Abstract
Motor skill learning induces long-lasting reorganization of dendritic spines, major sites of excitatory synapses, in the motor cortex. However, mechanisms that regulate these excitatory synaptic changes remain poorly understood. Here using in vivo two-photon imaging in awake mice, we found that learning-induced spine reorganization of L2/3 excitatory neurons occurs in the distal branches of their apical dendrites in L1 but not in the perisomatic dendrites. This compartment-specific spine reorganization coincided with subtype-specific plasticity of local inhibitory circuits. Somatostatin-expressing inhibitory neurons (SOM-INs) that mainly inhibit distal dendrites of excitatory neurons showed a decrease in axonal boutons immediately after the training begins, whereas parvalbumin-expressing inhibitory neurons (PV-INs) that mainly inhibit perisomatic regions of excitatory neurons exhibited a gradual increase in the axonal boutons during training. Optogenetic enhancement and suppression of SOM-IN activity during training destabilized and hyper-stabilized spines, respectively, and both manipulations impaired the learning of stereotyped movements. Our results identify SOM inhibition of distal dendrites as a key regulator of learning-related changes in excitatory synapses and the acquisition of motor skills.
INTRODUCTION
Motor skill learning involves changes in the motor cortex observed at multiple levels1–9. At the structural level, motor learning has been shown to induce reorganization of dendritic spines in the motor cortex, and the survival of learning-induced nascent spines is thought to be a basis for long-lasting motor memories10,11. However, little is known about the mechanisms that regulate the spatiotemporal specificity of these changes of excitatory synapses during motor learning. In other words, how does the circuit know when and where to modify synapses to encode a new motor skill? It is known that the excitability of dendrites plays a critical role in controlling the plasticity of excitatory circuits, raising an intriguing possibility that local inhibitory neurons are involved in regulating the specificity of learning-related changes of synaptic circuits during motor learning.
Cortical GABAergic inhibitory neurons display a great diversity based on differences in their morphology, anatomical connectivity, electrophysiological properties and marker expression12. Different subtypes of inhibitory neurons target different domains of excitatory neurons, affording them the ability to control the spatiotemporal activity of excitatory neurons. For example, somatostatin-expressing inhibitory neurons (SOM-INs) typically project their axons to the uppermost layer of cortex, L1, where they inhibit distal portions of apical dendrites of excitatory neurons. In contrast, parvalbumin-expressing inhibitory neurons (PV-INs) mainly target and inhibit somatic and perisomatic regions of excitatory neurons and regulate their spike output. There is accumulating evidence that inhibition plays an important role controlling the plasticity of excitatory circuits13–20. However, contributions of distinct subtypes of inhibitory neurons in adult learning are just beginning to be understood.
In this study, we used in vivo two-photon imaging in awake mice to chronically monitor the dynamics of dendritic spines of excitatory neurons and axonal boutons of SOM-INs and PV-INs throughout motor learning. Chronic imaging of dendritic spines in the distal branches of apical dendrites and the perisomatic dendrites of L2/3 excitatory neurons revealed dendritic compartment-specific reorganization of dendritic spines. Imaging the same axonal branches of SOM-INs or PV-INs throughout learning, we found that motor learning induces subtype-specific plasticity of inhibitory circuits in the motor cortex. Manipulation of SOM-IN activity affected the stability of dendritic spines and blocked the formation of stereotyped movements. Our results uncover an important role played by inhibitory neuron subtypes in regulating the spatiotemporal specificity of learning-related excitatory circuit plasticity.
RESULTS
Dendritic compartment-specific spine reorganization during motor learning
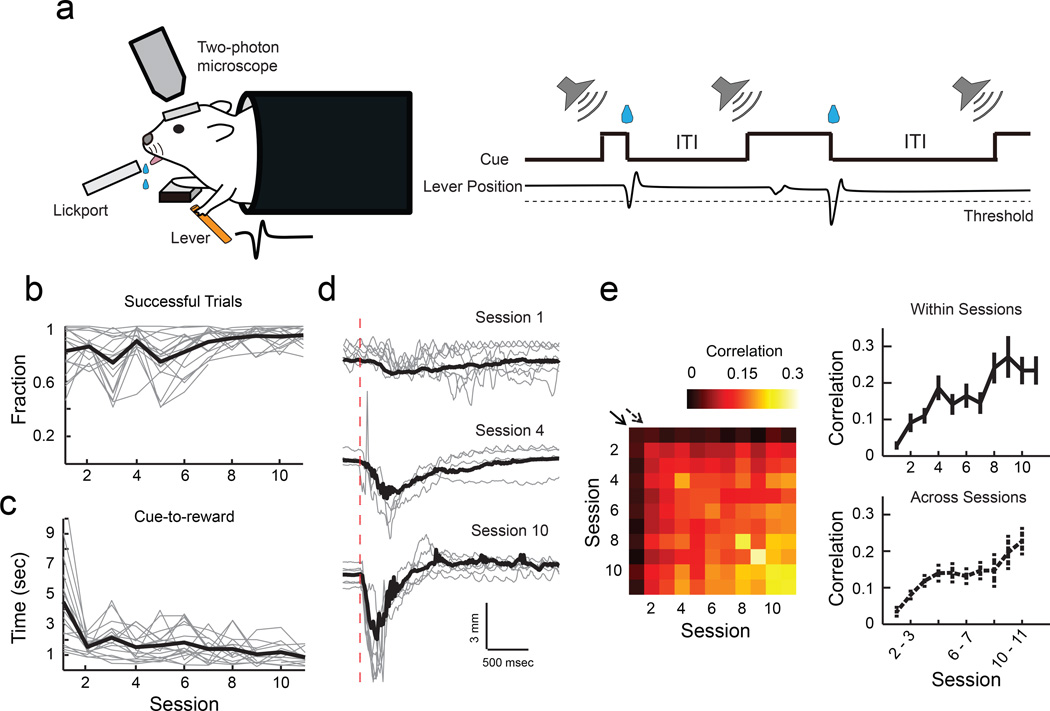
We adapted a cued lever-press task that we recently developed for mice to perform under a two-photon microscope1. In this task, mice under head-fixation learn to use their left forelimb to press the lever beyond the set threshold during an auditory cue to receive a water reward (Fig. 1a). Mice showed a gradual improvement in performance with training over 11 sessions, one session per day (Fig. 1b), and the time from cue onset to achieving the reward significantly decreased over time (Fig. 1c). Furthermore, their lever-press movements became more reproducible (Fig. 1d), shown by higher correlations of individual movements within and across later sessions (Fig. 1e). We recently showed that the motor cortex is required for the learning of stereotyped lever-press movements and that during learning, L2/3 excitatory neurons in the motor cortex acquire an activity pattern that is reproducible from trial to trial1. This led us to examine the synaptic changes within the motor cortex during this learning.
Fig. 1. Lever-press task for head-fixed mice.
(a) Schematic of experimental setup and task. (b) Fraction of successful trials improves with learning (P<0.001, 1-way ANOVA, n = 17 mice). Grey, individual animals; black, mean. (c) Time from cue onset to reward decreases with learning (P<0.001, 1-way ANOVA). Grey, medians of individual animals; black, mean. (d) Example lever movement traces from one animal aligned by movement onset, showing the emergence of movement stereotypy with learning. Grey, 10 individual trials; black, median of all trials; red dotted line, movement onset. (e), Left, trial-to-trial correlation of movement kinematics during learning. Each square represents the median value of the pairwise correlations of the rewarded movement traces of all trial pairs within the session pair, averaged across animals. Right, movement correlation (movement stereotypy) increases within and across sessions, corresponding to the diagonals shown by the solid and dotted arrows in the correlogram on the left (within sessions, P<0.001; across sessions, P<0.001, 1-way ANOVA, n = 17 mice).
