Abstract
RPE65 is a membrane-associated retinoid isomerase involved in the visual cycle responsible for sustaining vision. Many mutations in the human RPE65 gene are associated with distinct forms of retinal degenerative diseases. The pathogenic mechanisms for most of these mutations remain poorly understood. Here, we show that three Leber congenital amaurosis -associated RPE65 mutants (R91W, Y249C and R515W) undergo rapid proteasomal degradation mediated by the 26 S proteasome non-ATPase regulatory subunit 13 (PSMD13) in cultured human retinal pigment epithelium (RPE) cells. These mutant proteins formed cytosolic inclusion bodies or high molecular weight complexes via disulfide bonds. The mutations are mapped on non-active sites but severely reduced isomerase activity of RPE65. At 30°C, however, the enzymatic function and membrane-association of the mutant RPE65s are significantly rescued possibly due to proper folding. In addition, PSMD13 displayed a drastically decreased effect on degradation of the mutant proteins in the cells grown at 30°C. These results suggest that PSMD13 plays a critical role in regulating pathogenicity of the mutations and the molecular basis for the PSMD13-mediated rapid degradation and loss of function of the mutants is misfolding of RPE65.
Keywords: 26S proteasome non-ATPase regulatory subunit 13 (PSMD13), retinal degeneration, retinal pigment epithelium, retinoid isomerise, RPE65
RPE65 is a key retinoid isomerase (1–3) in the visual cycle essential for regenerating 11-cis retinaldehyde (11cRAL), the universal chromophore of the visual pigments in both cone and rod photoreceptors. This membrane-associated protein utilizes hydrophobic all-trans retinyl esters, such as all-trans retinyl palmitate, as its substrate for synthesizing 11-cis retinol (1, 2). The all-trans to 11-cis isomerization of retinoid is a rate-limiting step in the visual cycle (4). Mice lacking RPE65 (Rpe65−/− and rd12 mice) cannot synthesize 11-cis retinoids; therefore, photoreceptors in these mice lose sensitivity to light (5, 6).
More than 70 different missense mutations, including a dominant mutation, in the human RPE65 gene are associated with retinal degenerative diseases such as Leber congenital amaurosis (LCA), retinitis pigmentosa (RP), and childhood onset retinal dystrophy (Human Gene Mutation Database: www.hgmd.cf.ac.uk/ac/index.php; 7–12). Although most of these mutations have not been studied for their pathogenicity and disease mechanism, some mutations have been shown to abolish isomerase activity of RPE65 (1, 3, 13–17). Several mutations have also been shown to reduce expression levels of RPE65 in vitro (13–16, 18) and in mouse models (19–21). However, most of these mutations have not been studied for the molecular mechanisms underlying rapid degradation of the mutant proteins. Analysing the molecular pathway leading to rapid degradation of the mutant RPE65s is important for understanding the disease mechanisms of mutations and may lead to the development of a therapeutic intervention for the diseases.
The ubiquitin–proteasome pathway (UPP) is a highly selective proteolytic system that plays a pivotal role in cellular protein quality control by mediating degradation of misfolded proteins (22). Decreased proteasomal activity and proteasome overload are associated with multiple forms of inherited retinal degeneration (23, 24). PSMD13 is a positive regulator of UPP in yeast (25). The mouse PSMD13 is abundantly expressed in the retinal pigment epithelium (RPE; 18), and has been identified as a candidate for a negative regulator of RPE65 (26). We recently have shown that PSMD13 strongly promotes degradation of three disease-associated mutant RPE65s (L22P, T101I and L408P; 18). PSMD13 has been suggested to play a critical role in regulating pathogenicity of these mutations by mediating degradation of misfolded mutant RPE65s (18). Its impact on other RPE65 mutations, however, has not been studied.
Adeno-associated virus mediated therapy trials, which express wild-type RPE65 in patients’ RPE, have shown improvement in vision (27–30). However, a subsequent study reported that gene therapy did not stop the progressive retinal degeneration (31), suggesting that providing enzymatic activity alone may be not enough to block the pathological pathways leading to retinal degeneration. Recent identification of a dominant missense mutation in RPE65 of patients with RP (12) indicates that the mutated RPE65 has a dominant pathogenic effect. Some recessive missense mutations may also result in both loss of function and gain of cytotoxic function due to misfolding, mislocalization and aggregation of mutant RPE65 proteins (13, 14, 18). To prove this, further studies are needed.
Recently, we have shown that some disease-causing RPE65 mutants with mutations in non-active site can be rescued by helping their proper folding (18). In this study, we tried to extend our finding to other pathogenic mutations by testing additional three RPE65 mutants with a R91W, Y249C or R515W substitution. All these mutations are associated with LCA (9, 16, 32–34), the most severe form of retinal degeneration. The R91W substitution is one of the most frequent RPE65 mutations found in patients with LCA (9, 10, 35–38). We investigated these three RPE65 mutants with regard to their potential pathogenic mechanisms and rescue of their enzymatic function.
Materials and Methods
Cell culture, transfection and treatment
Cultured primary human RPE, ARPE-19 and 293 T-LC cells were maintained and transfected as described previously (1, 18, 39, 40). Transfected RPE cells were split into a 24-well culture plate without Millicell HA filters and incubated in modified Chee's medium to reduce expression levels of endogenous RPE65 (18, 40). Relative transfection efficiencies were determined by measuring activities of β-galactosidase from pRSV-LacZ (41) co-transfected with a wild-type or mutant RPE65 construct. For low temperature treatment, cells were maintained at 30°C under 5% CO2. For chemical treatment, cells were incubated overnight with MG132 (9 µM), MG115 (9 µM), pepstatin A (5 µM) or DMSO in the culture medium.
Site-directed mutagenesis
PCR using a QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Inc.) was carried out to introduce a mutation into the pRK5-RPE65 plasmid encoding the human RPE65 (16). Primers used for generating the mutant RPE65 constructs are shown in Table S1 in the Supplementary Material. Following mutagenesis, all sequences of the constructs were confirmed by DNA sequence analysis.
