Abstract
Heat shock protein 90α (Hsp90α), encoded by the HSP90AA1 gene, is the stress inducible isoform of the molecular chaperone Hsp90. Hsp90α is regulated differently and has different functions when compared to the constitutively expressed Hsp90β isoform, despite high amino acid sequence identity between the two proteins. These differences are likely due to variations in nucleotide sequence within non-coding regions, which allows for specific regulation through interaction with particular transcription factors, and to subtle changes in amino acid sequence that allow for unique post-translational modifications. This article will specifically focus on the expression, function and regulation of Hsp90α.
Keywords: Molecular Chaperone, Heat Shock Protein, Stress Response, Heat Shock Element, Post-translational Modification, Drug Target, Cancer, Gene Ontology, Gene Promoter, Interactome
Introduction
Heat shock protein 90 (Hsp90) is a well-characterized, conserved and essential chaperone protein in eukaryotes. Several cellular proteins, termed ‘clients’, rely on Hsp90 for their function. In humans there are several Hsp90 isoforms including the well-known cytoplasmic Hsp90α and Hsp90β, as well as the endoplasmic reticulum and mitochondrial isoforms Grp94 and TRAP1, respectively (Ammirante et al. 2006).
Hsp90 comprises 1–2% of cellular protein in unstressed cells and up to 4–6% in the presence of stress (Picard 2002; Whitesell and Lindquist 2005; Stravopodis et al. 2007; Taipale et al. 2010; Finka and Goloubinoff 2013). The two isoforms of Hsp90 best studied in mammalian cells are the constitutively-expressed Hsp90β and the stress-inducible Hsp90α. Hsp90α and Hsp90β isoforms are the result of gene duplication approximately 500 million years ago (Gupta 1995). These duplication events suggest the necessity of alternate forms of Hsp90 to perform distinct cellular functions in eukaryotes (bacteria express only one Hsp90-like protein, htpG). Despite sharing 86% amino acid sequence identity, Hsp90α and Hsp90β have unique functions during embryologic development and in the cell. Given that Hsp90α upregulation has been correlated with several diseases (see below), it is important to understand its unique functions and regulation.
Despite high amino acid identity, at the nucleotide level Hsp90α and Hsp90β differ more dramatically. This is especially true in the 3’ and 5’ non-coding regions (Rebbe et al. 1989; Zhang et al. 1999). The differences in these non-coding regions allow for specific regulation of Hsp90α expression by different transcription factors depending on cellular conditions. Further, slight variation in amino acid sequence of the two proteins allows for unique Hsp90α function. For example, Hsp90α is required for peptide-loaded MHC1 complex processing in spermatocyte maturation and hERG ion channel maturation (Kunisawa and Shastri 2006; Grad et al. 2010; Peterson et al. 2012). Hsp90α and Hsp90β also have opposing effects on the biogenesis of KCNQ4 channels and on endothelial nitric oxide (eNOS) activity (Cortes-Conzalez et al. 2010). Hsp90 contains three conserved domains: the amino-terminal ATP-binding domain, the middle domain, and carboxy-terminal dimerization domain. Each of these domains contain post-translational modification (PTM) sites. Within the Hsp90α protein, sequence specific post-translational modification sites are found (Taherian et al. 2008; Quanz et al. 2012; Muller et al. 2013). This allows for further regulation of Hsp90α-specific cellular functions.
Although Hsp90α has distinct functions from Hsp90β and its upregulation has been correlated with disease (see below), an Hsp90α-specific inhibitor has yet to be identified. Therefore, a better understanding of the unique functions of Hsp90α will be important for future isoform-specific drug design.
HSP90AA1 Gene Structure
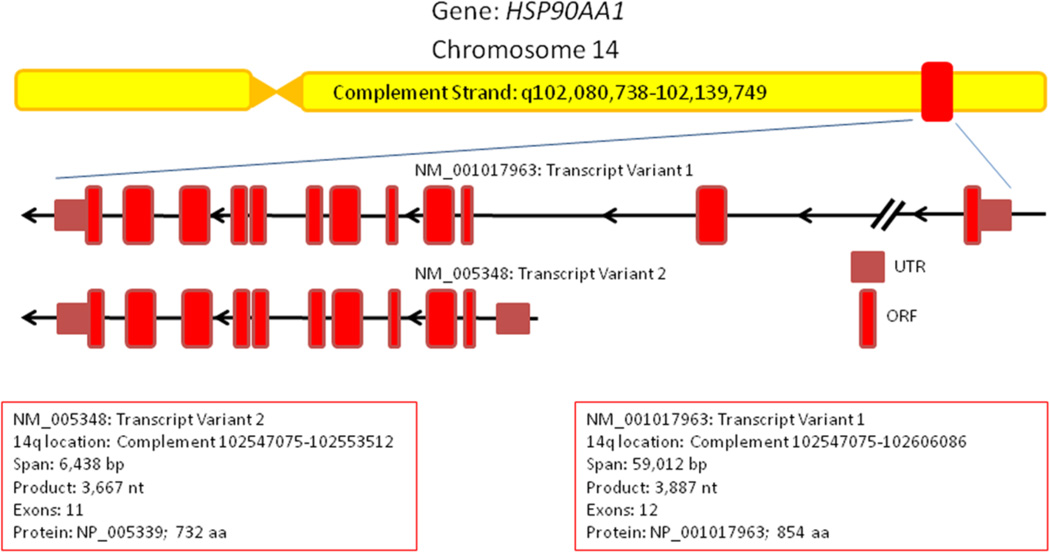
Human HSP90AA1 is encoded on the complement strand of Chromosome 14q32.33 and spans over 59 kbp. Several pseudogenes of HSP90AA1 exist throughout the human genome located on Chromosomes 3, 4, 11 and 14 (Ozawa et al. 1992; Chen et al. 2005). The HSP90AA1 gene encodes for two distinct mRNA transcripts initiated from separate transcription start sites (TSS). No mRNA splice variants of HSP90AA1 have presently been verified. Transcript variant 1 (TV1, NM_001017963.2) encodes the primate-specific 854 amino acid isoform 1 of Hsp90α (NP_001017963) from a 3,887 bp mRNA transcript containing 12 exons spanning 59, 012 bp (Tripathi and Obermann 2013). Transcript variant 1 is located directly adjacent to the WDR20 gene, which is transcribed from the opposite coding strand. Transcript variant 2 (TV2, NM_005348.3) encodes the well-studied 732 amino acid isoform 2 (NP_005339) from a 3,366 bp mRNA transcript containing 11 exons spanning 6,438 bp (Figure 1). DYNC1H1 encodes the gene product on the other side of HSP90AA1, which coincidentally has been found to interact with Hsp90α (Gano and Simon 2010). Hsp90α TV1 and TV2 are identical except for an additional 112 amino acids on the N-terminus of isoform 1 encoded by its first 2 exons. The function of the extended N-terminal domain on isoform 1 is currently not understood. This information was gathered from both NCBI Gene and the UCSC Genome Browser.
Figure 1.
Schematic of the human HSP90AA1 gene structure. Two transcript variants of Hsp90α protein are encoded on the complement strand of Chromosome 14. Information was obtained from NCBI Gene. Untranslated Region (UTR); ORF, Open Reading Frame (ORF).
Hsp90α expression
Despite sharing similar amino acid sequence, Hsp90α expression is regulated in a different manner than Hsp90β . Hsp90α is the stress-inducible isoform while Hsp90β is expressed constitutively. Several heat shock elements (HSE’s) are located upstream of Hsp90α allowing for its inducible expression upon binding of heat shock factor 1 (HSF1, the master transcriptional regulator of the heat shock response). Specifically, occupancy of the HSE located at −96/−60 enhances the expression of Hsp90α, while co-occupancy of the HSE complex at −1031/−1022 is required for a heat shock induction (Zhang et al. 1999; Sreedhar et al. 2004).
