Abstract
Although activation of sirtuin-1 (SIRT1) has been shown to protect the kidney from acute injury, its role in renal fibrosis remains controversial since both inhibition and activation of SIRT1 have been reported to attenuate renal fibrosis. To resolve this conflict, we further examined the effect of SIRT1 activators on the activation of renal interstitial fibroblasts and development of renal fibrosis in vivo and in vitro. In a murine model of renal fibrosis induced by unilateral ureteral obstruction, administration of SRT1720 (N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide), a potent activator of SIRT1, accelerated deposition of collagen fibrils and increased expression of fibroblast activation markers (α-smooth muscle actin [α-SMA], collagen I, and fibronectin) in the obstructive kidney of mice. In cultured rat renal interstitial fibroblasts (NRK-49F), exposure of cells to SRT1720 or YK-3-237 (B-[2-methoxy-5-[(1E)-3-oxo-3-(3,4,5-trimethoxyphenyl)-1-propen-1-yl]phenyl]-boronic acid), another SIRT1 activator, also resulted in enhanced expression of α-SMA and fibronectin. Mechanistic studies showed that augmentation of renal fibrogenesis by SRT1720 is associated with elevated phosphorylation of epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor β (PDGFRβ). SRT1720 treatment also increased the phosphorylation of signal transducer and activator of transcription 3 and protein kinase B in the fibrotic kidney and NRK-49F cells. However, SRT1720 treatment did not affect expression of proliferating cell nuclear protein, a proliferation marker and activation of extracellular signal regulated kinase 1/2 in vitro and in vivo. These results indicate that SIRT1-activating compounds can provoke renal fibrogenesis through a mechanism involved in the activation of EGFR and PDGFR signaling pathways and suggest that long-term use of SIRT1 activators risks the development and progression of chronic kidney disease.
Introduction
Tubulointerstitial fibrosis is a common event for the progression of chronic kidney disease regardless of the primary causes of renal disease (Tampe and Zeisberg, 2014). Renal fibrogenesis is characterized by activation of renal interstitial fibroblasts and subsequential production of an excessive amount of extracellular matrix (ECM) proteins. The transformation of fibroblasts into myofibroblasts is a core event for the development of fibrotic lesions in the kidney (Zeisberg and Neilson, 2010). Transforming growth factor (TGF)-β1 and other growth factors, such as epidermal and platelet-derived growth factors, are critically involved in the fibrotic process (Bonner, 2004; Liu et al., 2012, 2013). These cytokines/growth factors trigger their cellular events through binding to their receptors and subsequent activation of multiple downstream signaling pathways, such as signal transducer and activator of transcription 3 (STAT3), protein kinase B (AKT), and extracellular signal-regulated kinase 1/2 (ERK1/2) (Qin and Han, 2010; Liu et al., 2012, 2013; Kitada et al., 2013; Ponnusamy et al., 2013). It is evident that activation of epidermal growth factor receptors (EGFRs) and platelet-derived growth factor receptors (PDGFRs) contributes to activation of renal interstitial fibroblasts and development of renal fibrosis (Bonner, 2004; LeBleu and Kalluri, 2011; Liu et al., 2011, 2012).
Epigenetic modifications, such as acetylation/deacetylation, have also been linked to the pathogenesis of chronic kidney disease (Liu et al., 2013; Van Beneden et al., 2013; Tampe and Zeisberg, 2014). Protein acetylation is regulated by a network of enzyme systems, namely, histone acetyltransferases and histone deacetylases (HDACs). HDACs can modulate both acetylation of both histone and nonhistone proteins associated with fibrogenic process, such as STAT3 (Rombouts et al., 2002; Pang and Zhuang, 2010; Qin and Han, 2010). Among the four classes of HDACs, class I and II HDACs have been extensively studied for their role in tissue fibrogenesis (Pang et al., 2009, 2011; Pang and Zhuang, 2010). It is evident that small molecular inhibitors acting on these two classes of HDACs were effective in attenuating fibrosis in multiple organs including kidney (Pang et al., 2009; Liu et al., 2013; Van Beneden et al., 2013). Apart from class I and II HDACs, the role of sirtuins (SIRTs), a class III HDAC, has also been intensively studied in animal models of kidney diseases. Among the seven members of SIRTs, SIRT1 is ubiquitously expressed in renal tissue, and has been shown to be implicated in renal protection under ischemic, hypoxic, and calorie-restricted conditions (Fan et al., 2013; Kitada et al., 2013; Dong et al., 2014). However, the role of SIRT1 in renal fibrogenesis remains controversial even though its activity increases in the fibrotic kidney (Ponnusamy et al., 2014). Earlier studies have indicated that activation of SIRT1 by resveratrol, a polyphenol found in red wine, can alleviate renal fibrosis induced by unilateral ureteral obstruction (UUO) in mice (Li et al., 2010). However, our recent studies showed that inhibition of SIRT1 and 2 with sirtinol attenuated renal fibrosis in the same model. In addition, specific inhibition of SIRT1 by EX527 (6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide) or silencing of SIRT1 with siRNA suppressed activation of renal interstitial fibroblasts (Ponnusamy et al., 2014).These obviously conflicting results make it difficult to explain the functionality of SIRT1 in renal fibrogenesis and raise the issue of whether the antifibrotic effect of resveratrol is due to its nonspecific effects. It is well documented that this compound indeed has off-target effects (Pacholec et al., 2010). Indeed, a recent report from Venturelli et al. (2013) showed that resveratrol has an inhibitory effect on class I, II, and IV HDACs (Venturelli et al., 2013). With the view that blocking of class I/II HDACs can attenuate renal fibrosis (Pang et al., 2009; Liu et al., 2013), this suggests the possibility that resveratrol may alleviate renal fibrosis through a mechanism involved in the inhibition of class I/II HDACs rather than activation of SIRT1. Therefore, additional experiments are needed to characterize the role of SIRT1 activation in regulating renal fibrosis by using more specific SIRT1 pharmacological activators.
