Abstract
It is becoming increasingly clear that immunoactivation, which evolved as a system of host defense against pathogens, can become dysregulated and promote the pathogenesis of diverse diseases with both known and unknown etiologies (e.g., AIDS, age-related macular degeneration, cancer) as well as aging. Immunoactivation seems to be a “common denominator” or general mechanism of pathogenesis, and may explain the association and similarities in pathology among otherwise unrelated human diseases. Identification of general mechanisms of immunoactivation may lead to the development of new therapeutic strategies applicable to many diseases even before detailed knowledge of specific etiology and pathogenesis may be available.
Keywords: pathogens, viruses, inflammation, pathogenesis
Is immunoactivation a common driving force in human diseases?
All natural sciences evolved from one medieval root, Philosophia Naturalis, which considered Nature as an undivided entity and focused on understanding its most basic principles. Similarly, medicine considered the human body to be divided at most into four humors. With time, science underwent splitting into more-specialized areas. More recently, physics and chemistry developed increasingly unifying approaches to explain all the phenomena within one general theory, whereas medicine was focusing on specifics of particular diseases. This led to the progressive erosion of cross-disciplinary awareness, such that oncologists may know little about diseases of the eye ophthalmologists may be unfamiliar with eye-unrelated infections, and infectious disease experts may have only a superficial knowledge of cardiovascular disease. However, it seems that all these and many other diseases, including general aging, have one common driving mechanism, an inappropriate and dysregulated chronic immunoactivation.
The goal of this article is to emphasize the generality of this phenomenon, which is still not fully appreciated by the general biomedical community. Meanwhile, understanding the role of immunoactivation may explain clustering as well as some similarities in pathogesis in apparently unrelated diseases and suggests new therapeutic approaches.
Before discussing these general issues, I will first define the terms and next briefly consider four examples of unrelated diseases to illustrate how immunoactivation may be involved in the progression of a disease.
What is immunoactivation?
Since the terminology used in the literature is not always consistent, let us first define the subjects, at least in the framework of this review.
An encounter with a human pathogen that damages tissues first triggers an innate immune response, which includes interaction of cell surface pattern recognition receptors (PRRs) with common pathogen-associated molecular patterns (PAMPs), as well as other “nonspecific responses” such as release of “inflammatory” cytokines and of reactive oxygen species, vasodilatation, migration of leukocytes to infected tissue sites, expression of new proteins (“activation markers”) on cell surfaces, and cell proliferation. An innate response is followed by the adaptive immunity response, which may also contribute to inflammation. After the pathogens and their products are eliminated, most commonly in days or weeks, the immune system returns to its basic level.
Sometimes, however, acute inflammation fails to overcome the damage completely and is transformed into chronic inflammation with continuous upregulation of various cytokines, cell infiltrates, fibrosis, granuloma, and cell activation. This response can last from weeks to years.
The development of sensitive analytics has revealed that the immune system can remain activated for years and decades, but below the level of chronic inflammation. This may happen when the system does not return to the baseline completely, but also may develop de novo as a weak reaction to harmless foreign antigens. This state is associated with elevated levels of some cytokines, but not others, and with activation of some but not other cell populations; it has been designated “low-grade inflammation”, “para-inflammation”1, or “immunoactivation”, the term I will use here.
Immunoactivation and HIV disease
Unlike many other pathological conditions, human immunodeficiency virus (HIV) disease has a clear etiologic agent, (HIV-1). The very name of the virus indicates the nature of the disease: immunodeficiency. The damage caused by HIV-1 extends beyond the death of a subset of the infected cells to include death of uninfected cells or of abortively infected cells2 followed by the destruction of lymph node tissue3. Paradoxically, the driving force of this progressive immunodeficiency is immunoactivation4.
HIV-1 infects and replicates predominantly in activated CD4+ T cells. The immune system responds to infection by activating other lymphocytes, including uninfected CD4+T cells, thus creating new targets for the virus. This vicious cycle is facilitated by co-infections with other pathogens such as cytomegalovirus and other herpesviruses that are activated in HIV-1-infected individuals, as well by translocation of bacteria through the damaged gut mucosa5, further activating the immune system. Unable to eliminate HIV, the immune system becomes chronically activated, further facilitating HIV infection. Interestingly, some microbes that can reduce immune activation, e.g., human pegivirus (GB-virus C), improve survival of HIV-infected patients6.
Immunoactivation during HIV infection is evident from diverse immune system parameters, including activation phenotypes of cells, upregulation of selected cytokines7 and of C reactive protein, activation of matrix metalloproteinase, and deposition of collagen, which destroys lymph node cytoarchitecture8. It is immune activation rather than HIV-1 load that is a reliable predictor of disease progression9.
