Abstract
The mammalian immune system responds to eukaryotic glycan antigens during infections, cancer, and autoimmune disorders, but the immunological bases for such responses are unclear. Conjugate vaccines containing bacterial polysaccharides linked to carrier proteins (neoglycoconjugates) have proven successful, but these often contain repeating epitopes and the reducing end of the glycan is less important, unlike typical glycan determinants in eukaryotes, which are shorter in length and may include the reducing end. Here we have compared the effects of two linkage methods, one that opens the ring at the reducing end of the glycan, and one that leaves the reducing end closed, on the glycan specificity of the vaccine response in rabbits and mice. We immunized rabbits and mice with bovine serum albumin (BSA) conjugates of synthetic open- and closed-ring forms (OR versus CR) of a simple tetrasaccharide lacto-N-neo-tetraose (LNnT, Galβ1-4GlcNAcβ1-3Galβ1-4Glc), and tested reactivity to the immunogens and several related glycans in both OR and CR versions on glycan microarrays. We found that in rabbits the immune response to the CR conjugate was directed toward the glycan, whereas the OR conjugate elicited antibodies to the reducing end of the glycan and linker region but not specifically to the glycan itself. Unexpectedly, mice did not generate a glycan-specific response to the CR conjugate. Our findings indicate that the reducing end of the sugar is crucial for generation of a glycan-specific response to some eukaryotic vaccine epitopes, and that there are species-specific differences in the ability to make a glycan-specific response to some glycoconjugates. These findings warrant further investigation with regard to rational design of glycoconjugate vaccines.
Keywords: Glycoconjugate vaccine, lacto-N-neo-tetraose, anti-glycan antibodies, derivatized glycans, glycan microarrays
Introduction
Glycoconjugate vaccines are one of the most important innovations in preventative medicine. Vaccines against Haemophilus influenza, pneumococcus, and meningococcus dramatically decrease suffering caused by these organisms (reviewed in Trotter et al, 2008) 1. Licensed glycoconjugate vaccines are limited to those preventing bacterial infections. Pathogens such as fungi and parasites, and tumors, also induce immune responses to non-host-like or altered glycans, but no vaccines targeting eukaryotic glycans have yet been licensed. This gap in the field is likely due to multiple factors, including fundamental structural differences between relatively large microbial polysaccharides with repeating motifs versus smaller eukaryotic glycans without repeating motifs, and lack of knowledge about how to design immunogens that induce the desired glycan-specific response.
Conjugation of polysaccharide (PS) to a protein carrier greatly enhances the magnitude and longevity of the anti-glycan response, through interactions between cognate protein-specific T-cells and glycan-specific B-cells 2–4. Bacterial PS is often isolated from bacterial culture, hydrolyzed into oligosaccharides of heterogeneous length, and then chemically activated or derivatized with a linker molecule before conjugation to a protein carrier 5,6. Some common methods of PS activation and conjugation, such as periodate oxidation followed by reductive amination, have the disadvantage of destroying carbohydrate epitopes and/or creating neo-epitopes when the sugar rings are broken and then linked to other species 6,7. Linkage method can affect the immune response even with similar levels of glycan:protein loading 8. However, the repeating motifs within bacterial PS provide many modes of antigen presentation that may confer inherent immune-stimulatory properties. Thus, in spite of the crude methods used to conjugate bacterial PS to carrier proteins and the heterogeneity of the final product, conjugate vaccines have been successful at generating protective titers of antibody and long-term memory.
The antigenic glycans of eukaryotes, such as those targeted during parasite infection and tumor antigens, present unique challenges to glycoconjugate vaccine development. These epitopes are often non-repeating, may occur at the reducing (core) or non-reducing end (distal) of the glycan, and some parasite glycans have features in common with mammalian glycans (reviewed in Prasanphanich et al. 2013, van Die & Cummings 2009, van Diepen et al. 2012) 9–11. Interestingly, the linker used to generate the neoglycoconjugate can suppress antigenicity of the glycan epitope by creating an immunodominant neo-epitope 7,12–14. Recently, it was shown that glycopeptides from processed conjugate vaccines bind MHC, and glycopeptide-specific T-cells could be a major contributor to the vaccine response 15. Glycan-protein linkages could thus be B-cell as well as T-cell epitopes, which further underscores their importance in the immune response.
Several studies in the last decade have explored the structural principles of glycan-protein linkage that can optimize immune responses to both bacterial and eukaryotic glycans. For example, a protective vaccine against C. albicans was generated by linking β-mannan disaccharides to a protein via click chemistry, and it was found that stereo-diversification of the linker region with a mixture of anomers at the chiral carbons enhanced immunity to the proximal disaccharide portion 16,17. A synthetic vaccine containing the Tn-antigen glycopeptide along with a T-cell epitope covalently linked to a Toll-like Receptor ligand, where no artificial linkages other than peptide bonds are created, exhibited very promising results in mice 18. Many vaccine development efforts stand to benefit from these novel approaches to generating glycoconjugates to target immunity to eukaryotic glycan antigens.
In this regard, we have explored many types of conjugation chemistry in order to immobilize sugar epitopes on microarrays and/or attach them to protein carriers. One such method uses reductive amination to tag the glycan with either of the two fluorescent heterobifunctional linkers, 2-amino-N-(2-aminoethyl)-benzamide (AEAB) or p-nitrophenyl anthranilate (PNPA) 19,20. This process is facile, high-yielding, and results in homogeneous orientation of the glycan-protein epitopes. However, this method, like many others, requires reduction of the glycan, which can create a neo-epitope 12,13. We compared the binding properties of glycans, which were coupled to AEAB either through reductive amination (“open-ring”, OR) or acryloylation (“closed-ring”, CR) 20, and examined glycan recognition using glycan microarrays. For most glycan binding proteins (those targeting an epitope at the non-reducing end) binding was unaffected by the conjugation method, but antibody recognition of some epitopes was destroyed by reductive amination. For example, sialyl-Lewis X and type-2 H-antigens, were recognized by lectins, but not by monoclonal antibodies when the glycans were in the OR-derivatized form 20. Similarly, studies on the specificity of the rabbit response to human milk glycan-protein conjugates made using a different OR-linkage chemistry have shown that antisera heavily target the reducing-end/linker region, and may also possess specificity for the non-reducing end of the sugar, depending on which sugar is used 12,13,21. These studies suggest that chemical methods requiring ring opening of the reducing-end sugar of glycoconjugates can produce major alterations in glycan antigenicity, and may be unacceptable for making conjugate vaccines with relatively small eukaryotic glycan epitopes. However, to our knowledge, there are no studies that directly compare the effects of OR versus CR neoglycoconjugates on the immune response.
We tested the effect of OR- versus CR-linked LNnT (lacto-N-neo-tetraose, Galβ1-4GlcNAcβ1-3Galβ1-4Glc) BSA conjugates on the glycan-specificity of the immune response in immunized rabbits and mice. LNnT was chosen because it is a simple tetrasaccharide, and in the course of our studies we found that neither rabbit nor mouse sera have detectable natural antibodies to this glycan. We found that CR-, but not OR-linkage, enabled rabbits to make a glycan-specific response to LNnT. Mice, by contrast, made a barely-detectable glycan-specific response to LNnT-CR-BSA. These findings have important implications for the rational design of glycoconjugate vaccines using eukaryotic glycan antigens in the future.