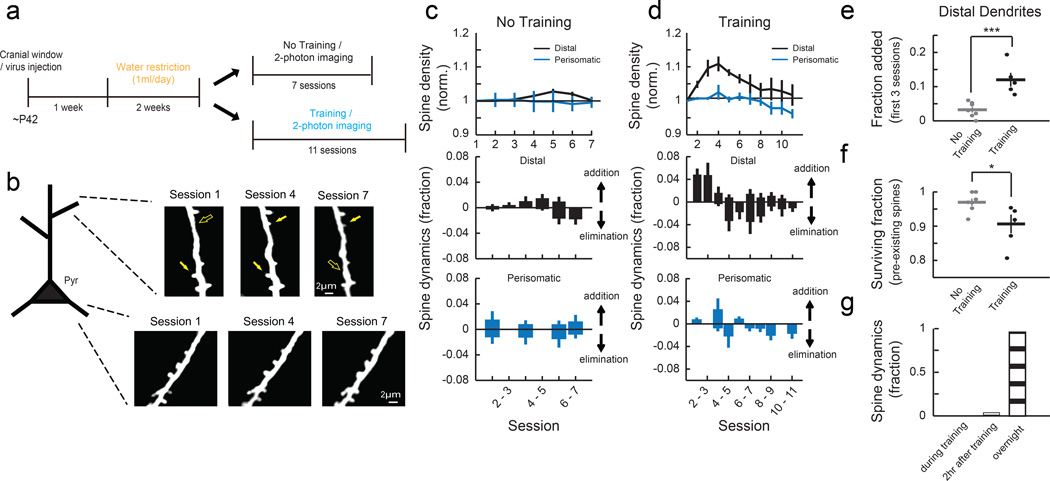
To monitor the dynamics of excitatory synapses in the motor cortex, we labeled a sparse set of L2/3 neurons by injecting a mixture of adeno-associated viral (AAV) vectors encoding Cre recombinase and Cre-dependent GFP in the forelimb area of the right motor cortex of wild-type mice and applied chronic two-photon imaging in awake head-fixed mice in every training session (Fig. 2a). Daily imaging of the same dendritic branches on L2/3 excitatory neurons revealed specific changes in dendritic spines during learning (Fig. 2b and Supplementary Fig. 1a-b). In the distal branches of apical dendrites located in L1, spine formation was significantly increased during the first 3 sessions of training compared to untrained controls (Fig. 2c-e), followed by an increased elimination of spines that existed at the beginning of training (‘pre-existing spines’, Fig. 2f). 75% (18 out of 24) of the spines that formed in the first 3 sessions of training were stable and remained until the end of the experiment. In contrast to the reorganization of distal spines, spines on perisomatic dendrites (<75 μm from soma) in L2/3 were relatively stable during learning (Fig. 2c-d). The spines on the distal dendrites in the hindlimb area of the motor cortex were also stable during learning (Supplementary Fig. 2). These results establish that the learning of the lever-press task induces an area-specific and subcellular compartment-specific reorganization of excitatory synapses in the motor cortex1,10,11.
Fig. 2. Motor learning induces compartment-specific reorganization of dendritic spines.
(a) Experimental timeline. (b) Repeated imaging of the distal portion of apical dendrites (L1, 0 – 50 μm from pia) and perisomatic dendrites (L2/3, 150 – 200 μm) of L2/3 pyramidal cells throughout learning. Filled and open arrows indicate present and absent dynamic spines, respectively. (c) Mean spine density normalized to the initial session (top) and daily spine dynamics (bottom) of distal dendrites (n = 7 mice, 269 spines) and perisomatic dendrites (n = 4 mice, 120 spines) in no training animals. (d) Mean spine density normalized to the initial session (top) and daily spine dynamics (bottom) of distal dendrites (n = 5 mice, 251 spines) and perisomatic dendrites (n = 5 mice, 206 spines) in training animals. Learning transiently increases spine density in distal but not perisomatic dendrites of L2/3 pyramidal cells (Distal: P<0.001, compared to no training; Perisomatic: P=0.246, compared to no training, 2-way ANOVA). (e) Learning increases the spine addition rate in the distal dendrites during the first 3 sessions. (f) Learning increases the elimination rate of pre-existing spines in the distal dendrites (fraction of pre-existing spines that remained until session 7). *P<0.05, ***P<0.001, one-tailed bootstrap test. (g) Spine formation and elimination in the distal dendrites rarely occurred during (0%, 0/25) or within two hours (4%, 1/25) of training sessions (n = 3 mice, 134 spines total). Error bars indicate SEM.
Subtype-specific plasticity of inhibitory circuits during motor learning
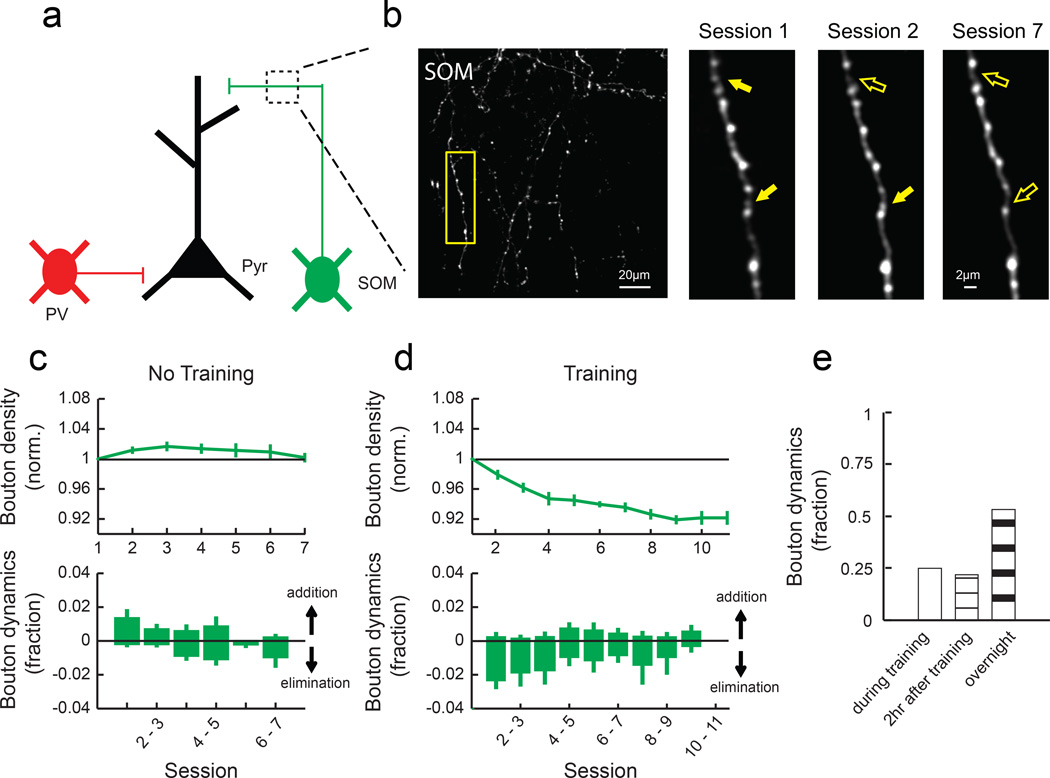
These observations suggest the existence of a mechanism that regulates the compartment-specificity of spine plasticity. Since a growing body of evidence suggests that inhibitory circuits play an important role in regulating local excitatory synaptic plasticity13–19, we asked whether motor learning induces plasticity of inhibitory circuits, focusing on SOM-INs and PV-INs due to their compartment-specific targeting. SOM-INs mainly inhibit distal dendrites of excitatory neurons, while PV-INs mainly inhibit perisomatic regions (Fig. 3a)12, and SOM- and PV-INs together compose about two thirds of cortical inhibitory neurons21,22. To monitor the dynamics of inhibitory synapses made by SOM- and PV-INs, we injected AAV encoding Cre-dependent GFP in the forelimb area of the right motor cortex of SOM-Cre23 or PV-Cre24 mice (Supplementary Fig. 3). In the GFP-labeled axons, boutons that presumably correspond to presynaptic terminals25 were clearly identifiable, and we could reliably follow the same axonal branches throughout learning. We imaged SOM-IN axons in L1, where they inhibit distal dendrites of excitatory neurons, repeatedly throughout 11 days of learning (Fig. 3b). We found that motor learning led to a significant reduction in the density of SOM boutons (Fig. 3d). However, bouton density was stable in the forelimb area of untrained mice and in the hindlimb area during training (Fig. 3c and Supplementary Fig. 2).
