Abstract
Purpose
Blockade of the programmed death-1 inhibitory cell-surface molecule on immune cells using the fully human immunoglobulin G4 antibody nivolumab mediates tumor regression in a portion of patients with advanced treatment-refractory solid tumors. We report clinical activity, survival, and long-term safety in patients with advanced renal cell carcinoma (RCC) treated with nivolumab in a phase I study with expansion cohorts.
Patients and Methods
A total of 34 patients with previously treated advanced RCC, enrolled between 2008 and 2012, received intravenous nivolumab (1 or 10 mg/kg) in an outpatient setting once every two weeks for up to 96 weeks and were observed for survival and duration of response after treatment discontinuation.
Results
Ten patients (29%) achieved objective responses (according to RECIST [version 1.0]), with median response duration of 12.9 months; nine additional patients (27%) demonstrated stable disease lasting > 24 weeks. Three of five patients who stopped treatment while in response continued to respond for ≥ 45 weeks. Median overall survival in all patients (71% with two to five prior systemic therapies) was 22.4 months; 1-, 2-, and 3-year survival rates were 71%, 48%, and 44%, respectively. Grade 3 to 4 treatment-related adverse events occurred in 18% of patients; all were reversible.
Conclusion
Patients with advanced treatment-refractory RCC treated with nivolumab demonstrated durable responses that in some responders persisted after drug discontinuation. Overall survival is encouraging, and toxicities were generally manageable. Ongoing randomized clinical trials will further assess the impact of nivolumab on overall survival in patients with advanced RCC.
INTRODUCTION
An improved understanding of renal cell carcinoma (RCC) biology has led to major advances in the treatment of patients with metastatic disease.1–4 Although agents that target the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathways prolong progression-free and likely overall survival, resistance invariably develops, often within the first year of therapy.1 For two decades, the clinical experience with high-dose interleukin-2 (IL-2) has provided proof of principle that immunotherapy can produce durable post-treatment responses in a small percentage of patients with RCC.5 However, the toxicity and limited efficacy of high-dose IL-2 have restricted its application. Developing agents that can induce a high proportion of durable tumor responses with acceptable toxicity profiles remains an unmet need for this patient population.
The inhibitory mechanisms that govern the interaction between an evolving tumor and the host immune response provide one explanation for why immunotherapies frequently fail to produce clinically relevant responses. A critical regulator of tumor-induced immune suppression is the programmed death-1 (PD-1) pathway.6 Many human solid tumors, including a proportion of RCC, express programmed death-ligand 1 (PD-L1; B7-H1), one of two ligands for PD-1.7 PD-L1 expression may be either constitutive, as a consequence of activation of an oncogenic pathway, or induced, as a consequence of infiltrating immune cell production of interferons.8,9 PD-1 engagement by its ligands (eg, PD-L1, PD-L2) inhibits T-cell proliferation, cytokine production, cytolytic function, and survival.10 Tumor-infiltrating lymphocytes typically express PD-1 and have impaired antitumor functionality in situ.11,12 PD-L1 expression on kidney tumor cells has been associated with higher tumor grade and worse prognosis, highlighting the potential clinical impact of this interaction.7 Phase I trials have been initiated with several monoclonal antibodies that block the binding of PD-1 to its ligands in an effort to restore immune function at the tumor site and induce antitumor activity without the significant toxicity associated with systemic cytokine administration.13–16
Nivolumab is a fully human immunoglobulin G4 PD-1 immune checkpoint–blocking antibody that specifically binds to PD-1 and disrupts negative signaling to restore T-cell antitumor function.17–19 In a first-in-human, dose-escalation safety trial, nivolumab was associated with clinical activity and a favorable safety profile in patients with several advanced solid tumors, including RCC.20 Further exploration of nivolumab in a multidose phase I study (ClinicalTrials.gov No. NCT00730639) showed objective tumor regressions in patients with advanced treatment-refractory melanoma (32%), non–small-cell lung cancer (17%), or RCC (29%).14
Here, we report the clinical activity, overall survival outcome, and long-term safety profile in patients with advanced RCC receiving nivolumab, with a minimum of 78 weeks since treatment initiation, some of whom completed the entire planned 96-week treatment course.
PATIENTS AND METHODS
Study Design
This dose-escalation, cohort-expansion study evaluated the safety and antitumor activity of nivolumab in patients with RCC, melanoma, non–small-cell lung cancer, colorectal cancer, and castration-resistant prostate cancer. The study design and methods, including protocol, amendments, and detailed statistical analysis plan, were previously published.14 This study was approved by local institutional review boards, and all patients or their legal representatives provided written informed consent before enrollment. Nivolumab was administered intravenously once every 2 weeks in an outpatient setting in 8-week treatment cycles, at 1, 3, or 10 mg/kg during the dose-escalation phase. The RCC population was treated with nivolumab 10 mg/kg in an initial expansion cohort, followed by a subsequent expansion cohort at 1 mg/kg. On the basis of observed objective responses, the protocol was further amended (January 23, 2012) to include the overall survival of patients as an exploratory end point.
Tumors were assessed radiographically after each 8-week treatment cycle. Treatment continued up to 96 weeks (12 cycles) or until patients experienced confirmed complete response, unacceptable toxicity, or progressive disease or withdrew consent. In clinically stable patients, treatment could continue beyond initial disease progression pending subsequent confirmation of progression, consistent with proposed immune response criteria.21 Patients with stable disease or an ongoing objective response (complete or partial) at the end of treatment were observed for up to 1 year and were offered the option of re-treatment for 1 additional year if disease progressed.