Results
Synthesis and characterization of LNnT-BSA glycoconjugate vaccines
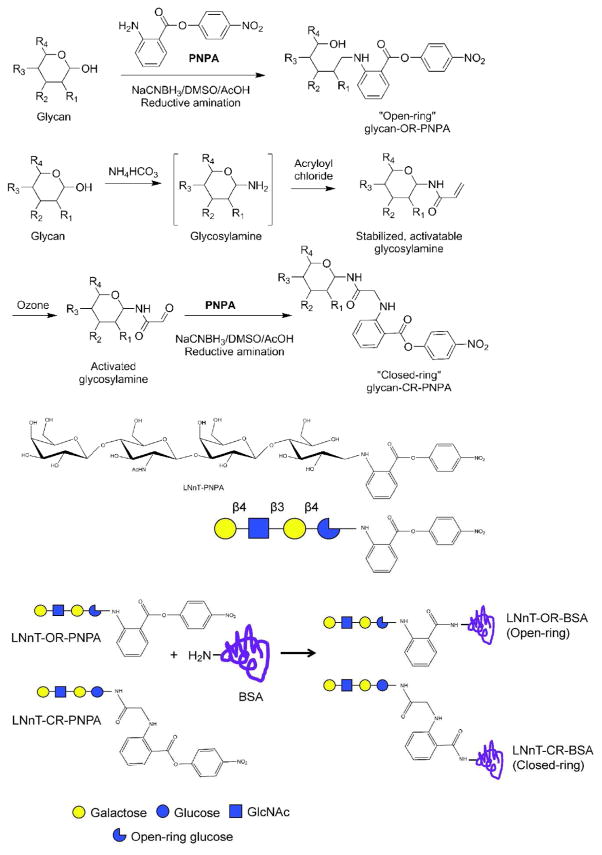
To generate closed-ring and open-ring sugar-protein conjugates, a milk glycan, lacto-N-neotetraose (LNnT, Galβ1-4GlcNAcβ1-3Galβ1-4Glc) was derivatized with p-nitrophenyl anthranilate (PNPA) via two different methods. Reductive amination alone results in the open-ring derivative, LNnT-OR-PNPA. An open-ring derivatized lactose (Lac, Galβ1-4Glc) was prepared as an additional control. For the preparation of LNnT-CR-PNPA by closed-ring derivatization, LNnT is treated with ammonium bicarbonate to form a glycosylamide, acryloylated with acryloyl chloride, activated with ozone and conjugated with PNPA by reductive amination, sequentially (Figure 1) 20. The derivatized LNnT and Lac were then conjugated with bovine serum albumin (BSA), resulting in Lac-OR-BSA, LNnT-BSA and LNnT-CR-BSA (Figure 1). MALDI-TOF studies showed that the conjugates had average sugar:protein ratios of 13:1 (Lac-OR-PNPA), 7:1 (LNnT-OR-PNPA), and 5:1 (LNnT-CR-PNPA).
Figure 1. Synthesis of open-ring and closed-ring LNnT-BSA conjugates.
Reaction scheme for synthesis of PNPA-derivatized sugars from free-reducing glycans via the open-ring and closed-ring methods (top), diagram of the open-ring LNnT-PNPA derivative used in this study, with monosaccharide code schematic drawn below (middle), and reaction scheme for synthesis of BSA glycoconjugates with the PNPA-derivatized, highlighting the similarities and differences between the open-ring and closed-ring conjugate structures in the linker region (bottom).
Closed-ring linkage induces greater glycan specificity than open-ring linkage in rabbits
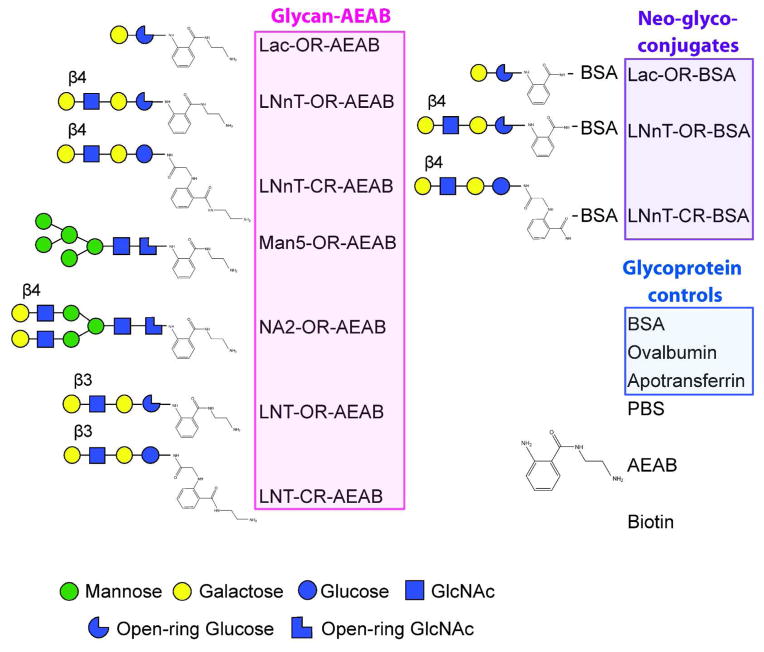
Each pair of rabbits was immunized subcutaneously with one of the three glycoconjugates, Lac-OR-BSA, LNnT-OR-BSA or LNnT-CR-BSA, in CFA once and IFA three more times. To assess the magnitude and specificity of the immune response, we analyzed the antisera using ELISA plates coated with the immunogens, as well as a custom glycan/glycoprotein microarrays (Figure 2). The glycan microarray contained 16 different derivatized compounds, each printed in hexareplicate spots on NHS slides. Open-ring Lac and LNnT, and closed-ring LNnT, as well as several other sugars, including LNT (lacto-neo-tetraose), which is identical to LNnT except for the β3 linkage at the non-reducing end, were printed to demonstrate glycan specificity. Note that AEAB, a linker related to PNPA but with an additional active amino group, was used for efficient printing on NHS-activated glass slides. The ring portion of the molecule attached to the sugar is shared between the two linkers, and when used for protein conjugation, the resulting conjugate is identical. The glycoconjugate immunogens and control glycoproteins were printed on the array as well as the AEAB linker alone.
Figure 2. BSA-PNPA Array.
List of glycan and glycoprotein structures printed on the BSA-PNPA array, used to screen immune rabbit and mouse sera in Figures 3 and 6.
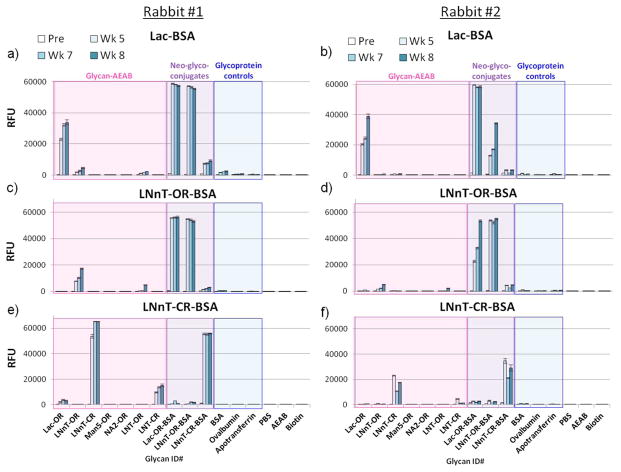
Both immunized rabbits produced a robust IgG response by week 5 and in most cases increasing through week 8 (Figure 3). The rabbits immunized with Lac-OR-BSA (Figure 3a–b) primarily targeted a combination of the open-ring derivatized lactose and linker-protein, as evidenced by the binding to Lac-OR-AEAB and Lac-OR-BSA and cross-reactivity to LNnT-OR-BSA, which contains the same open-ring Lac-linker-protein structure internally. We observed low reactivity with the closed-ring neoglycoconjugate or BSA alone. Rabbits immunized with the LNnT-OR-BSA neoglycoconjugate also most highly recognized Lac-OR-BSA and LNnT-OR-BSA, with some reactivity to the LNnT-OR-AEAB and a low response to LNnT-CR-BSA (Figure 3c–d). These data suggest that some combination of the non-reducing end sugar-linker-protein appears to be the primary epitope. The two rabbits also displayed varying levels of antibody to the open-ring LNT sugar indicating that a portion of the anti-glycan response was not specific for the reducing-end.