Efforts have been made to discover new molecules that are able to stimulate SIRT1 activities more specifically and potentially than resveratrol. SRT1720 (N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide) is structurally unrelated to resveratrol and does not share the off-target effects of resveratrol (Villalba and Alcain, 2012); therefore, it is a useful tool for verifying putative SIRT1-dependent effects in vivo. In this study, we investigated that the influence of SRT1720 on the progression of renal fibrosis in a murine model of renal fibrosis induced by UUO and on the activation of cultured renal fibroblasts.
Materials and Methods
Chemicals and Antibodies.
Antibodies to fibronectin, collagen I(A2), EGFR, glyceraldehyde-3-phosphate dehydrogenase, and proliferating cell nuclear antigen (PCNA) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All other antibodies used in this study were purchased from Cell Signaling Technology (Danvers, MA). SRT1720 was purchased from EMD Millipore (Billerica, MA). α-Smooth muscle actin (α-SMA) and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture and Treatments.
Rat renal interstitial fibroblasts (NRK-49F) were cultured in Dulbecco’s modified Eagle’s medium with F12 containing 5% fetal bovine serum and 0.5% penicillin and streptomycin in an atmosphere of 5% CO2 and 95% air at 37°C. To reduce the interference of growth factors in serum with SRT1720 activity, NRK-49F cells were grown in Dulbecco’s modified Eagle’s medium with F12 with 2.5% fetal bovine serum for 24 hours, and SRT1720 was directly added to subconfluent NRK-49F cells and incubated for 36 hours to determine the effects of SRT1720 on renal fibroblast activation.
Animals and Experimental Design.
The UUO model was established in 6–8 week old male C57 black mice that weighed 20–25 g (The Jackson Laboratory, Bar Harbor, ME) as described in previous studies (Pang et al., 2009, 2010). Briefly, the abdominal cavity was exposed via a midline incision and the left ureter was isolated and ligated. The contralateral kidney was used as a control. To examine the effects of SRT1720 on renal fibrosis after UUO injury, SRT1720 at 200 mg/kg body weight (prepared in 50 μl of dimethylsulfoxide) was intraperitoneally administered immediately after ureteral ligation and then given daily for 4 days. The dose of SRT1720 was selected based on a previous report (Imanishi et al., 2012). Control mice were injected with an equal volume of dimethylsulfoxide. The animals were sacrificed and the kidneys were collected at day 5 for protein analysis and histologic examination. All experimental procedures were performed according to the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The protocol (#0135-13) was approved by the Lifespan Animal Welfare Committee at Rhode Island Hospital. All surgery was performed under sodium pentobarbital anesthesia, and effort was made to minimize suffering.
Masson Trichrome Staining.
For assessment of renal fibrosis, Masson trichrome staining was performed according to the protocol provided by the manufacturer (Sigma-Aldrich). The semiquantitative analysis of the collagen tissue area (blue-colored area) was measured using ImageJ software developed at the National Institutes of Health (Bethesda, MD). The positive staining area from each microscopic field (200×) was calculated and graphed.
Immunoblot Analysis.
To prepare protein samples for western blotting, the kidney tissue samples were homogenized in the cell lysis buffer (Cell Signaling Technology) with protease inhibitor cocktail (Roche, Basel, Switzerland). After various treatments, cells were washed once with ice-cold phosphate-buffered saline and harvested in a cell lysis buffer mixed with a protease inhibitor cocktail. The protein level was measured by the bicinchoninic acid method and proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% nonfat milk for 1 hour at room temperature, the membranes were incubated with primary antibody overnight at 4°C and washed with Tris buffered saline with Tween-20. Then, the membranes were incubated with appropriate horseradish peroxidase–conjugated secondary antibody for 1 hour in room temperature. After washing the membrane with Tris buffered saline with Tween-20, bound antibodies were visualized by chemiluminescence detection using electrochemiluminescence solution obtained from GE Healthcare Life Sciences (Pittsburgh, PA).
Densitometry Analysis.
The semiquantitative analyses of different proteins were carried out by using the ImageJ software. Quantification is based on the intensity (density) of the band, which is calculated by the area and pixel value of the band. The quantification data are given as the ratio between the target protein and loading control (housekeeping protein) and the ratio between the phosphorylated protein and corresponding total protein.
Statistical Analysis.
Data are presented as mean ± S.D. and were subjected to one-way analysis of variance. Multiple means were compared using Tukey’s test, and the differences between two groups were determined by Student’s t test. P < 0.01 was considered statistically significant.
Results
SRT1720 Enhances Deposition of ECM Components and Activation of Renal Fibroblasts in the Kidney after UUO Injury.