Preparation of cell membrane pellets
293 T-LC cells expressing wild-type or mutant RPE65 were resuspended in ice-cold 10 mM HEPES buffer containing protease inhibitors (Roche Life Science) and homogenized in a glass-to-glass tissue grinder. This homogenate was used as the total cell lysate. A portion of the homogenates was centrifuged at 400 ×g for 10 min to remove the cell nuclei. The resulting supernatants were centrifuged for 1 h at 100,000 × g to pellet the membranes. The membrane pellets were resuspended in HEPES buffer containing 0.1% sodium dodecyl sulfate and used as the membrane fraction.
Analysis of high molecular weight complexes
ARPE-19 cells expressing WT or mutant RPE65 were incubated with 15 µM ubiquitin-activating enzyme (E1) inhibitor (UBEI-41) for 16 h. After washing with phosphate buffered saline (PBS), the cells were incubated in a lysis buffer containing or not containing reducing reagents (100 mM dithiothreitol and/or 2% 2-mercaptoethanol) for 30 min at room temperature or for 10 min at 70°C. The cell lysates were then subjected to immunoblot analysis.
Immunoblot analysis
Protein samples prepared in the lysis buffer with or without the DTT reducing reagent were separated in a 10 or 12% polyacrylamide gel by electrophoresis. The separated proteins were then transferred onto an Immobilon-P membrane (EMD Millipore Co.). The membrane was incubated in blocking buffer, primary antibody and horseradish peroxidase-conjugated anti-rabbit antibody (PerkinElmer Inc.). Antibodies against RPE65 (42), PSMD13 (Proteintech Inc.), β-actin (Sigma-Aldrich Co.) or E-cadherin (Santa Cruz Biotechnology, Inc.) were used as primary antibodies. Immunoblots were visualized with the enhanced ECL-Prime and quantified as described previously (18). To obtain clear chemiluminescence signals in linear range, exposure times of immunoblot membrane in the ImageQuant LAS 4000 (GE Healthcare Bio-Sciences) were adjusted between 5 and 90 s in 5–20 s intervals.
Immunoprecipitation
Cells expressing RPE65 and PSMD13-Flag were lysed in 50 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40 and protease inhibitors. Immunoprecipitation was carried out using an anti-FLAG M2 affinity gel (Sigma-Aldrich Co.) as described previously (43). The precipitated proteins were used for immunoblot analysis in the presence of reducing reagents.
Knockdown of PSMD13
PSMD13 depletion in ARPE-19 cells was performed by transfecting PSMD13 siRNA (OriGene Technologies Inc.) using PolyJet (SignaGen Laboratories). Scramble siRNA was used as a negative control. The PSMD13 siRNA targets exon 9 of the human PSMD13 gene. Forty-eight hours post transfection, the cells were used for immunoblot analysis.
Retinoid isomerase assay
The 293 T-LC cells transfected with construct encoding WT or mutant RPE65 were incubated with 5 µM of all-trans retinol (atROL) for 16 h at 30 or 37°C. Retinoids extracted from the cells were saponified and analysed by normal-phase HPLC as described previously (1, 26).
Mapping of mutation sites onto the crystal structure of RPE65
The ID (4F2Z) of the bovine RPE65 structure in a lipid environment (44) was obtained from the RCSB Protein Data Bank. Mapping of mutation sites onto a three-dimensional structure of RPE65 was done using the UCSF Chimera program.
Immunocytochemistry
ARPE-19 cells grown on a glass coverslip were fixed with 4% paraformaldehyde and incubated with 0.2% Triton X-100 in PBS for 15 min. After blocking with 10% fetal bovine serum (FBS) and 2% goat serum in PBS for 1 h, the cells were incubated with RPE65 antibody overnight at 4°C and then with Alexa Fluor 555-conjugated secondary antibody (Thermo Fisher Scientific Inc.) at room temperature for 1 h. Before and after incubating with antibodies, the cells were washed with 0.1% Tween 20 in PBS three times. Nuclei were labeled with DAPI (Sigma-Aldrich Co.). Images were captured with a Zeiss LSM510 Meta confocal microscope with a 40X oil-immersion objective.
Results
PSMD13 promoted degradation of mutant RPE65s in the proteasome
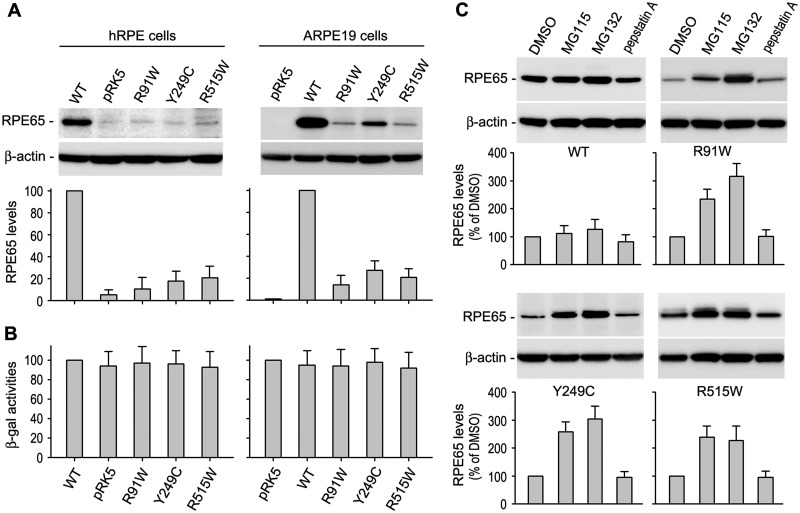
As a first step to analyse the disease mechanisms of the three RPE65 mutations (R91W, Y249C and R515W), we compared expression levels of wild-type and the mutant RPE65s in cultured human RPE cells, which retain many of the functional and morphological characteristics of RPE in vivo (40, 45–47). Immunoblot analysis of the RPE cells transfected with wild-type or mutant RPE65 construct showed that expression levels of the three mutant RPE65s were less than 30% of wild-type RPE65 (Fig. 1A). We obtained a similar result in ARPE-19 cells (Fig. 1A). Activities of β-galactosidase expressed from the pRSV-LacZ, which was co-transfected with WT or mutant RPE65 construct, were similar in all transfected cells (Fig. 1B), suggesting that the lower expression levels of the mutant RPE65s were due to post-transcriptional degradation rather than lower transfection efficiency of mutant RPE65s.
