Abstract
Vascular smooth muscle cells (VSMCs), are subjected to different types of mechanical forces within the vessel wall. Although it is known that VSMC undergo cell body reorientation in response to the mechanical stimulation, how this mechanical stretch is transduced within the cell into biochemical signals causing cytoskeleton reorganization remains unclear.
Cofilin, a protein that controls actin dynamics, is activated by Slingshot phosphatase-dependent Serine 3 dephosphorylation by redox dependent mechanisms. Nox4 is a main source of reactive oxygen species (ROS) in the vessel wall that localizes in association with the cytoskeleton. Therefore, we hypothesize that Nox4 mediates redox-dependent activation of cofilin which is required for cytoskeletal reorganization and cell reorientation after mechanical stimulation. In this study, we found that mechanical stretch stimulates ROS production in VSMCs and that the signaling that leads to cell reorientation required hydrogen peroxide but not superoxide. Indeed, mechanical stretch induces cofilin activation and stretch-induced cytoskeletal reorganization and cell reorientation is inhibited in cells where cofilin activity has been downregulated. Importantly, Nox4 deficient cells fail to activate cofilin and to undergo cell reorientation, a phenotype rescued by expression of a constitutive active cofilin mutant. Our results demonstrate that in VSMC mechanical stimulation activates cofilin by a Nox4-dependent mechanism and that this pathway is required for cytoskeleton reorganization and cell reorientation.
Keywords: Human Vascular Smooth Muscle Cells, Slingshot-1, Cofilin, Nox4, Hydrogen Peroxide
Introduction
Vascular smooth muscle cells (VSMCs), a main component of the vessel wall, are subjected to different types of mechanical forces. Indeed, in vitro [1] and in vivo [2] VSMC respond to biomechanical forces resulting in reorientation of the cell body, which has been proposed as one mechanism to counteract the strain. Although, it remains incompletely understood how mechanical forces are transduced within the cell into biochemical signals, it is expected that a variety of signaling pathways used for mechanotransduction share common elements at the cytoskeletal level.
Cofilin is member of the actin depolymerizing factor family of proteins that increase the rate of depolymerization of actin filaments (for review see [3, 4]). Cofilin activity, which is negatively regulated by phosphorylation on Ser3 by LIM kinases and reactivated by Slingshot 1L (SSH1L) phosphatase [5, 6], stimulates disassembly and severance of actin filaments at or near the pointed ends and thereby actin monomers are continuously supplied for polymerization and rapid turnover of actin filaments [7]. We have previously shown that PDGF-induced cofilin activation is regulated by a redox sensitive mechanism in VSMCs [6, 8, 9].
NADPH oxidases are main sources of O2·− and H2O2 in the vessel wall [10–12]. In all vascular cells, these oxidases are low output enzymes [13] that have been shown to modulate intracellular signaling pathways [11, 14–16]. NADPH oxidase activity in VSMC from large arteries in rodents is derived from the gp91phox homologues Nox1 and Nox4 [17]. While Nox1 is found in caveolae [18] and activated by growth factors and Angiotensin II to produce superoxide [17, 19, 20], Nox4 localizes to nucleus, stress fibers and focal adhesions (FAs), produces primarily hydrogen peroxide [19], and its activity seems to be required for cytoskeleton and FA stability [21].
In this work we test the hypothesis that cofilin is activated by Nox4-derived ROS after mechanical stimulation and that cofilin activity is required for VSMC reorientation during mechanical stress. Our results revealed that the cytoskeletal dynamics that support cell body reorientation after mechanical stimulation are redox sensitive and that the Nox4/cofilin axis is a key pathway in this response.
Materials and Methods
Cell culture and treatments
Human aortic smooth muscle cells (HASMCs) were purchased from Lonza (San Diego, CA) and cultured in Medium 231 with Smooth Muscle Growth Supplement-SMGS (Invitrogen-Life Technologies, Carlsbad, CA) and incubated in a 5% CO2 atmosphere at 37°C. HASMCs between passages 6 and 12 were seeded in 6-well BioFlex culture plate coated with type I collagen (Flexcell, Hillsborough, NC) at 80% confluency and were made quiescent in serum free media 24 hours before the stretching protocol. In some experiments, cells were pre-treated for 2 hours with 1200 I.U. of native or heat-denatured superoxide dismutase (Sigma S2515), or catalase (Sigma C3515). In same experiments, VSMC derived from Nox1KO animals (Nox1y/−) were prepared as we have done previously and culture in DMEM supplemented with 10% FBS.