HIV-triggered immunoactivation can continue for years, even after replication of the virus is suppressed, leading to various diseases and premature aging.
Immunoactivation and atherosclerosis
Atherosclerosis, the process that leads to formation of atherosclerotic plaques, is the major cause of various cardiovascular diseases. Evidence collected over more than 150 years supports the notion that activation of the immune system plays a major role in atherosclerosis (reviewed in10–12). Immune cells, in particular T lymphocytes and macrophages, but also B lymphocytes, dendritic cells, and mast cells, are found in large quantities in atherosclerotic plaques. In plaques, both T cells and macrophages are activated and produce pro-inflammatory cytokines such as interferon gamma and tumor necrosis factor, as well as various extra-cellular vesicles13 that also can contribute to cell activation and facilitate cytokine release. Although plaque T cells are blood-borne, in plaques they are much more activated than in blood14, indicating the presence of local antigens, as is also evident from the clonal expansion in the early lesions of apolipoprotein E -KO mice15. The nature of potential antigens in plaques has been debated for decades, and their list includes oxidized low-density lipoproteins, heat shock proteins, and debris of decomposed cells, as well as various infectious agents16. Not only local but also systemic immunoactivation constitutes a strong pro-atherosclerotic factor: for example in autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus17 or in end-stage renal disease18. In conclusion, although immunoactivation does not seem to be a cause of atherogenesis, it constitutes an important driver of disease progression from its initiation to thrombotic complications.
Immunoactivation and cancer
It was generally accepted until about two decades ago that the immune system is anti-tumorigenic, rapidly recognizing and eliminating continually evolving cancer cells (immuno-surveillance). Fortunately, the immune system only rarely fails to recognize and destroy the tumor cells, and even when it fails, it continues to fight the evolving tumor as evidenced by the abundance of immune cells in solid tumors. Therefore, it was assumed that a rational anti-cancer strategy would be to facilitate immune system activation, for example by introducing anti-cancer vaccines or by targeting negative regulators of immune system activation (e.g., cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and programmed cell death protein 1( PD-1)).
Although an anti-tumorigenic role for the immune system is still widely accepted, the contemporary view has become more nuanced with the appreciation, through increasing evidence, that the immune system also plays a strong pro-tumorigenic role19. Indeed, the risk of tumor formation in some tissues is significantly increased by local inflammation, for example mastitis in the case of breast cancer or ulcerative colitis in the case of colon cancer.
During the last decade it has become evident that the immune system can promote tumors in different ways (reviewed in19). First, the very same lymphocytes and macrophages that infiltrate most of the solid tumors secrete various cytokines that stimulate growth and inhibit apoptosis of pre-malignant and tumor cells. Some of these cytokines work through the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and signal transducer and activator of transcription 3 (STAT3)19, which are required for expression of several tumorogenic genes and of anti-apoptotic genes such as Bcl-XL and Bcl-2. Second, immune activation can create a microenvironment favorable for seeding and local proliferation of cancer cells. Third, local immunoactivation promotes tumor angiogenesis, without which solid tumor growth is limited by the diffusion of nutrients. Inactivation in mice of NF-kB and STAT-3, the key enzymes that inflammatory signal activates in the angiogenesis pathway, results in suppression of angiogenesis and of tumor growth20.
In conclusion, although immunoactivation may not initiate cell transformation, it is critical for modulating the development of cancer through integration and balance of anti-tumorigenic with pro-tumorigenic effects.
Immunoactivation and age-related macular degeneration (AMD)
Age-related macular degeneration, a progressive and irreversible loss of central vision caused by lesions and neovascularization of the retina, results in the death of macular retinal cells. The precise contribution of pro- and anti-inflammatory mediators to age-related macular degeneration is the subject of intense debate.
The retina is a highly metabolically active tissue with formation and turnover of various antigenic products of tissue metabolism. The immune status of a normal eye is characterized by low activation and an abundance of regulatory T cells that suppress activation. When normal intra-ocular immunosuppressive mechanisms fail to function properly in the eyes of age-related macular degeneration patients21, 22, the immunogenic products accumulated in the eyes may induce a local immune response with accumulation of activated immune cells that secrete inflammatory cytokines. Furthermore, autoantibodies against the products of retinal metabolism activate the complement system, creating complement-mediated damage23 accompanied by activation of immune cells.