In the presence of cellular stress, Hsp90α levels increase. Hsp90α is also expressed at differing levels in a tissue-specific manner and is up regulated in several cancers. In leukemia Hsp90α levels are upregulated and correlated with disease prognosis (Yufu et al. 1992; Tian et al. 2014). Increased levels of Hsp90α also suggest poor prognosis in breast and pancreatic cancers (Jameel et al. 1992; Gress et al. 1994). In human T-cells, HSP90AA1 expression is increased by the cytokines IL-2, IL-4 and IL-13 (Metz et al. 1996). Furthermore, Hsp90α levels are elevated in individuals suffering from chronic obstructive pulmonary disease (COPD) (Hacker et al. 2009). Thus, in some disease instances, elevated expression of Hsp90α potentially could serve as a prognostic indicator.
HSP90AA1 Promoter
Transcription of the HSP90AA1 gene is currently understood to be induced by stress through binding of the master transcription factor (TF) HSF1 to the HSP90AA1 promoter (Ciocca et al. 2013). However, several focused studies of the HSP90AA1 promoter along with extensive global analysis of the human genome indicate that various other transcription complexes regulate HSP90AA1 gene expression. Mammalian HSP90AA1 along with HSP90AB1 gene expression was first characterized in transformed mouse cells where it was shown that HSP90AB1 is constitutively expressed 2.5-fold higher than HSP90AA1 under normal conditions. However upon heat shock, HSP90AA1 expression increased 7.0-fold while HSP90AB1 increases only 4.5-fold (Ullrich et al. 1989). Detailed analysis of the HSP90AA1 promoter shows that there are 2 heat shock elements (HSE) within 1200 bp of the transcription start site (Zhang et al. 1999, Sreedhar et al. 2004). The distal HSE is required for heat shock induction and the proximal HSE functions as a permissive enhancer. This model is supported by ChIP-SEQ analysis of cells under normal conditions where HSF1 is found bound to the proximal HSE and not detected at the distal HSE. The proto-oncogene MYC is also found to induce HSP90AA1 gene expression and binds proximally to the TSS as verified by ChIP-SEQ. Depletion of Hsp90α expression indicates that HSP90AA1 is required for MYC-driven transformation (Teng et al. 2004). In breast cancer cells the growth hormone prolactin induces HSP90AA1 expression through STAT5 (Perotti et al. 2008). NF-kB or RELA also induces HSP90AA1 expression possibly explaining the pro-survival ability of NF-kB-driven transcription (Ammirante et al. 2008). Conversely, STAT1, the proto-tumor suppressor, is found to inhibit stress induced expression of HSP90AA1 (Chen et al. 2007). In addition to these findings, ChIP-SEQ analysis of the human genome indicates that at least 85 unique transcription factors bind to the promoter regions that drive the expression of both HSP90AA1 transcript variants (Hudson and Snyder 2006; Euskirchen et al. 2007), thus further indicating that HSP90AA1 gene expression may be highly regulated and complex.
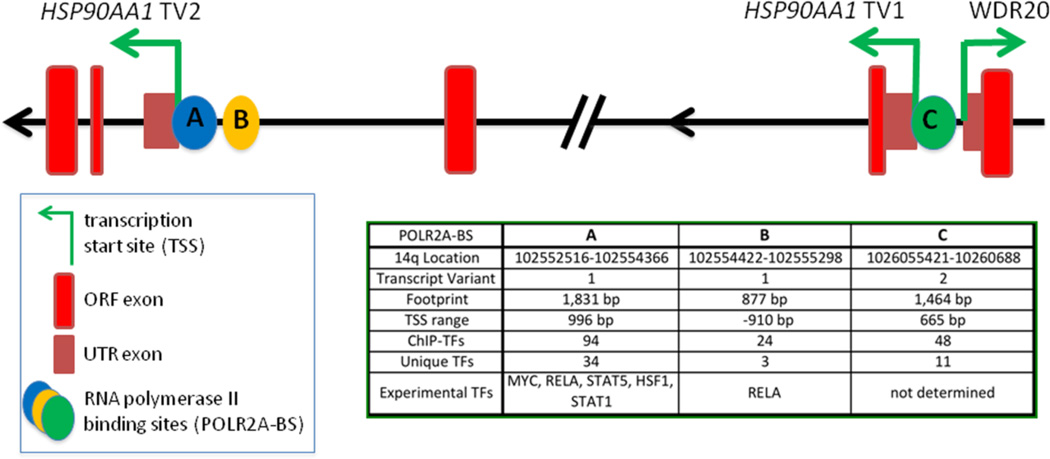
Examination of the HSP90AA1 gene structure shows that there are 3 separate RNA polymerase II (POLR2A) binding sites (Figure 2). Two of these sites (A and B) are associated with TV2 expression while one site (C) is associated with TV1 expression. Site A overlaps the TV2, TSS by 996 bp indicating that it is the primary site for transcription initiation. Site B is separated from site A by 76 bp and lies 910 bp away from the TSS. Site B also has a smaller footprint with fewer transcription factors associated with it, and therefore might serve as a secondary site for recruiting and pausing POLR2A. Site C overlaps the TSS of transcript variant 1 by 665 bp and does not cover the adjacent TSS of WDR20 which is encoded on the opposite DNA strand. This gene structure is consistent with the likelihood that HSP90AA1 expression may be regulated by multiple factors.
Figure 2.
Depiction of the HSP90AA1 transcription start sites. RNA polymerase II binds at three different sites on the HSP90AA1 gene; two sites associated with Transcript Variant 2 and one site associated with Transcript Variant 1. Information was obtained from UCSC Genome Browser and Encode.
The UCSC Genome Browser has gathered 161 ChIP-SEQ transcription factor profiles across the entire human genome providing a powerful resource for characterizing gene structure and regulation (Hudson and Snyder 2006; Euskirchen et al. 2007; Wang et al. 2013). Examination of the HSP90AA1 promoter indicates that 85 different TFs in total bind to one of the three RNA polymerase II (POLR2A) binding site footprints. Each site is bound by a unique set of TFs with site A being bound by 34 unique TFs and sites B and C being bound by 3 and 11 unique TFs, respectively (Figure 2). The unique TF profiles for each POLR2A binding sites suggest that specific biological processes are associated with the initiation of transcription of HSP90AA1 from each of these POLR2A sites. Using gene ontology, the bioinformatics initiative whose goal is to represent all gene and gene product attributes across all species, the biological processes, molecular functions or cellular components which a set of genes are most strongly associated with in a given context can be predicted. Indeed, gene ontology analysis of the complete TF set associated with each POLR2A site indicates that specific biological processes are related to each POLR2A site (Figure 3; Supplemental Table 1). Site A includes 180 unique gene ontology terms with the highest scoring biological processes terms related to regulation of cell death, apoptosis, viral processing and drug responses. Site B includes 23 unique terms with the highest scoring terms related to late viral transcription, regulation of transcription by glucose and carbon catabolites, nuclear organization and response to insulin. Overall, Site B seems to be influenced by energy supply and other metabolic processes along with viral transcription. Site C includes 31 unique terms with the highest scoring terms mainly related to regulation of cell differentiation and tissue development. This is of interest since site C controls the expression of TV1, which is not observed under most experimental conditions due to it possibly only being expressed in progenitor cells or highly specialized tissue. Together the comparison of the POLR2A binding sites along with all previous studies indicate that HSP90AA1 expression is associated with a variety of physiological functions and not solely with the heat shock response.