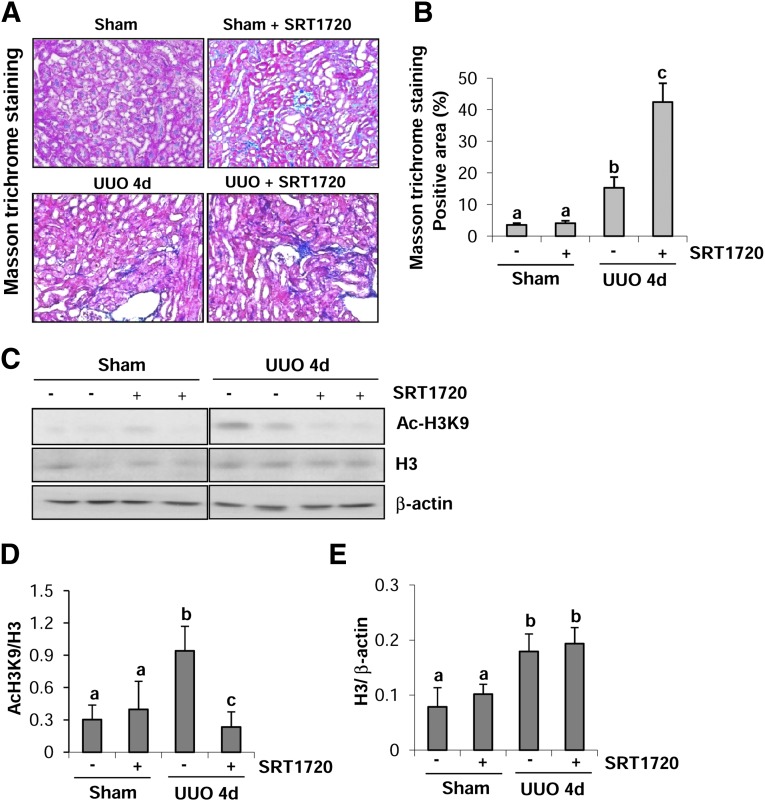
Progressive interstitial fibrosis is the result of excessive production of ECM components by activated fibroblasts (Zeisberg and Neilson, 2010). Ponnusamy et al. (2014) have shown that SIRT1 and 2 activities are increased during renal fibrosis induced by UUO injury and treatment with SIRT1/2 selective inhibitors can attenuate deposition of ECM proteins and inhibited activation of renal interstitial fibroblasts in the mouse model of renal fibrosis induced by UUO, suggesting that SIRT1/2 are critical regulators of renal fibrogenesis. If this conclusion is correct, SIRT1 activation would exert an opposite effect by enhancing renal fibrosis. To test this hypothesis, we collected the kidney after 4 days of UUO injury with or without SRT1720 treatment and then examined the effect of SRT1720 on renal fibrosis. Masson trichrome staining illustrates that the deposition and accumulation of ECM components were increased in the tubulointerstitial space as a consequence of myofibroblast activation, and administration of SRT1720 further increased the deposition of ECM components in the interstitial space (Fig. 1A). Semiquantitative analysis of Masson trichrome–positive areas revealed a 4-fold increase of ECM components in the obstructive kidney compared with the sham kidney. SRT1720 treatment increased ECM deposition by more than 3-fold compared with UUO injury alone (Fig. 1B). Immunoblot analysis of whole kidney tissue lysate indicated that acetylation of histone H3 at lysine 9 (Ac-H3K9) was increased in the injured kidney and its level was significantly decreased in the kidney of mice treated with SRT1720, indicating that the dose of SRT1720 was effective in elevating renal SIRT1 deacetylase activity (Fig. 1, C and D). In addition, there was also an increase in the expression of total H3 in UUO-injured kidney; however, its level was not affected by SRT1720 treatment (Fig. 1, C and E). Immunoblot analysis showed that UUO injury increased the expression of SIRT1 but that SRT1720 did not reduce its expression (Supplemental Fig. 1).
Fig. 1.
SRT1720 enhances the deposition of ECM and development of fibrosis in obstructed kidneys. (A) Photomicrographs illustrating Masson trichrome staining of kidney tissue after treatment with or without SRT1720. (B) The Masson trichrome–positive tubulointerstitial area (blue) relative to the whole area from 10 random cortical fields (200×) (mean ± S.D.) was analyzed. Data are represented as the mean ± S.D. Means with different lowercase letters are significantly different from one another (P < 0.01). (C) Kidney tissue lysates were subjected to immunoblot analysis with antibodies for acetyl-H3K9 (Ac-H3K9), total H3, or β-actin. The levels of Ac-H3K9 and total H3 were quantified by densitometry and normalized with β-actin (D and E). Values are mean ± S.D. (n = 6). Bars with different lowercase letters (a–c) are significantly different from one another (P < 0.01).
The expansion of renal interstitial fibrosis is classically manifested by an increase in the population of myofibroblasts, i.e., the phenotypically transformed fibroblasts that express α-SMA and produce ECM components (Meran and Steadman, 2011). By immunoblot analysis, we examined the expression of fibroblast activation and proliferation markers in UUO-injured kidney of mice treated with or without SRT1720. As shown in Fig. 2, increased expression of fibroblast activation markers (α-SMA, collagen I, and fibronectin), as well as proliferation markers (PCNA), were observed in the obstructed kidney. Administration of SRT1720 profoundly increased (about 2-fold) the expression levels of α-SMA, collagen I, and fibronectin (Fig. 2, A–D); however, PCNA expression was not affected by this compound (Fig. 2, E and F). Thus, these data indicate that SRT1720 can induce aggressive activation of fibroblasts and accumulation of ECM but not affect proliferation of interstitial fibroblast cells in the kidney after UUO injury.
Fig. 2.
Administration of SRT1720 increases renal interstitial fibroblast activation in obstructed kidneys. Kidney tissue lysates were subjected to immunoblot analysis with antibodies against α-SMA, collagen I, fibronectin, PCNA, or β-actin (A and E). Representative immunoblots from three independent experiments are shown. The levels of α-SMA, collagen I, fibronectin, and PCNA were quantified by densitometry and normalized with β-actin (B–D and F). Values are mean ± S.D. (n = 6). Bars with different lowercase letters (a–c) are significantly different from one another (P < 0.01).
Treatment with SIRT1 Activators Potentiates Cultured Renal Interstitial Fibroblast Activation.