Clinical and laboratory safety assessments were conducted in all treated patients at regular intervals during therapy and up to 70 days after last drug administration. Adverse event severity was graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).22 Immune-mediated adverse events with potential immunologic etiologies that might require more frequent monitoring or possible intervention with immune suppression or hormone replacement therapies were identified based on a prespecified list of Medical Dictionary for Regulatory Activities terms.
Participants
Eligibility criteria have been previously described.14 Patients had received at least one, but not more than five, prior systemic cancer therapies. Those with a history of autoimmune disease, prior therapy with T-cell modulating antibodies (eg, anti–PD-1, anti–PD-L1, anticytotoxic T-lymphocyte–associated antigen 4 [CTLA-4]), conditions requiring immunosuppression, or chronic infections were excluded.
Statistical Analyses
Tumor measurements were collected by investigators, and individual best responses were centrally assessed by the sponsor per modified RECIST (version 1.0) criteria.23 Objective response and stable disease rates were estimated with CIs using the Clopper-Pearson method.24 Time-to-event end points, including progression-free survival, overall survival, survival rates, and response duration, were estimated using the Kaplan-Meier method, with CIs for the medians based on the Brookmeyer-Crowley method and CIs for overall and progression-free survival rates based on the Greenwood formula. Survival data were collected retrospectively. Adverse events were coded using Medical Dictionary for Regulatory Activities (version 15.1). Efficacy analyses, including overall survival results for all patients, are reported as of September 2013. Baseline characteristics and adverse events are reported as of March 2013.
RESULTS
Patient Characteristics
Thirty-four patients with advanced RCC began treatment with nivolumab (1 mg/kg, n = 18; 10 mg/kg, n = 16) from November 2008 through January 2012. Baseline characteristics of these patients are listed in Table 1. Of note, 71% had received at least two prior systemic treatments for RCC; 71% had received antiangiogenic therapy; 71% had received immunologic, biologic, or hormone therapy; 32% had received an mTOR inhibitor; and 88% had a visceral metastatic lesion at baseline. The median duration of survival follow-up at the time of analysis was 45.2 months (range, 25.9 to 57.9 months).
Table 1.
Baseline Characteristics of All Treated Patients With RCC (N = 34)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 58 | |
| Range | 35-74 | |
| Sex | ||
| Male | 26 | 76 |
| Female | 8 | 24 |
| ECOG performance status* | ||
| 0 | 17 | 50 |
| 1 | 17 | 50 |
| No. of prior treatment regimens | ||
| 1 | 10 | 29 |
| 2 | 9 | 27 |
| 3 | 6 | 18 |
| ≥ 4 | 9 | 27 |
| Prior therapy | ||
| Surgery | 32 | 94 |
| Antiangiogenic agent | 24 | 71 |
| Hormonal, immunologic, or biologic | 24 | 71 |
| Chemotherapy | 19 | 56 |
| mTOR inhibitor | 11 | 32 |
| Radiotherapy | 10 | 29 |
| Lesions at baseline | ||
| Bone | 10 | 29 |
| Liver | 9 | 27 |
| Lung | 30 | 88 |
| Lymph node | 28 | 82 |
| Any visceral site | 30 | 88 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; mTOR, mammalian target of rapamycin; RCC, renal cell carcinoma.
Criteria as described in report by Oken et al.25
Response Rate, Duration of Response, Stable Disease, and Tumor Kinetics
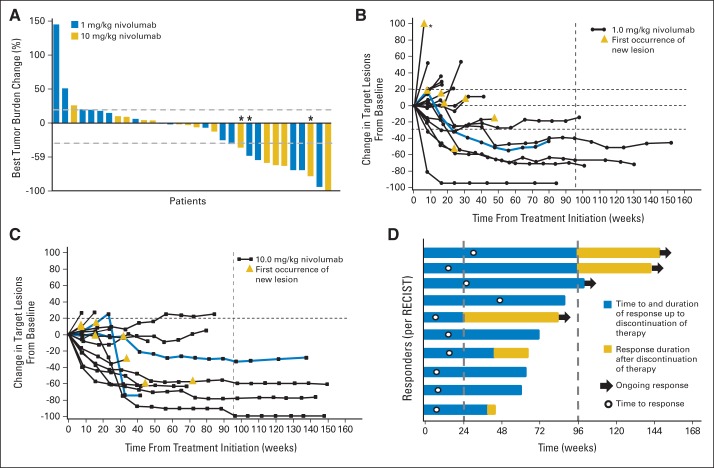
Objective responses (per RECIST) were observed in 29% of patients (10 of 34), with responses seen at both nivolumab doses tested; an additional 27% of patients (nine of 34) experienced stable disease lasting for at least 24 weeks (Table 2). Three additional patients (9%) experienced unconventional immune-related responses that did not fit RECIST criteria (eg, persistent reduction in target lesions in presence of new lesions or regression after initial progression; Figs 1A to 1C).21 Overall, 20 (63%) of 32 patients demonstrated some degree of tumor shrinkage (from 1% to 100%; Fig 1A).
Table 2.
