the Alternate Health Eating Index-2010 (AHEI-2010), and the Dietary Approaches to Stop Hypertension adherence scores for each participant. All scores emphasize fruits and vegetables, whole grains, and nuts, but they differ in score range and components such as dairy, sodium, and sweetened beverages. Regression models were used to examine 4-y changes in these scores and weight change within the same period, adjusting for lifestyle factors.
Results: Mean age at baseline was 49.4 y for NHS, 48.0 y for HPFS, and 36.3 y for NHS II. Baseline BMI (in kg/m2) was similar (23.7 for NHS, 24.7 for HPFS, and 23.0 for NHS II). We observed significantly less weight gain over 4-y periods with each SD increase of each diet quality score in both men and women. Results were significantly stronger in the younger cohort (NHS II) than in the older cohorts (e.g., −0.67 kg less weight gain in NHS II vs. −0.39 kg in NHS for each SD increase in AHEI-2010; P-heterogeneity: <0.001). Improvement of any of the diet scores benefited overweight (−0.27 to −1.08 kg less weight gain for each SD increase in score) more than normal-weight individuals (−0.10 to −0.40 kg; P-interaction: <0.001).
Conclusion: Improvement of diet quality is associated with less weight gain, especially in younger women or overweight individuals.
Keywords: weight change, overweight, nutrition, diet quality, food
Introduction
Excessive weight gain in adulthood is a risk factor for major chronic diseases such as diabetes and cardiovascular disease (1, 2). Weight gain during adulthood is often insidious, and individuals may not notice until substantial weight gain has occurred. Effective nonsurgical interventions for long-term weight loss and long-term weight maintenance are sparse, highlighting the importance of preventing weight gain throughout adulthood in both controlling the obesity epidemic and preventing chronic diseases.
Certain foods and food groups may be helpful for weight management. In particular, higher fiber foods or foods with lower glycemic effect have a greater ability to sustain satiety and may reduce overall food consumption (3). However, studies of specific food groups and weight change have shown mixed results. A meta-analysis of whole-grain intake showed no association with total, wheat, or oat, although a small weight reduction with high consumption of brown rice was noted (4). Short-term intervention trials with dairy suggest a modest reduction of body fat mass with increased dairy consumption (5). Fruit and vegetable intake had a weak inverse association with weight change only among individuals who quit smoking during follow-up (6). Diet composition may also influence the composition of the gut microbiome, which may in turn influence intake regulation and the availability of energy (7, 8).
Besides focusing on individual foods or nutrients, another approach to examining the relation between food and weight gain is to study overall eating patterns. This enables observation of the combined effects of consumption of multiple food groups on weight change. Many dietary recommendations emphasize increasing consumption of plant-based foods, such as fruits, vegetables, and whole grains. Adherence to these recommendations was operationalized into various dietary quality scores. In this regard, a number of studies have shown a favorable association between body weight and risk of obesity with adherence to indexes that reflect the Mediterranean-style diets (9–11) and healthy eating guidelines from the United States (12, 13) and France (14). Although these studies evaluate the association between diet quality at a single time point with subsequent weight gain, they do not provide insight into the potential benefit of improving one’s diet, especially among persons who already are overweight. We have previously observed decreases in long-term weight gain with increased consumption of water, vegetables, fruits, nuts, and yogurt over the same period of time (15, 16).
In this study, we examined 4-y changes in diet quality scores and weight change in the same 4-y period in middle-aged men and women with a follow-up of ≤20 y. The specific diet quality scores included were the Alternate Mediterranean Diet (aMed)12 the Alternate Healthy Eating Index-2010 (AHEI-2010), and the Dietary Approaches to Stop Hypertension (DASH) scores. Each of these scores reflects healthy eating guidelines and was previously associated with a lower risk of major chronic diseases (17–19).
Methods
Population.
Participants in this analysis were US men and women from the ongoing Nurses’ Health Study (NHS), Health Professionals Follow-Up Study (HPFS), and the Nurses’ Health Study II (NHS II). The NHS cohort began in 1976 with 121,000 female nurses aged 35–55 y (20), the HPFS cohort began in 1986 with 51,529 male health professions aged 40–75 y (21), and the NHS II began in 1989 with 116,671 female nurses aged 25–42 y. Every 2 y, a questionnaire was sent to each participant to assess lifestyle, health outcomes, and medication use. Every 4 y, a validated FFQ was sent to assess usual dietary intake (22).
In this analysis, follow-up began for NHS and HPFS participants in 1986 and NHS II participants in 1991. These dates were chosen because detailed diet information became available. Individuals with cardiovascular disease, diabetes, cancer, pulmonary disease, systemic lupus erythematosus, and ulcerative colitis were excluded at the beginning of follow-up. Individuals were also excluded if their FFQ reported implausible energy intake (>3500 kcal in women, >4000 kcal in men or <500 kcal in women, <900 kcal in men). If women reported a pregnancy in any biennial questionnaire, data from that 4-y period were not included in the analysis because our analysis examined changes in diet and weight over 4-y periods. We also censored individuals at 65 y of age because changes in body weight may reflect muscle loss due to aging. Individuals with BMI (in kg/m2) > 30 at the start of follow-up were also excluded because our purpose was to examine the potential of these scores for obesity prevention instead of treatment. After exclusions, 50,603 women from the NHS, 22,973 men from the HPFS, and 72,495 women from the NHS II were included in this analysis. This study was approved by the institutional review board at the Brigham and Women’s Hospital.
