Significance
Here we define the causative role of endoplasmic reticulum (ER) stress on selective modulation of pain signaling. High levels of ER stress and neuropathic pain in diabetic animals are reduced using ER stress blockers. In healthy animals, turning on the ER stress signal transduction cascade generates an immediate but lasting and site restricted painful phenotype, which is reversible by ER stress blockers. This previously unnoticed mechanism explains the broad lack of efficacy of available analgesics and should ignite the discovery of a new generation of therapeutics that do not directly quell ion channel or neurotransmitter activity.
Keywords: endoplasmic reticulum stress, pain, diabetes, tunicamycin, soluble epoxide hydrolase
Abstract
Despite intensive effort and resulting gains in understanding the mechanisms underlying neuropathic pain, limited success in therapeutic approaches have been attained. A recently identified, nonchannel, nonneurotransmitter therapeutic target for pain is the enzyme soluble epoxide hydrolase (sEH). The sEH degrades natural analgesic lipid mediators, epoxy fatty acids (EpFAs), therefore its inhibition stabilizes these bioactive mediators. Here we demonstrate the effects of EpFAs on diabetes induced neuropathic pain and define a previously unknown mechanism of pain, regulated by endoplasmic reticulum (ER) stress. The activation of ER stress is first quantified in the peripheral nervous system of type I diabetic rats. We demonstrate that both pain and markers of ER stress are reversed by a chemical chaperone. Next, we identify the EpFAs as upstream modulators of ER stress pathways. Chemical inducers of ER stress invariably lead to pain behavior that is reversed by a chemical chaperone and an inhibitor of sEH. The rapid occurrence of pain behavior with inducers, equally rapid reversal by blockers and natural incidence of ER stress in diabetic peripheral nervous system (PNS) argue for a major role of the ER stress pathways in regulating the excitability of the nociceptive system. Understanding the role of ER stress in generation and maintenance of pain opens routes to exploit this system for therapeutic purposes.
Following its discovery, endoplasmic reticulum (ER) stress and the ensuing unfolded protein response (UPR) proved to be major adaptive and homeostatic mechanisms that balance cells’ demand for proteins to their synthetic output (1–3). If and when disequilibrium in demand and synthesis cannot be overcome, ER stress leads to activation of cell death pathways. Activated ER stress pathways are observed in the pathogenesis of important diseases including diabetes and cancer (4). Specifically in the nervous system, key roles underlying multiple neurodegenerative diseases have been ascribed to ER stress. These include Alzheimer's and Parkinson's diseases, amyotrophic lateral sclerosis and prion diseases (5, 6). In these conditions disruption of homeostasis leads to plaque formation, neuronal loss and ultimately to dysfunction. However, beyond the progressive neurodegenerative diseases typically manifesting over the long term, little is known about how ER stress affects the nervous system. Regardless, current ideas on ER stress in the nervous system can be epitomized as a fundamental and sentient network modulating physiologic responses. As such, discovery of mechanisms governing ER stress in neurons should significantly enhance our basic understanding of normal physiology and etiology of diseases of the peripheral nervous system (PNS).
The diabetes induced neuropathic phenotype in rodents and humans displays progressively increasing pain in response to tactile stimulation and a loss of sensitivity to heat. First documented in the 19th century, its basis has been debated continuously since then. Over the past century extensive histological changes in the diabetic PNS are demonstrated (7). Paradoxically these changes include signs of both destructive and regenerative biological events. The main histopathological features include axonal swelling, degeneration of fibers beginning from distal ends, demyelination and degeneration, Schwann cell atrophy, signs of remyelination, distal sprouting of proximal nerve stumps. Moreover, cognitive decline and atrophy in the brain and spinal cord are frequently observed, suggesting that hallmark features extend to the central nervous system (8, 9). The distinctive sensory changes -also used to diagnose diabetes induced nerve damage- often coincide with characteristic features at the cellular level. These seem to occur in a selective manner, beginning from distal areas, and not all nerves are affected equally or with an identical time course. The mechanism(s) governing these changes are not fully understood but could facilitate the development of successful therapeutic interventions.
While studying EpFAs and sEH enzyme, we reported its up-regulation in the nervous system of diabetic rodents (10). Similarly sEH expression is elevated in heart, liver and adipose tissue arguing for a global increase in response to diabetes (11–13). The increase in activity contributes to dyslipidemia because sEH selectively degrades low-abundance but highly potent bioactive lipids that maintain homeostasis. These lipids, also termed epoxy fatty acids (EpFAs), are analgesic, anticonvulsant and antiinflammatory (14–18). When EpFAs are stabilized by inhibiting sEH in diabetic animals, neuropathic pain is effectively blocked (10, 18). A large number of biological effects have been attributed to EpFAs (19). The mechanism responsible for antinociception is conceivably different from other reported activities of EpFAs. An overarching molecular mechanism that could underlie numerous and seemingly independent effects could be suppression of ER stress. Genetic ablation or inhibition of sEH significantly blocks ER stress in the liver and adipose tissues of mice fed a high-fat diet (20). Inspired by these observations we asked if antinociceptive effects of inhibiting sEH are based on curtailing ER stress in peripheral nerves. Embedded in this hypothesis is the premise that ER stress is a causative factor in pain. Here we test both hypotheses and demonstrate causality between ER stress and diabetes induced neuropathic pain. Equally importantly, we demonstrate that inhibition of sEH as well as use of a standard chemical chaperone that assists and facilitates the correct folding of nascent proteins block both ER stress and pain.
Results
Active ER Stress Responses in Diabetic PNS Demystify Symptoms of Diabetic Polyneuropathy.