Clinical Activity of Nivolumab in Patients With RCC
| Dose (mg/kg) | Objective Response Rate* |
Duration of Response (months)† |
Stable Disease (weeks) |
PFS (months) |
OS (months) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 24 |
≥ 48 |
||||||||||||||
| No. of Patients | % | 95% CI | Median | Individual Durations | No. of Patients | % | 95% CI | No. of Patients | % | 95% CI | Median | 95% CI | Median | 95% CI | |
| Both doses | 10 of 34 | 29.4 | 15.1 to 47.5 | 12.9 | 8.4, 9.2, 11.4, 11.4+, 12, 13, 13, 17.5+, 26.9+, 29.1+ | 9 of 34 | 26.5 | 12.9 to 44.4 | 2 of 34 | 5.9 | 0.7 to 19.7 | 7.3 | 3.6 to 10.9 | 22.4 | 12.5 to NE |
| 1 | 5 of 18 | 27.8 | 9.7 to 53.5 | 12.9 | 9.2, 11.4, 11.4+, 13, 17.5+ | 4 of 18 | 22.2 | 6.4 to 47.6 | 1 of 18 | 5.6 | 0.1 to 27.3 | 4.7 | 1.9 to 10.9 | 29.3 | 11.5 to NE |
| 10 | 5 of 16 | 31.3 | 11.0 to 58.7 | 12.9 | 8.4, 12, 13, 26.9+, 29.1+ | 5 of 16 | 31.3 | 11.0 to 58.7 | 1 of 16 | 6.3 | 0.2 to 30.2 | 8.0 | 1.7 to 14.0 | 18.8 | 11.4 to NE |
Abbreviations: CR, complete response; NE, not estimable; OS, overall survival; PFS, progression-free survival; PR, partial response; RCC, renal cell carcinoma.
Objective response rates were calculated based on confirmed responses: ([CR + PR]/ No.) × 100. CIs were calculated using Clopper-Pearson method. Individual patient responses were adjudicated per RECIST (version 1.0) with modification. One CR was noted (in 10-mg/kg cohort).
Kaplan-Meier estimate; time from first response to time of documented progression, death, or (for censored data denoted by +) time to last tumor assessment.
Fig 1.
Characteristics of tumor regression in patients with renal cell carcinoma receiving nivolumab therapy. (A) Maximum reduction or minimum increase in sum of target lesion measurements compared with baseline in all treated patients with on-treatment tumor measurements (n = 32). Bar colors indicate nivolumab dose cohorts 1 or 10 mg/kg. Graph shows best individual change during study. Tumors were assessed after each cycle per RECIST (version 1.0) guidelines. Baseline tumor measurements were standardized to zero, and tumor burden was measured as sum of longest diameters of target lesions. Horizontal line at 20% indicates threshold for defining progressive disease according to RECIST; horizontal line at −30% indicates threshold for defining objective response (partial tumor regression) in absence of new lesions or nontarget disease progression according to RECIST. Objective responses were observed at both dose levels (1 and 10 mg/kg). Unconventional response patterns that did not meet RECIST criteria (eg, persistent reduction in target lesions in presence of new lesions, regression after initial progression) were observed in three patients (9%; indicated by asterisks). Response kinetics in patients receiving nivolumab (B) 1 or (C) 10 mg/kg. Baseline tumor measurements were standardized to zero. Tumor burden was measured as sum of longest diameters of target lesions. (B) Asterisk indicates off-scale value of 144%. Gold triangles indicate first occurrence of new lesion. (B, C) Vertical line at 96 weeks demonstrates maximum duration of planned continuous nivolumab therapy; horizontal line at −30% marks threshold for defining objective response (partial tumor regression) according to RECIST, and horizontal line at +20% indicates the threshold for defining progressive disease. Blue curves indicate unconventional immune-related response patterns that did not meet RECIST criteria in (B) one patient at 1-mg/kg dose and (C) two patients at 10-mg/kg dose. Objective responses, unconventional responses, and stable disease persisted after treatment discontinuation in some patients. According to RECIST criteria, 56% of patients (19 of 34) achieved objective response or disease stabilization exceeding 24 weeks (Table 2). (D) Durability of tumor regressions. Ten (29%) of 34 patients had objective tumor regressions, including five (28%) of 18 patients receiving nivolumab 1 mg/kg and five (31%) of 16 patients receiving 10 mg/kg. Blue bars indicate time to and duration of response during treatment; gold bars indicate response duration after treatment discontinuation; open circles indicate first evidence of objective response; arrows indicate ongoing response at time of analysis. Vertical line at 96 weeks indicates maximum duration of planned continuous nivolumab therapy. Reasons for treatment discontinuation with ongoing response included investigator-assessed complete response, attainment of maximum treatment duration, adverse events, investigator discretion, and withdrawal of patient consent.