Diet assessment and diet quality score computation.
Each FFQ contained ∼135 items, and each item had 9 frequency choices, ranging from <1 time/mo to ≥6 times/d. A standard portion size was also provided. Diet quality scores for each FFQ year were computed for each individual.
The AHEI-2010 included foods and nutrients that were shown to lower the risk of major chronic disease (17). It awards points for higher consumption of vegetables (excluding potatoes), whole fruits, whole grains, nuts and legumes, long-chain n–3 FAs, n–6 polyunsaturated fat and for lower consumption of sugar-sweetened beverages and fruit juices, red/processed meat, sodium, trans fat, and moderate alcohol consumption (5–10 g/d for women and 15–25 g/d for men). Each of the components has a range of 0–10 points, with a maximum overall score of 110 points.
The aMED score reflects food and nutrients common in traditional Mediterranean diets and awards 1 point for an intake greater than the cohort specific median for vegetables, legumes, fruits, nuts, whole grains, fish, and monounsaturated:saturated fat ratio and 1 point if intake was less than the cohort median for red and processed meat, and for alcohol intake between 5 and 15 g/d for women and 10–25 g/d for men (19). The possible range for the aMED score was 0–0 points.
The DASH score was developed on the basis of foods that were either emphasized or discouraged in the DASH trial (originally designed for blood pressure reduction) (18). It awards points for high intake of fruit, vegetables, nuts and legumes, low-fat dairy products, and whole grains and low intake of red/processed meats, sweets, and sodium. For healthy food groups or nutrients, participants were awarded 1 point if they were in the lowest quintile, 2 points if they were in the next intake quintile, and those in the highest quintile were assigned 5 points. Scoring was reversed for unhealthy food groups or nutrients. The score range is 8–40 points.
We had previously constructed an empirically derived food quality change score (FQCS) that strongly correlated with weight changes which is based on 4-y change in 17 foods such as red and processed meats, potatoes, refined grains, whole grains, fruits and vegetables, and low-fat dairy (15). Four-year change in each food group was classified into quintiles. Rankings for unhealthy food groups were reversed, and all quintile rankings were summed to an overall score with possible score range from 17 to 85. A summary of the food groups for each of the diet quality scores is given (Supplemental Table 1).
Body weight assessment.
Body weight was self-reported in each biennial questionnaire. Height was assessed in the first questionnaire for each cohort. A prior validation study in NHS showed a correlation coefficient of 0.96 between self-reported and technician-measured weight (23).
Assessment of lifestyle variables.
Smoking status and leisure-time physical activity were assessed in each cohort every 2 y. In each biennial questionnaire, physical activity was assessed by ≤10 questions of common leisure-time activities and converted to metabolic equivalent hours (METs) per week (24). Television watching was assessed in the NHS in 1992 and 2004; in the HPFS every 2 y, beginning in 1998; and in the NHS II in 1991, 1997, 2001, and 2005. Sleep duration was assessed in the NHS in 1986, 2000, and 2002; in the HPFS in 1987 and 2000; and in the NHS II in 2001.
Statistical analysis.
In this analysis, participants were followed from 1986 (baseline) to 2006 in the NHS and HPFS and from 1991 (baseline) to 2007 in the NHS II. Spearman correlation coefficients among diet quality scores were calculated. In this analysis, the exposure was 4-y changes in diet quality indexes, and the outcome was weight change over the same 4-y period. The relation between 4-y changes in diet quality and 4-y changes in weight was examined with multivariate linear regression (proc genmod in SAS; SAS Institute). A total of 5 4-y periods were included for NHS and HPFS, and 4 4-y time periods were included for NHS II. We applied an unstructured correlation matrix in the multiple regression procedure to account for within-person repeated measures of diet, weight, and lifestyle variables. We censored participants when they reached 65 y of age or 6 y before they reported a diagnosis of cancer, renal, liver, or pulmonary diseases. Missing data from categorical variables were assigned a missing indicator, and continuous variables were carried forward. Models were adjusted for the following potential confounders: age (continuous), BMI at the beginning of each 4-y period (continuous), change in smoking status in each 4-y period (stayed never smoker, stayed former smoker, stayed current smoker, change from past to current smoker, change from never smoker to current smoker), television watching (5 categories in NHS and a continuous variable in HPFS and NHS II), baseline and change in physical activity (quintiles of METs per week), baseline hours of sleeping (4 categories), and change in alcohol intake (continuous) for models with DASH because alcohol is not included in the DASH. In addition in women, we adjusted for parity (4 categories), menopausal status, and postmenopausal hormone use (4 categories). Weight change from all 3 cohorts was pooled to obtain a summary estimate by using inverse-variance weighted, random effects meta-analysis. This method allowed for heterogeneity of the cohort-specific effect estimate (25). Continuous variables with missing values were carried forward from the previous assessment, and an indicator variable was created for missing categorical variables.