Figure 3.
Gene Ontology (GO) comparisons of the HSP90AA1 POLR2A binding site profiles: GO terms were computed/gathered by compiling all the transcription factors (TFs) that were found within the POLR2A binding site footprints. The lists of TFs were then searched on Amigo2 Term Enrichment Service powered by PANTHER with the biological process and H. Sapiens parameters selected (Carbon et al. 2009). The resulting list of GO terms was ranked by p-value. GO terms that scored p <0.05 were accepted as significant. The resulting lists were then compared using VENNY. The top 10 unique GO terms for each POLR2A sites are listed.
HSP90α interactome
Combined, Hsp90α and Hsp90β are predicted to interact with 10% of the eukaryotic proteome (Zhao et al. 2005). In humans this represents a network of roughly 2,000 interacting proteins. Presently over 725 interactions have been experimentally documented for both Hsp90α and Hsp90β (Echeverria et al. 2011). This connectivity allows HSP90 to function as a network hub linking diverse protein interaction networks. Within these networks HSP90 primarily specializes in maintaining and regulating proteins involved in signal transduction or information processing. These include transcription factors that initiate gene expression, kinases that transmit information by post-translationally modifying other proteins and E3-ligases that target proteins for degradation via the proteosome. Indeed a recent study utilizing the LUMIER method has shown that human Hsp90β interacts with 7% of all transcription factors, 60% of all kinases and 30% of all E3-ligases (Taipale et al. 2014). Other studies have shown that HSP90 interacts with various structural proteins, ribosomal components and metabolic enzymes (Falsone et al. 2005; Skarra et al. 2011). Hsp90 has also been found to interact with a large number of viral proteins including those from HIV and EBOLA (Smith et al. 2010; Low and Fassati 2014). This is not to mention the numerous co-chaperones that modulate and direct Hsp90 activity.
Few studies have focused on discerning the unique protein interactions between Hsp90α and Hsp90β (Gano and Simon 2010; Hartson and Matts 2012). Work done in Xenopus eggs and yeast has shown that Hsp90α and Hsp90β differ in co-chaperone and client interactions (Taherian et al. 2008; Gong et al. 2009). However, little is understood concerning the unique functions delegated to each paralog. Fortunately, the Picard lab has aggregated all available Hsp90 interaction data into the Hsp90Int.DB website (Escheverria et al. 2011). This resource allows for the comparison of Hsp90α and Hsp90β interacting networks through its curation of 292 combined PubMed articles on both human paralogs: 128 articles focused on Hsp90α, 63 articles focused on Hsp90β and 101 articles covered both paralogs.
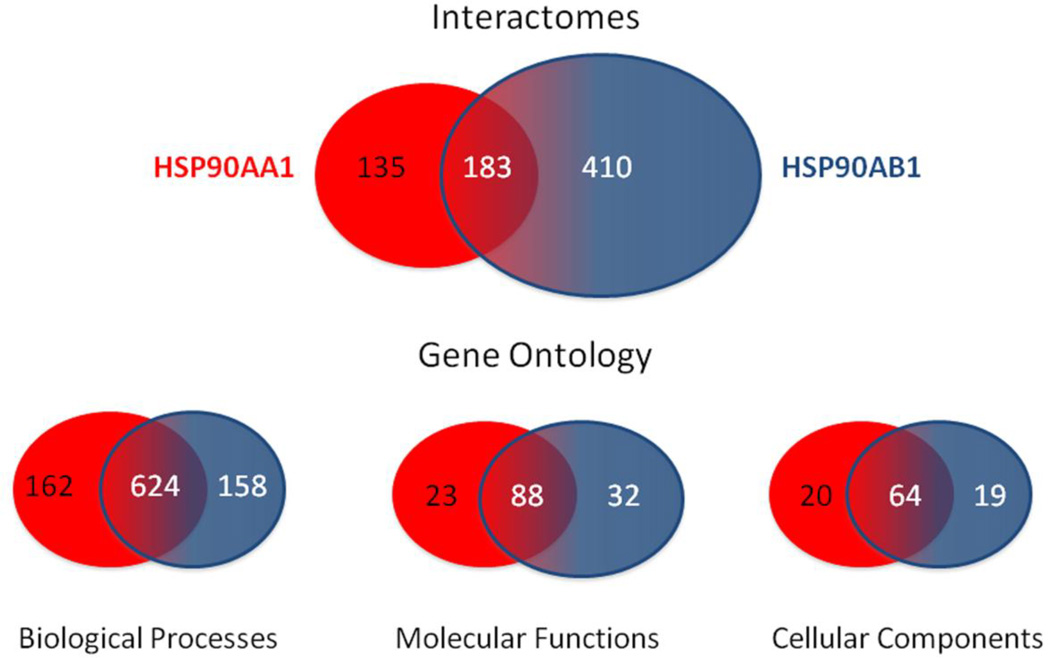
Including the massive LUMIER screen that compared the interactions of 630 proteins with only Hsp90β (Taipale et al. 2014), it can be observed that Hsp90α and Hsp90β interact with over 725 different proteins: Hsp90α exclusively interacting with 135 proteins, Hsp90β exclusively interacting with 410 proteins and both paralogs sharing 183 proteins (Figure 4). Collection of the gene ontology terms for the whole set of interacting proteins for Hsp90α or Hsp9β may allow one to predict the unique functions of each paralog. Comparing each list of gene ontology terms, it can be observed that Hsp90α is associated with 162 unique terms for biological processes while Hsp90β is associated with 158 unique terms; both paralogs share 624 terms for biological processes. Thus, although Hsp90β is currently found to interact with a larger number of proteins, Hsp90α is associated with a slightly larger number of biological processes. This is a particularly interesting observation in light of the fact Hsp90α is not required for mammalian viability while Hsp90β is essential (Voss et al. 2000; Grad et al. 2010; Kajiwara et al. 2012). This supports the idea that the biological processes associated with Hsp90β are more relevant to maintaining viability, while the processes associated with Hsp90α are more related to adaptation to stress or other specialized functions. It also demonstrates that Hsp90α and Hsp90β are redundant in contributing to a large number of biological functions. This is echoed in profiling the molecular function terms in which Hsp90α is associated with 23 unique terms, Hsp90β is associated with 32 unique terms and both paralogs share 88 molecular functions terms. This distribution further demonstrates that each paralog contributes to a unique set of basic molecular functions while overlapping in a large number of other functions. More telling is the profiling of cellular components terms where Hsp90α is associated with 20 unique terms, Hsp90β is associated with 19 unique terms and both paralogs share 64 cellular component terms. This provides information on the temporal-spatial distribution of each paralog within the cell and lends support to the role of Hsp90α as an extracellular agent (Eustace et al. 2004). Together this analysis demonstrates that HSP90α possesses unique biological functions discernible from Hsp90β. It also points to the need for a balanced analysis of the Hsp90 paralogs and a comprehensive screening of the Hsp90α interactome. All lists and terms were generated using Amigo2 and Venny (Supplemental Table 2) (Carbon et al. 2009; http://bioinfogp.cnb.csic.es/tools/venny/).
Figure 4.
Interactomes and Gene Ontology comparisons of HSP90AA1 and HSP90AB1. Venn diagram of the number of overlapping and unique protein interactors and gene ontology terms associated with each Hsp90 isoform is shown. Information was obtained from Hsp90Int.DB, Amigo2 and Venny. These values are expected to change over time as the interactomes of each Hsp90 isoforms are further defined.