The maximum change in the sum of target lesion dimensions from baseline is shown in Figure 1A, and tumor kinetics for both doses are shown in Figures 1B and 1C, respectively. In the 10 objective responders, the median duration of response was 12.9 months (range, 8.4 to 29.1+ months). Four (40%) of 10 responses were ongoing at the time of data analysis (Fig 1D), including three that persisted for approximately 1 year after treatment discontinuation. Some of these responses occurred rapidly, with four of the 10 responding patients achieving an objective response at the first radiographic assessment, 8 weeks after starting treatment (Figs 1B to 1D). The majority (70%) of responding patients demonstrated a response by the second assessment (16 weeks; Fig 1D), with a typical median time to response of 16 weeks (range, 8 to 48 weeks). Four of five patients who discontinued therapy for reasons other than disease progression were observed after treatment discontinuation. In these four patients, responses lasted an additional 19, 45+, 51+, and 59+ weeks after discontinuation. Tumor regression was observed at various anatomic sites, including lymph nodes, bone, and visceral lesions, and in primary as well as metastatic lesions (Fig 2).
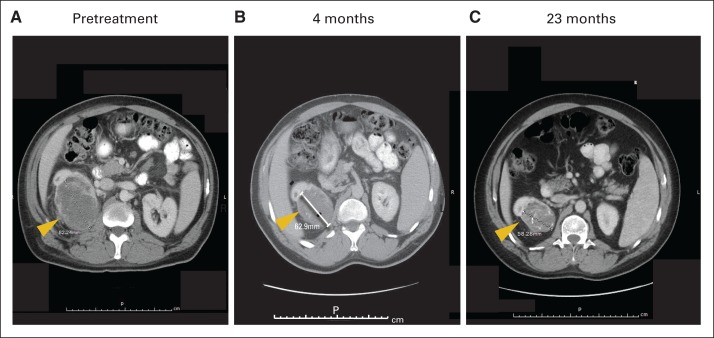
Fig 2.
Computed tomography scans showing partial response in primary tumor of metastatic renal cell carcinoma in patient treated with nivolumab; 48-year-old patient with low-volume but poorly differentiated disease who developed progressive disease after sunitinib, sorafenib, and thoracic surgery achieved partial response in primary tumor (indicated by arrows) after treatment with nivolumab 1 mg/kg. Treatment was held after three cycles, and response continued for 3 years after therapy.
Unconventional immune-related response patterns that did not meet RECIST criteria were observed in an additional three patients (9%); one received nivolumab at 1 mg/kg, and two at 10 mg/kg (Figs 1B and 1C). Unconventional responders were not included in calculations of objective response rate. Overall survival for the unconventional responders was 26.1+ at the 1-mg/kg dose and 11.6 and 43 months, respectively, at the 10-mg/kg dose.
Overall and Progression-Free Survival
On the basis of preliminary findings of durable responses in patients with RCC treated with nivolumab,14 an analysis of overall survival was performed. All patients initiated treatment at least 18 months before analysis. Median overall survival was 22.4 months (Table 2; Fig 3A). One-, 2-, and 3-year survival rates were 71%, 48%, and 44%, respectively (Fig 3A). Median progression-free survival was 7.3 months, with 1- and 2-year progression-free survival rates of 35% and 12%, respectively (Table 2; Fig 3B).
Fig 3.
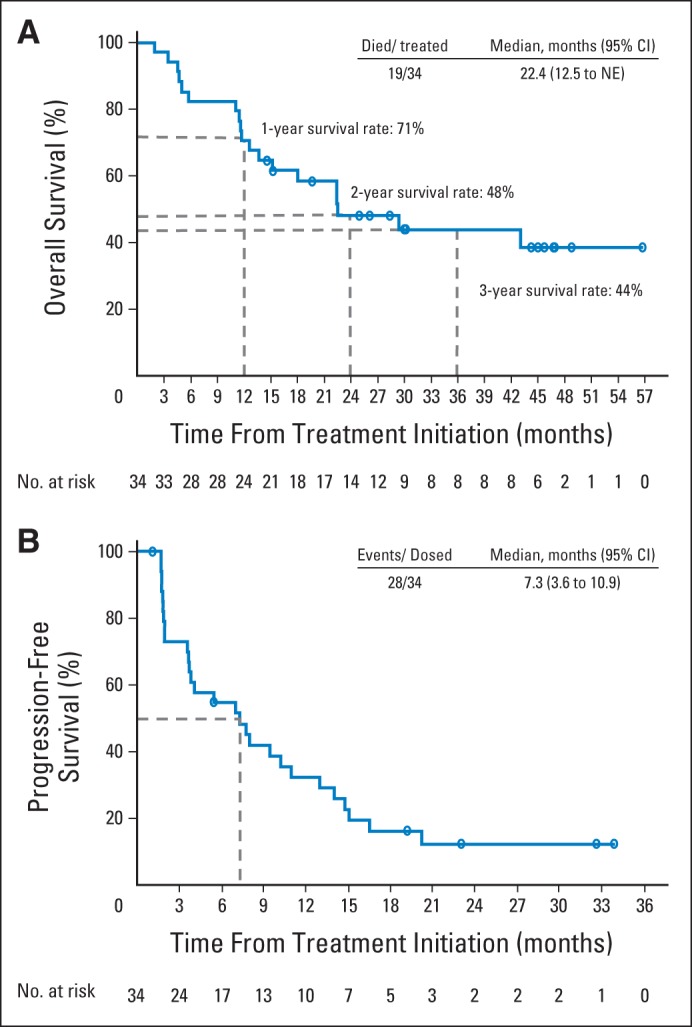
Efficacy outcomes in patients with renal cell carcinoma (RCC) receiving nivolumab. Kaplan-Meier curves of (A) overall and (B) progression-free survival in 34 nivolumab-treated patients with RCC. Analysis includes patients from both 1- and 10-mg/kg dose cohorts. (A) Patients with RCC had 1-, 2-, and 3-year overall survival rates of 71%, 48%, and 44%, respectively; median overall survival was 22.4 months. (B) Progression-free survival rates were 35% and 12% at 1 and 2 years, respectively; median was 7.3 months. Open circles indicate censored events, defined for overall survival as time to last known date alive before date of data analysis for patients without death, and defined for progression-free survival as time to last tumor assessment before date of data analysis for patients without disease progression or death. NE, not estimable.