Stretching protocol and assessment of cell alignment
Using a computer-controlled Flexcell FX-5000™ tension system (Flexcell, Hillsborough, NC), HASMCs were subjected to repeated biaxial stretching cycles of 10% with a frequency of 1 Hz for 24h. At the end of the stretching protocol, cells were fixed with 10% formaldehyde and stained with Phalloidin-Alexa Fluor-488 (Invitrogen-Life Technologies, Carlsbad, CA) and 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/mL). Images were acquired using an Olympus IX71 microscope coupled to a DP71 Camera. For each experiment, 5–6 images from the non-stretched groups and 7–10 images from the stretched groups were randomly taken in each quadrant of the well. Cell orientation was measured using the ImageJ software (rsbweb.nih.gov/ij/) and cell alignment calculated by determining the standard deviation (SD) of the sum of the angles that cells form with respect to an arbitrary reference. Higher values of the SD represent less aligned cells, while lower SD represents more aligned cells. The Coefficient of Alignment (CA) was estimated by the formula CA=100*(σ-|SD|)/σ. In this analysis, values approaching 100 indicate a higher degree of alignment, while values approaching 0 indicate a random distribution of the cells. For static controls, cells were cells seeded in the same bottom flexible flexcell plates and culture in the same incubator with the stretched cells, only the vacuum-mediated stretch was omitted.
Hydrogen peroxide quantification
We evaluate the effects of mechanical stretch on hydrogen peroxide production by VSMCs using 50 μM of Amplex Red (Invitrogen-Life Technologies, Carlsbad, CA) in phenol red free media containing 0.1 U/mL of horseradish peroxidase (HRP). At the end of each time-course, the content of hydrogen peroxide in the media was evaluated by quantification of the fluorescence emission at 580 nm after excitation at 530 nm and compared to the emission of the same number of cells in static condition.
Western blotting
For Nox4, cells were lysed in HEPES buffer pH 7.4 containing 0.5% of deoxycholate, 0.1% of SDS, 1% of Triton and 10% glycerol. After protein quantification using bicinchoninic acid assay (Pierce Chemical Company), the samples were reduced with SDS-buffer containing Tris (2-carboxyethyl) phosphine hydrochloride (TCEP, 150 mM). Samples were not boiled to avoid aggregation. For cofilin, cells were collected for western blots using warm laemmli buffer containing SDS and β-mercaptoethanol to avoid cofilin dephosphorylation during sample preparation. Protein samples were separated by electrophoresis and blotted onto PVDF. Membranes were incubated overnight with specific primary antibodies against Nox4 (Abcam, ab81967), α/β-tubulin (Cell Signaling 2148), p-Ser3cofilin (Cell Signaling 3311S). To evaluate total cofilin, p-cofilin-labeled membranes were stripped and rebloted with total cofilin antibody (Genetex GTX102156) followed by HRP-conjugated secondary antibodies. The intensity of the bands was revealed by ECL chemiluminescence using a Kodak scanner with Carestream software. Images were analyzed by densitometry using Image J software (rsbweb.nih.gov/ij/).
Small interfering RNA and plasmid transfection experiments
Cells were transfected with small interfering RNA (siRNA) or plasmids three days before the experiments using a Nucleofector ™ System (Amaxa Biosystems) set to the U25 program. To downregulate Nox4 or SSH1L phosphatase, 3 μg custom designed siRNA against each human sequence (5′-GCAUCUGUUCUUAACCUCA-3′ for Nox4 and 5′-ACUGAGGUACAGCUGGAUGUU-3′ for SH1L) was used to electroporate one million cells. All Stars negative control siRNA (Qiagen 1027281) was used as a control siRNA. To increase cofilin activity, cells were co-transfected with siRNA (negative control or against Nox4) and either 1.1 μg of a plasmid containing a constitutively active form of cofilin (GFP_S3A_cofilin, kind gift of Dr. Condeelis, Albert Einstein College of Medicine, NY) or 1.0 μg of GFP-empty vector as a control. Similarly, in order to intensify LIM kinase activity, cells were transfected with 2 μg of an EGFP-plasmid encoding a constitutively active LIM kinase mutant [6] or EGFP empty vector (Clontech) as a control.
RhoA activity
HASMCs cells were transfected with siRNA against Nox4 or with All Stars negative control siRNA using Nucleofector TM System set to U25 program. Cells were seeded on Collagen I-coated BioFlex plates and growth per 48h. Subsequently, cells were serum starved for 24h and subjected to repeated biaxial stretching cycles of 10% with a frequency of 1 Hz for 2h using Flexcell FX-5000TM tension system. Plates were immediately processed for GLISA-RhoA assay (Cytoskeleton, BK#124) following to the manufacturer instructions. Protein concentration was determined before the assay showing less than 10% of variation between conditions. Plate was read at 490nm using SynergyTM H1 System. Consequently, the absorbance values obtained were standardized by the protein concentration from each condition. Results are shown as mean ± SE of 3 biological replicates.
