Abstract
OBJECTIVE
To characterize epithelial cells of the small intestine and colon in horses without clinical gastrointestinal abnormalities with an emphasis on the stem cell niche constituents.
SAMPLE
Mucosal biopsy specimens from small and large intestines obtained from 12 horses euthanized for reasons unrelated to gastrointestinal disease or systemic disease.
PROCEDURES
Intestinal biopsy specimens were collected by sharp dissection immediately following euthanasia. Specimens were prepared for immunohistochemical, immunofluorescence, and transmission electron microscopic imaging to detect and characterize each epithelial cell type. Antibodies against protein biomarkers for cellular identification were selected on the basis of expression in other mammalian species.
RESULTS
Intestinal epithelial cell types were identified by means of immunostaining and morphological characterization with transmission electron microscopy. Some differences in biomarker expression and antibody cross-reactivity were identified in equine tissue, compared with other species. However, each known type of mucosal epithelial cell was identified in equine tissue.
CONCLUSIONS AND CLINICAL RELEVANCE
The methodology used can enhance detection of stem cells and progenitor cells as well as postmitotic cell lineages in equine intestinal tissues. Results may have relevance to regenerative potential of intestinal mucosa and survival in horses with colic.
Colic is a major cause of morbidity and death in horses. In 2005, a USDA National Animal Health Monitoring System report1 indicated that colic was second only to old age as the leading cause of death in horses. The intestine is a complex organ composed of multiple layers including the outer serosa, 2 muscular layers (an inner circular layer and outer longitudinal layer separated by fascia that contains the myenteric nerve plexus), the submucosa, and an innermost mucosal layer.2 Death in cases of colic is associated with breakdown of the mucosal barrier, of which the intestinal epithelial cells are an important component. These cells create a single layer that simultaneously forms a barrier, transports nutrients, and undergoes self-renewal.3 The glandular epithelium is arranged in structures called crypts of Lieberkühn. The small intestine is additionally composed of villi which extend into the intestinal lumen. This anatomic arrangement is referred to as the crypt-villus axis.2 At the base of the crypts are undifferentiated stem cells flanked by Paneth cells.4 Immediately adjacent to these cells are progenitor cells, and collectively, this region of the crypts is termed the stem cell niche.4 This population of cells is responsible for creating new epithelium every 3 to 5 days. The remaining epithelium is made up of mature, postmitotic cell types that include absorptive enterocytes, goblet cells, and Paneth cells. Severe mucosal injury likely compromises the proliferative cell population that resides within the glandular crypts. A study5 has shown that intestinal ischemic injury that denudes > 50% of the glandular epithelium, such as occurs with large colon volvulus, is associated with a poor prognosis for survival. However, research to explore this proliferative compartment of the intestinal mucosa in further detail has been lacking because, until recently, the technology to distinctly identify unique cell types did not exist. Protein biomarkers for intestinal epithelial stem cells have been identified and described in rodents since 20076 and in pigs in 1 recent study.7 Additionally, in these species and in humans, protein biomarkers have been similarly used to identify mature cell lineages.7–10 This is commonly based on a cell’s distinct function, although some cells are identified by use of uniquely expressed proteins whose role in cellular activity is incompletely understood. For example, epithelial cell adhesion molecule plays a role in cell-cell adhesion, is uniquely expressed by epithelial cells, and is therefore a useful target for cellular identification.11 Absorptive enterocytes in the small intestine and colon express digestive enzymes within the brush border that include sucrase isomaltase and carbonic anhydrase, respectively,12,13 allowing for targeted identification of these cell types. Finally, Paneth cells are a population of cells that exist only in the small intestine of certain mammalian species.7,14 These cells are commonly identified using lysozyme, an antibacterial enzyme, as the biomarker for identification.15 However, other biomarkers including c-KIT and UEA1 have also been used.14,16 To the authors’ knowledge, no study has fully characterized the equine intestinal epithelium by examination of protein biomarker expression and ultrastructural cellular appearance.
Recent advances in the field of intestinal stem cell biology have enabled detailed study of the stem cell niche as the potential source of novel therapeutic targets to enhance intestinal mucosal regeneration.17,18 The objective of the study reported here was to characterize epithelial cells of the small intestine and colon in horses without clinical gastrointestinal abnormalities, with an emphasis on the stem cell niche constituents. Our aim was to distinguish stem cells, partially differentiated cells, and postmitotic or fully differentiated epithelial cells by means of histologic evaluation, immunofluorescence, and electron microscopy.