Safety
A maximum-tolerated dose was not defined up to the maximum planned dose of 10 mg/kg. With extended observation since our initial report26 (range of time receiving treatment, 4 to 100 weeks), the spectrum and severity of treatment-related adverse events remained stable. Treatment-related adverse events of any grade were observed in 29 (85%) of 34 patients, the most common being fatigue (14 [41%] of 34), rash (nine [27%] of 34), diarrhea (six [18%] of 34), and pruritus (six [18%] of 34; Table 3). Six (18%) of 34 patients experienced grade 3 to 4 treatment-related adverse events. In the total treated patient population across all tumor types (N = 306), grade 3 to 4 drug-related adverse events occurred in 52 patients (17%).27
Table 3.
Treatment-Related AEs by Dose Level Occurring in ≥ 3% of All Treated Patients With RCC
| AE | Nivolumab Dose (mg/kg) |
Total (N = 34) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 18) |
10 (n = 16) |
|||||||||||
| All Grade |
Grade 3 to 4 |
All Grade |
Grade 3 to 4 |
All Grade |
Grade 3 to 4 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Any* | 15 | 83.3 | 2 | 11.1 | 14 | 87.5 | 2 | 25.0 | 29 | 85.3 | 6 | 17.6 |
| General disorders | ||||||||||||
| Fatigue | 6 | 33.3 | 0 | 0.0 | 8 | 50.0 | 0 | 0.0 | 14 | 41.2 | 0 | 0.0 |
| Pyrexia | 2 | 11.1 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Chills | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Influenza-like illness | 2 | 11.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Thirst | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Skin and cutaneous tissue disorders | ||||||||||||
| Rash | 7 | 38.9 | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | 9 | 26.5 | 0 | 0.0 |
| Pruritus | 4 | 22.2 | 0 | 0.0 | 2 | 12.5 | 1 | 6.3 | 6 | 17.6 | 1 | 2.9 |
| Dry skin | 3 | 16.7 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 4 | 11.8 | 0 | 0.0 |
| GI disorders | ||||||||||||
| Diarrhea | 5 | 27.8 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 6 | 17.6 | 0 | 0.0 |
| Abdominal pain | 2 | 11.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Dry mouth | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Nausea | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Investigations | ||||||||||||
| ALT increased | 2 | 11.1 | 0 | 0.0 | 2 | 12.5 | 1 | 6.3 | 4 | 11.8 | 0 | 0.0 |
| AST increased | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Blood alkaline phosphatase increased | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | 1 | 6.3 | 2 | 5.9 | 1 | 2.9 |
| Blood thyroid-stimulating hormone increased | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Hemoglobin decreased | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Weight decreased | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Metabolism and nutrition disorders | ||||||||||||
| Decreased appetite | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Hyperuricemia | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Hypophosphatemia | 1 | 5.6 | 0 | 0.0 | 2 | 12.5 | 2 | 12.5 | 3 | 8.8 | 2 | 5.9 |
| Musculoskeletal disorders | ||||||||||||
| Arthralgia | 2 | 11.1 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Pain in extremity | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Nervous system disorders | ||||||||||||
| Dysgeusia | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Headache | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Neuropathy peripheral | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Respiratory | ||||||||||||
| Dyspnea | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Allergic rhinitis | 1 | 5.6 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 5.9 | 0 | 0.0 |
| Endocrine disorders | ||||||||||||
| Hypothyroidism | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
Abbreviations: AE, adverse event; RCC, renal cell carcinoma.
Nos. reported within column may not add up to total No. reported in Any AE because patients who had > one AE were counted for each event but were counted only once for Any AE, and data for only those events reported in ≥ 3% of treated patient population are listed in this table.
Treatment-related immune-mediated adverse events, with potential immune-related causality (previously identified as adverse events of special interest14), of any grade were observed in 19 (56%) of 34 patients; the most common were rash (nine [27%] of 34), pruritus and diarrhea (six [18%] of 34 each), increased ALT (four [12%] of 34), and hypothyroidism (three [9%] of 34). Grade 3 to 4 treatment-related immune-mediated events were seen in three (9%) of 34 patients and included pruritus, macular rash, increased ALT, and acute respiratory failure (occurring in one patient each [2.9%]). Seven (21%) of 34 patients required management of immune-mediated adverse events with systemic glucocorticoids and/or other immunosuppressive agents; two (29%) of the seven patients resumed nivolumab therapy within 15 days of occurrence of the treatment-related adverse event, whereas the remaining patients discontinued therapy. Only one responder received systemic corticosteroids because of a drug-related adverse event, and this occurred long after the onset of response. One of the 34 patients with RCC experienced treatment-related pneumonitis (grade ≤ 2). No incidences of grade 3 to 4 treatment-related pneumonitis or treatment-related deaths resulting from any cause were observed in patients with RCC. In the total study population (N = 306), five patients (1%) had treatment-related grade ≥ 3 pneumonitis; four of these cases were fatal (three patients with non–small-cell lung cancer; one patient with colorectal cancer).27 No grade 3 to 4 treatment-related adverse events were documented within the protocol-specified observation period (100 days from last dose). One patient did develop grade 2 adrenal insufficiency 43 days after the last dose of nivolumab. This adverse event was treated with hormone-replacement therapy and resolved after 50 days.
