Abstract
BACKGROUND:
Stevens-Johnson syndrome (SJS) is an uncommon, sporadic disease and outbreaks are rare. In November 2013, an outbreak of SJS was identified at Children’s Hospital Colorado.
METHODS:
Outbreak cases were children aged 5–21 with a discharge diagnosis of SJS admitted from September 1 to November 30, 2013. Medical charts were reviewed using standardized data collection forms. Respiratory specimens were tested for viruses and Mycoplasma pneumoniae (Mp) by polymerase chain reaction (PCR). We conducted a separate 4-year retrospective case-control study comparing hospitalized SJS cases with and without evidence of Mp infection.
RESULTS:
During the outbreak, 8 children met SJS criteria. Median age was 11.5 years (range 8–16 years); 5 (63%) were boys and 5 (63%) were Mp-PCR–positive. Of the 5 PCR-positive children, none had preceding medication exposure, and all had radiographic pneumonia. All outbreak Mp isolates were macrolide susceptible. The retrospective case-control analysis showed that Mp-associated SJS episodes (n = 17) were more likely to have pneumonia (odds ratio [OR] 10.0, confidence interval [CI] 1.3–5.1), preceding respiratory symptoms (OR 30.0, CI 1.6–72.6), an erythrocyte sedimentation rate ≥35 mg/dL (OR 22.8, CI 2.1–244.9), and ≤3 affected skin sites (OR 4.5, CI 1.2–17.4) than non–Mp-associated SJS episodes (n = 23).
CONCLUSIONS:
We report the largest outbreak of SJS in children, which was also predominately associated with Mp infection. Mp-associated SJS was associated with a distinct clinical presentation that included less extensive skin disease, an elevated erythrocyte sedimentation rate, and evidence of a preceding respiratory infection.
What’s Known on This Subject:
Stevens-Johnson syndrome (SJS) is a rare and severe immunologic phenomenon characterized by rash and mucous membrane disease. SJS may be triggered by medications and, less commonly, by infections such as Mycoplasma pneumoniae (Mp). Outbreaks of SJS are exceedingly rare.
What This Study Adds:
We describe the largest SJS outbreak reported in children, which was also Mp-associated. In the first case-control study of this disease, we identify predictors of Mp-associated SJS versus non–Mp-associated SJS, including fewer skin lesions, pneumonia, and elevated erythrocyte sedimentation rate.
Stevens-Johnson syndrome (SJS) is an immune-mediated disease characterized by a prodromal illness followed by severe mucocutaneous symptoms.1 SJS can result in severe morbidity from scarring of mucosal surfaces, leading to blindness as well as urethral and esophageal strictures. The case-fatality rate for SJS is ∼10% in adults2,3 but may be less in children; up to 50% may develop long-term sequelae.4–6 Although the pathogenesis is incompletely understood, SJS and its more severe form, toxic epidermal necrolysis (TEN), are the result of an inflammatory response that results in keratinocyte necrosis and perivascular lymphocyte infiltration.7 SJS was classically ascribed to a medication hypersensitivity reaction; however, infectious etiologies, including Mycoplasma pneumoniae (Mp), are increasingly recognized as inciting agents.8–10
SJS has an estimated incidence of 1 to 7 cases per million person-years,11–15 although pediatric rates are not well described because of a lack of published information. Epidemiologic clusters of SJS are exceptionally rare and have been associated with both medications (including mebendazole and metronidazole)16 and infection such as Mp.9,17,18 SJS attributed to Mp represents only a small fraction of overall cases, and published information about the clinical characteristics of this condition is limited to small case series.
In November 2013, physicians observed an increase in the number of children admitted to a tertiary care children’s hospital with SJS associated with Mp infection. This clinical observation prompted a formal investigation, in collaboration with the Colorado Department of Public Health and Environment and Centers for Disease Control and Prevention (CDC), to improve our understanding of the epidemiology and clinical manifestations of Mp-associated SJS. This article characterizes the largest reported pediatric SJS outbreak and describes the clinical manifestations of Mp-associated SJS.
Methods
Setting
Children’s Hospital Colorado (CHCO) is a 553-bed, tertiary care hospital with 17 646 admissions in 2013. It is the primary children’s referral hospital for the state of Colorado, with a catchment population of 1.2 million children, and the surrounding states.
Case Definitions
A case of SJS during the outbreak was defined as a patient with an International Classification of Diseases, Ninth Revision (ICD-9) code for SJS, SJS-TEN, or TEN and meeting clinical criteria, including involvement of skin and at least 2 mucus membranes.19 Epidemiologic criteria for outbreak-associated cases of SJS were age 5 to 21 years and admission to CHCO between September 1 and November 30, 2013. The age range was selected to reflect both the patient population treated at CHCO and the population susceptible to Mp-associated SJS (extremely rare in infants, young children, and adults past middle age). The study period was selected to reflect both the long incubation period of Mp (2–4 weeks) and the time period during which clinicians reported an increase in SJS cases at CHCO. Additional hospitalized SJS cases were identified retrospectively to October 2008, when ICD-9 codes for SJS first came into use, to further characterize the spectrum of disease.
SJS cases were classified according to the likelihood of Mp as the underlying etiology. To be considered a “confirmed case,” a patient required a positive Mp result by polymerase chain reaction (PCR) on a respiratory tract specimen and no alternative etiology (eg, medication effect) identified. A “probable case” required positive Mp immunoglobulin (Ig)M (with or without positive IgG) with no alternative etiology more likely. A “possible case” was defined as an SJS patient with positive Mp laboratory testing (PCR or IgM) and an alternative etiology or symptoms consistent with Mp infection (radiographic pneumonia or fever and 1 of cough, shortness of breath, or hypoxia), but laboratory testing was negative or not performed. All others were considered non–Mp-associated cases.
