Abstract
Amblyopia is a neuro-developmental disorder of the visual cortex that arises from abnormal visual experience early in life. Amblyopia is clinically important because it is a major cause of vision loss in infants and young children. Amblyopia is also of basic interest because it reflects the neural impairment that occurs when normal visual development is disrupted. Amblyopia provides an ideal model for understanding when and how brain plasticity may be harnessed for recovery of function. Over the past two decades there has been a rekindling of interest in developing more effective methods for treating amblyopia, and for extending the treatment beyond the critical period, as exemplified by new clinical trials and new basic research studies. The focus of this review is on stereopsis and its potential for recovery. Impaired stereoscopic depth perception is the most common deficit associated with amblyopia under ordinary (binocular) viewing conditions (Webber & Wood, 2005). Our review of the extant literature suggests that this impairment may have a substantial impact on visuomotor tasks, difficulties in playing sports in children and locomoting safely in older adults. Furthermore, impaired stereopsis may also limit career options for amblyopes. Finally, stereopsis is more impacted in strabismic than in anisometropic amblyopia. Our review of the various approaches to treating amblyopia (patching, perceptual learning, videogames) suggests that there are several promising new approaches to recovering stereopsis in both anisometropic and strabismic amblyopes. However, recovery of stereoacuity may require more active treatment in strabismic than in anisometropic amblyopia. Individuals with strabismic amblyopia have a very low probability of improvement with monocular training; however they fare better with dichoptic training than with monocular training, and even better with direct stereo training.
Keywords: Amblyopia, Stereopsis, Perceptual learning, Videogames, Strabismus, Anisometropia
1. Introduction
Amblyopia is a neuro-developmental disorder of the visual cortex that arises from abnormal visual experience early in life, affecting between 1% and 4% of the general population (Ciuffreda, Levi, & Selenow, 1991; McKean-Cowdin et al., 2013; MEPEDS, 2009; Williams et al., 2008). Amblyopia usually has its onset within the first 3 years of life, and is thought to reflect alterations in the properties of neurons in early cortical areas (V1 and V2), possibly even as early as the LGN (Bi et al., 2011; Hess et al., 2009; Kiorpes, 2006; for a recent review of mechanisms see Levi, 2013). Accordingly, sensory deficits include a loss of visual acuity as well as of stereopsis, position acuity and contrast sensitivity, particularly at high spatial frequencies (Levi, 2006). Recent work suggests that the amblyopic deficit is then amplified downstream (Levi, 2006; Muckli et al., 2006). Thus amblyopes suffer not only from sensory deficits, but also from deficits not simply explained by low-level considerations (Farzin & Norcia, 2011; Kiorpes, 2006; Levi, 2006; Sharma, Levi, & Klein, 2000). These include second-order processing, contour integration, and temporal, spatial and/or capacity limits of attention. Thus, amblyopia leads to deficits in basic vision, and is also detrimental to many other aspects of visual cognition.
Amblyopia is clinically important because it is the most frequent cause of vision loss in infants and young children (Sachsenweger, 1968) aside from refractive error. Amblyopia is also of basic interest because it reflects the neural impairments that occur when normal visual development is disrupted, providing an ideal model for understanding when and how brain plasticity may be harnessed for recovery of function.
Brain plasticity is known to peak during a critical period in early childhood and to decrease thereafter (Bavelier et al., 2010; Movshon & Van Sluyters, 1981; Wiesel, 1982). While this highlights the effectiveness of early intervention to correct developmental deficits, the assumption that plasticity effectively ends after the critical period, has had a perverse effect in clinical practice. Amblyopic patients over the age of seven are often told that they will never be able to recover visual acuity or stereovision because their visual system is beyond the critical period for binocular vision. Young brains are certainly much more plastic than older ones, yet the last 15 years have shown that significant plasticity can still be induced beyond the critical period if appropriate input is provided (Baroncelli, Maffei, & Sale, 2011; Bavelier et al., 2010; Hess, Thompson, & Baker, 2014; Levi, 2012; Levi & Li, 2009; Levi & Polat, 1996; Morishita & Hensch, 2008; Wong, 2012).
Over the past two decades there has been a rekindling of interest in developing more effective methods for treating amblyopia, and for extending the treatment beyond the critical period, as exemplified by new clinical trials (Repka & Holmes, 2012) and new basic research studies (for recent reviews see Birch, 2013; Hess, Thompson, & Baker, 2014; Levi, 2012; Levi & Li, 2009). Concurrently, over the past decade, a number of studies have documented how rich forms of experience may trigger brain plasticity beyond the critical period (Bavelier et al., 2010; Hensch, 2005; Knudsen, 2004; Lillard & Erisir, 2011). This combination of factors is particularly exciting as treatment of amblyopia beyond the critical period appears within reach. Yet, it remains unknown which intervention is most efficient, which patients may benefit, and whether patients who have recovered have done so through similar mechanisms.
Much of the rehabilitation focus has been on restoring visual acuity, since reduced visual acuity is the sine qua non of amblyopia. However, many persons with amblyopia, particularly those with strabismus, also suffer from a large (sometimes complete) loss of stereoscopic depth perception. Recent reports of the dramatic effects of restored stereopsis have renewed interest in restoring stereopsis in affected adults. Susan Berry, a neuroscientist, recounts her recovery from strabismus and her amazement as she regained stereo-vision in her book, “Fixing My Gaze” (Barry, 2009). Vision scientist Bridgeman, who had been stereo deficient all his life also gives a vivid description of spontaneously recovering stereoscopic depth perception after viewing the 3D movie Hugo (Bridgeman, 2014) well into his sixth decade.
There is no shortage of reviews of various aspects of amblyopia over the last decade (Birch, 2013; Hess, Babu, et al., 2014; Hess, Thompson, et al., 2014; Kiorpes, 2006; Levi, 2006; Webber & Wood, 2005; Wong, 2012: Barrett, Bradley, & Candy, 2013; Grant & Moseley, 2011; Levi, 2012; Levi, 2013; Levi & Li, 2009; Repka & Holmes, 2012). The focus of this review is on stereopsis and its potential for recovery in persons with amblyopia, specifically, we address the following issues:
How is stereopsis compromised in amblyopia?
Why does stereopsis matter?
Can stereopsis be recovered in children and adults with amblyopia?
2. How is stereopsis compromised in amblyopia?
Under normal everyday viewing conditions, with both eyes open, the vision of persons with amblyopia is dominated by the strong eye. Thus, Webber and Wood (2005) suggest that the most common deficit associated with amblyopia under ordinary (binocular) viewing conditions is impaired stereoscopic depth perception. This is not surprising because it is well known that in normal vision, degrading the vision of one eye by blurring, filtering or reducing the contrast (Donzis et al., 1983; Legge & Gu, 1989; Menon, Bansal, & Prakash, 1997; Westheimer & McKee, 1980), results in reduced stereoacuity. Moreover, stereopsis is more degraded by monocular blur (or monocular contrast reduction) than by both eyes being blurred (Legge & Gu, 1989; Westheimer & McKee, 1980). Amblyopic patients, who we discuss here, face similarly degraded conditions.
2.1. Stereopsis and visual acuity
In individuals with amblyopia, the visual acuity of one eye is compromised; however, the relationship between the visual acuity of the amblyopic eye and stereoacuity is complex, as illustrated by Fig. 1, replotted from a large-scale study (Levi, McKee, & Movshon, 2011; McKee, Levi, & Movshon, 2003). Overall, worse visual acuity seems to correlate with worse stereo-acuity. However, upon close inspection this relationship seems mostly driven by anisometropic subjects (blue symbols). Indeed, over the entire range of amblyopic eye visual acuities, there are amblyopes who are essentially stereo-blind (red and gray symbols plotted along the top of the graph). These are mainly strabismic amblyopes, whether purely strabismic or mixed (strabismic and anisometropic). It is worth noting that constant strabismics with good acuity in both eyes are generally stereoblind.
Fig. 1.
Stereoacuity vs. visual acuity. The dotted lines show the upper and lower limits of the test. The data for strabismic anisometropes (gray squares) have been slightly displaced for clarity. Data replotted from Levi et al., 2011. The blue regression line suggests that worse visual acuity goes hand in hand with worse stereoacuity in anisometropic amblyopes; however this relationship does not hold in strabismic amblyopes or strabismic anisometropes. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Indeed, while the visual acuity of strabismic amblyopes (red diamonds) and strabismic-anisometropes (gray squares) varies over more than one log unit in Fig. 1, most were stereoblind, except for eight who showed stereoacuity of 2.33 arc min (140 arc s) or better. Clearly, strabismus, either with or without anisometropia, wreaks havoc on stereo acuity, independently of the visual acuity of the weak eye.
In contrast to strabismic amblyopes, many anisometropic amblyopes retain some stereopsis. McKee, Levi, and Movshon (2003) found that more than 50% of anisometropic amblyopes passed the Randot circles test, a standard test of stereopsis described below, compared with only about 10% of strabismic amblyopes. Holopigian, Blake and Greenwald (l986) found that anisometropic amblyopes have stereopsis at low, but not high, spatial frequencies, suggesting that while their stereoacuity is not as acute as normal, it is nevertheless functional. Among anisometropic subjects (blue symbols), there is a clear linear relationship between stereoacuity and the visual acuity of the weak eye, when plotted in log–log coordinates (blue dotted line in Fig. 1). Some inter-individual variance is clearly seen; for example, some anisometropic amblyopes have reduced visual acuity in the weak eye (up to 2.5 arc min – or 20/50), but excellent stereopsis (20 arc s), and some with stereo acuity better than 140 arc s have substantially reduced visual acuity (MAR up to 6 arc min or 20/120). Yet, the presence of a linear relationship between stereoacuity and visual acuity stands in contrast to the case of amblyopes with strabismus in which no such relationship is visible (red and gray symbols in Fig. 1).
2.2. Stereopsis and crowding
An important characteristic of amblyopia is crowding – the effect of nearby contours on object recognition (see Levi, 2008 for a review). Indeed, crowding limits object recognition in individuals with strabismic amblyopia (Levi, Song, & Pelli, 2007; Song, Levi, & Pelli, 2014). Interestingly, there appears to be a close linkage between high crowding and abnormal stereopsis. The amount of crowding distinguishes strabismic from purely anisometropic amblyopia, in nearly perfect agreement with lack of stereopsis (Fig. 2). Song, Levi, and Pelli (2014) found high agreement between the presence of strabismus, absence of stereopsis, and a high degree of crowding (as quantified by the spacing acuity (S/A) ratio). This linkage between crowding and stereoacuity has also been reported in amblyopic children (Greenwood et al., 2012), and we speculate that both the increased crowding and the reduced stereopsis may be related to when in the course of development, the impairment occurred (Levi & Carkeet, 1993). Whether crowding and stereopsis have some functional relationship in their underlying physiology is an interesting but unanswered question.
Fig. 2.
Stereoacuity and crowding, as quantified by the spacing:acuity ratio (S/A). Data points above the upper horizontal dotted line at stereoacuity = 6.67 min “fail” the test. The vertical dashed line, S/A = 1.84, divides amblyopic patients into two groups with large and small spacing:acuity ratio, or in other words high crowding or low crowding. High levels of crowding appear systematically associated with loss of stereo-acuity. Indeed, all but one amblyopic patient with a small S/A ratio pass the test, and all but one with a large S/A ratio fail. Data replotted from Song et al. (2014).