Fig. 1.
Mutant RPE65 proteins are degraded in the proteasome. (A) Immunoblot analysis of RPE65 in primary human RPE (hRPE) and ARPE-19 cells transfected with pRK5 mock vector or construct encoding wild-type (WT) or mutant (R91W, Y249C and R515W) RPE65. Beta-actin was detected as a loading control. Relative intensities of the RPE65 immunoblots were quantified, normalized by the β-actin levels, and expressed as percentage of WT RPE65. (B) Relative β-gal activities from pRSV-LacZ co-transfected with RPE65 constructs into the cells. (C) Immunoblot analysis of WT and the mutant RPE65s in ARPE-19 cells treated with inhibitors of the proteasome (MG115 and MG132) or lysosome (pepstatin A). Relative intensities of the immunoblots were quantified and expressed as percentage of RPE65 in the cells treated with DMSO. All error bars show SD (n = 3).
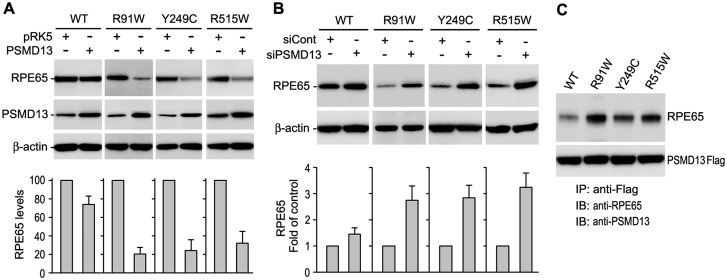
To identify the degradation pathway, we treated the cells with proteasome inhibitors (MG115, MG132) or a lysosome inhibitor (pepstatin A). Both proteasome inhibitors, but not the lysosome inhibitor, significantly increased expression levels of all three mutant RPE65s (Fig. 1C), suggesting that these mutant proteins are degraded in the proteasomes. To confirm this result, we tested the effects of PSMD13, a positive regulator of the proteasome (25), on expression levels of the mutant RPE65s. As shown in Fig. 2A, co-transfection of PSMD13 further reduced protein levels of the mutant RPE65s. Conversely, siRNA-mediated knockdown of PSMD13 significantly increased expression levels of the mutant proteins (Fig. 2B). Both overexpression and knockdown of PSMD13 exhibited mild effects on expression levels of wild-type RPE65 (Fig. 2A,B), suggesting that PSMD13 preferentially promotes degradation of mutant RPE65s. In support of this result, immunoprecipitation revealed that PSMD13 strongly interacted with mutant RPE65s (Fig. 2C).
Fig. 2.
PSMD13 mediated degradation of mutant RPE65 proteins. (A) Immunoblot analysis of WT or the indicated mutant RPE65 in ARPE-19 cells co-transfected with pRK5 mock vector or PSMD13 construct. PSMD13 expression was monitored by immunoblot analysis; β-actin was used as a loading control. Relative intensities of the RPE65 immunoblots were quantified and expressed as percentage of WT RPE65 in the histograms. (B) Immunoblot analysis showing increase in expression levels of the mutant RPE65s in ARPE-19 cells co-transfected with PSMD13 siRNA (siPSMD13). Histogram shows relative expression levels of RPE65 in the cells. (C) Immunoprecipitation showing strong interaction of PSMD13 with mutant RPE65 proteins. The 293 T-LC cells expressing PSMD13-Flag fusion protein and WT or mutant RPE65 were immunoprecipitated with a Flag antibody and the precipitates were probed with antibodies against RPE65 or PSMD13.
E1 Inhibitor increased accumulation of high molecular weight complexes of mutant RPE65s
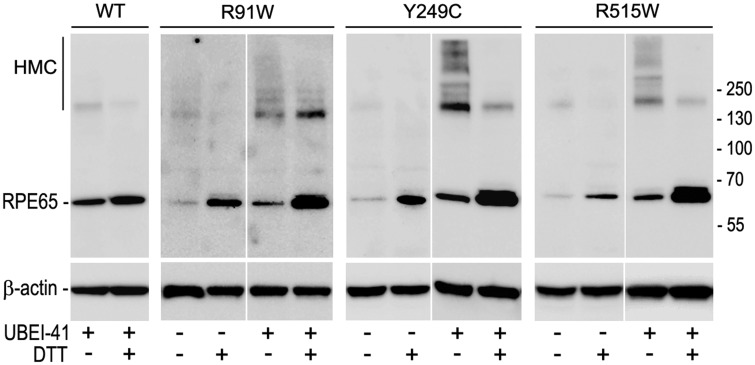
To test whether the mutant RPE65s are misfolded and undergo ubiquitination-dependent proteasomal degradation, we treated ARPE-19 cells expressing wild-type or mutant RPE65 with an inhibitor of ubiquitin-activating enzyme (E1). If ubiquitination is essential for degrading misfolded mutant RPE65, inhibition of ubiquitination should result in accumulation of misfolded RPE65. As shown in Fig 3, high molecular weight complexes (HMC) containing mutant RPE65s is significantly increased in the E1 inhibitor-treated cells compared with DMSO-treated cells. This HMC was significantly reduced when the cell lysates were treated with reducing reagent DTT (Fig. 3), suggesting that the mutant RPE65s formed HMC via disulfide bonds.
Fig. 3.