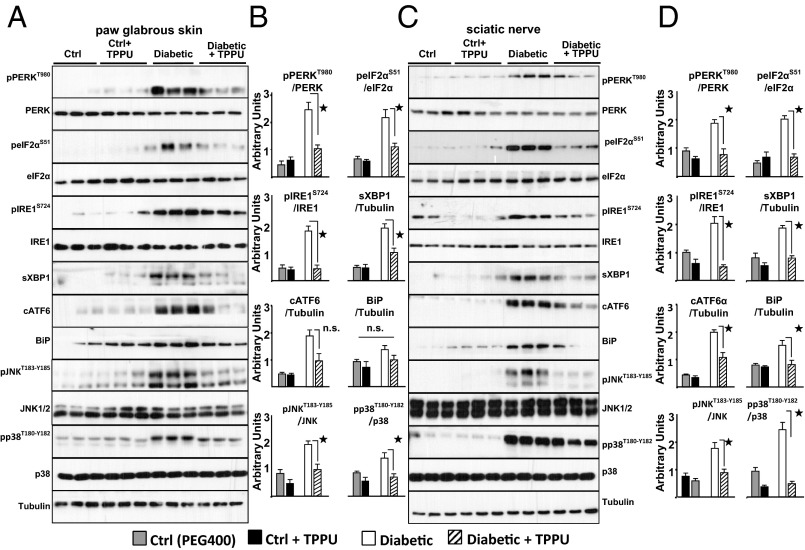
The sequelae in neuropathy could be driven by ER stress responses. Therefore, we first asked whether ER stress-mediated pathways are active in the PNS of type I diabetic rats. Streptozotocin induced type I diabetes results in neuropathy measured as increased sensitivity to touch (Fig. S1). Diabetic rats display sensitivity to tactile stimuli and exhibit activated ER stress responses in the hind paw and the sciatic nerve (Fig.1 and Figs. S2 and S3). Robust increases in activation of PKR-like endoplasmic reticulum-resident kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6), three key sensors of the ER stress signaling pathways are observed. Consistently, the levels of associated downstream targets are elevated suggesting full-scale activation of the ER stress pathways (21). Phosphorylation of eukaryotic initiation factor 2 subunit alpha (eIF2α) (Ser51), mediated by phospho-PERK, increased the mRNA level of its downstream target activating transcription factor 4 (Atf4). Phosphorylation of IRE1α led to a significant rise in total protein and mRNA levels of spliced X-box binding protein 1 (Xbp1), as well as the level of the ER chaperone binding immunoglobulin protein (BiP) in both tissues. Equally importantly, phosphorylation of p38 and c-jun NH2-terminal kinase (JNK) 1/2, two agreed upon kinase mediators of neuropathic pain, are significantly increased (22). Remarkably, age-matched healthy rats exhibit exceedingly low baseline levels of phosphorylated PERK, IRE1α, eIF2α, and cleaved ATF6, demonstrating the selectivity of these ER stress responses.
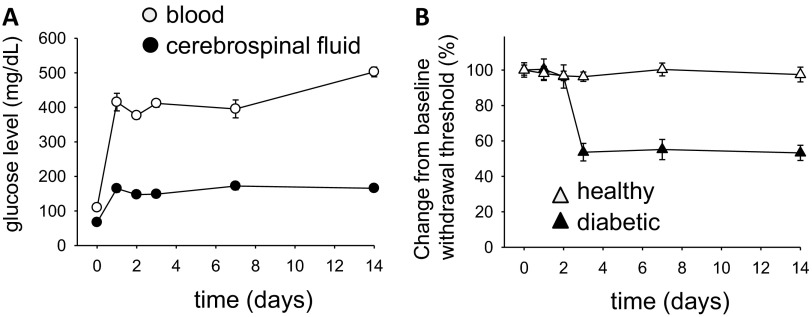
Fig. S1.
Following streptozocin administration (A) blood and CSF glucose levels rapidly rise and rats develop (B) sensitivity to mechanical stimuli measured by electronic von Frey test (n = 6).
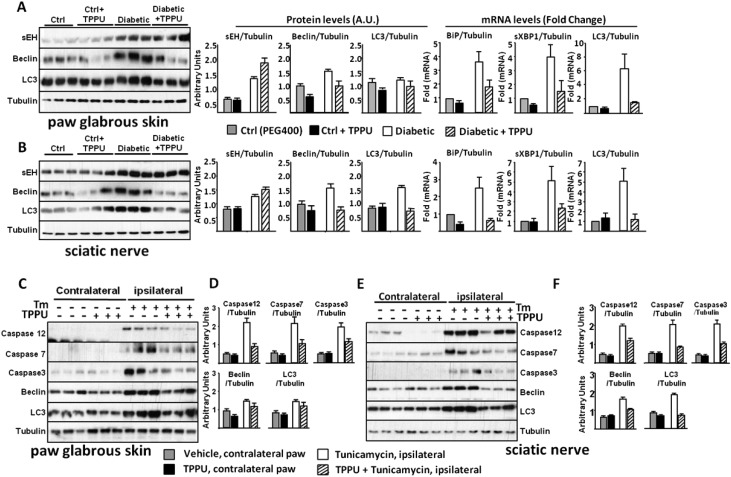
Fig. 1.
Elevated ER stress is a prominent feature in diabetic rats. TPPU blocks pain-related behavior and suppress markers of ER stress within 30 min. (A and B) Markers of ER stress from diabetic rats' paw skin measured by Western blotting. Representative immunoblots from 3 rats using total skin lysates of healthy, diabetic, TPPU (10 mg/kg, intraperitoneal) or vehicle treated animals are shown. Samples are immuno-blotted for proteins specified next to each row using Tubulin as a loading control. See Fig. S2 for all six rats. Bar graphs display phosphorylation level normalized to total protein expression. For nonphosphorylated proteins total protein is normalized to Tubulin (n = 6 per group, ★, P < 0.05, One-way ANOVA followed by Student-Newman–Keuls post hoc multiple comparison). (C and D) Markers of ER stress from sciatic nerve bundle of same rats. Total sciatic nerve lysates are immuno-blotted for targets as in A. See Fig. S3 for all rats. Bar graphs display phosphorylation level normalized to total protein expression and for nonphosphorylated proteins normalized to Tubulin (n = 6 per group, ★, P < 0.05, One-way ANOVA followed by Student Newman–Keuls post hoc multiple comparison). Data are presented as mean ± SEM in all subsequent figures throughout the text.