Fig. 3. SOM-IN axonal boutons are rapidly eliminated following training.
(a) SOM-INs mainly inhibit distal dendrites of excitatory neurons. (b) Left, a field of SOM-IN axons in L1 imaged throughout learning in vivo. Right, zoom of outlined area on the left. Filled and open arrows indicate present and absent dynamic boutons, respectively. (c) Mean normalized bouton density (top) and daily bouton dynamics (bottom) of SOM-INs in no training animals (n = 6 mice, 464 boutons). (d) Mean normalized bouton density (top) and daily bouton dynamics (bottom) of SOM-INs in training animals (n = 5 mice, 433 boutons). SOM boutons decreased with training (P<0.001, 2-way ANOVA with post hoc Tukey's test, compared to ʽno trainingʼ) due to an increase in bouton elimination (P<0.001, one-tailed bootstrap). (e) Many bouton formation and elimination events of SOM-INs occurred during (25%, 8/32) and within two hours (22%, 7/32) of training sessions (n = 3 mice, 258 boutons).
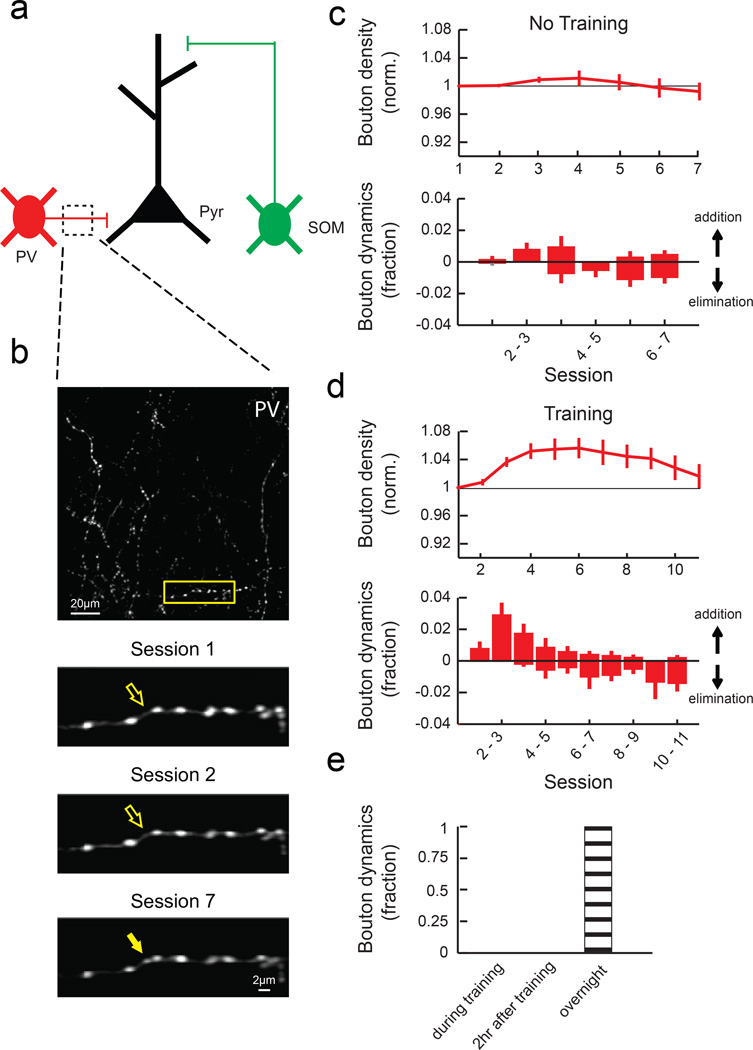
Next, we imaged PV-IN axons in L2/3, where they inhibit perisomatic regions of excitatory neurons (Fig. 4a). In contrast to SOM-IN boutons, motor learning induced a transient increase in the PV-IN bouton density compared to untrained controls (Fig. 4b-d). Taken together, these results indicate that motor learning induces opposing changes in SOM- and PV-INs, with a reduction in the density of boutons made by distally-targeting SOM-INs and an increase in the density of boutons made by perisomatically-targeting PV-INs.
Fig. 4. PV-IN axonal boutons transiently increase during learning.
(a) PV-INs mainly inhibit perisomatic regions. (b) Top, a field of PV-IN axons in L2/3. Bottom, zoom of outlined area above. (c) Mean normalized bouton density (top) and daily bouton dynamics (bottom) of PV-INs in no training animals (n = 6 mice, 488 boutons). (d) Mean normalized bouton density (top) and daily bouton dynamics (bottom) of PV-INs in training animals (n = 5 mice, 396 boutons). PV boutons increase with training (P<0.001, 2-way ANOVA with post hoc Tukey's test, compared to ʽno trainingʼ). (e) Bouton formation and elimination of PV-INs did not occur during or within two hours of training sessions (n = 3 mice, 12 dynamic boutons out of 215 total boutons). Error bars indicate SEM.
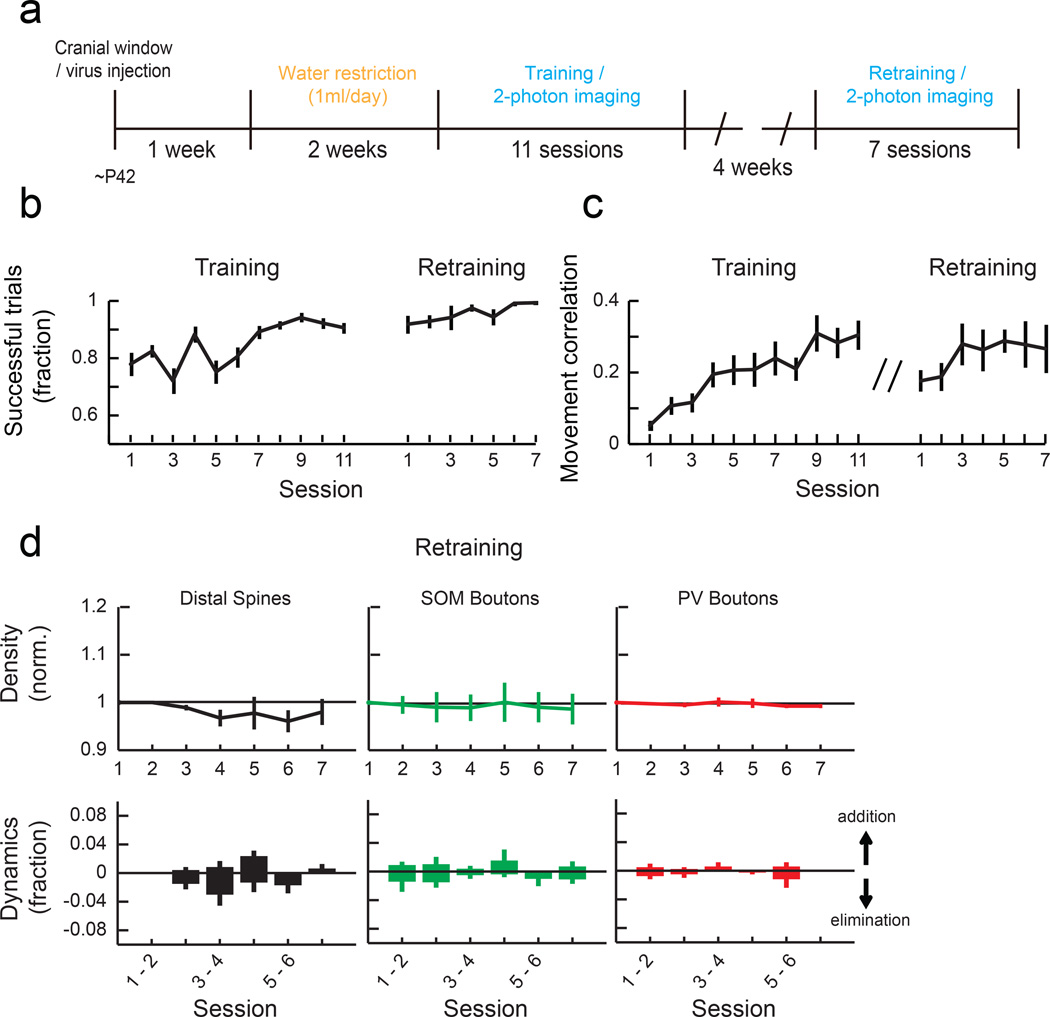
One of the hallmarks of motor learning is that once a skill is learned, it can be maintained for a long period of time without further training. Indeed, mice trained with the lever-press task maintained the skill one month after the original training, shown by high success rates and movement stereotypy (Fig. 5a-c). During this retraining, spines and SOM and PV boutons were stable (Fig. 5d), demonstrating that the reorganization of local synaptic circuits is specific to the initial acquisition of a new motor skill.