Hsp90α post-translational modifications
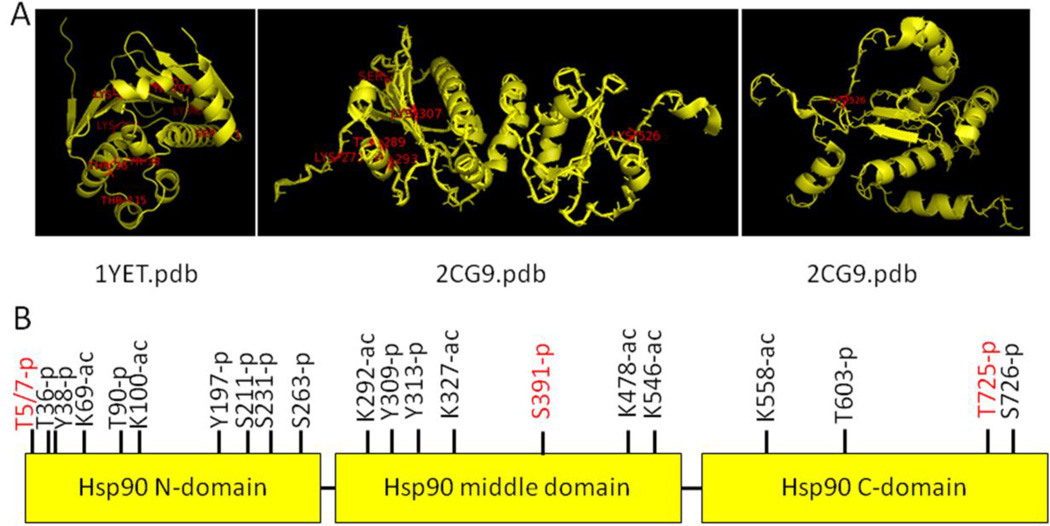
Post-translational modifications have a large impact on Hsp90 function and regulation (Mollapour and Neckers 2012). Phosphorylation, acetylation, S-nitrosylation, oxidation, methylation, sumoylation and ubiquitination are ways in which Hsp90 is modified in order to modulate its many functions. Furthermore, post-translational modification sites are found in all domains of Hsp90, consistent with a functional requirement in different regions of the protein. Twenty-eight sites within Hsp90α have been identified using site-specific mutation, while many more have been discovered using mass spectrometry (Figure 5). Many of these sites are conserved between Hsp90α and Hsp90β. However, there are a few distinctions between the two that allow for specific functions to be performed by Hsp90α.
Figure 5.
Hsp90 post-translational modification sites. A. Crystal structures of Hsp90 were obtained from RCSB protein databank; 1YET: human Hsp90α N-terminal domain bound to geldanamycin; 2CG9: yeast Hsp90 middle and C-terminal domains from full length structure bound to Sba1. Post-translational modification sites found on the PhosphoSite Plus website (http://www.phosphosite.org/homeAction.do;jsessionid=75252660BD94E928CEA59D49CC039052) are labeled in red using the PyMOL program. B. Display of Hsp90 post-translational sites. Unique phosphorylation sites for Hsp90α are in red.
Phosphorylation of Hsp90 has been shown to affect its binding to clients, cochaperones and nucleotide (Zhao et al. 2001; Mollapour et al. 2010; Mollapour et al. 2011; Xu et al. 2012; Quanz et al. 2012; Muller et al. 2013). Although Hsp90α and Hsp90β have several overlapping phosphorylation sites, some are specific to Hsp90α. These sites are highlighted in red in Figure 5B. An example of specific sites would be phosphorylation of Threonines 5 and 7 of Hsp90α. Phosphorylation of these sites happens in response to DNA damage and results in the co-localization of Hsp90 with DNA repair proteins at the sites of damage (Quanz et al. 2012 JBC; Solier et al. 2012). Specific phosphorylation of carboxy-terminal residues in Hsp70 and Hsp90α also act as a switch for co-chaperone interaction. The phosphorylation status of these residues allow Hop and Chip to recognize and bind or unbind Hsp70 and Hsp90α. Additionally, phosphorylation of the carboxy-terminus of both Hsp70 and Hsp90α is increased in primary cancers (Muller et al. 2013). This is not surprising as phosphorylation at this location increases Hop interaction with both proteins, which has been previously linked to faster tumor growth (Kubota et al. 2010; Ruckova et al. 2012).
Additional phosphorylation sites on Hsp90 are shared between the two paralogs, but have Hsp90α-specific consequences. For example, phosphorylation of Threonine 90 of Hsp90α increases its translocation to the cell surface and secretion (Lei et al. 2007; Wang et al. 2009). Secretion of Hsp90α has been suggested to be an essential prerequisite for proinvasiveness and tumor metastasis (Wang et al. 2009; McCready et al. 2010). Phosphorylation of Hsp90α at Serines 231 and 263 in the charged linker is found in leukemic cells. Phosphorylation of the same residues in Hsp90β did not occur in leukemic cells, but rather in untransformed cells (Lees-Miller et al. 1989; Kurokawa et al. 2008).
Hsp90α function is also regulated by acetylation. Treatment of cells with an HDAC inhibitor revealed seven acetylation sites that were hyperacetylated. The hyperacetylation of these sites increased Hsp90α secretion, leading to increased tumor invasiveness (Scroggins et al. 2007; Yang et al. 2008).
Hsp90α and cancer
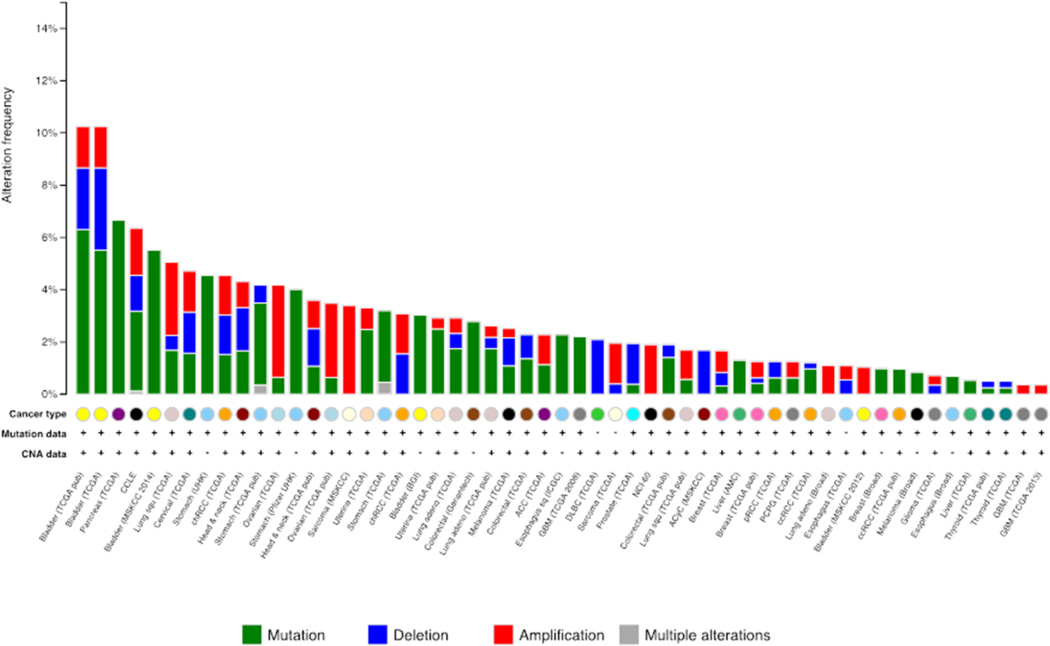
Over the last two decades HSP90 has emerged as an intriguing target in the war on cancer. HSP90 interacts and supports numerous proteins that promote oncogenesis, thus distinguishing Hsp90 as a cancer enabler as it is regarded as essential for malignant transformation and progression. Moreover, through their extensive interactomes, both paralogs are associated with each hallmark of cancer (Workman et al. 2007; Trepel et al. 2010). However, in contrast to changes in expression of Hsp90α protein (vide supra), the HSP90AA1 gene is not altered in a majority of tumors according to The Cancer Genome Atlas (TCGA). Currently bladder cancer is found to have the largest number of alterations at 10% of tumors sampled followed by pancreatic cancer at 6.7% (Gao et al. Science Signaling 2013; Cerami et al. Cancer Discovery 2012) (Figure 6). Additionally, whole genome sequencing across all tumor types and cancer cell lines reveals that there are presently 115 different mutations within the HSP90AA1 open reading frame (Supplemental Table 3). The effects of these mutations on HSP90α function, however, remain unknown. Remarkably, in a number of tumors the HSP90AA1 gene is homozygously deleted, suggesting that these tumors may have a reduced level of malignancy. This hypothesis is supported by a comparative genome-wide analysis of 206 gastric cancer patients that reported loss of HSP90AA1 is indeed associated with favorable outcomes after surgery alone (Buffart et al. 2012), and is in agreement with other studies reporting that the absence of Hsp90α in tumor biopsies may serve as a biomarker for positive clinical outcomes (Gallegos et al. 2008; Cheng et al. 2012).