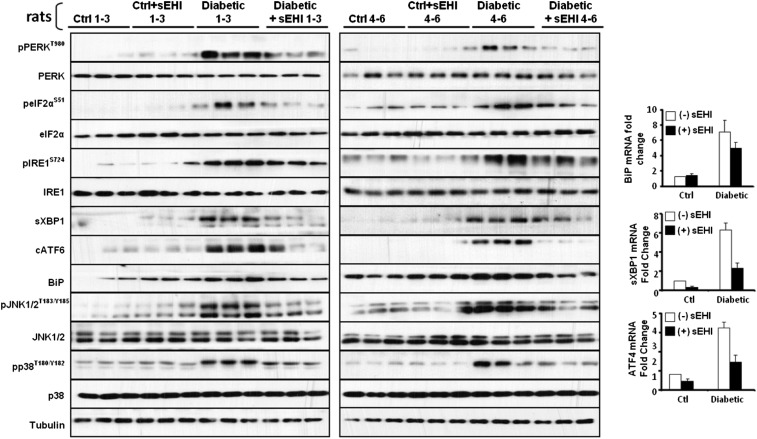
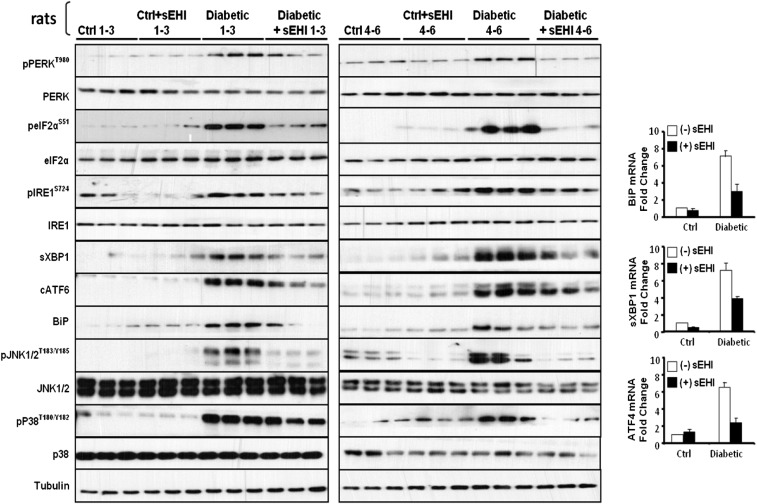
Fig. S2.
Western blots of paw skin samples of all six animals from Fig. 1A and bar graph of levels of mRNA expression for the key downstream targets of ER stress sensors. Expression of Bip, sXbp1, and Atf4 mRNA are significantly increased in diabetic rat paw skin and reduced by inhibition of sEH.
Fig. S3.
Western blots of sciatic nerve samples of all six animals from Fig. 1B and bar graph of levels of mRNA expression for the key downstream targets of ER stress sensors. Expression of Bip, sXbp1, and Atf4 mRNA are significantly increased in diabetic rat sciatic nerve bundle and reduced by inhibition of sEH.
Another hallmark of ER stress, autophagy, was apparent in diabetic rats, with microtubule-associated proteins 1A/1B light chains 3 (LC3) and beclin significantly increased in sciatic and skin samples, demonstrating a continuous and organized effort to replenish subcellular structures (Fig. S4).
Fig. S4.
ER stress leads to autophagy and apoptosis. (A) Expression of sEH and markers of autophagy are up-regulated in the skin of diabetic rats. (B) In parallel to the increases in the diabetic skin, expression of sEH and markers of autophagy are also up-regulated in the sciatic nerve of diabetic rats. (C–F) In healthy rats, inducing ER stress with intraplantar tunicamycin (Tm) rapidly leads to the rise of autophagy and apoptosis markers both locally in the skin and in the sciatic nerve. The levels of these molecular markers remain unchanged in the contralateral side samples demonstrating spatial restriction of the ER stress response.
Diverse Agents That Suppress ER Stress Responses Block Pain.
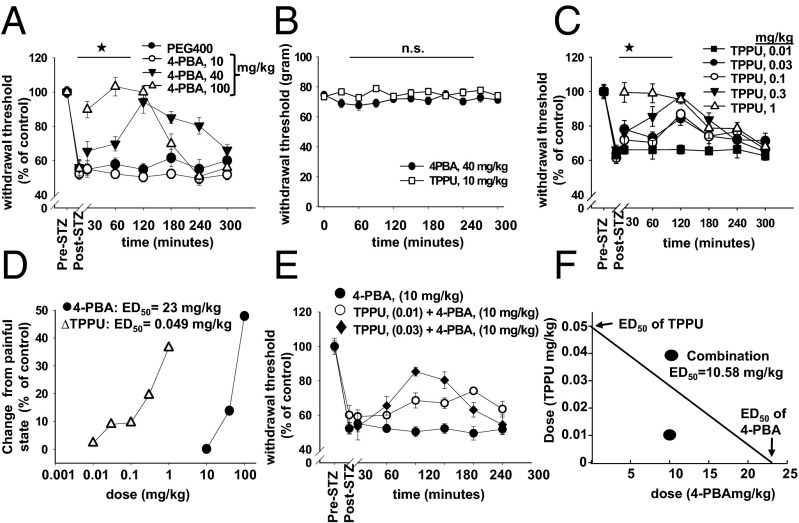
A similar observation on ER stress is described by Lupachyk et al. in type I diabetic rats whose pain related behavior was reduced using two chemical chaperones given for 12 wk at high doses (23). If there is a causal relationship between diabetes mediated neuropathic pain and ER stress, in particular through rapid phosphorylation, chemical chaperones that reduce ER stress should block pain and reduce the levels of ER stress markers immediately rather than following lengthy administration. Indeed, when 4-PBA (4-phenyl butyric acid, a small molecule proposed to facilitate the correct folding of nascent proteins) is given to diabetic rats, dose and time dependent antinociception occurs within minutes, whereas 4-PBA is without effect in healthy rats (Fig. 2 A and B). Of note, systemic delivery of 4-PBA (100 mg/kg) generates full efficacy and rapid onset. This outcome provides a rationale for the testing of FDA approved ER stress blockers against painful conditions.
Fig. 2.
A chemical chaperone and an sEH inhibitor synergistically block pain-related behavior in type I diabetic rats. (A) 4-PBA reduced pain dose dependently as quantified by von Frey assay. Withdrawal thresholds are reported as “% change from pre-diabetic baseline” (n = 6 per group, one-way ANOVA, followed by Student Newman–Keul's post hoc test, ★, P = 0.001). (B) Administration of 4-PBA (40 mg/kg) or TPPU to healthy rats does not result in changes in withdrawal threshold (one-way ANOVA, P = 0.88). (C) TPPU reduces pain dose dependently (n = 6 per group, one-way ANOVA, followed by Student Newman–Keul's test, ★, P = 0.001, 0.03–1 mg/kg vs. 0.001 mg/kg). Combination index is reported as the key parameter of the synergistic interaction. Drug reduction index for each compound demonstrates fold reduction in dose to attain similar efficacy (n = 6 per group in all panels). (D) Dose vs. efficacy at 60 min post administration. ED50 values are calculated using SigmaPlot software suite (n = 6 per group in all panels). (E) An ineffective dose of 4-PBA (10 mg/kg) is shown with combination doses. (F) The CompuSyn software constructed isobologram of the interaction. ED50 values for each drug are on x and y axis. The ED50 of the combination is lower than what would be expected if the two compounds did not interact.