Materials and Methods
Animals and sample collection
All animal experiments were approved by the Institutional Animal Care and Use Committee at North Carolina State University. Tissues were obtained from 12 healthy mares of various breeds that were euthanized for reasons unrelated to this study. Nine horses ranged in age from 3 to 13 years (mean ± SD, 7.5 ± 3.6 years), with 3 mature horses of unknown age. Immediately following euthanasia, full-thickness biopsy sections were sharply dissected from the following regions: the descending portion of the duodenum, the approximate middle of the jejunum, the ileum (10 cm orad to the ileocecal junction), the pelvic flexure for the proximal (ascending) colon, and the approximate middle of the distal (descending) small colon.
Histologic and immunofluorescence analyses
Tissues were immediately rinsed with 1X PBS solution and directly placed in either neutral-buffered 10% formalin or 4% paraformaldehyde solution. For immunohistochemical analysis, tissues were fixed in neutral-buffered 10% formalin solution for approximately 24 hours at room temperature (approx 21°C), embedded in paraffin, and sectioned (thickness, approx 5 to 8 μm). Slides were stained with H&E or toluidine blue to aid evaluation of crypt and villus morphology.
For immunofluorescence, rinsed tissues were fixed in paraformaldehyde solution for 14 to 18 hours at 4°C. The tissues were transferred to a 30% sucrose solution for ≥ 24 hours at 4°C, embedded in commercially available tissue freezing medium, frozen in liquid nitrogen, and stored at −80°C. Tissues were sectioned at thickness of 5 to 8 μm with a cryotome set at approximately −20°C and mounted on positively charged glass slides. Sections were washed 3 times with PBS solution to remove tissue freezing medium. Initially, all antibodies were tested with and without HIER to determine whether this step was necessary. Heat-induced epitope retrieval is a commonly used pretreatment procedure that modifies the molecular conformation of tissue proteins because the fixation process can cause protein cross-linking that makes epitopes unavailable for antibody binding.19 When required, HIER was performed by placing the slides into an antigen retrieval solutiona in a pressure cooker for 30 seconds at 120°C and then 90°C for 10 seconds. Following HIER, the slides were allowed to cool at room temperature for 20 minutes prior to continuing. Tissue permeabilization was performed on all slides with PBS solution containing a 0.3% concentration of nonionic surfactant for 20 minutes, washed twice with PBS solution, and incubated in blocking mediumb to prevent nonspecific binding.
Dilutions of functional primary antibodiesc–i used to identify specific cell types in the study with and without antigen retrieval are provided (Appendix). Primary antibodies were applied to the tissue sections in a commercially available antibody diluentb and incubated overnight at 4°C. All secondary antibodies (cyanine 3–labeled goat anti-mouse,j cyanine 3–labeled donkey anti-goat,j and yellow-green fluorescent dye-labeled goat anti-rabbitk) were diluted 1:500 and incubated for 45 minutes at approximately 21°C. Slides were counterstained with bisbenzamide Hoechst 33258 nuclear staine (diluted 1:1,000 in PBS solution).
Images were captured on an inverted fluorescence microscopel fitted with a digital camera.m The objective lenses used were 10X, 20X, and 40X with numerical apertures of 0.3, 0.45, and 0.6, respectively.n Immunohistochemically stained slides were imaged with a light microscopeo fitted with a digital camera.p The objective lenses used were 10X, 20X, 40X, and 60X (oil immersion lens) with numerical apertures of 0.3, 0.7, 0.6, and 1.35, respectively.q
Control slides were prepared with commercially available normal rabbit IgG or normal goat IgG isotype control primary antibodies.d Control slides were imaged to determine the amount of background staining. The exposure time used to accurately image the control slide was then used to image the slides being tested. Cells with fluorescence greater than background were considered positive. Additionally, localization from reports of immunofluorescence imaging of porcine and mouse studies4,6,7,20–23 as well as consistent repeatability of staining and appropriate localization along the crypt villus axis was used to support determination of accurate antibody specificity to cellular biomarkers. Autofluorescence was determined by imaging the tissue by use of cubes with filters of inappropriate excitatory and emission spectra and observing fluorescence.
Transmission electron microscopy
Transmission electron microscopy imaging was performed by the laboratory for Advanced Electron and Light Optic Methods at North Carolina State University. The tissue fixation, preparation, and image acquisition were performed as previously described.4,7,20,24 Briefly, tissue was fixed in a solution of formaldehyde and glutaraldehyde (4:1). The tissue was then dehydrated by immersion into a series of increasingly concentrated ethanol solutions. An epoxy resin was used to embed the tissue into blocks that were subsequently sectioned and stained for imaging.