Figure 3. Open- or closed-ring linkage impacts specificity of the rabbit response to LNnT-BSA conjugates.
Serum from two rabbits was diluted at 1:100 and screened on the BSA-PNPA glycan microarray. Serum was detected with anti-rabbit IgG-Alexa 488. Bars correspond to mean of hexareplicate spots +/− standard deviation. RFU, relative fluorescence units; Pre, pre-bleed; Wk 5 – Wk 8, Week 5 – week 8 bleeds. Pink, AEAB-derivatized glycans; purple, BSA neoglycoconjugates; blue, glycoprotein controls. X-axis labels correspond to the structures depicted in Figure 2. Note that signals reach the detection maximum near 60,000 RFU. Rabbit #1: a) Lac-BSA; b) LNnT-OR-BSA; c) LNnT-CR-BSA; Rabbit #2: d) Lac-BSA; e) LNnT-OR-BSA; f) LNnT-CR-BSA.
By contrast, rabbits immunized with the closed-ring conjugate (Figure 3e–f) specifically recognized LNnT-CR-AEAB as highly or higher than the LNnT-CR-BSA immunogen. They had no reactivity with BSA alone and very little cross-reactivity with the glycan-OR-AEAB structures or the glycan-OR-BSA neoglycoconjugates. The LNnT-CR-BSA response was therefore more specific to the immunogen, and more focused on the tetrasaccharide. There was also some degree of binding with LNT-CR-AEAB in each rabbit, suggesting that a small portion of the response to the closed-ring neoglycoconjugate was to an epitope internal to the sugar glycosylamide, or was impartial to the Gal-GlcNAc linkage at the non-reducing end of the sugar. However, LNnT-CR-AEAB was clearly the preferred epitope.
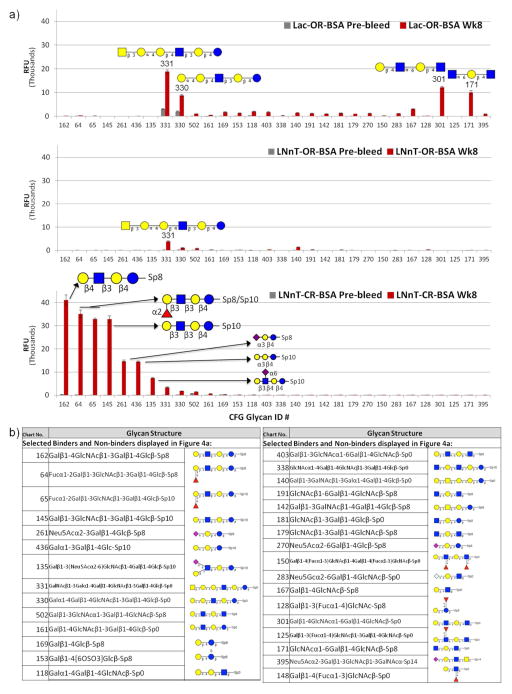
One rabbit from each group was also analyzed on the glycan microarray (version 4.2) from the Consortium for Functional Glycomics (CFG), which contains hundreds of synthetic glycans that are printed using a different closed-ring linkage that is unrelated to PNPA (Figure 4 and Table 1). Because the CFG linkers are distinct from those used in the immunization, reactivity with glycans on the CFG array would therefore be a better indication of glycan specificity. For clarity, a selection of relevant structures, i.e. those with LNnT- and Lac-related structures, are shown in Figure 4a. Pictorial representations of the structures included are shown in Figure 4b. The top 10 binders of each immunized rabbit screened are listed in Table 1, and the full dataset can be found online at http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp.
Figure 4. Immunized rabbits make a glycan-specific response to closed-ring LNnT-BSA.
a) Week 8 serum from one rabbit in each immunization group was diluted at 1:100 and screened on the CFG microarray. Selected binders and non-binders are shown here, sorted from high binding to low binding in the LNnT-CR-BSA serum, from left to right on the x-axis. Structures of the selected glycans are listed in b). The glycan ID#s on the X-axis correspond to CFG Chart ID#s. Serum was detected with anti-rabbit IgG-Alexa 488. RFU, relative fluorescence units. The top binding glycan IDs are listed in Table 1; a full listing of glycans on version 4.2 of the CFG can be found at http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml
Table 1. Top 10 CFG glycans bound by one rabbit serum from each immunization group.
The top binding glycans detected by CFG array screening of pooled rabbit serum are listed for each immunization, LNnT-CR-BSA (bottom), LNnT-OR-BSA (middle), Lac-OR-BSA (top); a full listing of glycans and linker abbreviations on version 4.2 of the CFG can be found at http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml
| Lac-OR-BSA | Week 8 | Pre-bleed | |||
|---|---|---|---|---|---|
|
| |||||
| Chart No. | Glycan Structure | Avg RFU | StDev | Avg RFU | StDev |
|
| |||||
| 400 | Galα1-4Galβ1-4GlcNAcβ1-2Manα1-3(Galα1-4Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAcβ-LVANKT | 32690 | 1550 | 15277 | 1667 |
| 331 | GalNAcβ1-3Galα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp0 | 18800 | 668 | 3069 | 183 |
| 123 | Galβ1-2Galβ-Sp8 | 14429 | 512 | 2 | 6 |
| 304 | GlcAβ1-3GlcNAcβ-Sp8 | 13407 | 1018 | 567 | 133 |
| 301 | Galβ1-4GlcNAcα1-6Galβ1-4GlcNAcβ-Sp0 | 12120 | 501 | 3 | 5 |
| 437 | Galβ1-4Galβ-Sp10 | 10452 | 454 | 4 | 4 |
| 171 | GlcNAcα1-6Galβ1-4GlcNAcβ-Sp8 | 9989 | 789 | 5 | 3 |
| 330 | Galα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp0 | 8809 | 424 | 1996 | 211 |
| 15 | GalNAcβ-Sp8 | 8416 | 1881 | 8 | 5 |
| 155 | Galβ1-4GalNAcβ1-3(Fucα1-2)Galβ1-4GlcNAcβ-Sp8 | 7659 | 528 | 20 | 11 |
|
| |||||
| LNnT-OR-BSA | |||||
|
| |||||
| 400 | Galα1-4Galβ1-4GlcNAcβ1-2Manα1-3(Galα1-4Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAcβ-LVANKT | 28494 | 344 | 365 | 48 |
| 304 | GlcAβ1-3GlcNAcβ-Sp8 | 3861 | 166 | 4 | 6 |
| 331 | GalNAcβ1-3Galα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp0 | 3756 | 298 | 8 | 5 |
| 211 | Manα1-3(Manα1-2Manα1-2Manα1-6)Manα-Sp9 | 2652 | 181 | 12 | 8 |
| 182 | GlcNAcβ1-4-MDPLys | 2512 | 158 | 23 | 9 |
| 140 | Galβ1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ-Sp0 | 1354 | 66 | 4 | 5 |
| 210 | Manα1-3(Manα1-6)Manα-Sp9 | 1005 | 86 | 9 | 9 |
| 330 | Galα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp0 | 1000 | 179 | 8 | 9 |
| 187 | GlcNAcβ1-4GlcNAcβ1-4GlcNAcβ-Sp8 | 839 | 92 | 298 | 62 |
| 502 | Galβ1-3GlcNAcα1-3Galβ1-4GlcNAcβ-Sp8 | 829 | 28 | 9 | 5 |
|
| |||||
| LNnT-CR-BSA | |||||
|
| |||||
| 162 | Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp8 | 40992 | 2178 | 233 | 25 |
| 64 | Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ-Sp8 | 35078 | 1646 | 60 | 34 |
| 65 | Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ-Sp10 | 32834 | 344 | 54 | 25 |
| 145 | Galβ1-3GlcNAcβ1-3Galβ1-4Glcβ-Sp10 | 32823 | 1436 | 59 | 6 |
| 400 | Galα1-4Galβ1-4GlcNAcβ1-2Manα1-3(Galα1-4Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAcβ-LVANKT | 20712 | 1447 | 1449 | 132 |
| 261 | Neu5Acα2-3Galβ1-4Glcβ-Sp8 | 14642 | 582 | 6 | 8 |
| 436 | Galα1-3Galβ1-4Glc-Sp10 | 14405 | 314 | 0 | 4 |
| 135 | Galβ1-3(Neu5Acα2-6)GlcNAcβ1-4Galβ1-4Glcβ-Sp10 | 7411 | 219 | 2 | 5 |
| 331 | GalNAcβ1-3Galα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp0 | 3396 | 175 | 124 | 42 |
| 8 | Rhaα-Sp8 | 2875 | 130 | 1305 | 190 |
The Lac-OR-BSA immunized rabbit displayed many antibody specificities in the week 8 serum, however only a few of the high binders contained Galβ1-4Glc motifs (#41, 42, 86, 330, 331, 243, 404), and most of the structures bound in the week 8 serum were also bound at lower levels in the pre-bleed serum (#400, 330, 331, 304) (Figure 4a, top panel and Table 1). The LNnT-OR-BSA immunized rabbit displayed a response that was similar in specificity to Lac-OR-BSA but lower in titer (Figure 4a, middle panel and Table 1). The pre-bleed serum was much lower in this animal, but again most of the binders in the week 8 serum were unrelated to LNnT. Two of the structures on the array containing an internal LNnT determinant as part of a larger structure, #330 (Galα1-4Galβ1-4 GlcNAcβ1-3Galβ1-4Glcβ-) and #331 (GalNAcβ1-3Galα1-4Galβ1-4 GlcNAcβ1-3Galβ1-4Glcβ-) were found at low levels in one or two of the pre-bleed sera and were boosted in all three immunization groups, thus these antibodies were probably not a result of the specific immunogens. Antibodies to a few unrelated structures (#400, #304, 502) were also boosted in all three groups; these could be natural antibodies that are boosted non-specifically by the immunization, and some of these specificities (#304; 330 – blood group P1 antigen) and antibodies to related antigens (#400, 331) have been previously reported in serum from healthy human donors 22.
