Abstract
The low-molecular weight isopropyl 2-acetamido-α-glucoside 16 (C34) inhibits toll-like receptor 4 (TLR4) in enterocytes and macrophages in vitro, and reduces systemic inflammation in mouse models of endotoxemia and necrotizing enterocolitis. We used a copper(II)-mediated solvolysis of anomeric oxazolines and an acid-mediated conversion of β-glucosamine and β-galactosamine pentaacetates to generate analogs of 16 at the anomeric carbon and at C-4 of the pyranose ring. These compounds were evaluated for their influence on TLR4-mediated inflammatory signaling in cultured enterocytes and monocytes. Their efficacy was confirmed using a NF-kB-luciferase reporter mouse, thus establishing the first structure-activity relationship (SAR) study in this series and identifying the more efficacious isopropyl 2-acetamido-α-galactoside 17.
Keywords: toll-like receptors (TLRs), anti-inflammatory, cabohydrates, eritoran, lipid A mimetics
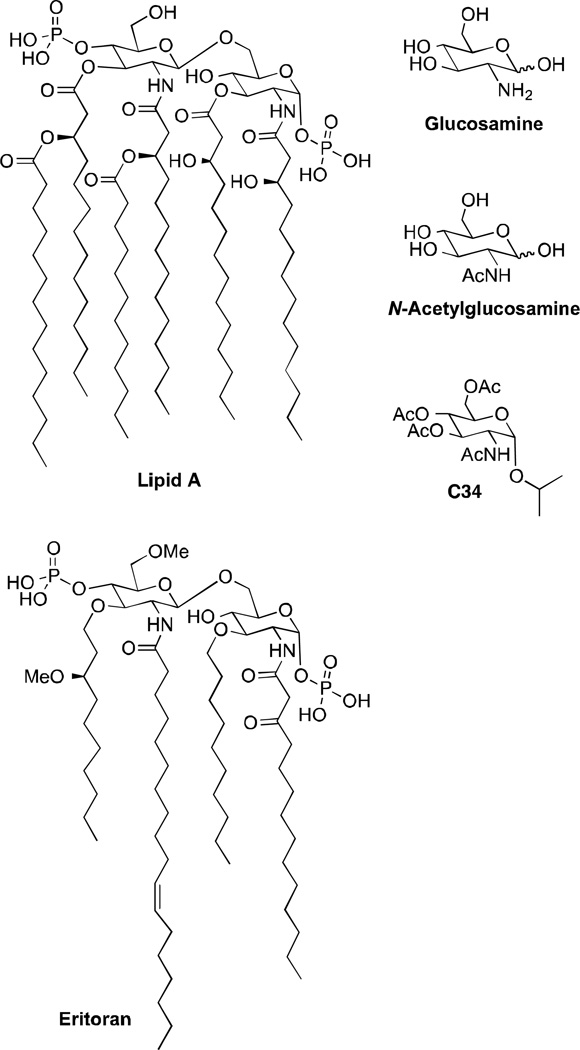
Carbohydrates are best known for their structural and energy storage functions in biological systems, but they demonstrate many other features, including the control of molecular and cellular recognition events (1, 2). Mono-, oligo- and polysaccharides can be found as conjugates of peptides, lipids, and secondary metabolites and modulate their physical and biological properties (1, 3). 2-Amino-2-deoxyglycosides, mainly glycosides of N-acetylglucosamine, are glycoconjugates present in human milk, blood, bacterial lipopolysaccharide antigens and plant root cells (4, 5). Glucosamine (Fig. 1) is a known treatment for pain maintenance in osteoarthritis and has no adverse side effects when compared to non-steroidal anti-inflammatory drugs (NSAIDs), nor does it affect glucose metabolism (6). Posttranslational modification of nuclear and cytoplasmic proteins by O-glycosidic attachment of β-N-acetylglucosamine is essential for cell viability, and improves cell survival under stress (7). Glucosamine- and N-acetylglucosamine-derived glycoconjugates have also shown promise as lead structures in drug development (8).
Figure 1.
Glycoconjugates.