Results
Identification of cells of epithelial origin
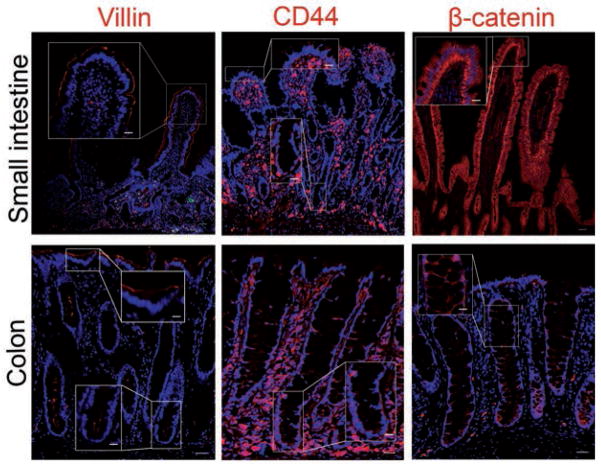
Overall background staining was deemed to be negligible, as determined by nonspecific staining of IgG isotype control slides. Cells of epithelial origin were distinguished from those of mesenchymal and hematopoietic origin in subjectively normal tissues of the equine small intestine and colon with anti-villin, anti-CD44, and anti-β-catenin antibodies. Binding of anti-villin antibody was localized to the apical brush border of intestinal epithelial cells. A gradient of expression was detected from the crypt bases toward the lumen (Figure 1). Epithelial cells were identified in small intestine and colon samples by expression of CD44 within the cell membrane; additional binding of anti-CD44 antibody was observed with intense staining throughout the lamina propria. Finally, the basolateral borders of epithelial cells along the entire crypt villous axis were positive for β-catenin expression.
Figure 1.
Photomicrographs of sections of equine small intestine and colon immunohistochemically stained for biomarkers (red) to detect epithelial cells. Immunostaining for villin, a protein associated with the microvillar actin filaments, is localized to the apical cellular border. A gradient of expression is observed from the crypt base toward the lumen. Immunostaining for CD44 and β-catenin, both membrane-bound proteins, is evident on the basolateral cellular border. Nuclei were counterstained with bisbenzamide (blue). Bar = 200 μm. Inset—Higher-magnification image of boxed area; bar = 50 μm.
Detection of proliferating and apoptotic cells
Detection of cells undergoing discrete stages of the cell cycle and confirmation of nuclear localization of proteins was completed with markers of cellular proliferation (PCNA, MCM2, and phosphohistone H3), apoptosis (cleaved caspase 3), and DNA (bisbenzamide). Compared subjectively with other markers of cellular proliferation, PCNA was the most widely distributed within the crypts (Figure 2). Cells in crypt bases expressed the G1 cell cycle marker MCM2. Phosphohistone H3 expression was localized to the fewest cells in equine intestinal mucosa. Cells in the small intestine and colon were also positive for cleaved caspase 3 expression; epithelial cells that tested positive were few in number and located near the luminal surface.
Figure 2.
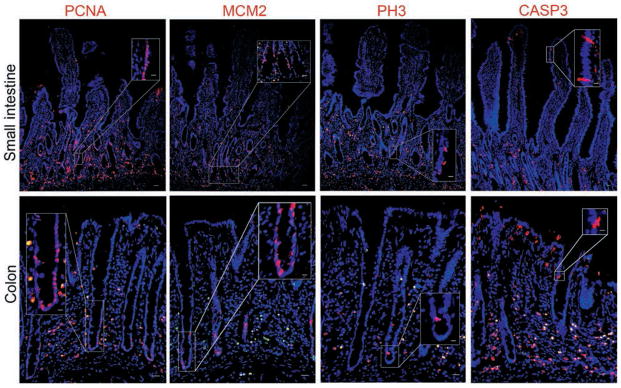
Photomicrographs of sections of equine small intestine and colon immunohistochemically stained for biomarkers (red) to detect proliferating cells in various cell cycle stages and apoptotic cells. Immunostaining for PCNA, a general marker of proliferation, identified the greatest number of cells along the crypt. Expression of MCM2, a marker of cells in the G1 stage, was restricted to the bases of crypts, whereas phosphohistone H3, a marker of cells between the G2 and M stages, identified the fewest number of cells. All markers of proliferation colocalized with bisbenzamide staining of the nuclei (blue). Presence of cleaved caspase 3, indicating apoptosis, was evident in a small number of cells near the luminal surfaces of the small intestine and colon. CASP3 = Cleaved caspase 3. PH3 = Phosphohistone H3. Bar = 200 μm. Inset—Higher-magnification image of boxed area; bar = 50 μm.