Fig. 5. Synaptic reorganization is not observed during performance of a previously learned task.
(a) Experimental timeline for retraining animals. (b) Fraction of successful trials in training (n = 17 mice) and retraining mice (n = 10 mice). (c) Median pairwise correlation of rewarded movements within each session in training and retraining mice. (d) Mean normalized density of distal spines, SOM boutons and PV boutons (top) and their daily dynamics in each session (bottom) during retraining (Spine: n = 3 mice, 181 spines, SOM: n = 3 mice, 196 boutons, PV: n = 3 mice, 254 boutons). The density of spines and boutons showed no significant changes (Spine: P=0.63, SOM boutons: P=0.99, PV boutons: P=0.91, 1-way ANOVA). Error bars indicate SEM.
To further understand the temporal dynamics of distal spines and inhibitory boutons with higher resolution, we next performed imaging at three time points each day (before training, immediately after training, and 2 hours after training) in the first 4 behavioral sessions. We found that the vast majority of spine changes occurred between 2 hours after training and the next day (Fig. 2g), consistent with a recent study26. PV bouton changes also followed a similar trend (Fig. 4e). However, about 50% of the changes in SOM boutons occurred during or within 2 hours after behavioral sessions (Fig. 3e), indicating that training induces a rapid elimination of SOM boutons.
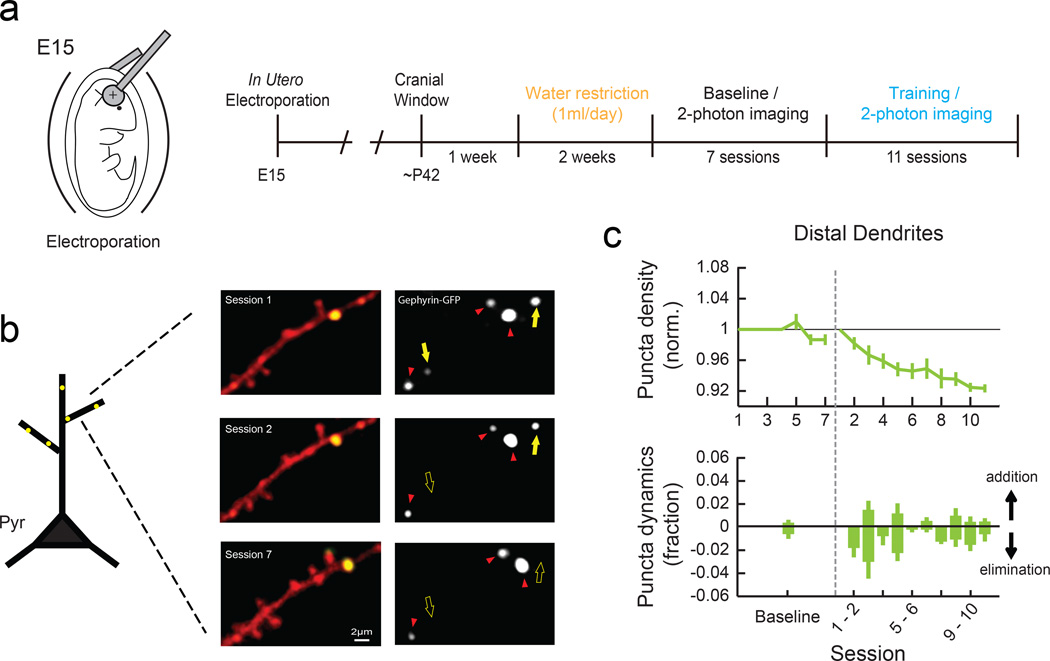
The observations of spine reorganization in distal dendritic branches and rapid loss of SOM-IN boutons during the initial phase of motor learning led us to hypothesize that the resulting reduction in dendritic inhibition creates a condition that allows learning-related changes in dendritic spines. However, SOM-INs inhibit not only excitatory neurons but also other inhibitory neuron types21, and therefore the reduction in SOM boutons does not necessitate a reduction in inhibitory synapses onto excitatory neuron dendrites. To address this issue, we expressed GFP-tagged Gephyrin, a postsynaptic scaffolding protein at inhibitory synapses, in L2/3 excitatory neurons using in utero electroporation (Fig. 6a). This allowed us to monitor the dynamics of inhibitory synapses16,17 in awake and behaving mice during motor learning. By repeatedly imaging the same distal branches of apical dendrites in L1 (Fig. 6b and Supplementary Fig. 1f), we found that GFP-positive puncta density decreased during learning, compared to untrained control (Fig. 6c). The time course and extent of Gephyrin-GFP dynamics mirrored those of SOM boutons. Thus, inhibitory synapses on distal dendrites of excitatory neurons are reduced during motor learning.
Fig. 6. Elimination of inhibitory synapses in the distal dendrites of L2/3 pyramidal neurons during learning.
(a) Schematic of in utero electroporation to express Gephyrin-GFP in neocortical L2/3 pyramidal neurons (left) and experimental timeline (right). (b) Left, Representative images of a distal dendritic branch in red with Gephyrin-GFP puncta shown in green. Right, the Gephyrin-GFP channel only. Yellow filled and open arrows indicate present and absent dynamic puncta, respectively. Red arrowheads indicate stable puncta. (c) Mean normalized Gephyrin-GFP puncta density (top) and daily dynamics (bottom) during baseline (7 sessions, n = 3 mice, 138 puncta) and learning (11 sessions, n = 4 mice, 339 puncta). For baseline, puncta dynamics from all sessions are combined. Black dotted line represents the beginning of the behavioral training. Gephyrin-GFP puncta are reduced with training (P<0.001, 2-way ANOVA with post hoc Tukey's test, compared to baseline) due to an increase in puncta elimination compared to the baseline (P<0.001, one-tailed bootstrap). Error bars indicate SEM.