We first examined the amount of weight change per 1 SD increase in each diet quality index in 4-y periods. Then, we ranked the amount of score change in each 4-y period and categorized into quintiles. Four-year weight change across quintiles of each diet quality index was then examined, using the third quintile (no change in diet quality index) as the reference group. To assess which diet quality score has the strongest association with weight change, we modeled 1 SD increase in scores pairwise in the same regression model and tested for the difference in weight change between 2 diet quality scores by the Wald chi-square test. We also compared each of the 3 diet quality scores with FQCS for weight change the same way.
Because physical activity is also associated with weight change, we formed a joint classification of physical activity (increasing METs, decreasing METs, or no change) with quintiles of change in AHEI-2010 score to model the combined relation of change in diet and change in physical activity with weight change. No change in diet and physical activity was the reference group. Finally, we conducted an analysis stratified by BMI (<25 vs. ≥25). All analyses were conducted with SAS version 9.2 (SAS Institute).
Results
Mean age ± SD at baseline was 49.4 ± 2.4 y in the NHS, 48.0 ± 2.4 y in the HPFS, and 36.3 ± 4.1 y in the NHS II (Table 1). Baseline mean BMI was 23.7 ± 1.4 in the NHS, 24.7 ± 1.1 in the HPFS, and 23.0 ± 2.5 in the NHS II. Mean weight increased in all 3 cohorts over time, with a mean of 0.5 ± 4.5 kg for each 4-y period in NHS to 2.2 ± 5.5 kg in NHS II. Over the course of the follow-up period (≤20 y in NHS and HPFS, and 16 y in NHS II), mean weight change was a gain of 5.4 ± 8.5 kg in NHS, 4.0 ± 7.2 kg in HPFS, and 8.5 ± 9.2 kg in NHS II. Physical activity was lowest in older women (NHS) and higher in the HPFS and in younger women (NHS II); however, mean hours of sleep (7 h) and diet quality indexes were similar across cohorts. Members of the 2 older cohorts (NHS and HPFS) modestly increased their fruit, vegetable, and whole-grain intake during follow-up, but little change was observed in younger women (NHS II). The 3 diet quality scores were moderately and significantly correlated with each other (Spearman correlation coefficients ranged from 0.38 to 0.48; all P < 0.001) (Supplemental Table 2).
TABLE 1.
Dietary and lifestyle characteristics of the men and women in the 3 cohorts1
| NHS (n = 50,603) |
HPFS (n = 22,973) |
NHS II (n = 72,495) |
||||
| Characteristic | Baseline (1986) | 4-y change | Baseline (1986) | 4-y change | Baseline (1989) | 4-y change |
| Age, y | 49.4 ± 2.4 | — | 48.0 ± 2.4 | — | 36.3 ± 3.7 | — |
| BMI, kg/m2 | 23.7 ± 1.4 | 0.2 ± 1.7 | 24.7 ± 1.1 | 0.3 ± 1.2 | 23.0 ± 2.5 | 0.8 ± 2.0 |
| Weight, kg | 64.0 ± 2.4 | 0.5 ± 4.5 | 79.5 ± 4.7 | 0.9 ± 4.0 | 62.6 ± 7.8 | 2.2 ± 5.5 |
| Baseline overweight, % | 31.0 | — | 46.5 | — | 23.8 | — |
| Physical activity, MET/wk | 14.9 ± 10.0 | 0.2 ± 1.9 | 23.0 ± 15.3 | 3.3 ± 3.7 | 21.9 ± 24.3 | 0.1 ± 20.0 |
| Smoking, % | ||||||
| Never | 45 | — | 54 | — | 66 | — |
| Past | 39 | — | 39 | — | 24 | — |
| Current | 16 | — | 7 | — | 10 | — |
| Alcohol,2 drinks/d | 0.5 ± 0.4 | 0.0 ± 0.4 | 0.8 ± 0.5 | 0.03 ± 0.6 | 0.2 ± 0.4 | 0.0 ± 0.4 |
| Sitting watching TV, h/wk | 12.0 ± 5.3 | — | 10.5 ± 4.0 | −0.2 ± 6.8 | 8.5 ± 6.8 | 0.3 ± 6.1 |
| Sleep, h/d | 7.0 ± 0.5 | — | 7.1 ± 0.5 | — | 7.0 ± 0.9 | — |
| Energy intake, kcal/d | 1798 ± 243 | −16 ± 419 | 1987 ± 278 | 3 ± 456 | 1802 ± 446 | 9 ± 434 |
| AHEI-2010 score | 50.3 ± 5.3 | 1.8 ± 8.4 | 51.7 ± 5.7 | 1.6 ± 7.8 | 48.9 ± 9.5 | 2.4 ± 8.7 |
| aMed score | 4.0 ± 0.9 | 0.03 ± 1.6 | 4.2 ± 1.0 | 0.01 ± 1.6 | 4.2 ± 1.6 | 0.1 ± 1.6 |
| DASH score | 23.6 ± 2.4 | 0.07 ± 3.5 | 23.5 ± 2.7 | 0.04 ± 3.7 | 23.8 ± 4.0 | 0.0 ± 3.4 |
| Vegetables, servings/d | 3.9 ± 1.0 | −0.3 ± 1.8 | 3.3 ± 0.9 | 0.07 ± 1.6 | 3.3 ± 1.7 | 0.2 ± 1.7 |
| Fruit, servings/d | 1.6 ± 0.5 | −0.04 ± 1.0 | 1.5 ± 0.6 | 0.06 ± 0.9 | 1.2 ± 0.8 | 0.1 ± 0.8 |
| Whole grains, servings/d | 1.1 ± 0.5 | 0.03 ± 1.0 | 1.4 ± 0.7 | 0.02 ± 2.0 | 1.4 ± 1.0 | 0.0 ± 0.9 |
Values are means ± SDs. AHEI-2010, Alternate Healthy Eating Index 2010; aMed, Alternate Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals’ Follow-Up Study; MET, metabolic equivalent hour; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
One drink is 148 mL of wine, 355 mL of beer, or 44 mL of liquor.