To confirm the role of SIRT1 in mediating renal fibroblast activation in the mouse model, we examined the effect of SRT1720 on the expression of fibrogenic markers in cultured renal interstitial fibroblast cells (NRK-49F). NRK-49F grown in reduced levels of serum (2.5% fetal bovine serum) was exposed to various concentrations (0.5–2 μM) of SRT1720 for 36 hours, and the expression levels of the fibroblast activation markers (α-SMA and fibronectin) were assessed. As shown in Fig. 3A, SRT1720 dose dependently increased expression of those proteins. Densitometry analysis demonstrated that SRT1720 increased the expression of α-SMA (Fig. 3B) and fibronectin (Fig. 3C) by approximately 2- to 3-fold at a dose of 1 μM, and their levels were further enhanced in fibroblasts exposed to 2 μM of SRT1720. To demonstrate that this increase is due to the activation of SIRT1 deacetylase activity, we also examined the level of Ac-H3K9. As expected, SRT1720 treatment decreased the level of Ac-H3K9 in a dose-dependent manner (0.5–2 μM). The level of Ac-H3K9 was significantly decreased (∼70%) at a concentration of 1 μM SRT1720 treatment and further decreased more than 90% at a dose of 2 μM (Fig. 3, A and D). However, SRT1720 treatment did not increase the level of PCNA (Fig. 3, E and F) and cell proliferation, even at the maximum concentration (2 μM), as indicated by cell counting (Fig. 3G) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Fig. 3H). Similarly, SRT1720 treatment did not increase the PCNA level in cultured renal proximal tubular cells (data not shown). These data are consistent with our in vivo data, indicating that SRT1720 triggers activation but not proliferation of renal interstitial fibroblasts.
Fig. 3.
SRT1720 treatment enhances activation of cultured renal interstitial fibroblasts. NRK-49F cells were cultured in 2.5% fetal bovine serum containing medium and incubated with different concentrations of SRT1720 (0–2 μM) for 36 hours. Then, cell lysates were prepared and subjected to immunoblot analysis with antibodies against α-SMA, fibronectin, Ac-H3K9, PCNA, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A and E). Representative immunoblots from three independent experiments are shown. The levels of Ac-H3K9, α-SMA, fibronectin, and PCNA were quantified by densitometry and normalized with GAPDH (B–D and F). NRK-49F cells were treated with the indicated concentration of SRT1720 for 36 hours and cells were randomly photographed in bright field (200×). Cell proliferation was measured by cell counting (G), or the MTT assay (H). Values are mean ± S.D. of three independent experiments. Bars with different lowercase letters (a–d) are significantly different from one another (P < 0.01).
We further examined the effect of YK-3-237 (B-[2-methoxy-5-[(1E)-3-oxo-3-(3,4,5-trimethoxyphenyl)-1-propen-1-yl]phenyl]-boronic acid), another potent activator of Sirt1 (Yi et al., 2013), on the activation of renal interstitial fibroblasts using NRK-49F cells. Our data demonstrated that exposure of cells to YK-3-237 also significantly reduced expression of α-SMA and fibronectin in a dose-dependent manner, with the maximum inhibition occurring at 10 μM. This dose of the inhibitor completely blocked expression of Ac-H3K9, suggesting its effectiveness in the activation of Sirt1 (Supplemental Fig. 2). Collectively, our data provide strong evidence that SIRT1 plays a critical role in mediating activation of renal interstitial fibroblasts.
SRT1720 Enhances UUO-Induced Phosphorylation of EGFR and PDGFRβ in Obstructed Kidneys.
Activation of growth factor signaling pathways is involved in the regulation of fibrosis development. Previous studies have shown that EGFR and PDGFRβ are two major contributors to renal fibroblast activation and renal fibrogenesis (Ludewig et al., 2000; Terzi et al., 2000; Bonner, 2004; Di Pascoli et al., 2013). To determine whether SRT1720 exerts its profibrotic effects through activation of these two receptors, we examined the effect of this agent on the phosphorylation of EGFR at Tyr1068 (Y1068) and PDGFRβ at Tyr751 (Y751). As shown in Fig. 4, the level of phospho-EGFR was increased by ∼4-fold after 4 days in the kidney with UUO injury, and SRT1720 treatment enhanced phospho-EGFR level up to 4-fold when compared with the UUO-injured kidney treated with the vehicle. Interestingly, SRT1720 administration also increased phospho-EGFR levels more than 8-fold in the sham-operated kidney compared with the control kidney (Fig. 4, A and B). In addition, UUO injury resulted in increased expression of total EGFR; however, SRT1720 treatment did not alter its expression levels (Fig. 4, A and C). Similarly, SRT1720 was also effective in potentiating PDGFRβ phosphorylation in both sham-operated and UUO-injured kidneys (Fig. 4, A and D); however, it was not effective in altering the level of total PDGFRβ (Fig. 4, A and E). Collectively, our data suggest that SRT1720 treatment enhances phosphorylation of both EGFR and PDGFRβ in the fibrotic kidney.
Fig. 4.
SRT1720 increases phosphorylation of EGFR and PDGFRβ in obstructed kidneys. Kidney tissue lysates were prepared and subjected to immunoblot analysis with antibodies against phospho-EGFR (Tyr1068), EGFR, phospho-PDGFRβ (Tyr751), PDGFRβ, or β-actin (A). The phospho-EGFR, phospho-PDGFRβ, EGFR, PDGFRβ, and β-actin were quantified by densitometry. The phospho-EGFR level was normalized to the total EGFR level (B) and the phospho-PDGFRβ level was normalized with total PDGFRβ (D). The levels of EGFR and PDGFRβ were normalized with β-actin (C and E). Values are mean ± S.D. (n = 6). Bars with different lowercase letters (a–d) are significantly different from one another (P < 0.01).
SRT1720 Enhances Phosphorylation/Activation of EGFR and PDGFRβ in Cultured Renal Fibroblasts.
To specifically demonstrate that SRT1720-induced enhancement of EGFR and PDGFRβ activation occurs in renal fibroblasts during UUO injury, we further examined the effect of SRT1720 on their phosphorylation status in cultured renal fibroblasts. Consistent with our in vivo data, exposure to SRT1720 also dose-dependently increased the phosphorylation level of EGFR and PDGFRβ in cultured NRK-49F cells (Fig. 5, A and B). In addition, SRT1720 increased the level of total EGFR, with the maximum effect occurring at 2 μM; however, this agent did not affect the level of total PDGFRβ (Fig. 5, A and C). Thus, SRT1720 can enhance EGFR and PDGFRβ phosphorylation in renal fibroblasts but with different effects on their total protein levels.
Fig. 5.