Manipulation of SOM-IN activity during learning affects spine stability
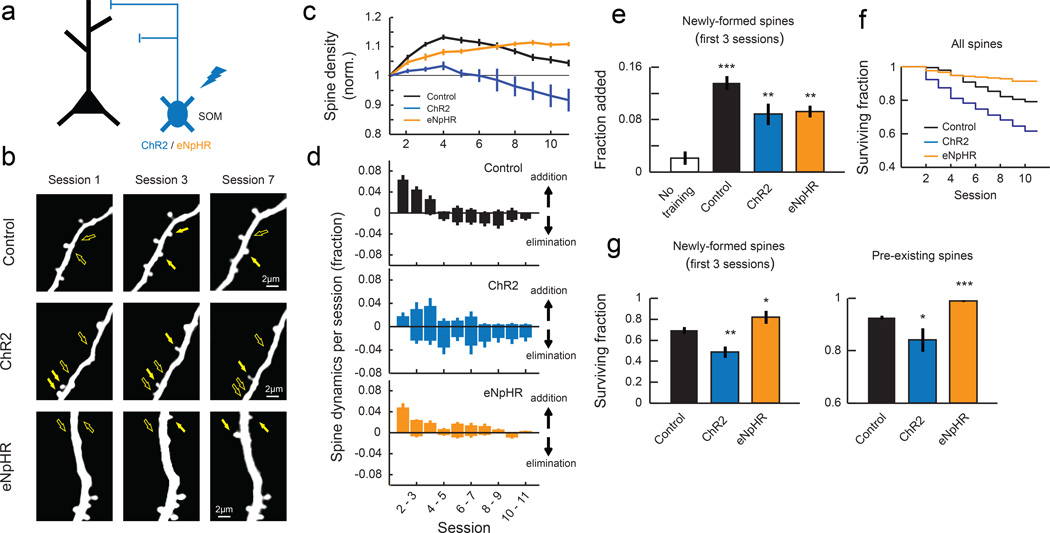
To test whether the reduced SOM inhibition during learning is essential for spine reorganization, we used optogenetics to activate SOM-INs during learning (Fig. 7a). We injected AAV encoding Cre-dependent channelrhodopsin-2 (ChR2) in SOM-Cre; Thy1-GFP-S double transgenic mice. In the Thy1-GFP-S line27, a sparse set of cortical neurons are labeled, which allows us to monitor dendritic spine dynamics without the use of Cre. We trained these mice and imaged the spines on distal dendrites in L1 daily as we mildly activated SOM-INs by delivering blue light (10 ms pulse at 3 Hz) through the imaging window during each training session (Fig. 7b and Supplementary Fig. 1c-d). This stimulation reliably evoked spiking of SOM-INs (Supplementary Fig. 4a-c), and repeated stimulation over days did not affect the survival of SOM-INs (Supplementary Fig. 4d-e). When ChR2 was activated in SOM-INs during training, a similar increase in spine formation rate was observed compared to control mice expressing tdTomato instead of ChR2 (sessions 1–3, Fig. 7d-e). However, SOM-IN activation prevented the stabilization of these learning-related nascent spines and also destabilized some of the pre-existing spines (Fig. 7f-g). As a result, the increase in spine density was abolished with SOM-IN activation (Fig. 7c). The spine dynamics were observed across multiple branches in both control and ChR2 animals (Supplementary Fig. 5). Neither ChR2 expression alone without blue light stimulation nor ChR2 stimulation without training affected spine dynamics (Supplementary Fig. 6a and d). These results suggest that the reduction in SOM-IN inhibition is an essential process regulating spine stabilization during learning.
Fig. 7. Manipulation of SOM-IN activity during training disrupts spine stability.
(a) ChR2 or eNpHR was expressed to activate or inactivate SOM-INs during training sessions, respectively. tdTomato was expressed in control animals. (b) Repeated imaging of L1 distal dendritic branches of excitatory neurons of control, ChR2, and eNpHR animals throughout learning. Filled and open arrows indicate present and absent dynamic spines, respectively. (c) Mean normalized spine density in control animals (ʽControlʼ, n = 12 mice, 665 spines), animals in which SOM-INs were activated during training (ʽChR2ʼ, n = 5 mice, 255 spines), and animals in which SOM-INs were inactivated during training (ʽeNpHRʼ, n = 6 mice, 397 spines). SOM-IN activation blocks learning-related increase of spine density (P<0.001, 2-way ANOVA with post hoc Tukey's test, compared to control), and SOM-IN inactivation extends the spine density increase (P<0.001, 2-way ANOVA with post hoc Tukey's test, compared to control). (d) Daily spine dynamics in control, ChR2, and eNpHR animals during training. (e) Training-induced spine formation in the first 3 sessions of control, ChR2, and eNpHR animals. **P<0.01, ***P<0.001, one-tailed bootstrap with Bonferroni correction compared to ʽNo trainingʼ. (f) Kaplan-Meier survival curves of all dendritic spines. Spines are less stable when SOM-INs are activated and more stable when SOM-INs are inactivated compared to control (P<0.001, log-rank test with Bonferroni correction). (g) Left, fraction of newly-formed spines in the first 3 sessions of training that remained until the end of the training. SOM-IN activation reduced the stability of learning-related new spines whereas SOM-IN inactivation hyperstabilized them. Right, fraction of pre-existing spines that remained until the end of training. *P<0.05, **P<0.01, ***P<0.001, one-tailed bootstrap test with Bonferroni correction compared to ʽControlʼ.
If the reduction in SOM-IN inhibition is essential for learning-related spine reorganization, would SOM-IN inactivation during learning further enhance spine reorganization? To address this question, we next inactivated SOM-INs during learning by injecting AAV encoding Cre-dependent halorhodopsin (eNpHR3.0) in SOM-Cre; Thy1-GFP-S mice and delivering amber light (10 ms pulse at 10 Hz) in each training session. In this condition, learning-related spine formation happened normally (Fig. 7d-e, Supplementary Fig. 6d) but spine elimination was almost completely abolished (Fig. 7d, f-g). This resulted in an increased spine density that was maintained until the end of training (Fig. 7c). Thus, spine dynamics is highly sensitive to the level of SOM inhibition.
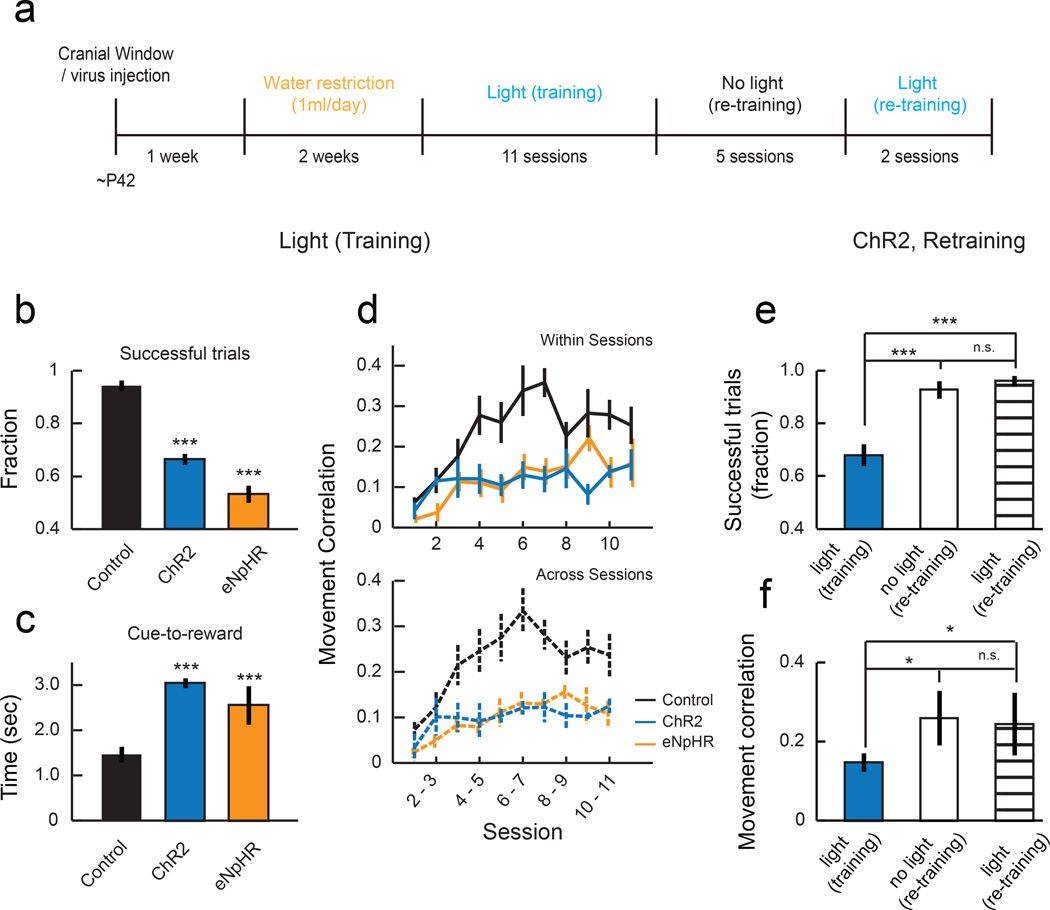
Manipulation of SOM-IN activity impairs learning
If proper spine reorganization is important for learning, it is predicted that the manipulations that changed spine dynamics also affect learning. Indeed, mice that had SOM-INs activated during training missed more trials than control mice (Fig. 8b) and took a significantly longer time to achieve the threshold for a reward from the cue onset (Fig. 8c). Furthermore, activation of SOM-INs blocked the formation of stereotyped movements, shown by the low trial-to-trial correlation of their movement kinematics throughout training (Fig. 8d). Similarly, SOM-IN inactivation also impaired motor learning, shown by a lower fraction of successful trials, longer time to achieve a reward, and lower correlation of movement kinematics (Fig. 8b-d). ChR2 expression alone without blue light stimulation had no effect on spine dynamics and learning (Supplementary Fig. 6e),
Fig. 8. Manipulation of SOM-IN activity impaired the formation of stereotyped movements.