Cell viability test
Cell suspension was diluted 1:1 in a 0.4% Trypan Blue solution. Cells were placed in a Neubauer chamber and counted under the microscope.
Statistical analysis
The results are expressed as means ± SE. Comparisons between groups were made by one-way or two-way analysis of variance followed by Dunnett’s multiple comparison post-hoc test using GraphPad Prism 6.0 for Windows. A probability value <0.05 was considered significant.
RESULTS
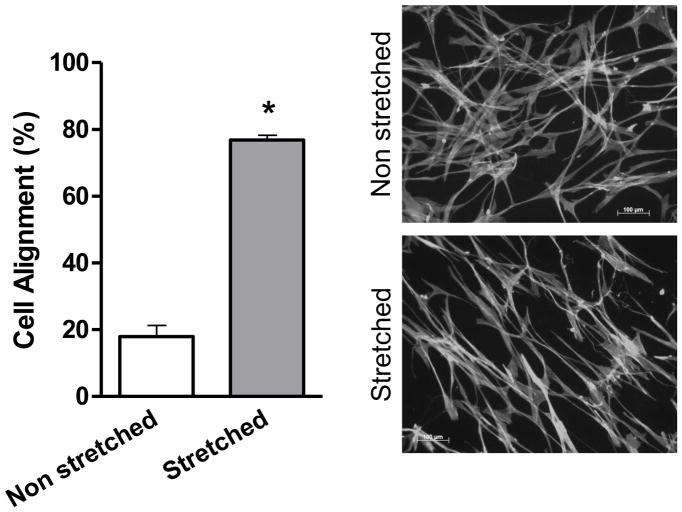
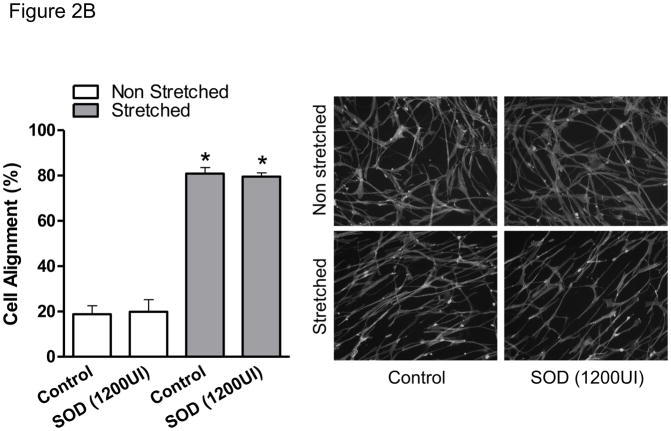
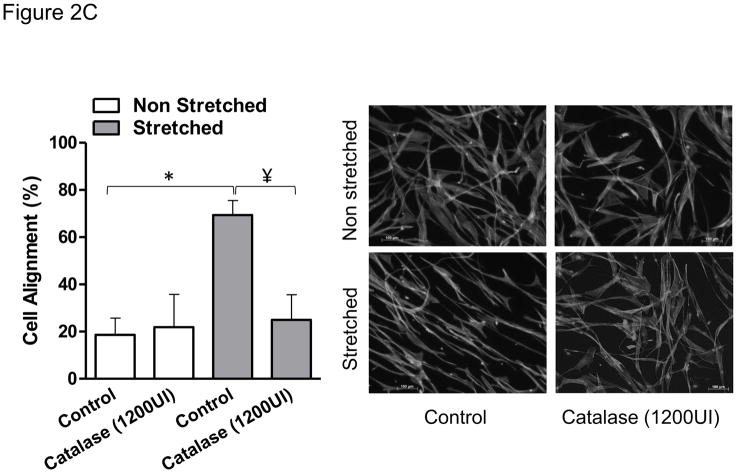
After 24 hours of mechanical stretch, HASMCs completely reorient their cell body perpendicular to the strain (Figure 1), this response was not dependent on confluency since cells between seeded at densities between 20–80% undergo similar degree of cell alignment (75±1.3–78±3%, p=0.54, Supplemental Figure 1) after mechanical stimulation. Because the cytoskeleton contains multiple proteins subject to redox modification [22], we investigated if the mechanism that produces cell body reorientation after mechanical stimulation is mediated by ROS. With that in mind, we first measured the production of ROS after mechanical stimulation. We observed that the accumulative hydrogen peroxide content, although increased over time in control cells, was significantly higher in cells subjected to mechanical stimulation (Figure 2A), an increase that is maintained and amplified throughout the experiment. In order to evaluate if this ROS production was required for cell reorientation and with the objective of discriminating the identity of the species that participate in this signaling pathway, cells were pretreated with either of two antioxidant enzymes: catalase and superoxide dismutase (SOD). Interestingly, our results showed that cell reorientation is not affected by the presence of SOD (Figure 2B), but is completely blocked in the presence of catalase (Figure 2C). These results demonstrate that hydrogen peroxide, and not superoxide, participates in the signaling pathway that leads to the reorganization of actin cytoskeleton after mechanical stretch.