SRT1720 enhances phosphorylation of EGFR and PDGFRβ in cultured renal interstitial fibroblasts. NRK-49F cells were cultured in 2.5% fetal bovine serum containing medium and then incubated with different concentrations of SRT1720 (0–2 μM) for 36 hours (A–C). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-EGFR (Tyr1068), EGFR, phospho-PDGFRβ (Tyr751), PDGFRβ, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A). Representative immunoblots from three experiments are shown. The phospho-EGFR, phospho-PDGFRβ, EGFR, PDGFRβ, and GAPDH were quantified by densitometry. (B) The phosphorylated EGFR and PDGFRβ were normalized to total protein levels. (C) The levels of EGFR and PDGFRβ were normalized with GAPDH. Values are mean ± S.D. of three independent experiments. Bars with different lowercase letters (a and b) are significantly different from one another (P < 0.01).
SRT1720 Enhances Phosphorylation of STAT3, but Not ERK1/2 in UUO-Injured Kidney and Cultured Renal Fibroblasts.
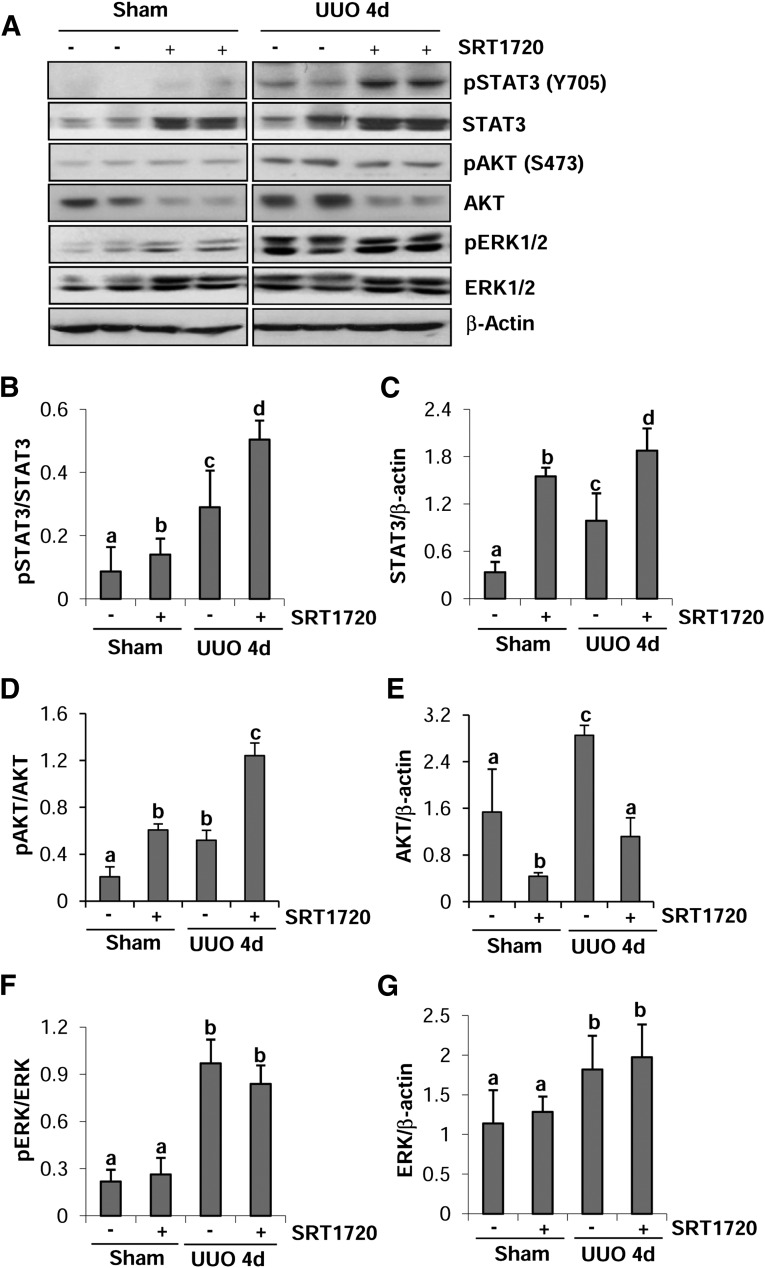
EGFR and PDGFRβ exert their biologic functions through activation of several intracellular signaling pathways including STAT3, AKT, and ERK1/2 (Ludewig et al., 2000; Liu et al., 2012; Tang et al., 2013), and these pathways are also associated with development of renal fibrosis (Pang et al., 2010; Lan and Du, 2015). Thus, we further examined the influence of SRT1720 on their phosphorylation in vivo and in vitro. As shown in Fig. 6, A–C, the phosphorylation levels of STAT3 (Y705) and total STAT3 were increased in the obstructed kidneys. Interestingly, SRT1720 administration further increased the levels of both phosphorylated and total STAT3 in UUO-injured and sham-operated kidneys. Compared with the sham-operated kidney, the UUO-injured kidney also showed an increase in the phosphorylation of AKT (S473) and SRT1720 treatment enhanced UUO-induced AKT phosphorylation as clearly indicated by the ratio of phospho-AKT/AKT. In sharp contrast to elevated phospho-AKT, administration of SRT1720 resulted in reduction in the level of AKT in both sham-operated and UUO-injured kidneys (Fig. 6, A, D, and E). Although increased levels of phosphorylated and total ERK1/2 were detected in the obstructed kidney, treatment with SRT1720 did not affect these responses (Fig. 6, F and G).
Fig. 6.
SRT1720 enhances STAT3 phosphorylation in obstructed kidneys. Kidney tissue lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-STAT3 (Tyr705), STAT3, phospho-AKT (Ser473), AKT, phospho-ERK1/2, ERK1/2, or β-actin (A). The levels of phosphorylated and total proteins were quantified by densitometry. The levels of phosphorylated proteins were normalized to their corresponding total protein (B, D, and F). The levels of STAT3, AKT, and ERK1/2 were normalized with β-actin (C, E, and G). Values are mean ± S.D. (n = 6). Bars with different lowercase letters (a–d) are significantly different from one another (P < 0.01).