(a) Experimental timeline. (b) Mean fractions of successful trials in sessions 7–11, showing that control animals achieved a reward in a larger fraction of trials than ChR2 or eNpHR animals. ***P<0.001, one-tailed bootstrap test with Bonferroni correction compared to ʽControlʼ. (c) Time from cue onset to achieve reward is longer in ChR2 and eNpHR animals compared to control. ***P<0.001, one-tailed bootstrap test with Bonferroni correction compared to ʽControlʼ. (d) Medians of trial-trial movement correlations. Values are lower in ChR2 and eNpHR animals, indicating their failure to form stereotyped movement patterns (within sessions: P<0.001; across sessions: P<0.001, compared to ʽControlʼ, 2-way ANOVA with post hoc Tukey's test). Error bars indicate SEM. (e) Mean fractions of successful trials of ChR2 animals in the last 2 sessions of training with light, retraining without light, and retraining with light (n = 5 mice). (f) Mean correlation of movements within sessions in all 3 conditions. Once the animals acquire the motor skill, SOM-IN stimulation did not impact the performance (P>0.1, ‘no light (re-training)’ vs. ‘light (retraining)’). *P<0.05, ***P<0.001, one-tailed bootstrap test with Bonferroni correction). Error bars indicate SEM.
The observed effects of SOM-IN manipulation on spine dynamics and learning were in stark contrast to when PV-INs were activated using the same protocol, which neither affected spine dynamics nor behavior (Supplementary Fig. 6b, d and e), indicating that the impairment of learning-related spine dynamics and motor learning was specific to SOM-IN activation. In addition, after the initial experiments, we trained the same SOM-IN ChR2 mice without blue light delivery, which led them to reach the expertise similar to control mice (Fig. 8a, e-f). Importantly, once the mice had acquired the skill, SOM-IN activation did not affect their performance (Fig. 8e-f). Thus, the effect of SOM-IN activation is specific to the acquisition of a new motor skill. Together, our results from SOM-IN activation and inactivation experiments suggest that the plasticity of SOM-INs during learning achieves the appropriate level of SOM-IN inhibition that is essential for the learning-related spine stabilization and elimination. They are also consistent with the notion that the stabilization of learning-related spines and elimination of some of the other, presumably unnecessary spines, ensured by an appropriate level of SOM inhibition, are indeed necessary for motor learning.
DISCUSSION
Previous studies showed that various sensory experience and learning paradigms cause a rearrangement of local synapses28–30. In particular, motor learning induces the establishment of new spines in the motor cortex1,10,31 which correlates with long-lasting motor memories11. Here we extend these studies by investigating spine plasticity in different dendritic compartments as well as bouton plasticity of two genetically-defined inhibitory neuron types in the motor cortex during motor learning. We find that learning-related spine plasticity occurs in L1 distal dendritic branches but not in perisomatic regions of L2/3 excitatory neurons. Coinciding with this compartment-specific spine reorganization is subtype-specific plasticity of inhibitory circuits, in which distally-targeting SOM-INs decrease their synapses in L1 and perisomatically-targeting PV-INs increase their synapses. Our results provide evidence for a novel mechanism by which subtype-specific inhibitory circuit plasticity regulates the spatiotemporal specificity of learning-related structural plasticity of excitatory synapses and thus the acquisition of motor skills.
Inhibitory control of excitatory circuit plasticity
We show that learning-related spine reorganization is restricted to the dendritic compartment which is inhibited by SOM-INs and that the number of SOM-IN boutons rapidly decreases during the initial phase of learning. Importantly, previous studies have implicated the involvement of N-methyl-D-aspartate receptor (NMDAR)-dependent LTP-like mechanisms in long-term stabilization of nascent dendritic spines in vitro32 and in motor learning in vivo33. Furthermore, the level of GABAergic inhibition can control dendritic excitability and synaptic plasticity, with more and less inhibition favoring synaptic depression and potentiation, respectively34–36. Interestingly, recent in vivo imaging studies showed that dendritic calcium events can predict the plasticity of response properties of hippocampal neurons37 and the synaptic plasticity in the motor cortex38. The latter study also showed that the branch specificity of dendritic calcium events is controlled by local inhibition. In light of these previous studies, we postulate that the reduced inhibition of distal dendrites by SOM-INs that we identify here during learning makes the local dendrites more depolarized, creating a condition that favors synaptic potentiation and the stabilization of learning-related spines.
The notion that subtype-specific inhibitory circuit plasticity regulates compartment-specific spine plasticity is an extension of previous studies showing inhibitory gating of excitatory plasticity13–20. For example, a study demonstrated that monocular deprivation induced dendritic branch retractions in L2/3 inhibitory neurons and a loss of inhibitory inputs onto neighboring pyramidal cells in the visual cortex of adult mice. It is proposed that the reduced inhibitory inputs enable excitatory plasticity to strengthen the inputs from the non-deprived eye20. Our observations on the rapid loss of SOM-IN boutons and the bidirectional effects of SOM-IN inhibition on the spine stability observed in the optogenetic manipulations further demonstrate that spine stability on distal dendrites is exquisitely sensitive to the level of SOM-IN inhibition; too much or too little SOM inhibition is detrimental to spine reorganization and motor learning. Manipulation of the activity of inhibitory neurons could in theory non-specifically affect circuit activity and plasticity. However, two lines of evidence argue against this possibility. First, SOM-IN activation after learning did not affect learned behavior (Fig. 8e-f). Second, activation of PV-INs during learning with the same protocol did not affect spine dynamics or learning (Supplementary Fig. 6). While we cannot claim that SOM-INs are the only circuit component whose manipulation affects spine dynamics and learning, these results support the unique role of SOM-INs in regulating learning-related plasticity in distal dendritic branches.
Contrary to SOM-INs, we observed a transient increase in PV-IN boutons during learning. PV-INs control the spike output of excitatory neurons by inhibiting their perisomatic regions, which are the regions we find to be relatively stable during motor learning. We speculate that this increase of PV-IN boutons is a homeostatic response to the learning-related reduction of SOM-IN inhibition and the resulting increase in the excitability of excitatory neurons. Indeed, homeostatic changes in inhibition have been found in PV-INs but not in SOM-INs39. Together, our results underscore the importance of intricate interactions between excitation and inhibition in learning-related circuit plasticity13–19.
Online Methods
Animals
All procedures were in accordance with protocols approved by UCSD institutional Animal Care and Use Committee and guidelines of the National Institute of Health. Mice were acquired from Jackson Laboratories (PV-Cre (008069), SOM-Cre (013044), Thy1-EGFP (011070)) and Charles River Laboratory (C57BL/6 wildtype). All animals before water restriction were group housed and all animals under water restriction were singly housed in disposable plastic cages with standard bedding in a reversed light cycle (12h-12h) room. Experiments were typically performed during the dark period. All animals used for imaging experiments were water restricted, regardless of whether they were trained or not.