Detection of specific epithelial cell types
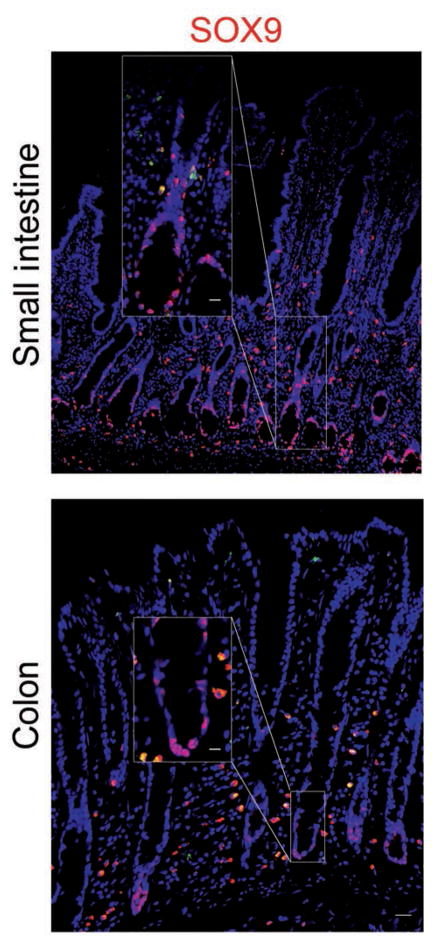
Stem cells and progenitor cells were identified deep within crypt bases with antibodies against SOX9. Nuclear expression of SOX9 was identified by colocalization with bisbenzamide in both small and large intestinal segments (Figure 3).
Figure 3.
Photomicrographs of sections of equine small intestine and colon immunohistochemically stained for SOX9 (red) to detect stem cells or progenitor cells in crypt bases. Immunostaining was localized to nuclei (counterstained with bisbenzamide [blue]). Bar = 200 μm. Inset—Higher-magnification image of boxed area; bar = 50 μm.
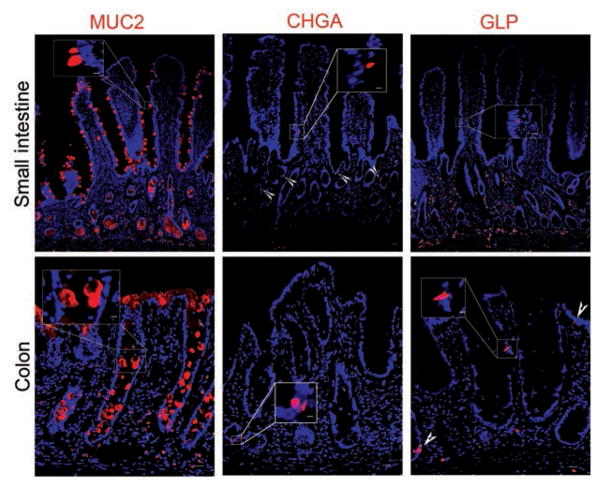
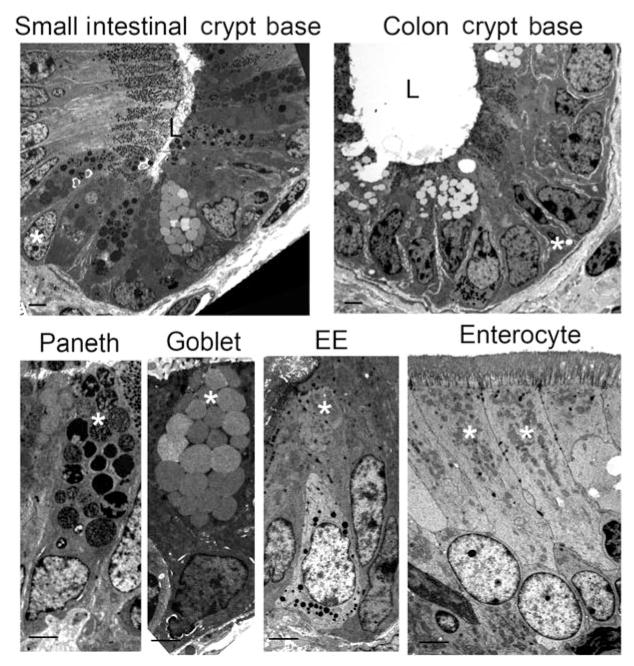
Antibodies against sucrase isomaltase in the small intestine and carbonic anhydrase II in the colon detected the proteins at the apical borders of absorptive enterocytes (Figure 4). Secretory enteroendocrine cells were identified by immunofluorescence labeling of chromogranin A within the cytoplasm of a small number of cells distributed throughout samples collected along the length of the intestinal tract (Figure 5). Further confirmation of this cell type in equine intestinal epithelium was achieved by evaluation of transmission electron microscopic images in which they were identified as cells with small, cytoplasmic, electron-dense granules located basolaterally (Figure 6). Anti-glucagon antibody binding provided evidence of glucagon-like peptide-secreting L cells, a subtype of enteroendocrine cell, in all intestinal segments.
Figure 4.