Laboratory Methods
Patients with SJS were tested for infectious etiologies at the discretion of their treating clinicians, usually at the time of hospital presentation. Testing for Mp infection at CHCO included PCR performed on throat swab specimens sent to a reference laboratory (Focus Diagnostics Inc, Cypress, CA), a respiratory pathogen PCR performed on the FilmArray (FA) system (BioFire Diagnostics, Salt Lake City, UT),20 and serology (IgM/IgG). FA is a US Food and Drug Administration–cleared multiplex PCR that can simultaneously detect 17 respiratory viruses and subtypes, as well as Mp, Chlamydia pneumoniae, and Bordetella pertussis in nasopharyngeal swabs; FA has been validated by the CHCO laboratory to also test nasopharyngeal wash and bronchoalveolar lavage specimens. Testing for herpes simplex virus by PCR and culture was also available.
At the time of the outbreak investigation, all available respiratory specimens from outbreak-associated SJS patients were collected from the CHCO and affiliated referral laboratories regardless of original Mp testing or test result. These specimens were sent to the Centers for Disease Control and Prevention (CDC) for confirmatory Mp PCR, culture, multiple-locus variable-number tandem-repeat analysis (MLVA) subtyping, and macrolide susceptibility profiling.21–24 MLVA typing was used to characterize the relatedness of circulating Mp strains. In addition, stored Mp-positive specimens from non-SJS patients in the preceding 1 year were also sent to CDC for testing as a comparison group.
Retrospective Case-Control Analysis
A separate retrospective case-control analysis was conducted to compare clinical characteristics of Mp-associated SJS episodes with non-Mp-associated SJS episodes. Medical charts were identified by ICD-9 discharge diagnosis of SJS, SJS/TEN, or TEN and reviewed for all children admitted to CHCO between October first, 2008, and November 30th, 2013. For this analysis, Mp-associated SJS episodes included those meeting confirmed or probable criteria as defined above, based on available data. Non-Mp associated episodes were those that did not meet Mp-associated SJS criteria. Episodes meeting possible criteria as defined above were excluded from the analysis since they lacked diagnostic certainty. Mp-associated SJS episodes were compared with non-Mp-associated SJS episodes for demographic, clinical, diagnostic, and treatment variables.
Statistical Analyses
Clinical, laboratory, medication, treatment, and outcome data of all SJS patients were recorded on a standardized case investigation form and entered into an Epi-Info version 7.1.3.0 (Atlanta, GA) database.
A rate 300% higher than the baseline event rate was predefined as the threshold for an SJS outbreak. To determine whether the excessive observed cases in the period from September 1 to November 30, 2013, corresponded to an outbreak with such scope, cumulative sum (CUSUM) analysis was applied to all cases of SJS by month from October 1, 2008, to November 30, 2013. A threshold corresponding to an average time to a “false alarm” (also known as average run length, or ARL0) of 170 months and an average time to outbreak detection of 4.5 months (ARL1) were calculated and added to the CUSUM plot. When the curve crosses the defined threshold, an outbreak is suggested.
Categorical variables were compared by using χ2 or Fisher’s exact analyses. Continuous variables were compared by using Wilcoxon rank-sum (Mann-Whitney U) analysis. P values <.05 were considered statistically significant. All analyses were performed by using Epi-Info version 7.1.3.0 and SAS version 9.3 (Cary, NC) software.
Ethical Approval
Outbreaks are reportable public health conditions in Colorado; as such, this outbreak investigation was not considered to be research that required review by an institutional review board or informed consent from the patients.
Results
Outbreak Investigation
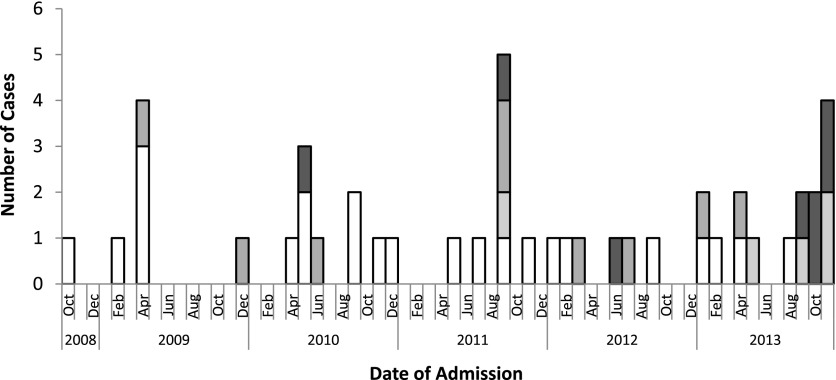
Eight SJS cases were admitted to CHCO between September 1 and November 30, 2013 (Fig 1). All had evidence of Mp infection; 5 (63%) were classified as confirmed Mp-associated SJS on the basis of positive Mp PCR results, and 3 (38%) were classified as possible Mp-associated SJS on the basis of clinical symptoms (Table 1). One child (13%) was also positive for herpes simplex virus (HSV) by PCR on an oral swab. Of 6 children tested by FA, 2 (33%) had a virus detected (1 rhino/enterovirus, 1 parainfluenza virus type 4) in addition to Mp. Both probable Mp-associated SJS cases in the outbreak were taking preceding medications: 1 (13%) child was taking trimethoprim-sulfamethoxazole for a sinus infection (starting 8 days before lesion onset) and azithromycin for pertussis postexposure prophylaxis (starting 13 days before lesion onset); 1 child was taking azithromycin for pneumonia (starting 6 days before lesion onset). Three (38%) children reported household contacts with respiratory symptoms. Of the 8 children, 5 (63%) were male and the median age was 11.5 years (range 8–16 years; Table 1).