The LNnT-CR-BSA immunized rabbit was the only sample tested which targeted LNnT (#162) and, with slightly lower magnitude, the isomer LNT (#145) and a fucosylated LNT (#64-65) (Figure 4a, bottom panel). These specificities were undetectable in pre-bleed serum. This antiserum also targeted three structures (#261, 436, 135) containing internal Galβ1-4Glcβ-moieties just distal to the linker, which were not targeted by the other immunized rabbits. However, the CFG contains many other structures having features in common with LNnT, including GlcNAcα1-4Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ- (#338), GlcNAcβ1-3Galβ1-4Glcβ- (#181) and many Galβ1-4GlcNAc-terminating (ex. #167) and/or Galβ1-4Glc-linked structures, which were negative in the LNnT-CR-BSA antiserum. Interestingly, a version of LNnT attached to a similar but shorter linker (#161) was bound about seventy-fold lower than #162. This could be a result of steric hindrance when the glycan is anchored closer to the slide.
Because glycans on the CFG array are printed in a closed-ring configuration using a linker unrelated to PNPA, these data demonstrate that a portion of the response to LNnT-CR-BSA is specific for LNnT and does not require the linker for binding. The antibodies also require the full tetrasaccharide for binding, but interestingly are permissive to some variations in the non-reducing end (Galβ1-4GlcNAc or Galβ1-3GlcNAc; α1-2 fucosylation) but not others (GlcNAcα1-4).
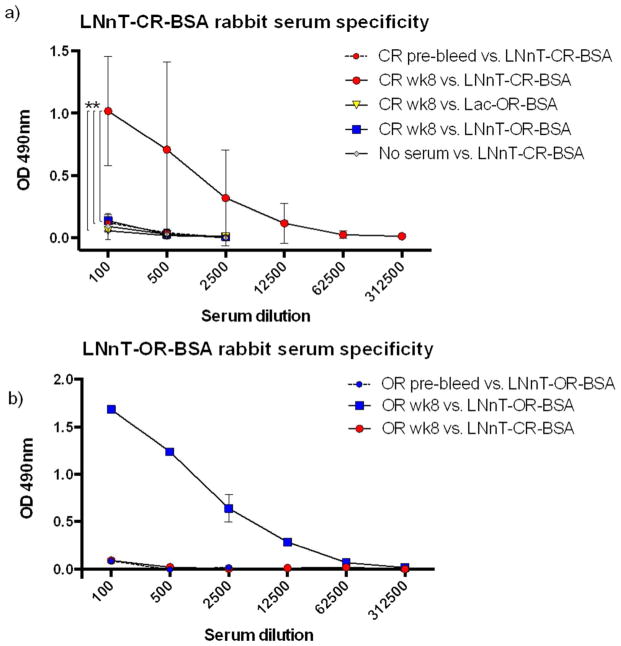
ELISA studies of the LNnT-CR-BSA-immunized (Figure 5a) and LNnT-OR-BSA-immunized (Figure 5b) rabbit serum against the various conjugates, where BSA reactivity has been blocked out, supported similar conclusions as the microarray studies. ANOVA with multiple comparisons showed that the week 8 LNnT-CR-BSA immunized rabbit serum was significantly elevated compared to pre-bleed, and was only significantly reactive with LNnT-CR-BSA among the three neoglycoconjugates coated on ELISA plates. Pooled serum from the LNnT-OR-BSA rabbits (Figure 6b) was likewise non-cross-reactive with the LNnT-CR-BSA conjugate.
Figure 5. Open- and closed-ring LNnT-BSA rabbit antisera are non-cross reactive with each other.
Open- and closed-ring LNnT-BSA rabbit antisera are non-cross reactive with each other. ELISA plates were coated with conjugates shown in the legend and interrogated with LNnT-CR-BSA antisera from each rabbit at decreasing concentrations (a). Plates were coated with the conjugates show in the legend and interrogated with LNnT-OR-BSA antisera pooled from two rabbits (b). 2-way ANOVA was performed for the 1:100-1:2500 dilutions, with Dunnett’s multiple comparison between the CR wk8 vs. CR pre-bleed, CR wk8 vs. LNnT-OR-BSA and CR wk8 vs. Lac-OR-BSA. ** = P< 0.005, which was significant for the serum vs. coating factor and for the three individual group comparisons shown with brackets. Part a) values are averaged from two rabbits which were each performed in duplicate and it was representative of two experiments. Part b) values are average of duplicates for sera pooled from 2 rabbits. One experiment was performed.
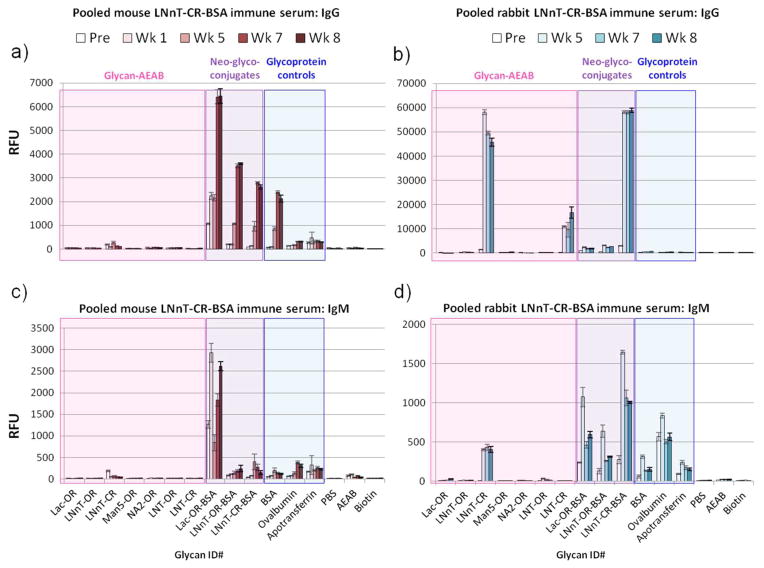
Figure 6. Mice lack glycan-specificity in the response to LNnT-CR-BSA, in contrast to rabbits.