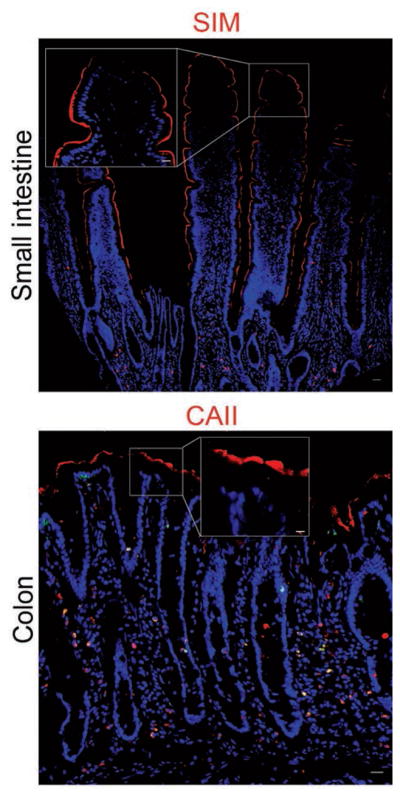
Photomicrographs of sections of equine small intestine and colon immunohistochemically stained for biomarkers (red) to detect absorptive enterocytes. Immunostaining for brush border digestive enzymes identified enterocytes of the small intestine (sucrase isomaltase) and colon (carbonic anhydrase II). Nuclei are visible (blue). CAII = Carbonic acid anhydrase II. SIM = Sucrase isomaltase. Bar = 200 μm. Inset—Higher-magnification image of boxed area; bar = 50 μm.
Figure 5.
Photomicrographs of sections of equine small intestine and colon immunohistochemically stained for biomarkers (red) to detect secretory cells. Mucus-producing goblet cells were positively identified by immunostaining for MUC2. Enteroendocrine cells were identified by immunostaining for chromogranin A and glucagon-like peptide (arrowheads); nuclei were counterstained with bisbenzamide (blue). CHGA = Chromagranin A. GLP = Glucagon-like peptide. Bar = 200 μm. Inset—Higher-magnification image of boxed area; bar = 50 μm.
Figure 6.
Photomicrographs illustrating ultrastructural appearance of equine intestinal epithelial cells in crypt bases of the small intestine and colon. Morphological characteristics of each cell type are clearly distinguishable. Crypt base columnar stem cells, Paneth cells, goblet cells, enteroendocrine, and absorptive enterocytes are marked (asterisks). Note that Paneth cells are not found in the colon. EE = Enteroendocrine. L = Lumen. Transmission electron microscopy; bar = 2 μm (top panels) or 5 μm (bottom panels).
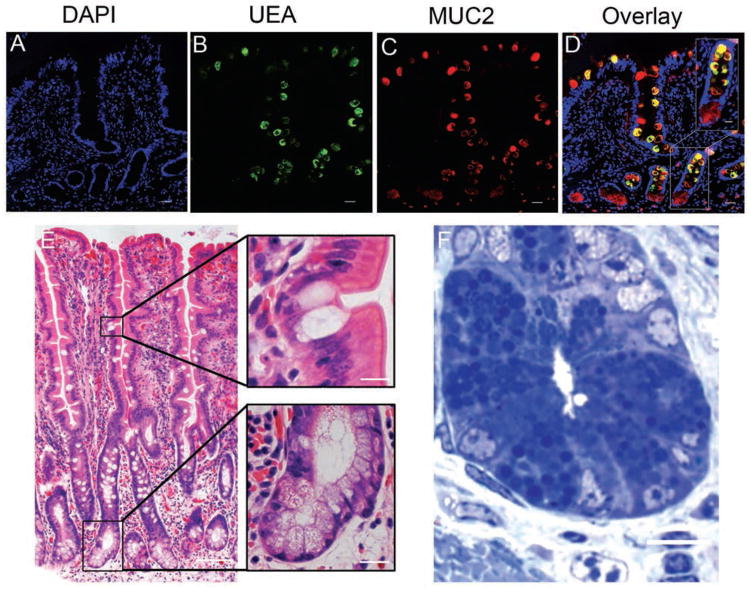
Cytoplasmic expression of MUC2 was detected in goblet cells of the small intestine and colon (Figure 5). However, positive immunostaining for MUC2 was also observed in cells within crypt bases, where goblet cells are uncommonly found. Examination of slides stained with H&E revealed that the degree of eosinophilic staining varied among horses; however, faint eosinophilic granular material could be identified (Figure 7). Paneth cell identification was further supported by identification of large granules within these cells that were strongly positive for toluidine blue staining, and was ultimately confirmed by means of transmission electron microscopy, which revealed cells containing large electron-dense granules adjacent to thin crypt-based stem cells25 (Figure 6). To more clearly distinguish goblet cells and Paneth cells, anti-UEA1 antibody binding was also evaluated. Only goblet cells in the small intestine appeared to bind this antibody, with no appreciable immunostaining within crypt bases where Paneth cells reside. Cells along the rest of the crypt villous axis were positive for UEA1, and samples from the colon tested negative. A colocalization experiment with antibodies against MUC2 and UEA1 revealed UEA1 colocalized with MUC2 in most of the cells along the crypt villous axis, except within the cells closest to the base of the crypt.