Results from Lupachyk et al. and this study support the idea that hyperglycemia mediated activation of ER stress occurs in peripheral and central nerves of diabetics (23, 24). These observations further support the idea that ER stress is involved in the etiology of diabetic neuropathy. In essence, the finding that one can modulate ER stress within minutes in vivo lends support to the hypothesis that pain and ER stress are functionally linked. Therefore, our results open routes to the development of novel probes and drug candidates on multiple targets in and around the canonical ER stress pathways while reiterating p38 and JNK as feasible therapeutic targets to address complex painful conditions (22).
Next, we asked if a different class of ER stress blocking compound would block neuropathic pain. Increasing the levels of EpFAs by inhibiting the enzyme sEH effectively blocks ER stress in the liver and adipose tissue of mice fed a high fat diet (20). In addition, sEH inhibitors are strong analgesics, and specifically in diabetic rats, they eliminate pain-related behavior in a dose dependent manner (10). Blood levels of sEH inhibitor, changes in epoxy fatty acids and antinociceptive activity triangulate to full target engagement. Here, an orally available inhibitor, TPPU, displayed higher efficacy than earlier inhibitors as would be expected from its higher in vitro potency, measured using baculovirus expressed recombinant rat sEH (Fig. 2C) (25). More to the point, full-blown ER stress and downstream responses are significantly reduced in the skin and largely normalized in the sciatic nerve by TPPU by 30 min post administration (Fig. 1). In the sciatic nerve, phosphorylation of PERK (Thr980), eIF2α (Ser51), and IRE1α (Ser727) as well as the induction of BiP are effectively blocked by TPPU along with significant decreases in sXBP1 and cleaved ATF6 expression. These follow the drastic decrease in the mRNA of Bip, sXbp1, and Atf4 (Fig. S2). Equally importantly, kinase mediators of neuropathic pain pp38 and pJNK are similarly normalized by TPPU as early as 30 min, reinforcing the role of ER stress in pain. Notably, in healthy animals, inhibition of sEH does not lead to changes in ER stress pathways, which is echoed in the absence of nociceptive threshold changes in healthy animals receiving sEH inhibitors.
Equivalent suppression of two UPR branches place epoxy fatty acids upstream of the ER stress sensors and argue for the use of EpFAs, their mimics, and sEH inhibitors as previously unidentified probes that modulate ER stress responses. Furthermore, these findings lend support to the hypothesis that a major role of EpFAs is modulation of ER stress and the mechanism of analgesia observed by sEH inhibitors is at least partially based on dampening ER stress.
Concurrent use of sEH Inhibitor and 4-PBA Synergistically Block Pain and ER Stress.
If sEH inhibition blocks pain by attenuating ER stress, synergistic reduction in pain should be seen when 4-PBA and sEH inhibitor are coadministered, in particular if these two agents independently converge on ER stress pathway and block its activation (26). Thus, the combination of subtherapeutic doses of 4-PBA (10 mg/kg) and TPPU (0.01, 0.03, and 0.1 mg/kg) were examined in diabetic rats (26). TPPU and 4-PBA synergistically block pain with significant combination index and drug reduction index values (Fig. 2 E and F and Table S1). TPPU is a potent inhibitor of the sEH enzyme with high-target occupancy as indicated by its Ki and the dissociation half life of TPPU (Table S2). These findings strengthen the hypothesis that 4-PBA treatment and sEH inhibition block pain by attenuating ER stress.
Table S1.
Parameters of synergistic reduction of pain by combination of 4-PBA and TPPU
| at ED50 | at ED97 | |
| Combination index (CI) | 0.63* | 0.25** |
| Drug reduction index for TPPU | 4.9 fold | 218 fold |
| Drug reduction index for 4-PBA | 2.2 fold | 3.9 fold |
CI < 1 indicates synergistic interaction, **CI < 0.3 indicates strong synergistic interaction.
Table S2.
Diverse Agents that Induce ER Stress Concurrently Induce Pain.
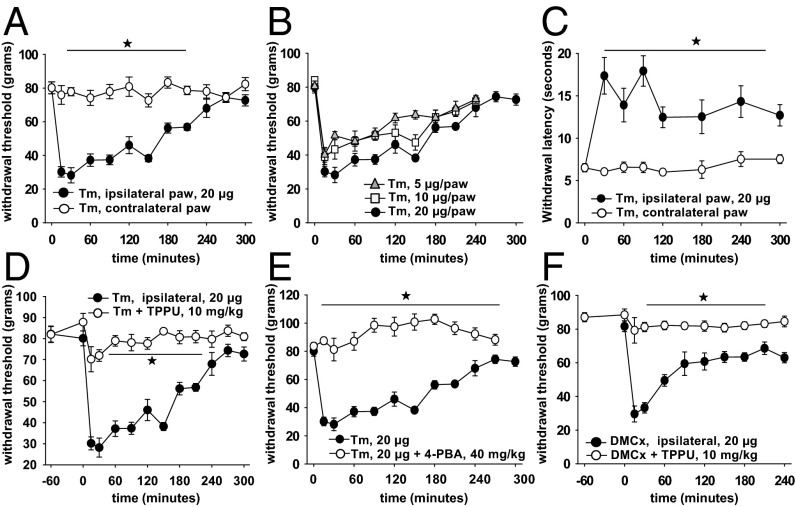
Although these results demonstrate high synchronicity between ER stress in the peripheral nerves and the pain modality, we asked if inducers of ER stress modulate pain sensation. Two well established inducers of ER stress include the glycosylation inhibitor tunicamycin (Tm) and the sarco/endoplasmic reticulum Ca2+-ATPase modulator dimethyl-celecoxib (DMCx) (27–29). Both induce a significant degree of pain when administered into the intraplantar region of the hind paw of healthy rats (Fig. 3). The effects are dose, time, and administration site dependent demonstrating specificity to the nociceptive system. Tm does not induce any immediate nocifensive behavior, however shortly after injection rats display discomfort, licking, shaking and guarding of the affected paw for several minutes after which guarding remains while other behaviors subside. A minimal amount of swelling occurs after Tm, although the mechanical withdrawal threshold quickly decreases to 25% of baseline levels (Fig. 3A). The speed and magnitude of decrease is notably fast and intense. This results in a larger assay dynamic range for quantification of antinociceptive compounds. Remarkably, the contralateral paw measurements demonstrate PNS restricted phenotype, at least during the first several hours. Surprisingly, Tm administration resulted in rapid and sustained loss of heat responses of the ipsilateral paw while animals become more sensitive to tactile stimuli similar to the rodent diabetic pain behavior profile (Fig. 3C). Importantly, sensitivity to mechanical stimuli is reversed using both TPPU and 4-PBA. Furthermore, a mechanistically different inducer of ER stress, DMCx generated a similar painful phenotype (Fig. 3F). DMCx was as potent as Tm, and effects were restricted to the ipsilateral paw and reversible by TPPU.