FIGURE 1.
Cases of SJS secondary to Mp at CHCO, October 2008–November 2013. CUSUM analysis indicated an SJS outbreak occurred during September 2011 and September to November 2013.  Confirmed Mp-associated SJS,
Confirmed Mp-associated SJS,  probable Mp-associated SJS,
probable Mp-associated SJS,  possible Mp-associated SJS, and
possible Mp-associated SJS, and  non–Mp-associated SJS.
non–Mp-associated SJS.
TABLE 1.
Demographic and Exposures Characteristics Among SJS Outbreak Cases in Colorado, September to November 2013
| ID | Age/Gender | Case Category | Mp PCR | Mp IgM | HSV PCR | HSV Culture | Preceding Medications |
|---|---|---|---|---|---|---|---|
| 1 | 14/F | Possible | NR | NR | NR | NR | AZM, TMP/SMX |
| 2 | 9/M | Confirmed | (+) | (+) | (–) | (–) | — |
| 3 | 9/M | Confirmed | (+) | (–) | NR | (–) | — |
| 4 | 16/M | Confirmed | (+) | NR | (+) | (–) | — |
| 5 | 8/M | Possible | (–) | NR | NR | NR | — |
| 6 | 9/F | Possible | (–) | NR | (–) | (–) | AZM |
| 7 | 15/F | Confirmed | (+) | NR | (–) | NR | — |
| 8 | 14/M | Confirmed | (+) | (+) | NR | (–) | — |
AZM, azithromycin; F, female; HSV, herpes simplex virus; M, male; NR, not recorded; TMP/SMX, trimethoprim/sulfamethoxazole; —, no medications.
All 8 children had oropharyngeal mucositis, 7 (88%) had conjunctival involvement, and 5 (63%) had vaginal/penile involvement (Table 2). All patients had skin involvement, and the median number of sites (head, trunk, arms, legs) involved was 2.5 (range 1–4), although the severity of skin involvement overall was mild. Mucosal disease severity varied, with 4 (50%) receiving amniotic membrane grafting to eyes, 2 (25%) requiring ICU admission, and 6 (75%) needing nasogastric feeds or total parenteral nutrition. One (13%) child did not require any of these interventions. All 8 children received azithromycin, including 2 (25%) who were put on a prolonged course (>1 year) with concern for development of bronchiolitis obliterans. All were discharged well.
TABLE 2.
Clinical Characteristics Among SJS Outbreak Cases in Colorado, September to November 2013
| Case Characteristic | All Cases | Confirmed Mp-Associated SJS | Possible Mp-Associated SJS |
|---|---|---|---|
| (n = 8) | (n = 5) | (n = 3) | |
| Conjunctival involvement, n (%) | 7 (88) | 5 (100) | 2 (67) |
| Oropharyngeal involvement, n (%) | 8 (100) | 5 (100) | 3 (100) |
| Vaginal/penile involvement, n (%) | 5 (63) | 4 (80) | 1 (33) |
| No. of skin sites involved, median (range) | 2.5 (1–4) | 1 (1−4) | 3 (2−4) |
| Fever, n (%) | 8 (100) | 5 (100) | 3 (100) |
| Radiographic pneumonia, n (%) | 6 (75) | 5 (100) | 1 (33) |
| Hospital days, median (range) | 6 (1−15) | 6 (4−15) | 6 (1−9) |
| ICU admission, n (%) | 2 (25) | 2 (40) | 0 (0) |
| Amniotic grafting, n (%) | 4 (50) | 3 (60) | 1 (33) |
| PCA or continuous analgesia, d, median (range) | 0 (0−10) | 3 (0−10) | 0 (0–0) |
| NG feeds or TPN, d, median (range) | 6.5 (0−9) | 7 (0−10) | 6 (0−7) |
| Household exposure, n (%) | 3 (38) | 2 (40) | 1 (33) |
| Maximum ESRa, median (range) | 57 (3−122) | 57 (18–122) | 59.5 (3−116) |
| Maximum CRPa, median (range) | 3.2 (0.5−34.6) | 23.4 (3.2−34.6) | 1.8 (0.5−3) |
| Treatment with IVIG, n (%) | 2 (25) | 2 (40) | 0 (0) |
| Treatment with systemic steroids, n (%) | 4 (50) | 2 (40) | 2 (67) |
| Treatment with azithromycin, n (%) | 6 (75) | 4 (80) | 2 (67) |
ESR and C-reactive protein were performed on 5 children, 3 with confirmed and 2 with possible Mp-associated SJS. IVIG, intravenous immunoglobulin; NG, nasogastric; PCA, patient-controlled analgesia; TPN, total parenteral nutrition.
The CUSUM exceeded the precalculated threshold in November 2013, indicating that an outbreak of SJS cases occurred during the study period (September 1 to November 30, 2013) (Fig 1). This finding also correlated with a large increase in Mp disease in the community.25
Mp Characterization
Banked Mp PCR-positive respiratory specimens were available from 48 children (including 5 children with outbreak-associated SJS and 43 with non-SJS disease) admitted to CHCO between January 1 and December 13, 2013, and were submitted to the CDC laboratory for further testing. Of these, 46 (96%) were confirmed as Mp-positive by the CDC PCR. Five MLVA types were observed among the specimens tested, including 3 among the 5 Mp-associated SJS cases: 1 of type 3-5-6-2, 2 of type 3-6-6-2, and 2 of type 4-5-7-2. Forty-three specimens (93%) were macrolide susceptible, and 3 (7%) were macrolide resistant. All Mp isolates from the SJS cases were macrolide susceptible.