Peptidoglycan fragments are recognized and induce signaling by toll-like receptors (TLRs), activating a pathway in innate immune cells that mediates an antimicrobial and proinflammatory response. In addition to common immunodisorders, TLRs have also been linked to carcinogenesis, and therefore they have evolved into major targets for drug discovery (9).
Several toll-like receptor 4 (TLR4) antagonists are under development as targeted therapeutics, including Eritoran from Eisai Pharmaceuticals (suspended in human Phase III trials), AV411 from Avigen (Phase II), and NI0101 from NovImmune (Preclinical) (9). Lipid A mimetics similar to Eritoran have become popular synthetic targets (10–12) and have sparked our interest in the use of glucosamine as a central scaffold for SAR elaboration of immunomodulating TLR ligands. As glucosamine is a component of the disaccharide core of both Lipid A and Eritoran (Fig. 1), we sought to mimic key structural features of these large liposaccharides, while reducing molecular weight and lipophilicity to generate more drug-like analogs. Using in silico drug discovery followed by in vivo testing, we recently discovered novel small molecule TLR4 inhibitory mono- and oligosaccharides (13). In particular, isopropyl 2-acetamidopyranoside C34 was found to inhibit TLR4 in enterocytes and macrophages in vitro and to reduce systemic inflammation in mouse models of endotoxemia and necrotizing enterocolitis (13). Subsequently, our strategy examined three areas for further structure-activity relationship (SAR) studies of this lead compound for TLR4-mediated inflammatory diseases: an α- vs β-glycosidic linkage, the configuration at C-4 of the pyranose (glucosamine vs galactosamine), and the length, size and hydrophilicity of the glycosyl chain. The pyranose hydroxyl and amino groups were O- and N-acetylated to mimic the longer acyl chains in Lipid A and Eritoran (Fig. 1).
The exploration of glycosylation methods for 2-amino-2-deoxyglycopyranosides was based on traditional literature protocols for carbohydrates, with some distinct differences due to the presence of the C-2 nitrogen atom (4). Glycosylation of 2-acetamido-2-deoxy-D-glucosamine (N-acetylglucosamine, GlcNAc) under standard Koenigs-Knorr conditions proceeded in good diastereoselectivity for the 1,2-trans-glycoside, but resulted in a large amount of the oxazoline side product (4). Furthermore, these conditions were limited to reactive glycosyl acceptors with a primary hydroxyl group which are often used in a large excess (4). If the N-acetyl group cyclizes to the oxazolinium intermediate and looses a proton to give the oxazoline, the glycosylation frequently stalls (4, 14). Because of the tendency of GlcNAc glycosyl donors to form the oxazoline side product, various alternatives to the N-acetyl group have been examined in glycosylation reactions (15), including protection with phthaloyl (16), tetrachlorophthaloyl (17, 18), 4,5-dichlorophthaloyl (19), dithiasuccinoyl (20, 21), trichloro- (22, 23), and trifluoroacetyl (22, 24), trichloroethoxycarbonyl (25–28), diacetyl (29), dimethylmaleoyl (30), and thiodiglycoloyl (31) groups, or a 2-azido group (5, 32–34).