Fig. 3.
ER stress inducer tunicamycin is rapid, intense and selective in generating pain behavior. (A) Tm is given into the midsection of one hind paw in a volume of 10 μL (20 μg). Withdrawal thresholds of ipsi- and contralateral paws are then monitored. The withdrawal data are presented as gram force required for inducing withdrawal reflex (n = 6 per group, one-way ANOVA, followed by Holm–Sidak's post hoc analysis, ★, P < 0.001, 30–210 min Tm vs. vehicle). (B) Decreasing doses of Tm generated less intense allodynia that subsided faster than the highest dose. (C) In contrast to the decrease in mechanical withdrawal threshold, Tm induced a notable increase in thermal withdrawal threshold. This hypoalgesia is restricted to the ipsilateral side (n = 6 per group, Kruskal–Wallis one way analysis of variance on ranks, ★, P < 0.05). (D) Intraplantar Tm induced pain is reversible with TPPU (i.p. route, 1 h before Tm, n = 6 per group, one-way ANOVA, followed by Holm–Sidak's post hoc analysis, ★, P < 0.001, 15–210 min Tm vs. TPPU+Tm). (E) Expectedly, this pain is reversible with chemical chaperone 4-PBA (n = 6 per group, one-way ANOVA, followed by Holm–Sidak's post hoc analysis, ★, P < 0.001, 15–210 min Tm vs. 4-PBA+Tm). (F) Mechanistically different ER stress inducer dimethylcelecoxib (DMCx) generates a rapid and intense pain phenotype. DMCx is given same as Tm. The phenotype is reversible with TPPU (i.p. route, 1h before DMCx, n = 6 per group, one-way ANOVA, followed by Holm–Sidak's post hoc analysis, ★, P < 0.001, 15–240 min DMCx vs. TPPU+DMCx). Neither 4-PBA nor TPPU alter nociceptive responses in the absence of an induced pain state as demonstrated in Fig. 2B.
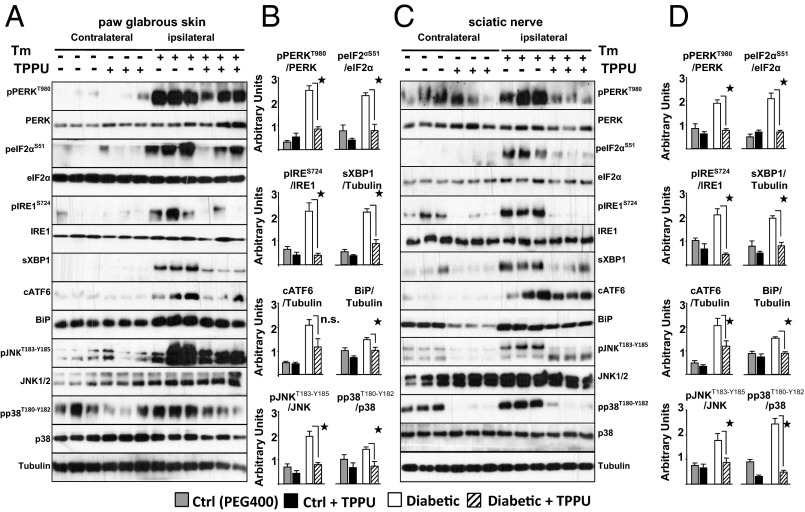
Concurrent with the Tm-induced changes in pain, molecular markers of ER stress are altered in a predictable way (Fig. 4 A–D). Major markers of ER stress are activated either as increased phosphorylated forms or as higher levels of transcripts and translated proteins. The acute sampling time after ER stress induction (30 min after Tm) demonstrates surprisingly synchronous pain and clear ER stress response occurring in an identical time frame. Quantification of contralateral paw ER stress markers concur with the results of pain measurements, a lack of activation on vehicle administered contralateral paw. This is also consistent with results from sEH inhibitor alone where no significant changes occur in the absence of induced pain or neuropathy. Consistent with results in diabetic rats, Tm-induced ER stress markers are reduced below detectable levels by inhibition of sEH. Similarly, inhibition of sEH seems to have equipotent effects on two major branches of the UPR. It is undetermined if EpFAs are directly modulating PERK and IRE1α branches or an upstream event. Surprisingly, the 30 min treatment with Tm is sufficient to initiate apoptotic and autophagic responses, both of which are blocked by inhibition of sEH at the level of skin and sciatic nerve (Fig. S4).
Fig. 4.
ER stress inducer Tm leads to activation of full range of ER stress responses within minutes. These are reversed by inhibition of sEH. Rats are given intraplantar Tm (20 μg) on the ipsilateral side and vehicle on the contralateral side with or without systemic TPPU (1 h before Tm, 10 mg/kg). Tissues are obtained 30 min post Tm, under deep anesthesia. (A and B) Quantification of markers of ER stress from Tm (ipsi-) and vehicle (contra) administered paw skin by Western blotting. Total skin lysates are immuno-blotted for targets specified next to each row using Tubulin as a loading control. Representative immunoblots of three rats from each group are shown. Bar graphs display expression of each target normalized to Tubulin or their respective unphosphorylated forms as indicated (n = 6 rats per group, Kruskal–Wallis one way analysis of variance on ranks followed by Student Newman–Keul's post hoc test, ★, P < 0.05). (C and D) Quantification of markers of ER stress in the sciatic nerve bundle of same rats as above by Western blotting. Total sciatic nerve lysates are immune-blotted for targets specified next to each row using Tubulin as a loading control. Representative immunoblots of three rats from each group are shown. Bar graph displays expression of each target normalized to Tubulin or their respective unphosphorylated forms as indicated (n = 6 rats per group, Kruskal–Wallis one way analysis of variance on ranks followed by Student Newman–Keul's post hoc test, ★, P < 0.05).
Discussion
The discovery reported here is important from a fundamental scientific perspective because a knowledge gap in the mechanism of nerve injury induced pain is filled. It is also important from an applied perspective because neuropathic pain remains a significant unmet clinical need. Painful diabetic neuropathy is one of the most challenging comorbidities of diabetes (30, 31). Its etiology and mechanisms are complex and poorly understood. Moreover, few therapeutic options exist. Currently approved drugs for neuropathic pain invariably address the increased hyperexcitability of nerves, a time-tested strategy. However, understanding the underlying cause of increased hyperexcitability and developing drugs that target these processes is an equally sought after strategy which should bring us steps closer to more selective and efficacious therapeutics that spare normal nerve function.