Mutant RPE65s formed high molecular weight complexes (HMC) via disulfide bonds. ARPE-19 cells expressing WT or the indicated mutant RPE65 were treated with inhibitor of ubiquitin-activating enzyme (UBEI-41) and subjected to immunoblot analysis in the presence or absence of reducing reagent (DTT).
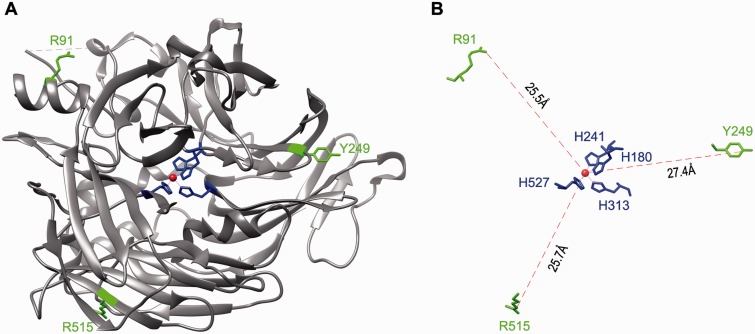
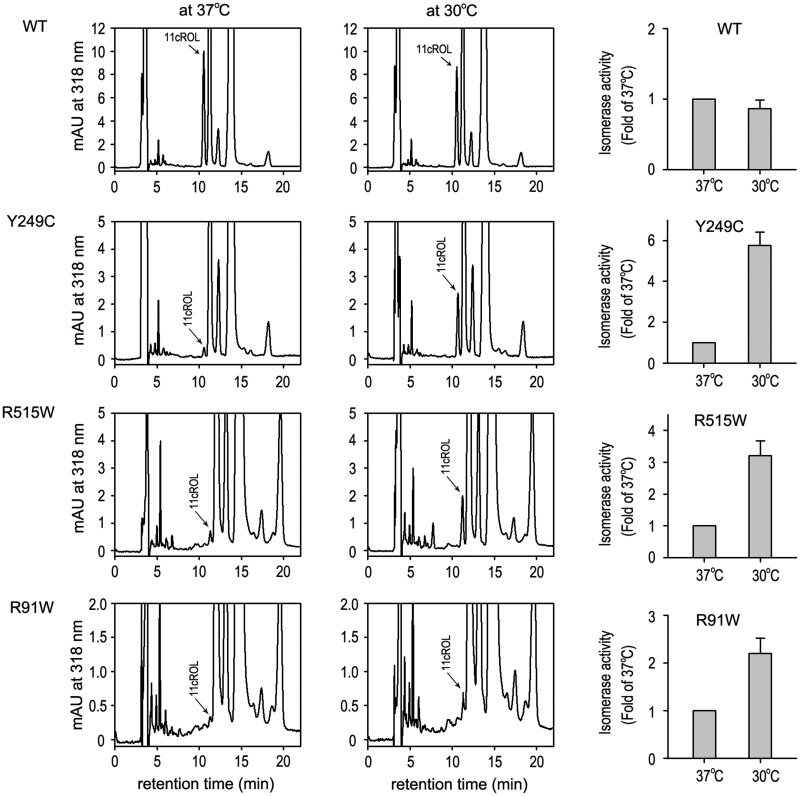
Low temperature increased isomerase activity of mutant RPE65s
By mapping the three mutation sites (R91, Y249 and R515) onto the crystal structure of the bovine RPE65, we found that these mutations are located far from the active site (Fig. 4A,B). We, therefore, tested if low temperature, which has been shown to help proper folding of many mutant proteins (18, 43, 48, 49), can rescue isomerase activity of the three mutant RPE65s. As shown in Fig 5, isomerase activities of the mutant RPE65s in the cells maintained at 30°C were increased at least 2.5-fold compared with their activities in the cells incubated at 37°C. The activity of wild-type RPE65, however, was not increased in the cells maintained at 30°C (Fig. 5).
Fig. 4.
Mapping of the three mutation sites on the crystal structure of RPE65. (A) A three-dimensional image of the crystal structure of the bovine RPE65. The catalytic site containing Fe2+ (brown sphere) and four iron-binding histidine residues is in the center of RPE65 structure. The three mutation sites (R91, Y249 and R515) shown in green are mapped in the non-active sites of RPE65. (B) A geometry showing the distances from the iron ion to the mutation sites. H180, H241, H313 and H527 are iron-binding histidine residues.
Fig. 5.
Low temperature dependent increase in isomerase activity of mutant RPE65s. Chromatograms of retinoids extracted from 293 T-LC cells expressing WT or the indicated mutant RPE65 at 37 °C or 30 °C. Peak of 11-cis retinol (11cROL) is marked by an arrow. The histograms show relative enzyme activity of WT and the mutant RPE65s at the indicated temperatures. All error bars show SD (n = 3).