Case-Control Analysis
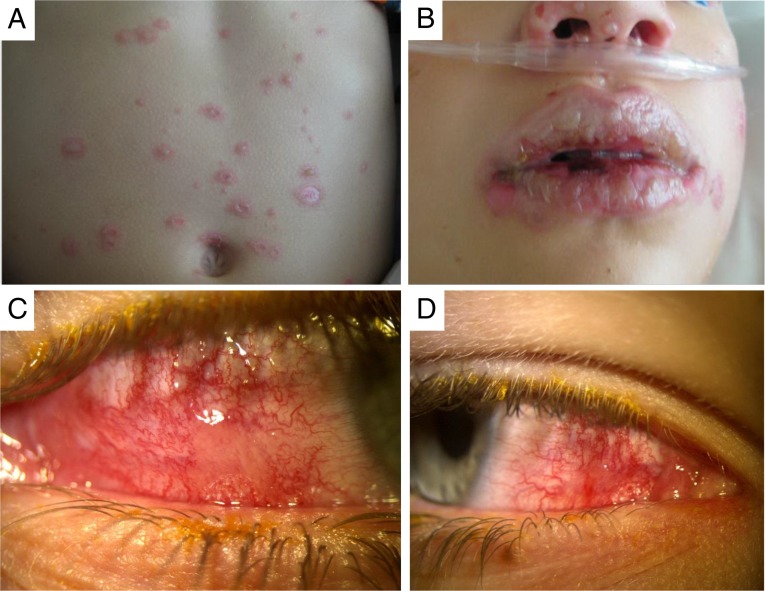
Twenty-two episodes of SJS were identified as confirmed (n = 8), probable (n = 9), or possible (n = 5) Mp-associated, and 23 were identified as non-Mp-associated between October 1, 2008, and November 30, 2013 (Table 3). The 5 possible Mp-associated SJS episodes were excluded from the analysis. Of the 23 non-Mp-SJS episodes, 17 (74%) had negative Mp PCR testing, and 6 (26%) did not receive Mp PCR testing. Demographic characteristics were similar between groups. Clinically, Mp-associated SJS episodes were significantly more likely than non-Mp-SJS episodes to have radiographic pneumonia (odds ratio [OR] 10, confidence interval [CI] 1.3–5.1) and to have preceding respiratory symptoms (OR 30.0, CI 1.6–72.6). Mp-associated SJS episodes had fewer skin sites involved (median 1, interquartile range [IQR] 1–4) than non-Mp-SJS episodes (median 4, IQR 2.5–4, P = .047). Mucous membranes distribution was similar between Mp-associated SJS episodes (median 3 sites, IQR 3–3) and non-Mp-SJS episodes (median 3, IQR 2.5–3). Subjectively, the physicians caring for hospitalized SJS patients reported less severe skin disease and more severe mucositis, including ocular disease (Fig 2), among the Mp-associated SJS group. Both groups had similar duration of SJS symptoms before hospitalization (median 3 days, IQR 2–5 days).
TABLE 3.
Comparison of Mp-Associated SJS and Non–Mp-Associated SJS, October 2008–November 2013
| Variable | Mp-SJS (n = 17) | Non–Mp-SJS (n = 23) | OR (CI) | P | All Inpatients (n = 35 964) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y, median (IQR) | 12.7 (10.0–14.5) | 11.7 (6.8–15.1) | — | NS | 6.8 ± 6.9 |
| Male, n (%) | 12 (71) | 14 (61) | 1.5 (0.6–2.9) | NS | 19 843 (55) |
| Race, nonwhite, n (%) | 2 (12) | 8 (35) | 0.3 (0.1–1.5) | .097 | 3356 (9) |
| Ethnicity, Hispanic, n (%) | 4 (24) | 6 (26) | 0.9 (0.4–2.2) | NS | 10 077 (28) |
| Clinical | |||||
| Fever (≥39.5°C), n (%) | 16 (94) | 20 (87) | 2.4 (0.3–10.1) | NS | — |
| Oral involvement, n (%) | 17 (100) | 23 (100) | N/A | N/A | — |
| Conjunctival involvement, n (%) | 16 (94) | 20 (87) | 2.4 (0.3–10.1) | NS | — |
| Genital involvement, n (%) | 14 (82) | 17 (74) | 1.6 (0.5–3.7) | NS | — |
| No. mucus membranes involved, median (IQR) | 3 (3–3) | 3 (2.5–3) | — | NS | — |
| ≥3 mucus membranes involved, n (%) | 13 (76) | 17 (74) | 1.1 (0.3–4.9) | NS | — |
| Skin involvement, n (%) | 13 (76) | 21 (91) | 0.3 (0.3–1.2) | NS | — |
| No. of skin sites involved, median (IQR) | 1 (1–4) | 4 (2.5–4) | — | .047 | — |
| ≤3 skin sites involved, n (%) | 12 (71) | 8 (35) | 4.5 (1.2–17.4) | — | |
| Recurrent SJS, n (%) | 4 (24) | 3 (13) | 2.1 (0.7–3.1) | NS | — |
| Pneumonia, n (%) | 9 (53) | 3 (13) | 10.0 (1.3–5.1) | .002 | — |
| Preceding antibiotics or AEDs,a n (%) | 0 (0) | 9 (39) | 1/infinity | <.001 | — |
| Preceding URI symptoms, n (%) | 16 (94) | 8 (35) | 30 (1.6–72.6) | <.001 | — |
| Household contacts with URI,b n (%) | 5 (50) | 4 (31) | 2.0 (0.6–3.9) | NS | — |
| ESR >35 mg/dL, n (%)c | 7 (64) | 1 (7) | 22.8 (2.1–244.9) | — | |
| Max CRP mg/dL, median (IQR)d | 7.5 (2.9–10.5) | 6.3 (2.8–11.8) | — | NS | — |
| Treatment | |||||
| Days hospitalized, median (IQR) | 9 (5–12) | 11 (5–12.5) | — | NS | — |
| NG feeding or TPN days, median (IQR) | 7 (0–9) | 5 (0–10) | — | NS | — |
| PCA/continuous opioid, d, median (IQR) | 0 (0–9) | 0 (0–9) | — | NS | — |
| ICU admission, n (%) | 5 (29) | 5 (22) | 1.5 (0.6–2.7) | NS | — |
| Amniotic graft, n (%) | 6 (35) | 7 (32) | 1.2 (0.5–2.4) | NS | — |
AED, antiepileptic drug; CRP, C-reactive protein; N/A, not applicable; NG, nasogastric; NS, nonsignificant; PCA, patient-controlled analgesia; TPN, total parenteral nutrition; URI, upper respiratory infection; —, not recorded.