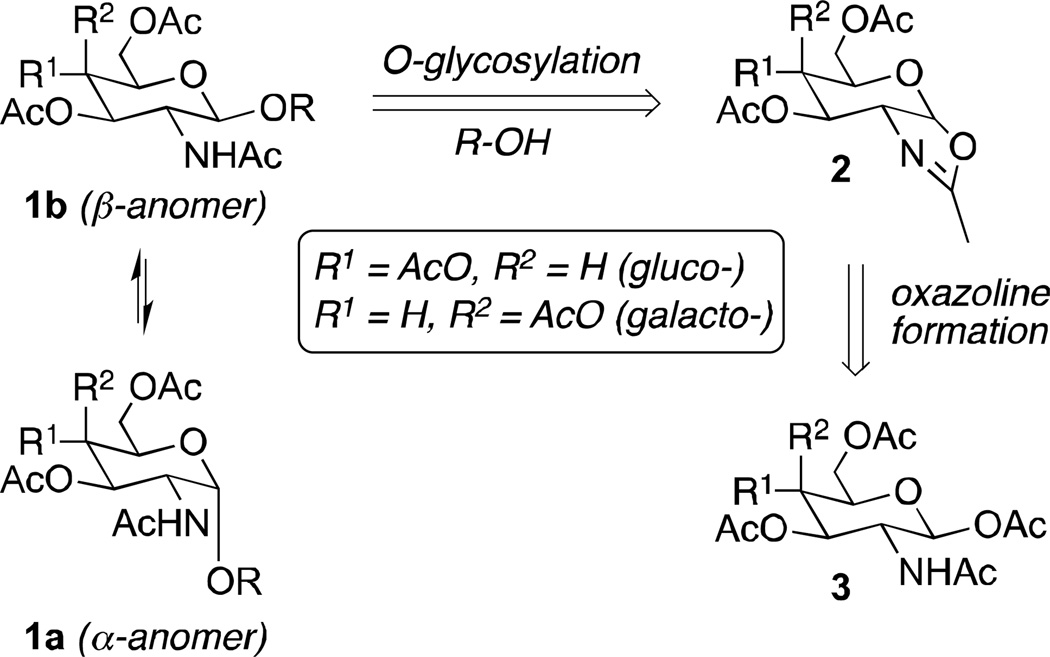
While these protective groups resolve the oxazoline issue, each requires additional steps for protection and subsequent substitution with the N-acetyl group in the desired analogs. Ring opening of aziridines such as those generated from iodosulfonamidation of glycals (35) or from the photolysis of triazolines (36) present an additional alternative for the formation of 2-amino-2-deoxyglycopyranosides, but these methods were unsuccessful in our hands. In contrast, the opening of oxazoline 2 with alcohols under mild, copper(II)-catalyzed conditions showed promise for formation of the β-glycosides 3,4,6-triacetyl-N-acetylglucosamine and 3,4,6-tri-acetyl-N-acetylgalactosamine 1b in our preliminary studies (13, 14, 37, Scheme 1). Furthermore, an acid catalyzed isomerization of the β-anomers 1b was envisioned to lead to the thermodynamically more stable α-anomers 1a, thus allowing a convergent approach from readily available pentaacetates 3.
Scheme 1.
Retrosynthetic analysis for α- and β-anomers 1 of 2-acetamido-2-deoxy-D-glucose and -galactose.
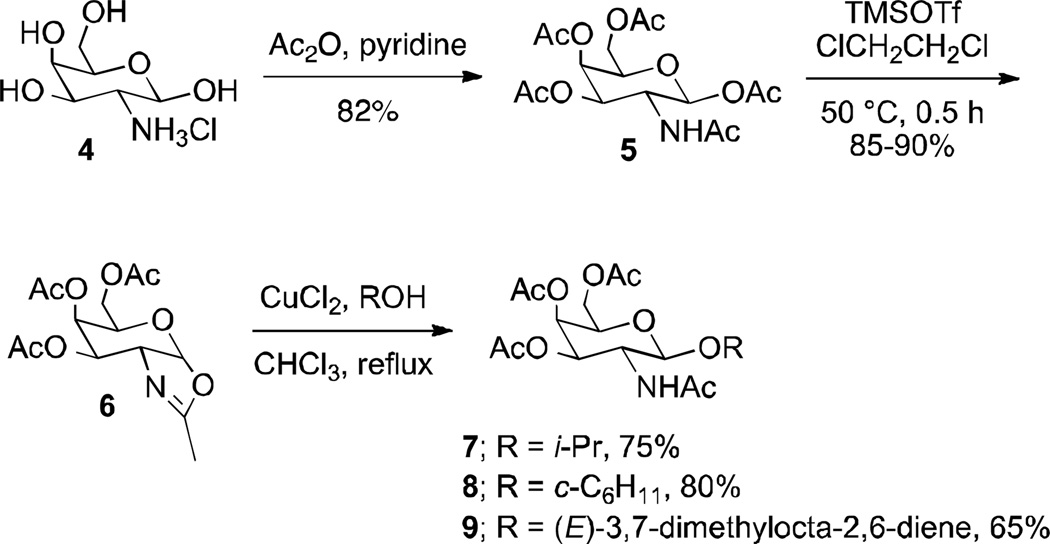
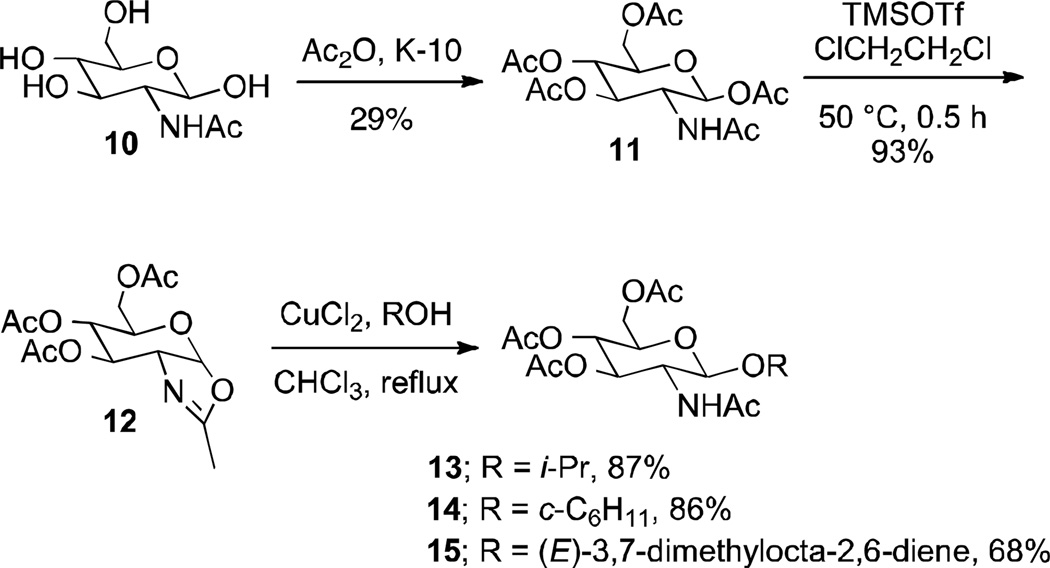