We further examined the effect of SRT1720 on the phosphorylation and expression of STAT3, AKT, and ERK1/2 in cultured renal fibroblasts. As shown in Fig. 7, SRT1720 treatment at 1 and 2 μM remarkably increased the phosphorylation level of STAT3 (Fig. 7, A and B). Similarly, SRT1720 at 2 μM also enhanced AKT phosphorylation (Fig. 7, A and C); however, phosphorylation of ERK1/2 was not affected by SRT1720 (Fig. 7, A and D). In addition, SRT1720 treatment resulted in a decrease of total AKT (Fig. 7, A and C), but not total STAT3 (Fig. 7, A and B) or ERK1/2 (Fig. 7, A and D), levels in cultured renal fibroblasts. Collectively, these data indicate that SIRT1 activation by SRT1720 regulates phosphorylation of STAT3 and AKT, but not ERK1/2, suggesting that SRIT1 has a diverse role in regulating activation of intracellular signaling pathways.
Fig. 7.
SRT1720 enhances STAT3 phosphorylation in cultured renal interstitial fibroblasts. NRK-49F cells were cultured in 2.5% fetal bovine serum containing medium and then with different concentrations of SRT1720 (0–2 μM) for 36 hours (A–D). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for phospho-STAT3 (Tyr705), STAT3, phospho-AKT (Ser473), AKT, phospho-ERK1/2, ERK1/2, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A). Representative immunoblots from three experiments are shown. The phosphorylated and total proteins were quantified by densitometry and phosphorylated protein levels were normalized to their corresponding total protein level. The levels of total STAT3, AKT, and ERK1/2 were normalized with GAPDH (B–D). Values are mean ± S.D. of three independent experiments. Bars with different lowercase letters (a and b) are significantly different from one another (P < 0.01).
Discussion
SIRT-activating compounds such as resveratrol have been widely studied for their ability to treat aging-related disorders in metabolic, cardiovascular, and neurodegenerative diseases (Stünkel and Campbell, 2011; Villalba and Alcain, 2012). However, due to the poor specificity and bioavailability of resveratrol, several molecules that are structurally unrelated to resveratrol have been developed to stimulate SIRT activities, which are more potent and specific than resveratrol. SRT1720 is a newly developed and effective SIRT1 activator that has been shown to have the potential to treat diabetes and other diseases (Dong, 2012). In this study, we examined the pharmacological effect of SRT1720 on the development of renal fibrosis and activation of renal fibroblasts in a mouse model of UUO and cultured renal interstitial fibroblasts. Our results showed that activation of SIRT1 with SRT1720 potentiated these pathologic processes. Moreover, exposure of cultured renal interstitial fibroblasts to YK-3-237, another SIRT1 activator, also promoted their activation as demonstrated by increased expression of α-SMA and fibronectin. These results provide strong evidence that SIRT1 plays an essential role in mediating renal fibrogenesis after chronic kidney injury.
Our findings are contrary to a previous report in which activation of SIRT1 was demonstrated to be an antifibrotic mechanism in the kidney by using resveratrol (Li et al., 2010). Currently, the molecular basis behind the striking inconsistencies generated by these two SIRT1 activators in terms of fibrosis regulation remains unclear. One possible explanation is that resveratrol acts on other targets rather than on SIRT1. In this regard, it has been reported that resveratrol has a property enabling it to inhibit the activity of class I, II, and IV HDACs (Venturelli et al., 2013). Because blocking class I/II HDACs with their specific inhibitors or siRNA have been shown to suppress renal fibroblast activation (Pang et al., 2009, 2011; Liu et al., 2013) and because those class I/II HDACs inhibitors are also effective in attenuating renal fibrosis (Pang et al., 2009; Liu et al., 2013), resveratrol-induced inhibition of these enzymes may override its role as the activator of SIRT1, thereby exhibiting antifibrotic effects. Structural and chemical studies indicate that SRT1720 is 100-fold more potent in stimulating SIRT1 activity when compared with resveratrol (Villalba and Alcain, 2012).
SRT1720-induced enhancement of the fibrotic process may be involved in the activation EGFR and PDGFRβ and their downstream signaling pathways. Previous studies from our laboratory and those from other groups have demonstrated that sustained activation of EGFR and PDGFRβ is associated with the activation of renal fibroblasts, expression of profibrotic factors, and uncontrolled accumulation of ECM in the interstitial space (Pang et al., 2010; LeBleu and Kalluri, 2011; Liu et al., 2011, 2012, 2013). Class I/II HDACs have been suggested to mediate activation of several growth factor receptor signaling pathways, including EGFR and STAT3 activity in the kidney after chronic injury (Bruzzese et al., 2011; Chou et al., 2011; Liu et al., 2013). Similar to this, inhibition of SIRT1 reduced the phosphorylation of EGFR and PDGFRβ in cultured renal fibroblasts and fibrotic kidneys (Ponnusamy et al., 2014). All these studies provide strong evidence that HDACs, including SIRT1, can function as a positive regulator of cellular membrane receptors associated with the fibrotic process. Thus, stimulation of SIRT1 by SRT1720 would result in the activation of these profibrotic signaling molecules. The mechanism by which SRT1720 enhances the phosphorylation of receptor tyrosine kinases remains unknown. One possibility is that SRT1720-induced SIRT1 activation reduces the activity of tyrosine phosphatase. In supporting this hypothesis, SIRT1 is found to suppress the expression of protein tyrosine phosphatase 1B (Sun et al., 2007; Gagarina et al., 2010), a member of the dephosphorylating protein family, which negatively regulates the activity of growth signaling molecules including EGFR and PDGFRβ (Gu et al., 2003; Ponnusamy et al., 2013). Nevertheless, we cannot rule out the possibility that SRT1720 can also stimulate renal fibrosis through stimulation of TGF-β signaling activation because it has been reported that activation of SIRT1 increased Smad reporter activity, enhanced transcription of TGF-β target genes, and promoted release of collagen, whereas knockdown of SIRT1 inhibited TGF-β/SMAD signaling and reduced collagen release in skin fibroblasts (Zerr et al., 2014). Further experiments are needed to examine the role of SIRT1 in the regulation of this signaling pathway in renal interstitial fibroblasts.