Figure 6.
Alterations of the HSP90AA1 gene across cancer. Data accumulated from the genomic characterization and analysis of tumor types according to The Cancer Genome Atlas. Chart was generated by cBioPrtal for Cancer Genomics.
Biologically, Hsp90α differs from Hsp90β in that Hsp90α is presently understood to function as a secreted extracellular agent in wound healing and inflammation in addition to its intracellular roles. These two processes are often hijacked by cancer allowing for malignant cell motility, metastasis and extravasion (Eustace et al. 2004). Current research in prostate cancer indicates that extracellular Hsp90α transduces signals that promote the chronic inflammation of cancer-associated fibroblasts. This reprogramming of the extracellular milieu surrounding malignant adenocarcinoma cells is understood to stimulate prostate cancer progression. Extracellular HSP90α induces inflammation through activation of the NF-kB (RELA) and STAT3 transcription programs that include the pro-inflammatory cytokines IL-6 and IL-8 (Bohonowych et al. 2014). Coincidentally NF-kB also induces expression Hsp90α (Ammirante et al. 2008), thus providing a model where newly-expressed Hsp90α would also be secreted from the stimulated fibroblast thereby creating positive autocrine and paracrine feedback loops resulting in an inflammatory storm at the site of malignancy. This concept requires further attention as it may explain the correlation of increased levels of Hsp90α in the plasma of patients whose cancers are in advanced stages of malignancy (Wang et al. 2009).
Hsp90 Inhibitors
As mentioned above, Hsp90 is exploited by cancer cells to support activated oncoproteins, including many kinases and transcription factors. These clients are often mutated, amplified or translocated in cancer, and Hsp90 works to buffer these and other cellular stresses induced by malignant transformation (Workman Ann NY Acad Sci 2007; Trepel 2010 Nat Rev Cancer). Inhibition of Hsp90 leads to the degradation or instability of many of its client proteins (Blagg BS Med Res Rev. 2006.). Thus, Hsp90 has become an attractive target for cancer therapy.
As with all ATPases, ATP binding and hydrolysis is essential for the chaperoning function of Hsp90 in vivo. Most Hsp90 inhibitors interfere with this cycle at its early stages by replacing ATP in the N-domain ATP binding site, leading to the regulated ubiquitination and proteasome-mediated degradation of most client proteins (Blagg and Kerr 2006.) (Figure 7). As such, the N-domain nucleotide binding pocket remains most amenable to drug targeting (Whitesell et al. 1994; Prodromou et al. 1997; Stebbins et al. 1997; Grenert et al. 1997; Sharma 1998, Schulte et al. 1998; Banerji et al. 2005; Banerji et al. 2005; Chiosis and Tao IDrugs 2006; Eccles 2008; Kummar et al. 2010; Lancet et al. 2010; Pacey et al. 2011; Patel et al. 2011; Jhaveri et al. 2012; Jego et al. 2013; Taldone et al. 2014). To date, there are 23 active Hsp90 inhibitor oncology trials, and 13 HSP90 inhibitors are currently undergoing clinical evaluation in cancer patients; 10 of these inhibitors have entered the clinic in the past few years (Neckers and Trepel 2014).
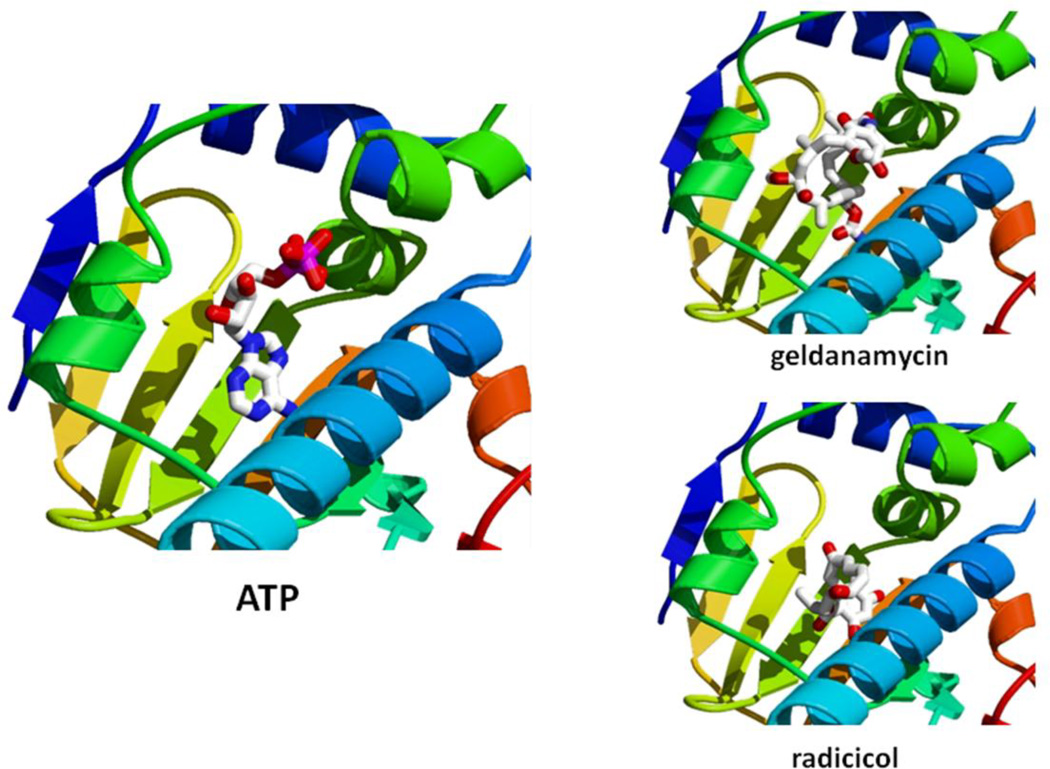
Figure 7.
Hsp90 N-terminal Inhibitors. Hsp90 N-terminal domain interaction with ATP is compared to interaction with Hsp90 inhibitors geldanamycin and radicicol using the PyMol program. 1YET.pdb (adapted from Roe et al. 1999).