Pooled serum from three mice (a,c) and two rabbits (b,d) was diluted at 1:100 and screened on the BSA-PNPA glycan microarray for IgG (a,b) and IgM (c,d). Serum was detected with goat anti-mouse IgG-Alexa 568, anti-mouse IgM-Alexa 488, and anti-rabbit-Alexa488 at 5μg/mL. Bars correspond to mean of hexareplicate spots +/− standard deviation. RFU, relative fluorescence units; Pre, pre-bleed; Wk 1 – Wk 8, Week 1 – week 8 bleeds. Pink, AEAB-derivatized glycans; purple, BSA neoglycoconjugates; blue, glycoprotein controls. X-axis labels correspond to the structures depicted in Figure 2. Note that signals reach the detection maximum near 60,000 RFU.
We additionally analyzed rabbit immune serum on an open-ring shotgun microarray of human milk glycans derivatized with AEAB 23. This array contains several structures that were defined in our previous publication using the metadata-assisted glycan sequencing (MAGS) approach, as well as many yet undefined human milk glycans. As predicted by the above experiments, only the LNnT-OR-BSA-immunized rabbit serum showed significant binding on this array (Supplemental Figure 1a–c). Almost all of the structures bound contained terminal or internal LNnT determinants, whereas the unbound structures primarily contained LNT (Supplemental Table 1). Some modifications of LNnT, including Lewis X determinants, appear to inhibit strong binding of the rabbit antisera, whereas several branched and linear substituents of LNnT, and a few branched type 2 poly-LN were tolerated (Supplemental Table 1). The rabbit antisera were also reactive with many of the undefined structures on the human milk glycan array, indicating the likely presence of LNnT determinants in these glycan fractions (Supplemental Figure 2). This data reinforces the idea that LNnT-OR-BSA and LNnT-CR-BSA immunizations produced non-cross reactive responses.
Mice immunized with closed-ring LNnT-BSA generate a muted glycan-specific response
As mice are commonly used in vaccine candidate testing, but have in the past been problematic subjects where generation of anti-glycan antibodies is concerned, we next asked whether mice showed a similar pattern of specificity when immunized with LNnT-BSA. We performed a small pilot study where three mice were immunized in the same fashion as the rabbits. Pooled mouse serum showed a muted IgG response to the sugar-linker compared to the high response made to neoglycoconjugates when immunized with LNnT-CR-BSA (Figure 6a), in contrast to rabbits (Figure 6b). A large portion of the IgG appears to be against BSA, with additional responses to the open-ring sugar and/or linker components of Lac-OR-BSA and LNnT-BSA. The IgM response in mice (Figure 6c) was similarly directed to Lac-OR-BSA and included non-specific anti-protein antibodies. While less focused than the IgG response, the rabbit IgM response (Figure 6d) also favored LNnT-CR-AEAB and LNnT-CR-BSA, with some IgM binding to the other neoglycoconjugates and glycoproteins as well. Thus, mice showed weak responses to the glycan determinants compared to the robust response seen in rabbits.
Discussion
The human immune system responds to eukaryotic glycan antigens during infections, cancer, and autoimmune disorders. However, there is little precedent on how to design vaccines targeting eukaryotic glycan epitopes, which are often non-repeating, may occur at the non-reducing (distal) or reducing end (core) of the glycan. Epitopes near the reducing end of a glycan, for example, might be disrupted by conventional glycoconjugate linkage methods such as reductive amination, which is commonly used for direct generation of glycan-protein conjugates with simple sugars. The purpose of our study was to use a common small glycan, lacto-N-neotetraose (LNnT), to compare the effect of two different linkage methods, one which opens the ring at the non-reducing end of the glycan (OR) and one which creates a longer linker, leaving the ring closed (CR), on the specificity of the vaccine response. We found that these two linkage methods elicited distinct immune responses, indicating that the reducing end of the sugar and linkage type is crucial for some glycan epitopes.
Rabbits immunized with OR forms of Lac- and LNnT- as well as CR forms of LNnT-BSA neoglycoconjugates displayed robust IgG responses that were different for each immunogen, as shown by glycan microarray studies. The OR-neoglycoconjugates induced a response that was specific for epitopes combining the reducing end sugar ring, the linker, and a portion of the protein carrier. This was demonstrated by their preferences for both of the OR-neoglycoconjugates over either of the Lac-OR-AEAB or LNnT-OR-AEAB, and near-complete lack of binding to LNnT-CR sugar derivatives on both glycan arrays. Our result is consistent with a previous study in which rabbits were immunized with LNT linked open-ring to BSA via a different linker. In that case the immune serum was strongly inhibited by the LNT in its open-ring form, both with and without the linker, but free-reducing LNT was a 1000-fold less potent inhibitor 13.
The LNnT-CR-BSA immunization, by contrast, resulted in a response that preferred the closed-ring tetrasaccharide and was not cross-reactive with the OR-sugars or conjugates on the BSA-PNPA array. Low-level binding to LNT on the BSA-PNPA suggests that a portion of the response targets the reducing end of the sugar and linker, or was impartial to the non-reducing end linkage. The LNnT-CR-BSA serum also bound LNnT and LNT on the CFG microarray, where a different closed-ring linker was used, indicating that the response was glycan-specific. Agalacto-LNnT was not bound, indicating that the tetrasaccharide backbone was necessary for antibody binding. Some modifications of the backbone, such as the LNT and fucosylated LNT isomers, were tolerated, but not all modifications of LNnT were bound. The tolerance of LNnT isomers/modifications could be a result of the polyclonal response, and it remains to be seen whether monoclonal antibodies with narrower specificity would be present if isolated from the animals. Taken together, the data indicate that LNnT-CR-BSA was the only neoglycoconjugate that induced a glycan-specific response to the full tetrasaccharide, which was the desired epitope.
LNnT is a human milk glycan, which contains the terminal disaccharide N-acetyllactosamine (LN), a common feature of mammalian glycans. Normal human serum and intravenous immunoglobulin (IVIG) derived from it have previously been shown to contain antibodies that recognize many self-like determinants, including LNnT and LNT 22,24,25. Interestingly, and by contrast, we did not detect such antibodies to LNnT in naïve rabbit serum, although it did contain antibodies to several of the CFG glycan determinants, and recognition against them was boosted after our immunization. This non-specific induction of anti-glycan antibodies could be a result of immune activation by the CFA/IFA adjuvant. Adjuvants can induce cytokine secretion and increased antigen presentation by antigen presenting cells, among other effects (reviewed in Reed, Orr & Fox 2013) 26, but the mechanism of boosting pre-existing serum antibodies is not known.
Furthermore, our ELISA, CFG, and human milk array studies indicated that the responses elicited by the OR- and CR- conjugates were not cross-reactive with each other. Thus, the reducing end of the sugar and linker has a profound effect on conjugate antigenicity for two very similar molecules to produce non-overlapping responses. Previous studies on rabbits immunized with OR- conjugates of sialylated human milk oligosaccharide and albumin showed that antisera often targets the reducing-end and linker region, and sometimes also possessed specificity for the non-reducing end of the sugar, depending on which sugar is used 12,13. For example, inhibition of binding of polyclonal anti-sialyllactose sera to its immunogen required a lactose sialylated in the same (either α2-3 or α2-6) linkage. However, immune serum to sialylated LNnT was effectively inhibited by derivatized lactose, which lacks the non-reducing end sialyl-LN 12. It was also shown by CR-linkage of oligosaccharide epitopes to a viral scaffold for immunization in chickens that the specificity of polyclonal responses was comparable to that of monoclonal antibodies 27. Thus, the CR- linkage of the glycan to protein carrier itself may be less antigenic, allowing the response to target the glycan epitope. However, some closed-ring conjugation strategies can also create an antigenic “neo-epitope” which suppresses the response to the glycan 7. Alternatively, in light of the recently-discovered ability of B-cells to present glycopeptide species from glycoconjugates to T-cells 15, perhaps OR- glycopeptides are less likely to be presented by MHC and result in a less robust T-dependent response, which would diminish affinity maturation of anti-glycan antibodies.