Figure 7.
Photomicrographs showing identification of equine Paneth cells with coimmunofluorescence (A to D), H&E (E), and toluidine blue (F) staining. Both goblet and Paneth cells were MUC2-positive (red) in equine small intestine. However, UEA1 immunostaining (green) was restricted to small intestinal goblet cells as demonstrated by colocalization of color (yellow; panel D) proximal to the crypt base. Nuclei in panels A to D are counterstained with bisbenzamide. Bar = 200 μm. Inset—Higher-magnification images of boxed area; bar = 50 μm.
Discussion
In the present study, each intestinal epithelial cell type was identified in subjectively normal intestine samples from horses by means of immunofluorescence, immunohistochemistry, and transmission electron microscopy. Multiple approaches to identify equine epithelial cell types were pursued because of the innate advantages and disadvantages of each technique. Antibody-based assays alone failed to positively identify all intestinal epithelial cell types. This is likely because all the antibodies that were used were commercially derived and raised against proteins in species other than horses. Although many cellular biomarkers are conserved between species, as indicated by cross-reactivity of several antibodies with equine proteins in this study, we tested other antibodies for use in our study that were deemed nonfunctional because positive staining was indistinguishable from background. This may have been attributable to variations in protein conformation between species or possibly differences in protein expression between cells of different species. At this time, development of antibodies raised against proteins specifically found in equine intestinal epithelial cells is arguably cost-prohibitive. However, establishing the existence and normal distribution of cell types within the intestinal mucosa, with the reagents and tools currently available, is important to provide the platform for future studies.
We initially aimed to generally identify cells of epithelial origin. Because an antibody against the commonly used biomarker for these cells, epithelial cell adhesion molecule, did not cross-react with equine tissue, antibodies raised against other biomarkers were tested. The CD44 cell surface receptor mediates cell-cell and cell-matrix interactions.26 In contrast to the finding in other species that CD44 expression is restricted to the stem cell zone,8 CD44 expression in equine small intestine and colon in the present study appeared to be evenly distributed along the entire crypt villous axis. However, the basolateral localization of CD44 in these cells was consistent with what has been described for other species.8 The broad expression of CD44 in nonepithelial cells of the lamina propria and submucosa in our study was also consistent with findings in other species and has been attributed to the antibody binding to lymphocytes.8 It is well-known that species variations in CD44 expression exist. However, the distribution pattern identified in equine intestinal tissue in our study is interesting, given that in other species CD44 expression is localized to a greater degree in crypt bases. This is attributed to its proposed role in stem cell maintenance. Through its interaction with the Wnt signaling pathway, β-catenin also plays an important role in stem cell niche homeostasis. This pathway interaction is thought to be critical for the maintenance of stem cells in an undifferentiated state.27 On Wnt pathway activation, β-catenin translocates from the cell membrane to the nucleus. Despite this, only basolateral membrane localization of this protein was detected in our immunofluorescence images of small intestine and colon samples, even in crypt base cells. Antibodies against CD44 and β-catenin may both prove invaluable in future studies to monitor stem cell regulatory pathways.
The ability to identify cells undergoing apoptosis serves as a useful tool in studies that examine cellular injury and regenerating tissue. This is because the balance between the addition and loss of cells is critical to normal epithelial function. An antibody against cleaved caspase 3 identified a few cells expressing the activated protein at the villus tip within the small intestine and luminal surface in the colon.9,28 Although this protein biomarker has been previously used for assessment of apoptosis in horses with gastrointestinal disease,28 our study confirmed the utility of cleaved caspase 3 antibody to identify apoptotic cells in the mucosa of the small intestine and colon from horses without known intestinal abnormalities.
Enterocytes comprise the predominant subtype of intestinal epithelial cells.4 These cells have the important function of absorbing nutrients, electrolytes, and water.4 We successfully identified enterocytes in the small intestine and colon. Additionally, cells of secretory cell lineage were also clearly identified. The secretory cell lineage is subdivided into enteroendocrine, goblet, and Paneth cells. Enteroendocrine cell hormone secretion serves a multitude of physiologically important functions, despite the fact that this population represents the fewest cells.10 They are identified along the entire length of the small and large intestine and secrete hormones packaged within small cytoplasmic granules located near the basement membrane,10 which is consistent with findings of the present study. Goblet cells, on the other hand, are broadly distributed and constitute the greatest cell number within the colon. The mucins that these cells produce are integral to normal intestinal function by providing protection for the epithelial surface as well as aiding in nutrient absorption.29 The anti-MUC2 antibody marked abundant numbers of mucus-producing goblet cells along the entire intestinal length but also labeled cells residing within crypt bases. We sought to identify the crypt-based mucin-producing cells with an antibody against UEA1. In murine and human tissue, UEA1 is used to identify both goblet and Paneth cells.16 Paneth cells are located immediately adjacent to stem cells within crypt bases and have been described to contain clear, mucinous appearing vacuoles.25,30 Paneth cells are secretory cells only found in the small intestine that have recently gained attention because of to their proposed role in intestinal stem cell maintenance, especially during times of injury.15,31 Interestingly, in horses, UEA1 antibody reactivity appeared to be restricted to goblet cells in the small intestine. To our knowledge, this finding has not been described for any other species. This may indicate a distinct difference in the expression of this epitope by equine intestinal epithelium, compared with that of other species in which other patterns have been identified.7,16 Unfortunately, we were not able to identify an antibody for solely Paneth cell identification because the antibodies against lysozyme and c-KIT that were tested did not cross-react with equine tissue. However, the presence of Paneth cells was confirmed in multiple ways, including colocalization with UEA1 and MUC2 immunostaining, evaluation of H&E-stained slides, and transmission electron microscopy.