DISCUSSION
Phase I trials of antibodies that block the PD-1/PD-L1 interaction in patients with solid tumors, including RCC, have reported encouraging preliminary safety and efficacy results.13–16 The prolonged follow-up period of our study (median, 45 months) enables the first evaluation to our knowledge of the potential impact of an anti–PD-1 antibody on tumor regression, response maintenance after treatment discontinuation, survival outcome, and treatment-associated toxicity in patients who initiated therapy at least 1 year earlier.
In this pretreated population of patients with RCC, among whom 44% received ≥ three prior therapies and 71% received prior antiangiogenic therapy, 29% experienced a confirmed objective response by RECIST criteria, 27% experienced stable disease lasting ≥ 24 weeks, and an additional 9% developed unconventional immune-related responses. Thus, the majority of patients may have received benefit from therapy. Among the 10 patients with objective responses, the median response duration was 12.9 months (based on Kaplan-Meier method). Patient characteristics were similar for responding and nonresponding patients. Overall, median progression-free survival was 7.3 months. These data are comparable to standard therapies used in the VEGF receptor tyrosine kinase inhibitor (TKI) –resistant setting. Results in patients with melanoma treated with the anti–CTLA-4 antibody ipilimumab have shown that tumor response together with a significant proportion of patients achieving prolonged stable disease and/or unconventional immune responses translates into a benefit in overall survival, so it is possible that there may be a clinical benefit with nivolumab in RCC that exceeds the effects on progression-free survival and tumor response noted in our study.28
Although VEGF or mTOR pathway inhibitors require continued treatment to maintain clinical benefit, a subset of patients in this nivolumab-treated population maintained their response status after discontinuation of PD-1 pathway blockade. Figure 1D depicts three responding patients who did not experience progression 45 weeks after stopping treatment. Further follow-up will be required to determine if this benefit remains durable, similar to the long-term survival outcomes seen after high-dose IL-2 therapy in patients with RCC5 or CTLA-4 blockade in patients with melanoma.28
These survival data for patients with RCC treated with nivolumab are comparable to results obtained with US Food and Drug Administration–approved agents when studied in similar treatment-refractory populations with advanced unresectable disease (VEGF receptor TKI–resistant patients). Overall survival rates in patients treated with nivolumab were 71%, 48%, and 44% at 1, 2, and 3 years, respectively, with median overall survival of 22.4 months. Although patient selection and improved poststudy therapeutic options may have played a role in this outcome, these results are consistent with the notion that PD-1 blockade may have a favorable impact on survival in this patient population and support ongoing studies in RCC. In recent years, standard approaches (VEGF or mTOR inhibition) have been studied in similar patient populations. For example, in a phase III trial enrolling patients with RCC whose disease progressed after VEGF receptor TKI therapy, everolimus was compared with placebo; median overall survival in that study was 14.8 versus 14.4 months, respectively.29,30 A phase III trial comparing sorafenib with temsirolimus in a sunitinib-refractory kidney cancer population yielded median overall survival of 16.6 and 12.3 months, respectively.31 In a phase III trial comparing axitinib with sorafenib as second-line treatment in patients with RCC, axitinib demonstrated median overall survival of 20.1 months.32 A potentially pivotal, randomized phase III trial is currently under way to prospectively compare overall survival in patients with RCC treated with either nivolumab or everolimus who had experienced progression during prior antiangiogenic therapy (ClinicalTrials.gov No. NCT01668784).
In the total treated patient population, across all tumor types (N = 306), grade 3 to 4 drug-related adverse events occurred in 17% of patients, but discontinuation of treatment as a result of drug-related adverse events occurred in only 5% of patients. All-grade and grade 3 to 4 treatment-related pneumonitis occurred in 4% (12 of 306) and 1% (four of 306) of patients, respectively. No drug-related deaths occurred in patients with RCC; however, four drug-related deaths occurred as a result of pneumonitis in patients with other disease types (three with non–small-cell lung cancer; one with colorectal cancer).27 In the RCC population, the safety profile was similar to that of the total treatment population, with grade 3 to 4 drug-related adverse events occurring in 18% of patients and no treatment-related deaths. Because the numbers of grade 3 to 4 treatment-related adverse events observed in the RCC cohort were few, a formal time-to-event analysis was not performed. However, it should be noted that in the melanoma cohort of this study (n = 107), the highest adverse event rate was observed within the first 6 months of treatment.33
Common toxicities were consistent with immune-related mechanisms and included fatigue, rash, diarrhea, and endocrinopathies. High-grade toxicities were generally manageable in an outpatient setting using protocol-specific algorithms involving the use of glucocorticoids and/or drug discontinuation. This favorable toxicity profile should facilitate the exploration of PD-1 antibody–based combination and adjuvant regimens. Only one responder in the RCC cohort received glucocorticoid treatment for a drug-related adverse event, which occurred long after response. Future studies will assess the potential impact and efficacy of glucocorticoid use in adverse event management.