PSMD13 exhibited a reduced effect on degradation of the mutant RPE65s at 30°C
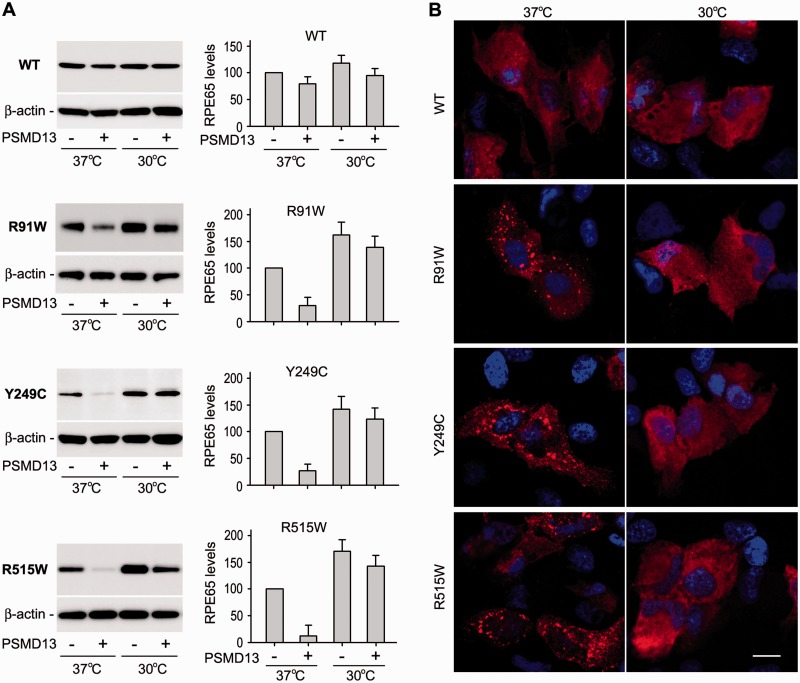
The results described above suggest that low temperature can rescue the non-active site mutant RPE65s by promoting proper folding of RPE65. If PSMD13 mediates degradation of misfolded RPE65 protein, PSMD13 should exhibit a reduced effect on degradation of mutant RPE65s at 30°C. We tested this possibility by co-expressing mutant RPE65 with PSMD13 in ARPE-19 cells grown at 30 and 37°C. At normal temperature, expression levels of the mutant RPE65s in PSMD13-co-transfected cells were reduced at least 60% compared with those in mock vector-co-transfected cells (Fig. 6A). However, expression levels of the mutant RPE65s at 30°C were significantly increased in both cells co-transfected with PSMD13 or mock vector (Fig. 6A). At this low temperature, expression levels of the mutant RPE65s in PSMD13-co-transfected cells were similar to those in mock vector-co-transfected cells (Fig. 6A), suggesting that PSMD13 mediated degradation of misfolded mutant RPE65s and low temperature promoted proper folding of the mutant RPE65s. In support of this possibility, PSMD13 displayed a mild effect on expression levels of wild-type RPE65 at both 37 and 30°C (Fig. 6A).
Fig. 6.
Decrease in PSMD13-mediated degradation and inclusion body formation of the mutant RPE65s at low temperature. (A) ARPE-19 cells co-transfected with RPE65 and pPSMD13 or pRK5 were grown at 37 or 30 °C and subjected to immunoblot analysis using antibodies against RPE65 or β-actin. Histograms show relative expression levels of WT and the mutant RPE65 proteins in the pPSMD13- or the mock vector-co-transfected cells grown at 37 or 30 °C. (B) Representative confocal microscopic images of ARPE-19 cells transfected with WT or mutant RPE65 construct. The cells were incubated at 37 or 30 °C, and stained with a RPE65 antibody. Scale bars denote 10 µm.
Low temperature inhibited inclusion body formation of mutant RPE65s
Since the three mutant RPE65s are misfolded at 37°C, we tested whether the mutant RPE65s form aggregates in the cells at 37°C. We expressed the mutant RPE65s in ARPE-19 cells at 37°C and then performed immunocytochemistry. As predicted, the mutant RPE65s formed numerous cytoplasmic inclusion bodies in the cells grown at 37°C (Fig. 6B). To test whether low temperature can reduce the formation of inclusion bodies, we performed the same experiment on the cells maintained at 30°C. Immunocytochemistry showed that cytoplasmic inclusion body formation of the mutant RPE65s were significantly reduced at 30°C (Fig. 6B).
Low temperature promoted association of the mutant RPE65s with membranes
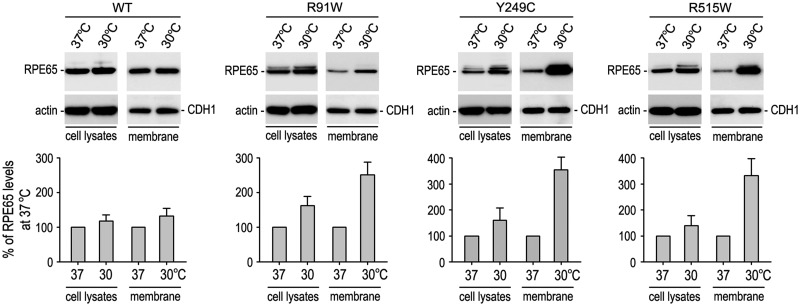
It has been shown that association of RPE65 with membranes is important for its isomerase activity (42, 50–52). We, therefore, tested if low temperature might enhance association of the mutant RPE65s with membranes. We analysed the relative content of wild-type and mutant RPE65s in membrane fractions and total homogenates of 293 T-LC cells grown at 37 or 30°C. Low temperature exhibited a mild effect on association of wild-type RPE65 with membranes (Fig. 7). In contrast, membrane-associated mutant RPE65s were increased 220–340% in the cells maintained at 30°C compared with the cells kept at 37°C (Fig. 7). The three mutant RPE65s were also increased in total homogenates of the cells maintained at 30°C compared with the homogenates of the cells kept at 37°C (Fig. 7). However, the increased rates were only 40–80% (Fig. 7), suggesting that membrane-associated mutant RPE65s may be more stable.
Fig. 7.
Increased membrane-association of the mutant RPE65s at low temperature. Total cell lysates and membrane fractions of 293 T-LC cells expressing WT or mutant RPE65 were analyzed by immunoblot analysis using antibodies against RPE65, β-actin or E-cadherin (CDH1). Histograms show relative contents of RPE65 proteins in the indicated fractions of cells maintained at 37 versus 30 °C. Contents of RPE65 in the cell lysates and membrane fractions were normalized by β-actin or CDH1 levels, respectively. Error bars show SD (n = 3).
Discussion
Based on our recent study on some disease-causing mutant RPE65s (18), we hypothesized that the majority of pathogenic mutant RPE65s with non-active site mutations can be rescued. The purpose of this study is to consolidate our hypothesis by further analysing additional three RPE65 mutants (R91W, Y249C and R515W) associated with LCA. We observed that all three mutant RPE65s undergo rapid proteasomal degradation most likely due to misfolding. This misfolding induced rapid degradation appears to be a common and critical molecular basis for their loss of enzymatic function. Consistent with this possibility, retinoid isomerase activity of the three mutant RPE65s was significantly rescued at low temperature, which inhibited degradation of the mutant proteins possibly by helping their proper folding.