Finally, like other diseases in which immunoactivation plays a role, age-related macular degeneration is associated not only with local but also with systemic immunoactivation. In the serum of age-related macular degeneration patients there is a significant increase of interleukin (IL)-2224, which is also expressed in other chronic inflammatory conditions including psoriasis and rheumatoid arthritis, where its upregulation often correlates with increased disease activity. Also upregulated is IL-1724 a pro-inflammatory cytokine that controls extracellular pathogens and induces matrix destruction and neovascularization.
Thus, although it remains unclear what triggers age-related macular degeneration, immunoactivation appears to play a critical role in its progression.
Immunoactivation as a common denominator of human pathologies?
These few examples superficially described above illustrate that highly diverse human diseases may have one common feature, immunoactivation. This may be the basis for the association between apparently unrelated pathologies. For example, immunoactivation may link rheumatoid arthritis25, type 2 diabetes26, or end-stage renal disease18 to atherosclerosis.
It is possible that immunoactivation caused by irritation of the airway by cigarette smoke may explain why smoking is a common risk factor not only in cancer of the lung, where the products of smoking are deposited, but also in many apparently unrelated pathologies: cardiovascular diseases, preeclampsia, type 2 diabetes, and others.
Also, the well-known link of stress to cardiovascular disease seems to involve immunoactivation, as suggested earlier (see27), and was evidenced by a recently described stress-induced activation of haemopoetic stem cells and release of high numbers of neutrophils, monocytes, and lymphocytes into blood28.
Not only the links between apparently unrelated diseases but also similarities in the pathogenic details may be explained in the framework of immunoactivation. For example, as mentioned above, induction of angiogenesis is typical for solid tumors. Similarly, abnormal angiogenesis, albeit of leaky vessels, is the main feature of the wet form of age-related macular degeneration. In an apparent recognition of this similarity, the same angiogenesis inhibitors are currently used in treatment of age-related macular degeneration and of some solid tumors.
Also, rheumatoid arthritis is associated with high levels of active metalloproteinases as well as of other collagen-degrading enzymes. Metalloproteinase was reported to be increased in aortas of stressed mice28. An increase in metalloproteinases may lead to instability and collapse of atherosclerotic plaques in atherosclerosis. Activation of metalloproteinases is involved in cancer cell invasion and contributes to the disruption of lymph node cytoarchitecture in HIV-1-infected patients, Similarly, activation of metalloproteinases leads to liver fibrosis in the chronic phase of hepatitis Bvirus infection, characterized by immunoactivation. Thus, immunoactivation, which is common to all these human diseases, is associated with an abnormal activatation of the same enzymes, which leads to pathologies, albeit specific for each disease.
Immunoactivation can be triggered in healthy individuals by endogenous agents (e.g., microbiota), or by invasion of low-pathogenic viruses or bacteria. This may pre-dispose to various diseases that may not progress otherwise.
The important role of immunoactivation in various diseases makes it a potential target for therapy. Successful attempts to use various immune suppressants have been undertaken in HIV disease, in age-related macular degenerationE, in diabetes, and in atherosclerosis. Moreover, some approved drugs with known mechanisms of action have been found to have a general anti-immunoactivation property, which may explain unexpected treatment effects. For example, statins, which were introduced for cholesterol-lowering therapy in atherosclerosis, have been found to suppress immunoactivation and are now prescribed as prophylaxis for cardiovascular disease as well as for patients with type 2 diabetes.
Aging may also be mediated by immunoactivation, as best illustrated in HIV disease. Many physiological aspects of HIV-1-infected individuals resemble those that in uninfected individuals are associated with aging29. Initially, they were ascribed to ongoing HIV-1 replication. However, as a result of efficient anti-HIV therapy many HIV-infected patients have now been living without detectable presence of HIV for about two decades. Nonetheless, these patients acquire various diseases typical of aging (including cancer, atherosclerosis, arthritis, and general fragility) approximately 15 years earlier than a control group9. The immune systems of these patients are more activated than those of the controls9.
The early appearance of age-related diseases in HIV-infected but successfully treated individuals confirms the role of immunoactivation in these diseases but also constitutes another piece of evidence of the role of immunoactiavtion in general aging. Also, premature aging has been linked to immunoactivation in rheumatoid arthritis30 and implicated in aging in a Swedish cohort of old individuals (85+, with some reaching 100)31.
As with other cases of immune activation, in HIV-infected patients it is not clear what supports immunoactivation for many years after HIV-1 is fully suppressed. One of the candidates is cytomegalovirus , a common human herpesvirus that remains reactivated at a low level even years after HIV-1 has been suppressed32, 33. Interestingly, cytomegalovirus also was shown to be a negative factor in the Swedish cohor}. Although cytomegalovirus has been suggested as a driving force in cardiovascular diseases34, this association remains controversial16.