We observed an inverse association between an increase in diet quality score and weight change. After adjusting for potential confounders and simultaneous changes in other lifestyle factors, each 1-SD increase in the AHEI-2010 over 4 y was associated with less weight gain in the same period. This ranged from −0.35 kg less weight gain (95% CI: −0.37, −0.32) in the HPFS to −0.66 kg less weight gain (95% CI: −0.68, −0.64) in the NHS II (Table 2). Results of the DASH score were similar to the AHEI-2010. Each SD increase in aMed in 4-y periods was also significantly associated with less weight gain, but the amount appeared to be less than the AHEI-2010 or the DASH score. Compared with individuals with no change in diet quality indexes, individuals in the highest category of score improvement (i.e., quintile 5) in AHEI-2010 over 4 y had significantly less weight gain (Table 3). However, individuals with a reduction of diet quality score (i.e., decrease in diet quality) compared with individuals with no change had substantially greater weight gain in the same time period.
TABLE 2.
Relation between change in diet quality score (1-SD increase) and weight change in 4-y periods in the NHS, HPFS, and NHS II cohorts1
| NHS (n = 50,603) | HPFS (n = 22,973) | NHS II (n = 72,495) | Pooled | |
| AHEI-2010 | ||||
| Age adjusted | −0.40 (−0.42, −0.38) | −0.35 (−0.37, −0.32) | −0.66 (−0.68, −0.64) | −0.47 (−0.66, −0.28)* |
| Multivariable2 | −0.39 (−0.41, −0.36) | −0.34 (−0.37, −0.32) | −0.67 (−0.69, −0.65) | −0.47 (−0.67, −0.26)* |
| aMed | ||||
| Age adjusted | −0.19 (−0.21, −0.17) | −0.21 (−0.24, −0.19) | −0.30 (−0.32, −0.27) | −0.23 (−0.30, −0.17)* |
| Multivariable2 | −0.18 (−0.20, −0.16) | −0.21 (−0.23, −0.18) | −0.30 (−0.32, −0.28) | −0.23 (−0.30, −0.16)* |
| DASH | ||||
| Age adjusted | −0.40 (−0.42, −0.37) | −0.32 (−0.34, −0.29) | −0.53 (−0.55, −0.51) | −0.41 (−0.53, −0.29)* |
| Multivariable2 | −0.40 (−0.42, −0.37) | −0.32 (−0.34, −0.29) | −0.53 (−0.56, −0.51) | −0.42(−0.54, −0.29)* |
Values are weight change (95% CI), kg. *P-heterogeneity among the 3 cohorts: <0.0001. AHEI-2010, Alternate Healthy Eating Index 2010; aMed, Alternate Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals’ Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
Adjusted for age, baseline BMI, smoking, baseline and change in physical activity, hours of sleep, hours of sitting, alcohol (DASH score only), and for women only, parity, menopausal status, and postmenopausal hormone use.
TABLE 3.