While the N-terminal nucleotide-binding pocket of Hsp90 is most widely studied and thus targeted, recent studies have suggested that a second nucleotide-binding site is located in the Hsp90 C-terminus (Marcu J Biol Chem 2001; Garnier J Biol Chem 2002; Soti J. Biol Chem 2002; Soti Eur J Biochem 2003; review by Donnelly et al, Curr Med Chem 2008). Targeting of this region is reported to result in specifically reduced Hsp90-steroid hormone receptor interactions and has been shown to influence Hsp90 binding to the N-domain nucleotide pocket (Sreedhar Biochim Biophys Acta 2004; Rosenhagen Mol Endocrinol 2003). Although none of the C-terminal Hsp90 inhibitors have yet to enter the clinic, the use of both N- and C-terminal Hsp90 inhibitors in combination represents an exciting new strategy for chemotherapy.
Although many of the previously mentioned inhibitors share the same Hsp90 binding site (either N- or C-terminal), it has been shown that some of these drugs preferentially access distinct Hsp90 populations, which can be differentiated by the extent of their post-translational modification (Moulick Nat Chem Biol 2011; Beebe Oncotarget 2013). Though no published inhibitor has yet to distinguish between Hsp90α and Hsp90β, a recent study has shown that phosphorylation of a particular residue in the Hsp90 N-terminus can provide isoform specificity to inhibitor binding (Beebe Oncotarget 2013), thus providing an additional level of regulation for optimal Hsp90 targeting.
Future directions for HSP90α
In the era of precision medicine, continued investigation of the HSP90AA1 gene, in terms of function, regulation and drug targeting, should prove beneficial. Further characterization of the HSP90AA1 promoter could be useful in predicting the expression of Hsp90α in specific scenarios related to disease. Determining the effects of individual and combined sets of TFs on the transcription of HSP90AA1 may provide insights into how cancer utilizes Hsp90α expression to promote malignancy. An investigation into the expression profile of TV1 may also be useful in better understanding HSP90AA1 biology. Moreover, through the use of advanced proteomic and molecular biology techniques, a better understanding of the Hsp90α interactome across all tissues and disease states should provide valuable insights into how the stress response is hijacked in cancer and other disorders. This knowledge may also aid in designing drug combinations to combat cancer that anticipate and circumvent the development of drug resistance. Continued examination of the effects of PTMs on Hsp90α function will yield important information on chaperone regulation and drug specificity in normal and disease states. PTM profiling will also discern what protein populations of Hsp90α are preferentially targeted by drugs. Conversely, the induction of the stress response, including Hsp90α expression, may prove useful in addressing neurological pathologies related to protein aggregation and aging.
Supplementary Material
Research Highlights.
HSP90AA1 is not only transcriptionally regulated by HSF1 but also by other factors
HSP90AA1 and HSP90AB1 are differentially regulated at the transcriptional level
The protein products of HSP90AA1 and HSP90AB1 are homologs but with unique function
Hsp90α and Hsp90β are differentially regulated by posttranslational modification
HSP90AA1 transcript and Hsp90α protein levels may have prognostic value in disease
Acknowledgements
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM083924). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The authors also would like to acknowledge support from the Intramural Research Program of the National Cancer Institute (grants ZIA BC011032 and ZIA SC010074 to LN). The corresponding Gene Wiki entry for this review can be found here: http://en.wikipedia.org/wiki/Heat_shock_protein_90kDa_alpha_%28cytosolic%29,_member_A1
Abbreviations
- Hsp90
heat shock protein 90
- Grp94
glucose regulated protein 94
- TRAP1
tumor necrosis factor associated protein 1
- MHC1
major histocompatibility complex 1
- PTM
posttranslational modification
- TSS
transcription start site
- HSE
heat shock element
- TF
transcription factor
- HSF1
heat shock factor 1
- POLR2
RNA polymerase 2
- TV
transcript variant
- LUMIER
LUminescence-based Mammalian IntERactome
- TCGA
The Cancer Genome Atlas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ammirante M, Rosati A, Gentilella A, Festa M, Petrella A, Marzullo L, Pascale M, Belisario MA, Leone A, Turco MC. The activity of hsp90α promoter is regulated by NF-κB transcription factors. Oncogene. 2008;27:1175–1178. doi: 10.1038/sj.onc.1210716. [DOI] [PubMed] [Google Scholar]
- 2.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. Journal of clinical oncology. American Society of Clinical Oncology. 2005;23(18):4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 3.Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clinical cancer research. 2005;11(19 Pt 1):7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 4.Beebe K, Mollapour M, Scroggins B, Prodromou C, Xu W, Tokita M, Taldone T, Pullen L, Zierer BK, Lee MJ, Trepel J, Buchner J, Bolon D, Chiosis G, Neckers L. Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors. Oncotarget. 2013;4(7):1065–1074. doi: 10.18632/oncotarget.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagg BS, Kerr TD. Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006 May;26(3):310–338. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 6.Bohonowych JE, Hance MW, Nolan KD, Defee M, Parsons CH, Isaacs JS. Extracellular Hsp90 mediates an NF-kappaB dependent inflammatory stromal program: implications for the prostate tumor microenvironment. The Prostate. 2014;74(4):395–407. doi: 10.1002/pros.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffart TE, Carvalho B, van Grieken NC, van Wieringen WN, Tijssen M, Kranenbarg EM, et al. Losses of chromosome 5q and 14q are associated with favorable clinical outcome of patients with gastric cancer. The oncologist. 2012;17(5):653–662. doi: 10.1634/theoncologist.2010-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, et al. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25(2):288–289. doi: 10.1093/bioinformatics/btn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012 May;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family genes in the human genome: insights into their divergence and evolution. Genomics. 2005;86(6):627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen XS, Zhang Y, Wang JS, Li XY, Cheng XK, Zhang Y, Wu NH, Shen YF. Diverse effects of Stat1 on the regulation of hsp90alpha gene under heat shock. J. Cell Biochem. 2007;102(4):1059–1066. doi: 10.1002/jcb.21342. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Q, Chang JT, Geradts J, Neckers LM, Haystead T, Spector NL, Lyerly HK. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012;14(2):R62. doi: 10.1186/bcr3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiosis G, Tao H. Purine-scaffold Hsp90 inhibitors. IDrugs. 2006;9(11):778–782. [PubMed] [Google Scholar]