Figure 1. HASMCs reorient after mechanical stimulation.
HASMCs between passages 6 and 12 were seeded in BioFlex culture plates coated with type I collagen. Cells were subjected to repeated biaxial stretching cycles of 10% with a frequency of 1 Hz for 24h and them fixed and stained. Cell alignment was estimated using a fluorescence microscope as is described in Material and Methods. Representative image and bar graphs (mean ± SE) of at least 5–7 independent experiments performed in duplicate are shown. (*) indicates P<0.05.
Figure 2. H2O2 mediates HASMC reorientation after mechanical stimulation.
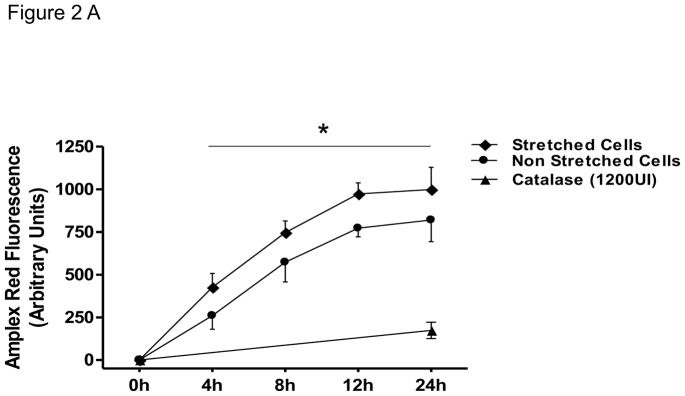
HASMCs were seeded in BioFlex culture plates coated with type I collagen. A) Cells were subjected to repeated biaxial stretching cycles of 10% with a frequency of 1 Hz for 24h in phenol-red free media supplemented with 50 μM Amplex and 0.1 U/mL HRP. At the indicated times, H2O2 was quantified using a fluorometer as indicated in Materials and Methods. B and C) Cells were serum deprived and pretreated for four hours with SOD (B) or catalase (C) prior to 24 hours of cyclic stretch. After fixation and staining, cell alignment was estimated as indicated in Materials and Methods. Representative image and bar graphs (mean ± SE) of at least 5–7 independent experiments performed in duplicate are shown. * and ¥ Indicates P<0.05 for stretch and catalase, respectively.
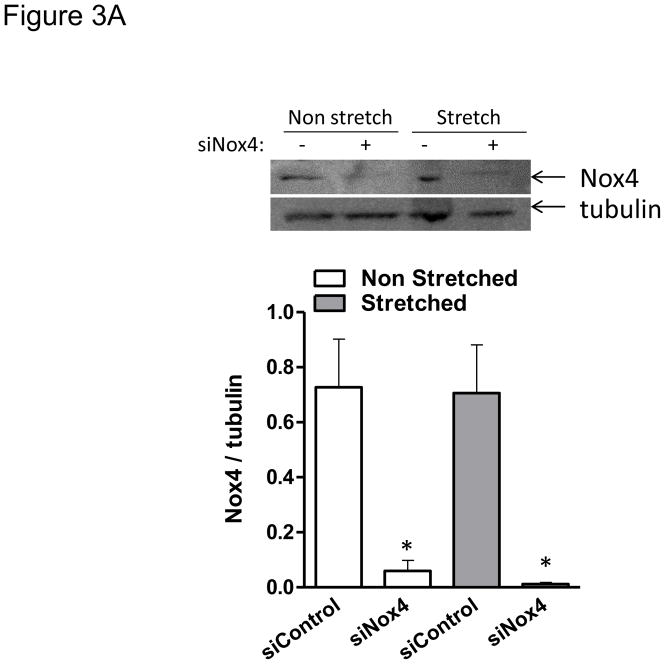
Nox4 is a unique member of the NADPH family of oxidases, first because it associates with the cytoskeleton but more importantly because it is the only catalytic homologue that directly produces hydrogen peroxide [19, 22]. Therefore, we investigated the specific role of this enzymatic subunit in the redox-sensitive cellular responses to mechanical stimulation in smooth muscle. Cells in which Nox4 was effectively downregulated using siRNA (Figure 3A) were unable to reorient after 24 hours of cyclic stretch (Figure 3B). On the contrary, cells that are deficient in Nox1 were able to orient as the wild type (from 22.9±4.3 to 85.9±0.4 in wild type vs. 21.1±2.3 to 84.5±2.1 in Nox1y/− p=0.54). These results indicate that Nox4 activity is required for cell reorientation in response to mechanical forces.