Did not include azithromycin as a preceding antibiotic.
Seven cases and 10 controls with missing data.
Six cases and 9 controls with missing data.
Four cases and 4 controls with missing data.
FIGURE 2.
Images of lesions in patients with Mp-associated Stevens-Johnson syndrome. Skin manifestations (A) were mild, with severe oropharyngeal (B) and conjunctival (C and D) disease.
ESR was significantly higher in Mp-associated SJS episodes (median 57 [IQR 23–105] mm/hour, reference range 0–20 mm/hour) compared with non-Mp-associated SJS episodes (18 [IQR 13–28] mm/hour, P = .008), but C-reactive protein was similar (7.5 [IQR 2.9–10.5] mg/dL vs 6.3 [IQR 2.8–11.8] mg/dL, reference range 0–1.0 mg/dL, P = .730]. An ESR ≥35 mm/hour was strongly predictive of Mp-associated SJS (OR 22.8, CI 2.1–244.9). Treatments, duration of hospitalization (median 9 vs 11 days), ICU admissions (29% vs. 22%), and ocular amniotic membrane grafting (35% vs 32%) were similar between groups. All children were discharged well except 1 non-Mp-associated SJS child who died due to complications unrelated to SJS.
Discussion
We describe the largest reported outbreak of SJS in children, which was predominantly associated with Mp infection. The disease was characterized by extensive mucositis, especially ocular disease, and less severe skin manifestations than non–Mp-associated SJS. In our retrospective case-control analysis of SJS hospitalizations, children with Mp-associated SJS were significantly more likely to have preceding respiratory symptoms, radiographic pneumonia, and elevated ESR than children with non–Mp-associated SJS; they also had fewer skin sites affected.
Mp-associated SJS is not well described in the literature, likely due to lack of sensitive diagnostic testing. Recently, PCR testing for Mp has begun to replace or accompany serology, allowing greater diagnostic accuracy.26 Interestingly, several case series from the 1940s may be the first descriptions of Mp-associated SJS,17,27–31 although molecular diagnostic testing was unavailable at the time. Often referred to as erythema multiforme major or exudativum, Mp-associated SJS disease, diagnosed primarily by serology, was characterized by extensive mucosal lesions and minimal skin involvement, with an atypical “psittacosis-like” pneumonia.10,17,19,32 Furthermore, a recent, systematic review of the literature suggests that Mp-associated disease might be a clinical entity distinct from drug-induced SJS. These authors proposed renaming this entity to Mp-induced rash and mucositis due to the minimal cutaneous involvement.33 Our data support this finding of mucositis-predominant disease, but in contrast to the conclusion of their review, the majority of our patients had severe disease necessitating prolonged hospitalizations and surgical intervention. Outbreaks of SJS are exceedingly rare with only 2 confirmed Mp-associated SJS clusters reported (10 cases total).9,18 Although 5 children in our outbreak had positive Mp testing by PCR, all 8 met Mp-associated SJS case definitions and had additional findings supportive of an Mp etiology including radiographic pneumonia, elevated ESR, and history of exposure to household contacts with respiratory symptoms.
We offer 2 likely explanations for the recent outbreak. Our initial hypothesis was that a new Mp strain with a greater predisposition to induce SJS was circulating in the community. In support of this hypothesis, of the 5 Mp-SJS cases with available MLVA typing, 3 had a similar subtype, despite the fact that multiple MLVA subtypes were circulating in the community during this time. These subtypes, however, have not been previously associated with SJS disease and have been circulating across the United States for several years.34 MLVA typing may also not be a reliable measure of clonality because multiple MLVA types may circulate in a single outbreak setting.35 A second, more likely hypothesis is that there was a large outbreak of Mp in the community, which resulted in an increased rate of SJS due to an overall increased rate of exposure to Mp. This is supported by a community-wide investigation, which found a significant increase in atypical pneumonia diagnoses, macrolide prescriptions, and positive Mp testing.25 Epidemics of Mp pneumonia are known to occur in 3- to 7-year cycles,36 and Mp outbreaks have been previously observed in Colorado.37
The clinical spectrum of disease in our pediatric outbreak and retrospective case-control study was similar to previous published case series of Mp-associated SJS.8,38,39 Prodromal respiratory illness and fever were common.8 Mucositis was predominant, with multiple mucus membranes involved, including ocular lesions.38,39 We observed a less severe distribution of skin involvement in the Mp-SJS group. Mucous membrane involvement was similar between groups, but severity and distribution were more difficult to quantify. Other studies also report less severe skin manifestations and organ dysfunction in patients with Mp-associated SJS, including some who describe a Mp-associated SJS-like disease with mild or no skin lesions, referred to as Mp-associated mucositis (MPAM),10,40–43 atypical SJS,9,44–46 incomplete SJS,47 or Fuch syndrome.48–50 Several of our SJS cases had previous episodes of SJS, as well as previous mucositis-only episodes that did not meet our case definitions.51 Many experts consider these various exanthemas separate manifestations along a spectrum of SJS.9,52,53 In general, skin involvement is common with Mp infection, with Mp pneumonia said to be associated with rash in 3% to 33% of patients.54,55 Mp has also been cultured from blisters of patients with SJS.56 Several of our patients with Mp-associated SJS had severe disease, necessitating ICU admission, ventilator support, amniotic membrane grafting, and other supportive measures. Although data were limited, it was not clear that treatment with intravenous immunoglobulin (IVIG), corticosteroids, or macrolide antibiotics led to improved outcome, which is consistent with previous literature.7
The significant differences between Mp-associated SJS and non-Mp-associated SJS are not surprising. Mp is highly transmissible57 and may cause a spectrum of disease including atypical pneumonia and upper respiratory infections and may incite asthma exacerbations.36,54 ESR is known to be falsely elevated with Mp infection because of the presence of cold agglutinins,58–60 and the significantly elevated ESR among Mp-SJS patients suggests its use as a potential biomarker of Mp-associated disease.