The fully protected galactosamine β-pentaacetate 5 (38) and glucosamine β-pentaacetate 11 (39) were prepared from tetrols 4 and 10, respectively, using pertinent literature procedures (Schemes 2 and 3). The corresponding oxazolines 6 and 12 were subsequently obtained in high yields with TMSOTf in 1,2-dichloroethane (14, 40–42). Other literature methods to access glyco-oxazolines, including SnCl4 in dichloromethane, did not lead to the desired products (43, 44). Oxazolines 6 and 12 were then heated at reflux in CHCl3 in the presence of CuCl2 and isopropanol, cyclohexanol, and geraniol as glycosyl acceptors to give the corresponding C-2 glycosides in good yields as pure β-anomers after recrystallization or chromatographic purification (14). Six different derivatives were prepared with the goal to vary lipophilicity, with 3 epimeric pairs differing only by their configuration at C-4. Glycosylation of isopropanol gave pyranosides 7 and 13 with a clog P of −0.5, whereas the cyclohexyl derivatives 8 and 14 were more lipophilic (clog P 0.6). The geraniol glycosides 9 and 15 (clog P 1.8) represented the most lipophilic small molecule analogs of Lipid A and Eritoran in this series (45).
Scheme 2.
Synthesis of three β-glycosides of 2-acetoamido-2-deoxy-D-galactose (7–9).
Scheme 3.
Synthesis of three β-glycosides of 2-acetoamido-2-deoxy-D-glucose (13–15).