Figure 3. Nox4 is required for HASMC reorientation after mechanical stimulation.
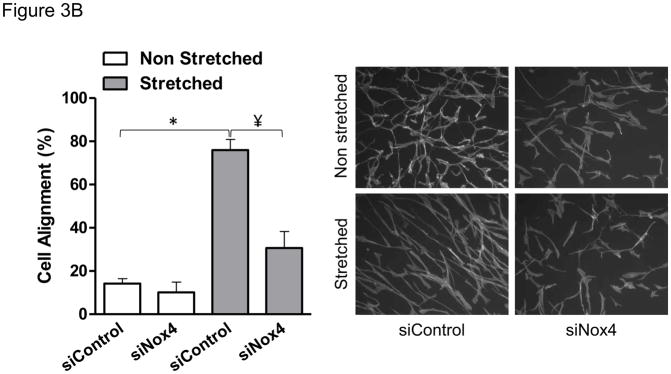
HASMCs were transfected with siRNA against Nox4 or control non-targeting siRNA 3 days before mechanical stimulation. After 24 hours of stretch, cells were either collected for western blots to verify the downregulation of Nox4 (A), or fixed and stained to estimate cellular alignment (B) as indicated in Materials and Methods. Representative image and bar graphs (mean ± SE) of at least 5–7 independent experiments performed in duplicate are shown. * and ¥ Indicate P<0.05 for siRNA against Nox4 and stretch, respectively.
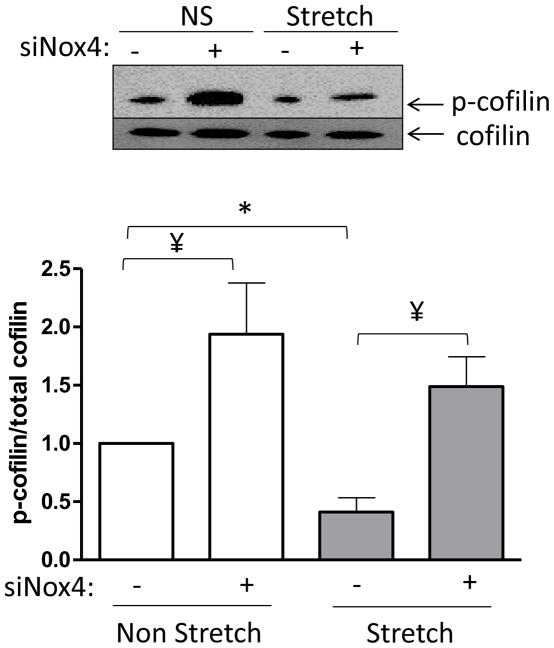
Regarding the target of Nox4, our own work has demonstrated that the actin binding/severing protein cofilin is activated in a redox dependent manner in VSMC during PDGF-induced migration [23]. Therefore, we reasoned that cofilin may be the target of Nox4-produced H2O2 required for reorientation after mechanical stimulation. To evaluate this possibility, we measured cofilin activation/dephosphorylation after 2 hours of mechanical stretch, the time at which we observed the initial changes at the cytoskeleton level (data not shown), in cells transfected with control siRNA or siRNA to Nox4. As expected, we observed that in control cells cofilin is activated to a similar extent as that which we have reported to occur during VSMC migration [6] (Figure 4). Interestingly, the data also confirmed that in the absence of Nox4, stretch-induced cofilin dephosphorylation is blunted (Figure 4). Together, these results support the idea that cofilin is required for the cytoskeletal organization that occurs after mechanical stimulation and that it is activated by a Nox4-dependent mechanism.
Figure 4. Cofilin is activated by a Nox4 dependent mechanism.
HASMCs were transfected with siRNA against Nox4 or non-targeting siRNA 3 days before initiating 2 hours of cyclic mechanical stimulation or static control conditions. Samples were processed for western blotting using specific antibodies for cofilin and p-cofilin. Representative image and bar graphs (mean ± SE) of at least 5 independent experiments performed are shown. * and ¥ Indicate P<0.05 for stretch and siNox4, respectively.