An interesting and potentially useful aspect of the study was the relatively high degree of specificity of LNnT-OR-BSA immunized rabbit sera for OR-LNnT determinants on the human milk array. This sera could be a helpful reagent for MAGS of shotgun array glycans in the future, such as the yet undefined human milk glycans in Yu et al. (2014) 23, as there are no other antibodies specific for LNnT determinants at present.
In contrast to rabbits, three mice of two different strains did not make a glycan-specific response to the closed-ring conjugate. A large portion of the IgG appears to be against BSA, with the remainder preferring the Lac-OR-BSA conjugate, suggesting that the primary epitopes are the aromatic amine-amide-BSA linkage and against the BSA molecule itself. It is possible that the preference for Lac-OR-BSA over LNnT-BSA is due to its somewhat higher sugar:protein ratio in our preparations, or that the antibodies bind preferentially to a terminal lactose. The mice generated an IgM in response to LNnT-CR-BSA, which preferred Lac-OR-BSA and was not sugar-specific, in contrast to rabbits where both IgG and IgM demonstrated specificity for the closed-ring linked tetrasaccharide. Thus, the inability of mice to respond to the sugar is not due to an inability to class-switch, but could be due to an absence of this specificity in the immune repertoire, a difference in presentation of the glycoconjugate, or other yet undiscovered immune factors. This result remains to be confirmed in a more highly-powered experiment and should be investigated in different mouse strains. Some investigators have had success at inducing glycan-specific antibodies in mice immunized with BSA conjugates where bacterial PS were used or smaller epitopes were linked in closed-ring fashion 28–31, however mice have also failed to generate glycan-specific responses in several instances, as discussed below.
Interspecies (goat vs. mouse, rabbit vs. mouse) as well as mouse inter-strain differences in the ability to mount a glycan-specific antibody response to both native glycoproteins and synthetic glycoconjugates have been previously noted in the literature 17,32–37. One explanation for the observed differences between rabbits and mice could be a differentially tolerized immune repertoire due to differences between murine and rabbit milk oligosaccharide composition. The oligosaccharide content of mammalian milk varies drastically among species, with human milk oligosaccharides such as LNnT occurring at 100–1000-fold lower levels in cow’s milk, for example (reviewed in Bode 2012) 38. Alternatively, there could be gaps in the murine immune repertoire for other reasons, or mechanistic deficits in the ability to respond to oligosaccharide conjugates. Though we used both outbred Swiss Webster and inbred C57BL/6 mice in our studies, a deficit in the ability to present certain types of glycopeptides could conceivably result from decreased variation at the MHC locus, as is seen in inbred mice, which are more commonly used for glycoconjugate immunization studies. Understanding these strain- and species-specific differences, and how they are similar or different from the human immune response, should be a priority as the choice of animals for testing can determine the success or failure of vaccine candidates.
In summary, we have demonstrated that the type of linkage between a glycan epitope and its carrier protein can profoundly impact the specificity of the immune response to LNnT in rabbits and that mice only weakly respond to the CR conjugate of LNnT. Particular attention should be paid when the glycan epitope is small and opening of the non-reducing end ring could result in destruction or alteration of the desired epitope. Closed-ring linkage was preferable for preservation of the antigenicity of the LNnT tetrasaccharide, and further research studies should address whether this is more generally applicable to eukaryotic glycans in the context of other glycoconjugate vaccines. Additionally, novel strategies of glycoconjugate vaccine synthesis, such as recombinant production, should be pursued in order to present glycan antigens more naturally and avoid the problem of antigenic linkers altogether.
Experimental Procedures
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification unless otherwise specified. All HRP-conjugated antibodies were purchased from KPL (Gaithersburg, Maryland). All Alexa-conjugated antibodies were purchased from Invitrogen/Molecular Probes (Eugene, OR).
Preparation of conjugate vaccines
Derivatization of open-ring (reductively aminated) LNnT (lacto-N-neo-tetraose, Galβ1-4GlcNAcβ1-3Galβ1-4Glc)-PNPA (p-nitrophenyl anthranilate) conjugate was performed as previously described 19. Derivatization of closed-ring (acryloylated) LNnT-PNPA conjugate was performed similarly to our previously published work, with some modifications 20. Briefly, free-reducing LNnT was dissolved in water. An excess of ammonium bicarbonate (Acros Organics, NJ) was added and after heating at 55°C for 1.5 hours, the reaction was cooled and diluted with water. The resulting mixture was purified in batches, on a series of two pre-conditioned 1g carbograph SPE columns (Waters, Milford, MA) and eluted with 50% acetonitrile (Fisher Scientific, Fair Lawn, NJ)/10mM ammonium bicarbonate. After removal of organic solvent, the glycosylamine derivative of LNnT was snap-frozen and lyophilized. On ice, 600mg of sodium bicarbonate, followed by 2mL of ice cold saturated sodium bicarbonate, followed immediately by 200μl of acryloyl chloride were added to each batch. Reactions were vortexed for 10 minutes with venting, 5mL of water was added, and the resulting glycosylamide was purified on carbograph columns as described previously, using 50% acetonitrile/0.1% TFA to elute. The samples were dried and molecular weights determined using a Bruker Daltonics Ultraflex II MALDI-TOF/TOF in RP mode, which were consistent with the expected product. Samples were reduced with sodium borohydride, followed by acetic acid at 4°C to reduce any residual reducing glycan to prevent formation of contaminating open-ring products, and again desalted on carbograph columns. The stabilized glycosylamide was dried and dissolved in 2mL methanol and cooled to −78°C. Ozone from an ozone generator was bubbled through the mixture until reactions remained blue and they were allowed to remain uncovered in the fume hood to return to room temperature. Methyl sulfide (300μl) was then added to each batch and incubated overnight at 4°C. The activated glycosylamide was dried completely under nitrogen gas and immediately derivatized with PNPA.
To make the open-ring (LNnT-OR-PNPA and Lac-OR-PNPA) and closed-ring PNPA (LNnT-CR-PNPA) derivatives, respectively, free-reducing LNnT, free-reducing lactose, and the activated glycosylamide LNnT were each mixed with 0.35M freshly prepared PNPA (Matrix Scientific, Columbia, SC) and 1M sodium cyanoborohydride at a ratio of 10:1 PNPA:glycan, in a solution of 7:3 (v/v) DMSO:acetic acid. The mixtures were heated at 65°C for 2hr and quenched by addition of 10 volumes of acetonitrile. After 2 hr at −20°C, the glycan derivatives precipitated from the acetonitrile solution and were collected after centrifugation at 4,000rpm. To remove excess PNPA, the supernatant was discarded and the pellets were washed with cold acetonitrile and then re-dissolved in water and centrifuged to remove particulate material from the soluble products. The LNnT-OR-PNPA and LNnT-CR-PNPA were stored at −80°C until conjugation with protein. Aliquots were spotted 1:1 with DHB (2,5-dihydroxybenzoic acid) matrix for MALDI-TOF analysis in RP mode. Theoretical molecular weights of the PNPA conjugates are 949 and 1004g/mol, but observed molecular weights are at 958 and 1013g/mol, respectively.