Since stereopsis in normal vision is degraded by monocular blur, it is not surprising that in anisometropic amblyopes, the loss of stereopsis depends on the amount of anisometropia. Fig. 3 shows the effect of unequal refractive error in the two eyes on stereopsis. Specifically, this figure shows the cumulative probability of stereoacuity being 40 arc s or worse (a factor of two worse than “normal” for this test). With 3 diopters of pure anisometropia (blue circles), 40% of both hyperopic and myopic anisometropes have reduced stereopsis. For hyperopic anisometropes, increased anisometropia results in an increasing proportion of the population with reduced stereopsis. In contrast, the data for the myopic anisometropes saturate, so that even with as much as 10 D of anisometropia, more than 50% of the myopic anisometropes retain stereoacuity of better than 40 arc s. In contrast, 99% of strabismic anisometropes (gray squares) fail to meet the 40 arc s criterion regardless of the amount of anisometropia. Thus, the loss of stereoacuity appears to be a general feature accompanying strabismus, while it occurs in anisometropia only when there is substantial unilateral defocus.
Fig. 3.
The cumulative probability of stereo-acuity being 40 arc s or worse. Cumulative probabilities for positive and negative values of vector blur anisometropia were computed separately, beginning at 0. Data replotted from Levi et al. (2011).
2.3. Stereopsis and suppression
For more than a century, suppression, or inhibition of the amblyopic eye by the strong eye has been implicated as a feature, and possibly a cause, of amblyopia (Worth & Chevasse) and loss of stereopsis, and there is strong clinical (von Noorden, 1996), psychophysical (Levi, Harwerth, & Smith, 1979; Levi, Harwerth, & Smith, 1980; Baker, Meese, & Hess, 2008; Harrad & Hess, 1992; Hess, 1991; Maehara, Thompson, Mansouri, Farivar, & Hess, 2011; Mansouri, Thompson, & Hess, 2008; Smith, Levi, Manny, Harwerth, & White, 1985; Ding, Klein & Levi, 2013; Ding & Levi, 2014; Hess, Babu, et al., 2014; Hess, Thompson, et al., 2014; Levi, 2013) and physiological (Bi et al., 2011; Sengpiel & Blakemore, 1996; Harrad, Sengpiel & Blakemore, 1996) evidence for this point of view. However, the role, occurrence and nature of suppression in amblyopia has been somewhat controversial (Barrett, Panesar, Scally, & Pacey, 2012; Holopigian, Blake, & Greenwald, 1986). Moreover, it has been suggested that suppression may take on different forms in anisometropia and strabismus – passive in anisometropia (where the amblyopic eye's image is blurred) but active in strabismus, in order to avoid diplopia.
Some of the disagreements over the role of suppression undoubtedly reflect the many different types of tests used to measure suppression. It is well known in the clinical literature that the artificial situations used to test suppression will often influence the very suppression that one is attempting to measure (von Noorden, 1996). Moreover, suppression may depend strongly on the nature of the targets and their similarity in the two eyes, target locations in the visual field, and other factors (Hess, 1991; Schor, 1977).
While there are several new approaches to quantifying suppression (e.g., Ding, Klein & Levi, 2013; Huang, Zhou, Lu, & Zhou, 2011; Levi, 2013; Mansouri et al., 2008), it seems important to develop a battery of psychophysical tests that might allow one to better quantify the range and diversity of suppression most relevant to every day functioning. To be of clinical relevance, these tests should be developed with constraints from the clinic in mind. We are encouraged by several recent efforts in this direction (Kwon et al., 2014; Li, Hess, et al., 2013; Li, Thompson, et al., 2013; Narasimhan, Harrison, & Giaschi, 2012).
3. Why does stereopsis matter?
We review here the functional consequences of the loss of stereopsis for individuals with amblyopia, drawing on the extant literature. Stereopsis seems to provide a unique sensation of depth in the world, as evidenced by normal observer's experience when viewing 3D displays or movies and by the remarkable changes in the qualia of depth perception reported by people who have recovered stereopsis. However, stereopsis is just one of many cues that the brain uses to infer 3D spatial relationships in visual scenes (Howard & Rogers, 2008). We first review the role of stereopsis in normal vision. For persons with normal binocular vision, binocular depth thresholds in natural scenes can be a factor of 10 better than monocular thresholds (McKee & Taylor, 2010). This difference in performance is due to stereopsis.
In observers with normal binocular vision, studies of visual cue integration consistently demonstrate that stereoscopic disparities contribute strongly to depth and shape perception when presented in conjunction with others depth cues (Hillis, Watt, Landy, & Banks, 2004; Johnston, Cumming, & Parker, 1993; Knill & Saunders, 2003; Lovell, Bloj, & Harris, 2012; Vuong, Domini, & Caudek, 2006). Despite these laboratory demonstrations, the functional importance of stereopsis remains much debated.
The most studied behavior in relation to stereopsis is probably driving. While early studies seemed to show some correlation between stereoscopic acuity and accident rates (Gresset & Meye, 1994; Humprhiss, 1987), more recent studies have found little correlation between stereopsis (or more generally, intact binocular vision) and driving performance (Bauer et. al. 2001; McKnight, Shinar, & Hilburn 1991; Oladehinde et. al. 2007). Thus it remains unclear just how important stereopsis is for safe driving. Interestingly, the emerging story is different for visually guided control of one's own body movements.
In humans with normal binocular vision, visually guided hand movements are significantly impaired when viewing is restricted to one eye (Fielder & Moseley, 1996; Melmoth & Grant, 2006; O'Connor et al., 2010; Servos, Goodale, & Jakobson, 1992). Movements take longer and are less accurate under monocular viewing. For example, movements took on average 100 ms longer, and subjects made about three times as many corrective movements under monocular conditions (Melmoth & Grant, 2006). These differences between monocular and binocular conditions were highly significant. Planning hand movements in depth is clearly more uncertain under monocular viewing, since visual information about the distance of a target from the observer is significantly degraded when stereoscopic information is removed.
Online visual feedback from the moving hand is also critical to motor control (Connolly & Goodale, 1999; Keele & Posner, 1968; Saunders & Knill, 2004; Saunders & Knill, 2005). A recent study showed that, even when monocular cues about the position and movement of the hand in depth are available, online corrections to hand movements in depth are significantly impaired under monocular viewing (Hu & Knill, 2011). Online corrections effectively disappeared during the fast phase of movements. Thus, deficits in both planning and online control likely contribute to impairments in motor control caused by the removal of binocular information. This is almost certainly due to the removal of stereoscopic information.
Walking performance is also significantly degraded, slower by about 10%, in normal subjects under monocular vs. binocular conditions (Hayhoe, Gillam, Chajka, & Vecellio 2009).
While the evidence relating binocular vision and stereo information (not necessarily stereoacuity) to visuomotor performance in normally sighted subjects is strong, the relationship between the impairment in visually guided hand movements and stereoacuity remains somewhat controversial. For example, Read et al. (2013) report that subjects (aged 7–82) performed manual dexterity tasks faster and more accurately with both eyes open than with one eye occluded, but the binocular advantage was not significantly correlated with their stereoacuity. Similarly, Murdoch, McGhee, and Glover (1991) reported that while individuals with no stereopsis have difficulty in performing a task with 3D clues, there are some individuals (post-fellowship ophthalmologists) who “have better manual dexterity than one might anticipate on the basis of stereoacuity testing alone”. Clearly there are substantial individual differences in manual dexterity performance, and it seems plausible that some individuals with poor stereopsis maybe able to compensate, while others, with excellent stereoacuity, may be “klutzes”. However, a recent large-scale study (O'Connor et al., 2010) showed that performance on motor skills pegboard and bead tasks was related to the subject's stereoacuity with those with normal stereoacuity performing best.
These results are mirrored in amblyopic patients. A number of studies have shown that amblyopes with impaired stereopsis show deficits in visually-guided hand movements similar to those caused by occluding vision of one eye in normally-sighted subjects. These deficits are thought to be due to impaired stereopsis, rather than to reduced visual acuity (Grant, Melmoth, Morgan, & Finlay, 2007; Melmoth, Finlay, Morgan, & Grant, 2009; Niechwiej-Szwedo et al., 2012; Suttle et al., 2011; Wong, 2012), fixation instability (Subramanian, Jost & Birch, 2013), or impaired vergence control (Melmoth, Storoni, Todd, Finlay, & Grant, 2007). We acknowledge that given the co-occurrence of strabismus, amblyopia and reduced stereopsis in many of the subjects in these studies, it is not possible to conclusively link these visuomotor deficits to reduced stereopsis per se. However, Hrisos et al. (2006) showed that reduced binocularity significantly predicted visuomotor deficits in their patients, whereas the depth of amblyopia did not. Moreover, Melmoth et al. (2009) showed similar visuomotor deficits in amblyopic patients whose visual acuity had been successfully corrected but stereoacuity remained impaired.
Consistent with the findings in normally sighted adults, poor stereoacuity in amblyopic patients seems to particularly impair visual feedback control of movements, leading to significantly longer and less accurate hand movements (Grant et al., 2007). The effects of losing stereopsis extend beyond hand movements. In addition, adaptations to changes in terrain (e.g., steps) are significantly less accurate without stereopsis both in normally sighted subjects viewing monocularly, and in subjects with amblyopia and reduced stereoacuity or absent stereopsis (Buckley et al., 2010; Helbostad, Vereijken, Hesseberg, & Sletvold, 2009).
While most of these studies focus on adults, the results suggest that impaired stereopsis may also negatively affect everyday activity in amblyopic children (Webber, Wood, Gole, & Brown, 2008a), as well as also limit career and job options. For example, surgeons, pilots or architects are all professions in which excellent stereoacuity is vital. In addition, while quantitative studies are needed, it has been suggested that expert athletes such as soccer players or tennis players rely heavily on their ability to properly estimate depth, as they predict the trajectory of the ball they just impacted. Finally, we should not forget the impact of amblyopia in young children and stigmatizing cost of being labeled as a clumsy kid with a patch (Webber, Wood, Gole, & Brown, 2008b). Interestingly, parents of strabismic children whose eyes have been surgically aligned sometimes report improvements in their child's visuomotor skills (von Noorden, 1996; Webber & Wood, 2005). Whether this is due to improved stereoacuity or to other factors remains unknown, and is an important topic for future studies.
4. Can stereopsis be recovered in children and adults with amblyopia?
In children with amblyopia, having some measurable stereopsis (vs. having none) significantly influences the outcome of treatment. Children with no measurable stereopsis have a more than twofold increase in risk for persistent amblyopia (Birch, 2013). Thus the status of stereopsis and whether it can be recovered appear critical when considering treatment.
It is important to note that stereopsis is not a single entity. First, there are thought to be distinct mechanisms for processing coarse vs. fine (or first vs. second-order) disparity signals (Wilcox & Allison, 2009; Tsutsui, Taira & Sakata, 2005) and for processing motion in depth (e.g., Rockers, Cormack & Huk, 2009). Second, stereoscopic functions vary along a number of important stimulus dimensions in the normally-sighted population, including eccentricity and spatial frequency (Siderov & Harwerth, 1995). Third, the multi-faceted nature of stereopsis is reflected by variability in patient etiology. Some patients who are categorized as stereo-blind using standard clinical tests evidence a variety of residual stereoscopic functions, including preserved sensitivity to second-order disparity signals (McColl, Ziegler & Hess, 200; Harris et al., 2000), preserved sensitivity to motion in depth in peripheral vision (Sireteanu, Fronius, & Singer, 1981). Recent work suggests that coarse stereopsis may be selectively spared in stereo deficient children with a history of amblyopia (Giaschi et al., 2013). In the sections below we focus primarily on stereopsis measured with standard, static, clinical tests. However, it would clearly be helpful to study stereopsis, and its recovery, using methods that tap the wide range of stereoscopic capacities.