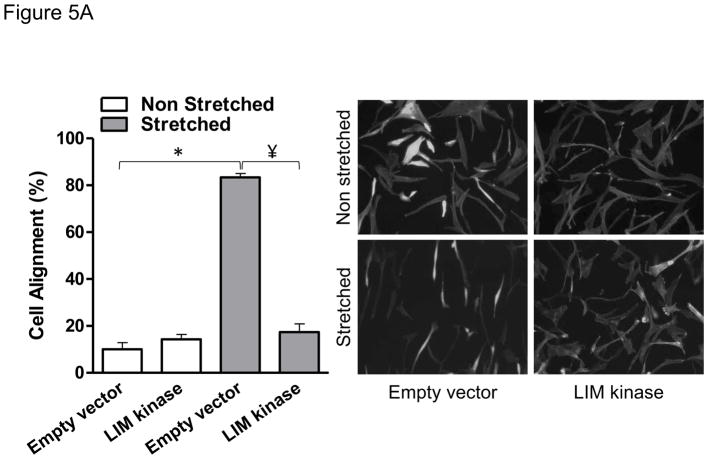
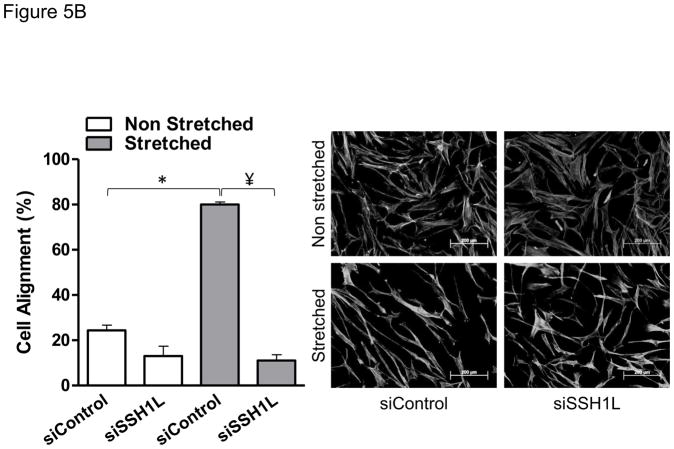
To test the role of cofilin in cell reorientation, we manipulated cofilin activity by altering the expression levels of its upstream regulators: LIM-kinase and the SSH1L phosphatase as we have done previously [6]. As shown in Figure 5, the inhibition of cofilin activity by either knocking down SSH1L phosphatase (Figure 5A) or expression of a constitutively active form of LIM kinase (Figure 5B) abolished cell reorientation after mechanical stretch. These results indicate that cofilin activity is required for the rearrangements of the cytoskeleton that support cell reorientation in response to mechanical forces. Finally, in order to confirm that Nox4-activated cofilin mediates cell reorientation after mechanical stimulation, we tested the ability of a constitutive active form of cofilin to restore the cell response to strain lost in Nox4-deficient cells. As anticipated, constitutively active cofilin S3A mutant, an unphosphorylated mimetic that cannot be inactivated by phosphorylation, is capable of restoring the response to mechanical stimulation in Nox4 deficient cells (Figure 6). This demonstrates that Nox4 is responsible for cofilin activation/dephosphorylation during cofilin-mediated cell alignment after mechanical stimulation. Interestingly, the Nox4-mediated activation of cofilin during mechanical stimulation does not seem to be mediated by changes in RhoA activity (Supplemental Figure 2).
Figure 5. Cofilin activity is required for HASMC reorientation after mechanical stimulation.
HASMCs were transfected with a plasmid containing a constitutive active form of LIMK or empty vector 48 hours before mechanical stimulation (A) or siRNA against Slingshot-1L phosphatase or non-targeting siRNA (B) 3 days before initiating mechanical stimulation. After 24 hours of stretch, cells were fixed and stained to estimate cellular alignment as indicated in Materials and Methods. Representative image and bar graphs (mean ± SE) of 4 independent experiments performed in duplicate are shown. (*) Indicates P<0.05 for the effect of stretch and ¥ indicates P<0.05 for the effect of LIMK and siSSH in A and B, respectively.
Figure 6. A constitutively active cofilin mutant reverses the phenotype induced by the lack of Nox4.
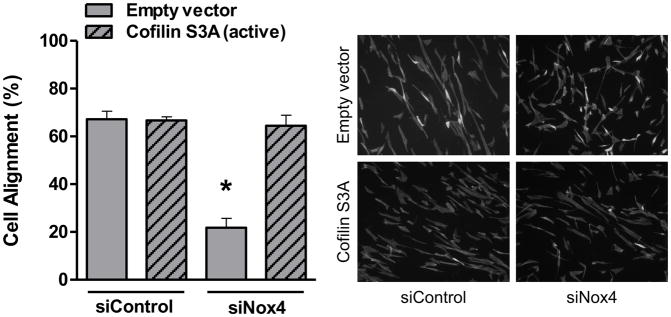
HASMCs were co-transfected with siRNA against Nox4 or a non-targeting siRNA and with an expression vector for cofilin S3A or empty vector 48 hours before mechanical stimulation. After 24 hours of stretch, cells were fixed and stained to estimate cellular alignment as indicated in Materials and Methods. Representative image (B) and bar graphs (mean ± SE) (A) of at least 5–7 independent experiments performed in duplicate are shown. (*) Indicates P<0.05.