Ultimately, we believe that the knowledge gained from this study is critical to future research and the future of equine medicine in the treatment of colic. The ability to monitor the preservation or loss of stem cells and progenitor cells and to assess changes in stem cell regulatory pathways and postmitotic cell lineages will likely become useful tools in appraisal of mucosal regenerative potential and prognostication for horses with colic.
Acknowledgments
Supported by an NIH training grant (No. T32OD011130 [LMG]) and the North Carolina Horse Council.
The authors thank Monica Mattmuller and Dr. Jeanette Shipley-Phillips for technical support.
ABBREVIATIONS
- HIER
Heat-induced epitope retrieval
- MCM2
Minichromosome maintenance complex 2
- MUC2
Mucin 2
- PCNA
Proliferating cell nuclear antigen
- SOX9
Sex determining region Y-box 9
- UEA1
Ulex europaeus agglutinin 1
Appendix. Functional antibodies used in a study to identify epithelial cells of discrete lineages in samples of apparently healthy small intestine and colon collected from 12 adult horses
| Cell type | Antibody | Dilution | HIER |
|---|---|---|---|
| Epithelial | Goat anti-human villind | 1:500 | Yes |
| Rat anti-human CD44f | 1:400 | Yes | |
| Mouse anti-human β-catening | 1:200 | Yes | |
| Any (markers for proliferation, cell cycle, or apoptosis) | Mouse anti-rat PCNAc | 1:100 | Yes |
| Mouse anti-rat PCNAh | 1:500 | Yes | |
| Goat anti-human MCM2d | 1:200 | Yes | |
| Rabbit anti-human phosphohistone H3c | 1:500 | Yes | |
| Rabbit anti-human cleaved caspase 3g | 1:400 | Yes | |
| Intestinal epithelial stem cell | Rabbit anti-human SOX9c | 1:1,000 | Yes |
| Absorptive enterocyte | Goat anti-rat sucrase isomaltased | 1:500 | Yes |
| Goat anti-mouse carbonic anhydrase IId | 1:250 | Yes | |
| Enteroendocrine | Rabbit anti-porcine chromogranin Ai | 1:1,000 | Yes |
| Rabbit anti-human glucagond | 1:250 | Yes | |
| Goblet | Rabbit anti-human MUC2d | 1:1,000 | No |
| Anti-horse lectin (UEA1)-atto 488 conjugatee | 1:500 | No |
Horses were euthanized for reasons unrelated to gastrointestinal disease or systemic disease. Full-thickness biopsy sections were collected from the following regions immediately after euthanasia: the descending portion of the duodenum, the approximate middle of the jejunum, the ileum (10 cm orad to the ileocecal junction), the pelvic flexure for the proximal (ascending) colon, and the approximate middle of the distal (descending) small colon.
Footnotes
Reveal Decloaker, Biocare Medical, Concord, Calif.
Protein Block Serum-Free, Dako, Carpinteria, Calif.
Millipore, Temecula, Calif.
Santa Cruz Biotechnology, Santa Cruz, Calif.
Sigma-Aldrich, St Louis, Mo.
Biolegend, San Diego, Calif.
Cell Signaling Technology Inc, Danvers, Mass.
Abcam, Cambridge, Mass.
Immunostar, Hudson, Wis.
Jackson ImmunoResearch Laboratories, West Grove, Pa.
Invitrogen, Life Technologies, Grand Island, NY.
Olympus IX81 Microscope, Olympus, Tokyo, Japan.
Hamamatsu Digital Camera, ORCA-flash 4.0 or -03G, Hamamatsu Digital Camera, Hamamatsu, Japan.
Olympus LUC Plan FLN, Olympus, Tokyo, Japan.
Olympus Bx45 Microscope, Olympus, Tokyo, Japan.
Olympus DP72 Digital Camera, Olympus, Tokyo, Japan.