4.1. Quantifying stereopsis
In order to address recovery, it is important to briefly discuss the methods used for measuring and quantifying stereopsis. As Westheimer (2013) notes, it is critical to make the distinction between “stereopsis and the ability to judge the three-dimensional disposition of objects in the visual field from other cues.” Unfortunately, many of the clinical tests fail to fully eliminate cues to such judgment. Consider for example, the widely used Randot “Circles” test (Stereo Optical Co., Chicago, IL), a test recommended by Simons (1981) for use with amblyopic patients. The Randot circles test, like most clinical stereopsis tests, is a test of the ability to distinguish differences in perceived distance of static targets – in this case circles – based on the relative disparities of the targets. Polarized targets and polarizing viewers provide separate images of the targets to the two eyes. The Randot Circles test presents contoured circles at 10 discrete disparity levels (from 20 to 400 arc s). The patient task is to choose which of the 3 circles at each disparity level appears closer than the other two – a simple 3 alternative forced choice. Note that this is not a cyclopean (Julesz, 1963) random dot stereogram. The circles are presented on a background of random dots, but are highly visible monocularly, which may be helpful for amblyopic patients with poor vision (Simons, 1981). Despite the random dot background, for large disparities, there are monocular cues, based on the image displacement that creates the retinal disparity. Indeed, Fawcett and Birch (2003) found that stereoacuity scores derived using the Randot Circles test showed good agreement with those measured with random-dot stereo-grams (with no monocular contours) when stereoacuity was 160 s of arc or better, but the Randot Circles test progressively overestimated stereoacuity for poorer random-dot stereoacuity scores. Whether this is due to subjects using the monocular cues or because stereograms with monocularly visible contours and cyclopean stereograms are processed by different neural mechanisms (e.g., coarse vs fine) is unclear.
There are other clinical tests (e.g., the Frisby test – see Simons, 1981 for a comparison of clinical tests); however, all of these have caveats. For example, all of the tests have a maximum disparity. Subjects who initially fail to detect the largest disparity are often labeled as “stereoblind”, or as having a stereo sensitivity (1/stereo threshold) equal to zero. For these subjects, quantifying the amount of improvement that may occur as a result of treatment is problematic, since the “zero” may not actually be zero! Clinical tests also have a smallest disparity, and thus may underestimate improvements in stereo acuity, since patients may improve beyond the test's finest disparity.
Some consider the appreciation of depth in genuine random-dot stereograms to be the gold standard for stereopsis because the stereograms contain no monocular information (Julesz, 1963). On the other hand, failure to achieve stereopsis with random dot stereo-grams may occur because the dots are small and dense, low in contrast, and static, making them less than optimal for a strabismic observer to detect depth (Ding & Levi, 2011; Simons, 1981; Westheimer, 2013). We note that McKee et al. (2003) reported a nearly perfect agreement between passing (or failing) the Randot circles test (described above) and a psychophysical measure of binocular function known as the binocular motion integration in a large group of amblyopic subjects.
Although measures of stereopsis and stereo-acuity are not without weaknesses, there is enough convergence to ask whether stereopsis when absent can be recovered or stereo-acuity when poor retrained.
4.2. Is it possible to recover stereopsis?
Several recent reports suggest that it may indeed be possible to recover stereopsis, even in adulthood. As noted in the Introduction, Susan Barry acquired stereoscopic vision following successful unconventional visual therapy begun at 48 years of age, resulting in a dramatic improvement of her perception of depth or the appreciation of “the space between” objects (Barry, 2009). Her new stereoscopic vision brought much more to her life than just depth perception: Objects became clearer, motion perception more veridical, her ability to move around the world more confident. Even more dramatic is the experience of Bruce Bridgeman who recovered stereopsis after watching the 3-D movie Hugo (Bridgeman, 2014). Whether this sort of immersive experience with very large disparities, along with many other depth cues will be an effective treatment for abnormal stereopsis, remains to be tested. Moreover, we note that neither Barry nor Bridgeman were amblyopic. However, these case studies, along with lab studies of perceptual learning resulting in the recovery of stereopsis (Ding, 2011 – discussed further below), call into question the notion that recovery of stereopsis can only occur during a “critical period” of development when the visual system is still plastic. This idea, dating back to the last century, led a number of practitioners to tell Susan and her mother that “nothing can be done” about her vision (and one to suggest that she might need a psychiatrist). Since binocular neurons are present in the visual cortex of primates within the first week of life, Barry surmises that some of the innate wiring of her binocular connections remained intact, and that vision therapy taught her to move her eyes into position for stereovision, “finally giving these neurons the information they were wired to receive”. Although this is one possible explanation, other plausible explanations exist, including compensatory mechanism and new wiring giving rise to the recovery of binocular information through different pathways.
Below we review studies of recovery of stereopsis in both children and adults with amblyopia. We present analyses of extant studies (below – see Table 1 and Fig. 5) where we consider: (i) reports of any improvement in stereopsis; (ii) reports of patients with no measurable stereopsis prior to treatment who have measurable stereopsis following treatment; and (iii) reports of at least a 2-level improvement on a test (e.g., from 200 arc s to 100 arc s on the Randot Circles) and a post training stereoacuity of 160 arc s or better. We regard the latter criterion as providing reasonable evidence for genuine recovery of stereoacuity. Our analysis is based on published studies in which we were able to identify data (stereo thresholds or stereo sensitivity) for individual subjects (adults and children) that could be identified as anisometropic or strabismic amblyopes. Note that in the following sections we have combined purely strabismic amblyopes and amblyopes with both anisometropia and strabismus, referring to them as “strabismic”.
Table 1.
The study of (left column) the mean age (in years), duration of training (hours) and the number of anisometropia, strabismic and all subjects out of the total number in that category who show: (i) any improvement in stereopsis; (ii) no measurable stereopsis prior to treatment and who have measurable stereopsis following treatment; and (iii) at least a 2-level improvement on a test (e.g., from 200 arc s to 100 arcs on the Randot circles) and a stereoacuity of 160 arcs or better.
| Study | N subjects | Age | Duration (hours) |
Any improvement |
Stereo post/not pre |
2 steps + 160″ or better |
Task | Stereo test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N aniso | N strab | ALL | N aniso | N strab | ALL | N aniso | N strab | ALL | ||||||
| Patching | ||||||||||||||
| Vedamurthy et al. (submitted for publication) | 16 | 39 | 40 | 3/7 | 4/9 | 7/16 | 0/7 | 0/9 | 0/16 | 3/7 | 2/9 | 5/16 | Supervised patching | RDC |
| Monocular training | ||||||||||||||
| Perceptual learning | ||||||||||||||
| Polat (2009) | 5 | 7 | 33 | 1/5 | 1/5 | 1/5 | 1/5 | Contrast sensitivity | RDS | |||||
| Li, Provost, and Levi (2007) | 2 | 11 | 100 | 1/1 | 1/1 | 2/2 | 1/1 | 1/1 | 2/2 | 1/1 | 1/1 | 2/2 | Position discrimination | RDC |
| Liu et al. (2011) NPT | 13 | 12 | 53 | 9/11 | 9/11 | 0/9 | 0/2 | 1/13 | 7/9 | 0/4 | 7/13 | Grating acuity | RDC | |
| Li and Levi (2004) | 7 | 37 | 20 | 1/7 | 1/7 | 1/2 | 0/5 | 1/7 | 1/2 | 0/5 | 1/7 | Position discrimination | RDC | |
| Liu et al. (2011) PT | 10 | 12 | 53 | 3/9 | 3/9 | 0/9 | 0/1 | 0/10 | 4/9 | 0/1 | 4/10 | Grating acuity | RDC | |
| Zhang et al. (2014) | 19 | 19-27 | >40 | 13/13 | 6/6 | 19/19 | 4/13 | 3/6 | 7/19 | 8/13 | 0/6 | 8/19 | Multiple TPE | RDC |
| Total % | 56 | 27/41 | 8/12 | 35/53 | 6/34 | 4/22 | 11/51 | 21/34 | 2/22 | 23/56 | ||||
| 66 | 67 | 66 | 18 | 18 | 22 | 62 | 9 | 41 | ||||||
| Action VGP (monocular) | ||||||||||||||
| Li et al. (2011) (MOH) | 20 | 30 | 40 | 5/5 | 0/15 | 5/20 | 1/1 | 0/2 | 1/3 | 3/5 | 0/14 | 3/19 | Action videogame | RDC |
| Monocular total % | 76 | 32/46 | 8/27 | 40/73 | 7/35 | 4/24 | 12/54 | 24/39 | 2/36 | 26/75 | ||||
| 70 | 30 | 55 | 20 | 17 | 22 | 62 | 6 | 35 | ||||||
| Dichoptic training | ||||||||||||||
| Perceptual learning | ||||||||||||||
| Knox et al. (2012) | 14 | 9 | 5 | 7/12 | 7/14 | 0/1 | 3/7 | 3/8 | 0/2 | 2/7 | 2/14 | Dichoptic tetris | TNO/Frisby | |
| Hess, Mansouri, and Thompson, (2010a, 2010b) | 9 | 40 | 48 | 8/9 | 8/9 | 0/0 | 6/9 | 6/9 | 0/0 | 6/9 | 6/9 | Dichoptic motion | RDC | |
| Li, Hess, et al. (2013); Li, Thompson, et al. (2013) | 9 | 22 | 10 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Dichoptic tetris | Randot preschool |
| Ooi et al. (2013) | 3 | 29 | 19 | - | 3/3 | 3/3 | - | - | 0 | 0/0 | 1/3 | 1/3 | Push-pull | RDS & CS |
| Hess, Babu, et al. (2014); Hess, Thompson, et al. (2014) | 14 | 33 | 10-30 | - | - | 11/14 | N/A | N/A | 2/5 | N/A | N/A | 5/14 | IPOD | RDC |
| Total % | 49 | 18/24 | 29/38 | 0/1 | 9/16 | 11/22 | 0/2 | 9/19 | 14/40 | |||||
| 75 | 76 | - | 56 | 50 | 0 | 47 | 35 | |||||||
| VGP (dichoptic) | ||||||||||||||
| To et al. (2011) | 9 | 36 | 10-20 | - | 5/9 | - | 3/6 | 3/6 | N/A | 3/9 | 3/9 | Dichoptic motion | RDC | |
| Cleary et al. (2007) | 12 | 8 | 4 | - | 4/12 | 4/12 | N/A | 0/1 | 0/1 | N/A | 3/12 | 3/12 | Dichoptic video and game | N/A |
| Vedamurthy et al. (submitted for publication) | 23 | 39 | 40 | 5/10 | 3/13 | 8/23 | 2/9 | 1/13 | 3/22 | 5/9 | 2/13 | 7/22 | Dichoptic videogame | RDC |
| Li et al. (2014) | 45 | 4-12 | 16-32 | N/A | N/A | 5/45 | N/A | N/A | N/A | N/A | N/A | N/A | ||
| Total % | 89 | 5/10 | 7/25 | 22/89 | 2/9 | 4/20 | 6/29 | 5/9 | 8/34 | 13/43 | ||||
| 50 | 28 | 25 | 22 | 20 | 21 | 56 | 24 | 30 | ||||||
| Dichoptic total % | 138 | 5/10 | 25/49 | 51/127 | 2/10 | 13/36 | 17/51 | 5/11 | 17/53 | 27/83 | ||||
| 50 | 51 | 40 | 20 | 36 | 33 | 45 | 32 | 33 | ||||||
| Stereo training | ||||||||||||||
| Ding and Levi (2011) | 5 | 25 | >40 | 1/1 | 4/4 | 5/5 | 0/0 | 4/4 | 4/4 | 1/1 | 4/4 | 5/5 | Stereo PL | RDC & custom |
| Vedamurthy et al. (2014b) | 11 | 35 | 2/2 | 4/9 | 6/11 | 0/0 | 1/3 | 1/3 | 2/2 | 4/9 | 6/11 | VR “bug squashing” | RDC | |
| Astle, McGraw, and Webb, (2011) | 2 | 27 | 2 | 2/2 | 0/0 | 2/2 | 0/0 | 0/0 | 2/2 | 2/2 | Monocular & stereo PL | Custom | ||
| Xie et al. (2014) | 11 | 21 | <10 | 10/10 | - | 11/11 | 0/0 | 0/0 | 7/10 | 8/11 | ||||
| Total % | 29 | 15/15 | 8/13 | 13/18 | 0/0 | 5/7 | 5/7 | 12/15 | 8/13 | 21/29 | ||||
| 100 | 62 | 83 | 71 | 71 | 80 | 62 | 72 | |||||||
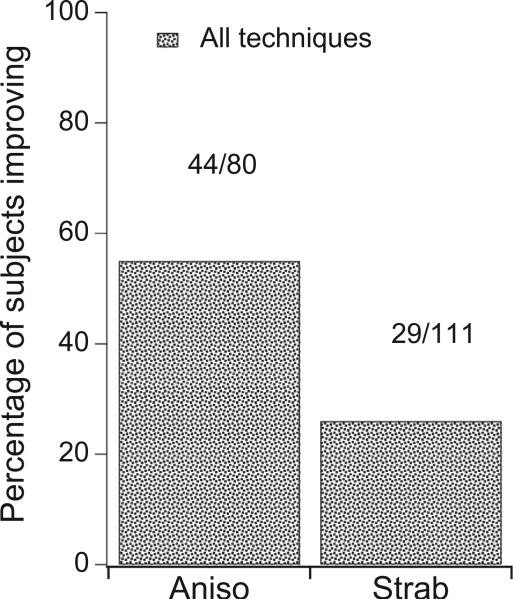
| Grand total % | 259 | 55/78 | 45/100 | 122/247 | 9/52 | 22/76 | 34/128 | 44/80 | 29/111 | 79/203 | ||||
| 71 | 45 | 49 | 17 | 29 | 27 | 55 | 26 | 39 | ||||||
Fig. 5.