Our study has several limitations. Despite the greater accuracy of Mp PCR testing compared with serology, there remains a small risk of false-positive detection of the organism related to asymptomatic carriage or prolonged shedding (up to 4 months), both of which have been described.61 Thus, it is possible that a greater community Mp burden along with prolonged shedding led to a large number of positive tests among SJS patients who were unrelated to their SJS. However, such detections would not explain the increased number of SJS patients admitted during our outbreak window. Another limitation is that most of our SJS cases were identified retrospectively with data limited to that available in the medical records. The use of retrospective data also made it difficult to accurately quantify the severity of clinical disease. Despite some evidence of less severe skin manifestations and more severe mucositis among Mp-SJS cases in both the outbreak and retrospective case-control study, our ability to quantify these differences was limited. In addition, it is possible that during the outbreak study period, there was potential for greater awareness of Mp and SJS in the community, leading to increased testing and detection of cases. However, this increased awareness would most likely have only affected increased detection of Mp disease but not SJS because the severity of SJS in children usually necessitates admission to the hospital. The introduction of FA testing for Mp in the year preceding our outbreak may also have led to a greater case detection of Mp-SJS, although again this would likely only affect rates of Mp infection and not SJS.
Conclusions
We report the largest outbreak of SJS in children. We hypothesize that the SJS outbreak was due to a large community-wide outbreak of Mp.25 The spectrum of Mp-associated SJS disease was consistent with previous reports, including severe mucositis and milder skin manifestations. Several children suffered significant morbidity during their hospitalizations. The presentation of Mp-associated SJS was clinically distinct from non-Mp associated SJS and clinicians should have a high suspicion for Mp-associated SJS when disease is characterized by radiographic pneumonia, preceding respiratory symptoms, fewer skin manifestations, and elevated ESR. If future clusters of Mp-associated SJS are identified or detected, we recommend prospective collection of respiratory samples for Mp culture and typing, as well as further evaluation of treatment measures.
Glossary
- CDC
Centers for Disease Control and Prevention
- CHCO
Children’s Hospital Colorado
- CI
confidence interval
- CUSUM
cumulative sum analysis
- ESR
erythrocyte sedimentation rate
- FA
FilmArray
- ICD-9
International Classification of Diseases, Ninth Revision code
- Ig
immunoglobulin
- IQR
interquartile range
- MLVA
multiple-locus variable-number tandem-repeat analysis
- Mp
Mycoplasma pneumoniae
- OR
odds ratio
- PCR
polymerase chain reaction
- SJS
Stevens-Johnson syndrome
- TEN
toxic epidermal necrolysis
Footnotes
Drs Olson, Watkins, Glodé, Kutty, and Dominguez conceptualized and designed the study and drafted the initial manuscript; Drs Demirjian, Lin, Robinson, Foo, and Mrs Mason, and Lauper contributed significantly to the acquisition and analysis of data and drafting and revising the article; Mrs Pretty, Mr Benitez, and Drs Winchell, Diaz, Miller, Kupfer, and Kennedy contributed to the acquisition and analysis of data and revision of the article for important intellectual content; and all authors approved the final manuscript as submitted.
This work was presented as an oral presentation at ID Week 2014; October 7–12, 2014; Philadelphia, PA.