Multivariable adjusted results for weight change in 4-y periods according to quintiles of diet quality score change in the NHS (n = 50,603), HPFS (n = 22,973), and NHS II (n = 72,495) cohorts1
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| AHEI-2010 | |||||
| Median score change2 | −8 | −2 | 0 | 5 | 12 |
| NHS | 0.51 (0.45, 0.58) | 0.17 (0.11, 0.24) | Reference | −0.05 (−0.11, 0.01) | −0.54 (−0.61, −0.48) |
| HPFS | 0.44 (0.36, 0.52) | 0.05 (−0.04, 0.14) | Reference | −0.07 (−0.15, 0.01) | −0.47 (−0.55, −0.39) |
| NHS II | 0.73 (0.67, 0.80) | −0.05 (−0.12, 0.02) | Reference | −0.25 (−0.32, −0.18) | −1.06 (−1.13, −1.00) |
| Pooled | 0.56 (0.39, 0.74)* | 0.06 (−0.08, 0.20)* | Reference | −0.12 (−0.25, −0.01)* | −0.69 (−1.06, −0.32)* |
| aMed | |||||
| Median score change2 | −2 | −1 | 0 | 1 | 2 |
| NHS | 0.24 (0.18, 0.30) | 0.10 (0.03, 0.77) | Reference | −0.10 (−0.16, −0.04) | −0.26 (−0.32, −0.20) |
| HPFS | 0.29 (0.22, 0.35) | 0.15 (0.05, 0.25) | Reference | −0.03 (−0.11, 0.05) | −0.30 (−0.38, −0.23) |
| NHS II | 0.48 (0.41, 0.54) | 0.15 (0.09, 0.22) | Reference | −0.16 (−0.22, −0.10) | −0.47 (−0.54, −0.41) |
| Pooled | 0.33 (0.19, 0.48)* | 0.13 (−0.09, 0.17) | Reference | −0.10 (−0.17, −0.03)* | −0.35 (−0.48, −0.21)* |
| DASH | |||||
| Median score change2 | −4 | −1 | 0 | 1 | 4 |
| NHS | 0.51 (0.44, 0.57) | 0.17 (0.11, 0.24) | Reference | −0.13 (−0.20, −0.06) | −0.51 (−0.57, −0.45) |
| HPFS | 0.39 (0.32, 0.47) | 0.12 (0.04, 0.20) | Reference | −0.05 (−0.13, 0.03) | −0.43 (−0.50, −0.35) |
| NHS II | 0.67 (0.61, 0.73) | 0.15 (0.08, 0.21) | Reference | −0.22 (−0.29, −0.15) | −0.72 (−0.79, −0.66) |
| Pooled | 0.52 (0.37, 0.68) | 0.15 (0.11, 0.19) | Reference | −0.14 (−0.23, −0.04)* | −0.55 (−0.73, −0.38)* |
Values are weight change (95% CI), kg. *P-heterogeneity: <0.05. Adjusted for age, baseline BMI, smoking, physical activity, hours of sleep, hours of sitting, alcohol (DASH score only), and, for women only, parity, menopausal status, and postmenopausal hormone use. AHEI-2010, Alternate Healthy Eating Index 2010; aMed, Alternate Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals’ Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; Q, quintile.
Average of all 3 cohorts.
When we examined heterogeneity of results, comparing NHS with NHS II and HPFS with NHS II, we found that the inverse association between diet quality scores and weight change was stronger in the younger NHS II cohort than in the 2 older cohorts (each P-heterogeneity: <0.001). When we stratified the analysis of the 2 older cohorts by age, we found that individuals aged <55 y had less weight gain than individuals aged ≥55 y for each SD increase in each of the diet quality score (Supplemental Table 3). For example, for each 1-SD increase in AHEI-2010, weight change in women aged <55 y was −0.49 kg compared with −0.31 kg in women aged ≥55 y (P-interaction: <0.0001).
When we stratified the analysis by BMI at the beginning of each 4-y period, we observed significantly less weight gain with greater increases in diet quality scores, regardless of the score, cohort, or BMI status (Table 4). However, the inverse association between increase in diet quality score and weight change was stronger in individuals with a BMI of ≥25.0 than individuals with normal BMI. For example, in the NHS II, weight change was −1.08 kg (95% CI: −1.13, −1.05) less for every 1-SD increase in the AHEI-2010 among overweight individuals and −0.40 kg (95% CI: −0.42, −0.38) among individuals with normal BMI. Similar results, although with varying magnitude of weight change, were observed for all diet quality scores across all 3 cohorts.
TABLE 4.
Multivariable adjusted results for the association between 1 SD increase in diet quality score and weight change in 4-y periods, stratified by BMI in the NHS, HPFS, and NHS II cohorts1
| NHS2 | HPFS3 | NHS II4 | Pooled | |
| AHEI-2010 | ||||
| BMI < 25 | −0.22 (−0.24, −0.19) | −0.21 (−0.24, −0.17) | −0.40 (−0.42, −0.38) | −0.27 (−0.40, −0.14)* |
| BMI ≥ 25 | −0.61 (−0.65, −0.57) | −0.43 (−0.47, −0.39) | −1.08 (−1.13, −1.05) | −0.71 (−1.08, −0.34)* |
| P-interaction | <0.0001 | <0.0001 | <0.0001 | — |
| aMed | ||||
| BMI < 25 | −0.10 (−0.12, −0.08) | −0.14 (−0.17, −0.10) | −0.17 (−0.19, −0.15) | −0.13 (−0.18, −0.09)* |
| BMI ≥ 25 | −0.27 (−0.31, −0.23) | −0.25 (−0.29, −0.21) | −0.48 (−0.52, −0.43) | −0.33 (−0.47, −0.20)* |
| P-interaction | <0.0001 | <0.001 | <0.0001 | — |
| DASH | ||||
| BMI < 25 | −0.25 (−0.27, −0.23) | −0.21 (−0.24, −0.18) | −0.30 (−0.32, −0.28) | −0.26 (−0.31, −0.20)* |
| BMI ≥ 25 | −0.57 (−0.61, −0.53) | −0.37 (−0.41, −0.33) | −0.86 (−0.90, −0.82) | −0.60 (−0.87, −0.33)* |
| P-interaction | <0.0001 | <0.0001 | <0.0001 | — |
Values are weight change (95% CI), kg. *P-heterogeneity: <0.05. Adjusted for age, baseline BMI (in kg/m2), smoking, physical activity, hours of sleep, hours of sitting, alcohol (DASH score only), and, for women only, parity, menopausal status, and postmenopausal hormone use. AHEI-2010, Alternate Healthy Eating Index 2010; aMed, Alternate Mediterranean Diet Score; DASH, Dietary Approaches to Stop Hypertension; HPFS, Health Professionals’ Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
NHS: BMI < 25, n = 36,713; BMI ≥ 25, n = 26,490.