DISCUSSION
The goal of the current study was to begin to elucidate the signaling pathways used by vascular smooth muscle to transduce mechanical perturbation. FAs serve as the structural link between the cytoskeleton and the extracellular matrix. Therefore, we anticipated that the pathways that are used to transduce signals triggered by strain are initiated at the FA level and subsequently alter cytoskeleton dynamics. In this regard, ten years ago Hilenski and collaborators reported that Nox4 was found in stress fibers and FA [18], suggesting a role for this enzyme in the regulation of the actin cytoskeleton that was later confirmed [21, 24]. However, the downstream signaling molecules that mediate Nox4-dependent cytoskeleton regulation are far to be clear. Taking into account our previous work about the redox regulation of the small actin binding protein cofilin [6], in this study we tested the hypothesis that Nox4 participates in cytoskeleton reorganization induced by mechanical stretch by a mechanism that involves redox regulation of cofilin. Our results showing that Nox4-dependent activation of cofilin is a key signaling pathway in the cellular responses to mechanical stimulation provide an explanation for how Nox4 regulates the organization of the cytoskeleton and offer an additional mechanism by which this oxidase is relevant to vascular biology.
The induction of mechanical strain has previously been linked to increased ROS production, in particular superoxide and H2O2 [25]. In this study, we found a significant and sustained increase in H2O2 during the 24-hour stretch regimen. Although we cannot rule out the possibility that there is a contribution of superoxide-derived H2O2 produced in response to strain, the ability of catalase to completely block VSMC realignment (Figure 2C) demonstrates that H2O2 is the major signaling molecule in our system. This is not surprising due its characteristic of higher stability and lower reactivity when compare to other reactive oxygen species [26]. Furthermore, because SOD had no effect on cell alignment (Figure 2B), there appears to be no contribution of superoxide-derived H2O2 in this signaling pathway, indicating that a direct source of H2O2, such as Nox4, regulates alignment after stretch in VSMC.
Published studies support the idea that Nox proteins are sensitive to mechanical perturbation, although the exact response varies greatly among cell type and strain/stress regimens. In fact, in bovine aortic endothelial cells, oscillatory shear stress has been shown to upregulate Nox2 and Nox4 mRNA expression. In contrast, pulsatile flow downregulates both Nox2 and Nox4 mRNA expression [27]. On the other hand, when mouse endothelial cells are subjected to oscillatory shear stress, Nox1 is increased while Nox2 and Nox4 are decreased [28]. Human umbilical vein endothelial cells downregulate Nox4 after 24 hours of cyclic strain [29]. In VSMCs, mechanical stretch in combination with Angiotensin II synergistically increases Nox1 expression (33), which mediates the expression of metalloproteinase-2 [30].
Nox4 activity has been thought to be constitutive and mainly activated by an increase in enzymatic units after upregulation of protein expression. Incongruous with that view, it is well reported in the literature that Nox4-dependent ROS production after agonist stimulation occurs in a time frame too short to depend upon de novo protein synthesis. However, the mechanisms that govern acute activation of Nox4 are still undefined. Here, we demonstrate a clear role for Nox4-derived reactive oxygen species in cofilin activation (Figure 4) and cell alignment after mechanical stimulation (Figure 3B) with no increase in Nox4 protein expression during the 24 hours of stretch (Figure 3A). This opens up the interesting possibility that Nox4 may be activated by a mechanically-induced integrin dependent mechanism, as it has been previously proposed during cell adhesion [31].
Multiple proteins that participate in the regulation of actin, such as cofilin, are redox sensitive [32]. In fact, our own work has shown that after stimulation with PDGF, the activation of cofilin occurs by a Nox1-dependent mechanism in VSMC. However, and in agreement with the data implicating a source that directly generates H2O2, we observed that VSMC-derived from aorta of Nox1y/− mice undergo comparable cell alignment than wild type cells after 24 hours of mechanical stretch. This raises the intriguing possibility that redox-dependent cofilin activation occurs in a delimited subcellular localization that may increases specificity in such a way that when PDGFR is activated in the caveolae [25], Nox1 will activate cofilin while Nox4 will be responsible for its activation within the FA after mechanical stimulation. In this regard, a limitation of our study is that the forces in the well are not homogenous [33]. Therefore, our data regarding cell alignment and cell signaling represent an average of the changes induced by mechanical stimulation that varies across a population of cells. Studies at the single cell level will be required to investigate in more detail how compartmentalization is affected by force generation.