The percentage of anisometropic and strabismic amblyopes achieving at least a two-level improvement in stereoacuity and a stereoacuity of 160” or better with all methods of treatment (based on the studies in Table 1). The numbers above each bar show the number of subjects achieving this improvement/the number of participants in that category.
4.3. Standard clinical treatment
The standard clinical treatment for amblyopia for the last two centuries consists of: (i) correcting any refractive error, and (ii) patching or “penalizing” the strong eye, in order to “force” the weak eye to do the work. This treatment is almost exclusively applied to children, adults being considered past their critical period for recovery of vision.
In young children, simply correcting the refractive error results in improved visual acuity and stereo acuity. For example, Richardson, Wright, Hrisos, Buck, and Clarke (2005), found that refractive correction alone resulted in an 30 arc s improvement in stereoacuity in non-strabismic amblyopic children between the ages of 3 and 4 years, 9 months. This improvement in stereopsis from refractive correction alone (often referred to as refractive adaptation) has been confirmed by other studies (Stewart et al., 2013). Importantly the improvement is not limited to anisometropic amblyopia, but also extends to strabismic amblyopia.
Patching or penalization also results in improved stereoacuity both in young children (less than 7 – Agervi et al., 2009; Lee & Isenberg, 2003; Steele et al. 2006; Wallace et al., 2011) and in older children (7–12 years of age – PEDIG, 2008). Specifically, for the older group, combining patching and penalization (using atropine to blur the strong eye at near) treatment resulted in an improvement of 2 or more levels on the Randot Preschool stereoacuity test in about 22% of the patients. Fig. 4 shows a small decrease in the percentage of pediatric patients with very poor stereo acuity (800 arc s or worse) – and a modest increase in the percentage of patients with good stereo acuity (100 arc s or better) after patching – 12% of anisometropic amblyopes and 5% of strabismics.
Fig. 4.
The effect of patching on stereopsis recovery in children. The percentage of patients with very poor or no stereopsis (800 arc s or >) decreases, and there is a modest increase in the percentage of patients with good stereo acuity (100 arc s or <). Based on data in PEDIG (2008).
Combining data from seven PEDIG (Pediatric Eye Disease Investigator group) clinical trials, Wallace et al. (2011) evaluated stereoacuity before and after treatment in a large sample (633) of anisometropic children amblyopes. As expected, even before treatment, amblyopes with better initial visual acuity and less anisometropia had better stereoacuity. Better post-treatment stereoacuity was associated with better base-line stereoacuity and better post-treatment visual acuity in their amblyopic eyes; however, among patients with normal or nearly normal visual acuity following treatment, stereoacuity remained impaired compared to children of the same age with normal vision.
Early onset strabismus is a major obstacle to the development of good stereoacuity. In an extensive review, Birch and Wang (2009) reported that only about 30% of infantile esotropes who underwent early surgery in the first year of life showed coarse stereopsis (100–3000 arc s) at age 5, and less than 0.5% of this cohort developed normal stereoacuity. Early botulinum toxin treatment resulted in a better outcome, with 50% showing coarse stereo, and 20% achieving stereo acuity of less than 60 arc s. Yet this still means that half of the patients fail to recover coarse stereopsis. How to best treat those individuals that do not respond to patching has been the focus of the experimental treatments considered below.
4.4. Experimental treatments
The results of the clinical treatment presented to date are in pediatric populations because adults have generally been considered to be beyond the critical period for recovery. In the following sections we review data based on experimental treatments in adults as well as children. These data suggest that while adults are more difficult to treat, there are solid reasons to believe that adult treatment can be effective. Note that here we only review those studies where we are able to assign individual data to amblyopic subjects that could be identified as either anisometropic or strabismic. Thus, our survey (Table 1 and Figs. 5 and 7) does not reflect the adult data of Li, Hess, et al., 2013; Li, Thompson, et al., 2013 where only average stereo sensitivity data are provided, or the children's data of Li et al. (2014) where only 5/45 (11%) of the subjects improved, but it is unclear whether these 5 were anisometropic or strabismic, or by how much they improved. Nonetheless, taken together, these studies reveal greater plasticity in stereopsis than previously thought. They also point to the greater advantage of dichoptic approaches when it comes to retraining stereo vision.
Fig. 7.
The percentage of anisometropic amblyopes (A) and strabismic amblyopes (B) showing improved stereopsis with various methods of treatment. The selected criterion for stereopsis improvement is achieving at least a two-level improvement in stereopsis and a stereoacuity of 16000 or better (data plotted based on the studies in Table 1).
Combined across all of the experimental studies reviewed below (see Table 1), we find that 55% of anisometropic amblyopes and 26% of strabismic amblyopes show substantial improvement in stereoacuity after intervention (Fig. 5). Below we look in more detail at the specific classes of treatment.
4.4.1. Monocular treatment
Traditionally, the aim of amblyopia treatment (both clinical and experimental) has been to improve first and foremost visual acuity of the amblyopic eye and check other visual functions.
4.4.1.1. Supervised patching
To date there are no randomized clinical trials of patching in adults with amblyopia; however, the study of Vedamurthy et al. (submitted for publication) included a control group who watched action movies with their amblyopic eye, while the strong eye was patched. Surprisingly, 43% (3/7) of their aniso-metric amblyopes, and 22% (2/9) of strabismic amblyopes met our criterion for improvements in stereopsis, that is at least a 2 level improvement and a stereoacuity of 160 arc s (considered to be clinically significant – Fawcett & Birch, 2003).
4.4.1.2. Monocular perceptual learning (PL)
It is well known that practicing challenging visual tasks can lead to dramatic and long-lasting improvements in performing them, i.e., practice makes perfect! In adults with normal vision, practice can improve performance on a variety of visual tasks (see Sagi, 2011 for a recent review). This learning can be quite specific (to the trained task, orientation, eye, etc., – but recent work shows that this apparently specific learning can be made to generalize using the appropriate training protocol (Xiao et al., 2008a; Xiao et al., 2008b; Zhang et al., 2010) even in amblyopic patients (Zhang, Cong, Klein, Levi, & Yu, 2014). Over the last two decades or so, there has been a great deal of interest in applying PL to patients with amblyopia, and to date there have been more than thirty published studies, involving more than 400 amblyopic subjects and a wide range of tasks.
Most of these studies have been conducted in adult amblyopes and involve monocular PL with the amblyopic eye while the strong eye is patched. The results of many of these studies have been reviewed elsewhere (Levi, 2012; Levi & Li, 2009), with the focus on the amount of learning in the trained task, and any transfer to the visual acuity of the amblyopic eye. On average, these studies show that amblyopic subjects improve by about a factor of two on the trained task, and that their visual acuity also improves by about a factor of ≈1.6, roughly two lines on a LogMAR acuity chart (Levi, 2012; Levi & Li, 2009). Unfortunately few of these studies report on transfer of learning to stereopsis. A few mention gains in stereopsis in passing (e.g., Li & Levi, 2004), but in many other published studies it not clear whether stereoacuity was measured before and after training in all subjects, or only in some. One recent exception is the study of Zhang et al., (2014) who performed extensive monocular PL in a group of 19 adult amblyopes. Their results, summarized in Fig. 6, show substantial improvements in stereoacuity in adults with both anisometropic and strabismic amblyopia, including several who were “stereoblind” (i.e., unable to see a disparity of 500 arc s, the largest disparity tested – data in the blue rectangle) initially.
Fig. 6.
The effect of extensive monocular PL on stereoacuity in adult amblyopes. Both anisometropic (blue) and strabismic (red) amblyopes, including several who were “stereoblind” (i.e., unable to see a disparity of 500 arc s, the largest disparity tested – data in the turquoise rectangle) initially show improved stereo sensitivity after PL (replotted from Zhang et al., 2014). These subjects were arbitrarily assigned a threshold value of 600 arc s., which was used in the calculation of % improvement. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Based on the data reported in the extant studies (Table 1), roughly 18% of both strabismic and anisometropic amblyopes with no measurable stereopsis initially, demonstrated measurable stereopsis following monocular PL. Importantly, more than 60% of anisometropic amblyopes met or surpassed our criterion for improvements in stereopsis by the end of training. In contrast only 9% of strabismic amblyopes achieved the same criterion, despite showing equivalent improvement in visual acuity as anisometropic amblyopes (Table 1 and Fig. 7A). Thus simply improving monocular visual processing may result in improved stereopsis in anisome-tropic, but much less so in strabismic amblyopes.