HPFS: BMI < 25, n = 13,417; BMI ≥ 25, n = 14,756.
NHS II: BMI < 25, n = 56,879; BMI ≥ 25, n = 36,701.
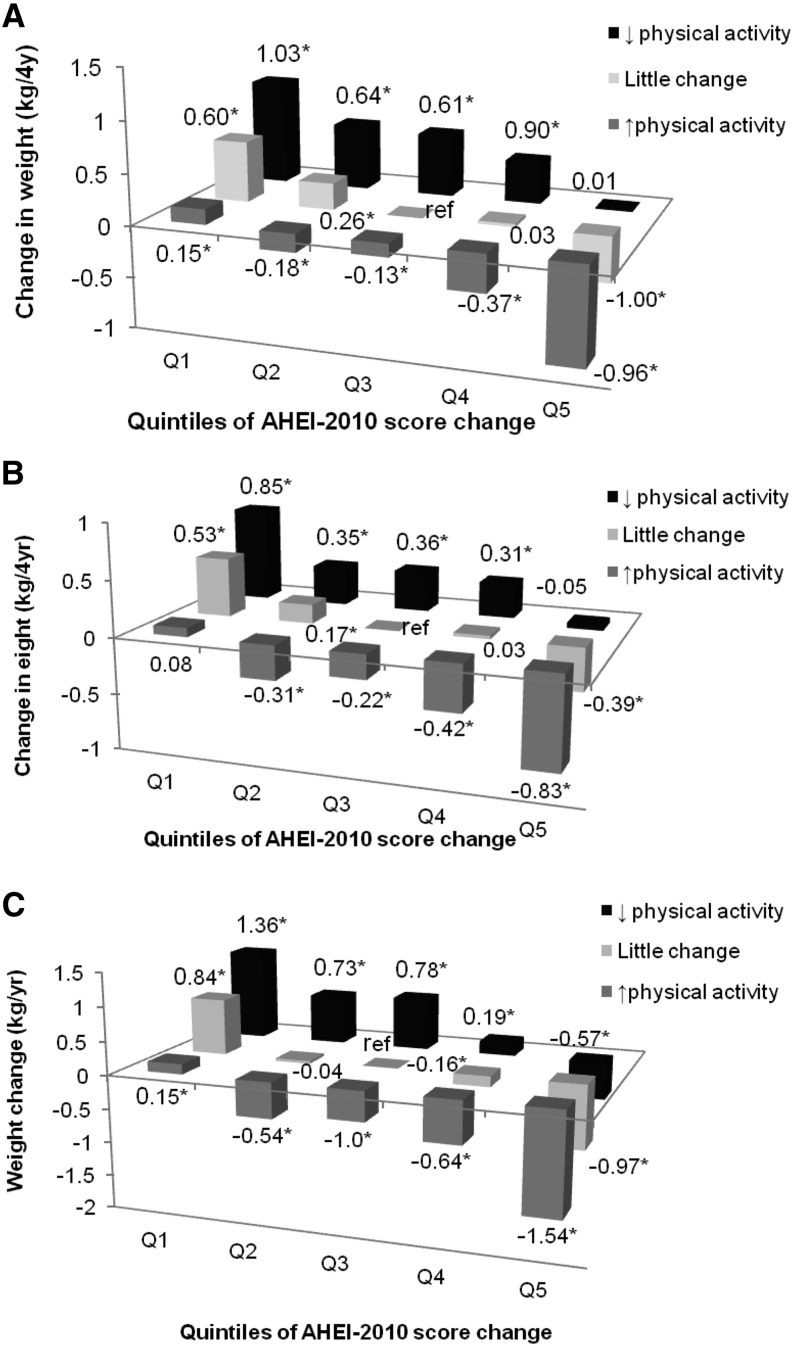
When we jointly classified participants by their change in AHEI-2010 score and changes in physical activity, individuals in the highest category of AHEI-2010 score increase whom also increased their physical activity over the same 4 y had less weight gain than individuals who did not change their diet score or physical activity (−0.96 kg for NHS, −0.83 kg for HPFS, −1.54 kg for NHS II; all P < 0.0001) (Figure 1). However, individuals in the lowest quintile of AHEI-2010 change with simultaneous reduction of physical activity gained significantly more weight over 4 y (1.03 kg in the NHS, 0.85 kg in the HPFS, 1.36 kg in the NHS II; all P < 0.001) than individuals without change in either factor.
FIGURE 1.