- 14.Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2013;87(1):19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortez-Gonzalez C, Barrera-Chimal J, Ibarra-Sanchez M, Gilbert M, Gamba G, Zentella A, Flores ME, Bobadilla NA. Opposite effect of Hsp90alpha, Hsp90beta on eNOS ability to produce nitric oxide or superoxide anion in human embryonic kidney cells. Cell Physiol. Biochem. 2010;26:657–668. doi: 10.1159/000322333. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly A, Blagg BS. Novobiocin additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr. Med. Chem. 2008;15(26):2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, et al. NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer research. 2008;68(8):2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 18.Echeverria PC, Bernthaler A, Dupuis P, Mayer B, Picard D. An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PloS one. 2011;6(10):e26044. doi: 10.1371/journal.pone.0026044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Euskirchen GM, Rozowsky JS, Wei CL, Lee WH, Zhang ZD, Hartman S, Emanuelsson O, Stolc V, Weissman S, Gerstein MB, Ruan Y, Snyder M. Mapping of Transcription Factor Binding Regions in Mammalian Cells by Chip: Comparison of Array- and Sequencing-Based Technologies. Genome Res. 2007;17(6):898–909. doi: 10.1101/gr.5583007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eustace BK, Sakurai T, Stewart JK, Yimlamai D, Unger C, Zehetmeier C, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nature cell biology. 2004;6(6):507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 21.Falsone SF, Gesslbauer B, Tirk F, Piccinini AM, Kungl AJ. A proteomic snapshot of the human heat shock protein 90 interactome. FEBS letters. 2005;579(28):6350–6354. doi: 10.1016/j.febslet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Finka A, Goloubinoff P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones. 2013;18(10):591–605. doi: 10.1007/s12192-013-0413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallegos RM, Floor K, Roepman P, Rodriguez JA, Meijer GA, Mooi WJ, Jassem E, Niklinski J, Muley T, van Zandwijk N, Smit EF, Beebe K, Neckers L, Yistra B, Giaccone G. Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One. 2008;3(3):e0001722. doi: 10.1371/journal.pone.0001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gano JJ, Simon JA. A proteomic investigation of ligand-dependent HSP90 complexes reveals CHORDC1 as a novel ADP-dependent HSP90-interacting protein. Molecular & cellular proteomics : MCP. 2010;9(2):255–270. doi: 10.1074/mcp.M900261-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013 Apr 2;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnier C, Lafitte D, Tsvetkov PO, Barbier P, Leclerc-Devin J, Millot JM, Briand C, Makarov AA, Catelli MG, Peyrot V. Binding of ATP to heat shock protein 90: evidence for an ATP-binding site in the C-terminal domain. J. Biol. Chem. 2002;277(14):12208–12214. doi: 10.1074/jbc.M111874200. [DOI] [PubMed] [Google Scholar]; Söti C, Rácz A, Csermely P. A Nucleotide-dependent molecular switch controls ATP binding at the C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a C-terminal binding pocket. J. Biol. Chem. 2002 Mar 1;277(9):7066–7075. doi: 10.1074/jbc.M105568200. [DOI] [PubMed] [Google Scholar]
- 27.Gong Y, Kakihara Y, Krogan N, Greenblatt J, Emili A, Zhang Z, et al. An atlas of chaperone-protein interactions in Saccharomyces cerevisiae: implications to protein folding pathways in the cell. Molecular systems biology. 2009;5:275. doi: 10.1038/msb.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grad I, Cederroth CR, Walicki J, Grey C, Barluenga S, Winssinger N, De Massy B, Nef S, Picard D. The molecular chaperon Hsp90alpha is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One. 2010;5:e15770. doi: 10.1371/journal.pone.0015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. Journal of Biol. Chem. 1997;272(38):23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 30.Gress TM, Muller-Pillasch F, Weber C, Lerch MM, Friess H, Buchler M, Beger HG, Adler G. Differential expression of heat shock proteins in pancreatic carcinoma. Cancer Res. 1994;54(2):547–551. [PubMed] [Google Scholar]
- 31.Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences, an examination of the relationship among animals, plants, fungi species. Mol. Biol. Evol. 1995;12(6):1062–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- 32.Hacker S, Lambers C, Hoetzenecker K, Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T, Zimmermann M, Tghavi S, Klepetko W, Ankersmit HJ. Elevated Hsp27, Hsp70 and Hsp90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55(1–2):31–40. [PubMed] [Google Scholar]
- 33.Hartson SD, Matts RL. Approaches for defining the Hsp90-dependent proteome. Biochimica et biophysica acta. 2012;1823(3):656–667. doi: 10.1016/j.bbamcr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. http://bioinfogp.cnb.csic.es/tools/venny/
- 35. http://genome.ucsc.edu/
- 36. http://www.ncbi.nlm.nih.gov/gene.
- 37.Hudson ME, Snyder M. High-throughput methods of regulatory element discovery. Biotechniques. 2006;41(6):673, 675, 677. doi: 10.2144/000112322. [DOI] [PubMed] [Google Scholar]
- 38.Jameel A, Skilton RA, Campbell TA, Chander SK, Coombes RC, Lugmani YA. Clinical and biological significance of HSP89 alpha in human breast cancer. Int J Cancer. 1992;50(3):409–415. doi: 10.1002/ijc.2910500315. [DOI] [PubMed] [Google Scholar]
- 39.Jego G, Hazoume A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer letters. 2013;332(2):275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90(Hsp90) inhibitors in cancers. Biochem. Biophys. Acta. 2012;1823(3):742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajiwara C, Kondo S, Uda S, Dai L, Ichiyanagi T, Chiba T, et al. Spermatogenesis arrest caused by conditional deletion of Hsp90alpha in adult mice. Biology open. 2012;1(10):977–982. doi: 10.1242/bio.2012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubota H, Yamamoto S, Itoh E, Abe Y, Nakamura A, Izumi Y, Okada H, Iida M, Nanjo H, Itoh H, Yamamoto Y. Increased expression of co-chaperone HOP and HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress Chaperones. 2010;15(6):1003–1011. doi: 10.1007/s12192-010-0211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, et al. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. European journal of cancer. 2010;46(2):340–347. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Kurokawa M, Zhao C, Reya T, Kornbluth S. Inhibition of apoptosome formation by suppression of Hsp90beta phosphorylation in tyrosine-induced leukemias. Mol Cell Biol. 2008;28:5494–5506. doi: 10.1128/MCB.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24(4):699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 47.Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989;264:17275–17280. [PubMed] [Google Scholar]
- 48.Lei H, Venkatakrishnan A, Yu S, Kazluauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 49.Low JS, Fassati A. Hsp90: a chaperone for HIV-1. Parasitology. 2014;141(9):1192–1202. doi: 10.1017/S0031182014000298. [DOI] [PubMed] [Google Scholar]
- 50.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275(47):37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 51.McCready J, Sims JD, Chan D, Jay D. Secretion of extracellular hsp90α via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metz K, Ezernieks J, Sebald W, Duschl A. Interleukin-4 upregulates the heat shock protein Hsp90alpha and enhances transcription of a reporter gene coupled to a single heat shock element. FEBS lett. 1996;385(1–2):25–28. doi: 10.1016/0014-5793(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 53.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochimica et biophysica acta. 2012;1823(3):648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mollapour M, Tsutsumi S, Neckers L. Hsp90 phosphorylation, Wee1 and the cell cycle. Cell Cycle. 2010;9(12):2310–2316. doi: 10.4161/cc.9.12.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mollapour M, Tsutsumi S, Truman AW, Xu W, Vaughan CK, Beebe K, Konstantinova A, Vourganti S, Panaretou B, Piper PW, Trepel JB, Prodromou C, Pearl LH, Neckers L. Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell. 2011;41(6):627–681. doi: 10.1016/j.molcel.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, Vu LP, Zhao X, Zatorska D, Taldone T, Smith-Jones P, Alpaugh M, Gross SS, Pillarsetty N, Ku T, Lewis JS, Larson SM, Levine R, Erdjument-Bromage H, Guzman ML, Nimer SD, Melnick A, Neckers L, Chiosis G. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7(11):818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Land DP, Vojtesek B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 58.Neckers L, Trepel JB. Stressing the development of small molecules targeting HSP90. Clinical cancer research. 2014;20(2):275–277. doi: 10.1158/1078-0432.CCR-13-2571. [DOI] [PubMed] [Google Scholar]
- 59.Ozawa K, Murakami Y, Eki T, Soeda E, Yokoyama K. Mapping of the gene family for the human heat-shock protein 90 alpha to chromosomes 1, 4, 11, and 14. Genomics. 1992;12(2):214–220. doi: 10.1016/0888-7543(92)90368-3. [DOI] [PubMed] [Google Scholar]