FINANCIAL DISCLOSURE: Dr Glodé is a member of a Pfizer data safety monitoring board for an unrelated vaccine. Dr Robinson served on a 1-day Scientific Advisory Board to provide input into Biofire’s Gastroenteritis Pathogen polymerase chain reaction panel; Biofire markets a Respiratory Pathogen Panel that was used to detect the Mycoplasma pneumoniae infections described in this article. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Olson is supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors' sole responsibility and do not necessarily represent official NIH views. Funded by the National Institutes of Health.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Stevens AMJ. F. A new eruptive fever associated with stomatitis and ophthalmia: report of two cases in children. Am J Dis Child. 1922;24:526 [Google Scholar]
- 2.Atanasković-Marković M, Medjo B, Gavrović-Jankulović M, Ćirković Veličković T, Nikolić D, Nestorović B. Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatr Allergy Immunol. 2013;24(7):645–649 [DOI] [PubMed] [Google Scholar]
- 3.Sekula P, Dunant A, Mockenhaupt M, et al. RegiSCAR study group . Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197–1204 [DOI] [PubMed] [Google Scholar]
- 4.Singalavanija S, Limpongsanurak W. Stevens-Johnson syndrome in Thai children: a 29-year study. J Med Assoc Thai. 2011;94(suppl 3):S85–S90 [PubMed] [Google Scholar]
- 5.Forman R, Koren G, Shear NH. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years’ experience. Drug Saf. 2002;25(13):965–972 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein Y, Soon GS, Acuna P, et al. Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics. 2011;128(4):723–728 [DOI] [PubMed] [Google Scholar]
- 7.Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med. 2011;39(6):1521–1532 [DOI] [PubMed] [Google Scholar]
- 8.Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome. Report of eight cases and review of the literature. Clin Pediatr (Phila). 1991;30(1):42–49 [DOI] [PubMed] [Google Scholar]
- 9.Ravin KA, Rappaport LD, Zuckerbraun NS, Wadowsky RM, Wald ER, Michaels MM. Mycoplasma pneumoniae and atypical Stevens-Johnson syndrome: a case series. Pediatrics. 2007;119(4). Available at: www.pediatrics.org/cgi/content/full/119/4/e1002 [DOI] [PubMed] [Google Scholar]
- 10.Schalock PC, Dinulos JG. Mycoplasma pneumoniae–induced Stevens-Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol. 2005;52(2):312–315 [DOI] [PubMed] [Google Scholar]
- 11.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126(1):43–47 [PubMed] [Google Scholar]
- 12.Naldi L, Locati F, Marchesi L, Cainelli T. Incidence of toxic epidermal necrolysis in Italy. Arch Dermatol. 1990;126(8):1103–1104 [DOI] [PubMed] [Google Scholar]
- 13.Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML, Girre JP. Toxic epidermal necrolysis (Lyell syndrome). Incidence and drug etiology in France, 1981–1985. Arch Dermatol. 1990;126(1):37–42 [DOI] [PubMed] [Google Scholar]
- 14.Schöpf E, Stühmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome. An epidemiologic study from West Germany. Arch Dermatol. 1991;127(6):839–842 [DOI] [PubMed] [Google Scholar]
- 15.Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127(6):831–838 [PubMed] [Google Scholar]
- 16.Chen KT, Twu SJ, Chang HJ, Lin RS. Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan. Am J Public Health. 2003;93(3):489–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finland M, Jolliffe LS, Parker F, Jr. Pneumonia and erythema multiforme exudativum; report of four cases and three autopsies. Am J Med. 1948;4(4):473–492 [DOI] [PubMed] [Google Scholar]
- 18.Salmon P, Rademaker M. Erythema multiforme associated with an outbreak of Mycoplasma pneumoniae function. N Z Med J. 1993;106(966):449–450 [PubMed] [Google Scholar]
- 19.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96 [PubMed] [Google Scholar]
- 20.Robinson CC, Pretty K, Olson D, Dominguez SR. Evaluation of the FilmArray respiratory panel for detection of Mycoplasma pneumoniae and respiratory viruses in multiple specimen types. Presented at the American Society for Microbiology 30th Clinical Virology Symposium; April 2014; Daytona Beach, FL [Google Scholar]
- 21.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011;70(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter ND, Grant GB, Bandy U, et al. Community outbreak of Mycoplasma pneumoniae infection: school-based cluster of neurologic disease associated with household transmission of respiratory illness. J Infect Dis. 2008;198(9):1365–1374 [DOI] [PubMed] [Google Scholar]
- 23.Benitez AJ, Diaz MH, Wolff BJ, et al. Multilocus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical isolates from 1962 to the present: a retrospective study. J Clin Microbiol. 2012;50(11):3620–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff BJ, Thacker WL, Schwartz SB, Winchell JM. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob Agents Chemother. 2008;52(10):3542–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francois-Watkins LK, Olson D, Diaz MH, Benitez AJ, Winchell JM, Robinson CC, et al. Epidemiology and molecular characteristics of Mycoplasma pneumoniae (Mp) during an outbreak of Mp-associated Stevens-Johnson syndrome—Colorado, 2013. Presented at ID Week; October 7–12, 2014; Philadelphia, PA
- 26.Thurman KA, Walter ND, Schwartz SB, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis. 2009;48(9):1244–1249 [DOI] [PubMed] [Google Scholar]
- 27.Commission on Acute Respiratory Diseases . Association of pneumonia with erythema multiforme exudativum. Arch Intern Med. 1946;78(6):687–710 [DOI] [PubMed] [Google Scholar]
- 28.Costello MJ. Erythema multiforme exudativum (erythema bullosum malignans; pluriorificial type); personal observations of cases in Willard Parker Hospital for contagious diseases (1932–1946). J Invest Dermatol. 1947;8(3):127–144 [PubMed] [Google Scholar]