Surgery and virus injection
Surgical procedures were performed as previously described1. Adult mice (6 weeks or order, male and female) were anaesthetized with isofluorane and injected with Baytril (10 mg/kg), dexamethasone (2 mg/kg), and buprenorphine (0.1 mg/kg) subcutaneously at the beginning to prevent infection, inflammation and discomfort. A custom head-plate was glued and cemented to the skull. Craniotomy (~3 mm) was performed over the right caudal forelimb area (300 μm anterior and 1,500 μm lateral from bregma). For the hindlimb area, craniotomy was made at 1,500 μm poster and 1,500 μm lateral from bregma. All coordinates were based on previous microstimulation experiments40–43. Sparse labeling of L2/3 neurons was achieved by injecting viral solutions in the center of craniotomy at three locations (~500 μm apart), 20–30 nL at each site (~250 μm depth). For imaging distal branches of apical dendrites of L2/3 pyramidal cells, a mixture of AAV2/1-CAG-FLEX-GFP (1:1) and AAV2/1-CMV-PI-Cre (1:5,000) diluted in saline was injected into C57BL/6 wild-type mice. For imaging perisomatic dendrites of L2/3 pyramidal cells, a more diluted mixture (AAV2/1-CAG-FLEX-GFP (1:1) and AAV2/1-CMV-PI-Cre (1:15,000)) was used for sparser labeling. For L2/3 PV or SOM axonal bouton imaging, AAV2/1-CAG-FLEX-GFP (1:100) diluted in saline was injected into PV-Cre or SOM-Cre mice, respectively. All viruses were purchased from UPenn Vector Core. Following the virus injections, a glass window was implanted over the craniotomy. The edges between the window and the skull were filled with 1.5% agarose and the window was secured with dental acrylic.
In utero electroporation
Surgical procedures were performed as previously described44. To target neocortical L2/3 neurons, timed-pregnant female C57BL/6 mice (E15) were anaesthetized with isofluorane and injected with Baytril (10 mg/kg), dexamethasone (2 mg/kg), and buprenorphine (0.1 mg/kg) subcutaneously at the beginning to prevent infection, inflammation and discomfort. Embryos were injected with ~1–2 μL of a mixture of plasmid, gephyrin-GFP (0.5 μg/μL, gift from C. Levelt) and tdTomato (2 μg/μL), into the left lateral ventricle. Five electric pulses (intensity = 40 V, duration = 50 msec, frequency = 1 Hz) were delivered, targeting the motor cortex, using a square wave electroporator (Harvard Apparatus).
Immunohistochemistry
Coronal sections (50 μm) were cut with a microtome, blocked in 4% normal goat serum in PBS, and incubated overnight at 4°C with primary antibodies diluted in blocking solution. After washing, sections were incubated in Alexa Fluor-conjugated secondary antibodies (Life Technologies) for 2 hours at room temperature, mounted, and imaged (Zeiss Axio Imager). The primary and secondary antibodies and dilutions used were: Rabbit anti-PV (1:3000, Swant, PV27), Rabbit anti-SOM (1:250, Immunostar, 20089), Alexa 350 goat-anti-rabbit (1:200, A21068).
Behavior
Two weeks after surgery, mice were water-restricted at 1 mL / day. After ~14 days of water restriction, mice were trained 1 session / day for 11 sessions under a two-photon microscope. The hardware and software used for behavioral training have been previously described1. Lever position was continuously monitored through a piezoelectric flexible force transducer. A 6-kHz tone marked the cue period (up to 30 sec in the first session, up to 10 sec in subsequent sessions) during which a successful lever-press was rewarded with water (~10 μL per trial) paired with a 500 msec, 12 kHz tone, followed by an intertrial interval (variable duration of 2 – 4 sec in the first session and 5 – 10 sec in subsequent sessions). A successful lever-press was defined as crossing of two thresholds (upper threshold ~0.5 mm, lower threshold ~1.5 mm in sessions 1 – 2, ~3.0 mm in sessions 3 – 4, and ~4.5 mm in sessions 5 – 11) within 200 msec. The upper threshold ensured that the animal did not hold the lever near the lower threshold. The lower lever thresholds were incrementally increased to encourage the learning of a novel movement. Failure of passing the thresholds during the cue period resulted in a loud white noise sound and the start of an intertrial interval. Lever presses during the intertrial interval were neither rewarded nor punished. Each animal typically performed ~100 – 150 trials / day and received ~1 mL of water reward. Each training session was terminated when the animal reached 100 successful trials or when it stopped performing. Retraining was performed 4 weeks after the initial training. Mice were trained 1 session / day for 7 sessions using the final threshold (~4.5 mm).
Movement analysis
Voltage traces from the piezoelectric lever were parsed into movement and quiescent bouts and down-sampled from 10 kHz to 1 kHz. Lever trajectories on different trials were compared from the onset of the rewarded movement to 2 sec after the onset of movement. Similarity of lever trajectories across trials was computed by Pearson correlation. Rewarded movements which started before the onset of the cue stimulus were excluded from analysis. Movement bouts were identified as previously described1; in brief, movement was detected by a velocity threshold, and the onset and offset of movement bouts were refined by the position of the lever respectively leaving or entering a stable state.
Imaging and image processing
Imaging was performed in awake mice at the beginning of each training session, and in a subset of animals, also at the end of each training session and 2 hours after the end of the training session. Images were acquired using a commercial two-photon microscope (B-Scope, Thorlabs) with a 16X objective (NIKON) with excitation at 925 nm (Ti-Sa laser, Newport) at ~28 Hz, 20 frames / plane, 80 – 120 planes per animal with a 1 μm z-axis step size. For spine imaging, distal dendritic branches were located within 100 μm from the pia surface (L1) and perisomatic dendrites were between ~150 – 250 μm (L2/3). For axonal bouton imaging, axonal branches of SOM-INs and PV-INs were located within 100 μm (L1) and at ~150 – 250 μm (L2/3) from the pia surface, respectively. For Gephyrin-GFP puncta imaging, the baseline group was a subset of mice that were later used for training, but the images were taken on separate dendritic branches. All dendritic branches for Gephyrin-GFP puncta imaging were located within 100 μm from the pia surface (L1). Images were acquired at the resolution of 512 × 512 pixels encompassing ~94 × 85 μm (distal dendrites and Gephyrin-GFP), ~54 × 40 μm (perisomatic dendrites), and ~138 × 128 μm (boutons). Distal dendrites in 4 no training mice were imaged at ~54 × 40 μm so that they could be scored blindly with the images of perisomatic dendrites. Lateral motion for each image plane (20 frames) was corrected by full-frame cross-correlation image alignment (Turboreg45 plugin in ImageJ), using the average of the five most consistent consecutive frames as the reference image. Following this lateral motion correction, all 20 frames within a plane were averaged, and different image planes were then aligned using recursive alignment of stacks of images using Stackreg (ImageJ). Represented images shown in figures are projections from 3D image stacks containing the dendritic/axonal segments of interest. The images were simply processed with linear smoothing and look-up-table adjustments for presentation using ImageJ.