- 60.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clinical cancer research. 2011;17(6):1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel HJ, Modi S, Chiosis G, Taldone T. Advances in the discovery and development of heat-shock protein 90 inhibitors for cancer treatment. Expert opinion on drug discovery. 2011;6(5):559–587. doi: 10.1517/17460441.2011.563296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perotti C, Liu R, Parusel CT, Bocher N, Schultz J, Bork P, Pfitzner E, Groner B, Shemanko CS. Heat shock protein-90-alpha, a prolactin-STAT5 target gene identified in breast cancer cells, is involved in apoptosis regulation. Breast Cancer Res. 2008;10(6):R94. doi: 10.1186/bcr2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson LB, Eskew JD, Vielhauer GA, Blagg BS. The hERG channel is dependent upon the Hsp90alpha isoform for maturation, trafficking. Mol. Pharm. 2012;9:1841–1846. doi: 10.1021/mp300138n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59(10):1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90(1):65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 66.Quanz M, Herbette A, Sayarath M, de Koning L, Dubois T, Sun JS, Dutreix M. Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci. J Biol Chem. 2012;287(12):8803–8815. doi: 10.1074/jbc.M111.320887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rebbe NG, Hickman WS, Ley TJ, Stafford DW, Hickman S. Nucleotide sequence and regulation of a human 90-kDa heat shock protein gene. J Biol Chem. 1989;264(25):15006–15011. [PubMed] [Google Scholar]
- 68.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 69.Rosenhagen MC, Soti C, Schmidt U, Wochnik GM, Hartl FU, Holsboer F, Young JC, Rein T. The heat shock protein 90-targeting drug cisplatin selectively inhibits steroid receptor activation. Mol. Endocrinol. 2003;17(10):1991–2001. doi: 10.1210/me.2003-0141. [DOI] [PubMed] [Google Scholar]
- 70.Ruckova E, Muller P, Nenutil R, Voitesek B. Alterations of the Hsp70/Hsp90 chaperone and the HOP/CHIP co-chaperone system in cancer. Cellular and Molecular Biology Letters. 2012;17(3):446–458. doi: 10.2478/s11658-012-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, et al. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell stress & chap. 1998;3(2):100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol cell. 2007;25(1):151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma SV, Agatsuma T, Nakano H. Targeting of the protein chaperone, HSP90, by the transformation suppressing agent, radicicol. Oncogene. 1998;16(20):2639–2645. doi: 10.1038/sj.onc.1201790. [DOI] [PubMed] [Google Scholar]
- 74.Skarra DV, Goudreault M, Choi H, Mullin M, Nesvizhskii AI, Gingras AC, et al. Label-free quantitative proteomics and SAINT analysis enable interactome mapping for the human Ser/Thr protein phosphatase 5. Proteomics. 2011;11(8):1508–1516. doi: 10.1002/pmic.201000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith DR, McCarthy S, Chrovian A, Olinger G, Stossel A, Geisbert TW, Hensley LE, Connor JH. Inhibition of heat-shock protein 90 reduces Ebola virus replication. Antiviral Res. 2010;87(2):187–194. doi: 10.1016/j.antiviral.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solier S, Kohn KW, Scroggins B, Xu W, Trepel J, Neckers L, et al. Heat shock protein 90alpha (HSP90alpha), a substrate chaperone of DNA-PK necessary for the apoptotic response. Proc. Nat.Acad. 2012;109(32):12866–12872. doi: 10.1073/pnas.1203617109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soti C, Vermes A, Haystead TA, Csermely P. Comparative analysis of the ATP-binding sites of Hsp90 by nucleotide affinity cleavage: a distinct nucleotide specificity of the C-terminal ATP-binding site. Eur. J. Biochem. 2003;270(11):2421–2428. doi: 10.1046/j.1432-1033.2003.03610.x. [DOI] [PubMed] [Google Scholar]
- 78.Sreedhar AS, Kalmar E, Csermely P, Shen Y. Hsp90 isoforms: functions, expression and clinical importance. FEBS Letters. 2004;1–3:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 79.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89(2):239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 80.Stravopodis DJ, Margaritis LH, Voutsinas GE. Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem. 2007;14:3122–3138. doi: 10.2174/092986707782793925. [DOI] [PubMed] [Google Scholar]
- 81.Taherian A, Krone PH, Ovsenek N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochem. Cell Biol. 2008;86(1):37–45. doi: 10.1139/o07-154. [DOI] [PubMed] [Google Scholar]
- 82.Taherian A, Krone PH, Ovsenek N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochemistry and cell biology. 2008;86(1):37–45. doi: 10.1139/o07-154. [DOI] [PubMed] [Google Scholar]
- 83.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nature Rev. Mol. Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 84.Taipale M, Tucker G, Peng J, Krykbaeva I, Lin ZY, Larsen B, et al. A quantitative chaperone interaction network reveals the architecture of cellular protein homeostasis pathways. Cell. 2014;158(2):434–448. doi: 10.1016/j.cell.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taldone T, Ochiana SO, Patel PD, Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends in pharmacological sciences. 2014;35(11):592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teng SC, Chen YY, Su YN, Chou PC, Chiang YC, Tseng SF, Wu KJ. Direct activation of HSP90A transcription by c-Myc contributes to c-Myc-induced transformation. J Biol Chem. 2004;279(15):14649–14655. doi: 10.1074/jbc.M308842200. [DOI] [PubMed] [Google Scholar]
- 87.Tian WL, He F, Fu X, Lin JT, Tang P, Huang YM, Guo R, Sun L. High expression of heat shock protein 90 alpha and its significance in human acute leukemia cells. Gene. 2014;542(2):122–128. doi: 10.1016/j.gene.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 88.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tripathi V, Obermann WM. A primate specific extra domain in the molecular chaperone Hsp90. PLoS One. 2013;8:e71856. doi: 10.1371/journal.pone.0071856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ullrich SJ, Moore SK, Appella E. Transcriptional and translational analysis of the murine 84- and 86-kDa heat shock proteins. J. Biol. Chem. 1989;264(12):6810–6816. [PubMed] [Google Scholar]
- 91.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127(1):1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 92.Wang J, Chen B, Wang Y, Wang N, Garbey M, Tran-Son-Tay R, Berceli SA, Wu R. Reconstructing regulatory networks from dynamic plasticity of gene expression by mutual information. Nucleic Acids Res. 2013;41(8):e97. doi: 10.1093/nar/gkt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Song X, Zhou W, Fu Y, Shi H, Liang Y, Tong M, Chang G, Luo Y. The regulatory mechanism of Hsp90 {alpha} secretion and its function in tumor malignancy. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 95.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. PNAS. 1994;91(18):8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction, tumor stress. Ann. N. Y. Acad .Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 97.Xu W, Mollapour M, Prodromou C, Wang S, Scroggins BT, Palchick Z, Beebe K, Siderius M, Lee MJ, Couvillon A, Trepel JB, Miyata Y, Matts R, Neckers L. Dynamic tyrosine phosphorylation modulates cycling of the Hsp90-P50(CDC37)-AHA1 chaperone machine. Mol Cell. 2012;47(3):434–443. doi: 10.1016/j.molcel.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y, Rehka R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K. Role of acetylation and extracellular location of heat shock protein 90α in tumor cell invasion. Cancer Research. 2008;68:4833. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yufu Y, Nishimura J, Nawata H. High constituitive expression of heat shock protein 90 alpha in human acute leukemia cells. Leuk Res. 1992;16(6–7):597–605. doi: 10.1016/0145-2126(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 100.Zhang S, Yu J, Cheng X, Ding L, Heng F, Wu N, Shen Y. Regulation of human Hsp90α gene expression. FEBS Letters. 1999;444:130–135. doi: 10.1016/s0014-5793(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 101.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120(5):715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y, Gilmore R, Leone G, Coffey MC, Weber B, Lee PWK. Hsp90 phosphorylation is linked to its chaperoning function assembly of the reovirus cell attachment protein. J Biol Chem. 2001;276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.