The importance of cytomegalovirus for the human immune system seems to be underappreciated, since although humans encounter hundreds of pathogens throughout their life, at an advanced age a disproportionally high fraction of T lymphocytes is specific for this virus. The clonal expansion of cytomegalovirus -specific T cells with age may shrink the repertoire of other T cells, thus contributing to increased susceptibility to various infections that become chronic, triggering persistent immunoactivation.
Concluding remarks
The idea that immunoactivation contributes to pathogenesis and disease progression (the “immunoactivation hypothesis”) is not new. At different times, it was introduced in various fields of medicine. For example, with regard to atherosclerosis it can be traced to Rudolf Virchow in the nineteenth century, whereas with regard to type 2 diabetes it was introduced in the early 1990s. However, in most cases the concept of damaging immunoactivation remained confined to the field of research where it was formulated. Now, it is becoming clear that the immunoactivation hypothesis is applicable generally.
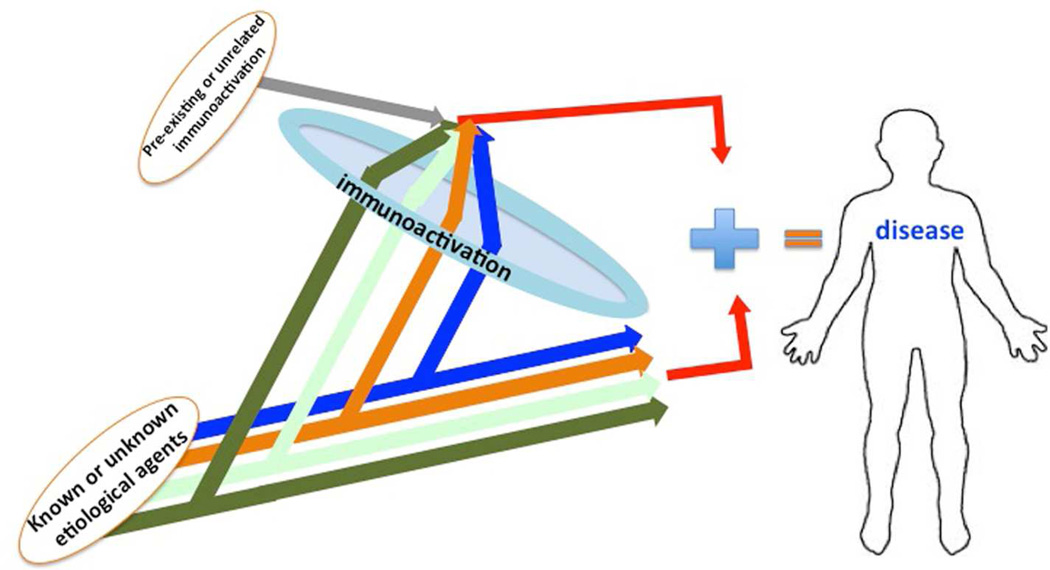
It is known that many important biological processes require two signals to progress. It seems that for various diseases, immunoactivation is one of the signals (Figure 1).
Figure 1. Immunoactivation hypothesis.
Human diseases are triggered by various etiological agents: viruses, bacteria, parasites, or unknown factors causing specific pathologies in the human organism. However, disease progression also requires immunoactivation, which is largely similar for all of them. This immunoactivation can be caused by the same agents or may have been developed prior to and independently of the etiological agent.
For example, although HIV-1 is the primary etiological agent of the acquired immune deficiency syndrome (AIDS), it is immunoactivation that makes the disease progress to immunodeficiency. Studies of sooty mangabeys have shown that simian immunodeficiency virus infection is not accompanied by immunoactivation and the infection leaves the animals healthy, whereas simian immunodeficiency virus infection of macaques that is accompanied by immunoactivation leads to AIDS-related disease35. Immunoactivation may play a similar role in diseases for which primary etiological agents are not known, such as atherosclerosis or cancer.
Nowadays, immunoactivation is becoming a new therapeutic target leading to new therapeutic approaches based on anti-immunoactivation strategies.
Clinical Significance.
Immunoactivation, which evolved as a system of host defense against pathogens, can become dysregulated and promote the pathogenesis of diverse diseases with both known and unknown etiologies and even be an important factor in general aging. Identification of general mechanisms of immunoactivation may lead to the development of new therapeutic strategies applicable to many diseases even before detailed knowledge of their specific etiology and pathogenesis may be available.