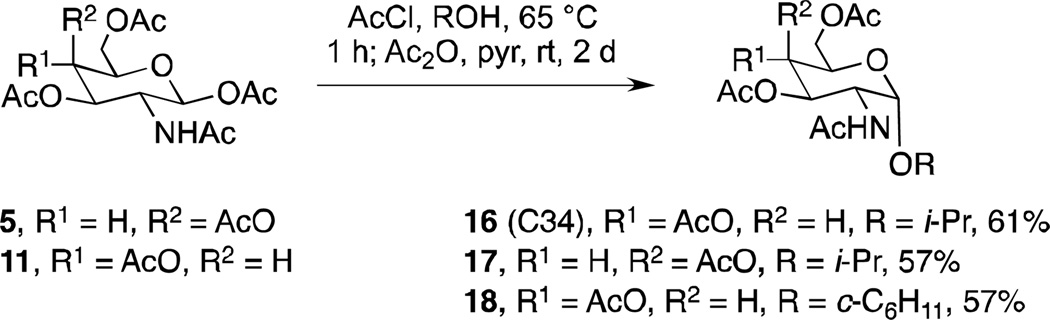
The corresponding α-glycosides in the galactosamine and glucosamine series proved more difficult to synthesize, and at first all previously envisioned acid catalyzed isomerization conditions starting with the corresponding β-glycosides were unsuccessful. Finally, heating the β-glucosamine and β-galactosamine pentaacetates 11 and 5, respectively, in in situ prepared 5% HCl in i-propanol and cyclohexanol, in analogy to literature conditions used for the synthesis of methyl α-D-glucosamine from D-glucosamine pentaacetate,(46) provided a 3:1 mixture of partly deacylated α:β anomers (Scheme 4). After reacetylation with acetic anhydride in pyridine, the mixture of anomers was separated by chromatography on SiO2 to give the desired α-anomeric glycosides 16–18 in moderate yields. Extensive NMR analyses were performed to assign the configurations of all glycoside products (47).
Scheme 4.
Acid-mediated tandem glycosylation and β- to α-anomeric equilibration.
As discussed in the introduction, many chronic inflammatory diseases and certain cancers trace their origin to increased signaling from the innate immune receptor TLR4, and our preliminary studies had identified 16 (C34) as a potent anti-inflammatory agent in mouse models of endotoxemia and necrotizing enterocolitis (13). In order to investigate the SAR of 16 and determine potential therapeutic benefits of analog structures, we screened compounds 7–9, 13–15, and 17–18 for the response on TLR4-mediated inflammatory signaling in cultured enterocytes (non-transformed rat small intestinal IEC-6 cells) and monocytes (mouse RAW 264.7 cells). Efficacy was subsequently confirmed using the NF-kB-luciferase reporter mouse, followed by assessment of pro-inflammatory cytokines IL6, IL1β and TNFα via qRT-PCR (48).
As shown in Table 1, isopropyl-β-galactoside 7, isopropyl-α-glucoside 16 and cyclohexyl-α-glucoside 18 produced the strongest anti-inflammatory suppression of LPS-induced cytokine mRNA levels in IEC6 cells, whereas geranyl-β-galactoside 9 and β-glucosides 13–15 were comparatively ineffective. These results, in combination with the intermediate level of activity of cyclohexyl-β-galactoside 8 and isopropyl-α-galactoside 17, indicate that a syn-configuration of acetate groups at C-4 and anomeric substituents at C-1 are preferred with regard to suppression of the pro-inflammatory IL6, IL1β and TNFα. The larger, more lipophilic geranyl group at the anomeric carbon in 9 and 15 also appears detrimental to bioactivity. The analogous measurements in RAW cells are consistent with these data (Table 1).
Table 1.
Biological evaluation of anti-inflammatory activities
| Compound | Suppression of LPS-induced IL6, IL1β, and TNFα mRNA levels in IEC6 cellsa |
Suppression of LPS-induced IL6, IL1β, and TNFα induction in RAW cellsb |
Protection of IL6, IL1β, and TNFα induction in micec |
|---|---|---|---|
| 7 | 58.0% (p<0.01) | 50% (p<0.01) | <64% (p<0.01) |
| 8 | 44% (p<0.01) | 50% (p<0.05) | <60% (p<0.01) |
| 9 | <20% | <20% | <20% |
| 13 | <20% | <20% | <20% |
| 14 | <20% | <20% | 48% |
| 15 | 30% | 32% | <20% |
| 16 (C34; ref. 13) | 55% (p<0.01) | 50% (p<0.01) | 69% (p<0.01) |
| 17 | 43% (p<0.01) | 50% (p<0.01) | 78% (p<0.01) |
| 18 | 54% (p<0.05) | 50% (p<0.01) | 64% (p<0.01) |
Treatment and dosages:
IEC6 cells (LPS 25 µg/mL, compounds 5 µg/mL, 30 min pretreatment).