Lac-OR-PNPA, LNnT-OR-PNPA, and LNnT-CR-PNPA were quantified via reverse phase HPLC on a Vydac C18 analytical column (Grace, Columbia, MD) to calculate molar amounts of the glycans, and these were added to a 3:2:5 (v/v/v) mixture of water:saturated sodium bicarbonate:BSA 20mg/mL (Fisher Scientific, Fair Lawn, NJ) at a glycan:protein molar ratio estimated to be about 20:1. These were rotated at 37°C for 3 days with 0.05% sodium azide and then dialyzed against water for over 24 hrs. The resulting Lac-OR-BSA, LNnT-OR-BSA and LNnT-CR-BSA neoglycoconjugates were spotted in sinapinic acid matrix (20mg/mL in 50% acetonitrile, 0.1%TFA) for MALDI-TOF analysis in order to estimate the resulting conjugation ratio. Purity was assessed by SDS-PAGE. The glycoconjugates were dried and stored at −20°C until use.
Immunizations
Glycoconjugates were dissolved at 10mg/mL in sterile phosphate-buffered saline (PBS). This mixture was diluted 1:5 in PBS and then emulsified at a 1:1 ratio with complete Freund’s adjuvant (CFA) for a final antigen concentration of 1mg/mL. The initial immunization of rabbits and mice consisted of 200μg injected subcutaneously per animal, spread out over the neck and hind flank for mice. Boosters of 100μg per animal in incomplete Freund’s adjuvant (IFA) were administered at 2, 4, and 6 weeks. Animals were bled via facial vein puncture one day before immunization (pre-bleed), and at weeks 5, 7, and 8. Two rabbits and three mice (one Swiss-Webster, two C57BL/6, all adult females) were used for these pilot studies. Mouse immunizations were performed under an approved IACUC protocol at Emory University. Rabbit immunizations were performed by ProSci Incorporated (Poway, CA).
Preparation and analysis of glycan microarrays
Microarrays were prepared, assayed, and analyzed as described in Heimburg-Molinaro et al. 39 with the following modifications. Lac-OR-AEAB, LNnT-OR-AEAB and LNnT-CR-AEAB were prepared as described [22] and the glycoprotein conjugates described above were printed in hexareplicate spots on NHS-activated glass slides (Schott, Tempe, AZ) using a Piezo Printer (Piezorray, PerkinElmer). Derivatized glycans were printed with 2 drops (666pL) of 100μM solutions and glycoproteins were printed with 2 drops of 0.5mg/mL solutions, in phosphate buffer, pH 8.0. Binding assays were performed as previously described 39. Arrays were quality-controlled via binding assays with biotinylated lectins (Vector Labs, Burlingame, CA) at 1μg/mL and cy5-streptavidin (Invitrogen/Zymed, Carlsbad, CA) at 0.5μg/mL. For serum binding assays, serum was diluted from 1:50 to 1:500 in binding buffer (TSM with BSA and 0.05% Tween-20) and applied to microarrays and incubated for 1 hr at RT. Wells containing individual microarrays were washed 3 times with 200μl TSM wash buffer, and 3 times with 200μl TSM buffer with 5 minutes of shaking for each wash. The same washing procedure was used after secondary incubations. The secondary antibodies, goat anti-mouse IgG-Alexa 568, anti-mouse IgM-Alexa 488, and anti-rabbit IgG-Alexa 488 were used at 5μg/mL. Slides were scanned on a PerkinElmer Proscanner XL4000 and ScanArray Express software was used to align spots, remove background, and quantify fluorescence. Microsoft excel was used to average the 6 replicate spots for each glycan ID #, and determine SEM, SD, and %CV.
CFG binding assays were conducted as described 39 with the following modifications: incubating slides with rabbit sera at 1:100 for the primary incubation and goat anti-rabbit IgG-Alexa 488 at 5μg/mL for the secondary incubation.
Human milk glycan arrays were prepared as described 23 and binding assays were performed as described above with detection by anti-rabbit IgG-Alexa 488.
ELISA
Glycoproteins were diluted to 1μg/mL in 0.05M carbonate/bicarbonate (Fisher, Fair Lawn, NJ) buffer pH 9.6 and 50μl were coated in each well of a clear, flat-bottom, non-tissue culture treated 96-well plate (Greiner Bio-One, Monroe, NC) overnight at 4°C. Excess coating solution was removed and the plate was blotted and washed three times with PBS-T (1X PBS with 0.05% Tween-20 (Fisher, Fair Lawn, NJ)) using a squirt bottle. All remaining incubations were 1 hour at room temperature on a slowly shaking orbital shaker, and all washes were with PBS-T. To each well, 200μl of blocker was added. Because of background issues in mouse serum ELISAs, assays were replicated with two different blockers: 3% BSA in PBS-T (with 1% BSA in serum and antibody dilutions) and non-animal protein NAP blocker (G-Biosciences, St. Louis, MO) 1:2 in PBS-T (with NAP 1:4 in serum and antibody dilutions). The blocker was discarded and plates were washed three times. The serum was diluted appropriately and 50μl was added to each well. After incubation the serum was discarded and the plate was washed five times. The secondary antibody, goat anti-rabbit IgG or anti-mouse IgG linked to HRP, was diluted 1:1000 and 50μl were added to each well. After incubation the unbound antibody was discarded and the plate was washed five times. The plate was then covered while O-phenylenediamine dihydrochloride was dissolved to 0.5mg/mL in stable peroxide buffer (Thermo Scientific, Rockford, IL). When the substrate was fully dissolved, 50μl were added to each well. Plates were incubated standing in the dark for 20 minutes. The reaction was quenched with 25μl per well of 3N sulfuric acid. Bubbles were popped and the absorbance of the plate was read at 490nm in a Victor plate reader (Perkin Elmer).
Supplementary Material
Acknowledgments
The authors would like to thank the Consortium for Functional Glycomics for use of the mammalian glycan array. This work was supported by NIH grants to RDC (AI101982, P41GM103694 and GM098791).