4.4.1.3. Monocular videogame play
PL clearly can result in improved visual capacities even in adults with amblyopia, however, there are two major drawbacks to PL for clinical use – specificity and tedium. PL is often highly specific to the stimulus, task, retinal location etc. However, a crucially important goal in rehabilitation is to have the learning generalize. While there are learning protocols that do aid in generalizing learning (e.g., double training – Xiao et al., 2008a; Xiao et al., 2008b; Zhang et al., 2010, 2014), they still require several thousands of trials. Moreover, standard PL is highly repetitive and considered tedious by many subjects. Thus, an alternative approach is to use highly engaging and motivating videogames.
In the first such study (Li, Ngo, Nguyen, & Levi, 2011), 20 adult amblyopes played an off the shelf videogame (Medal of Honor) with their amblyopic eye (AE), while the non-amblyopic eye (NAE) was patched. Both strabismic and anisometropic amblyopes showed improved visual acuity. Stereopsis, however, improved in the five anisometropic amblyopes, but in none of the 15 strabismics participants (Fig. 7B).
4.4.2. Dichoptic treatment
A more recent trend involves dichoptic treatment, in which different images are presented to the two eyes at the same time. The key aim of this approach is to try to eliminate or reduce interocular suppression.
4.4.2.1. Dichoptic perceptual learning (PL)
Hess and his colleagues have used several variants of a dichoptic motion coherence task in a series of studies in adults (Hess, Mansouri, & Thompson, 2010a; Hess, Mansouri, & Thompson, 2010b; Hess, Mansouri, & Thompson, 2011; Hess, Babu, et al., 2014; Hess, Thompson, et al., 2014; Li, Hess, et al., 2013; Li, Thompson, et al., 2013) and children (Birch, 2013; Knox et al., 2012; Li et al., 2014). The method essentially consists of presenting ‘signal’ dots, all moving coherently in the same direction to one eye, and ‘noise’ dots, all moving in random directions, to the other. For amblyopic subjects, threshold was determined either by the ratio of signal to noise dots required to determine the coherent motion direction (Hess, Mansouri, and Thompson, 2010a, 2010b), or by determining the ratio of AE to NAE contrast required to determine the coherent motion direction (Li, Hess, et al., 2013; Li, Thompson, et al., 2013). As is evident in Fig. 7C, none of the anisometropic amblyopes showed improved stereopsis; however, we note that the number of anisometropic amblyopes in the dichoptic PL category is very small (only 2, and one showed excellent stereoacuity at Pre-Test). However, dichoptic PL appears to be substantially more effective than monocular PL in improving stereopsis in strabismic amblyopes (compare Fig. 7A with C). More than 40% of strabismic amblyopes showed improved stereopsis, compared with less than 10% reported for monocular PL. For an extensive review of these studies see Hess, Babu, et al., 2014; Hess, Thompson, et al., 2014.
Ooi, Su, Natale, and He (2013) used a different approach – a sensory dominance “push–pull” task, to achieve the same goal (reducing suppression), in three adults with amblyopia. Their push–pull protocol is designed to “excite the weak eye, while completely inhibiting the strong eye's perception to recalibrate the interocular balance of excitatory and inhibitory interactions.” They report improved contrast thresholds and stereopsis in their three subjects; however only one (S2) met our 2-level/160 arc s criterion. That subject showed an impressive ≈fourfold improvement in stereo acuity.
4.4.2.2. Dichoptic videogame play
One of the earliest dichoptic videogame studies used the I-BiT system, which “invokes a three-dimensional image in those with normal single binocular vision by stimulating both eyes simultaneously”. Cleary, Moody, Buchanan, Stewart, and Dutton (2009) tested this system in 12 amblyopic children who did not comply or respond to occlusion. Specifically, in each of 8 sessions, the children viewed a 20-min video clip with the “detail” viewed by the amblyopic eye and the surrounding frame by the fellow eye, and spent 5 min playing an interactive videogame with the detail of the visual scene split between the two eyes. The authors report that the subjects perceived the images projected to the two eyes. Seven of the 12 children showed improvement in high contrast visual acuity and 8 showed improvement in low contrast visual acuity (3–18 months after the treatment). Most interestingly, 4 of the 12 children showed an improvement in stereoacuity (one from 400 to 40 arc s)!
A variant of the dichoptic PL method described above, is a Tetris-like dichoptic videogame, which requires players to arrange falling blocks into a pattern.1 Some of the blocks are seen by the amblyopic eye at high contrast and others to the strong eye at a lower contrast, tailored to each patient's level of suppression (Li et al., 2014; To et al., 2011;). This method has been applied to both children and adults with amblyopia. In their recent review, Hess, Babu, et al. (2014), Hess, Thompson, et al. (2014) report that averaged across all of the studies acuity improved by 2 lines, a result that mirrors the outcome of most interventions studies. Interestingly, one-third of patients showed improved stereopsis regardless of amblyopia type (anisometropic, 31%; strabismic, 37%).
A different approach, described in this issue (Bayliss, Vedamurthy, Bavelier, Nahum, & Levi, 2012; Bayliss, Vedamurthy, Nahum, Levi, & Bavelier, 2013; Vedamurthy, Bayliss, Knill, Bavelier, & Levi, 2012a; Vedamurthy et al., submitted for publication), uses a customized dichoptic action video game designed to reduce suppression, promote fusion and increase attention by the amblyopic eye under binocular conditions. The game, viewed in a stereoscope, presents identical images to the two eyes, with the luminance/contrast of the image seen by the strong eye decreased to perceptually match that of the weak (amblyopic) eye. This is an effective method for balancing the input to the two eyes (Baker et al., 2008; Ding & Levi, 2014; Zhou, Jia, Huang, & Hess, 2013), and frequent alignment and suppression checks ensured successful fusion. Following 40 h of videogame play, observers showed similar improvements in visual acuity to those seen with monocular videogame play, but stereoacuity improved in about half of study participants, with average overall improvements being significant for the videogame group (Table 1).
Combined across the dichoptic videogame studies, 56% of anisometropic amblyopes and 24% of strabismic amblyopes showed an improvement of at least 2-steps and stereoacuity better than 160 arc s. (Fig 7D).
4.4.3. Direct stereo training
A recent case report documented substantial improvements in two anisometropic adults who had undergone refractive adaptation and monocular PL followed by stereo training (Astle, McGraw & Web, 2011), and several laboratory studies support the notion that it is possible to improve stereopsis in adults with abnormal binocular visual experience through visual training or perceptual learning of stereopsis per se. For example, Nakatsuka et al. (2007) reported that adult monkeys reared with prisms had mild stereo deficiencies that improved through PL after 10,000– 20,000 trials.
Ding and Levi (2011) provided the first evidence for the recovery of stereopsis through perceptual learning in human adults long deprived of normal binocular vision. They used a novel training paradigm that combined monocular cues that were perfectly correlated with the disparity cues. Fol lowing PL (thousands of trials) with stereo scopic gratings, adults who were initially stereoblind or stereoanomalous showed substantial recovery of stereopsis. Importantly, these subjects reported that depth “popped out” in real life, and they were able to enjoy 3-D movies for the first time, similar to the experiences of Stereo Sue and Bruce Bridgeman. Their recovered stereopsis is based on perceiving depth by detecting binocular disparity, but has reduced resolution and precision. Similar improvements were recently reported in a group of anisometropic and ametropic amblyopes who were trained with anaglyphic textures with different disparities (Xi et al., 2014).
More recently, Vedamurthy et al. (in preparation) have developed a virtual-reality (VR) system for training stereopsis in amblyopes (both anisometropic and strabismic) and in strabismics (both with and without amblyopia) that embeds the training in a natural visuo-motor task whereby patients have to squash a small virtual bug with a hand-held cup. Some stimuli contained monocular texture cues to slant as well as stereoscopic cues, some contained only stereoscopic cues and some contained conflicting monocular and stereo cues, enabling Vedamurthy et al. to compute the relative weights given to stereo and monocular slant cues. Following training, 8 of 11 subjects gave increased weight to stereo cues relative to monocular cues, 6 showed significant improvement on separate stereopsis tests, 5 showed improved visual acuity, and all 11 showed reduced suppression.
Combined across studies, the gains for direct stereo training (Fig. 7E) appear to be greater than the gains obtained through either monocular or dichoptic (2D) training, particularly for strabismic amblyopes. This can be more clearly seen in Fig. 8, which compares monocular training, dichoptic training (combining PL and VGP studies) and direct stereo training. This figure shows clearly that patients with strabismic amblyopia have a very low probability of improvement with monocular training; however they fare better with dichoptic training than with monocular training, and even better with direct stereo training.
Fig. 8.
Experimental training summary. The percentage of anisometropic (left 3 bars) and strabismic (right three bars) achieving the criterion improvement in stereopsis based on monocular training (combining PL & VGP – black bars), dichoptic training (combining PL & VGP – gray bars) and direct stereopsis training (blue bars). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.4.4. Mechanisms of improvement
A discussion of the mechanisms of improvement is beyond the scope of this review. A good deal of evidence, both physiological and behavioral, suggests that changing the balance of neural excitation and inhibition by either reducing inhibition or boosting excitation may be crucial in recovery of visual functions (Baroncelli et al., 2011; Bavelier et al., 2010; Morishita & Hensch, 2008). All approaches to retraining the amblyopic eye, whether monocular videogame play (Li et al., 2011), perceptual learning (Levi & Li, 2009), the “push–pull” method (Ooi et al., 2013) or dichoptic training (Hess, Babu, et al., 2014; Hess, Thompson, et al., 2014; Knox et al., 2012; Vedamurthy et al., submitted for publication) seek to achieve this altered balance by increasing signal, reducing noise, or modulating attention in the amblyopic eye.
One possible explanation for the greater success of dichoptic and stereo training in the recovery of stereopsis, particularly in strabismic patients, is that these techniques also train binocular fusion, by placing the images on corresponding areas in the two eyes. Indeed, Ding and Levi (2011) reported on one subject who recovered stereopsis following extensive fusion training.
Finally, we note that training stereopsis directly (Astle, McGraw & Web, 2011; Ding, 2011; Vedamurthy et al., in preparation) may provide a useful scaffold for integrating information from the two eyes, and may therefore present a more efficient way to restore stereovision in amblyopic patients, while simultaneously fostering improved visual acuity.
5. Summary, caveats, and conclusions
Figs. 5, 7 and 8 and Table 1 summarize the reported improvements in stereopsis for each of the approaches discussed above, based on the extant studies for which we had access to individual subject stereo data – a total of more than two hundred subjects. Across all methods, more than one fourth of amblyopes with no measurable stereopsis prior to training showed at least some measurable stereopsis after training (Table 1), and more than 50% of anisometropic and about 26% of strabismic amblyopes showed at least a 2-level improvement in visual acuity and stereoacuity of 160 arc s or better (Fig. 5).
There are many caveats that should be kept in mind. First, the numbers of subjects in each study are generally small, and we have only included those studies that provide the individual results, as opposed to just mean results. Second, different studies use different tests to quantify stereopsis and many of the tests have such a limited range of tested disparities, that patients may be labeled as “stereoblind” simply because the test did not provide sufficiently coarse disparities (see quantifying stereopsis above). In addition, for many clinical stereo tests, test–retest reliability is often poor, with 95 confidence intervals of 1 to 2 octaves (e.g., Adams, Leske, Hatt, & Holmes, 2009; Fawcett & Birch, 2000). While some studies include control groups (e.g., Li et al., 2014; Li, Hess, et al., 2013; Li, Thompson, et al. 2013; Vedamurthy et al., submitted for publication), most do not, making it difficult to interpret the changes in stereoacuity. Clearly, there is a need for better stereo tests that can be applied to patients.