Olympus UPlan FLN, Olympus, Tokyo, Japan.
This manuscript represents a portion of a thesis submitted by Dr. Gonzalez to the North Carolina State University Department of Clinical sciences, as partial fulfillment of the requirements for a Doctor of Philosophy degree.
Presented as a podium presentation at the American College of Veterinary Surgeons Surgery Summit, San Antonio, Texas, October 2013; and the Colic Research Symposium, Dublin, July 2014.
References
- 1.USDA APHIS Veterinary Services Centers for Epidemiology and Animal Health. Part I: baseline reference of equine health and management. Fort Collins, Colo: USDA; 2005. [Google Scholar]
- 2.Dellmann H. Textbook of veterinary histology. 3. Philadelphia: Lea & Febiger; 1987. [Google Scholar]
- 3.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461–479. doi: 10.1002/aja.1001410403. [DOI] [PubMed] [Google Scholar]
- 5.Van Hoogmoed L, Snyder JR, Pascoe JR, et al. Use of pelvic flexure biopsies to predict survival after large colon torsion in horses. Vet Surg. 2000;29:572–577. doi: 10.1053/jvet.2000.17836. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez LM, Williamson I, Piedrahita JA, et al. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS ONE. 2013;8:e66465. doi: 10.1371/journal.pone.0066465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gracz AD, Fuller MK, Wang F, et al. CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells. 2013;31:2024–2030. doi: 10.1002/stem.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster DM, Stauffer SH, Stone MR, et al. Proteasome inhibition of pathologic shedding of enterocytes to defend barrier function requires X-linked inhibitor of apoptosis protein and nuclear factor kappa B. Gastroenterology. 2012;143:133–144. doi: 10.1053/j.gastro.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Ahlman H, Nilsson O. The gut as the largest endocrine organ. Ann Oncol. 2001;12:S63–S68. doi: 10.1093/annonc/12.suppl_2.s63. [DOI] [PubMed] [Google Scholar]
- 11.Trzpis M, McLaughlin PM, de Leij LM, et al. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez IR, Taravel FR, Whelan WJ. Characterization and function of pig intestinal sucrase-isomaltase and its separate subunits. Eur J Biochem. 1984;143:575–582. doi: 10.1111/j.1432-1033.1984.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 13.Amasaki T, Amasaki H, Nagasao J, et al. Immunohistochemical localization of carbonic anhydrase isoenzymes in salivary gland and intestine in adult and suckling pigs. J Vet Med Sci. 2001;63:967–970. doi: 10.1292/jvms.63.967. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg ME, Nusse Y, Kalisky T, et al. Identification of a ckit+ colonic crypt base secretory cell that supports Lgr5+ stem cells in mice. Gastroenterology. 2012;142:1195–1205. doi: 10.1053/j.gastro.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong VW, Stange DE, Page ME, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin SA, Barker N. Gastrointestinal stem cells in self-renewal and cancer. J Gastroenterol. 2011;46:1039–1055. doi: 10.1007/s00535-011-0424-8. [DOI] [PubMed] [Google Scholar]
- 18.Markel TA, Crisostomo PR, Lahm T, et al. Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg. 2008;43:1953–1963. doi: 10.1016/j.jpedsurg.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krenacs L, Krenacs T, Stekovics E, et al. Heat-induced antigen retrieval for immunohistochemical reactions in routinely processed paraffin sections. Methods Mol Biol. 2010;588:103–119. doi: 10.1007/978-1-59745-324-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 21.West AB, Isaac CA, Carboni JM, et al. Localization of villin, a cytoskeletal protein specific to microvilli, in human ileum and colon and in colonic neoplasms. Gastroenterology. 1988;94:343–352. doi: 10.1016/0016-5085(88)90421-0. [DOI] [PubMed] [Google Scholar]
- 22.Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–G1118. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 24.Dykstra MJ. Biological electron microscopy: theory, techniques, and troubleshooting. 2. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 25.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat. 1974;141:521–535. doi: 10.1002/aja.1001410406. [DOI] [PubMed] [Google Scholar]
- 26.Goodison S, Urquidi V, Train D. CD44 cell adhesion molecule. Mol Pathol. 1999;52:18–96. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto D, Gregorieff A, Begthel H, et al. Canonical wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe EL, White NA, Buechner-Maxwell V, et al. Detection of apoptotic cells in intestines from horses with and without gastrointestinal tract disease. Am J Vet Res. 2003;64:982–988. doi: 10.2460/ajvr.2003.64.982. [DOI] [PubMed] [Google Scholar]
- 29.Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh Y, Yamano M, Matsuda M, et al. Ultrastructure of paneth cells in the intestine of various mammals. J Electron Microsc Tech. 1990;16:69–80. doi: 10.1002/jemt.1060160109. [DOI] [PubMed] [Google Scholar]
- 31.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]