Abstract
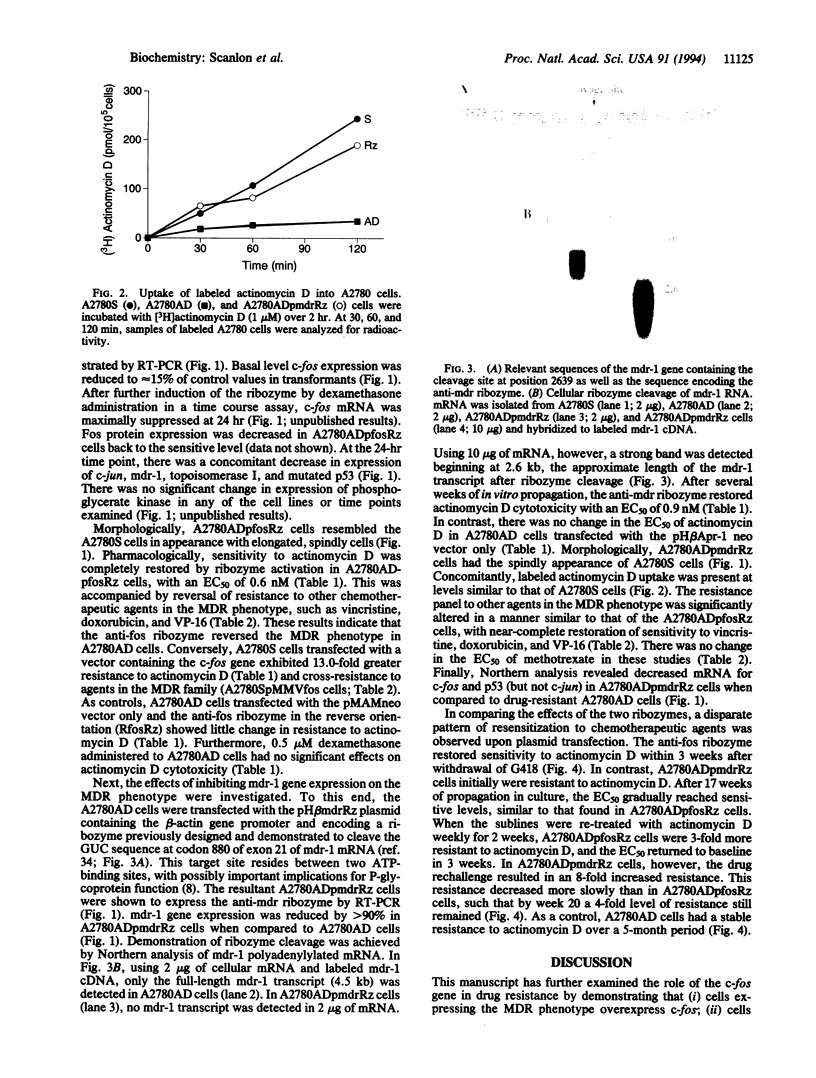
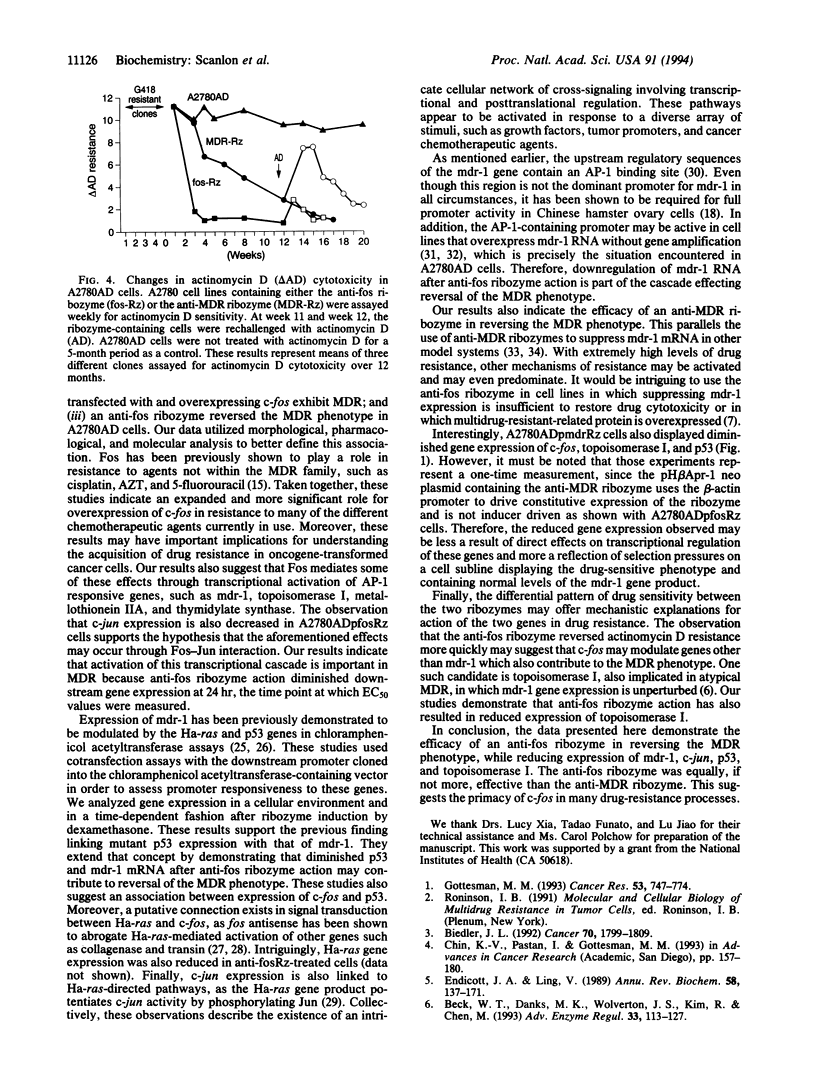
This study examined the effects of suppressing c-fos oncogene expression on multidrug resistance (MDR). A2780S human ovarian carcinoma cells with resistance to actinomycin D were isolated and the resultant A2780AD cells exhibited the MDR phenotype. A hammerhead ribozyme designed to cleave fos RNA cloned into the pMAMneo plasmid was transfected into A2780AD cells. Induction of the ribozyme resulted in decreased expression of c-fos, as well as that of the MDR gene (mdr-1), c-jun, and mutant p53. The transformants displayed altered morphology and restored sensitivity to chemotherapeutic agents comprising the MDR phenotype. An anti-mdr ribozyme separately expressed in A2780AD cells efficiently degraded mdr-1 mRNA. However, reversal of the MDR phenotype by the anti-mdr ribozyme occurred one-fourth as rapidly as that induced by the anti-fos ribozyme. These results reinforce the central role played by c-fos in drug resistance through its participation in signal transduction pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T., Danks M. K., Wolverton J. S., Kim R., Chen M. Drug resistance associated with altered DNA topoisomerase II. Adv Enzyme Regul. 1993;33:113–127. doi: 10.1016/0065-2571(93)90012-3. [DOI] [PubMed] [Google Scholar]
- Biedler J. L. Genetic aspects of multidrug resistance. Cancer. 1992 Sep 15;70(6 Suppl):1799–1809. doi: 10.1002/1097-0142(19920915)70:4+<1799::aid-cncr2820701623>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Pastan I., Gottesman M. M. Function and regulation of the human multidrug resistance gene. Adv Cancer Res. 1993;60:157–180. doi: 10.1016/s0065-230x(08)60825-8. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Funato T., Yoshida E., Jiao L., Tone T., Kashani-Sabet M., Scanlon K. J. The utility of an anti-fos ribozyme in reversing cisplatin resistance in human carcinomas. Adv Enzyme Regul. 1992;32:195–209. doi: 10.1016/0065-2571(92)90017-t. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Holm P. S., Scanlon K. J., Dietel M. Reversion of multidrug resistance in the P-glycoprotein-positive human pancreatic cell line (EPP85-181RDB) by introduction of a hammerhead ribozyme. Br J Cancer. 1994 Aug;70(2):239–243. doi: 10.1038/bjc.1994.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani-Sabet M., Funato T., Florenes V. A., Fodstad O., Scanlon K. J. Suppression of the neoplastic phenotype in vivo by an anti-ras ribozyme. Cancer Res. 1994 Feb 15;54(4):900–902. [PubMed] [Google Scholar]
- Kashani-Sabet M., Funato T., Tone T., Jiao L., Wang W., Yoshida E., Kashfinn B. I., Shitara T., Wu A. M., Moreno J. G. Reversal of the malignant phenotype by an anti-ras ribozyme. Antisense Res Dev. 1992 Spring;2(1):3–15. doi: 10.1089/ard.1992.2.3. [DOI] [PubMed] [Google Scholar]
- Kashani-Sabet M., Wang W., Scanlon K. J. Cyclosporin A suppresses cisplatin-induced c-fos gene expression in ovarian carcinoma cells. J Biol Chem. 1990 Jul 5;265(19):11285–11288. [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Dorai T., Holland J. F., Ohnuma T. Reversal of drug sensitivity in multidrug-resistant tumor cells by an MDR1 (PGY1) ribozyme. Cancer Res. 1994 Mar 1;54(5):1271–1275. [PubMed] [Google Scholar]
- Koizumi M., Kamiya H., Ohtsuka E. Ribozymes designed to inhibit transformation of NIH3T3 cells by the activated c-Ha-ras gene. Gene. 1992 Aug 15;117(2):179–184. doi: 10.1016/0378-1119(92)90727-7. [DOI] [PubMed] [Google Scholar]