Abbreviations
- OR
open-ring
- CR
closed-ring
- BSA
bovine serum albumin
- LNnT
lacto-N-neo-tetraose (LNnT, Galβ1-4GlcNAcβ1-3Galβ1-4Glc)
- Lac
Lactose (Galβ1-4Glc)
- LNT
lacto-neo-tetraose (Galβ1-3GlcNAcβ1-3Galβ1-4Glc)
- PS
polysaccharide
- AEAB
2-amino-N-(2-aminoethyl)-benzamide
- PNPA
p-nitrophenyl anthranilate
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- NHS
N-Hydroxysuccinimide
- CFG
Consortium for Functional Glycomics
- LN
N-acetyllactosamine (Galβ1-4GlcNAc)
- ELISA
enzyme-linked immune assay
- ANOVA
analysis of variance
- MAGS
metadata-assisted glycan sequencing
Footnotes
Supporting Information Available: Two Figures and 1 Table. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, Hombach J, Kieny MP. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–45. doi: 10.1016/j.vaccine.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 2.Guttormsen H, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect Immun. 1999;67:6375–84. doi: 10.1128/iai.67.12.6375-6384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic Memory Induced by a Glycoconjugate Vaccine in a Murine Adoptive Lymphocyte Transfer Model. Infect Immun. 1998;66:2026. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, Characterization, and Immunogenicity of Haemophilus influenzae Type b Polysaccharide Protein Conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc Natl Acad Sci U S A. 1999;96:5194–7. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frasch CE. Preparation of bacterial polysaccharide-protein conjugates: analytical and manufacturing challenges. Vaccine. 2009;27:6468–70. doi: 10.1016/j.vaccine.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis(y) may be suppressed by a bifunctional cross-linker required for coupling to a carrier protein. Chemistry. 2004;10:3517–24. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 8.Mawas F, Niggemann J, Jones C, Corbel MJ, Kamerling JP, Johannes F, Vliegenthart G, Vliegenthart JFG. Immunogenicity in a Mouse Model of a Conjugate Vaccine Made with a Synthetic Single Repeating Unit of Type 14 Pneumococcal Polysaccharide Coupled to CRM197. Infect Immun. 2002;70:5107–5114. doi: 10.1128/IAI.70.9.5107-5114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in Host-Helminth Interactions. Front Immunol. 2013;4:240. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Diepen A, Van der Velden NSJ, Smit CH, Meevissen MHJ, Hokke CH. Parasite glycans and antibody-mediated immune responses in Schistosoma infection. Parasitology. 2012;139:1219–30. doi: 10.1017/S0031182012000273. [DOI] [PubMed] [Google Scholar]
- 11.Van Die I, Cummings RD. Glycomics in unraveling glycan-driven immune responses by parasitic helminths. Handbook of Glycomics. 2009:367–396. [Google Scholar]
- 12.Smith DF, Ginsburg V. Antibodies against sialyloligosaccharides coupled to protein. J Biol Chem. 1980;255:55–59. [PubMed] [Google Scholar]
- 13.Zopf DA, Tsai C, Ginsburg V. Antibodies against Oligosaccharides Coupled to Proteins: Characterization of Carbohydrate Specificity by Radioimmune Assay. Arch Biochem Biophys. 1978;185:61–71. doi: 10.1016/0003-9861(78)90144-3. [DOI] [PubMed] [Google Scholar]
- 14.Smith DF, Zopf DA, Ginsburg V. Carbohydrate Antigens: Coupling of Oligosaccharide Phenethylamine-Isothiocyanate Derivatives to Bovine Serum Albumin. Methods Enzymol. 1978;50:169–171. doi: 10.1016/0076-6879(78)50017-7. [DOI] [PubMed] [Google Scholar]
- 15.Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17:1602–9. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundle DR, Nycholat C, Costello C, Rennie R, Lipinski T. Design of a Candida albicans Disaccharide Conjugate Vaccine by Reverse Engineering a Protective Monoclonal Antibody. ACS Chem Biol. 2012;7:1754–63. doi: 10.1021/cb300345e. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski T, Luu T, Kitov PI, Szpacenko A, Bundle DR. A structurally diversified linker enhances the immune response to a small carbohydrate hapten. Glycoconj J. 2011;28:149–64. doi: 10.1007/s10719-011-9331-8. [DOI] [PubMed] [Google Scholar]
- 18.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat Chem Biol. 2007;3:663–7. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luyai A, Lasanajak Y, Smith DF, Cummings RD, Song X. Facile preparation of fluorescent neoglycoproteins using p-nitrophenyl anthranilate as a heterobifunctional linker. Bioconjug Chem. 2009;20:1618–24. doi: 10.1021/bc900189h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song X, Lasanajak Y, Xia B, Smith DF, Cummings RD. Fluorescent Glycosylamides Produced by Microscale Derivatization of Free Glycans for Natural Glycan Microarrays. ACS Chem Biol. 2009;4:741–750. doi: 10.1021/cb900067h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffrey AM, Zopf DA, Ginsburg V. Affinity chromatography of carbohydrate-specific immunoglobulins: Coupling of oligosaccharides to sepharose. Biochem Biophys Res Commun. 1975;62:608–613. doi: 10.1016/0006-291x(75)90442-8. [DOI] [PubMed] [Google Scholar]
- 22.Bovin N, Obukhova P, Shilova N, Rapoport E, Popova I, Navakouski M, Unverzagt C, Vuskovic M, Huflejt M. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim Biophys Acta. 2012;1820:1373–82. doi: 10.1016/j.bbagen.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Lasanajak Y, Song X, Hu L, Ramani S, Mickum ML, Ashline DJ, Prasad BVV, Estes MK, Reinhold VN, et al. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol Cell Proteomics. 2014;5962 doi: 10.1074/mcp.M114.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS. Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J Allergy Clin Immunol. 2009;123:1268–76. e15. doi: 10.1016/j.jaci.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, Miescher S, Simon HU, Pashov A, Vassilev T, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med. 2015;7:269ra1. doi: 10.1126/scitranslmed.3010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 27.Kaltgrad E, Sen Gupta S, Punna S, Huang CY, Chang A, Wong CH, Finn MG, Blixt O. Anti-carbohydrate antibodies elicited by polyvalent display on a viral scaffold. Chembiochem. 2007;8:1455–62. doi: 10.1002/cbic.200700225. [DOI] [PubMed] [Google Scholar]
- 28.Helling F, Shang A, Calves M, Zhang S, Ren S, Yu RK, Oettgen HF, Livingston PO. GD3 Vaccines for MelanomaY: Superior Immunogenicity of Keyhole Limpet Hemocyanin Conjugate Vaccines. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 29.Kudryashov V, Kim HM, Ragupathi G, Danishefsky SJ, Livingston PO, Lloyd KO. Immunogenicity of synthetic conjugates of Lewis y oligosaccharide with proteins in mice: towards the design of anticancer vaccines. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins JB, Kubler-Kielb J, Vinogradov E, Mocca C, Pozsgay V, Shiloach J, Schneerson R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc Natl Acad Sci U S A. 2009;106:7974–8. doi: 10.1073/pnas.0900891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pozsgay V, Kubler-Kielb J, Schneerson R, Robbins JB. Effect of the nonreducing end of Shigella dysenteriae type 1 O-specific oligosaccharides on their immunogenicity as conjugates in mice. Proc Natl Acad Sci U S A. 2007;104:14478–82. doi: 10.1073/pnas.0706969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin C, Bencúrová M, Borth N, Ferko B, Jensen-Jarolim E, Altmann F, Hantusch B. Immunoglobulin G specifically binding plant N-glycans with high affinity could be generated in rabbits but not in mice. Glycobiology. 2006;16:349–57. doi: 10.1093/glycob/cwj071. [DOI] [PubMed] [Google Scholar]
- 33.Hino S, Umeda F, Inumaru S, Aoki N, Sato C, Okajima T, Nadano D, Matsuda T. IgG2 dominancy and carbohydrate recognition specificity of C3H/He mouse antibodies directed to cross-reactive carbohydrate determinants (CCDs) bearing beta-(1,2)-xylose and alpha-(1,3)-fucose. Immunol Lett. 2010;133:28–34. doi: 10.1016/j.imlet.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Alonso de Velasco E, Verheul AF, Van Steijn AM, Dekker HA, Feldman G, Kamerling JP, Vliegenthart JF, Snippe H. Epitope Specificity of Rabbit Immunoglobulin G (IgG) Elicited by Pneumococcal Type 23F Synthetic Oligosaccharide- and Native Polysaccharide-Protein Conjugate Vaccines Y: Comparison with Human Anti-Polysaccharide 23F IgG. Infect Immun. 1994;62:799–808. doi: 10.1128/iai.62.3.799-808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardor M, Faveeuw C, Fitchette AC, Gilbert D, Galas L, Trottein F, Faye L, Lerouge P. Immunoreactivity in mammals of two typical plant glyco-epitopes, core alpha(1,3)-fucose and core xylose. Glycobiology. 2003;13:427–34. doi: 10.1093/glycob/cwg024. [DOI] [PubMed] [Google Scholar]
- 36.Jansen WTM, Hogenboom S, Mark JL, Kamerling JP, Johannes FG, Verhoef J, Snippe H, Verheul AFM. Tetrasaccharide-Protein Conjugates Contain Pneumococcal Type 6A and 6B Common and 6B- Specific Epitopes That Elicit Protective Antibodies in Mice Synthetic 6B Di-, Tri-, and Tetrasaccharide-Protein Conjugates Contain Pneumococcal Type 6A and 6B Common a. Infect Immun. 2001;69:787–793. doi: 10.1128/IAI.69.2.787-793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mccool TL, Schreiber JR, Greenspan NS. Genetic Variation Influences the B-Cell Response to Immunization with a Pneumococcal Polysaccharide Conjugate Vaccine. Infect Immun. 2003;71:5402–5406. doi: 10.1128/IAI.71.9.5402-5406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–62. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. Preparation and Analysis of Glycan Microarrays, in. Current Protocols in Protein Science. 2011:1–33. doi: 10.1002/0471140864.ps1210s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.