Multivariable-adjusted joint classification of change in AHEI-2010 score and change in physical activity and 4-y weight change (in kg) in the NHS (n = 50,603; A), HPFS (n = 22,973; B), and NHS II (n = 72,495; C) cohorts. *P < 0.05. Adjusted for age, baseline BMI, smoking, baseline and change in physical activity, hours of sleep, hours of sitting, alcohol (DASH score only), and, for women only, parity, menopausal status, and postmenopausal hormone use. AHEI-2010, Alternate Healthy Eating Index 2010; HPFS, Health Professionals’ Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
We also compared the diet quality indexes with each other on their strength in influencing weight changes by including 2 indexes in the same regression model. One-SD improvement in the AHEI-2010 was associated with substantially less weight gain than 1-SD improvement of the aMed. The AHEI-2010 and DASH scores were not different from each other in terms of their associations with weight change (Supplemental Table 4). When the 3 diet quality scores were compared with FQCS, improving the FQCS had stronger inverse association with lower weight gain than improving any of the other diet quality scores.
Discussion
In this analysis we assessed changes in diet quality scores and change in weight over the same time period and observed that improving a person’s diet, as reflected by an increase in diet quality scores, is associated with less long-term weight gain. Stronger associations were observed in younger individuals than in older individuals and in overweight and obese participants compared with their normal-weight counterparts.
The AHEI-2010, aMed, and DASH are moderately correlated with each other because they all emphasized higher consumption of fruits, vegetables, and whole grains and de-emphasized red and processed meat consumption. However, substantial difference were also found among them, such as not having a sodium component in aMed, not having a dairy component in AHEI-2010, not having an alcohol component in DASH, and the long-chain n–3 FAs and trans fat components in the AHEI-2010. Among these 3 scores, the AHEI-2010 has the greatest potential score range (0–110 points), followed by the DASH score (5–40 points), and the aMed with a 9-point range. A wider score range would allow for greater discrimination of dietary differences among cohort participants and less misclassification of diet characteristics. Thus, it is not surprising that the AHEI-2010 showed the strongest association. However, less weight gain was also observed for increasing aMed and DASH scores, suggesting increased adherence to any of these healthful diets could be beneficial for prevention of long-term weight gain.
Several possible mechanisms may explain how healthy diets may reduce weight gain (15, 26–28). The most common one centers on their effect on hunger and satiety and hence total energy intake. A prevailing characteristic shared by many dietary recommendations is an emphasis on fruits, vegetables, and whole grains and a de-emphasis on refined grains, sweets, and red/processed meats. Our previous study in the NHS, HPFS, and NHS II cohorts showed that an increased consumption of vegetables, fruits, and whole grains was associated with less weight gain, whereas increased intake of starchy foods and refined grains was associated with weight gain (15). Low-energy-density foods such as fruits and vegetables were shown to sustain satiety longer, reduce food intake (28), and thus may reduce total energy intake. However, the magnitude of this effect may vary, depending on what else is consumed in the same meal (29). In addition, studies have also shown that foods with a lower glycemic index may delay the onset of hunger and therefore overall food intake (26, 27). Less weight gain may also be in part because lower amounts of insulin (an anabolic hormone) are secreted in response to consuming meals with a lower glycemic index (3). More recently, data from a weight-loss trial showed that individuals who consumed isocaloric diets lower in glycemic index or higher in fat have higher resting energy expenditures than individuals who consumed low-fat diets (30).
However, many refined grains, sweets, and snacks are high in sugar and fat. Fructose was shown to increase dopamine secretion and therefore is a higher hedonic food than lower-sugar foods (31, 32). This may in turn drive overconsumption, as observed in animal studies. At the same time, highly palatable foods, which often contain substantial amounts of fat and sugar, also reduce dopamine receptors in the brain, which were noted in addiction physiology. Specific foods or nutrients may also influence weight gain through alteration of the gut microbiota. Dietary fat favors the growth of Firmicutes bacteria that has greater capacity to convert polysaccharides not digested by humans into monosaccharides and SCFAs that can be absorbed by humans (7, 8, 33). This may increase energy available to humans from food and may also alter gut hormone signaling that would normally limit food intake.
The most recent literature on overall diet quality and weight change focuses on the influence of baseline eating pattern and weight change, and our results are in agreement with those data. In particular, studies from various countries that used different indexes to measure adherence to the Mediterranean diet have generally shown favorable results. Prospective studies from Spain showed less weight gain (9) and a smaller waist circumference (34) among individuals with higher Mediterranean diet scores at baseline. Data from the European Prospective Investigation into Cancer and Nutrition showed 0.16 kg less weight gain over 5 y among individuals with better adherence to the Mediterranean diet (top one-third of the score range vs. the bottom one-third) (10). In the United States, data from the Framingham Heart Study showed less gain in waist circumference with a higher Mediterranean diet score (11). In France, higher diet scores that reflected healthy dietary guidelines from France, the United States, and the Mediterranean diet were all associated with a lower risk of developing obesity among men in 13 y of follow-up (35). In the American population, adherence to the 2005 Dietary Guidelines for Americans, was associated with lower BMI and waist circumference in a multi-ethnic cohort in the United States, but less weight gain was only observed in white men (12).