Activation of the ER stress responses has long been reported in diabetes and metabolic disease models and patients (24, 32). Here, we extended these findings to the PNS and distinctively demonstrate a rapidly observable functional change occurring within minutes as a pain phenotype in response to ER stress. In the diabetic PNS, ER stress is prominent and appear causal to the painful phenotype. Suppression of ER stress using a chemical chaperone or EpFA stabilizing probe led to rapid reduction of pain and ER stress. Coadministration of these two agents synergistically block pain and ER stress suggesting that the two classes of compounds independently converge on ER stress pathways. Activation of ER stress by independent methods, inducing systemic hyperglycemia, artificially by blocking correct protein folding and generating intracellular calcium imbalance in the hind paw tissue invariably result in strong painful phenotypes. Notably, this phenotype is site specific with no changes observed on the contralateral side. These suggest selectivity of the ER stress responses to nociceptive neuronal firing rather than neuronal activity. Accordingly, in healthy animals, 4-PBA or sEH inhibitors do not modulate ER stress markers or baseline nociceptive thresholds. Overall a causal association between ER stress responses and pain is proposed. This outcome, if true, opens routes to examining multiple potential ER stress related therapeutic targets with existing probes for complex pain problems in man and companion animals.
The effects on phosphorylation of p38 and JNK, two well recognized pain-modulating kinases add to the hypothesis that ER stress is an underlying mechanism in multiple painful syndromes. Our findings that they take part in the orchestration of ER stress responses provide insights into their upstream regulators in addition to highlighting ER stress as a common denominator for pain. Activation of MAPKs is important in mediating signal transduction from the ER to the cell nucleus. Specifically, p38-MAPK is activated by an array of ER stress inducing agents including tunicamycin. ER stress-induced activation of p38 is associated with inflammation and mediates cytokine production through a variety of mechanisms. Activation of JNK in response to ER stress seems to be IRE1- and TNF-receptor-associated factor 2 (TRAF2) dependent. Active IRE1 recruits adaptor molecule TRAF2 which activates the apoptosis-signal-regulating kinase (ASK1). This mitogen-activated kinase transmits various stress signals to downstream MAPKs including JNK and p38 (33). Increased phosphorylation and activation of p38 and JNK in spinal astrocytes and microglia after nerve injury has been observed. Furthermore, intrathecal injections of selective inhibitors of p38, and JNK attenuated inflammatory and neuropathic pain in rats and mice (22). Overall these findings suggest that p38 and JNK have pivotal roles in ER stress signaling during neuropathic pain and that inhibition of ER stress would be an effective strategy to alleviate neuropathic pain. Aside from these two kinases other players in the ER stress pathways are well characterized (34). Although these targets have not been considered in the context of pain our results strongly argue for their exploration. Specifically for diabetic neuropathy, our findings are consistent with knowledge generated over the past 50 years. For example, easily recognized morphological changes previously described in neuropathic pain suggest activation of autophagic and apoptotic cascades (i.e., loss of distal ends of primary afferents). During diabetes different classes of primary afferents go through a continuum of different stages i.e., cells transmitting heat related information first become more sensitive then over time their responses diminish (35). A similar pattern is captured here using Tm (tunicamycin). Intraplantar Tm leads to rapid mechanical hyperalgesia and thermal hypoalgesia while the phenotype is maintained on the ipsilateral side. Both hyperglycemia and Tm increase ER stress, autophagy and apoptosis markers, and are reduced by 4-PBA and sEH inhibition. Changes observed in different types of primary afferents could stem from yet unknown susceptibility factors or could be related to exposure of cells to stressors. Regardless, the complexity in the etiology of diabetic neuropathy may originate from the diversity of ER stress responses in different afferent classes and cells supporting or communicating with these neurons, ultimately generating a complex picture. Excellent examples of this type of selectivity and diversity among nociceptors in response to injured adjacent ganglia are reported (36, 37). Nerve damage induced pain causes selective changes in uninjured C-, Aδ, and Aβ nociceptors demonstrating their diverse levels of susceptibility to insult. Overall our observations provide a system wide view of the diabetic PNS where key cellular and molecular events are driven by ER stress. There is epistemic uncertainty in the two new models of pain based on ER stress. The remedy for reducing uncertainty is increasing the diversity of available models of pain. Our findings argue that ER stress based models of pain could be appropriate in evaluating novel compounds. On the other hand many of the currently used models may already have a strong ER stress based component as exemplified here with the STZ model.
The EpFAs are potent and short lived bioactive lipid mediators. Although their discovery dates back several decades, initial studies required laborious steps to obtain sufficient quantities for bioassays which were mostly restricted to in vitro and ex vivo systems (38). Subsequently, the realization that the sEH is a major contributor to their short half life in vivo drove the discovery of inhibitors with increasing potency and drug like properties (39, 40). Current probes, including TPPU, are easy to administer orally or in drinking water, have high rates of exposure and are exceptionally potent on sEH (25). Taking advantage of selective probes and mass spectroscopy technology to monitor EpFAs, we hypothesized that inhibition of sEH would have antiinflammatory effects (41). Surprisingly, EpFAs not only block inflammation due to sepsis and associated pain but they are independently analgesic, being effective in models of nerve injury and PGE2 induced pain (15). The kinase mediators of neuropathic pain, p38 and JNK, are effectively blocked by inhibition of sEH as early as 30 min after induction of ER stress. This predicts that targeting sEH is a therapeutic approach for neuropathic pain with one example recently reported in a horse suffering from terminal laminitis (42). Much of the work performed with the EpFAs and sEHI results in similar outcomes, whereas sEHI have more profound and sustained activity compared with the EpFAs themselves (43). This is not surprising because sEHI stabilize EpFAs. However, even without sEHI, administration of EpFAs to the site of inflammation or by intraspinal or i.c.v. routes result in direct pain and seizure blocking effects. Overall these are encouraging findings, although bioactive lipids as mediators of analgesia are not well understood. The EpFAs and endocannabionoids certainly do not fit the criteria of classical neurotransmitters. Although the endocannabinoids produce a more recognizable phenotype, the behavioral profile of EpFAs is more subtle and difficult to fit into known classes of compounds. Despite this difficulty in classification, inhibition of sEH has several key advantages in pain therapy, including better efficacy than existing analgesics, lack of narcotic and addictive effects and lack of gastrointestinal and cardiac side effects. There are clear and multiple mechanisms of action of EpFAs and sEHI in reducing pain related behavior. In regard to the ER stress pathways current data do not demonstrate a branch that is strongly blocked by EpFAs over the other branches. This suggests the target(s) of EpFAs may be at the level of or upstream to the ER stress sensors. Another possibility, however unlikely, is that EpFAs modulate the conformation of these protein sensors by way of altering ER-membrane physical properties. The rapid effects of EpFAs and sEHI suggest it is more likely that phosphorylation of key targets may be altered by EpFAs. Although these pending questions about details of mechanism of action of EpFAs remain to be understood, stronger evidence points toward positive modulation of the GABAergic signaling because sEHI and EpFAs are anticonvulsant in models of seizures, only when GABA antagonists are used (16). Furthermore, their efficacy is reversible by blockage of the steroid and neurosteroid synthetic pathways at distinct steps. Currently it is unknown if the sEHI augments GABAergic signaling through its ability to block ER stress and render the GABA system functional under neuropathic conditions, which is known to reduce the activity of GABAergic signaling (44). Regardless, investigating if and how classical pain targets respond to ER stress or its alleviation is an area of future interest.