RPE65 is an abundant protein in the RPE (53, 54). It has been estimated to account for 10% of total microsomal proteins in the bovine RPE (53). In contrast, its catalytic activity as the retinoid isomerase is significantly lower than those of other visual cycle enzymes (4, 55), partially due to expression of its negative regulators in the RPE (26). The high abundance of RPE65, therefore, is necessary for compensating for its low enzymatic activity in the RPE. In this study, we observed that expression levels of the three LCA-associated mutant RPE65s are at least 70% less than that of wild-type RPE65 in both primary human RPE and ARPE-19 cells (Fig. 1A). This lower expression level is one of the reasons causing loss of RPE65 function. Treatment of the cells with proteasome inhibitors (MG115 and MG132), but not a lysosome inhibitor (pepstatin A), significantly increased expression levels of the mutant RPE65s (Fig. 1C), suggesting that the decreased protein levels are mainly due to rapid degradation of mutant proteins in the proteasome. Consistent with the result, co-expression of PSMD13, a positive regulatory subunit of the proteasome (25), further reduced expression levels of the mutant RPE65s (Fig. 2A) most likely through enhancing the proteolytic function of the proteasome. In support of this possibility, knockdown of PSMD13 significantly increased expression levels of the mutant proteins (Fig. 2B). These results suggest that PSMD13 mediates degradation of the mutant RPE65s in the proteasome of the cells.
Importantly, PSMD13 appears to mediate degradation of misfolded, but not properly folded, RPE65. In yeast, PSMD13 homolog (Rpn9) interacts with multiubiquitin receptor (25). Deletion of Rpn9 results in accumulation of multiubiquitinated proteins, suggesting that PSMD13/Rpn9 mediates proteasomal degradation of multiubiquitinated proteins via direct or indirect interaction (25). In this study, we observed that PSMD13 strongly interacted with mutant RPE65 proteins (Fig. 2C), which might be ubiquitinated due to misfolding (Fig. 3). We also observed that the three RPE65 mutants formed numerous cytoplasmic inclusion bodies (Fig. 6B), suggesting that the mutant RPE65s are misfolded. The interaction of PSMD13 with mutant RPE65s might mediate degradation of the misfolded mutant proteins in the proteasome at normal temperature. At 30°C, however, PSMD13 exhibited a significantly reduced effect on degradation of these mutant RPE65s (Fig. 6A). At this low temperature, not only inclusion body formation is reduced (Fig. 6B) but also enzymatic function of the mutant RPE65s is significantly rescued (Fig. 5), suggesting that low temperature promoted proper folding of these mutant RPE65s. In addition, PSMD13 exhibited a mild effect on degradation of wild-type RPE65 at both 30 and 37°C (Figs 2, 6A). These results suggest that PSMD13 plays an important role in protein quality control by mediating degradation of misfolded protein. Formation of the HMC and cytoplasmic inclusion bodies (Figs 3 and 6B) suggests that the mutant RPE65 proteins may cause acute or chronic cellular stress. PSMD13 is, therefore, a critical player in eliminating or reducing the cytotoxic effect of misfolded mutant RPE65s.
We recently suggested that whether a mutation is mapped on the active or near the active site is a critical factor in determining if the enzymatic function of the mutant RPE65 can be rescued at low temperature (18). As expected, the isomerase activities of the three mutant RPE65s, whose mutation sites are localized on the non-active sites (Fig. 4), are significantly increased at 30°C (Fig. 5). Inclusion body formation of these mutant RPE65s is also significantly reduced at 30°C (Fig. 6B). These results agree with the effects of low temperature on reversing cellular mislocalization and rescuing the function of some other mutant proteins (43, 48, 49). Myocilin and the interphotoreceptor retinoid-binding protein (IRBP) are secretory proteins. However, the glaucoma-causing mutant myocilin and the retinitis pigmentosa-associated IRBP cause endoplasmic reticulum (ER) stress due to misfolding and subsequent accumulation in the ER (43, 56). At low temperature, these mutant proteins can be secreted and the ER stress is significantly reduced (43, 49). Similarly, we recently showed that low temperature not only rescued the isomerase activity but also reduced aggregate formation of three other disease-causing mutant RPE65s (18).
Previous studies have shown that association of RPE65 with membranes is important for its substrate-binding and isomerase function (42, 50–52, 57). Misfolding and subsequent mislocalization of the mutant RPE65s might disrupt their membrane-association, substrate-binding and enzymatic function. We observed that low temperature significantly increased association of the three mutant RPE65s with membranes (Fig. 7). This increased membrane association is most likely due to proper folding and might contribute to the increased isomerase activities of the mutant RPE65s at low temperature (Fig. 5).
Although experimental confirmation is needed, our results strongly suggest that many pathogenic mutant RPE65s with non-active site mutations can be rescued for their enzymatic function by physical and chemical approaches. Since the majority of disease-causing missense mutations in RPE65 are non-active site mutations, this study provides important clues for further investigation of the pathogenic mechanism and functional rescue strategy for these mutant RPE65s associated with retinal degenerative diseases.
Supplementary Data
Supplementary Data are available at JB Online.
Funding
This work was supported by grants from National Institutes of Health (EY021208 to M. J.; EY017280 to S. G. J.; GM103340 to Louisiana State University Neuroscience COBRE facilities), Macula Vision Research Foundation (to D. B. and to S.G.J.), Research to Prevent Blindness (to Louisiana State University Department of Ophthalmology) and Louisiana Lions Eye Foundation (to Louisiana State University Department of Ophthalmology). DB holds the Dolly Green Chair at the David Geffen School of Medicine, University of California, Los Angeles.