Image analysis
Spine and axonal bouton dynamics were manually scored and tracked over the entire 11 sessions in three dimensions using a custom program in IGOR (J. Boyd and K. Haas, University of British Columbia). The program provides a platform for manual scoring by spatially aligning the image stacks in 3D. Dendritic spines, axonal boutons, or Gephyrin puncta were then identified, measured, and tracked in 3D stacks manually across all time points based on the published criteria17,25,46. In all the optogenetics experiments, the experimenter was blinded to the condition (opsin vs. control), and the scorer was again blinded to both the condition (opsin vs. control), behavioral result of the animal (learned, not learned, vs. not trained) as well as session numbers. For other experiments, the scorer was blinded to the session number of each image, which was randomized. This excludes the possibility of subjective bias favoring addition or elimination of spines/boutons/puncta. After the blind scoring, session numbers were revealed and scoring was corrected with the following criteria - if a spine/bouton/punctum was scored as absent in one session (session X) and present in the immediately preceding (session X-1) and following (session X+1) sessions, then it was called present on session X. Furthermore, if a spine was scored as present in one session (session X) and absent in the immediately preceding (session X-1) and following (session X+1) sessions, then it was called absent on session X. No more than one correction was applied on any given spine, bouton or punctum. If a spine/bouton/punctum contained these gaps after one correction, then it was excluded from following analyses. In total, 6.7% of spines/boutons/puncta were corrected (487 of 7215), and only 0.6% were excluded (45 of 7215). While this corrected for mistakes in scoring, we may be slightly underestimating the dynamics. The blind scoring results matched those from independent scoring of the same data set without shuffling (data not shown). The total density of spines/boutons/puncta scored in each session was normalized to the initial session. The number of mice, spine/bouton/punctum, branch number, branch length, and density analyzed in all experimental conditions are summarized in Supplementary Table 1.
Optogenetic experiments
For ChR2 or eNpHR expression in SOM-INs, AAV2/1-CAG-FLEX-ChR2-tdTomato (UPenn Vectore Core) or AAV2/1-CAG-FLEX-eNpHR3.0-mCherry (Neurophotonic Center, University of Lavel), respectively, was injected at five sites (~500 μm apart), 100 nL at each site (~250 μm depth) in the right caudal forelimb area of SOM-Cre:Thy1-EGFP mice. For ChR2 expression in PV-INs, AAV2/1-CAG-FLEX-ChR2-tdTomato was injected at five sites (~500 μm apart), 100 nL at each site (~250 μm depth) in the right caudal forelimb area of PV-Cre:Thy1-EGFP mice. Control mice of the same genotype were injected with AAV2/1-CAG-FLEX-tdTomato (UPenn Vector Core). Experimenters were blinded to the virus prior to surgeries and each set of experiments contained both opsin and tdTomato animals. Two weeks after the surgery, animals underwent water restriction at 1 mL / day for 14 days. Images of dendritic spines were acquired at the beginning of each behavior session. For ChR2 experiments, blue light pulses from an LED (10 msec pulses, 3 Hz, ~40 mW, 470 nm, Doric Lenses) were delivered directly onto the center of the glass window throughout each behavior session (~30 min). For eNpHR experiments, amber light pulses (10 msec pulses, 10 Hz, ~25 mW, 590 nm, Doric Lenses) were delivered. Control mice received either blue or amber light stimulation in each behavior session and animals were pooled together for analysis. The light power was measured at the tip of the optic fiber (~200 μm in diameter) using a laser power meter.
Two-photon guided cell-attached recording
ChR2-expressing SOM-IN mice with an imaging window were prepared as above. On the day of the experiment, mice were anaesthetized under isofluorane and the glass window was removed. The animals were anesthetized with ketamine (100 mg/kg of body weight) and head-fixed under a two-photon microscope. Loose-patch recordings were performed with glass pipettes (~5 – 7 MΩ) filled with 200 μM Alexa Fluor 488 in saline. ChR2-tdTomato expressing SOM-INs were identified and targeted for recording. Signals were amplified 100–500X by the Axon CNS amplifier (Molecular Devices), filtered at 2 kHz, and recorded using Ephus (Matlab) at 10 kHz. In all 15 targeted neurons, blue light stimulation (10 msec pulses, 3 Hz) was applied with alternating blocks of 5 sec baseline and 5 sec stimulation for up to 20 min. In one of these neurons, after 10 min of this initial recording, blue light stimulation (10 msec pulses, 3 Hz) was applied continuously for additional 10 min.
Statistical analyses
All data points are presented as mean ± s.e.m except in supplementary Fig. 5a, data points are presented as median. No statistical tests were used to predetermine sample sizes but our sample sizes are similar to other previously reported studies14,47. Data in all optogenetics experiments were collected and analyzed blind to the condition of the experiments. In other experiments, only the analyses were performed in blind conditions. Detailed descriptions on the blinding procedure are in the ‘Image Analysis’ section. No specific randomization was used for data collection and analyses, but animals were assigned to each experiment without any bias, and both genders were used in all experiments. Data distribution was assumed to be normal in all experiments but this was not formally tested, and all statistical analyses were performed with Matlab. Statistical significance was determined by bootstrap test, one-way, or two-way ANOVA with post hoc corrections and two-tailed tests were used for all comparisons unless indicated.
Supplementary Material
Acknowledgements
We thank C. Levelt for the Gephyrin-GFP construct, B. Bloodgood, R. Malinow and members of the Komiyama lab for comments and discussions. This work was supported by grants from Japan Science and Technology Agency (PRESTO), Pew Charitable Trusts, Alfred P. Sloan Foundation, David & Lucile Packard Foundation, Human Frontier Science Program, McKnight Foundation, NIH (R01 NS091010A), UCSD Center for Brain Activity Mapping and New York Stem Cell Foundation to TK. SXC is a Human Frontier Science Program postdoctoral fellow. AJP is supported by the Neuroplasticity of Aging Training Grant (AG000216). TK is a NYSCF-Robertson Investigator.
Footnotes
Supplementary Information is linked to the online version of the paper.
Author Contributions SXC and TK conceived the project. AJP and TK developed the task. ANK performed the PV-IN experiments. AJP performed the in vivo cell-attached recordings. All other experiments were performed by SXC with assistance by ANK. SXC and TK analyzed the data with assistance by AJP and wrote the manuscript with inputs from AJP and ANK.
References
- 1.Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- 2.Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 3.Huber D, et al. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 6.Komiyama T, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 7.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 8.Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007;54:653–666. doi: 10.1016/j.neuron.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci. 2013;16:1340–1347. doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 13.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 14.Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 15.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 16.Chen JL, et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Versendaal D, et al. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 2012;74:374–383. doi: 10.1016/j.neuron.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlman SJ, et al. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JL, et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nature neuroscience. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi H, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Yang G, et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng G, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 28.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 29.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–490. doi: 10.1038/nrn3258. [DOI] [PubMed] [Google Scholar]
- 31.Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill TC, Zito K. LTP-induced long-term stabilization of individual nascent dendritic spines. J Neurosci. 2013;33:678–686. doi: 10.1523/JNEUROSCI.1404-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan MT, et al. Role of motor cortex NMDA receptors in learning-dependent synaptic plasticity of behaving mice. Nat Commun. 2013;4:2258. doi: 10.1038/ncomms3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayama T, et al. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat Neurosci. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gidon A, Segev I. Principles governing the operation of synaptic inhibition in dendrites. Neuron. 2012;75:330–341. doi: 10.1016/j.neuron.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Steele PM, Mauk MD. Inhibitory control of LTP and LTD: stability of synapse strength. Journal of neurophysiology. 1999;81:1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- 37.Sheffield ME, Dombeck DA. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 2015;517:200–204. doi: 10.1038/nature13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520:180–185. doi: 10.1038/nature14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li CX, Waters RS. Organization of the mouse motor cortex studied by retrograde tracing and intracortical microstimulation (ICMS) mapping. Can J Neurol Sci. 1991;18:28–38. doi: 10.1017/s0317167100031267. [DOI] [PubMed] [Google Scholar]
- 41.Pronichev IV, Lenkov DN. Functional mapping of the motor cortex of the white mouse by a microstimulation method. Neurosci Behav Physiol. 1998;28:80–85. doi: 10.1007/BF02461916. [DOI] [PubMed] [Google Scholar]
- 42.Ayling OG, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods. 2009;6:219–224. doi: 10.1038/nmeth.1303. [DOI] [PubMed] [Google Scholar]
- 43.Tennant KA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- 45.Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 46.Holtmaat A, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nature neuroscience. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.