- 29.Fletcher MW, Harris RC. Erythema exudativum sultiforme (Hebra) bullous type. J Pediatr. 1945;27:465–479 [DOI] [PubMed] [Google Scholar]
- 30.Soll SN. Eruptive fever with involvement of the respiratory tract, conjunctivitis, stomatitis and balanitis; an acute clinical entity, probably of infectious origin; report of twenty cases and review of the literature. Arch Intern Med. 1947;79(5):475–500 [DOI] [PubMed] [Google Scholar]
- 31.Stanyon JH, Warner WP. Mucosal respiratory syndrome. Can Med Assoc J. 1945;53(5):427–434 [PMC free article] [PubMed] [Google Scholar]
- 32.Dresner E. Erythema multiforme exudativum; the Stevens-Johnson syndrome. Lancet. 1949;2(6588):1036–1038, illust [DOI] [PubMed] [Google Scholar]
- 33.Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72(2):239–245 [DOI] [PubMed] [Google Scholar]
- 34.Diaz MH, Benitez AJ, Winchell JM. Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance, 2006–2013. J Clin Microbiol. 2015;53(1):124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waller JL, Diaz MH, Petrone BL, et al. Detection and characterization of Mycoplasma pneumoniae during an outbreak of respiratory illness at a university. J Clin Microbiol. 2014;52(3):849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.From the Centers for Disease Control and Prevention . From the Centers for Disease Control and Prevention. Outbreak of community-acquired pneumonia caused by Mycoplasma pneumoniae—Colorado, 2000. JAMA. 2001;285(16):2073–2074 [PubMed] [Google Scholar]
- 38.Kunimi Y, Hirata Y, Aihara M, Yamane Y, Ikezawa Z. Statistical analysis of Stevens-Johnson syndrome caused by Mycoplasma pneumonia infection in Japan. Allergol Int. 2011;60(4):525–532 [DOI] [PubMed] [Google Scholar]
- 39.Wetter DA, Camilleri MJ. Clinical, etiologic, and histopathologic features of Stevens-Johnson syndrome during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2010;85(2):131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figueira-Coelho J, Lourenço S, Pires AC, Mendonça P, Malhado JA. Mycoplasma pneumoniae–associated mucositis with minimal skin manifestations. Am J Clin Dermatol. 2008;9(6):399–403 [DOI] [PubMed] [Google Scholar]
- 41.Latsch K, Girschick HJ, Abele-Horn M. Stevens-Johnson syndrome without skin lesions. J Med Microbiol. 2007;56(pt 12):1696–1699 [DOI] [PubMed] [Google Scholar]
- 42.Schalock PC, Dinulos JG. “Atypical” Stevens-Johnson syndrome? Pediatrics. 2007;120(2):451–452, author reply 452 [DOI] [PubMed] [Google Scholar]
- 43.Vanfleteren I, Van Gysel D, De Brandt C. Stevens-Johnson syndrome: a diagnostic challenge in the absence of skin lesions. Pediatr Dermatol. 2003;20(1):52–56 [DOI] [PubMed] [Google Scholar]
- 44.Birch J, Chamlin S, Duerst R, Jacobsohn D. Mycoplasma pneumoniae and atypical Stevens-Johnson syndrome in a hematopoietic stem cell transplant recipient. Pediatr Blood Cancer. 2008;50(6):1278–1279 [DOI] [PubMed] [Google Scholar]
- 45.McGouran DC, Petterson T, McLaren JM, Wolbinski MP. Mucositis, conjunctivitis but no rash—the “atypical Stevens-Johnson syndrome.” Acute Med. 2011;10(2):81–82 [PubMed] [Google Scholar]
- 46.Zipitis CS, Thalange N. Intravenous immunoglobulins for the management of Stevens-Johnson syndrome with minimal skin manifestations. Eur J Pediatr. 2007;166(6):585–588 [DOI] [PubMed] [Google Scholar]
- 47.Ramasamy A, Patel C, Conlon C. Incomplete Stevens-Johnson syndrome secondary to atypical pneumonia. BMJ Case Rep; 2011;Oct 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li K, Haber RM. Stevens-Johnson syndrome without skin lesions (Fuchs syndrome): a literature review of adult cases with Mycoplasma cause. Arch Dermatol. 2012;148(8):963–964 [DOI] [PubMed] [Google Scholar]
- 49.Meyer Sauteur PM, Gansser-Kälin U, Lautenschlager S, Goetschel P. Fuchs syndrome associated with Mycoplasma pneumoniae (Stevens-Johnson syndrome without skin lesions). Pediatr Dermatol. 2011;28(4):474–476 [DOI] [PubMed] [Google Scholar]
- 50.Havliza K, Jakob A, Rompel R. Erythema multiforme majus (Fuchs syndrome) associated with Mycoplasma pneumoniae infection in two patients. J Dtsch Dermatol Ges. 2009;7(5):445–448 [DOI] [PubMed] [Google Scholar]
- 51.Olson D, Francois-Watkins LK, Demirjian A, Lin X, Robinson CC, Glodé MP, et al. Recurrent episodes of Stevens Johnson syndrome (SJS): clinical and epidemiologic characteristics. Presented at ID Week; October 7–12, 2014; Philadelphia, PA
- 52.Letko E, Papaliodis DN, Papaliodis GN, Daoud YJ, Ahmed AR, Foster CS. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94(4):419–436; quiz 436-418, 456 [DOI] [PubMed]
- 53.Ayangco L, Rogers RS, III. Oral manifestations of erythema multiforme. Dermatol Clin. 2003;21(1):195–205 [DOI] [PubMed] [Google Scholar]
- 54.Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, Steinbach WJ. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 7th ed. Philadelphia, PA: Elsevier/Saunders; 2014 [Google Scholar]
- 55.Feigin RD. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Philadelphia, PA: Saunders/Elsevier; 2009 [Google Scholar]
- 56.Meseguer MA, de Rafael L, Vidal ML. Stevens-Johnson syndrome with isolation of Mycoplasma pneumoniae from skin lesions. Eur J Clin Microbiol. 1986;5(2):167–168 [DOI] [PubMed] [Google Scholar]
- 57.Foy HM, Grayston JT, Kenny GE, Alexander ER, McMahan R. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA. 1966;197(11):859–866 [PubMed] [Google Scholar]
- 58.Biberfeld G, Johnsson T, Jonsson J. Studies on Mycoplasma Pneumoniae infection in Sweden. Acta Pathol Microbiol Scand. 1965;63:469–475 [DOI] [PubMed] [Google Scholar]
- 59.Copps SC, Allen VD, Sueltmann S, Evans AS. A community outbreak of Mycoplasma pneumonia. JAMA. 1968;204(2):123–128 [PubMed] [Google Scholar]
- 60.Jansson E, von Essen R, Tuuri S. Mycoplasma pneumoniae pneumonia in Helsinki 1962–1970. Epidemic pattern and autoimmune manifestations. Scand J Infect Dis. 1971;3(1):51–54 [DOI] [PubMed] [Google Scholar]
- 61.Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med. 2013;10(5):e1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]