Interestingly, SRT1720 administration increased the level of phosphorylated and total STAT3 in both sham-operated and fibrotic kidney. The structural analysis of STAT3 revealed that both phosphorylation (Tyr705) and acetylation (Lys685) are located in the SH2 domain of the STAT3 protein. Therefore, acetylation and phosphorylation may work mutually to regulate STAT3 activity (O’Shea et al., 2005). As such, SRT1720-mediated increase of SIRT1 activity may promote the phosphorylation of STAT3 by inhibiting its acetylation in addition to its influence on the protein tyrosine phosphatase system. On the other hand, SRT1720-mediated SIRT1 activation may regulate STAT3 through suppressing the activity/expression of suppressor of cytokine signaling (SOCS) family members such as SOCS1 and SOCS3, the negative regulators of STAT3. In fact, these two SOCS not only promote dephosphorylation but also ubiquitination and degradation of the STAT family of proteins (Kile et al., 2002; Croker et al., 2008). Therefore, SIRT1-mediated inhibition of SOCS1/3 may abolish the STAT3 degradation process, thereby increasing the stability of STAT3. As a result, both phosphorylated and total STAT3 levels are increased. A further detailed study should be carried out to unveil the mechanism of SRT1720-induced up-regulation of phosphorylated and total STAT3 levels.
It is quite surprising that SRT1720 treatment reduced the total AKT level in both fibrotic and sham-operated kidneys without affecting the phosphorylation of AKT (Ser473), which results in an increase in the ratio of pAKT to AKT. In general, phosphorylation of Ser473 at the hydrophobic motif site is necessary for full activation of the AKT molecule, and it is also the major site engaged in many physiologic and pathologic functions of AKT (Liao and Hung, 2010). Recent studies indicated that the AKT activity is controlled by the negative feedback loop, which is triggered by hyperactivation of AKT, and this hyperactivation has the capacity to regulate AKT stability and/or expression (Hart and Vogt, 2011; Wu et al., 2011). In supporting this, Wu et al. (2011) found that consecutive activation of AKT by Ser473 phosphorylation promotes its rapid degradation by a polyubiquitination-dependent proteosomal pathway, thereby turning off the AKT signaling (Wu et al., 2011). Given that SIRT1 promotes AKT phosphorylation through deacetylation (Horio, 2012; Li et al., 2013), SRT1720-induced SIRT1 activity might stimulate sustained activation of AKT, which in turn leads to degradation of AKT and reduction of total AKT levels.
SIRT activity has been linked to metabolic control, cell survival, DNA repair, development, neuroprotection, and healthy aging (Stünkel and Campbell, 2011; Villalba and Alcain, 2012). Because SIRT activation could have beneficial effects on human diseases, there is growing interest in the discovery of small molecules able to stimulate SIRT activity. Several small molecule activators of SIRT1 that are structurally unrelated to resveratrol have been developed. Among them, SRT1720 is one of the most effective SIRT1 activators. The role of SIRT1720 in treating insulin resistance, diabetes, and other diseases has been tested in animal models, and drugs similar to SRT1720 are currently in human clinical trials (Villalba and Alcain, 2012). However, due to the fibrosis-promoting effect of SIRT activators, large doses and long-term application should be avoided when they are used clinically in the future.
In summary, this is the first study to demonstrate that activation of SIRT1 by SIRT1720 potentiates renal fibroblast activation and renal fibrogenesis. The profibrotic effects of SIRT1720 are associated with activation of EGFR and PDGFRβ and their downstream signaling molecules STAT3 and AKT. On this basis, caution should be used in the application of SIRT1 activators in order to avoid induction of kidney fibrotic disorders or acceleration of pre-existing chronic kidney disease when they are used for renal diseases.
Supplementary Material
Abbreviations
- Ac-H3K9
acetylation of histone H3 at lysine 9
- AKT
protein kinase B
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular signal-regulated kinase 1/2
- EX527
6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide
- HDAC
histone deacetylase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCNA
proliferating cell nuclear antigen
- PDGFR
platelet-derived growth factor receptor
- SIRT1
sirtuin-1
- SRT1720
N-[2-[3-(piperazin-1-ylmethyl)imidazo[2,1-b][1,3]thiazol-6-yl]phenyl]quinoxaline-2-carboxamide
- α-SMA
α-smooth muscle actin
- SOCS
suppressor of cytokine signaling
- STAT3
signal transducer and activator of transcription 3
- TGF
transforming growth factor
- UUO
unilateral ureteral obstruction
- YK-3-237
B-[2-methoxy-5-[(1E)-3-oxo-3-(3,4,5-trimethoxyphenyl)-1-propen-1-yl]phenyl]-boronic acid
Authorship Contributions
Participated in research design: Ponnusamy, Zhao, S. Zhuang.
Conducted experiments: Ponnusamy, Zhou, Tolbert.
Contributed new reagents or analytic tools: Ponnusamy, Tolbert.
Performed data analysis: Ponnusamy, Zhou.