We note too that the studies considered here represent a wide range of ages, study durations, training methods and measurements. However, even confining our analysis to only those studies that tested adult patients, we find that 43% showed the criterion improvement. In addition, some of the studies may have confounded treatments. For example, as noted above, in children, spectacle correction alone can improve visual acuity and stereoacuity (Richardson et al., 2005; Stewart et al., 2013). Amblyopic subjects, upon entry to a study are often refracted and given an updated spectacle correction. It is unclear whether spectacle correction has the same benefit in adults, and most adult PL studies do not include a refractive adaptation period. Additionally, monocular PL studies generally involve patching the strong eye, and few studies include a control for patching.
Another important issue is that many of the studies do not provide details about the angle of strabismus during treatment and testing. Proper binocular alignment is critical for stereopsis in strabismic subjects, and it seems important to know whether this was achieved and how. Finally, it is quite likely that negative results (failure to find improvements) are either underreported, or not reported at all. Despite these caveats, overall the results appear promising. Perhaps in the not too distant future eye doctors will tell their adult patients with amblyopia and impaired stereopsis that something can be done.
Bearing in mind the many caveats (discussed below), it is interesting to compare the effects of each of the different approaches, and to compare anisometropic vs strabismic amblyopes (Fig. 7). Between ≈40% and 80% of anisometropic amblyopes, achieve at least a 2-level/160” or better improvement for all approaches, except for dichoptic PL (this is probably an artifact of the small N). Direct stereo training results in the highest percentage of both strabismic and anisometropic amblyopes reaching this level.
Unsurprisingly, strabismic amblyopes do not fare as well as anisometropes in recovering stereoacuity. Whether the apparent difference between strabismic and anisometropic amblyopes reflects differences in their baseline stereopsis (i.e., many strabismic subjects fail the test or have very poor stereopsis initially), is unclear. Based on standard clinical treatment, the PEDIG studies suggest that better post-treatment stereoacuity was associated with better base-line stereoacuity and better post-treatment visual acuity in their amblyopic eyes (Wallace et al., 2011). Clearly strabismic amblyopes require an approach that is more actively aimed at normalizing binocular interactions and/or directly targeting disparity sensitive mechanisms in order to regain stereopsis, than do anisometropic amblyopes.
In order to look into this question more closely, we have replotted data from several of our studies, involving 94 subjects and multiple training approaches, as post- vs. pre-training thresholds (Fig. 9). What seems clear from this figure is that: (i) many more anisometropic (blue) than strabismic (red) amblyopes improve after training (symbols below the gray unity line). (ii) Many more strabismic (40/57 – 70%) than anisometropic (12/37 – 32%) amblyopes have no measurable stereopsis both before and after training, and, (iii) there are both anisometropic and strabismic amblyopes at all levels of pre-training stereoacuity (including no measurable stereopsis) who show improvements following training, some achieving stereoacuity of 140 arc s or better (as indicated by the horizontal dashed lines). This figure, based on 94 adults, shows clearly that despite the dogma, many adults with amblyopia can recover, at least partially, stereoacuity.
Fig. 9.
Post vs. pre-training stereo thresholds. This figure replots data from several of our studies, involving 94 subjects and multiple training approaches. Blue symbols – anisometropic amblyopes; red symbols – strabismic amblyopes. The diagonal gray line indicates no improvement. Symbols below the line show improved performance following training. Data below the dashed horizontal lines indicate a post-training stereothreshold of 140 arc s or better. Data within the turquoise rectangle indicate no measurable pre-training stereopsis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our review of the literature suggests that impaired stereoscopic depth perception is the most common deficit associated with amblyopia under ordinary (binocular) viewing conditions (Webber & Wood, 2005), and that this impairment may have a substantial impact on visuomotor tasks, difficulties in playing sports in children and locomoting safely in older adults. Furthermore, impaired stereopsis may not only negatively impact everyday activity, but may also limit career options for amblyopes. Stereopsis is much more impacted in strabismic than in anisometropic amblyopia, and recovery may require more active treatment in strabismic than in anisometropic amblyopia. Importantly however, despite the many caveats, the present review shows there are reasons to be optimistic. Clearly, recovery of at least some degree of stereopsis in patients with amblyopia, even beyond the critical period, is possible. Indeed, this is in line with a number of recent animal studies showing that recovery of visual function can be extended well beyond the critical period by a variety of methods (Baroncelli et al., 2011; Bavelier et al., 2010; Morishita & Hensch, 2008; Kaneko & Stryker, 2014; Duffy & Mitchell, 2013; Montey, Eaton, & Quinlan, 2013). Thus, the time may have come to re-evaluate patching as the standard clinical approach, as treatment with close to a 50% success in adults is likely to be even more successful in young children. In parallel, the large variance in outcome across patients certainly calls for further studies to unpack the factors that may predict treatment success.
Acknowledgments
This research was supported by Grants RO1EY020976 and RO1EY016880 from the National Eye Institute to D.L., D.K. and D.B., and a grant from the Swiss National Foundation (100014_140676) to D.B. We thank Suzanne McKee and Adrien Chopin for their invaluable comments and suggestions on an earlier version of this manuscript.
Our dear friend and colleague David Knill died tragically while this manuscript was under review. We, and indeed the entire vision community, will miss him sorely, and we dedicate this paper to his memory.
Footnotes
We note that the boundary between dichoptic PL and dichoptic videogames is not always clear.
6. Uncited references
Green and Bavelier (2012), Harrad et al. (1996), McKee (1983), Vedamurthy et al. (2012b), Watanabe (2014), Worth and Chevasse (1950).
References
- Adams WE, Leske DA, Hatt SR, Holmes JM. Defining real change in measures of stereoacuity. Ophthalmology. 2009;116:281–285. doi: 10.1016/j.ophtha.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agervi P, Kugelberg U, Kugelberg M, Simonsson G, Fornander M, Zetterström C. Treatment of anisometropic amblyopia with spectacles or in combination with translucent Bangerter filters. Ophthalmology. 2009;116(8):1475–1480. doi: 10.1016/j.ophtha.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Astle AT, McGraw PV, Webb BS. Recovery of stereo acuity in adults with amblyopia. BMJ Case Reports. 2011 doi: 10.1136/bcr.07.2010.3143. http://dx.doi.org/10.1136/bcr.07.2010.314. [DOI] [PMC free article] [PubMed]
- Baker DH, Meese TS, Hess RF. Contrast masking in strabismic amblyopia: Attenuation, noise, interocular suppression and binocular summation. Vision Research. 2008;48(15):1625–1640. doi: 10.1016/j.visres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Maffei L, Sale A. New perspectives in amblyopia therapy on adults: A critical role for the excitatory/inhibitory balance. Frontiers in Cellular Neuroscience. 2011;5:25. doi: 10.3389/fncel.2011.00025. http://dx.doi.org/10.3389/fncel.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett BT, Bradley A, Candy TR. The relationship between anisometropia and amblyopia. Progress in Retinal and Eye Research. 2013;36:120–158. doi: 10.1016/j.preteyeres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett BT, Panesar GK, Scally AJ, Pacey IE. A limited role for suppression in the central field of individuals with strabismic amblyopia. PLoS ONE. 2012;7(5):e36611. doi: 10.1371/journal.pone.0036611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SO. Fixing my gaze: A scientist's journey into seeing in three dimensions. Basic Books; New York, NY: 2009. [Google Scholar]
- Bauer A, et al. The relevance of stereopsis for motorists: A pilot study. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2001;239(6):400–406. doi: 10.1007/s004170100273. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. The Journal of Neuroscience. 2010;30(45):14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss JD, Vedamurthy I, Bavelier D, Nahum M, Levi D. Lazy eye shooter: A novel game therapy for visual recovery in adult amblyopia.. 4th International IEEE Consumer Electronics Society – Games Innovation Conference (IGIC2012).2012. [Google Scholar]
- Bayliss JD, Vedamurthy I, Nahum M, Levi DM, Bavelier D. Lazy eye shooter: Making a game therapy for visual recovery in adult amblyopia usable. HCI International 2013. 2013 [Google Scholar]
- Bi H, Zhang B, Tao X, Harwerth RS, Smith EL, 3rd, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cerebral Cortex. 2011;21(9):2033–2045. doi: 10.1093/cercor/bhq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE. Amblyopia and binocular vision. Progress in Retinal and Eye Research B. 2013:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Wang J. Stereoacuity outcomes after treatment of infantile and accommodative esotropia. Optometry and Vision Science. 2009;86(6):647–652. doi: 10.1097/OPX.0b013e3181a6168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman B. Restoring adult stereopsis: A vision researcher's personal experience. Optometry and Vision Science. 2014;91(6):e135–e139. doi: 10.1097/OPX.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Buckley JG, Panesar GK, MacLellan MJ, Pacey IE, Barrett BT. Changes to control of adaptive gait in individuals with long-standing reduced stereoacuity. Investigative Ophthalmology & Visual Science. 2010;51(5):2487–2495. doi: 10.1167/iovs.09-3858. http://dx.doi.org/10.1167/iovs.09-3858. [DOI] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and clinical aspects. Butterworth-Heinemann; Stoneham, MA: 1991. [Google Scholar]
- Cleary M, Moody AD, Buchanan A, Stewart H, Dutton GN. Assessment of a computer-based treatment for older amblyopes: The glasgow pilot study. Eye. 2009;23:124–131. doi: 10.1038/sj.eye.6702977. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA. The role of visual feedback of hand position in the control of manual prehension. Experimental Brain Research. 1999;125(3):281–286. doi: 10.1007/s002210050684. [DOI] [PubMed] [Google Scholar]
- Ding J, Levi DM. Recovery of stereopsis through perceptual learning in human adults with abnormal binocular vision. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):E733–E741. doi: 10.1073/pnas.1105183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Levi DM. Rebalancing binocular vision in amblyopia. Ophthalmic and Physiological Optics. 2014;34(2):199–213. doi: 10.1111/opo.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Klein SA, Levi DM. Binocular combination in abnormal binocular vision. Journal of Vision. 2013;13(2):14. doi: 10.1167/13.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzis PB, Rappazzo JA, Burde RM, Gordon M. Effect of binocular variations of Snellen’s visual acuity on Titmus stereoacuity. Archives of Ophthalmology. 1983;101:930–932. doi: 10.1001/archopht.1983.01040010930016. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Mitchell DE. Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Current Biology. 2013;23:382–386. doi: 10.1016/j.cub.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Farzin F, Norcia AM. Impaired visual decision-making in individuals with amblyopia. Journal of Vision. 2011;11(14) doi: 10.1167/11.14.6. http://dx.doi.org/10.1167/11.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Validity of the Titmus and Randot circles tasks in children with known binocular vision disorders. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2003;7:333–338. doi: 10.1016/s1091-8531(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Interobserver test-retest reliability of the Randot preschool stereotest. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2000;4:354–358. doi: 10.1067/mpa.2000.110340. [DOI] [PubMed] [Google Scholar]