Conflict of Interest
None declared.
Supplementary Material
Glossary
Abbreviations
- 11cRAL
11-cis retinaldehyde
- 11cROL
11-cis retinol
- DAPI
4',6-diamidino-2-phenylindole
- DMSO
Dimethyl sulfoxide
- DTT
Dithiothreitol
- ER
endoplasmic reticulum
- HMC
high molecular weight complexes
- HPLC
high-performance liquid chromatography
- PSMD13
26
S proteasome non-ATPase regulatory subunit 13
- RPE
retinal pigment epithelium
- SD
standard deviation
- UPP
ubiquitin-proteasome pathway
References
- 1.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winston A, Rando RR. Regulation of isomerohydrolase activity in the visual cycle. Biochemistry. 1998;37:2044–2050. doi: 10.1021/bi971908d. [DOI] [PubMed] [Google Scholar]
- 5.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 6.Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, Noorwez SM, Malhotra R, McDowell JH, Kaushal S, Hauswirth WW, Nusinowitz S, Thompson DA, Heckenlively JR. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA) Mol. Vis. 2005;11:152–162. [PubMed] [Google Scholar]
- 7.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 8.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber's congenital amaurosis. Nat. Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 9.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc. Natl. Acad. Sci. USA. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson DA, Gyurus P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, Sieving PA, Gal A. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- 11.Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Heon E, Levin A, Jan J, Lam B, Carr RE, Franklin A, Radha S, Andorf JL, Sheffield VC, Stone EM. Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch. Ophthalmol. 2000;118:538–543. doi: 10.1001/archopht.118.4.538. [DOI] [PubMed] [Google Scholar]
- 12.Bowne SJ, Humphries MM, Sullivan LS, Kenna PF, Tam LC, Kiang AS, Campbell M, Weinstock GM, Koboldt DC, Ding L, Fulton RS, Sodergren EJ, Allman D, Millington-Ward S, Palfi A, McKee A, Blanton SH, Slifer S, Konidari I, Farrar GJ, Daiger SP, Humphries P. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur. J. Hum. Genet. 2011;19:1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Moiseyev G, Takahashi Y, Ma JX. Impacts of two point mutations of RPE65 from Leber's congenital amaurosis on the stability, subcellular localization and isomerohydrolase activity of RPE65. FEBS Lett. 2006;580:4200–4204. doi: 10.1016/j.febslet.2006.06.078. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi Y, Chen Y, Moiseyev G, Ma JX. Two point mutations of RPE65 from patients with retinal dystrophies decrease the stability of RPE65 protein and abolish its isomerohydrolase activity. J. Biol. Chem. 2006;281:21820–21826. doi: 10.1074/jbc.M603725200. [DOI] [PubMed] [Google Scholar]
- 15.Bereta G, Kiser PD, Golczak M, Sun W, Heon E, Saperstein DA, Palczewski K. Impact of retinal disease-associated RPE65 mutations on retinoid isomerization. Biochemistry. 2008;47:9856–9865. doi: 10.1021/bi800905v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philp AR, Jin M, Li S, Schindler EI, Iannaccone A, Lam BL, Weleber RG, Fishman GA, Jacobson SG, Mullins RF, Travis GH, Stone EM. Predicting the pathogenicity of RPE65 mutations. Hum. Mutat. 2009;30:1183–1188. doi: 10.1002/humu.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolaeva O, Takahashi Y, Moiseyev G, Ma JX. Negative charge of the glutamic acid 417 residue is crucial for isomerohydrolase activity of RPE65. Biochem. Biophys. Res. Commun. 2010;391:1757–1761. doi: 10.1016/j.bbrc.2009.12.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Izumi T, Hu J, Jin HH, Siddiqui AA, Jacobson SG, Bok D, Jin M. Rescue of Enzymatic Function for Disease-associated RPE65 Proteins Containing Various Missense Mutations in Non-active Sites. J. Biol. Chem. 2014;289:18943–18956. doi: 10.1074/jbc.M114.552117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samardzija M, von Lintig J, Tanimoto N, Oberhauser V, Thiersch M, Reme CE, Seeliger M, Grimm C, Wenzel A. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum. Mol. Genet. 2008;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- 20.Won J, Shi LY, Hicks W, Wang J, Hurd R, Naggert JK, Chang B, Nishina PM. Mouse model resources for vision research. J. Ophthalmol. 2011;2011:391384. doi: 10.1155/2011/391384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright CB, Chrenek MA, Foster SL, Duncan T, Redmond TM, Pardue MT, Boatright JH, Nickerson JM. Complementation test of Rpe65 knockout and tvrm148. Invest. Ophthalmol. Vis. Sci. 2013;54:5111–5122. doi: 10.1167/iovs.13-12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang F, Taylor A. Role of the ubiquitin-proteasome in protein quality control and signaling: implication in the pathogenesis of eye diseases. Prog. Mol. Biol. Transl. Sci. 2012;109:347–396. doi: 10.1016/B978-0-12-397863-9.00010-9. [DOI] [PubMed] [Google Scholar]
- 23.Lobanova ES, Finkelstein S, Skiba NP, Arshavsky VY. Proteasome overload is a common stress factor in multiple forms of inherited retinal degeneration. Proc. Natl. Acad. Sci. USA. 2013;110:9986–9991. doi: 10.1073/pnas.1305521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando R, Noda K, Tomaru U, Kamoshita M, Ozawa Y, Notomi S, Hisatomi T, Noda M, Kanda A, Ishibashi T, Kasahara M, Ishida S. Decreased proteasomal activity causes photoreceptor degeneration in mice. Invest. Ophthalmol. Vis. Sci. 2014;55:4682–4690. doi: 10.1167/iovs.13-13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeuchi J, Fujimuro M, Yokosawa H, Tanaka K, Toh-e A. Rpn9 is required for efficient assembly of the yeast 26S proteasome. Mol. Cell. Biol. 1999;19:6575–6584. doi: 10.1128/mcb.19.10.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Lee J, Zhou Y, Gordon WC, Hill JM, Bazan NG, Miner JH, Jin M. Fatty acid transport protein 4 (FATP4) prevents light-induced degeneration of cone and rod photoreceptors by inhibiting RPE65 isomerase. J. Neurosci. 2013;33:3178–3189. doi: 10.1523/JNEUROSCI.2428-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 28.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl. Acad. Sci. USA. 2013;110:E517–525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson RH, Waseem N, Searle R, van der Spuy J, Russell-Eggitt I, Bhattacharya SS, Thompson DA, Holder GE, Cheetham ME, Webster AR, Moore AT. An assessment of the apex microarray technology in genotyping patients with Leber congenital amaurosis and early-onset severe retinal dystrophy. Invest. Ophthalmol. Vis. Sci. 2007;48:5684–5689. doi: 10.1167/iovs.07-0207. [DOI] [PubMed] [Google Scholar]