- Ransone L. J., Verma I. M. Nuclear proto-oncogenes fos and jun. Annu Rev Cell Biol. 1990;6:539–557. doi: 10.1146/annurev.cb.06.110190.002543. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Jiao L., Funato T., Wang W., Tone T., Rossi J. J., Kashani-Sabet M. Ribozyme-mediated cleavage of c-fos mRNA reduces gene expression of DNA synthesis enzymes and metallothionein. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10591–10595. doi: 10.1073/pnas.88.23.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M. Elevated expression of thymidylate synthase cycle genes in cisplatin-resistant human ovarian carcinoma A2780 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):650–653. doi: 10.1073/pnas.85.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M., Tone T., Funato T. Cisplatin resistance in human cancers. Pharmacol Ther. 1991 Dec;52(3):385–406. doi: 10.1016/0163-7258(91)90033-i. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Newman E. M., Lu Y., Priest D. G. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Vendrell M., Hayward M., Baker S. J., Miao G. G., Schilling K., Robertson L. M., Curran T., Morgan J. I. Continuous c-fos expression precedes programmed cell death in vivo. Nature. 1993 May 13;363(6425):166–169. doi: 10.1038/363166a0. [DOI] [PubMed] [Google Scholar]
- Teeter L. D., Eckersberg T., Tsai Y., Kuo M. T. Analysis of the Chinese hamster P-glycoprotein/multidrug resistance gene pgp1 reveals that the AP-1 site is essential for full promoter activity. Cell Growth Differ. 1991 Sep;2(9):429–437. [PubMed] [Google Scholar]
- Ueda K., Pastan I., Gottesman M. M. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J Biol Chem. 1987 Dec 25;262(36):17432–17436. [PubMed] [Google Scholar]
- Zastawny R. L., Salvino R., Chen J., Benchimol S., Ling V. The core promoter region of the P-glycoprotein gene is sufficient to confer differential responsiveness to wild-type and mutant p53. Oncogene. 1993 Jun;8(6):1529–1535. [PubMed] [Google Scholar]