Although the early results seen with PD-1 pathway–blocking antibodies in patients with RCC have been encouraging, translational research efforts will be essential to optimize this approach. Importantly, the significant heterogeneity seen in kidney cancers may make the development of predictive biomarkers challenging.34 For example, preliminary correlative studies have demonstrated that although tumor PD-L1 expression by immunohistochemistry may increase the likelihood of benefit with anti–PD-1, it fails to identify all responders.14,15,35,36 A more comprehensive understanding of why some patients with PD-L1–negative tumors respond to PD-1 pathway blockade, while many with PD-L1–positive tumors fail to do so, will be critical to improving patient selection and developing anti–PD-1–based combination strategies for RCC.
The impressive clinical impact of concurrent inhibition of both CTLA-4 and PD-1 recently reported in patients with melanoma37 has led to the exploration of combination immunotherapy approaches in other solid tumors, including RCC (ClinicalTrials.gov No. NCT01472081). In the melanoma trial,37 responses to combined checkpoint blockade were seen with equal frequency in PD-L1–positive and –negative tumors, suggesting that the addition of anti–CTLA-4 may alter factors in the tumor microenvironment, possibly rendering PD-L1–negative tumors more susceptible to anti–PD-1 blockade.35 A trial comparing clinical activity of the combination of nivolumab and ipilimumab with standard TKI therapy in advanced RCC has been initiated (ClinicalTrials.gov No. NCT01472081). Indeed, it is possible that treatment with PD-1 pathway–blocking agents in the treatment-naive setting may yield even better results and obviate the need for subsequent lines of therapy.
Although the advent of VEGF and mTOR pathway–targeted therapies (eg, sorafenib, sunitinib, and everolimus) have significantly improved outcomes for patients with RCC, disease progression is inevitable, and therapy must be continued to maintain efficacy, leading to continued toxicities.38 The results of our study suggest that nivolumab can be administered safely in an outpatient setting to pretreated patients with RCC and demonstrate durable clinical activity. Blockade of the PD-1 pathway may represent an important new target for RCC therapy.16 Efforts to rationally refine this approach with biomarker, combination, and clinical registration trials of nivolumab in patients with RCC are under way.
Supplementary Material
Acknowledgment
We thank the patients who participated in this study, clinical faculty, and personnel, including Cherylann Carr, Tianna Dauses, Robert Gray, Marina Laiko, Evan Lipson, Alice Pons, and Pritish John, Johns Hopkins University School of Medicine and Sidney Kimmel Comprehensive Cancer Center; Rose Marujo, James Mier, Elizabeth Buchbinder, and Bryan Marion, Beth Israel Deaconess Medical Center; Anne Caldwell and Matthew Burke, Yale Cancer Center; Suzanne Burke, Kim Feldhaus, Elaine Granch, Nabeela Iqbal, and Prathima Koppolu, University of Michigan; Jahleen Byers, Bryan Greene, Jill Hinson, Eric Keller, and Lori Lipocky, Carolina BioOncology Institute; Serena Rucker, Vanderbilt University Medical Center; John Gerks, Susan Vigen, Ye Zhou, Mark Salvati, and Nils Lonberg, Bristol-Myers Squibb; and medical writers Mark Palangio and Lou Passador, StemScientific.
Footnotes
Supported by Bristol-Myers Squibb, which also funded writing assistance provided by StemScientific; by Ono Pharmaceutical; and by the National Cancer Institute of the National Institutes of Health, and a Specialized Programs of Research Excellence grant under award number P50CA101942 (D.F.M., T.K.C., M.B.A., and F.S.H).
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT0730639.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: David F. McDermott
Provision of study materials or patients: Toni K. Choueiri, David C. Smith, Igor Puzanov, Michael B. Atkins
Collection and assembly of data: David F. McDermott, Mario Sznol, Toni K. Choueiri, Hans J. Hammers, F. Stephen Hodi, Jon M. Wigginton, Georgia D. Kollia, Ashok Gupta, Dan McDonald, Vindira Sankar, Michael B. Atkins
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech/Roche, Pfizer
Research Funding: Prometheus Laboratories
Charles G. Drake
Stock or Other Ownership: ImmuneXcite, Compugen
Consulting or Advisory Role: Amplimmune, Bristol-Myers Squibb, Compugen, Dendreon, ImmuneXcite, NexImmune, Newlink Genetics, Merck, Roche/Genentech, Potenza Therapeutics
Research Funding: Aduro Biotech, Janssen Pharmaceuticals, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Amplimmune
Mario Sznol
Stock or Other Ownership: Amphivena, Adaptive Biotechnologies
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, Amgen, AstraZeneca/MedImmune, Symphogen, Merus, Immune Design, Anaeropharma, Kyowa-Hakko Kirin, Lion Biotechnologies, Nektar, Novartis, Prometheus, Seattle Genetics, Astellas Pharma, Neostem, Pierre Fabre
Other Relationship: Haymarket Media
Toni K. Choueiri
Consulting or Advisory Role: Pfizer, Bayer, GlaxoSmithKline, Novartis, Merck, Bristol-Myers Squibb
Research Funding: Pfizer (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), Merck (Inst)
John D. Powderly
Employment: Carolina BioOncology Institute
Leadership: BioCytics
Stock or Other Ownership: BioCytics
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Amplimmune
Speakers' Bureau: Bristol-Myers Squibb, Dendreon, Merck Sharp & Dohme