From a translational perspective, development of novel pain therapeutics has been slow compared with other drugs. Remarkably few drug candidates with novel mechanisms of action are currently under development despite significant clinical need for new drugs to treat pain. The discovery that pain is largely regulated by ER stress should if true will raise the hopes of developing a new generation of effective therapeutics in the form of inhibitors of sEH or of other ER stress regulators that potentially address diverse painful states in patients. These compounds, in theory, may have a lesser degree of mechanistic toxicity if nociceptive neurons are more susceptible to ER stress as argued here.
Materials and Methods
Details of the experimental protocols are given in SI Materials and Methods.
Animals and Animal Care.
All procedures were in agreement with standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals. All procedures were performed according to institutional guidelines for animal experimentation and were approved by the Animal Resource Services of the University of California, Davis, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Rats were housed under standard conditions, 12-h light–dark cycle and ad libitum food and water.
Models, Behavioral Tests, Treatments, and Biochemical Analyses.
Male SD rats weighing 250 g were from Charles River Laboratories and maintained under standard conditions. All procedures were in agreement with standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals. Study was performed according to institutional guidelines for animal experimentation and was approved by the Animal Resource Services of the University of California, Davis, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Diabetes was induced by a single i.v. injection of STZ (55 mg/kg) as described (45). Behavioral tests were done using a modified von Frey assay (10). All data are presented as mean ± SE. TPPU and 4-PBA were administered using PEG400 as the vehicle in a volume of 1 mL/kg, by i.p route. Tm and Dmcx were administered into the plantar surface of one hind paw. Standard methods were used for Western blotting detailed in the online SI (20). Antibodies were from Santa Cruz Biotechnology, Cell Signaling Technology and Abcam. Total RNA was extracted using TRIzol (Invitrogen). cDNA was generated using high-capacity cDNA synthesis kit (Applied Biosystems). Bip, sXbp1, and Atf4 transcripts were assessed by SYBR Green qRT-PCR using SsoAdvanced Universal SYBR Green Supermix (iCycler, BioRad). Relative gene expression was quantified using the ∆CT method and normalized to Tata-box binding protein.
Statistical Analyses.
Data were analyzed using the SigmaPlot analysis package (Systat).
SI Materials and Methods
Chemicals and Reagents.
Antibodies for tubulin, p-PERK (Thr980), PERK, p-eIF2α (Ser51), eIF2α, sXBP1, cATF6, and IRE1α, were from Santa Cruz Biotechnology. Antibodies for p38, pp38 (Thr180/Tyr182), BiP, JNK, p-JNK (Thr183/Tyr185), and Cox 2 were obtained from Cell Signaling Technology. Antibodies for p-IRE1α (Ser724) was purchased from Abcam. PVDF membranes and protein standards were obtained from BIO-RAD. The ECL Western blotting system was from Thermo Fisher Scientific. All other reagents were from the highest quality available and were purchased from Sigma.
Western Blotting.
Tissues were homogenized in radio-immunoprecipitation assay buffer [RIPA, 10 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 0.1% (wt/vol) SDS, 1% (wt/vol) Triton X-100, 1% sodium deoxycholate, 5 mM EDTA, 1 mM NaF, 1 mM sodium orthovanadate, and protease inhibitors]. Homogenates were centrifuged at 13,000 × g for 10 min, the supernatant collected, and protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce Chemical) according to the manufacturer's instructions. Aliquots of total cell lysates containing 25–40 μg of protein were denatured with Laemmli buffer, resolved by SDS/PAGE and transferred to PVDF membranes. Membranes were blotted for 1 h in 5% (wt/vol) BSA and subsequently incubated in the presence of corresponding primary antibodies (1:1,000 dilution for all of the antibodies except pJNK, JNK, pp38, and p38 which were 1:5,000) overnight at 4 °C. After incubation for 60 min at room temperature in the presence of the HRP-conjugated secondary antibody (1:10,000 dilution), the reacting bands were visualized using the ECL Western blotting system. Pixel intensities of immunoreactive bands were quantified using FluorChem Q Imaging software (Alpha Innotech). For phosphorylated proteins, data are presented as normalized signal of phosphorylated form to total target protein signal for each animal individually. For nonphosphorylated proteins, normalization was performed using Tubulin for each animal individually. The graphs display mean signal intensity ± SEM (20).
Quantitative RT-PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen). cDNA was generated using a high-capacity cDNA synthesis Kit (Applied Biosystems). Bip, sXbp1, and Atf4 were assessed by SYBR Green quantitative real-time PCR using SsoAdvanced Universal SYBR Green Supermix (iCycler, BioRad). Relative gene expression was quantified using the ∆CT method with appropriate primers and normalized to Tata-box binding protein (Tbp). Briefly, the threshold cycle (Ct) was determined and relative gene expression was calculated as follows: fold change = 2-∆(∆Ct), where ∆Ct = Ct target gene-Ct Tbp (cycle difference) and ∆(∆Ct) = Ct (treated rats)-/Ct (control rats).
Nociceptive Models and Behavioral Tests.
The STZ (55 mg/kg, i.v.) induced rat neuropathic pain model is extensively described (45). For the tunicamycin and dimethylcelecoxib induced models these compounds were administered by intraplantar injection in a volume of 10 µL saline. Standard nociceptive measurements were performed in all experiments. For the von Frey test an electronic rigid tip instrument was used (IITC). For the thermal nociceptive thresholds the modified Hargreaves' method was used. The baseline withdrawal thresholds before STZ administration were taken as 100% response and all mean nociceptive thresholds were converted to percentage values. Inhibitors were administered by i.p. route after completely dissolving in vehicle PEG400 (1 mL/kg). All data are presented as mean ± SE of mean (10).
Statistical Analyses.
Data were analyzed by parametric and nonparametric one-way ANOVA followed by post hoc tests suggested by the SigmaPlot analysis package (Systat Software). Results are depicted as mean ± SEM. CompuSyn software package was used to quantify the synergy between drugs.