RhoA activates the cofilin kinase LIMK through ROCK [34]. In addition, it has been reported that both uniaxial mechanical stretch [35] and the Nox4 positive regulator, Poldip2, increase RhoA activity [21]. Therefore, we sought to evaluate if the RhoA/LIMK pathway contributes to the Nox4-mediated cofilin activation after biaxial strain that we reported. Although our results showed that RhoA activity is unchanged at the time of cofilin activation, we do not believe that this implies that RhoA activation occurs in response to uniaxial strain but not biaxial strain; rather, it is likely that its activation occurs at a different time or in a discrete subcellular localization that we were not able to detect when using total lysates.
In contrast to previous studies that emphasized the detrimental consequences of stretch-induced ROS production, our results present an additional and normal physiological role for ROS production during mechanical stimulation that may be lost or overridden during the establishment of pathology. Indeed, perpendicular alignment to strain seems to be a normal and conserved feature of VSMCs in vivo [2] and additional research is required to understand if the loss of this response contributes the vascular dysfunction in pathological conditions such as hypertension. Because VSMC is a cell type that is constantly under tension, understanding the signaling pathways that act as mechanical sensors is key and may be particularly relevant during diseases that are accompanied by increased vessel wall tension.
Supplementary Material
Highlights.
We examine the cytoskeleton-mediated responses of VSMC to mechanical stimulation
Mechanical stimulation activates de actin binding protein cofilin by a Nox4-dependent mechanism
Nox4-mediated activation of cofilin is required for VSMC reorientation after mechanical stimulation
Acknowledgments
This work was supported by NIH grants HL093115 and HL113167.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Standley PR, Cammarata A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol. 2002;283:H1907–1914. doi: 10.1152/ajpheart.01043.2001. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell MK, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, Dalman RL, Zarins CK, Denk W, Taylor CA. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol. 2008;27:171–181. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 4.Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- 5.Alexander RW. Theodore Cooper Memorial LectureHypertension, the pathogenesis of atherosclerosis, Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 6.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by LIM kinase and Slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res. 2008;102:432–438. doi: 10.1161/CIRCRESAHA.107.158923. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci. 2000;25:19–23. doi: 10.1016/s0968-0004(99)01511-x. [DOI] [PubMed] [Google Scholar]
- 8.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.San Martin ALM, Griendling KK. Novel Nox1-mediated mechanism of SSH1L activation in VSMC: Role in cell migration. Atherosclerosis Thrombosis and Vascular Biology. 2008;28:e109. [Google Scholar]
- 10.Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. American Journal of Physiology. 1994;267:L815–822. doi: 10.1152/ajplung.1994.267.6.L815. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. Journal of Laboratory & Clinical Medicine. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 14.Tummala PE, Chen XL, Sundell CL, Laursen JB, Hammes CP, Alexander RW, Harrison DG, Medford RM. Angiotensin II induces vascular cell adhesion molecule-1 expression in rat vasculature: A potential link between the renin-angiotensin system and atherosclerosis. Circulation. 1999;100:1223–1229. doi: 10.1161/01.cir.100.11.1223. [DOI] [PubMed] [Google Scholar]
- 15.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circulation Research. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 16.Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic, oxidative stress response to angiotensin II in mice [see comment] Circulation Research. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 17.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation, redox-sensitive signaling pathways [see comment] Circulation Research. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 18.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1, Nox4 in vascular smooth muscle cells [see comment] Arteriosclerosis, Thrombosis & Vascular Biology. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 19.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, San Martin A. Platelet-derived growth factor (PDGF) regulates Slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem. 2011;286:35430–35437. doi: 10.1074/jbc.M111.268284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxidants & redox signaling. 2009;11:1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gough DR, Cotter TG. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell death & disease. 2011;2:e213. doi: 10.1038/cddis.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 29.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxidants & redox signaling. 2009;11:2385–2397. doi: 10.1089/ars.2009.2561. [DOI] [PubMed] [Google Scholar]
- 30.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 31.de Rezende FF, Martins Lima A, Niland S, Wittig I, Heide H, Schroder K, Eble JA. Integrin alpha7beta1 is a redox-regulated target of hydrogen peroxide in vascular smooth muscle cell adhesion. Free radical biology & medicine. 2012;53:521–531. doi: 10.1016/j.freeradbiomed.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Go YM, Orr M, Jones DP. Actin cytoskeleton redox proteome oxidation by cadmium. American journal of physiology. Lung cellular and molecular physiology. 2013;305:L831–843. doi: 10.1152/ajplung.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vande Geest JP, Di Martino ES, Vorp DA. An analysis of the complete strain field within Flexercell membranes. Journal of biomechanics. 2004;37:1923–1928. doi: 10.1016/j.jbiomech.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 35.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. Journal of cell science. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.