Contributed to the writing of the manuscript: Ponnusamy, M. A. Zhuang, Bayliss, S. Zhuang.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant 5R01-DK085065]; the National Nature Science Foundation of China [Grants 81270778 and 81470920]; and the Key Discipline Construction Project of the Pudong Health Bureau of Shanghai [Grant PWZ2014-06].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bonner JC. (2004) Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15:255–273. [DOI] [PubMed] [Google Scholar]
- Bruzzese F, Leone A, Rocco M, Carbone C, Piro G, Caraglia M, Di Gennaro E, Budillon A. (2011) HDAC inhibitor vorinostat enhances the antitumor effect of gefitinib in squamous cell carcinoma of head and neck by modulating ErbB receptor expression and reverting EMT. J Cell Physiol 226:2378–2390. [DOI] [PubMed] [Google Scholar]
- Chou CW, Wu MS, Huang WC, Chen CC. (2011) HDAC inhibition decreases the expression of EGFR in colorectal cancer cells. PLoS ONE 6:e18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, Kiu H, Nicholson SE. (2008) SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol 19:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pascoli M, Diví M, Rodríguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, Bosch J, García-Pagán JC. (2013) Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol 58:904–910. [DOI] [PubMed] [Google Scholar]
- Dong XC. (2012) Sirtuin biology and relevance to diabetes treatment. Diabetes Manag (Lond) 2:243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YJ, Liu N, Xiao Z, Sun T, Wu SH, Sun WX, Xu ZG, Yuan H. (2014) Renal protective effect of sirtuin 1. J Diabetes Res 2014:843786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Yang HC, You L, Wang YY, He WJ, Hao CM. (2013) The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int 83:404–413. [DOI] [PubMed] [Google Scholar]
- Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ. (2010) SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum 62:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Dubé N, Kim JW, Cheng A, Ibarra-Sanchez MdeJ, Tremblay ML, Boisclair YR. (2003) Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol 23:3753–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JR, Vogt PK. (2011) Phosphorylation of AKT: a mutational analysis. Oncotarget 2:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y. (2012) Diabetes: insulin signal meets SIRT1 at AKT. Nat Rev Endocrinol 8:131–132. [DOI] [PubMed] [Google Scholar]
- Imanishi S, Hayashi R, Ichikawa T, Suzuki K, Sasahara M, Kondo T, Ogawa H, Tobe K. (2012) SRT1720, a SIRT1 activator, aggravates bleomycin-induced lung injury in mice. Food Nutrition Sci 3:157–163. [Google Scholar]
- Kile BT, Schulman BA, Alexander WS, Nicola NA, Martin HM, Hilton DJ. (2002) The SOCS box: a tale of destruction and degradation. Trends Biochem Sci 27:235–241. [DOI] [PubMed] [Google Scholar]
- Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. (2013) Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond) 124:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan A, Du J. (2015) Potential role of Akt signaling in chronic kidney disease. Nephrol Dial Transplant 30:385–394. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Kalluri R. (2011) Blockade of PDGF receptor signaling reduces myofibroblast number and attenuates renal fibrosis. Kidney Int 80:1119–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. (2010) Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 177:1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XH, Chen C, Tu Y, Sun HT, Zhao ML, Cheng SX, Qu Y, Zhang S. (2013) Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol 48:490–499. [DOI] [PubMed] [Google Scholar]
- Liao Y, Hung MC. (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2:19–42. [PMC free article] [PubMed] [Google Scholar]
- Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. (2012) Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23:854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, Bayliss G, Zhao TC, Yan H, Zhuang S. (2013) Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS ONE 8:e54001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. (2011) Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol 22:1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig D, Kosmehl H, Sommer M, Böhmer FD, Stein G. (2000) PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res 299:97–103. [DOI] [PubMed] [Google Scholar]
- Meran S, Steadman R. (2011) Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Kanno Y, Chen X, Levy DE. (2005) Cell signaling. Stat acetylation—a key facet of cytokine signaling? Science 307:217–218. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. (2010) SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285:8340–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. (2009) Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297:F996–F1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. (2010) A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78:257–268. [DOI] [PubMed] [Google Scholar]
- Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H, Zhuang S. (2011) Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem 112:2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang M, Zhuang S. (2010) Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 335:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy M, Ma L, Zhuang S. (2013) Necrotic renal epithelial cell inhibits renal interstitial fibroblast activation: role of protein tyrosine phosphatase 1B. Am J Physiol Renal Physiol 304:F698–F709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy M, Zhou X, Yan Y, Tang J, Tolbert E, Zhao TC, Gong R, Zhuang S. (2014) Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther 350:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Han YP. (2010) Epigenetic repression of matrix metalloproteinases in myofibroblastic hepatic stellate cells through histone deacetylases 4: implication in tissue fibrosis. Am J Pathol 177:1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts K, Niki T, Greenwel P, Vandermonde A, Wielant A, Hellemans K, De Bleser P, Yoshida M, Schuppan D, Rojkind M, et al. (2002) Trichostatin A, a histone deacetylase inhibitor, suppresses collagen synthesis and prevents TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp Cell Res 278:184–197. [DOI] [PubMed] [Google Scholar]
- Stünkel W, Campbell RM. (2011) Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen 16:1153–1169. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319. [DOI] [PubMed] [Google Scholar]
- Tampe B, Zeisberg M. (2014) Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol Dial Transplant 29 (Suppl 4):iv72–iv79. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R, Bayliss G, Yan H, Zhuang S. (2013) Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G. (2000) Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beneden K, Mannaerts I, Pauwels M, Van den Branden C, van Grunsven LA. (2013) HDAC inhibitors in experimental liver and kidney fibrosis. Fibrogenesis Tissue Repair 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S, Berger A, Böcker A, Busch C, Weiland T, Noor S, Leischner C, Schleicher S, Mayer M, Weiss TS, et al. (2013) Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS ONE 8:e73097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM, Alcaín FJ. (2012) Sirtuin activators and inhibitors. Biofactors 38:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Ouyang W, Lazorchak AS, Liu D, Shen HM, Su B. (2011) mTOR complex 2 targets Akt for proteasomal degradation via phosphorylation at the hydrophobic motif. J Biol Chem 286:14190–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi YW, Kang HJ, Kim HJ, Kong Y, Brown ML, Bae I. (2013) Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells. Oncotarget 4:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. (2010) Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21:1819–1834. [DOI] [PubMed] [Google Scholar]
- Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, Schett G, Distler JH. (2014) Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis DOI:10.1136/annrheumdis-2014-205740 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.