Raw cells (LPS, 10 ng/mL, compounds 5 µg/mL, 30 min pretreatment).
Mice (LPS 2.5 mg/kg, compounds 2.5 mg/kg, 30 min pre-pretreatment).
A more complex pattern emerges in the in vivo biological assessment. While acetamidopyranosides 9, 13, and 15 are still inactive, protection of cytokine expression in mice is most effective with 2.5 mg/kg of isopropyl-α-galactoside 17 (78%), slightly surpassing isopropyl-β-galactoside 7 (64%), isopropyl-α-glucoside 16 (69%) and cyclohexyl-α-glucoside 18 (64%). Furthermore, cyclohexyl-β-glucoside 14 picks up moderate protective activity. This slight discrepancy from the cell-based biological activities is likely due to different rates of in vivo absorption, metabolism and excretion of these compounds and illustrates the importance of a multi-tiered assay strategy.
In conclusion, we have established a viable synthetic strategy to access configurationally diverse 2-acetamidopyranoside derivatives and used a small set of analogs to establish a preliminary SAR for our previous lead structure, TLR4 inhibitor 16 (C34). Thus, we were able to identify analogs that were equipotent to 16 in cell-based models. Most significantly, we also discovered an analog 17 that showed a significantly higher efficacy in an in vivo rodent model of inflammatory disease. Further characterization of the biological and therapeutic potential of these inhibitors of cytokine release will be reported in due course.
Supplementary Material
Acknowledgments
The authors thank Mr. Pete Chambers and Ms. Taber Lewis for QC analyses by LCMS, and the University of Pittsburgh Technology Commercialization Consortium (TCC) and the NIH (NS081744 and DK79307) for financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedicated to the memory of Prof. Harry Wasserman, in deep appreciation of his many insightful contributions to Organic Chemistry.
Supplementary Material
Synthetic procedures, spectroscopic data, and assay conditions.
References and notes
- 1.Filice M, Palomo JM. RSC Advances. 2012;2:1729–1742. [Google Scholar]
- 2.Wang Z, Du J, Che P-L, Meledeo MA, Yarema KJ. Curr. Opin. Chem. Biol. 2009;12:565–572. doi: 10.1016/j.cbpa.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weymouth-Wilson AC. Nat. Prod. Rep. 1997;14:99–110. doi: 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]
- 4.Banoub J, Boullanger P, Lafont D. Chem. Rev. 1992;92:1167–1195. [Google Scholar]
- 5.Herzner H, Reipen T, Schultz M, Kunz H. Chem. Rev. 2000;100:4495–4538. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JW, Nicolosi RJ, Borzelleca JF. Food Chem. Tox. 2005;43:187–201. doi: 10.1016/j.fct.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Darley-Usmar VM, Ball LE, Chatham JC. J. Mol. Cell. Cardiol. 2012;52:538–549. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paraskar AS, Soni S, Chin KT, Chaudhuri P, Muto KW, Berkowitz J, Handlogten MW, Alves NJ, Bilgicer B, Dinulescu DM, Mashelkar RA, Sengupta S. Proc. Nat. Acad. Sci. USA. 2010;107:12435–12440. doi: 10.1073/pnas.1007026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Connolly DJ, O’Neill LAJ. Curr. Opin. Pharmacol. 