- Fielder AR, Moseley MJ. Does stereopsis matter in humans? Eye. 1996;10(2):233–238. doi: 10.1038/eye.1996.51. [DOI] [PubMed] [Google Scholar]
- Giaschi D, Lo R, Narasimhan S, Lyons C, Wilcox LM. Sparing of coarse stereopsis in stereodeficient children with a history of amblyopia. Journal of Vision. 2013;13(10):17. doi: 10.1167/13.10.17. http://dx.doi.org/10.1167/13.10.17. [DOI] [PubMed] [Google Scholar]
- Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus. 2011;19(3):119–128. doi: 10.3109/09273972.2011.600423. [DOI] [PubMed] [Google Scholar]
- Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Investigative Ophthalmology & Visual Science. 2007;48(3):1139–1148. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Learning, attentional control, and action video games. Current Biology. 2012;22(6):R197–206. doi: 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Tailor VK, Sloper JJ, Simmers AJ, Bex PJ, Dakin SC. Visual acuity, crowding, and stereo-vision are linked in children with and without amblyopia. Investigative Ophthalmology & Visual Science. 2012;53:7655–7665. doi: 10.1167/iovs.12-10313. [DOI] [PubMed] [Google Scholar]
- Gresset JA, Meye FM. Risk of accidents among elderly car drivers with visual acuity equal to 6/12 or 6/15 and lack of binocular vision. Ophthalmic and Physiological Optics. 1994;14:33–37. doi: 10.1111/j.1475-1313.1994.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision Research. 1992;32(11):2135–2150. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- Harrad R, Sengpiel F, Blakemore C. Physiology of suppression in strabismic amblyopia. British Journal of Ophthalmology. 1996;80(4):373–377. doi: 10.1136/bjo.80.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe M, Gillam B, Chajka K, Vecellio E. The role of binocular vision in walking. Visual Neuroscience. 2009;26(1):73–80. doi: 10.1017/S0952523808080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbostad JL, Vereijken B, Hesseberg K, Sletvold O. Altered vision destabilizes gait in older persons. Gait & Posture. 2009;30(2):233–238. doi: 10.1016/j.gaitpost.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hess RF, Babu RJ, Clavagnier S, Black J, Bobier W, Thompson B. The iPod binocular home-based treatment for amblyopia in adults: Efficacy and compliance. Clinical and Experimental Optometry. 2014;97(5):389–398. doi: 10.1111/cxo.12192. http:// dx.doi.org/10.1111/cxo.12192. [DOI] [PubMed] [Google Scholar]
- Hess RF. The site and nature of suppression in squint amblyopia. Vision Research. 1991;31(1):111–117. doi: 10.1016/0042-6989(91)90078-j. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: Antisuppression therapy. Optometry and Vision Science. 2010a;87(9):697–704. doi: 10.1097/OPX.0b013e3181ea18e9. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restorative Neurology and Neuroscience. 2010b;28(6):793–802. doi: 10.3233/RNN-2010-0550. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19(3):110–118. doi: 10.3109/09273972.2011.600418. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: Structure, suppression and plasticity. Ophthalmic and Physiological Optics. 2014;34(2):146–162. doi: 10.1111/opo.12123. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Gole G, Mullen KT. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. European Journal of Neuroscience. 2009;29:1064–1070. doi: 10.1111/j.1460-9568.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis JM, Watt SJ, Landy MS, Banks MS. Slant from texture and disparity cues: Optimal cue combination. Journal of Vision. 2004;4(12):967–992. doi: 10.1167/4.12.1. http://dx.doi.org/10.1167/4.12.1. [DOI] [PubMed] [Google Scholar]
- Holopigian K, Blake R, Greenwald MJ. Selective losses in binocular vision in anisometropic amblyopes. Vision Research. 1986;26:621–630. doi: 10.1016/0042-6989(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Howard IP, Rogers BJ. Seeing in depth. Oxford University Press; 2008. [Google Scholar]
- Hu B, Knill DC. Binocular and monocular depth cues in online feedback control of 3-D pointing movements. Journal of Vision. 2011;7(11):1–13. 23. doi: 10.1167/11.7.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CB, Zhou J, Lu ZL, Zhou Y. Deficient binocular combination reveals mechanisms of anisometropic amblyopia: Signal attenuation and interocular inhibition. Journal of Vision. 2011;11(6) doi: 10.1167/11.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphriss D. Three South African studies on the relation between road accidents and drivers’ vision. Ophthalmic and Physiological Optics. 1987;7:73–79. doi: 10.1016/0275-5408(87)90183-9. [DOI] [PubMed] [Google Scholar]
- Johnston EB, Cumming BG, Parker AJ. Integration of depth modules: Stereopsis and texture. Vision Research. 1993;33(5):813–826. doi: 10.1016/0042-6989(93)90200-g. [DOI] [PubMed] [Google Scholar]
- Julesz B. Stereopsis and binocular rivalry of contours. Journal of the Optical Society of America. 1963;53:994–999. doi: 10.1364/josa.53.000994. [DOI] [PubMed] [Google Scholar]
- Keele SW, Posner MI. Processing of visual feedback in rapid movements. Journal of Experimental Psychology. 1968;77:155–158. doi: 10.1037/h0025754. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: Animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Knill DC, Saunders J. Do humans optimally integrate stereo and texture information for judgments of surface slant? Vision Research. 2003;43(24):2539–2558. doi: 10.1016/s0042-6989(03)00458-9. [DOI] [PubMed] [Google Scholar]
- Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: Prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Investigative Ophthalmology & Visual Science. 2012;53(2):817–824. doi: 10.1167/iovs.11-8219. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kwon M, Lu ZL, Miller A, Kazlas M, Hunter DG, Bex PJ. Assessing binocular interaction in amblyopia and its clinical feasibility. PLoS One. 2014;9(6):e100156. doi: 10.1371/journal.pone.0100156. http://dx.doi.org/10.1371/journal.pone.0100156 [eCollection 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Isenberg SJ. The relationship between stereopsis and visual acuity after occlusion therapy for amblyopia. Ophthalmology. 2003;110(11):2088–2092. doi: 10.1016/S0161-6420(03)00865-0. [DOI] [PubMed] [Google Scholar]
- Legge GE, Gu YC. Stereopsis and contrast. Vision Research. 1989;29:989–1004. doi: 10.1016/0042-6989(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Levi DM. Visual processing in amblyopia: Human studies. Strabismus. 2006;14(1):11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding – An essential bottleneck for object recognition: A mini-review. Vision Research. 2008;48(5):635–654. doi: 10.1016/j.visres.2007.12.009. http://dx.doi.org/10.1016/ j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM. Prentice award lecture 2011: Removing the brakes on plasticity in the amblyopic brain. Optometry and Vision Science. 2012;89(6):827–838. doi: 10.1097/OPX.0b013e318257a187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM. Linking assumptions in amblyopia. Visual Neuroscience. 2013;30:277–287. doi: 10.1017/S0952523813000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Carkeet A. Amblyopia: A consequence of abnormal visual development. In: Simons K, editor. Early visual development, normal and abnormal. Oxford University Press; 1993. pp. 391–408. [Google Scholar]
- Levi DM, Harwerth RS, Smith EL., 3rd Humans deprived of normal binocular vision have binocular interactions tuned to size and orientation. Science. 1979;206(4420):852–854. doi: 10.1126/science.493988. [DOI] [PubMed] [Google Scholar]
- Levi DM, Harwerth RS, Smith EL., 3rd Binocular interactions in normal and anomalous binocular vision. Documenta Ophthalmologica. 1980;49(2):303–324. doi: 10.1007/BF01886623. [DOI] [PubMed] [Google Scholar]
- Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research. 2009;49(21):2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Research. 2011;51:48–57. doi: 10.1016/j.visres.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(13):6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Song S, Pelli DG. Amblyopic reading is crowded. Journal of Vision. 2007;7(2):21.1–21.17. doi: 10.1167/7.2.21. http://dx.doi.org/10.1167/7.2.21. [DOI] [PubMed] [Google Scholar]
- Li J, Hess RF, Chan LY, Deng D, Yang X, Chen X, et al. Quantitative measurement of interocular suppression in anisometropic amblyopia: A case– control study. Ophthalmology. 2013;120:1672–1680. doi: 10.1016/j.ophtha.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Li J, Thompson B, Lam CS, Deng D, Chan LY, Maehara G, et al. The role of suppression in amblyopia. Investigative Ophthalmology & Visual Science. 2011;52(7):4169–4176. doi: 10.1167/iovs.11-7233. [DOI] [PubMed] [Google Scholar]
- Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Current Biology. 2013;23(8):R308–R309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Li SL, Jost RM, Morale SE, Stager DR, Dao L, Stager D, et al. A binocular iPAD treatment for amblyopic children. Eye. 2014 doi: 10.1038/eye.2014.165. http://dx.doi.org/10.1038/eye.2014.165. [DOI] [PMC free article] [PubMed]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision. 2004;4(6):476–487. doi: 10.1167/4.6.7. http://dx.doi.org/10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Investigative Ophthalmology & Visual Science. 2007;48:5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology. 2011;9(8):e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillard AS, Erisir A. Old dogs learning new tricks: Neuroplasticity beyond the juvenile period. Developmental Review. 2011;31(4):207–239. doi: 10.1016/j.dr.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Zhang T, Jia YL, Wang NL, Yu C. The therapeutic impact of perceptual learning on juvenile amblyopia with or without previous patching treatment. Investigative Ophthalmology & Visual Science. 2011;52(3):1531–1538. doi: 10.1167/iovs.10-6355. [DOI] [PubMed] [Google Scholar]
- Lovell PG, Bloj M, Harris JM. Optimal integration of shading and binocular disparity for depth perception. Journal of Vision. 2012;12(1):1. doi: 10.1167/12.1.1. [DOI] [PubMed] [Google Scholar]
- Maehara G, Thompson B, Mansouri B, Farivar R, Hess RF. The perceptual consequences of interocular suppression in amblyopia. Investigative Ophthalmology & Visual Science. 2011;52(12):9011–9017. doi: 10.1167/iovs.11-7748. http://dx.doi.org/10.1167/iovs.11-7748. [DOI] [PubMed] [Google Scholar]
- Mansouri B, Thompson B, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Research. 2008;48(28):2775–2784. doi: 10.1016/j.visres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, Wen G, Kim J, Borchert M, et al. Prevalence of amblyopia or strabismus in asian and non-Hispanic white preschool children: Multi-ethnic pediatric eye disease study. Ophthalmology. 2013;120:2117–2124. doi: 10.1016/j.ophtha.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- McKee SP. The spatial requirements for fine stereoacuity. Vision Research. 1983;23(2):191–198. doi: 10.1016/0042-6989(83)90142-6. [DOI] [PubMed] [Google Scholar]
- McKee SP, Taylor DG. The precision of binocular and monocular depth judgments in natural settings. Journal of Vision. 2010;10(10):5. doi: 10.1167/10.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight AJ, Shinar D, Hilburn B. The visual and driving performance of monocular and binocular heavy-duty truck drivers. Accident Analysis & Prevention. 1991;23(4):225–237. doi: 10.1016/0001-4575(91)90002-m. [DOI] [PubMed] [Google Scholar]
- Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) Group Prevalence and causes of visual impairment in African–American and Hispanic preschool children: The multi-ethnic pediatric eye disease study. Ophthalmology. 2009;116:1990–2000. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmoth DR, Grant S. Advantages of binocular vision for the control of reaching and grasping. Experimental Brain Research. 2006;171(3):371–388. doi: 10.1007/s00221-005-0273-x. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Finlay AL, Morgan MJ, Grant S. Grasping deficits and adaptations in adults with stereo vision losses. Investigative Ophthalmology & Visual Science. 2009;50(8):3711–3720. doi: 10.1167/iovs.08-3229. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Storoni M, Todd G, Finlay AL, Grant S. Dissociation between vergence and binocular disparity cues in the control of prehension. Experimental Brain Research. 2007;183(3):283–298. doi: 10.1007/s00221-007-1041-x. [DOI] [PubMed] [Google Scholar]
- Menon V, Bansal A, Prakash P. Randot stereoacuity at various binocular combinations of Snellen acuity. Indian Journal of Ophthalmology. 1997;45:169–171. [PubMed] [Google Scholar]
- Montey KL, Eaton NC, Quinlan EM. Repetitive visual stimulation enhances recovery from severe amblyopia. Learning & Memory. 2013;20:311–317. doi: 10.1101/lm.030361.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: Impact on vision. Current Opinion in Neurobiology. 2008;18(1):101–107. doi: 10.1016/j.conb.2008.05.009. http://dx.doi.org/10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Van Sluyters RC. Visual neural development. Annual Review of Psychology. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Muckli L, et al. Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Research. 2006;46:506–526. doi: 10.1016/j.visres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Murdoch JR, McGhee CN, Glover V. The relationship between stereopsis and fine manual dexterity: Pilot study of a new instrument. Eye (London) 1991;5(Pt. 5):642–643. doi: 10.1038/eye.1991.112. [DOI] [PubMed] [Google Scholar]
- Nakatsuka C, Zhang B, Watanabe I, Zheng J, Bi H, Ganz L, et al. Effects of perceptual learning on local stereopsis and neuronal responses of V1 and V2 in prism-reared monkeys. Journal of Neurophysiology. 2007;97(4):2612–2626. doi: 10.1152/jn.01001.2006. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Harrison ER, Giaschi DE. Quantitative measurement of interocular suppression in children with amblyopia. Vision Research. 2012;66:1–10. doi: 10.1016/j.visres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Kennedy SA, Colpa L, Chandrakumar M, Goltz HC, Wong AM. Effects of induced monocular blur versus anisometropic amblyopia on saccades, reaching, and eye–hand coordination. Investigative Ophthalmology & Visual Science. 2012;53(8):4354–4362. doi: 10.1167/iovs.12-9855. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Investigative Ophthalmology & Visual Science. 2010;51(4):2019–2023. doi: 10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- Oladehinde MK, et al. Visual functions of commercial drivers in relation to road accidents in Nigeria. Indian Journal of Occupational and Environmental Medicine. 2007;11(2):71. doi: 10.4103/0019-5278.34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi TL, Su YR, Natale DM, He ZJ. A push–pull treatment for strengthening the ‘lazy eye’ in amblyopia. Current Biology. 2013;23(8):R309–310. doi: 10.1016/j.cub.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDIG Patching vs atropine to treat amblyopia in children aged 7 to 12 years. Archives of Ophthalmology. 2008;126:1634–1642. doi: 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U. Making perceptual learning practical to improve visual functions. Vision Research. 2009;49(21):2566–2573. doi: 10.1016/j.visres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Read JC, Begum SF, McDonald A, Trowbridge J. The binocular advantage in visuomotor tasks involving tools. i-Perception. 2013;4(2):101–110. doi: 10.1068/i0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Holmes JM. Lessons from the amblyopia treatment studies. Ophthalmology. 2012;119(4):657–658. doi: 10.1016/j.ophtha.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Richardson SR, Wright CM, Hrisos S, Buck D, Clarke MP. Stereoacuity in unilateral visual impairment detected at preschool screening: Outcomes from a randomized controlled trial. Investigative Ophthalmology & Visual Science. 2005;46:150–154. doi: 10.1167/iovs.04-0672. [DOI] [PubMed] [Google Scholar]
- Sachsenweger R. Problems of organic lesions in functional amblyopia. In: Arruga H, editor. International strabismus symposium. S. Karger A.G.; 1968. p. 63. [Google Scholar]
- Sagi D. Perceptual learning in vision research. Vision Research. 2011;51(13):1552–1566. doi: 10.1016/j.visres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Saunders J, Knill DC. Visual feedback control of hand movements. Journal of Neuroscience. 2004;24(13):3223–3234. doi: 10.1523/JNEUROSCI.4319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control both the direction and distance of pointing movements. Experimental Brain Research. 2005;162(4):458–473. doi: 10.1007/s00221-004-2064-1. [DOI] [PubMed] [Google Scholar]
- Schor CM. Visual stimuli for strabismic suppression. Perception. 1977;6(5):583–593. doi: 10.1068/p060583. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye. 1996;10(Pt 2):250–258. doi: 10.1038/eye.1996.54. [DOI] [PubMed] [Google Scholar]
- Servos P, Goodale MA, Jakobson LS. The role of binocular vision in prehension: A kinematic analysis. Vision Research. 1992;32(8):1513–1521. doi: 10.1016/0042-6989(92)90207-y. [DOI] [PubMed] [Google Scholar]
- Sharma V, Levi DM, Klein SA. Undercounting features and missing features: Evidence for a high-level deficit in strabismic amblyopia. Nature Neuroscience. 2000;3(5):496–501. doi: 10.1038/74872. [DOI] [PubMed] [Google Scholar]
- Simons K. A comparison of the Frisby, Random-Dot E, TNO, and Randot circles stereotests in screening and office use. Archives of Ophthalmology. 1981;99(3):446–452. doi: 10.1001/archopht.1981.03930010448011. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Fronius M, Singer W. Binocular interaction in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision Research. 1981;21(7):1065–1074. doi: 10.1016/0042-6989(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Levi DM, Manny RE, Harwerth RS, White JM. The relationship between binocular rivalry and strabismic suppression. Investigative Ophthalmology & Visual Science. 1985;26(1):80–87. [PubMed] [Google Scholar]
- Song S, Levi DM, Pelli DG. A double dissociation of the acuity and crowding limits to letter identification, and the promise of improved visual screening. Journal of Vision. 2014;14(5):3. doi: 10.1167/14.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AL, Bradfield YS, Kushner BJ, France TD, Struck MC, Gangnon RE. Successful treatment of anisometropic amblyopia with spectacles alone. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2006;10:37–43. doi: 10.1016/j.jaapos.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Wallace MP, Stephens DA, Fielder AR, Moseley MJMOTAS, Cooperative The effect of amblyopia treatment on stereoacuity. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2013;17:166–173. doi: 10.1016/j.jaapos.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Investigative Ophthalmology & Visual Science. 2013;54(3):1998–2003. doi: 10.1167/iovs.12-11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Investigative Ophthalmology & Visual Science. 2011;52(3):1851–1864. doi: 10.1167/iovs.10-6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To L, Thompson B, Blum JR, Maehara G, Hess RF, Cooperstock JR. A game platform for treatment of amblyopia. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2011;19(3):280–289. doi: 10.1109/TNSRE.2011.2115255. [DOI] [PubMed] [Google Scholar]
- Vedamurthy I, Bayliss JA, Knill DC, Bavelier D, Levi DM. A novel active game therapy for visual recovery in adult amblyopia. ARVO Abstract. 2012a [Google Scholar]
- Vedamurthy I, Huang S, Levi DM, Bavelier D, Knill DC. Recovery of stereopsis in adults through training in a virtual reality task.. Optical Society of America, Fall Vision Meeting; Rochester, NY, USA. 2012b. [Google Scholar]
- Vedamurthy I, Nahum M, Huang S, Zheng F, Bayliss J, Bavelier D, Levi D. A dichoptic custom-made action video game as a treatment for adult amblyopia. D.M. (submitted for publication). [DOI] [PMC free article] [PubMed]
- von Noorden GK. Binocular vision and ocular motility. Theory and management of strabismus. 5th ed. C.V. Mosby; St. Louis: 1996. [Google Scholar]
- Vuong Q, Domini F, Caudek C. Disparity and shading cues cooperate for surface interpolation. Perception. 2006;35:145–155. doi: 10.1068/p5315. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Melia M, Birch EE, Holmes JM, Hopkins KB, et al. Stereoacuity in children with anisometropic amblyopia. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2011;15:455–461. doi: 10.1016/j.jaapos.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sasaki Y. Perceptual learning: Toward a comprehensive theory. Annual Review of Psychology. 2014 Sep 10; doi: 10.1146/annurev-psych-010814-015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber AL, Wood J. Amblyopia: Prevalence, natural history, functional effects and treatment. Clinical and Experimental Optometry. 2005;88:365–375. doi: 10.1111/j.1444-0938.2005.tb05102.x. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood J, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Investigative Ophthalmology & Visual Science. 2008a;49:594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood J, Gole GA, Brown B. Effect of amblyopia on self-esteem in children. Optometry and Vision Science. 2008b;85:1074–1081. doi: 10.1097/OPX.0b013e31818b9911. [DOI] [PubMed] [Google Scholar]
- Westheimer G, McKee SP. Stereogram design for testing local stereopsis. Investigative Ophthalmology & Visual Science. 1980;19:802–809. [PubMed] [Google Scholar]
- Westheimer G. Clinical evaluation of stereopsis. Vision Research. 2013;90:38–42. doi: 10.1016/j.visres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Wiesel T. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wilcox LM, Allison RS. Coarse-fine dichotomies in human stereopsis. Vision Research. 2009;49:2653–2665. doi: 10.1016/j.visres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: Data from the ALSPAC study. British Journal of Ophthalmology. 2008;92:959–964. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- Wong AM. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Canadian Journal of Ophthalmology. 2012;47:399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Worth CA, Chevasse FB, editors. Squint: Its causes, pathology and treatment. Blakiston; Philadelphia: 1950. [Google Scholar]
- Xi J, Jia WL, Feng LX, Lu ZL, Huang CB. Perceptual learning improves stereoacuity in amblyopia. Investigative Ophthalmology & Visual Science. 2014 Apr 15;55(4):2384–2391. doi: 10.1167/iovs.13-12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Current Biology. 2008a;18(24):1922–1926. doi: 10.1016/j.cub.2008.10.030. http://dx.doi.org/10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao LQ, Zhang JY, Wang R, Klein SA, Levi DM, Yu C. Complete transfer of perceptual learning across retinal locations enabled by double training. Current Biology. 2008b;18(24):1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Cong LJ, Klein SA, Levi DM, Yu C. Perceptual learning improves adult amblyopic vision through rule-based cognitive compensation. Investigative Ophthalmology & Visual Science. 2014;55(4):2020–2030. doi: 10.1167/iovs.13-13739. http://dx.doi.org/10.1167/iovs.13-13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Zhang GL, Xiao LQ, Klein SA, Levi DM, Yu C. Rule-based learning explains visual perceptual learning and its specificity and transfer. Journal of Neuroscience. 2010;30(37):12323–12328. doi: 10.1523/JNEUROSCI.0704-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0704-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jia W, Huang CB, Hess RF. The effect of unilateral mean luminance on binocular combination in normal and amblyopic vision. Scientific Reports. 2013;3:2012. doi: 10.1038/srep02012. [DOI] [PMC free article] [PubMed] [Google Scholar]