The availability of repeated dietary assessment allowed us to take advantage of the long-term follow-up to assess change in diet over several years. Because we have detailed lifestyle information, we were able to control for simultaneous change in lifestyle factors, including sleep duration. However, some level of measurement error cannot be avoided because of the self-reported nature of diet and lifestyle information. Our study did not measure body composition; thus, we could not differentiate change in lean vs. fat mass. Compared with the AHEI-2010, the narrower score range in the aMed and DASH limited their ability to differentiate highly healthy eating patterns from moderately healthy ones. This might result in an artificially lower magnitude of association with weight change with these scores. Nevertheless, our data are consistent in the direction of association with weight change and showed that improving one’s diet in mid-life can benefit weight management. In particular, the absolute weight change was greater among individuals with higher BMI. Therefore, in the context of the current obesity epidemic, these data provide support for the importance of adopting a diet high in fruits, vegetables, and whole grains and low in refined grains, sweets, and both red and processed meats in preventing adult weight gain. In addition, our finding that weight change was greater among younger women speaks to the importance of preventing unnecessary weight gain in young adults to reduce the risk of obesity-related diseases later in life. Preventing the development of overweight and obesity in this age group is also paramount to controlling the obesity epidemic.
In conclusion, improvement of diet quality is associated with less weight gain, especially in younger women and overweight individuals.
Acknowledgments
TTF, SEC, DM, WCW, and FBH designed the research; TTF, AP, and TH conducted the research and analyzed the data; DKT provided technical advice in analysis and writing. TTF was responsible for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AHEI-2010, Alternate Healthy Eating Index 2010; aMed, Alternate Mediterranean Diet; DASH, Dietary Approaches to Stop Hypertension; FQCS, food quality change score; HPFS, Health Professionals’ Follow-Up Study; MET, metabolic equivalent hour; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
References
- 1.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol 2014;179:1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens J, Erber E, Truesdale KP, Wang CH, Cai J. Long- and short-term weight change and incident coronary heart disease and ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2013;178:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig DS. The glycemic index. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 4.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2013;98:872–84. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergnaud AC, Norat T, Romaguera D, Mouw T, May AM, Romieu I, Freisling H, Slimani N, Boutron-Ruault MC, Clavel-Chapelon F, et al. Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am J Clin Nutr 2012;95:184–93. [DOI] [PubMed] [Google Scholar]
- 7.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem 2013;24:1663–77. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014;146:1525–33. [DOI] [PubMed] [Google Scholar]
- 9.Beunza JJ, Toledo E, Hu FB, Bes-Rastrollo M, Serrano-Martinez M, Sanchez-Villegas A, Martinez JA, Martinez-Gonzalez MA. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr 2010;92:1484–93. [DOI] [PubMed] [Google Scholar]
- 10.Romaguera D, Norat T, Vergnaud AC, Mouw T, May AM, Aqudo A, Buckland G, Slimani N, Rinaldi S, Couto E, et al. Mediterranean dietary patterns and prospective weight change in participants of the EPIC-PANACEA project. Am J Clin Nutr 2010;92:912–21. [DOI] [PubMed] [Google Scholar]
- 11.Rumawas ME, Meigs JB, Dwyer JT, McKeown NM, Jacques PF. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. Am J Clin Nutr 2009;90:1608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao SK, Beresford SA, Frank LL, Schreiner PJ, Burke GL, Fitzpatrick AL. Modifications to the Healthy Eating Index and its ability to predict obesity: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2008;88:64–9. [DOI] [PubMed] [Google Scholar]
- 13.Zamora D, Gordon-Larsen P, Jacobs DR, Jr., Popkin BM. Diet quality and weight gain among black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study (1985–2005). Am J Clin Nutr 2010;92:784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesse-Guyot E, Castebon K, Estaquio C, Czernichow S, Galan P, Hercberg S. Association between the French nutritional guideline-based score and 6-year anthropometric changes in a French middle-aged adult cohort. Am J Epidemiol 2009;170:757–65. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Hou T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan A, Malik VS, Hou T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013;37:1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010;92:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 22.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med 1995;333:677–85. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr., Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 Suppl):S498–504. [DOI] [PubMed]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 26.Chang KT, Lampe JW, Schwarz Y, Breymeyer KL, Noar KA, Song X, Neuhouser ML. Low glycemic load experimental diet more satiating than high glycemic load diet. Nutr Cancer 2012;64:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keogh J, Atkinson F, Eisenhauer B, Inamdar A, Brand-Miller J. Food intake, postprandial glucose, insulin and subjective satiety responses to three different bread-based test meals. Appetite 2011;57:707–10. [DOI] [PubMed] [Google Scholar]
- 28.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009;97:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams RA, Roe LS, Rolls BJ. Assessment of satiety depends on the energy density and portion size of the test meal. Obesity (Silver Spring) 2014;22:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012;307:2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 2012;71:478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig RH. Fructose: it’s “alcohol without the buzz”. Adv Nutr 2013;4:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 2014;5:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funtikova AN, Benitez-Arciniega AA, Gomez SF, Fitó M, Elosua R, Schroder H. Mediterranean diet impact on changes in abdominal fat and 10-year incidence of abdominal obesity in a Spanish population. Br J Nutr 2014;111:1481–7. [DOI] [PubMed] [Google Scholar]
- 35.Lassale C, Fezeu L, Andreeva VA, Hercberg S, Kengne AP, Czernichow S, Kesse-Guyot E. Association between dietary scores and 13-year weight change and obesity risk in a French prospective cohort. Int J Obes (Lond) 2012;36:1455–62. [DOI] [PubMed] [Google Scholar]