- 33.Kondo H, Qin M, Mizota A, Kondo M, Hayashi H, Hayashi K, Oshima K, Tahira T, Hayashi K. A homozygosity-based search for mutations in patients with autosomal recessive retinitis pigmentosa, using microsatellite markers. Invest. Ophthalmol. Vis. Sci. 2004;45:4433–4439. doi: 10.1167/iovs.04-0544. [DOI] [PubMed] [Google Scholar]
- 34.Katagiri S, Hayashi T, Kondo M, Tsukitome H, Yoshitake K, Akahori M, Ikeo K, Tsuneoka H, Iwata T. RPE65 Mutations in Two Japanese Families with Leber Congenital Amaurosis. Ophthalmic Genet. 2014:1–9. doi: 10.3109/13816810.2014.991931. [DOI] [PubMed] [Google Scholar]
- 35.Lorenz B, Gyurus P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- 36.El Matri L, Ambresin A, Schorderet DF, Kawasaki A, Seeliger MW, Wenzel A, Arsenijevic Y, Borruat FX, Munier FL. Phenotype of three consanguineous Tunisian families with early-onset retinal degeneration caused by an R91W homozygous mutation in the RPE65 gene. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:1104–1112. doi: 10.1007/s00417-005-0096-2. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, Wilson JM, Aguirre GD, Stone EM, Palczewski K. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. USA. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seong MW, Kim SY, Yu YS, Hwang JM, Kim JY, Park SS. Molecular characterization of Leber congenital amaurosis in Koreans. Mol. Vis. 2008;14:1429–1436. [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye. Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- 41.Shirasawa H, Jin MH, Shimizu K, Akutsu N, Shino Y, Simizu B. Transcription-modulatory activity of full-length E6 and E6*I proteins of human papillomavirus type 16. Virology. 1994;203:36–42. doi: 10.1006/viro.1994.1452. [DOI] [PubMed] [Google Scholar]
- 42.Jin M, Yuan Q, Li S, Travis GH. Role of LRAT on the retinoid isomerase activity and membrane association of Rpe65. J. Biol. Chem. 2007;282:20915–20924. doi: 10.1074/jbc.M701432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Yang Z, Hu J, Gordon WC, Bazan NG, Haas AL, Bok D, Jin M. Secretory defect and cytotoxicity: the potential disease mechanisms for the retinitis pigmentosa (RP)-associated interphotoreceptor retinoid-binding protein (IRBP) J. Biol. Chem. 2013;288:11395–11406. doi: 10.1074/jbc.M112.418251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiser PD, Farquhar ER, Shi W, Sui X, Chance MR, Palczewski K. Structure of RPE65 isomerase in a lipidic matrix reveals roles for phospholipids and iron in catalysis. Proc. Natl. Acad. Sci. USA. 2012;109:E2747–2756. doi: 10.1073/pnas.1212025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlson A, Bok D. Promotion of the release of 11-cis-retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992;31:9056–9062. doi: 10.1021/bi00152a049. [DOI] [PubMed] [Google Scholar]
- 46.Carlson A, Bok D. Polarity of 11-cis retinal release from cultured retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1999;40:533–537. [PubMed] [Google Scholar]
- 47.Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, Dvash T, Yang XJ, Travis GH, Williams DS, Bok D, Fan G. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum. Mol. Genet. 2010;19:4229–4238. doi: 10.1093/hmg/ddq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 49.Vollrath D, Liu Y. Temperature sensitive secretion of mutant myocilins. Exp. Eye Res. 2006;82:1030–1036. doi: 10.1016/j.exer.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaeva O, Takahashi Y, Moiseyev G, Ma JX. Purified RPE65 shows isomerohydrolase activity after reassociation with a phospholipid membrane. FEBS J. 2009;276:3020–3030. doi: 10.1111/j.1742-4658.2009.07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi Y, Moiseyev G, Ablonczy Z, Chen Y, Crouch RK, Ma JX. Identification of a novel palmitylation site essential for membrane association and isomerohydrolase activity of RPE65. J. Biol. Chem. 2009;284:3211–3218. doi: 10.1074/jbc.M807248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golczak M, Kiser PD, Lodowski DT, Maeda A, Palczewski K. Importance of membrane structural integrity for RPE65 retinoid isomerization activity. J. Biol. Chem. 2010;285:9667–9682. doi: 10.1074/jbc.M109.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bavik CO, Busch C, Eriksson U. Characterization of a plasma retinol-binding protein membrane receptor expressed in the retinal pigment epithelium. J. Biol. Chem. 1992;267:23035–23042. [PubMed] [Google Scholar]
- 54.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J. Biol. Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 55.Saari JC, Bredberg DL. CoA- and non-CoA-dependent retinol esterification in retinal pigment epithelium. J. Biol. Chem. 1988;263:8084–8090. [PubMed] [Google Scholar]
- 56.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 57.Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J. Biol. Chem. 2004;279:635–643. doi: 10.1074/jbc.M310042200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.