Acknowledgements
I am grateful to my colleagues, who advised me on the specifics of the diseases of their expertise: Drs. Michael Lederman, Philip Murphy, Robert Nussenblatt, Alexander Shpektor, and Elena Vasilieva.
Funding source: Intramural Program Eunice Kennedy-Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: None
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives cd4 t-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting cd4+ t cells and limiting reconstitution in HIV-1 and siv infections through damage to the secondary lymphoid organ niche. Seminars in Immunology. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nature medicine. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunology. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattarai N, McLinden JH, Xiang J, Landay AL, CHIVero ET, Stapleton JT. GB virus C particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J Immunol. 2013;190:6351–6359. doi: 10.4049/jimmunol.1300589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederman MM, Margolis L. The lymph node in HIV pathogenesis. Seminars in Immunology. 2008;20:187–195. doi: 10.1016/j.smim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Immune mechanisms in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 12.Frostegard J. Immunity, atherosclerosis and cardiovascular disease. BMC Medicine. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. Journal of Thrombosis and Haemostasis : JTH. 2006;4:1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 14.Grivel JC, Ivanova O, Pinegina N, Blank PS, Shpektor A, Margolis LB, Vasilieva E. Activation of t lymphocytes in atherosclerotic plaques. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:2929–2937. doi: 10.1161/ATVBAHA.111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulsson G, Zhou X, Tornquist E, Hansson GK. Oligoclonal t cell expansions in atherosclerotic lesions of apolipoprotein e-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:10–17. doi: 10.1161/01.atv.20.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Alpert JS. The role of infection in the genesis and complications of atherosclerosis. Current Cardiology Reports. 2002;4:173–175. doi: 10.1007/s11886-002-0045-1. [DOI] [PubMed] [Google Scholar]
- 17.Sherer Y, Shoenfeld Y. Mechanisms of disease: Atherosclerosis in autoimmune diseases. Nature Clinical Practice. Rheumatology. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 18.Nusair MB, Rajpurohit N, Alpert MA. Chronic inflammation and coronary atherosclerosis in patients with end-stage renal disease. Cardiorenal Medicine. 2012;2:117–124. doi: 10.1159/000337082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. The Journal of Clinical Investigation. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitcup SM, Sodhi A, Atkinson JP, Holers VM, Sinha D, Rohrer B, Dick AD. The role of the immune response in age-related macular degeneration. Int J Inflam. 2013;2013:348092. doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: Implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature Medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, Chakrabarty S, Agron E, Chan CC, Klein ML, Chew E, Ferris F, Nussenblatt RB. Complement component c5a promotes expression of il 22 and il-17 from human t cells and its implication in age-related macular degeneration. J Transl Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasceri V, Yeh ET. A tale of two diseases: Atherosclerosis and rheumatoid arthritis. Circulation. 1999;100:2124–2126. doi: 10.1161/01.cir.100.21.2124. [DOI] [PubMed] [Google Scholar]
- 26.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 27.Dhabhar FS. Effects of stress on immune function: The good, the, bad, and the beautiful. Immunologic Research. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 28.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nature Medicine. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 30.Straub RH, Scholmerich J, Cutolo M. The multiple facets of premature aging in rheumatoid arthritis. Arthritis and Rheumatism. 2003;48:2713–2721. doi: 10.1002/art.11290. [DOI] [PubMed] [Google Scholar]
- 31.Strindhall J, Nilsson BO, Lofgren S, Ernerudh J, Pawelec G, Johansson B, Wikby A. No immune risk profile among individuals who reach 100 years of age: Findings from the swedish nona immune longitudinal study. Experimental Gerontology. 2007;42:753–761. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific t cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PloS one. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appay V, Fastenackels S, Katlama C, Ait-Mohand H, Schneider L, Guihot A, Keller M, Grubeck-Loebenstein B, Simon A, Lambotte O, Hunt PW, Deeks SG, Costagliola D, Autran B, Sauce D. Old age and anti-cytomegalovirus immunity are associated with altered T-cell reconstitution in HIV-1-infected patients. AIDS. 2011;25:1813–1822. doi: 10.1097/QAD.0b013e32834640e6. [DOI] [PubMed] [Google Scholar]
- 34.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt PW, Deeks SG, Hodis HN, Kaplan RC. Cytomegalovirus immunoglobulin g antibody is associated with subclinical carotid artery disease among HIV-infected women. The Journal of Infectious Diseases. 2012;205:1788–1796. doi: 10.1093/infdis/jis276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]