Determination of Inhibitor Potency.
TPPU was synthesized in house but is also available commercially. Enzyme residence time refers to the dissociation rate constant (koff) on a target enzyme. Recent work demonstrates that residence time is an important parameter that can better predict in vivo efficacy (46). In particular, we demonstrated that inhibitors of sEH with longer residence time display more efficacy in in vivo tests than those with shorter time of residence in the enzyme, using the diabetes mediated model of neuropathic pain (47, 48). These properties for TPPU used in this study include excellent inhibitory potency with a long enzyme residence time and are displayed in Table S2.
Acknowledgments
This work was supported by US National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.), NIEHS Superfund Research Program P42 ES004699, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R21 AR062866 (to B.I. and B.D.H.), the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke (NINDS) U54 NS079202 (to B.I. and B.D.H.), NIEHS ES025598-01A1 (to B.D.H.), and 1K99ES024806 (to K.S.S.L.). Research in F.G.H. laboratory is funded by NIH research Grants R01DK090492 and R01DK095359 (to F.G.H.) and K99DK100736 (to A.B.).
Footnotes
Conflict of interest statement: B.I., K.S.S.L., and B.D.H. are co-inventors on patents related to sEH by the University of California. B.D.H. and B.I. are co-founders of EicOsis LLC.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510137112/-/DCSupplemental.
References
- 1.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 2.Hummasti S, Hotamisligil GS. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res. 2010;107(5):579–591. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197(7):857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle KM, et al. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15(10):2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stetler RA, et al. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92(2):184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas PK, Lascelles RG. Schwann-cell abnormalities in diabetic neuropathy. Lancet. 1965;1(7400):1355–1357. doi: 10.1016/s0140-6736(65)92154-9. [DOI] [PubMed] [Google Scholar]
- 8.Strachan MWJ, Price JF. Diabetes. Cognitive decline and T2DM—a disconnect in the evidence? Nat Rev Endocrinol. 2014;10(5):258–260. doi: 10.1038/nrendo.2014.38. [DOI] [PubMed] [Google Scholar]
- 9.Reske-Nielsen E, Lundbaek K. Pathological changes in the central and peripheral nervous system of young long-term diabetics. II. The spinal cord and peripheral nerves. Diabetologia. 1968;4(1):34–43. doi: 10.1007/BF01241031. [DOI] [PubMed] [Google Scholar]
- 10.Inceoglu B, et al. Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes. Proc Natl Acad Sci USA. 2012;109(28):11390–11395. doi: 10.1073/pnas.1208708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas H, Schladt L, Knehr M, Oesch F. Effect of diabetes and starvation on the activity of rat liver epoxide hydrolases, glutathione S-transferases and peroxisomal beta-oxidation. Biochem Pharmacol. 1989;38(23):4291–4297. doi: 10.1016/0006-2952(89)90528-5. [DOI] [PubMed] [Google Scholar]
- 12.De Taeye BM, et al. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring) 2010;18(3):489–498. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewey S, Lai X, Witzmann FA, Sohal M, Gomes AV. Proteomic analysis of hearts from Akita mice suggests that increases in soluble epoxide hydrolase and antioxidative programming are key changes in early stages of diabetic cardiomyopathy. J Proteome Res. 2013;12(9):3920–3933. doi: 10.1021/pr4004739. [DOI] [PubMed] [Google Scholar]
- 14.Inceoglu B, et al. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inceoglu B, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108(12):5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inceoglu B, et al. Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PLoS ONE. 2013;8(12):e80922. doi: 10.1371/journal.pone.0080922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inceoglu B, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105(48):18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014;15(9):907–914. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piomelli D, Hohmann AG, Seybold V, Hammock BD. A lipid gate for the peripheral control of pain. J Neurosci. 2014;34(46):15184–15191. doi: 10.1523/JNEUROSCI.3475-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettaieb A, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288(20):14189–14199. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 22.Ji RR, Gereau RW, 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Brain Res Rev. 2009;60(1):135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lupachyk S, Watcho P, Obrosov AA, Stavniichuk R, Obrosova IG. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Exp Neurol. 2013;247:342–348. doi: 10.1016/j.expneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose TE, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53(19):7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Watowich SS, Morimoto RI. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol Cell Biol. 1988;8(1):393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kardosh A, et al. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt’s lymphoma in vitro and in vivo. Cancer Biol Ther. 2005;4(5):571–582. doi: 10.4161/cbt.4.5.1699. [DOI] [PubMed] [Google Scholar]
- 29.Pyrko P, et al. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007;6(4):1262–1275. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- 30.Peltier A, Goutman SA, Callaghan BC. Painful diabetic neuropathy. BMJ. 2014;348:g1799. doi: 10.1136/bmj.g1799. [DOI] [PubMed] [Google Scholar]
- 31.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456–2465. doi: 10.2337/dc12-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregor MF, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 35.Malik RA. Pathology of human diabetic neuropathy. Handb Clin Neurol. 2014;126:249–259. doi: 10.1016/B978-0-444-53480-4.00016-3. [DOI] [PubMed] [Google Scholar]
- 36.Boada MD, et al. 2014. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol, jn 00506 02014.
- 37.Djouhri L, Fang X, Koutsikou S, Lawson SN. Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain. 2012;153(9):1824–1836. doi: 10.1016/j.pain.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capdevila J, et al. The oxidative metabolism of arachidonic acid by purified cytochromes P-450. Biochem Biophys Res Commun. 1981;101(4):1357–1363. doi: 10.1016/0006-291x(81)91597-7. [DOI] [PubMed] [Google Scholar]
- 39.Chacos N, et al. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223(2):639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 40.Morisseau C, et al. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96(16):8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmelzer KR, et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102(28):9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guedes AG, et al. Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis. Vet Anaesth Analg. 2013;40(4):440–448. doi: 10.1111/vaa.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morisseau C, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 45.Aley KO, Levine JD. Rapid onset pain induced by intravenous streptozotocin in the rat. J Pain. 2001;2(3):146–150. doi: 10.1054/jpai.2001.21592. [DOI] [PubMed] [Google Scholar]
- 46.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov. 2006;5(9):730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 47.Lee KS, et al. Förster resonance energy transfer competitive displacement assay for human soluble epoxide hydrolase. Anal Biochem. 2013;434(2):259–268. doi: 10.1016/j.ab.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KS, et al. Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J Med Chem. 2014;57(16):7016–7030. doi: 10.1021/jm500694p. [DOI] [PMC free article] [PubMed] [Google Scholar]