2012;12:510–518. doi: 10.1016/j.coph.2012.06.002. [DOI] [PubMed] [Google Scholar]; (b) Neve JE, Wijesekera HP, Duffy S, Jenkins ID, Ripper JA, Teague SJ, Campitelli M, Garavelas A, Nikolapoulos G, Le PV, De A, Leone P, Pham NB, Shelton P, Fraser N, Carroll AR, Avery VM, Mccrae C, Williams N, Quinn RJ. J. Med. Chem. 2014;57:1252–1275. doi: 10.1021/jm401321v. [DOI] [PubMed] [Google Scholar]; (c) Peri F, Calabrese V. J. Med. Chem. 2014;57:3612–3622. doi: 10.1021/jm401006s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Bazin HG, Murray TJ, Bowen WS, Mozaffarian A, Fling SP, Bess LS, Livesay MT, Arnold JS, Johnson CL, Ryter KT, Cluff CW, Evans JT, Johnson DA. Bioorg. Med. Chem. Lett. 2008;18:5350–5354. doi: 10.1016/j.bmcl.2008.09.060. [DOI] [PubMed] [Google Scholar]; (b) Artner D, Oblak A, Ittig S, Garate JA, Horvat S, Arrieumerlou C, Hofinger A, Oostenbrink C, Jerala R, Kosma P, Zamyatina A. ACS Chem. Biol. 2013;8:2423–2432. doi: 10.1021/cb4003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Stöver AG, Da Silva Correia J, Evans JT, Cluff CW, Elliot MW, Jeffery EW, Johnson DA, Lacy MJ, Baldridge JR, Probst P, Ulevitch RJ, Persing DH, Hershberg RM. J. Biol. Chem. 2004;279:4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]; (b) Lee K-H, Liu Y-J, Biswas A, Ogawa C, Kobayashi KS. J. Biol. Chem. 2011;286:5727–5735. doi: 10.1074/jbc.M110.108001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ainge GD, Martin WJ, Compton BJ, Hayman CM, Larsen DS, Yoon S-I, Wilson IA, Harper JL, Painter GF. J. Med. Chem. 2011;54:7268–7279. doi: 10.1021/jm2008419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. Sci. Signal. 2012;5:ra13. doi: 10.1126/scisignal.2001963. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dunn-Siegrist I, Tissières P, Drifte G, Bauer J, Moutel S, Pugin J. J. Biol. Chem. 2012;287:16121–16131. doi: 10.1074/jbc.M112.348383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Peri F, Marinzi C, Barath M, Granucci F, Urbano M, Nicotra F. Bioorg. Med. Chem. 2006;14:190–199. doi: 10.1016/j.bmc.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 13.Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T, Ma C, Branca M, Ozolek J, L BJ, Wipf P, Hackam DJ. PLoS One. 2013;8:e65779. doi: 10.1371/journal.pone.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittmann V, Lennartz D. Eur. J. Org. Chem. 2002:1363–1367. [Google Scholar]
- 15.Debenham J, Rodebaugh R, Fraser-Reid B. Ann. 1997:791–802. [Google Scholar]
- 16.Lemieux RU, Takeda T, Chung BY. ACS Symp. Ser. 1977;39:90–115. [Google Scholar]
- 17.Debenham JS, Madsen R, Roberts C, Fraser-Reid B. J. Am. Chem. Soc. 1995;117:3302–3303. [Google Scholar]
- 18.Castro-Palomino JC, Schmidt RR. Tetrahedron Lett. 1995;36:5343–5346. [Google Scholar]
- 19.Shimizu H, Ito Y, Matsuzaki Y, Iijima H, Ogawa T. Biosci. Biotech. Biochem. 1996;60:73–76. doi: 10.1271/bbb.60.73. [DOI] [PubMed] [Google Scholar]
- 20.Meinjohanns E, Meldal M, Paulsen H, Bock K. J. Chem. Soc., Perkin Trans. 1995;1:405–415. [Google Scholar]
- 21.Jensen KJ, Hansen PR, Venugopal D, Barany G. J. Am. Chem. Soc. 1996;118:3148–3155. [Google Scholar]