Research Funding: Bristol-Myers Squibb, Genentech, AstraZeneca, Merck Serono, Amplimmune, Eli Lilly, Incyte, Macrogenics
David C. Smith
Research Funding: AstraZeneca (Inst), OncoMed (Inst), Exelixis (Inst), MedImmune (Inst), Tekmira (Inst), Atterocor (Inst), ImClone Systems (Inst), Incyte (Inst), Celgene (Inst), Aragon Pharmaceuticals (Inst), Bristol-Myers Squibb/Medarex (Inst), Teva (Inst), Bayer (Inst), Eisai (Inst), Millennium Pharmaceuticals (Inst), Debiopharm Group (Inst), Boston Biomedical (Inst), Eli Lilly (Inst), Oncogenex (Inst), PSMA Development (Inst), Abraxis BioScience (Inst)
Julie R. Brahmer
Consulting or Advisory Role: Merck
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myer Squibb
Richard D. Carvajal
Consulting or Advisory Role: AstraZeneca, Aura Biosciences
Research Funding: Bristol-Myers Squibb
Other Relationship: Aura Biosciences
Hans J. Hammers
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Igor Puzanov
Consulting or Advisory Role: Genentech, Roche
F. Stephen Hodi
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Genentech/Roche
Harriet M. Kluger
Honoraria: Merck, Prometheus
Research Funding: Bristol-Myers Squibb, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Suzanne L. Topalian
Stock or Other Ownership: Jounce (I), Potenza Therapeutics (I)
Consulting or Advisory Role: Jounce Therapeutics, GlaxoSmithKline, Five Prime Therapeutics, sanofi-aventis
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: MedImmune (I), Bristol-Myers Squibb (I), Potenza Therapeutics (I)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, sanofi-aventis, Five Prime Therapeutics
Drew M. Pardoll
Consulting or Advisory Role: sanofi-aventis, AstraZeneca, ImmuneXcite, Jounce, Neximmune
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Amplimmune (I)
Jon M. Wigginton
Employment: Bristol-Myers Squibb, MacroGenics
Leadership: Macrogenics
Stock or Other Ownership: Bristol-Myers Squibb, Macrogenics
Patents, Royalties, Other Intellectual Property: Bristol-Myers Squibb (Inst)
Georgia D. Kollia
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Ashok Gupta
Employment: Bristol-Myers Squibb, MedImmune
Stock or Other Ownership: Bristol-Myers Squibb, AstraZeneca/MedImmune
Dan McDonald
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Vindira Sankar
Employment: Bristol-Myers Squibb
Jeffrey A. Sosman
Honoraria: GlaxoSmithKline, Amgen
Consulting or Advisory Role: GlaxoSmithKline, Genentech
Research Funding: Bristol-Myers Squibb, Novartis, GlaxoSmithKline
Michael B. Atkins
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Merck, Genentech, Pfizer, Novartis, GlaxoSmithKline, Nektar, X4 Pharma, Eli Lilly
REFERENCES
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:210–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179:50–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 9.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–780. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 11.Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7–H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:244–245. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho DC, Sosman JA, Sznol M, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;31(suppl 15s):285s. abstr 4505. [Google Scholar]
- 16.McDermott DF, Drake CD, Sznol M, et al. Clinical activity and safety of antiprogrammed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients (pts) with previously treated, metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2012;30(suppl):278s. abstr 4505. [Google Scholar]
- 17.Nurieva RI, Liu X, Dong C. Molecular mechanisms of T-cell tolerance. Immunol Rev. 2011;241:133–144. doi: 10.1111/j.1600-065X.2011.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 20.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (version 3.0) http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 26.Drake CG, McDermott DF, Sznol M, et al. Survival, safety and response duration results of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in a phase 1 trial in patients with previously treated metastatic renal cell carcinoma (mRCC): Long-term patient follow-up. J Clin Oncol. 2013;31(suppl 15s):287s. abstr 4514. [Google Scholar]
- 27.Topalian SL, Sznol M, Brahmer JR, et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with advanced solid tumors: Survival and long-term safety in a phase I trial. J Clin Oncol. 2013;31(suppl 15s):173s. abstr 3002. [Google Scholar]
- 28.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: Final results and analysis of prognostic factors. Cancer. 2010;116:4256–4426. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 31.Hutson T, Escudier B, Esteban E, et al. Temsirolimus vs sorafenib as second line therapy in metastatic renal cell carcinoma: Results from the INTORSECT trial. Ann Oncol. 2012;(suppl 9):23. abstr LBA22. [Google Scholar]
- 32.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 33.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan MK HC, Curran MA, Hollman T, et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. J Clin Oncol. 2013;31(suppl 15s):173s. abstr 3003. [Google Scholar]
- 36.Grosso J HC, Inzunza D, Cardona DM, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) J Clin Oncol. 2013;31(suppl 15s):177s. abstr 3016. [Google Scholar]
- 37.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkins MB, Bukowski RM, Escudier BJ, et al. Innovations and challenges in renal cancer: Summary statement from the Third Cambridge Conference. Cancer. 2009;115(suppl):2247–2251. doi: 10.1002/cncr.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.