- 22.Wolfrom ML, Bhat HB. J. Org. Chem. 1967;32:1821–1823. doi: 10.1021/jo01281a025. [DOI] [PubMed] [Google Scholar]
- 23.Blatter G, Beau J-M, Jacquinet J-C. Carbohydr. Res. 1994;260:189–202. doi: 10.1016/0008-6215(94)84038-5. [DOI] [PubMed] [Google Scholar]
- 24.zu Reckendorf WM, Wassiliadou-Micheli N. Chem. Ber. 1970;103:1792–1796. doi: 10.1002/cber.19701030614. [DOI] [PubMed] [Google Scholar]
- 25.Imoto M, Yoshimura H, Shimamoto T, Sakaguchi N, Kusumoto S, Shiba T. Bull. Chem. Soc. Jpn. 1987;60:2205–2214. [Google Scholar]
- 26.Paulsen H, Krogmann C. Ann. 1989:1203–1213. [Google Scholar]
- 27.Ellervik U, Magnusson G. Carbohydr. Res. 1996;280:251–260. doi: 10.1016/0008-6215(95)00318-5. [DOI] [PubMed] [Google Scholar]
- 28.Dullenkopf W, Castro-Palomino JC, Manzoni L, Schmidt RR. Carbohydr. Res. 1996;296:135–147. doi: 10.1016/s0008-6215(96)00237-6. [DOI] [PubMed] [Google Scholar]
- 29.Castro-Palomino JC, Schmidt RR. Tetrahedron Lett. 1995;36:6871–6874. [Google Scholar]
- 30.Aly MRE, Castro-Palomino JC, Ibrahim E-SI, El-Ashry E-SH, Schmidt RR. Eur. J. Org. Chem. 1998:2305–2316. [Google Scholar]
- 31.Castro-Palomino JC, Schmidt RR. Tetrahedron Lett. 2000;41:629–632. [Google Scholar]
- 32.Lemieux RU, Ratcliffe RM. Can. J. Chem. 1979;57:1244–1251. [Google Scholar]
- 33.Paulsen H. Angew. Chem. Int. Ed. Engl. 1982;21:155–173. [Google Scholar]
- 34.Schmidt RR, Kinzy W. Adv. Carbohydr. Chem. Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 35.Griffith DA, Danishefsky SJ. J. Am. Chem. Soc. 1990;112:5811–5819. [Google Scholar]
- 36.Dahl RS, Finney NS. J. Am. Chem. Soc. 2004;126:8356–8357. doi: 10.1021/ja0319238. [DOI] [PubMed] [Google Scholar]
- 37.Eyer BR. MS Thesis. University of Pittsburgh; 2013. [Google Scholar]
- 38.Traar P, Belaj F, Francesconi KA. Austr. J. Chem. 2004;57:1051–1053. [Google Scholar]
- 39.Knapp S, Huhn RA, Amorelli B. Org. Synth. 2007;84:68–76. [Google Scholar]
- 40.Nakabayashi S, Warren CD, Jeanloz RW. Carbohydr. Res. 1986;150:c7–c10. doi: 10.1016/0008-6215(86)80028-3. [DOI] [PubMed] [Google Scholar]
- 41.Norberg O, Deng L, Aastrup T, Yan M, Ramström O. Anal. Chem. 2010;83:1000–1007. doi: 10.1021/ac102781u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Routenberg Love K, Andrade RB, Seeberger PH. J. Org. Chem. 2001;66:8165–8176. doi: 10.1021/jo015987h. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava VK. Carbohydr. Res. 1982;103:286–292. [Google Scholar]
- 44.Hesek D, Suvorov M, Morio K-i, Lee M, Brown S, Vakulenko SB, Mobashery S. J. Org. Chem. 2004;69:778–784. doi: 10.1021/jo035397e. [DOI] [PubMed] [Google Scholar]
- 45.Instant JChem was used for physicochemical property calculations; Instant JChem 6.2. 2014 ChemAxon ( http://www.chemaxon.com). [Google Scholar]
- 46.Takeda R, Ryu SY, Park JH, Nakanishi K. Tetrahedron. 1990;46:5533–5542. [Google Scholar]
- 47.Eyer BR. MS Thesis. University of Pittsburgh; 2013. [Google Scholar]
- 48.For a detailed description of assay conditions, see the Supplementary Material.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.